ABSTRACT
Background: Omicron had swept the mainland China between December 2022 and January 2023, while SARS-CoV-2 still continued to evolve. To fully prepare for the next wave, it’s urgent to evaluate the humoral immune response post BA.5/BF.7 breakthrough infection against predominant sub-lineages among existing vaccination strategies and the elders. Method: This study enrolled a longitudinal young-adult cohort from 2/3-dose vaccination to 1 month after breakthrough infection, and an elder cohort at 1 month after breakthrough infection. Seral samples were collected and tested for humoral immune response to SARS-CoV-2 subvariants including WT, BA.2, BA.5, BF.7, BQ.1.1, CH.1.1, XBB.1.5. Results: BA.5/BF.7 breakthrough infection induced higher neutralization activity than solely vaccination in all SARS-CoV-2 strains, while the latest Omicron subvariants, BQ.1.1, CH.1.1, XBB.1.5, exhibited the strongest neutralization evasion ability. There was a negative correlation between age and humoral immune response in WT, BA.5, BQ.1.1, and XBB.1.5. Compared to non-vaccination groups, breakthrough infection in two-dose vaccination groups had significantly higher neutralizing antibody against WT, BA.2, BA.5, BF.7 but not to BQ.1.1, CH.1.1, XBB.1.5 while booster dose against the prototype prior-breakthrough would not further significantly enhance individual’s humoral responses against the latest Omicron subvariants. Conclusions: Newer variants manifest increasing immune evasion from neutralization and repeated prototype-based booster vaccines may not further enhance neutralizing antibody against emerging new variants. Older adults have lower levels of neutralizing antibody. Future vaccination strategies should aim to enhance effective neutralization to contemporary variants.
KEYWORDS: Omicron, SARS-CoV-2, humoral immune, breakthrough infection, vaccination
Introduction
Since the late 2019, the novel coronavirus, known as SARS-CoV-2, has rapidly spread across the globe [1]. Since November 2021, the Omicron strain has currently become the predominant variant of concerns of SARS-CoV-2 in most countries. The Omicron variant kept mutating during the past years and the subvariants including BA.1, BA.2, BA.4, BA.5, BF.7, etc. as well as the latest XBB.1.5 swept the world, causing thousands of deaths and huge loss. It’s necessary to keep evaluating risk population and the efficacy of existing vaccination strategies against the latest Omicron subvariants.
At the beginning of 2021, the Chinese government started to encourage the public to get vaccinated against SARS-CoV-2 in China. As of January 13th 2023, a total of 3,488.05 million doses of COVID-19 vaccines have been administered [2]. During December 2022 and January 2023, large amounts of residents in China experienced BA.5/BF.7 breakthrough infections within a short period of time, with a total of 99,229,372 confirmed cases reported according to the World Health Organization [3]. Currently, most patients have already recovered from the illness.
CoronaVac and BBIBP-CorV are two inactivated virus vaccines developed in China, which have demonstrated good efficacy in protecting against COVID-19 [4,5]. Both vaccines have been approved for use in several countries and regions to combat the COVID-19 pandemic. In a vaccine efficacy study conducted in Argentina on elderly individuals aged 60 and above, it was found that after receiving two doses of BBIBP-CorV, the vaccine had an 85.0% preventive effect against death [6]. In 2021, a clinical study on the safety and immunogenicity of a third booster dose of inactivated whole-virus particle vaccines (CoronaVac or BBIBP-CorV) in healthy adults suggested that booster vaccination can provide additional protection in the next 6 months [7]. Two-dose inactivated vaccines with or without homologous or heterologous booster doses have become the main vaccination strategies for Chinese residents during the past 2 years. Evaluating the effect of Chinese vaccine strategies on humoral response after BA.5/BF.7 breakthrough infection can provide significant evidence for disease control and prevention.
Several studies have suggested that advanced age was the risk factor for infection and illness progression in SARS-CoV-2, both in wild-type and Omicron subvariants [8–10]. Chen et.al suggested that advanced age would lead to longer virus nucleic acid positivity duration and higher severity rate [11]. Timothy et.al raised that age negatively correlates with antibody response after vaccination alone, but no correlation in breakthrough infection or hybrid immune group among participants who received mRNA vaccines [12]. A nationwide study in the United Kingdom demonstrated the rate of reinfection for the elder is 15.86%, two times higher than 6.46% for the younger [13], and the consequences of reinfection in elderly patients are more serious [14–17]. To protect this fragile population from repeated infection and illness progression, there is an urgent need to evaluate the immune response of the elders against new subvariants after BA.5/BF.7 breakthrough infection and work on future vaccination strategies.
Based on the synchronous vaccination and infection background above, this study focused on the individual humoral response before and after the Omicron BA.5/BF.7 breakthrough infection during December 2022 and January 2023 in China, among different vaccination strategies and different aged groups. A longitudinal young-adult cohort from 2-dose or 3-dose vaccination to breakthrough infection and another elder cohort 1 month post breakthrough infection was enrolled into this study. Neutralization efficacy was evaluated using pseudovirus neutralization assays as previously described [18].
Method
Study design and sample collection
Participants in this study were infected with Omicron BA.5/BF.7 subvariants during the wave between December 2022 and January 2023 in Shanghai, China and formed into two cohorts, the young-adult cohort and the elder cohort. The young-adult cohort enrolled participants <65yrs while the elder cohort ≥65yrs. Patients who had autoimmune disease, organ failure (liver, kidney, respiratory, etc.), active cancer, long-term bedridden, etc. were excluded. Baseline information, SARS-CoV-2 infection history and severity, vaccination history as well as current health status were collected through questionnaire. Homogenous booster vaccination was defined as 3-dose inactivated vaccines. Heterogenous booster vaccination was defined as recombinant protein subunit vaccine booster following two-dose inactivated vaccines, as previously reported [7].
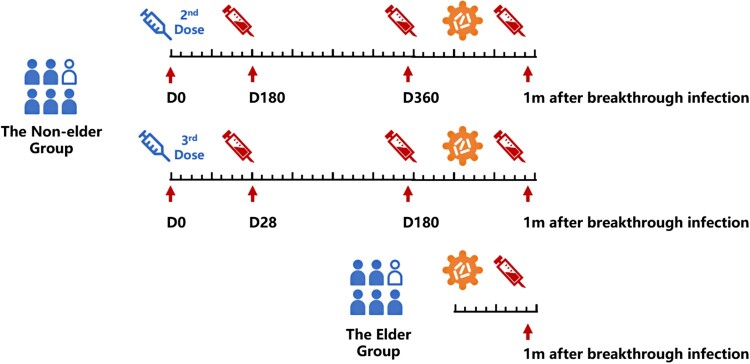
Peripheral blood samples were collected from individuals at Huashan Hospital, Tongren Hospital and Community Health Service of Qingpu District in Shanghai. For those received 3-dose vaccination in the young-adult cohort, serial seral samples were collected at day 0, 28, 180 after the third vaccination and 1 month after breakthrough infection. For those who received 2-dose vaccination in the young-adult cohort, serial seral samples were collected at day 180, 360 after the second vaccination and 1 month after breakthrough infection. For the elder cohort, seral samples were only collected at 1 month after breakthrough infection. Seral was isolated after centrifuged with 3000 rpm 10 min and stored at −80°C (Figure 1).
Figure 1.
Study design and sample collection.
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the Ethics Committee of Huashan Hospital (KY2022-596).
Cell lines
HEK293T cells (Cat# CRL-3216), Vero E6 cells (cat# CRL-1586) were from ATCC and cultured in 10% Fetal Bovine Serum (FBS, GIBCO cat# 16140071) supplemented Dulbecco’s Modified Eagle Medium (DMEM, ATCC cat# 30-2002) at 37°C, 5% CO2. I1 mouse hybridoma cells (ATCC, cat# CRL-2700) were cultured in Eagle’s Minimum Essential Medium (EMEM, ATCC cat# 30-2003) with 20% FBS.
Construction and production of variant pseudoviruses
Plasmids encoding the WT (D614G) SARS-CoV-2 spike and Omicron sub-lineage spikes were constructed. HEK293 T cells were transfection with the indicated spike gene using Polyethylenimine (Polyscience). Cells were cultured overnight at 37°C with 5% CO2 and VSV-G pseudotyped ΔG-luciferase (G*ΔG-luciferase, Kerafast) was used to infect the cells in DMEM at a multiplicity of infection of 5 for 4 h before washing the cells with 1 × DPBS three times. The next day, the transfection supernatant was collected and clarified by centrifugation at 3000 g for 10 min. Each viral stock was then incubated with 20% I1 hybridoma (anti-VSV-G; ATCC, CRL-2700) supernatant for 1 h at 37°C to neutralize the contaminating VSV-G pseudotyped ΔG-luciferase virus before measuring titres and making aliquots to be stored at −80°C.
Pseudovirus neutralization assays
Neutralization assays were performed by incubating pseudoviruses with serial dilutions of sera, and scored by the reduction in luciferase gene expression. In brief, Vero E6 cells were seeded in a 96-well plate at a concentration of 2 × 104 cells per well. Pseudoviruses were incubated the next day with serial dilutions of the test samples in triplicate for 30 min at 37°C. The mixture was added to cultured cells and incubated for an additional 24 h. The luminescence was measured by Luciferase Assay System (Beyotime). ID50 was defined as the dilution at which the relative light units were reduced by 50% compared with the virus control wells (virus + cells) after subtraction of the background in the control groups with cells only. The ID50 values were calculated using nonlinear regression in GraphPad Prism (Supplementary Figure).
Id50 cumulative distribution analysis
The ID50 cumulative distributions of different sub-lineages were determined by the proportion of samples at or above a given titre at different infection statuses. The max ID50 on the Cumulative Distribution figure was assumed as 1.1-fold of the maximum titre for each sub-lineage.
Quantitative and statistical analysis
The statistical analyses for the pseudovirus virus neutralization assessments were performed using GraphPad Prism for the calculation of mean value and SEM for each data point. Each specimen was tested in triplicate. Antibody neutralization IC50 values were calculated using a five-parameter dose–response curve in GraphPad Prism. Geometric mean and 95% CI were calculated for neutralization results. Spearman rank test was performed to describe the relationship between age and neutralization results after logarithmization. Kruskal–Wallis test with Dunn’s multiple comparison correction was performed comparing the neutralization IC50 values. Adjusted two-tailed p values are reported. No statistical methods were used to determine whether the data met the assumptions of the statistical approach.
Result
Baseline characteristics of study participants
A total of 134 participants were finally enrolled and tested for pseudovirus neutralization assay, including 53 young adults and 81 elders. In the young-adult cohort, 21 (39.6%) were male and the median age was 33 (IQR: 27-42), with 12 (22.6%) having a smoking habit, excessive drinking, or obesity and 51 (96.2%) receiving at least 2 dose vaccination prior to breakthrough infection. In the elder cohort, 46 (56.8%) were male and the median age was 80 (IQR: 69-88). Hypertension (40 [49.4%]), heart conditions (40 [36.4%]), and cerebrovascular disease (23 [28.4%]) were the most common comorbidities. Forty (49.4%) received at least 2 doses before breakthrough infection (Table 1).
Table 1.
Baseline characteristics of study participants one month after breakthrough infection.
| All | The young-adult Cohort | The Elder Cohort | |||
|---|---|---|---|---|---|
| All the elders | Fully vaccinated | Not–fully Vacccinated | |||
| N | 134 | 53 | 81 | 40 | 41 |
| Gender, male (%) | 67 (50) | 21 (39.6) | 46 (56.8) | 21 (52.5) | 25 (61) |
| Age, median (IQR) | 69 (39–81) | 33 (27–42) | 80 (69–88) | 75.5 (69–81.25) | 83 (69–91) |
| Comorbidities, N (%) | 87 (64.9) | 12 (22.6) | 75 (92.6) | 36 (90) | 39 (95.1) |
| Smoke, N (%) | 16 (11.9) | 3 (5.7) | 13 (16) | 10 (25) | 3 (7.3) |
| Alcohol Abuse, N (%) | 12 (9) | 3 (5.7) | 9 (11.1) | 8 (20) | 1 (2.4) |
| Obesity BMI > 28, N (%) | 11 (8.2) | 6 (11.3) | 5 (6.2) | 3 (7.5) | 2 (4.9) |
| hypertension, N (%) | 45 (33.6) | 5 (9.4) | 40 (49.4) | 24 (60) | 16 (39) |
| Heart Conditions, N (%) | 36 (26.9) | 0 (0) | 36 (44.4) | 17 (42.5) | 19 (46.3) |
| Cardiovascular Disease, N (%) | 23 (17.2) | 0 (0) | 23 (28.4) | 10 (25) | 13 (31.7) |
| Chronic Lung Disease, N (%) | 6 (4.5) | 0 (0) | 6 (7.4) | 2 (5) | 4 (9.8) |
| Vaccination ≥ 2doses, N (%) | 91 (67.9) | 51 (96.2) | 40 (49.4) | 40 (100) | 0 (0) |
| Heterogenous Booster | – | 21 (39.6) | – | – | – |
| Homogenous Booster | – | 19 (35.8) | – | – | – |
| Median (IQR) Days From Last Vaccination to Breakthrough Infection | 384.5(126.75–490) | 481(380–500) | 172(66–243) | 182(79–228.5) | 124.5(31.5–267.75) |
| WT, GMT [95%CI] | 489 (383–623) | 1182 (950–1470) | 274 (199–378) | 745 (560–991) | 104 (71–151) |
| BA.2, GMT [95%CI] | 726 (601–877) | 717 (561–915) | 733 (558–962) | 1071 (770–1490) | 506 (335–765) |
| BA.5, GMT [95%CI] | 585 (478–716) | 716 (569–901) | 513 (381–692) | 744 (530–1046) | 357 (222–574) |
| BA.7, GMT [95%CI] | 503 (427–593) | 570 (469–692) | 464 (364–592) | 605 (467–782) | 358 (239–537) |
| BQ.1.1, GMT [95%CI] | 236 (193–289) | 353 (267–467) | 181 (138–238) | 233 (172–314) | 142 (91–223) |
| CH.1.1, GMT [95%CI] | 258 (209–318) | 196 (154–250) | 308 (227–420) | 303 (208–441) | 314 (190–519) |
| XBB.1.5, GMT [95%CI] | 157 (132–187) | 222 (178–277) | 125 (99–159) | 149 (113–196) | 106 (72–156) |
Abbreviations: IQR: Interquartile Rangel; WT wild type; GMT: Geometric Mean Titre; CI: Confidence Interval.
Dynamic humoral responses increases post vaccination and breakthrough infection
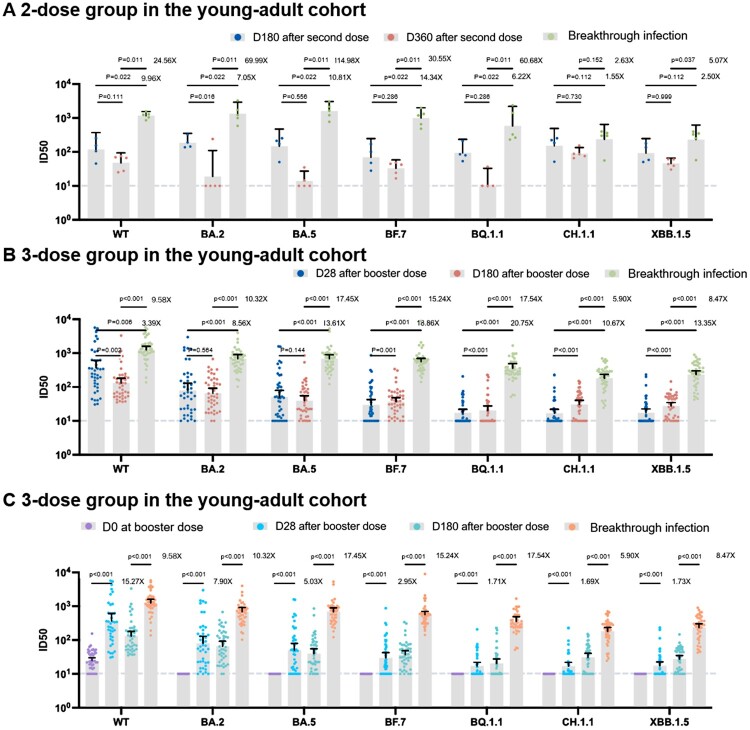
We analysed the serial neutralizing responses among 53 individuals from the young-adult cohort. For the two-dose vaccination group, we calculated the ratio of the geometric mean fold rise (GMFR) for the geometric mean titres (GMTs) at 1 month after breakthrough infection to 360 days after the second dose. The GMFRs against the WT, BA.2, BA.5, BF.7, BQ.1.1, CH.1.1, and XBB.1.5 variants varied between 2.63 and 114.98 fold (Figure 2(A), supplement table 1). For the booster dose vaccination group, we calculated the GMFR of the GMTs at 1 month after breakthrough infection to 180 days after the booster dose. The neutralization GMTs against the WT, BA.2, BA.5, BF.7, BQ.1.1, CH.1.1, and XBB.1.5 was approximately 5.90–17.54 fold. BA.5/BF.7 breakthrough infection significantly enhanced the neutralization activities in both 2 vaccination strategies (Figure 2(B), supplement table 1).
Figure 2.
Humoral response following vaccination alone and breakthrough infection in the young-adult cohort. (A) Humoral response following 2-dose vaccination alone and breakthrough infection (B) Humoral response following 3-dose vaccination alone and breakthrough infection. (C) The ratio of geometric mean fold rise (GMFR) for the geometric mean titres (GMTs) of booster vaccination and breakthrough infection. Dashed Line: The Lower Limit of Detection.
We then compared the increases in GMFR of the GMTs between breakthrough infection and booster vaccination groups. We found that the GMFR against BA.5, BF.7, BQ.1.1, CH.1.1, and XBB.1.5 was significantly increased after the breakthrough infection compared to that after the booster dose, indicating that BA.5/BF.7 breakthrough infection could stimulate a much higher humoral response than the booster vaccination against prototyped strain among Omicron strains, especially the latest subvariants BQ.1.1, CH.1.1, and XBB.1.5 (Figure 2(C), supplement table 1).
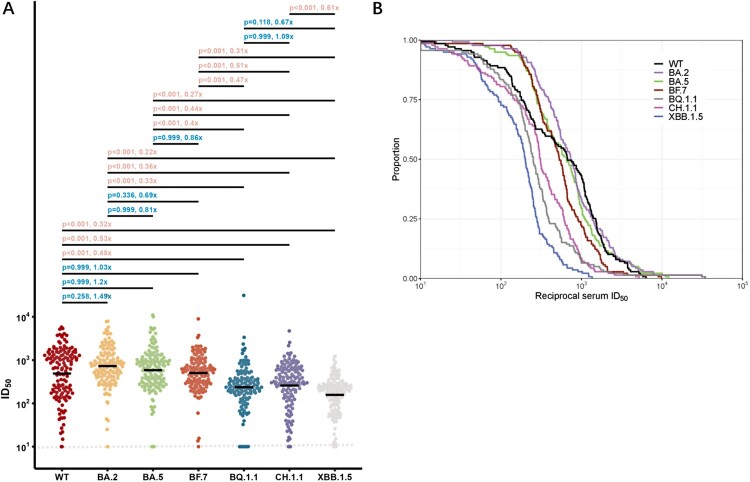
Immune escape among different Omicron subvariants
To compare the neutralization evasion abilities of Omicron subvariants, we integrated the data of neutralizing titre for WT and Omicron subvariants from all participants with BA.5/BF.7 breakthrough infections, and summarized here in Figure 3. We performed a statistical comparison of the resistance of these emerging Omicron subvariants to seral samples from 134 individuals who had more recently had BA.5/BF.7 breakthrough infections. Near all sera from the Omicron BA.5/BF.7 breakthrough infection had neutralizing antibody titres above LOD (Limit of detection). Most of the participants had fairly high neutralizing titres for WT and Omicron subvariants, which could be attributed to the high antigenic similarity of BA.5/BF.7 as well as the short sample collection time post-infection. We observed BA.5/BF.7 breakthrough infection induced similar degree of neutralizing titres against WT, BA.2, BA.5, and BF.7, with no significant difference. The GMTs for BA.5 and BF.7 were all above 400, even for the BA.2 or BA.5 descendant viruses, such as BQ.1.1, CH.1.1, and XBB.1.5 exhibited significant evasion from the BA.5/BF.7 breakthrough infection sera, the GMTs were all above 100 or higher (Figure 3A). Overall, these results indicated that most people infected in the current wave in China should have certain protection against the currently circulating and newly-emerging SARS-CoV-2 viruses. We also conducted cumulative distribution analysis utilizing data from all the BA.5/BF.7 breakthrough infection sera. The decrease trends of curve are similar between WT, BA.2, BA.5, and BF.7. On contrast, BQ.1.1, CH.1.1, and XBB.1.5 shifting to the left, which further indicated the enhanced neutralization evasion potency (Figure 3(B)).
Figure 3.
Neutralization of the WT and Omicron subvariants at 1 month after BA.5/BF.7 breakthrough infection. (A) Neutralization of pseudotyped WT and Omicron sub-lineage viruses at 1 month after breakthrough infection with Omicron BA.5/BF.7. P value was compared using Kruskal–Wallis test with Dunn’s multiple comparison correction and the fold-change was calculated by comparing the left geometric mean to the right one. (B) Cumulative distribution function plots of titres against WT and Omicron subvariants. The proportion indicated samples at or above a given titre. Dashed Line: The Lower Limit of Detection.
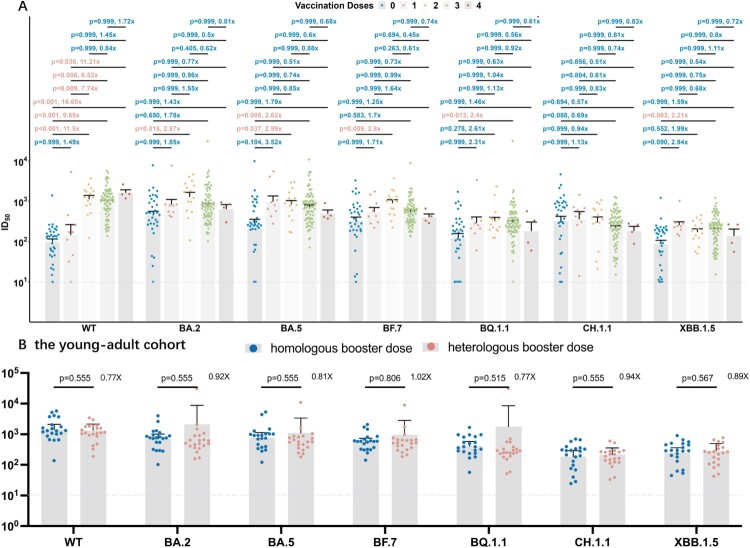
Correlation between different vaccination strategies and post breakthrough humoral responses
To figure out the efficacy of different vaccination doses and booster strategies after BA.5/BF.7 breakthrough infection, 134 participants were grouped according to the doses (Figure 4(A)), and individuals who received 3 doses in the young-adult cohort were further grouped according to homologous or heterologous booster vaccination (Figure 4(B)).
Figure 4.
Correlation between vaccination doses and strategies and neutralization antibodies against WT strain and the Omicron subvariants by breakthrough infection sera. Correlation between vaccination doses and neutralization antibodies. P value was compared using Kruskal–Wallis test with Dunn’s multiple comparison correction and the fold-change was calculated by comparing the left geometric mean to the right one. Correlation between homologous and heterologous booster strategies and neutralization antibodies in the young-adult cohort. P-value was compared using Kruskal–Wallis test with Dunn’s multiple comparison correction and the fold-change was calculated by comparing the left geometric mean to the right one. Dashed Line: The Lower Limit of Detection.
A significant increase of reciprocal ID50 against WT was observed after 2, 3, 4-dose vaccination, suggesting a satisfying efficacy for vaccination against wild-type SARS-CoV-2 strain. 2-dose vaccination could improve the neutralization titres compared with unvaccinated individuals in early Omicron subvariants including BA.2, BA.5, BF.7 but not in the latest variants, BQ.1.1, CH.1.5, XBB.1.5, while additional booster vaccination, neither homologous or heterologous, cannot further enhance the neutralizing responses after BA.5/BF.7 breakthrough infection (Figure 4(A,B)).
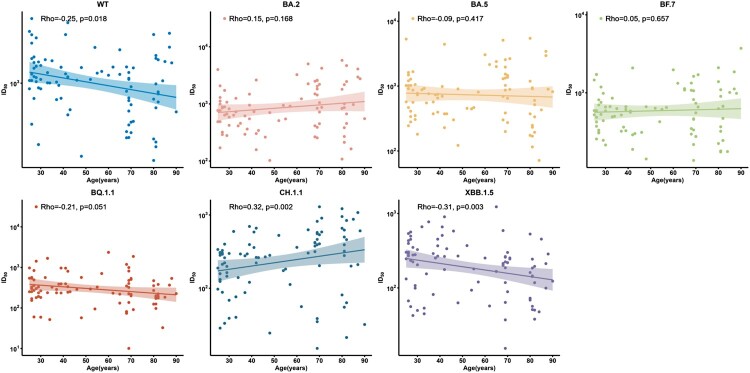
Correlation between age and humoral responses to different SARS-CoV-2 strains
We next analysed the correlation between neutralization titres and age using Spearman rank test among 91 individuals with at least 2-dose vaccination against WT and Omicron subvariants. We observed that the reciprocal seral ID50 were negatively correlated to age in WT (rho = −0.25, p = 0.018), BA.5 (rho = −0.09, p = 0.417), BQ.1.1 (rho = −0.21, p = 0.051), and XBB.1.5 (rho = −0.31, p = 0.003) subvariants, while CH.1.1 showed a contrary trend (rho = 0.32, p = 0.002) (Figure 5), indicating that the elder were still more vulnerable after BA.5/BF.7 breakthrough infection.
Figure 5.
Correlation between age and neutralization antibodies against WT strain and the Omicron subvariants at 1 month after BA.5/BF.7 breakthrough infection among full vaccination subgroup. 91 participants with full vaccination were included for analysis. Spearman rank correlation coefficients and two-tailed P-values were shown at the left upper.
Discussion
This study evaluated the dynamic humoral responses against WT and Omicron subvariants after different vaccination regimens and breakthrough infections. Overall, BA.5/BF.7 breakthrough infection post vaccination induced higher neutralization activity than sole vaccination in all SARS-CoV-2 strains, while the latest Omicron subvariants, BQ.1.1, CH.1.1, XBB.1.5 exhibited the strongest neutralization evasion. There was a negative correlation between age and seral neutralizing activities. Compared to non-vaccination groups, full vaccination could significantly increase neutralizing antibodies against WT, BA.2, BA.5, BF.7 but not to BQ.1.1, CH.1.1, XBB.1.5 after BA.5/BF.7 breakthrough infection. But as the Omicron strains continuously mutate, heterologous or homologous booster dose against the original strain prior-breakthrough would not further significantly enhance individual’s humoral responses against the latest Omicron subvariants.
Omicron breakthrough infections can elicit potent, broad, and durable neutralizing antibody responses against all SARS-CoV-2 subvariants than vaccination alone [19–22]. This phenomenon was also observed in the latest SARS-CoV-2 subvariants BQ.1.1, CH.1.1, and XBB.1.5, suggesting a similar immunogenicity during virus evolution. However, our data also indicated a much lower humoral neutralizing capacity against BQ.1.1, CH.1.1, and XBB.1.5 than the prototype [22–24]. The continuous immune evasion along with virus mutation was in accordance with an increased risk of reinfection during Omicron infection [25]. This highlighted the importance to keep dynamic surveillance on virus mutation and antigen shift.
Instead of focusing on vaccination or breakthrough infection alone in previous studies [26–28], this study focused on the neutralization status at the recovery stage among different ages and vaccination doses. The elders were at high risk for SARS-CoV-2 infection and illness progression [11]. Previous studies have identified that humoral neutralizing activities decayed with aging after vaccination alone [12,29–31]. Our study showed that even after breakthrough infection, the neutralizing antibody titre also decreased along with aging among most subvariants including XBB.1.5 [32], explaining the clinical phenomenon that the elder are still more vulnerable to reinfection than the younger. As the variant continuously to circulate globally, it’s necessary to protect these fragile population from repeated infections and vaccine selection is the most critical step.
Vaccination can enhance the neutralization activities after BA.5/BF.7 breakthrough infection against most subvariants including WT, BA.2, BA.5, BF.7 but not to BQ.1.1, CH.1.1, XBB.1.5 [19,33], proving the effectiveness of our vaccination strategies during the past 2 years. However, booster doses, neither homologous or heterologous, failed to provide further protection as new variants emerged [34,35]. The phenomenon could be possibly explained by the “original antigenic sin” doctrine previously described in influenza [34]. Repeated booster doses against wild type might not continue to provide effective immune response on the latest Omicron subvariants and new vaccine strategies based on antigens and sequences should be carefully selected. Interestingly, we noted a significant difference in neutralization activities between D28 and D180 in 3-dose group with D180 slightly higher than D28 in BF.7, BQ.1.1, CH.1.1, and XBB.1.5. However, despite the statistical significance, the distance between the average GMT of these 2 groups was not far, which was not considered clinically significant for stronger protection (supplement table 1).
This study used VSV pseudovirus to detect neutralization activity, which was widely applied in our previous study [18,36,37]. There are also two backbones pseudovirus systems that simultaneously used in the neutralization assays for SARS-CoV-2 [38,39]. In reference studies, there was no significant difference between the two pseudovirus systems. This study didn’t collect nasopharyngeal swabs during this wave. The BA.5/BF.7 wave was defined according to previously published data reporting the primary endemic subvariants between December 2022 and January 2023 [40]. Besides, the significant difference in vaccine doses among two groups might lead to bias when analysing the correlation between vaccine and neutralization activities. However, our study has proved that age and vaccine doses would not influence the titres against most Omicron subvariants, while vaccine effect against WT has been proved by previous studies [12,21]. In future, all the participants will be followed up to 12 months persistently after the breakthrough infection, to further assess the attenuation of neutralization activities, as well as the incidence of reinfection and post-acute sequelae of SARS-CoV-2 infection (PASC).
Overall, our data highlighted the need for exploring new vaccination strategies, as the efficacy of booster vaccine regimen based on prototype is continuously weakened against the emerging new variants like CH.1.1 and XBB.1.5. Additionally, although breakthrough infection can boost humoral responses against all variants, elder groups would still be more susceptible for reinfections. As SARS-CoV-2 variants continue to emerge and evolve, understanding the interplay between different vaccine-regimen efficacy and virus evolution will be crucial for optimizing future vaccination strategies among different populations.
Supplementary Material
Acknowledgements
We thank all the patients who volunteered for this trial and the study site personnel for their contributions.
Funding Statement
This work is supported by the Shanghai Municipal Science and Technology Major Project [HS2021SHZX001], Shanghai Science and Technology Committee [21NL2600100, 20dz2260100], National Natural Science Foundation of China [92169212, 82041010], Research Fund of Shanghai Tongren Hospital, Shanghai Jiaotong University School of Medicine [TRYJ2021LC10], the National Natural Science Foundation of China [32270142 to P.W], Shanghai Rising-Star Program [22QA1408800 to P.W.], National Key Research and Development Program of China [2022YFC2009801].
Note
Overall, our data highlighted the need for exploring new vaccination strategies, as the efficacy of booster vaccine regimen based on prototype is continuously weakened against the emerging new variants like CH.1.1 and XBB.1.5. Additionally, although breakthrough infection can boost humoral responses against all variants, elder groups would still be more susceptible for reinfections. As SARS-CoV-2 variants continue to emerge and evolve, understanding the interplay between different vaccine-regimen efficacy and virus evolution will be crucial for optimizing future vaccination strategies among different populations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.China CDC . The vaccination status against SARS-CoV-2 in China. [cited 2023 Jul 2]. Available from: https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_12208/202301/t20230114_263378.html.
- 3.People’s Republic of China . WHO Coronavirus Disease (COVID-19) Dashboard with vaccination data. [cited 2023 Apr 11]. Available from: https://covid19.who.int.
- 4.Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet Lond Engl. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva-Valencia J, Soto-Becerra P, Escobar-Agreda S, et al. Effectiveness of the BBIBP-CorV vaccine in preventing infection and death in health care workers in Peru 2021. Travel Med Infect Dis. 2023;53:102565. doi: 10.1016/j.tmaid.2023.102565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rearte A, Castelli JM, Rearte R, et al. Effectiveness of rAd26-rAd5, ChAdOx1 nCoV-19, and BBIBP-CorV vaccines for risk of infection with SARS-CoV-2 and death due to COVID-19 in people older than 60 years in Argentina: a test-negative, case-control, and retrospective longitudinal study. Lancet Lond Engl. 2022;399:1254–1264. doi: 10.1016/S0140-6736(22)00011-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ai J, Zhang H, Zhang Q, et al. Recombinant protein subunit vaccine booster following two-dose inactivated vaccines dramatically enhanced anti-RBD responses and neutralizing titers against SARS-CoV-2 and Variants of Concern. Cell Res. 2021. Feb;590(7844):140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140–145. doi: 10.1038/s41586-020-2918-0 [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Klein SL, Garibaldi BT, et al. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65:101205. doi: 10.1016/j.arr.2020.101205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Li C, Liu F, et al. Age-associated SARS-CoV-2 breakthrough infection and changes in immune response in a mouse model. Emerg Microbes Infect. 2022;11:368–383. doi: 10.1080/22221751.2022.2026741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Wang H, Ai J, et al. Identification of CKD, bedridden history and cancer as higher-risk comorbidities and their impact on prognosis of hospitalized Omicron patients: a multi-centre cohort study. Emerg Microbes Infect. 2022;11:2501–2509. doi: 10.1080/22221751.2022.2122581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bates TA, McBride SK, Leier HC, et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci Immunol. 2022;7:eabn8014. doi: 10.1126/sciimmunol.abn8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mensah AA, Lacy J, Stowe J, et al. Disease severity during SARS-COV-2 reinfection: a nationwide study. J Infect. 2022;84:542–550. doi: 10.1016/j.jinf.2022.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lafaie L, Célarier T, Goethals L, et al. Recurrence or relapse of COVID-19 in older patients: a description of three cases. J Am Geriatr Soc. 2020;68:2179–2183. doi: 10.1111/jgs.16728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravioli S, Ochsner H, Lindner G.. Reactivation of COVID-19 pneumonia: a report of two cases. J Infect. 2020;81:e72–e73. doi: 10.1016/j.jinf.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunsinger D, Kutti Sridharan D, Rokkam D, et al. COVID-19 reinfection in an immunosuppressed patient without an antibody response. Am J Med Sci. 2021;362:103. doi: 10.1016/j.amjms.2021.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowe B, Xie Y, Al-Aly Z.. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med. 2022;28:2398–2405. doi: 10.1038/s41591-022-02051-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ai J, Wang X, He X, et al. Antibody evasion of SARS-CoV-2 Omicron BA.1, BA.1.1, BA.2, and BA.3 sub-lineages. Cell Host Microbe. 2022;30:1077–1083.e4. doi: 10.1016/j.chom.2022.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walls AC, Sprouse KR, Bowen JE, et al. SARS-CoV-2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses. Cell. 2022;185:872–880.e3. doi: 10.1016/j.cell.2022.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collier AY, Brown CM, McMahan KA, et al. Characterization of immune responses in fully vaccinated individuals following breakthrough infection with the SARS-CoV-2 delta variant. Sci Transl Med 2022;eabn6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergwerk M, Gonen T, Lustig Y, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021. Oct 14;385(16):1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Ai J, Li X, et al. Neutralization of Omicron BA.4/BA.5 and BA.2.75 by booster vaccination or BA.2 breakthrough infection sera. Cell Discov. 2022;8:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehmsen S, Pedersen RM, Bang LL, et al. BQ.1.1, XBB.1, and XBB.1.5 neutralization after bivalent mRNA COVID-19 booster in patients with cancer. Cancer Cell. 2023;41:649–650. doi: 10.1016/j.ccell.2023.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu A, Wei P, Man M, et al. Antigenic characterization of SARS-CoV-2 Omicron subvariants XBB.1.5, BQ.1, BQ.1.1, BF.7 and BA.2.75.2. Signal Transduct Target Ther. 2023;8:125. doi: 10.1038/s41392-023-01391-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulliam JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science 2022;eabn4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yue C, Song W, Wang L, et al. ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5. Lancet Infect Dis. 2023;23:278–280. doi: 10.1016/S1473-3099(23)00010-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Iketani S, Li Z, et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186:279–286.e8. doi: 10.1016/j.cell.2022.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Y, Jian F, Wang J, et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature. 2023;614:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bates TA, Leier HC, Lyski ZL, et al. Age-Dependent neutralization of SARS-CoV-2 and P.1 variant by vaccine immune serum samples. JAMA. 2021;326:868–869. doi: 10.1001/jama.2021.11656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tillmann F-P, Figiel L, Ricken J, et al. Effect of third and fourth mRNA-based booster vaccinations on SARS-CoV-2 neutralizing antibody titer formation, risk factors for non-response, and outcome after SARS-CoV-2 Omicron breakthrough infections in patients on chronic hemodialysis: a prospective multicenter cohort study. J Clin Med. 2022;11:3187. doi: 10.3390/jcm11113187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itamochi M, Yazawa S, Inasaki N, et al. Neutralization of Omicron subvariants BA.1 and BA.5 by a booster dose of COVID-19 mRNA vaccine in a Japanese nursing home cohort. Vaccine. 2023;41:2234–2242. doi: 10.1016/j.vaccine.2023.02.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia S, Jiao F, Wang L, et al. SARS-CoV-2 Omicron XBB subvariants exhibit enhanced fusogenicity and substantial immune evasion in elderly population, but high sensitivity to pan-coronavirus fusion inhibitors. J Med Virol. 2023;95:e28641. doi: 10.1002/jmv.28641 [DOI] [PubMed] [Google Scholar]
- 33.Lartigau M, Ouattara E, Tumiotto C, et al. Post-vaccination SARS-cov-2 infection in nursing home residents, Bordeaux, France. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2022;149:105134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao B, He L, Bao Y, et al. Repeated vaccination of inactivated SARS-CoV-2 vaccine dampens neutralizing antibodies against Omicron variants in breakthrough infection. Cell Res. 2023;33:258–261. doi: 10.1038/s41422-023-00781-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suntronwong N, Yorsaeng R, Puenpa J, et al. COVID-19 breakthrough infection after inactivated vaccine induced robust antibody responses and cross-neutralization of SARS-CoV-2 variants, but less immunity against Omicron. Vaccines. 2022;10:391. doi: 10.3390/vaccines10030391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Zhao X, Song J, et al. Homologous or heterologous booster of inactivated vaccine reduces SARS-CoV-2 Omicron variant escape from neutralizing antibodies. Emerg Microbes Infect. 2022;11:477–481. doi: 10.1080/22221751.2022.2030200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Jiang S, Jiang S, et al. Neutralization of SARS-CoV-2 BQ.1.1, CH.1.1, and XBB.1.5 by breakthrough infection sera from previous and recent waves in China. Cell Discov. 2023;9:64. doi: 10.1038/s41421-023-00569-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walls AC, Miranda MC, Schäfer A, et al. Elicitation of broadly protective sarbecovirus immunity by receptor-binding domain nanoparticle vaccines. Cell. 2021;184:5432–5447.e16. doi: 10.1016/j.cell.2021.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moriyama S, Anraku Y, Taminishi S, et al. Structural delineation and computational design of SARS-CoV-2-neutralizing antibodies against Omicron subvariants. Nat Commun. 2023;14:4198. doi: 10.1038/s41467-023-39890-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu G, Ling Y, Jiang M, et al. Primary assessment of the diversity of Omicron sublineages and the epidemiologic features of autumn/winter 2022 COVID-19 wave in Chinese Mainland. Front Med. 2023. Mar 31:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.