Abstract
Objective:
Certain treatments have demonstrated acute efficacy for binge-eating disorder (BED) but many patients who receive “evidence-based” interventions do not derive sufficient benefit. Given the dearth of controlled research examining treatments for patients who fail to respond to initial interventions, this study tested the efficacy of cognitive-behavioral therapy (CBT) for patients with BED who do not respond to initial acute treatments.
Methods:
Prospective randomized double-blind placebo-controlled single-site trial, conducted August 2017-December 2021, tested 16-weeks of therapist-led CBT for non-responders to initial treatment (naltrexone/bupropion and/or behavioral therapy) for BED with obesity. Thirty-one patients (mean age 46.3 years, 77.4% women, 80.6% White, mean BMI 38.99 kg/m2) who were non-responders to initial acute treatments were randomized to CBT (N=18) or no-CBT (N=13), in addition to continuing double-blinded pharmacotherapy. Independent assessments were performed at baseline, throughout treatment, and posttreatment; 83.9% completed posttreatment assessments.
Results:
Intention-to-treat remission rates were significantly higher for CBT (61.1%; N=11/18) than no-CBT (7.7%; N=1/13). Mixed models of binge-eating frequency (assessed using complementary methods) converged revealing a significant interaction between CBT and time and a significant main effect of CBT. Binge-eating frequency decreased significantly with CBT but did not change significantly with no-CBT. Since only 4 patients received behavioral treatment during the acute treatments, we performed “sensitivity-type” analyses restricted to the 27 patients who received pharmacotherapy during the acute treatment and found the same pattern of findings for CBT versus no-CBT.
Conclusions:
Adult patients with BED who fail to respond to initial pharmacological treatments should be offered CBT.
Keywords: eating disorders, binge eating, cognitive-behavioral therapy, behavior therapy, treatment non-responder, obesity
Binge-eating disorder (BED), a prevalent (1,2) and costly public health problem (3), is associated with elevated risk for psychiatric and medical disorders and with psychosocial impairments (2,4). BED, a psychiatric diagnosis, is also associated strongly with obesity (1,2); BED and obesity have distinct behavioral, psychological, and neurobiological features despite their frequent comorbidity (5). The combination of an eating disorder and obesity – each difficult to treat – poses a challenge for clinicians and patients, one that has long been highlighted (6).
Although controlled treatment research has identified specific behavioral/psychological (7) and pharmacological (8) treatments effective for BED, roughly half of patients who receive evidence-based interventions do not derive sufficient benefit (9). Treatment studies have found that combining behavioral/psychological and pharmacological treatments for BED (and other eating disorders) have generally not enhanced outcomes (10). Research to date on moderators of response to different treatments for BED (11) have yielded few findings for informing prescription of specific treatments (i.e., which treatments for whom) (12) as a strategy for improving outcomes (13). Identifying treatments that benefit non-responders to initial treatments attempted for BED represents a pressing clinical and research need.
Certain treatment guidelines for BED, such as those of the National Institute for Health and Clinical Excellence (NICE) (14), suggest that “specialist” CBT be pursued when initial efforts with self-help or guided-self-help versions of CBT prove ineffectual (cf; 15). Research with representative “real world” samples indicates that most people with BED do not seek treatment, and when they do seek help it rarely involves treatments with empirical support (16). Clinical logic is that “specialist” treatments for BED be pursued when initial “generalist” treatments sought by patients fail to provide sufficient benefit.
While there is a growing literature on treatments with efficacy for BED, there is a dearth of controlled research examining treatments for patients who fail to respond to initial interventions. We are aware of only three studies to date — two controlled trials (6,17) and one uncontrolled trial (18) — that have evaluated treatments for patients with BED who failed to respond to initial therapies. Grilo and colleagues (17), in an adaptive “sequential multiple assignment randomized trial,” switched 49 patients with BED who did not benefit sufficiently from a generalist behavioral treatment after one month to guided-self-help-CBT and one of two obesity medications (sibutramine or orlistat) or placebo. While there was some benefit to the addition of sibutramine, but not to orlistat, for reducing the frequency of binge-eating, the guided-self-help CBT intervention did not appear to provide much overall additional benefit to the non-responders. This suggested that a more intensive second treatment might be needed. The two other studies examined non-responders to specialist CBT treatments. Agras and colleagues (6) tested interpersonal psychotherapy (IPT; N=13) versus an assessment-only control condition (N=17) in 30 patients who failed to respond to CBT and found that IPT was not associated with any further improvement. Eldredge and colleagues (18), in an open extension study of CBT (without a comparison condition), reported that extending CBT for an additional 12 weeks showed a “strong trend” to produce improvements in binge eating amongst initial non-responders to CBT (i.e., 6 of the 14 patients no longer met criteria for BED).
Taken together, the field needs controlled research on treatments for non-responders to acute treatments for BED. This report describes a prospective, randomized controlled test of the efficacy of CBT for patients with BED comorbid with obesity who failed to respond to acute “generalist” treatments delivered in a randomized double-blind placebo-controlled trial (19). Specifically, the acute treatment trial tested naltrexone/bupropion and behavioral therapy, alone and combined, for BED comorbid with obesity (19). The putative mechanisms of naltrexone/bupropion combination, an FDA-approved medication for the treatment of obesity (20), are relevant for BED. Briefly, the bupropion stimulates pro-opiomelanocortin (POMC) neurons and the naltrexone blocks endogenous feedback that inhibits POMC activity (21,22). The trial included a placebo arm that also yielded non-responders; since placebo response is a well-established phenomenon in pharmacotherapy trials for BED (10,11) non-responders were also eligible for the present trial. Finally, the behavioral treatment (behavioral weight loss (BWL), a “generalist” and broadly disseminable intervention) has been shown to produce acute outcomes in patients with BED that approximate those with “specialist” psychological treatments (such as CBT and IPT (17,23,24).
Patients categorized as “non-responders” (defined as less than 65% reduction in binge-eating frequency) following acute 16-week treatments in the acute treatment “Stage 1” trial (19) were randomized to either CBT or to no-CBT for 16 weeks in the present “Stage 2” trial, in addition to continuing double-blinded pharmacotherapy (see Table 1). Thus, the design tests whether, amongst non-responders, adding CBT enhances on-going pharmacotherapy (naltrexone/bupropion or placebo) which despite not producing much benefit does nonetheless represent an important “real-world” clinical reality (i.e., practitioners tend to continue pharmacotherapy when they add psychotherapeutic interventions) plus a strong methodological control in the case of no-CBT. We hypothesized that CBT would have higher remission rates and greater reductions in binge-eating frequency than the no-CBT condition.
TABLE 1.
Demographic characteristics and treatment variables of the overall sample and by treatment condition.
| Overall | no CBT | CBT | ||||||
|---|---|---|---|---|---|---|---|---|
| N=31 | n=13 | n=18 | Test statistic | p value | ||||
|
| ||||||||
| Age, N, M (SD) | 46.29 | 13.06 | 45.08 | 12.72 | 47.17 | 13.60 | 0.19 | 0.67 |
| Sex, n (%) | 1.05 | 0.59 | ||||||
| Male | 6 | 19.4% | 2 | 15.4% | 4 | 22.2% | ||
| Female | 24 | 77.4% | 11 | 84.6% | 13 | 72.2% | ||
| Other | 1 | 3.2% | 0 | 0.0% | 1 | 5.6% | ||
| Race, n (%) | 2.62 | 0.45 | ||||||
| White | 25 | 80.6% | 11 | 84.6% | 14 | 77.8% | ||
| Asian | 1 | 3.2% | 1 | 7.7% | 0 | 0.0% | ||
| Black | 4 | 12.9% | 1 | 7.7% | 3 | 16.7% | ||
| Multiracial | 1 | 3.2% | 0 | 0.0% | 1 | 5.6% | ||
| Ethnicity, n (%) | 0.54 | 0.46 | ||||||
| Hispanic or Latinx | 4 | 12.9% | 1 | 7.7% | 3 | 16.7% | ||
| Not Hispanic or Latinx | 27 | 87.1% | 12 | 92.3% | 15 | 83.3% | ||
| Sexual Orientation, n (%) | 4.31 | 0.23 | ||||||
| Heterosexual | 26 | 83.9% | 13 | 100.0% | 13 | 72.2% | ||
| Gay or Lesbian | 3 | 9.7% | 0 | 0.0% | 3 | 16.7% | ||
| Bisexual | 1 | 3.2% | 0 | 0.0% | 1 | 5.6% | ||
| Other | 1 | 3.2% | 0 | 0.0% | 1 | 5.6% | ||
| Education, n (%) | 0.29 | 0.96 | ||||||
| High School | 2 | 6.5% | 1 | 7.7% | 1 | 5.6% | ||
| Some college | 16 | 51.6% | 6 | 46.2% | 10 | 55.6% | ||
| College | 2 | 6.5% | 1 | 7.7% | 1 | 5.6% | ||
| More than college | 11 | 35.5% | 5 | 38.5% | 6 | 33.3% | ||
|
Treatment Variables*** Stage 1 Initial Treatment, n (%) |
0.06 | >0.99 | ||||||
| Received placebo | 14 | 45.2% | 6 | 46.2% | 8 | 44.4% | ||
| Received NB | 13 | 41.9% | 7 | 53.8% | 6 | 33.3% | ||
| Received BWL+placebo | 2 | 6.5% | 0 | 0.0% | 2 | 11.1% | ||
| Received BWL+NB | 2 | 6.5% | 0 | 0.0% | 2 | 11.1% | ||
|
Stage 2 Continuing Medication |
0.35 | 0.68 | ||||||
| Continued placebo | 14 | 45.2% | 6 | 46.2% | 8 | 44.4% | ||
| Continued NB | 9 | 29.0% | 5 | 38.4% | 4 | 22.2% | ||
| None (due to adverse event) | 8 | 25.8% | 2 | 15.4% | 6 | 33.3% | ||
Note: Test statistic = chi-square for categorical variables and ANOVAs for dimensional variables.
M = mean. SD = standard deviation. N = number; P values are for two-tailed tests.
CBT = cognitive behavioral therapy
The acute (Stage 1) treatment was a 2X2 balanced factorial design, and when considered separately, the N=31 participants in this trial testing CBT had received one of the following four initial acute (Stage 1) treatment conditions: placebo, naltrexone/bupropion (NB), behavioral weight loss (BWL) plus placebo, or behavioral weight loss plus naltrexone/bupropion (BWL+NB). The treatment variables summarized in this Table show the distributions of the four “Stage 1 Initial Treatment” conditions and show the “Stage 2 Continuing Medication” conditions (i.e., continued placebo, continued naltrexone/bupropion, or no medication because discontinued due to adverse event), which did not differ, separately for CBT and no-CBT.
METHODS
This RCT, approved by the Yale Institutional Review Board, included a data safety and monitoring plan with physician safety monitor. Participants provided written informed consent.
Participants
Participants for this controlled “Stage 2” trial were eligible if they participated in the initial acute Stage 1 trial and were categorized as “non-responders” following initial acute 16-week treatments in a randomized double-blind study testing naltrexone/bupropion and behavioral therapy, alone and combined, for BED in people with obesity described previously (19). When patients initially enrolled in the Stage 1 treatment trial, they were informed that the study involved two treatment stages and they consented to two treatment stages – i.e., the acute treatment stage and a second stage that would test, in the case of “non-responders” the utility of CBT, and in the case of “responders” the utility of pharmacotherapy maintenance. Thus, participation in this treatment study was anticipated when participants consented to the two-stage treatment study without a second consent.
Eligibility for the initial acute Stage 1 trial required DSM-5 (25) criteria for BED, ages 18–70 years old, and a body mass index (BMI) ≥30.0 and ≤50.0 (or ≥27.0 with obesity-related comorbidity). The initial trial employed minimal exclusion criteria (to enhance generalizability) comprising clinical issues that, regardless of clinical setting, would require alternative treatments or represent contraindications to naltrexone/bupropion. Exclusionary criteria for the initial acute trial and for the present CBT trial included: concurrent treatment for eating/weight disorders, taking contraindicated medications (e.g., opiates), uncontrolled medical conditions or contraindications to naltrexone/bupropion (e.g., seizure history, bulimia nervosa or anorexia nervosa history, cardiovascular disease, psychosis/bipolar disorder, systolic blood pressure>160mmHg, diastolic blood pressure>100mmHg, or heart rate>100 beats/minute), and pregnancy/breastfeeding.
“Non-response” to the initial acute 16-week treatments was defined as less than 65% reduction in binge-eating frequency at posttreatment. The 65% reduction threshold as the definition for “response” to treatment was previously used in an adaptive stepped-care treatment trial (17). The 65% definition was chosen following a series of studies reporting that this cut-point, originally defined empirically using signal detection methods, reliably predicted treatment outcomes for BED, including reductions in both binge-eating and weight through 12-month follow-ups (26). Binge-eating frequency was assessed using the Eating Disorder Examination Interview (EDE; 27) as part of the comprehensive posttreatment evaluation. The EDE was administered by doctoral-level assessors who were blinded to treatment conditions. The Stage 1 posttreatment evaluation was performed immediately following completion of the initial acute treatments and eligible participants were randomized and began this Stage 2 treatment study within one week.
The 31 participants had mean age of 46.29 (SD=13.06) years and mean BMI of 38.99 (SD=5.14) kg/m2; 77.4% (N=24) were female, 42.0% (N=13) attained college or advanced degrees, an in terms of race, 80.6% (N=25) identified as White and in terms of ethnicity 12.9% identified as Hispanic/Latinx. Table 1 summarizes the participants’ sociodemographic characteristics (and reports their “Stage 1” treatment conditions) and Table 2 summarizes their clinical characteristics.
TABLE 2.
Clinical measures by treatment condition.
|
|
no CBT | CBT | ||
|---|---|---|---|---|
| n=13 | n=18 | |||
| M | SD | M | SD | |
| Binge-Eating Frequency (EDE) | ||||
| Pre-Treatment | 13.31 | 8.17 | 17.94 | 24.90 |
| Post-Treatment | 14.38 | 9.65 | 0.80 | 1.61 |
| Change | 0.50 | 12.87 | −18.47 | 26.68 |
| Binge-Eating Frequency (EDE-Q) | ||||
| Pre-Treatment | 13.62 | 14.87 | 8.94 | 9.64 |
| Post-Treatment | 15.40 | 16.37 | 2.67 | 3.06 |
| Change | 2.10 | 5.07 | −6.57 | 11.01 |
| Weight (pounds) | ||||
| Pre-Treatment | 232.16 | 28.89 | 236.09 | 51.57 |
| Post-Treatment | 223.11 | 22.68 | 225.40 | 60.24 |
| Change | −2.98 | 11.23 | 0.13 | 10.90 |
| % Change | −1.31% | 4.87 | −0.16% | 4.27 |
Note: CBT = cognitive behavioral therapy; EDE = Eating Disorder Examination Interview;
EDE-Q = Eating Disorder Examination - Questionnaire
M = mean; SD = standard deviation
Assessments
Assessment procedures were performed by trained/monitored doctoral-level research-clinicians who were independent from and blinded to treatments. The Eating Disorder Examination Interview (EDE; 16th-edition; 27) was administered to assess binge-eating frequency and eating-disorder psychopathology at baseline and post-treatment. The EDE has demonstrated good inter-rater and test-retest reliability in studies with BED (28). The Eating Disorder Examination-Questionnaire (29), which has good test-retest reliability (30) and converges significantly with the EDE interview (31), was used to obtain binge-eating frequency data during the past 28 days at monthly assessments throughout the course of treatment. Weight and height were measured at baseline and weight was measured monthly and at post-treatment.
Randomization and Blinding Procedures
The randomization schedule, developed by a biostatistician, assigned participants categorized as “non-responders” randomly in equal proportions, using stratified blocked randomization with the initial Stage 1 treatment as a stratifying variable, to either CBT or to no-CBT for 16-weeks (Figure 1). All participants continued their double-blinded pharmacotherapy without changes from the acute treatment trial unless they had adverse events necessitating withdrawal. The schedule comprised random block sizes of two and four to obviate secular trends and to yield approximately equal proportions. Participants and clinicians remained blinded as to whether they had received naltrexone/bupropion or placebo in their initial Stage 1 treatment stage and to their continued medication condition during this Stage 2 trial. Assessors of outcomes were also blinded to whether participants had received BWL during their prior Stage 1 treatment in addition to whether they were assigned to CBT or no-CBT in this Stage 2 trial for non-responders.
Figure 1. Participant flow throughout the study.
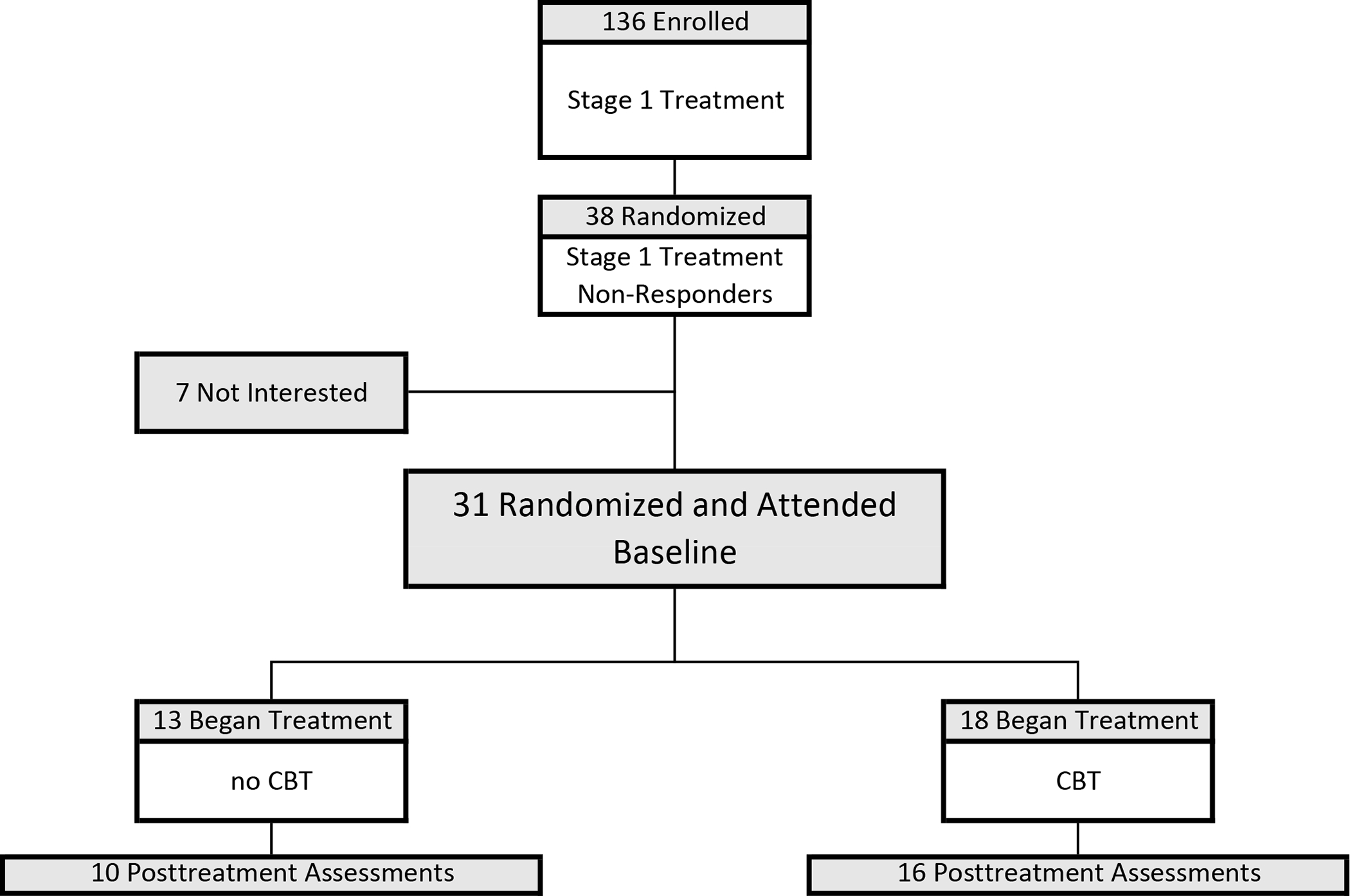
Participant flow through this randomized controlled trial (RCT) testing CBT versus no-CBT in addition to continuing double-blinded pharmacotherapy from Stage 1 initial acute trial (either naltrexone/bupropion (NB) or placebo) for patients with binge-eating disorder who were non-responders to acute treatments (Stage 1). Stage 1 treatment (N=136) was a RCT testing naltrexone/bupropion and behavioral weight loss (BWL), alone and together, using a 2×2 balanced factorial design, described previously (19). Of the 136 participants receiving Stage 1 treatments, 38 were categorized as treatment non-responders and were eligible for this Stage 2 trial for non-responders. “Randomization” (N=38) was the point at which the continuing (double-blinded) medication was ordered from the pharmacy once a participant was categorized as a “non-responder.” Participants were then scheduled for their “baseline session” at which time they were informed of their randomization (CBT or no-CBT) plus instructed to continue their medication, which was immediately available. Of the 38 randomized, 7 were excluded because of not attending baseline session or “not interested.” For this Stage 2 trial, the “intention-to-treat” sample (N=31) was defined as randomized plus attended the baseline session.
Of the N=31 randomized plus attended baseline session in this trial, 18 received cognitive-behavioral therapy (CBT) and 13 did not receive cognitive-behavioral therapy (no-CBT). Posttreatment assessments were obtained for 88.9% (N=16/18) of those receiving CBT and for 76.9% (N=10/13) of those in the no-CBT condition. Table 1 summarizes the Stage 1 treatments (a stratifying variable in the randomization schedule) received by the participants in the CBT and no-CBT conditions. See Supplemental Figure 1 for additional detailed description of the history of Stage 1 treatments for the participants at each step of this Stage 2 study.
Treatments
Cognitive-behavioral Therapy (CBT).
The CBT was delivered by research-clinicians following CBT treatment manuals developed initially at Oxford which were adapted further and specifically for BED and used in our previous treatment trials (24,32). The CBT manuals provide detailing session-by-session procedures for the clinicians and patients for this “specialist” focal treatment consisting of three overlapping phases. Phase one involves establishing a collaborative therapeutic relationship while focusing on educating the patient about the nature of binge eating and factors thought to maintain the problem. Specific behavioral strategies (e.g., self-monitoring) are used to help patients identify problematic eating patterns while establishing a normal structured eating pattern. Phase two integrates cognitive restructuring procedures, where patients learn to identify and challenge maladaptive cognitions regarding eating and weight/shape and thoughts that trigger binge eating. Phase three focuses on maintenance of change and relapse prevention.
Pharmacotherapy.
Pharmacotherapy involved continuing the double-blind medication (naltrexone/bupropion or placebo) from the acute Stage 1 treatment trial (19). Naltrexone/bupropion combination full dosing comprised naltrexone-sustained-release (32 mg/day) plus bupropion-sustained-release (360 mg/day) as in previous trials with obesity (33,34). Two doses, each containing 8mg naltrexone and 90mg bupropion, were taken twice daily. Placebo was taken in capsules matched in appearance and frequency (i.e., two capsules twice daily).
Study physicians administered the pharmacotherapy, which focused on medication management (compliance, safety, and side-effects) without any additional psychotherapeutic or behavioral interventions. Monthly medication refills were accompanied by re-reviewing medication compliance and dosing schedules; pill bottles were returned for pill counts at post-treatment. Side-effect and safety checklists were performed monthly by research clinicians.
Statistical Analysis
Analyses to compare treatments were all intention-to-treat and were performed for all randomized patients who attended the first treatment session. The primary outcome variable was binge eating, which was analyzed in two complementary ways – i.e., as a categorical variable (remission) and as a continuous variable (monthly frequency).
For the categorical variable, binge-eating remission was defined as zero episodes during the previous 28 days on the Eating Disorder Examination interview at posttreatment; any missing data were imputed as failure (i.e., non-remission). Fisher’s exact tests were used to compare proportion of participants classified with remission in the CBT versus no-CBT conditions.
For the continuous variable, binge-eating frequency was assessed using two complementary assessment methods: the primary method was the Eating Disorder Examination Interview given at baseline and posttreatment (Figure 2-A) and the secondary method was the Eating Disorder Examination-Questionnaire, which was given at baseline, monthly during treatment, and at posttreatment (Figure 2-B). The questionnaire data were used as a “convergent” method to the interview data and to illustrate the time course of changes in binge-eating frequency throughout treatment. Intention-to-treat analyses used all available data in mixed models without imputation. Variables not conforming to normality were log-transformed prior to analysis. Mixed effects models were fitted with fixed factors including CBT (yes, no) time (all relevant time points), and interactions between CBT and time. In each model, we considered different error structures and selected the best-fitting structure using the Schwarz’s Bayesian Criterion. Tests of effect slices and focused comparisons of least square means were used to explain significant effects in the models.
Figure 2. Binge-eating across treatment conditions.
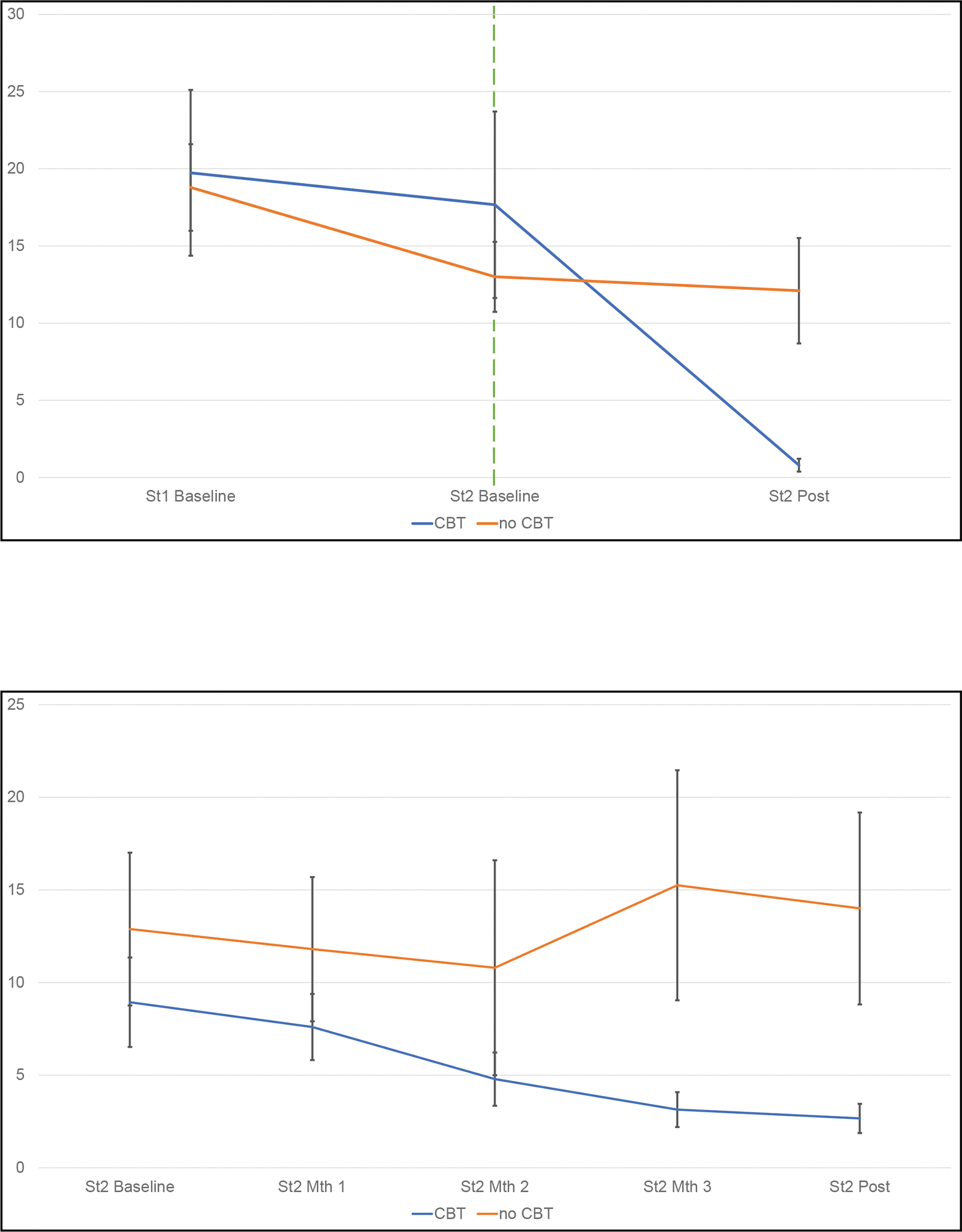
A Frequency of binge eating during the last 28 days shown at baseline and at post-treatment (assessed using the Eating Disorder Examination interview). The two lines to the right of the vertical line show the raw binge-eating frequencies separately for CBT and no-CBT conditions during this Stage 2 trial for non-responders to the initial (Stage 1) treatments. For context, to the left of the vertical line, the lines show the frequencies of binge-eating during the initial acute (Stage 1) treatments for the participants who were eventually classified as “non-responders” and subsequently randomized to either CBT or no-CBT. Error bars indicate standard error.
B Frequency of binge eating during the last 28 days shown at baseline, monthly, and at post-treatment (assessed using the Eating Disorder Examination-Questionnaire administered monthly) for this Stage 2 trial. The two lines show the raw binge-eating frequencies separately for CBT and no-CBT conditions during this Stage 2 trial for non-responders to the initial (Stage 1) treatments. Error bars indicate standard error.
Weight (measured) was analyzed as a secondary continuous outcome (percent weight-loss from the beginning of this Stage 2 trial for initial non-responders) using intention-to-treat mixed-models structured like those for binge eating frequency described above.
We explored whether time of measurement (before or during the COVID pandemic) affected the results by including an indicator for timing of measurement as a covariate. The results did not change substantively and therefore the final models are not adjusted for COVID.
RESULTS
Randomization and Participant Characteristics
Figure 1 (CONSORT) summarizes participant flow throughout the study. Of the 136 participants who began acute Stage 1 treatments (19), 38 were categorized as treatment non-responders and eligible for this Stage 2 trial for non-responders. The point of “randomization” (N=38) was when the continuing (double-blinded) medication was ordered from the pharmacy once a participant was categorized as a “non-responder.” This design decision was made in order to ensure a smooth transition to Stage 2 (with CBT or no-CBT) without any disruption in the continuing on-going medication regimen or dosing. Participants were then scheduled for their Stage 2 “baseline session” at which time they were informed of their randomization (CBT or no-CBT) plus instructed to continue their medication, which was immediately available. Of the 38 randomized, 7 were excluded because of not attending baseline session or “not interested.” For this Stage 2 trial, the “intention-to-treat” sample (N=31) was defined as randomized plus attended the baseline session.
Of the 31 participants, N=18 received CBT and N=13 were assigned to no-CBT; participants continued double-blinded pharmacotherapy from the acute Stage 1 treatment. Post-treatment assessments were obtained for 83.9% (N=26/31) of participants (88.9% (N=16/18) of those receiving CBT and 76.9% (N=10/13) of those in the no-CBT condition). Table 1 summarizes the Stage 1 treatments (a stratifying variable) received by the participants randomized to CBT and no-CBT conditions. Supplemental Figure 1 augments Figure 1 by providing the additional description of the history of Stage 1 treatments received by the participants as they progressed through the randomization, treatment, and outcome assessment components of this Stage 2 study.
Outcomes
Binge-eating Remission.
Intention-to-treat remission rates (based on the EDE Interview) were significantly higher for CBT (61.1% (N=11/18) versus no-CBT (7.7% (N=1/13) (Fisher’s exact test p=0.003).
We performed two additional “sensitivity-type” analyses examined binge-eating remission, both which converged with the primary intention-to-treat findings above. First, remission rates for “completers” (i.e., without failure imputed for missing data) were significantly higher for CBT (73.3% (N=11/15) versus no-CBT (12.5% (N=1/8) (Fisher’s exact test p=0.009). Second, since only 4 of the 31 patients received behavioral treatment during the Stage 1 trial, an analysis restricted to the 27 patients who received only pharmacotherapy in Stage 1 was performed. Remission rates were significantly higher for CBT than no-CBT in both the intention-to-treat sample (78.6% (N=11/14) versus 7.7% (N=1/13); Fisher’s exact test p=0.0003) and in the “completer” sub-sample (91.7% (N=11/12) versus 12.5% (N=1/18); Fisher’s exact test p=0.0008) of those who received pharmacotherapy alone in Stage 1.
Binge-eating Frequency.
Table 2 shows descriptive statistics for binge-eating frequency outcomes and Figure 2 illustrates the changes in binge-eating frequency over time in the CBT versus no-CBT conditions. Mixed models analyses of binge-eating frequency (episodes during the past month assessed with the Eating Disorder Examination interview) revealed a significant interaction between CBT and time (F(1,28.8)=18.07, p=0.0002) and also significant main effects of CBT (F(1,28.2)=19.68, p=0.0001) and time (F(1,28.8)=23.86, p<.0001). Figure 2-A illustrates binge-eating frequency decreased significantly for those who received CBT (F(1,25.7)=54.09, p<.0001) but did not change significantly for those who did not receive CBT (F(1,30.8)=0.16, p=0.69). Mixed models of binge-eating frequency (determined monthly with the Eating Disorder Examination–Questionnaire) revealed a trend-level interaction between CBT and time (F(4,82.1)=2.35, p=0.06) and a significant main effect of CBT (F(1,30.7)=5.02, p=0.03). Figure 2-B illustrates those who received CBT had significantly lower binge-eating frequency than those without CBT at month three (F(1,62.3)=7.90, p=0.007) and at posttreatment (F(1,55.6)=10.7, p=.002).
A “sensitivity-type” analyses restricted to the 27 patients who received only pharmacotherapy during Stage 1 treatment was performed. The findings converged with the primary intention-to-treatment findings for binge-eating frequency outcomes above. Mixed models of binge-eating frequency during the past month assessed with the EDE interview revealed a significant interaction between CBT and time (F(1,23.5)=23.07, p<0.0001), main effect of CBT (F(1,23.5)=18.84, p=0.0002), and time (F(1,23.5)=29.63, p<.0001); those who received CBT F(1,20.9)=59.35, p<.0001) but not those with no-CBT (F(1,25.7)=0.18, p=.67) decreased significantly. Mixed models of binge-eating frequency during the past month assessed with the EDE-Questionnaire revealed a significant interaction between CBT and time (F(4,69.7)=2.65, p=.04) and main effect of CBT (F(1,25.3)=5.92, p=0.02). Those who received CBT had significantly lower binge-eating frequency than those without CBT at month three (F(1,49.9)=8.16, p=0.006) and at posttreatment (F(1,45.0)=12,84, p=.0008).
Percent Weight Loss.
Table 2 shows weight values at baseline and post-treatment and changes. Mixed models of percent weight loss measured monthly and at posttreatment revealed no significant interaction effects between CBT and time (F(3,28)=0.13, p=0.94) and no significant main effects of CBT (F(1,23.2)=0.03, p=0.87) or of time (F(3,28)=2.62, p=0.07).
DISCUSSION
The present prospective randomized controlled treatment study of adults with BED and obesity, only the third controlled trial for non-responders to initial treatments (6,17), found that 16-week therapist-led CBT resulted in significant and robust improvements in binge eating. Patients who failed to respond during an initial 16-week placebo-controlled treatment trial testing naltrexone/bupropion and behavioral treatments, alone and combined (19), were randomized to CBT or no-CBT for 16 weeks in addition to continuing their double-blinded initial pharmacotherapy. Findings for binge-eating — assessed using complementary assessment methods — converged to indicate that CBT was associated with significantly superior outcomes than the control condition. The outcomes with CBT were robust: intention-to-treat remission rates were significantly higher for CBT than no-CBT (61.1% versus 7.7%, respectively) and mixed models analyses of two continuous measures of binge-eating frequency revealed that CBT resulted in substantial reductions.
Only 4 of the 31 patients in this trial had received behavioral treatment during the Stage 1 trial. This distribution follows the Stage 1 overall findings that while both behavioral therapy and naltrexone/bupropion were associated with significant improvements in BED, behavioral treatment showed a stronger and more consistent pattern of efficacy (19). Thus, we performed a series of “sensitivity-type” analyses restricted to the 27 patients who received pharmacotherapy (i.e., excluding the 4 who had received behavioral therapy) during Stage 1 treatments and the analyses revealed the same pattern of significant findings for CBT versus no-CBT. Thus, while our analyses indicate that patients with BED with co-existing obesity who fail to respond to initial pharmacological treatment (specifically, naltrexone/bupropion) should be offered CBT, our findings cannot speak to whether CBT benefits those who fail to respond to behavioral therapy.
These findings, which indicate that therapist-led CBT can produce robust improvements in binge eating following failure to benefit from initial pharmacological treatments for BED, are particularly compelling when considered with the context of the very clear non-responsiveness to the initial 16-week interventions. Non-responsive was defined as less than 65% reduction in binge-eating frequency (17,26) and inspection of the course of binge-eating frequency (considered by both investigator-based interview and monthly self-report questions) illustrated in Figure 2, revealed a basically “flat” (i.e., unchanging frequency) during the initial 16-week trial followed by a clear response in those who received CBT in this trial versus those who did not receive CBT and, in turn, showed no significant reductions. For broader context, we note that the observed 61.1% remission rate for this patient group of non-responders compares very favorably to the CBT literature for BED (9). The 61.1% remission rate is nearly identical to those reported for guided-self-help CBT and for therapist-led IPT by Wilson and colleagues in their 2-site trial (23) and exceeds the rates observed in our prior trials of therapist-led CBT for BED (24,32).
We highlight some broader issues regarding help-seeking and treatment availability as context for our findings. CBT, a “specialist” treatment with efficacy for eating and other psychiatric disorders, is not widely available (35). Research with representative samples of people with BED (and other eating disorders) have found that evidence-based “specialist” treatments are rarely sought (16,36). The development of “scalable” guided-self-help CBT interventions — which have shown efficacy (9) — may increase treatment availability and decrease some potential barriers to use (e.g., cost, time, and privacy issues). Indeed, certain treatment guidelines such as NICE (14) have highlighted guided self-help CBT as a potential first step when seeking treatment for BED. We offer, however, some cautionary considerations for non-responders to initial treatments, for whom guided self-help CBT might not be the ideal next step (i.e., rather than therapist-led CBT). A recent re-analysis of aggregated data from controlled trials evaluating CBT interventions for BED revealed that after statistical adjustment for demographic and clinical characteristics, therapist-led CBT was associated with significantly better outcomes than guided self-help methods (15). Relatedly, in a stepped care trial for BED, when patients who did not have a rapid response to initial treatment with BWL were switched to guided self-help CBT, little additional benefit was observed (17). Thus, a circumspect approach would be that “specialist” psychological treatments (33,38) - such as therapist-led CBT for BED - be sought when initial interventions do not provide sufficient benefit.
We note methodological strengths and limitations as context for interpreting our study’s findings. Study strengths include complex two-stage treatment delivery allowing us to rigorously ascertain non-responsive to initial treatments that have utility for BED (19) immediately preceding this trial, the prospective randomized controlled design to test CBT versus no-CBT with the context of continued double-blind pharmacological treatment delivered by trained/monitored faculty-level study physicians, independent assessments using well-validated measures administered by trained/monitored doctoral research clinicians, minimal exclusionary criteria intended to enhance generalizability, and high retention rates. A notable limitation is the lack of control for therapist attention and time; although both the CBT and no-CBT groups continued with double-blind pharmacological treatment and associated visits, these contacts differed considerably from the more frequent and highly structured CBT, thus precluding firm conclusions that CBT has a “specific” effect. A related methodological point is that while the assessors were blinded to whether patients received CBT, the patients knew whether they were receiving CBT or not, and this might have influenced their reporting in some fashion. The generalizability of the findings to different clinical settings, to people with different sociodemographic (our patient group was 77.4% female, 80.6% White, and 42% had college degrees) and/or clinical characteristics (39–41), or to patients who have relapsed following beneficial interventions or failed to derive benefit from these or different interventions in their past, is uncertain. The sample size was limited but this was overcome by the high retention and the robust clinical effects that were clearly statistically significant.
With these methodological considerations as context, we conclude that adult patients with BED with co-existing obesity who fail to respond to initial pharmacological treatments for BED should be offered CBT.
Supplementary Material
Funding:
National Institutes of Health grant R01 DK49587 (Grilo), K23 DK115893 (Lydecker) and UL1 TR001863. The funding agency (National Institutes of Health) played no role in the content of this paper.
Footnotes
Potential Conflicts of interest: The authors declare no conflicts of interest. Dr. Grilo reports broader interests, which did not influence this research, including Honoraria for lectures, and Royalties from Guilford Press and Taylor & Francis Publishers for academic books. Dr. Lydecker reports Honoraria for lectures. Dr. Gueorguieva reports royalties from Taylor & Francis Publishers for an academic book.
Authorship CrediT: Carlos M. Grilo: Conceptualization, methodology, investigation, supervision, analysis, writing - original draft, writing – review and editing, funding acquisition. Janet Lydecker: investigation, data curation, analysis, visualization, supervision, project administration, writing – review; Ralitza Gueorguieva: formal analysis, writing - review
Clinicaltrials.gov registration: NCT03063606
(Behavioral and Pharmacologic Treatment of Binge Eating and Obesity: Specialist Treatment)
Contributor Information
Carlos M. Grilo, Department of Psychiatry, Yale University School of Medicine, New Haven
Janet A. Lydecker, Department of Psychiatry, Yale University School of Medicine, New Haven
Ralitza Gueorguieva, Department of Biostatistics, Yale School of Public Health, New Haven
Data Sharing:
De-identified data will be provided in response to reasonable written request to achieve goals in an approved written proposal (from non-commercial academic researchers).
REFERENCES
- 1.Udo T, Grilo CM: Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of US adults. Biol Psychiatry 2018;84:345–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund PA, Chiu WT, et al. : The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol Psychiatry 2013;73:904–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Streatfeild J, Hickson J, Austin SB, et al. : Social and economic cost of eating disorders in the United States: evidence to inform policy. Int J Eat Disord 2021;54:851–868 [DOI] [PubMed] [Google Scholar]
- 4.Udo T, Grilo CM: Psychiatric and medical correlates of DSM-5 eating disorders in a nationally representative sample of adults in the United States. Int J Eat Disord 2019;52:42–50 [DOI] [PubMed] [Google Scholar]
- 5.Boswell RG, Potenza MN, Grilo CM: The neurobiology of binge-eating disorder compared with obesity: implications for differential therapeutics. Clin Ther 2021;43:50–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agras WS, Telch CF, Arnow B, et al. : Does interpersonal therapy help patients with binge eating disorder who fail to respond to cognitive-behavioral therapy? J Consult Clin Psychol 1995;63:356–360 [DOI] [PubMed] [Google Scholar]
- 7.Grilo CM. Psychological and behavioral treatments for binge-eating disorder. J Clin Psychiatry 2018;78(S1): 20–24. [DOI] [PubMed] [Google Scholar]
- 8.McElroy SL: Pharmacologic treatments for binge-eating disorder. J Clin Psychiatry 2017;78S1:14–19 [DOI] [PubMed] [Google Scholar]
- 9.Hilbert A, Petroff D, Herpertz S, et al. : Meta-analysis of the efficacy of psychological and medical treatments for binge-eating disorder. J Consult Clin Psychol 2019;87:91–105 [DOI] [PubMed] [Google Scholar]
- 10.Reas DL, Grilo CM: Psychotherapy and medications for eating disorders: better together? Clin Ther 2021;43:19–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grilo CM, Masheb RM, Crosby RD: Predictors and moderators of response to cognitive behavioral therapy and medication for the treatment of binge eating disorder. J Consult Clin Psychol 2012;80:897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindardon J, Piedad la Garcia X, Brennan L: Predictors, moderators, and mediators of treatment outcome following manualised cognitive-behavioural therapy for eating disorders: A systematic review. Eur Eat Disorders Rev 2017;25:3–12. [DOI] [PubMed] [Google Scholar]
- 13.Wilson GT, Grilo CM, Vitousek KM: Psychological treatment of eating disorders. Am Psychol 2007;62:199–216 [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Health and Clinical Excellence (NICE): Eating disorders: recognition and treatment (NICE Guideline NG69), 2017 [PubMed] [Google Scholar]
- 15.Grilo CM, Thompson-Brenner H, Shingleton RM, Thompson DR, Franko DL: Clinical moderators and predictors of cognitive-behavioral therapy by guided-self-helkp versus therapist-led for binge-eating disorder: analysis of aggregated clinical trials. Int J Eat Disord 2021;54”1875–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffino JA, Udo T, Grilo CM: Rates of help-seeking in U.S. adults with lifetime DSM-5 eating disorders: Prevalence across diagnoses and sex and ethnic/racial differences. Mayo Clin Proc 2019;94:1415–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grilo CM, White MA, Masheb RM, et al. : Randomized controlled trial testing the effectiveness of adaptive “SMART” stepped-care treatment for adults with binge-eating disorder comorbid with obesity. Am Psychologist 2020;75:204–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eldredge KL, Agras WS, Arnow B, et al. : The effects of extending cognitive-behavioral therapy for binge eating disorder among initial treatment nonresponders. Int J Eat Disord 1997;21:347–352 [DOI] [PubMed] [Google Scholar]
- 19.Grilo CM, Lydecker JA, Fineberg SK, et al. : Naltrexone plus bupropion combination medication and behavior therapy, alone and combined, for binge-eating disorder: randomized double-blind placebo-controlled trial. Am J Psychiatry 2022;179:927–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanovski SZ, Yanovski JA: Naltrexone extended-release plus bupropion extended-release for treatment of obesity. JAMA, 2015;313:1213–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billes SK, Greenway FL: Combination therapy with naltrexone and bupropion for besity. Expert Opin Pharmacother 2011;12:1813–26 [DOI] [PubMed] [Google Scholar]
- 22.Cowley MA, Smart JL, Rubinstein M, et al. : Leptin activates anorexigenic POMC neurons through neural network in the arcuate nucleus. Nature 2001;411(6836):480–484 [DOI] [PubMed] [Google Scholar]
- 23.Wilson GT, Wilfley DE, Agras WS, et al. : Psychological treatments of binge eating disorder. Arch Gen Psychiatry 2010;67:94–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grilo CM, Masheb RM, Wilson GT, et al. : Cognitive-behavioral therapy, behavioral weight loss, and sequential treatment for obese patients with binge-eating disorder: a randomized controlled trial. J Consult Clin Psychol 2011;79:675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Psychiatric Association: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association, 2013 [Google Scholar]
- 26.Grilo CM, White MA, Wilson GT, Gueorguieva R, Masheb RM: Rapid response predicts 12-month post-treatment outcomes in binge-eating disorder: theoretical and clinical implications. Psychol Med 2012;42:807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fairburn CG, Cooper Z, O’Connor M: Eating Disorder Examination (16.0D). In Fairburn CG. Cognitive Behavior Therapy and Eating Disorders. NY: Guilford Press, 2008 [Google Scholar]
- 28.Grilo CM, Masheb RM, Lozano-Blanco C, et al. : Reliability of the Eating Disorder Examination in patients with binge eating disorder. Int J Eat Disord 2004;35:80–85 [DOI] [PubMed] [Google Scholar]
- 29.Fairburn CG, Begin SJ: Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord 1994;16:363–370 [PubMed] [Google Scholar]
- 30.Reas DL, Grilo CM, Masheb RM: Reliability of the eating disorder examination-questionnnaire in patients with binge eating disorder. Behav Res Ther 2006;44:43–51 [DOI] [PubMed] [Google Scholar]
- 31.Grilo CM, Masheb RM, Wilson GT: A comparison of different methods for assessing the features of eating disorders in patients with binge eating disorder. J Consult Clin Psychol 2001;69:317–322 [DOI] [PubMed] [Google Scholar]
- 32.Grilo CM, Masheb RM, Wilson GT: Efficacy of cognitive behavioral therapy and fluoxetine for the treatment of binge eating disorder: a randomized double-blind placebo-controlled comparison. Biol Psychiatry 2005;57:301–309 [DOI] [PubMed] [Google Scholar]
- 33.Greenway FL, Fujioka K, Plodkowski RA, et al. : Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multi-centre randomized double-blind placebo-controlled phase 3 trial. Lancet 2010;376:595–605 [DOI] [PubMed] [Google Scholar]
- 34.Wadden TA, Foreyt JP, Foster GD, et al. : Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity 2011;19:110–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shafran R, Clark CM, Fairburn CG, et al. : Mind the gap: improving the dissemination of CBT. Behav Res Ther 2009;47:902–909. [DOI] [PubMed] [Google Scholar]
- 36.Marques L, Alegra M, Becker AE, et al. : Comparative prevalence, cxorrelates of impairment, and service utilization for eating disorders across US ethnic groups: implications for reducing ethncic disparities in health care access for eating disorders. Int J Eat Disord 2011;44:412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Comer JS, Barlow DH: The occasional case against broad dissemination and implementation: retaining a role for specialty care in the delivery of psychological treatments. Am Psychol 2014;69:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grilo CM: Smertphone-assisted delivery of cognitive-behavioral guided self-help for binge eating: cautionary musings of implications given the importance of comparsion groups. Am Psychiatry 2020;177:110–112 [DOI] [PubMed] [Google Scholar]
- 39.Franko DL, Thompson-Brenner H, Thompson DR, et al. : Racial/ethnic differences in adults in randomized clinical tials of binge eating disorder. J Consult Clin Psychol 2012;80:186–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lydecker J, Grilo CM: Different yet similar: examining race and ethnicity in treatment seeking dults with binge-eating disorder. J Consult Clin Psychol 2016;84:88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lydecker J, Gueorguieva R, Masheb R, et al. : Examining race as a predictor and moderator of treatment outcomes for binge-eating disorder: analysis of aggregated randomized controlled trials. J Consult Clin Psychol 2019;87:530–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data will be provided in response to reasonable written request to achieve goals in an approved written proposal (from non-commercial academic researchers).


