Abstract
Background:
Acute lymphoblastic leukemia (ALL) accounts for 80% of all leukemias diagnosed in children. Although ALL age patterns are consistent across racial/ethnic groups, their incidence and mortality rates are highly variable.We assessed the age-standardized ALL incidence and mortality rates of Puerto Rican Hispanic (PRH) children and compared them with those of US mainland Hispanics (USH), non-Hispanic whites (NHW), non-Hispanic blacks (NHB) and Non-Hispanic Asian or Pacific Islanders (NHAPI).
Methods:
Differences between racial/ethnic groups were assessed by estimating the Standardized Rate Ratio (SRR) for 2010–2014. Secondary data analyses of the Puerto Rico Central Cancer Registry and the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) databases were performed for the 2001–2016 period.
Results:
PRH children had 31% lower incidence rates than USH, but 86% higher incidence rates than NHB. In addition, the incidence trends of ALL increased significantly from 2001 to 2016 among PRH and USH, with 5% and 0.9% per year, respectively. Moreover, PRH have a lower 5-year overall survival (81.7%) when compared to other racial/ethnic groups.
Conclusions:
PRH children were found to have disparities in ALL incidence and mortality rates compared to other racial/ethnic groups in the US. Additional research is warranted to identify the genetic and environmental risk factors that may be associated with the disparities observed.
Impact:
This is the first study reporting the incidence and mortality rates of childhood ALL for PRH and making comparisons with other racial/ethnic groups in the US.
Keywords: Childhood leukemia, Puerto Rico, Hispanic, epidemiology, leukemia subtypes
INTRODUCTION
Acute Lymphoblastic Leukemia (ALL) is the most common cancer in children and adolescents in the United States (US). This malignancy accounts for approximately 26% of all cancers occurring in children younger than 20 years (>3,000 new cases per year)1–4 and causes approximately 25% of cancer-related deaths in children5. According to the Centers for Disease Control and Prevention (CDC), the overall incidence of pediatric ALL in the US during 2001–2014 was 34.0 cases per million persons. In the past decades, ALL incidence rates have increased by approximately 1% per year for all races/ethnicities in the US, suggesting that risk factors may have become more prevalent1, 6.
The incidence and mortality rates of childhood leukemia vary according to the molecular subtype, race/ethnicity, age at diagnosis, sex and socioeconomic status7–15. US Hispanics have the highest incidence rates of childhood ALL and have poorer survival rates than other non-Hispanic ethnic groups7, 9, 13, 16–18. However, the term Hispanic is often used as a broad category to refer to subpopulations of Mexican, Cuban, Puerto Rican, South or Central America, Dominican, or other individuals of Spanish descent living in mainland US18, 19. According to 2021 data from the US Census, people of Mexican origin were the largest Hispanic group (63.0% of total Hispanic population), followed by other Hispanic (14.9%) and Puerto Rican (8.8%)20. Therefore, the cancer data reported for Hispanics are aggregated, which may mask important differences between these subpopulations. However, because the Puerto Rico Cancer Registry (PRCCR) is not a part of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program, the Hispanic cancer data reported by SEER in the US does not include data from Hispanics living in Puerto Rico.
During 2010–2014, the Puerto Rico Cancer Registry (PRCCR) reported that leukemia was the most common cancer diagnosed in Puerto Rican Hispanic children, accounting for 26.0% and 26.4% of all cancers diagnosed in boys and girls, respectively21. From 2000 to 2016, childhood cancer incidence rates in boys increased by 3% per year, whereas in girls, they increased by an average of 4.8% per year in Puerto Rico. Higher cancer incidence rates were observed in children between 15 and 19 years of age than in children between 5 and 14 years of age. Moreover, children aged 15–19 years were reported to have higher mortality rates than children under 9 years of age21. Unfortunately, the PRCCR report combined all leukemia cases. Therefore, incidence and mortality data from childhood ALL cases, the most common type of leukemia diagnosed among children, in Puerto Rican Hispanic children are lacking. This study is the first to address this knowledge gap by assessing the incidence and mortality of childhood ALL for Puerto Rican Hispanics and to compare this data with ALL incidence and mortality rates among racial/ethnic groups in mainland US.
MATERIALS AND METHODS
This study involved secondary data analysis of the Puerto Rico Central Cancer Registry (PRCCR) and the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. The PRCCR gave us access to ALL pathology reports to extract information, including genetic tests, when available. Data from PRCCR were provided to us without personal identifiers and through a Secure File Transfer Protocol. Data from the SEER program are of the public domain, contain no personal identifiers of the cancer cases included, and are available online on the following website: http://seer.cancer.gov/. This study was approved by the Institutional Review Board of the University of Puerto Rico, Medical Sciences Campus (protocol number 0150118).
Data sources
Puerto Rico Central Cancer Registry (PRCCR):
Incident cases of childhood ALL in Puerto Rico for the period 2008–2016 were obtained from the PRCCR. PRCCR (RRID: SCR_023507), one of the oldest population-based cancer registries in the world, is responsible for collecting, analyzing, and publishing information on all cancer cases diagnosed and/or treated in Puerto Rico. Reporting of cancer cases to the PRCCR by public and private medical institutions is required by law. Since 1997, the PRCCR has been part of the CDC’s National Program of Cancer Registries and uses the Surveillance, Epidemiology, and End Results (SEER) Program and the North American Association of Central Cancer Registries (NAACCR) standards for coding data. Partially supported by the CDC’s National Program of Cancer Registries, the PRCCR must meet national standards like completeness of case ascertainment and information recorded, limits on death certificate only cases, duplicate primary cases, and passing standard edits. The PRCCR acquires information from island-wide hospitals, outpatient clinics, pathology laboratories, and radiotherapy/chemotherapy sites. In addition, linking the PRCCR database to Medicaid, Medicare, and private insurance data for PR residents, the PRCCR-Health Insurance Linkage Database (HILD) provides information about treatment, medical procedures, comorbidities, costs, and provider information. Over the years, the PRCCR improved data collection of cancer cases through electronic reporting, achieving a completeness of more than 95% of all cases since 2010, an important achievement that resulted in obtaining the NAACCR’s Gold Certification and maintaining the PR cancer data in the CDC’s report “United States Cancer Statistics”22. Mortality information for PR from 2008–2016 was obtained from the PRCCR, as reported by death certificates prepared by the Demographic Registry of the Puerto Rico Department of Health.
Incident cases of childhood ALL for US Hispanic (USH), non-Hispanic White (NHW), non-Hispanic Black (NHB) and Non-Hispanic Asian or Pacific Islanders (NHAPI) individuals in the US for the period 2008–2016 were obtained from the SEER*Stat 8.3.4 software (National Cancer Institute Surveillance Program, Bethesda, MD). The SEER program is a national cancer surveillance database that collects and reports incidence and survival data from a sample of the US population. Data collected by SEER include demographic characteristics, anatomical and histological characteristics of the specific cancer, stage of diagnosis, diagnostic techniques used, treatment received within four months of diagnosis, and patient outcomes. Cancer mortality information for NHW, NHB, USH and NHAPI from 2008–2016 was obtained from the SEER program as reported by the National Center for Health Statistics (NCHS). Puerto Rico mortality cases were not included in the NCHS data source.
Study Population:
All reported cases of childhood (0–19 years) Acute Lymphoblastic Leukemia (ALL) in Puerto Rican Hispanics (PRH) and in the US, specifically for the NHW, NHB, and USH groups, during 2008–2016 were analyzed.
Statistical Analysis
Incidence and mortality rates:
For each racial/ethnic group, we applied the indirect method to compute childhood ALL age-standardized incidence and mortality rates per 100,000 persons during 2012–2016, using the incidence and mortality of 2000 US rates as standard risks. These rates were identified by the IASI for incidence and IASM for mortality, as follows:
where C indicates the crude incidence or mortality in the study population; indicates the total number of incident cases or deaths in our study population with the -th ethnic group; indicates the age-specific incidence or mortality rate in the -th age group of the standard population; and indicates the number of persons in the -th age group for -th ethnic group. IASI and IASM incidence trends were summarized annual percent change (APC).
Trends:
Age-standardized incidence (IASI) and age-standardized mortality (IASM) incidence trends for the period 2001–2016 were summarized with the annual percent change (APC) and estimated by ethnic group. The APCs were calculated using the Joinpoint Regression Program of the NCI23, 24.
Risk differences:
To assess racial/ethnic group differences, the IASI/IASM were grouped from 2012 to 2016 as follows:. Where is the proportion of children in the -th age group of the US 200 standard population, is the number of cases (new cases or deaths) in the -th age group for the -th ethnic group in the -th year, and is the population in the -th age group of the -th ethnic group in the -th year.
Then, the ratio of two standardized rates between two different groups was estimated with their 95% confidence interval,21 to assess significant differences in ALL children incidence and mortality rates between USH, NHW, NHB, and NHAPI compared with PRH. This ratio was denoted as the Standardized Rate Ratio (SRR), and we used USH, NHW, NHB, and NHAPI as the reference racial/ethnic groups.
Relative Survival:
One, three, and five-year relative survival rates were calculated for children with PRH. Follow-up was performed to 2017. The relative survival rate represents the ratio of the observed survival of cancer patients divided by the expected survival for a group of people in the general population that is similar to that of the patient group with respect to sex, age, and calendar period of observation.
Data Availability
The datasets generated and analyzed during the current study are not publicly available due to the confidentiality policy of the Puerto Rico Central Cancer Registry but are available from the corresponding author on reasonable request.
RESULTS
Incidence and Mortality
To assess racial/ethnic group differences of Puerto Rican Hispanic (PRH) children with ALL with US Hispanics (USH), non-Hispanic-white (NHW), non-Hispanic-black (NHB) and non-Hispanic Asian or Pacific Islander (NHAPI), we estimated the incidence and mortality Standardized Rate Ratio (SRR) for 2012–2016. The overall Age-standardized rates (ASR) of childhood ALL incidence and mortality per 100,000 by racial/ethnic group from 2012–2016 are shown in Table 1. USH had the highest age-standardized incidence rate overall, followed by NHW, PRH, NHAPI, and NHB. Similar trends were observed when analyzing age-standardized incidence according to sex, with the exception that PRH girls had higher incidence than NHW. For PRH and NHAPI, we observed that girls had a slightly higher incidence of ALL than boys, in contrast to the other racial/ethnic groups. Our data showed that overall, PRH children had a 31% lower incidence of ALL (SRR = 0.69, 95% CI = 0.57, 0.81) and 86% higher incidence (SRR = 1.86, 95% CI = 1.50, 2.28) than USH and NHB, respectively. PRH male children had a 39% lower incidence (SRR = 0.61, 95% CI = 0.47, 0.77) compared to USH, but a 69% higher incidence (SRR = 1.69, 95% CI = 1.25, 2.24) compared to NHB. PRH girls had an 86% higher incidence (SRR = 1.86, 95% CI = 1.50, 2.28) compared to NHB. No significant differences in incidence rates were observed between PRH and NHW or NHAPI. For age-standardized mortality rate overall, USH had the highest rate, followed by PRH, NHAPI, NHW, and NHB. The same trend was observed when analyzing age-standardized mortality according to sex, with the exception that NHW and NHB girls had the same value. In addition, no significant differences (p<0.05) were observed in mortality rates between PRH children and those from other racial/ethnic groups in the US.
Table 1.
Age-standardized* Incidence and Mortality Rates (per 100,000) for Children with Acute Lymphoblastic Leukemia (ALL) from 2012 to 2016
| Age-standardized rates | SRR (95% CI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PRH | NHW | USH | NHB | NHAPI | US overall | PRH vs NHW | PRH vs USH | PRH vs NHB | PRH vs NHAPI | PRH vs US overall | |
| Incidence | |||||||||||
| Overall | 3.21 | 3.32 | 4.68 | 1.73 | 3.15 | 3.55 | 0.97 (0.80–1.15) | 0.69 (0.57–0.81) | 1.86 (1.50–2.28) | 1.02 (0.83–1.24) | 0.90 (0.75–1.07) |
| Boys | 3.16 | 3.59 | 5.19 | 1.87 | 3.12 | 3.87 | 0.88 (0.68–1.12) | 0.61 (0.47–0.77) | 1.69 (1.25–2.24) | 1.01 (0.76–1.33) | 0.82 (0.63–1.03) |
| Girls | 3.26 | 3.03 | 4.14 | 1.58 | 3.19 | 3.23 | 1.08 (0.82–1.37) | 0.79 (0.60–1.00) | 1.86 (1.50–2.28) | 1.02 (0.76–1.35) | 1.01 (0.78–1.28) |
| Mortality | |||||||||||
| Overall | 0.29 | 0.21 | 0.40 | 0.19 | 0.25 | 0.25 | 1.38 (0.73–2.21) | 0.73 (0.38–1.17) | 1.48 (0.77–2.45) | 1.16 (0.59–2.00) | 1.13 (0.60–1.81) |
| Boys | 0.34 | 0.24 | 0.46 | 0.22 | 0.28 | 0.30 | 1.41 (0.61–2.51) | 0.74 (0.32–1.32) | 1.57 (0.66–2.90) | 1.20 (0.49–2.39) | 1.16 (0.50–2.05) |
| Girls | 0.23 | 0.17 | 0.33 | 0.17 | 0.21 | 0.21 | 1.33 (0.43–2.63) | 0.70 (0.23–1.39) | 1.37 (0.43–2.83) | 1.08 (0.34–2.46) | 1.09 (0.35–2.14) |
Age-standardized rates using the US 2000 standard population;
SSR indicated standardized rate ratio with 95% confidence interval. PRH=Puerto Rican Hispanics, NHW=non-Hispanic White, USH=US Hispanics, NHB=non-Hispanic Black, NHAPI=non-Hispanic Asian or Pacific Islander, US overall=All US racial/ethnic groups.
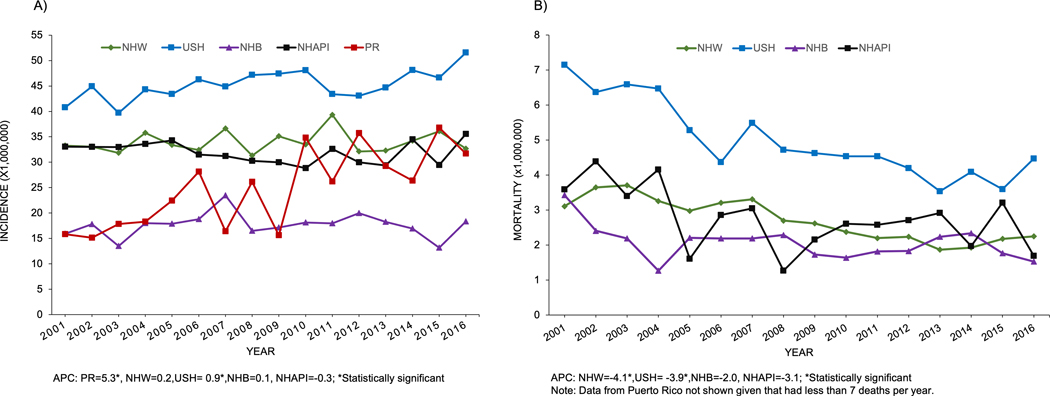
From 2001 to 2016, distinct patterns in ALL incidence trends were observed according to race/ethnicity (Figure 1A). The incidence of ALL increased significantly (p<0.05) from 2001 to 2016 among children with ALL for PRH and USH, with 5% and 0.9% per year, respectively. The mortality rates for NHW and USH decreased significantly during this period, with APC=−4.1% and −3.92%, respectively (Figure 1B). The mortality rate for PRH could not be calculated for this period because there were fewer than seven deaths per year.
Figure 1.
Trends for age-standardized (using 2000 US population) incidence (A) and mortality (B) rates (per 1,00,000) for children (0–19 years) with acute lymphoblastic leukemia (ALL) for Puerto Rican Hispanics (PRH) and among Non-Hispanic Whites (NHW), Non-Hispanic Blacks (NHB), US Hispanics (USH) and Non-Hispanic Asian or Pacific Islanders (NHAPI), 2001–2016.
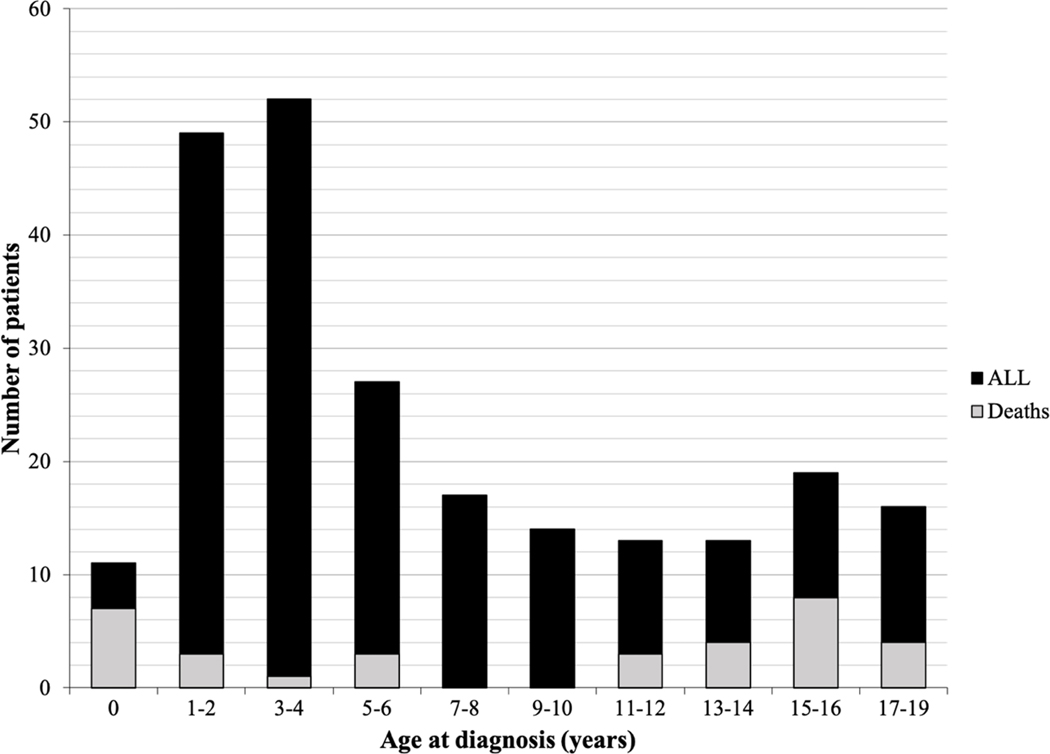
Table 2 shows the 1-, 3- and 5-year survival estimate for PRH children with ALL for the 2008–2012 period, with overall survival estimate of 92.4% (95% CI, 86.2% - 95.9%), 83.2% (95% CI, 75.6% - 88.6%), and 81.7% (95% CI, 73.9% - 87.4%), respectively. Males have higher 1-year survival estimates when evaluated by age, however the 3- and 5-year survival rates were similar. The age distribution and number of deaths of PRH children diagnosed with ALL between 2008–2016 are shown in Figure 2. During this period, 231 cases of childhood Acute Lymphoblastic Leukemia were identified and a total of thirty-three deaths were recorded.
Table 2.
Net Survival (%) at 1, 3, and 5 years after diagnosis for Puerto Rican Hispanic children (0–19 years) diagnosed with Acute Lymphoblastic Leukemia for the 2008–2012 period.
| Year | Survival (%) | 95% CI | |
|---|---|---|---|
| Overall | 1 | 92.4 | 86.2 – 95.9 |
| 3 | 83.2 | 75.6 – 88.6 | |
| 5 | 81.7 | 73.9 – 87.4 | |
| Boys | 1 | 96.1 | 88.3 – 98.8 |
| 3 | 83.0 | 72.5 – 89.8 | |
| 5 | 81.8 | 71.1 – 88.9 | |
| Girls | 1 | 87.1 | 74.8 – 93.7 |
| 3 | 83.4 | 70.5 – 91.1 | |
| 5 | 81.6 | 68.4 – 89.7 |
Figure 2.
Age distribution of Puerto Rican Hispanics ALL patients at diagnosis (n = 231 patients, diagnosed during 2008–2016 period). Black columns represent patients diagnosed with ALL by age; gray columns represent reported deaths by age.
DISCUSSION
Acute lymphoblastic leukemia (ALL) accounts for 80% of all leukemias diagnosed in children25. In the United States (US), ALL accounts for 27% of cancers diagnosed in children aged 0–19 years, disproportionately affecting children between 2 and 5 years4. Even though the age patterns of ALL are consistent across racial and ethnic groups, the incidence and survival rates are highly variable7, 26. Several studies have reported higher incidence and lower survival rates for some Hispanic subpopulations in the US 1, 3, 18, 19, 27. However, to date, there have been no reports on childhood ALL in Hispanics living in Puerto Rico (PRH). According to the United States Census Bureau Puerto Rico: 2020 Census, the population of Puerto Rico is 98.9% Hispanic or Latino (RRID: SCR_011587). In this study, we report the first estimates of childhood acute lymphoblastic leukemia incidence, mortality, and survival rates in PRH and compare them with other racial/ethnic groups in the US. The information revealed in our study will serve as a guide for future research on this minority population.
Consistent with what has been published, the majority of the diagnosed cases of ALL in PRH were between the ages of 2 and 4 years 4, 27. In addition, we observed that PRH girls had a slightly higher incidence of ALL than PRH boys, which is in contrast with other racial/ethnic groups in this study and what has been previously reported4, 9, 28, 29. The reasons behind sex differences in childhood ALL risk are unknown, but studies have suggested that sex-specific factors and single nucleotide polymorphism (SNPs) in the regulatory regions of RASSF2 and HLA-DQB1 genes may explain sex-specific effects30, 31. However, further studies are needed to evaluate genetic variation in PRH children and how they may explain the slightly higher incidence observed in girls from Puerto Rico.
The overall ALL age-standardized incidence reported in this study for PRH children is similar to that of non-Hispanic White (NHW) children and non-Hispanic Asian or pacific Islander (NHAPI) living in the US. Nevertheless, there are significant differences in childhood ALL incidence between PRH children and US Hispanics (USH) and non-Hispanic Blacks (NHB). PRH children have a lower risk (31%) of ALL compared to USH. This is not unexpected given that several studies have reported that USH children have the greatest incidence of childhood leukemia in the country, with incidence rates more than 20% higher than those for non-Hispanic children1, 32, 33. For instance, Marcotte et al. evaluated the incidence by single year of age and reported that USH children have a 46% higher risk of developing ALL than NHW, whereas NHB and NHAPI have a lower risk than NHW33. Similarly, Giddings et al. reported that Hispanics in California had 32% higher risk (32%) of ALL when compared to NHW, while NHB and NHAPI had 45% and 9% lower risk than NHW, respectively29. In contrast, PRH children have a higher risk (86%) of ALL than NHB children, which have the lowest childhood ALL incidence in the US1, 33.
This report also documents an increasing incidence of childhood ALL in PRH and USH children during the 2001–2016 period, with PRH children having the highest annual percent change (5.3% versus 0.9%, respectively). Childhood leukemia cases in Puerto Rico, like those on the mainland, are diagnosed using the National Comprehensive Cancer Network (NCCN) guidelines, and patients have been participating in National Cancer Institute-sponsored clinical trials since 1980 (Cancer Therapy Evaluation Program (CTEP) ID: PR018 and CTEP ID: PR038), ruling out the possibility that the high annual percent change for PRH is due to differences in diagnosis methodologies. Siegel et al.1, stated that although there was an increasing trend in childhood ALL rates during 2001–2008 for USH, it was followed by a subsequent period (2008–2014) of stable trends. We included data of cases of childhood ALL for USH from two additional years (2015–2016) that may explain the slight increase in incidence observed for this group. Similarly, previous reports have described the increased in incidence among USH during the past decades28. Even though the underlying basis of the increased risk of childhood leukemia in USH remains unknown, GWAS performed with Hispanic in California identified a risk loci in IKZF1 which was significantly associated with both global and local Indigenous American ancestry34. Among the risk factors for childhood ALL are cesarean delivery, advanced maternal age, infant birthweight, and as well as germline variants located within or near cancer predisposition genes35–38. Furthermore, occupational and/or residential exposure to organic solvents and pesticides has been linked to an increased risk of childhood leukemia in the United States and other Latin American countries39–42. However, none of these factors have been studied in PRH. Due to the small number of deaths per year, it was not possible to assess the mortality trend for PRH children for the 2001–2016 period, but a significant decrease in mortality was observed for the NHW and USH.
Despite the fact that the 5-year survival for ALL has improved significantly over the past decades due to increased participation of patients in clinical trials, improved supportive care, and risk stratification implementations2, 9, 43–50, this study reported a lower 5-year overall survival (OS) for PRH children (81.7%) when compared to the ones reported for USH, NHB, NHAPI and NHW in other studies. For instance, Khan et al.18 reported that the 5-year OS rates for USH and non-Hispanic patients from 2005 to 2011 were 89.2% and 92.7%, respectively. On the other hand, for the 2000–2005 period, Hunger et al.17 estimated that the 5-year OS rates for USH, NHW, and NHB were 87.6%, 91.4%, and 87.4%, respectively. In the US, the 5-year OS rate is slightly higher for girls than for boys, and survival rates vary according to age group and leukemia subtype4, 51, 52. In contrast to other ethnic groups living in the US, where survival rates have been generally higher for girls, PRH girls and boys have similar 5-year OS rates of 81.6% vs. 81.8%, respectively. Additionally, PRH girls and boys have lower 5-year OS rates than those reported for other groups in the US: 85.1% and 83.0% for USH girls and boys, 85.6 % and 82.7% for NHB girls and boys, 89.3 % and 86.3% for NHAPI girls and boys, and 91.6 % and 88.9% for NHW girls and boys, respectively15. There was also a marked difference in the 1-year survival rates for PRH boys (96.1%) and girls (87.1%), which differs from the US, where the reported 1-year net survival rates for boys and girls are 95.9% and 95.5%, respectively52. Age at diagnosis has been demonstrated to be an important prognostic factor for both the incidence and survival of pediatric ALL and has been incorporated into the NCI risk group classification. Most of the deaths in children with ALL in PR were reported for infants <1 year of age, followed by children >12 years of age. This is in agreement with what has been reported in the literature for childhood ALL survival distribution by age in the US, where children diagnosed during infancy have higher mortality rates, followed by those diagnosed between ages 10–14 and 15–192, 51, 52. Treatment failure is one of the factors associated with a decrease in ALL survival, since one out of five children with ALL experience relapse and have an overall survival rate of only 30%53–59. It has been reported that USH and NHB have a higher likelihood of relapse and lower 5-year disease-free survival than other ethnic groups16. An increased risk of neurotoxicity as a result of methotrexate chemotherapy for USH has been reported, which is associated with relapse, hospitalization, and changes in leukemia therapy60.
It is known that the genetic composition of different racial and ethnic groups varies dramatically, and ancestry-related genetic variations may contribute to the racial and ethnic disparities observed in childhood ALL incidence and mortality 27–29, 61–63. Hispanics are known to have a complex population structure resulting from more than 500 years of genetic admixture of European, Native American, and African individuals, and at the same time, these populations exhibit ancestry variation within countries themselves64–66. PRH have an average ancestral composition of 15.2%, 21.2%, and 63.7% from Native American, African, and European populations, respectively66. Correlations between ancestry and risk of ALL have been established for Hispanic populations 64, indicating that a higher proportion of Native American genetic ancestry is associated with a higher risk of ALL26, 67–69. For example, for B-ALL (that accounts for 80–85 % of childhood ALL46), higher proportions of African and Native American genetic ancestry have been correlated with lower survival for NHB and USH, respectively70. Moreover, genetic ancestry has been associated with ALL molecular subtypes showing that Native American ancestry is associated with CRLF2 rearrangements and ETV6-RUNX1-like ALL. African ancestry has been associated with T-cell ALL and TCF3-PBX1 gene fusion. All of these genetic alterations are linked with poor prognosis based on the functional effect on the affected genes and other coexisting mutations71–74. Higher levels of Native American ancestry have also been linked to an increased risk of relapse in children with ALL of self-declared Hispanic ethnicity26. However, for PRH children diagnosed with ALL, further analysis must be conducted to assess how ancestral genetic composition may be associated with the incidence and lower survival rates reported in this study.
The genetic basis of childhood ALL susceptibility is likely polygenic75. Cumulative evidence suggests that genetic variation among Hispanic subpopulations is correlated with an increased risk of childhood ALL. For example, several studies have identified that variations in certain genes 62, 69, 76–80 are associated with an increased risk of childhood ALL development in USH. Quiroz et al.67 carefully postulated that the differential ancestral proportions among Central and South America are correlated with the variable incidence of childhood ALL, stating that regions with higher indigenous ancestry have higher incidence, while those with higher African American ancestry have lower incidence. Furthermore, variability in the incidence and mortality rates of colorectal, endometrial, breast, prostate, liver, thyroid, and cervical cancers between Puerto Ricans (living in the US and on the main island) and other Hispanic subpopulations has also been observed8, 81–84. Studies focused on the molecular biology of childhood ALL based on the ancestral proportions among Hispanic subpopulations are needed to shed light on the disparities observed in incidence and mortality rates for children with PR.
In conclusion, the disparities observed among children with PR when compared to other ethnic groups in the US, especially with USH, may be attributed to factors such as the frequency of high-risk leukemia subtypes, environmental exposures, reduced access to care, and compliance to chemotherapy3, 7, 16. However, ancestry-related genetic variations may also play an important role in the risk of childhood ALL. The increasing evidence of how ancestral contributions among the admixed Hispanic subpopulations correlate with the incidence and survival of childhood ALL underscores the need for further studies to assess the impact of genetics in the Puerto Rican pediatric population to attain personalized treatment to improve survival.
ACKNOWLEDGEMENTS
We acknowledge the Comprehensive Cancer Center of the University of Puerto Rico for institutional support.
Financial support:
This study was supported in part by RCMI grant U54MD007600 (National Institute on Minority Health and Health Disparities), the Hispanic Alliance for Clinical and Translational Research (National Institute for General Medical Sciences Award number U54GM133807), the University of Puerto Rico/ MD Anderson Cancer Center: Partnership for Excellence in Cancer Research (NCI award # CA096297/CA096300), and the Puerto Rico Central Cancer Registry (CDC grant # NU58DP006318).
Footnotes
Conflict of interest disclosure: No conflict of interest to disclose
REFERENCES
- 1.Siegel DA; Henley SJ; Li J; Pollack LA; Van Dyne EA; White A, Rates and Trends of Pediatric Acute Lymphoblastic Leukemia - United States, 2001–2014 MMWR Morb Mortal Wkly Rep 2017, 66, 950–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hossain MJ; Xie L; McCahan SM, Characterization of pediatric acute lymphoblastic leukemia survival patterns by age at diagnosis. J Cancer Epidemiol 2014, 2014, 865979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirtane K; Lee SJ, Racial and ethnic disparities in hematologic malignancies. Blood 2017, 130 (15), 1699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward E; DeSantis C; Robbins A; Kohler B; Jemal A, Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014, 64 (2), 83–103. [DOI] [PubMed] [Google Scholar]
- 5.Curtin SC; Minino AM; Anderson RN, Declines in Cancer Death Rates Among Children and Adolescents in the United States, 1999–2014. NCHS Data Brief 2016, 257, 1–8. [PubMed] [Google Scholar]
- 6.Wiemels J, Perspectives on the causes of childhood leukemia. Chem Biol Interact 2012, 196 (3), 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim JYS; Bhatia S; Robison LL; Yang JJ, Genomics of Racial and Ethnic Disparities in Childhood Acute Lymphoblastic Leukemia. Cancer 2014, 120, 955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinheiro PS; Callahan KE; Siegel RL; Jin H; Morris CR; Trapido EJ, et al. , Cancer Mortality in Hispanic Ethnic Groups. Cancer Epidemiol Biomarkers Prev 2017, 26 (3), 376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadan-Lottick NS; Ness KK; Bhatia S; Gurney JG, Survival Variability by Race and Ethnicity in Childhood Acute Lymphoblastic Leukemia. JAMA 2003, 290 (15), 2008–14. [DOI] [PubMed] [Google Scholar]
- 10.Kahn JM; Keegan TH; Tao L; Abrahao R; Bleyer A; Viny AD, Racial disparities in the survival of American children, adolescents, and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia, and Hodgkin lymphoma. Cancer 2016, 122 (17), 2723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lilljebjorn H; Fioretos T, New oncogenic subtypes in pediatric B-cell precursor acute lymphoblastic leukemia. Blood 2017, 130 (12), 1395–401. [DOI] [PubMed] [Google Scholar]
- 12.Pelpola JS, Disparities in Incidence and Mortality of Childhood Leukemias. Stanford Journal of Public Health 2013. [Google Scholar]
- 13.Quiroz E; Venkateswaran AR; Nelson R; Aldoss I; Pullarkat V; Rego E, et al. , Immunophenotype of acute lymphoblastic leukemia in minorities- analysis from the SEER database. Hematol Oncol 2022, 40 (1), 105–10. [DOI] [PubMed] [Google Scholar]
- 14.Williams LA; Richardson M; Marcotte EL; Poynter JN; Spector LG, Sex ratio among childhood cancers by single year of age. Pediatr Blood Cancer 2019, 66 (6), e27620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore KJ; Barragan F; Williams LA, Survival disparities for childhood cancers exist when defined by race/ethnicity and sex. Cancer Epidemiol 2022, 81, 102262. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia S, Disparities in cancer outcomes: lessons learned from children with cancer. Pediatr Blood Cancer 2011, 56 (6), 994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunger SP; Lu X; Devidas M; Camitta BM; Gaynon PS; Winick NJ, et al. , Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol 2012, 30 (14), 1663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn JM; Cole PD; Blonquist TM; Stevenson K; Jin Z; Barrera S, et al. , An investigation of toxicities and survival in Hispanic children and adolescents with ALL: Results from the Dana-Farber Cancer Institute ALL Consortium protocol 05–001. Pediatr Blood Cancer 2017, 65 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel RL; Fedewa SA; Miller KD; Goding-Sauer A; Pinheiro PS; Martinez-Tyson D, et al. , Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin 2015, 65 (6), 457–80. [DOI] [PubMed] [Google Scholar]
- 20.Scherer Z; Mayol-García Y, Half of People of Dominican and Salvadoran Origin Experienced Material Hardship in 2020. U. S. Census Bureau: 2022; Vol. 2023. [Google Scholar]
- 21.Zavala-Zegarra DE; Tortolero-Luna G; Torres-Cintrón CR; Alvarado-Ortiz M; Román-Ruiz BS; Ortiz-Ortiz KJ, Cancer en Puerto Rico: 2012–2016. Puerto Rico Central Cancer Registry. San Juan, Puerto Rico 2020. [Google Scholar]
- 22.Zavala-Zegarra DE; Tortolero-Luna G; Torres-Cintrón CR; Alvarado-Ortiz M; Román-Ruiz BS; Ortiz-Ortiz KJ, Cancer ein Puerto Rico: 2010–2014. Puerto Rico Central Cancer Registry. San Juan, Puerto Rico 2017. [Google Scholar]
- 23.Joinpoint Regression Program, Version 4.8.0.1 - April 2020; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. [Google Scholar]
- 24.Kim HJ; Fay MP; Feuer EJ; Midthune DN, Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000, 19 (3), 335–51. [DOI] [PubMed] [Google Scholar]
- 25.Steliarova-Foucher E; Colombet M; Ries LAG; Moreno F; Dolya A; Bray F, et al. , International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol 2017, 18 (6), 719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang JJ; Cheng C; Devidas M; Cao X; Fan Y; Campana D, et al. , Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nat Genet 2011, 43 (3), 237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrington-Trimis JL; Cockburn M; Metayer C; Gauderman WJ, Rising rates of acute lymphoblastic leukemia in Hispanic children: trends in incidence from 1992 to 2011. BLOOD 2015, 125 (19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrington-Trimis JL; Cockburn M; Metayer C; Gauderman WJ; Wiemels J; McKean-Cowdin R, Trends in childhood leukemia incidence over two decades from 1992 to 2013. Int J Cancer 2017, 140 (5), 1000–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giddings BM; Whitehead TP; Metayer C; Miller MD, Childhood leukemia incidence in California: High and rising in the Hispanic population. Cancer 2016, 122 (18), 2867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams LA; Richardson M; Kehm RD; McLaughlin CC; Mueller BA; Chow EJ, et al. , The association between sex and most childhood cancers is not mediated by birthweight. Cancer Epidemiol 2018, 57, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh SK; Lupo PJ; Scheurer ME; Saxena A; Kennedy AE; Ibrahimou B, et al. , A childhood acute lymphoblastic leukemia genome-wide association study identifies novel sex-specific risk variants. Medicine (Baltimore) 2016, 95 (46), e5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Cancer Society. Cancer Facts & Figures for Hispanics/Latinos 2018–2020. Atlanta: American Cancer Society, Inc. 2018. [Google Scholar]
- 33.Marcotte EL; Domingues AM; Sample JM; Richardson MR; Spector LG, Racial and ethnic disparities in pediatric cancer incidence among children and young adults in the United States by single year of age. Cancer 2021, 127 (19), 3651–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith A. J. d.; Wahlster L; Jeon S; Yu F; Black S; Gazal S, et al. , Racial and Ethnic Disparities in Childhood Acute Lymphoblastic Leukemia Risk Due to an IKZF1 Noncoding Regulatory Variant. Blood 2022, 140 8880–81. [Google Scholar]
- 35.Marcotte EL; Thomopoulos TP; Infante-Rivard C; Clavel J; Petridou ET; Schuz J, et al. , Caesarean delivery and risk of childhood leukaemia: a pooled analysis from the Childhood Leukemia International Consortium (CLIC). Lancet Haematol 2016, 3 (4), e176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petridou ET; Georgakis MK; Erdmann F; Ma X; Heck JE; Auvinen A, et al. , Advanced parental age as risk factor for childhood acute lymphoblastic leukemia: results from studies of the Childhood Leukemia International Consortium. Eur J Epidemiol 2018, 33 (10), 965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Neill KA; Murphy MF; Bunch KJ; Puumala SE; Carozza SE; Chow EJ, et al. , Infant birthweight and risk of childhood cancer: international population-based case control studies of 40 000 cases. Int J Epidemiol 2015, 44 (1), 153–68. [DOI] [PubMed] [Google Scholar]
- 38.Kratz CP; Stanulla M; Cave H, Genetic predisposition to acute lymphoblastic leukemia: Overview on behalf of the I-BFM ALL Host Genetic Variation Working Group. Eur J Med Genet 2016, 59 (3), 111–5. [DOI] [PubMed] [Google Scholar]
- 39.Hyland C; Gunier RB; Metayer C; Bates MN; Wesseling C; Mora AM, Maternal residential pesticide use and risk of childhood leukemia in Costa Rica. Int J Cancer 2018, 143 (6), 1295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuniga-Venegas LA; Hyland C; Munoz-Quezada MT; Quiros-Alcala L; Butinof M; Buralli R, et al. , Health Effects of Pesticide Exposure in Latin American and the Caribbean Populations: A Scoping Review. Environ Health Perspect 2022, 130 (9), 96002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metayer C; Scelo G; Kang AY; Gunier RB; Reinier K; Lea S, et al. , A task-based assessment of parental occupational exposure to organic solvents and other compounds and the risk of childhood leukemia in California. Environ Res 2016, 151, 174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunier RB; Kang A; Hammond SK; Reinier K; Lea CS; Chang JS, et al. , A task-based assessment of parental occupational exposure to pesticides and childhood acute lymphoblastic leukemia. Environ Res 2017, 156, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X; Rastogi P; Shah B; Zhang L, B lymphoblastic leukemia/lymphoma: new insights into genetics, molecular aberrations, subclassification and targeted therapy. Oncotarget 2017, 8 (39), 66728–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeoh E-J; Ross ME; Shurtleff SA; Williams WK; Patel D; Mahfouz R, et al. , Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell 2002, 1, 133–43. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y; Miller S; Roulston D; Bixby D; Shao L, Genome-Wide Single-Nucleotide Polymorphism Array Analysis Improves Prognostication of Acute Lymphoblastic Leukemia/Lymphoma. J Mol Diagn 2016, 18 (4), 595–603. [DOI] [PubMed] [Google Scholar]
- 46.Tasian SK; Hunger SP, Genomic characterization of paediatric acute lymphoblastic leukaemia: an opportunity for precision medicine therapeutics. Br J Haematol 2017, 176 (6), 867–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linabery AM; Ross JA, Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer 2008, 113 (9), 2575–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith MA; Altekruse SF; Adamson PC; Reaman GH; Seibel NL, Declining Childhood and Adolescent Cancer Mortality. Cancer 2014, 120 (16), 2497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pui CH; Evans WE, A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol 2013, 50 (3), 185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.American Cancer Society. Cancer Facts & Figures 2020. Atlanta: American Cancer Society; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma H; Sun H; Sun X, Survival improvement by decade of patients aged 0–14 years with acute lymphoblastic leukemia: a SEER analysis. Sci Rep 2014, 4, 4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tai EW; Ward KC; Bonaventure A; Siegel DA; Coleman MP, Survival among children diagnosed with acute lymphoblastic leukemia in the United States, by race and age, 2001 to 2009: Findings from the CONCORD-2 study. Cancer 2017, 123 Suppl 24, 5178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma X; Edmonson M; Yergeau D; Muzny DM; Hampton OA; Rusch M, et al. , Rise and fall of subclones from diagnosis to relapse in pediatric B-acute lymphoblastic leukaemia. Nat Commun 2015, 6, 6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrison CJ, Genomic analysis drives tailored therapy in poor risk childhood leukemia. Cancer Cell 2012, 22 (2), 139–40. [DOI] [PubMed] [Google Scholar]
- 55.Kuiper RP; Schoenmakers EF; van Reijmersdal SV; Hehir-Kwa JY; van Kessel AG; van Leeuwen FN, et al. , High-resolution genomic profiling of childhood ALL reveals novel recurrent genetic lesions affecting pathways involved in lymphocyte differentiation and cell cycle progression. Leukemia 2007, 21 (6), 1258–66. [DOI] [PubMed] [Google Scholar]
- 56.Mullighan CG; Phillips LA; Su X; Ma J; Miller CB; Shurtleff SA, et al. , Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science 2008, 322 (5906), 1377–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmah J; Fedders B; Panzer-Grumayer R; Fischer S; Zimmermann M; Dagdan E, et al. , Molecular characterization of acute lymphoblastic leukemia with high CRLF2 gene expression in childhood. Pediatr Blood Cancer 2017, 64 (10). [DOI] [PubMed] [Google Scholar]
- 58.Zhang HH; Wang HS; Qian XW; Fan CQ; Li J; Miao H, et al. , Genetic variants and clinical significance of pediatric acute lymphoblastic leukemia. Ann Transl Med 2019, 7 (14), 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberts KG; Mullighan CG, How new advances in genetic analysis are influencing the understanding and treatment of childhood acute leukemia. Curr Opin Pediatr 2011, 23 (1), 34–40. [DOI] [PubMed] [Google Scholar]
- 60.Taylor OA; Brown AL; Brackett J; Dreyer ZE; Moore IK; Mitby P, et al. , Disparities in Neurotoxicity Risk and Outcomes among Pediatric Acute Lymphoblastic Leukemia Patients. Clin Cancer Res 2018, 24 (20), 5012–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Symanski E; Tee Lewis PG; Chen TY; Chan W; Lai D; Ma X, Air toxics and early childhood acute lymphocytic leukemia in Texas, a population based case control study. Environ Health 2016, 15 (1), 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu H; Yang W; Perez-Andreu V; Devidas M; Fan Y; Cheng C, et al. , Novel susceptibility variants at 10p12.31–12.2 for childhood acute lymphoblastic leukemia in ethnically diverse populations. J Natl Cancer Inst 2013, 105 (10), 733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim JY; Bhatia S; Robison LL; Yang JJ, Genomics of Racial and Ethnic Disparities in Childhood Acute Lymphoblastic Leukemia. Cancer 2014, 120 (7), 955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bryc K; Velez C; Karafet T; Moreno-Estrada A; Reynolds A; Auton A, et al. , Colloquium paper: genome-wide patterns of population structure and admixture among Hispanic/Latino populations. Proc Natl Acad Sci U S A 2010, 107 Suppl 2, 8954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mersha TB; Abebe T, Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum Genomics 2015, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Via M; Gignoux CR; Roth LA; Fejerman L; Galanter J; Choudhry S, et al. , History shaped the geographic distribution of genomic admixture on the island of Puerto Rico. PLoS One 2011, 6 (1), e16513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quiroz E; Aldoss I; Pullarkat V; Rego E; Marcucci G; Douer D, The emerging story of acute lymphoblastic leukemia among the Latin American population - biological and clinical implications. Blood Rev 2019, 33, 98–105. [DOI] [PubMed] [Google Scholar]
- 68.Walsh KM; Chokkalingam AP; Hsu LI; Metayer C; de Smith AJ; Jacobs DI, et al. , Associations between genome-wide Native American ancestry, known risk alleles and B-cell ALL risk in Hispanic children. Leukemia 2013, 27 (12), 2416–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perez-Andreu V; Roberts KG; Xu H; Smith C; Zhang H; Yang W, et al. , A genome-wide association study of susceptibility to acute lymphoblastic leukemia in adolescents and young adults. Blood 2015, 125 (4), 680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barragan FA; Mills LJ; Raduski AR; Marcotte EL; Grinde KE; Spector LG, et al. , Genetic ancestry, differential gene expression, and survival in pediatric B-cell acute lymphoblastic leukemia. Cancer Med 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee SHR; Antillon-Klussmann F; Pei D; Yang W; Roberts KG; Li Z, et al. , Association of Genetic Ancestry With the Molecular Subtypes and Prognosis of Childhood Acute Lymphoblastic Leukemia. JAMA Oncol 2022, 8 (3), 354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iacobucci I; Mullighan CG, Genetic Basis of Acute Lymphoblastic Leukemia. J Clin Oncol 2017, 35 (9), 975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tirado CA; Shabsovich D; Yeh L; Pullarkat ST; Yang L; Kallen M, et al. , A (1;19) translocation involving TCF3-PBX1 fusion within the context of a hyperdiploid karyotype in adult B-ALL: a case report and review of the literature. Biomark Res 2015, 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lilljebjorn H; Henningsson R; Hyrenius-Wittsten A; Olsson L; Orsmark-Pietras C; von Palffy S, et al. , Identification of ETV6-RUNX1-like and DUX4-rearranged subtypes in paediatric B-cell precursor acute lymphoblastic leukaemia. Nat Commun 2016, 7, 11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Enciso-Mora V; Hosking FJ; Sheridan E; Kinsey SE; Lightfoot T; Roman E, et al. , Common genetic variation contributes significantly to the risk of childhood B-cell precursor acute lymphoblastic leukemia. Leukemia 2012, 26 (10), 2212–5. [DOI] [PubMed] [Google Scholar]
- 76.Xu H; Cheng C; Devidas M; Pei D; Fan Y; Yang W, et al. , ARID5B genetic polymorphisms contribute to racial disparities in the incidence and treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol 2012, 30 (7), 751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reyes-Leon A; Ramirez-Martinez M; Fernandez-Garcia D; Amaro-Munoz D; Velazquez-Aragon JA; Salas-Labadia C, et al. , Variants in ARID5B gene are associated with the development of acute lymphoblastic leukemia in Mexican children. Ann Hematol 2019, 98 (10), 2379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garcia-Hernandez SC; Meneses-Sanchez P; Porchia LM; Torres-Rasgado E; Perez-Fuentes R; Gonzalez-Mejia ME, Differential effects of the methylenetetrahydrofolate reductase polymorphisms (C677T and A1298C) on hematological malignancies among Latinos: a meta-analysis. Genet Mol Biol 2019, 42 (3), 549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qian M; Xu H; Perez-Andreu V; Roberts KG; Zhang H; Yang W, et al. , Novel susceptibility variants at the ERG locus for childhood acute lymphoblastic leukemia in Hispanics. Blood 2019, 133 (7), 724–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harvey RC; Mullighan CG; Chen IM; Wharton W; Mikhail FM; Carroll AJ, et al. , Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood 2010, 115 (26), 5312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tortolero-Luna G; Torres-Cintron CR; Alvarado-Ortiz M; Ortiz-Ortiz KJ; Zavala-Zegarra DE; Mora-Pinero E, Incidence of thyroid cancer in Puerto Rico and the US by racial/ethnic group, 2011–2015. BMC Cancer 2019, 19 (1), 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ortiz AP; Soto-Salgado M; Calo W; Nogueras G; Tortolero-Luna G; Hebl S, et al. , Disparities in breast cancer in Puerto Rico and among Hispanics, non-Hispanic whites, and non-Hispanics blacks in the United States, 1992–2004. Breast J 2010, 16 (6), 666–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soto-Salgado M; Suárez E; Torres-Cintrón M; Pettaway CA; Colón V; Ortiz AP, Prostate Cancer Incidence and Mortality among Puerto Ricans: An Updated Analysis Comparing Men in Puerto Rico with US Racial/Ethnic Groups. Puerto Rico health sciences journal 2015, 31 (2), 107–13. [PubMed] [Google Scholar]
- 84.Soto-Salgado M; Suarez E; Calo W; Cruz-Correa M; Figueroa-Valles NR; Ortiz AP, Incidence and mortality rates for colorectal cancer in Puerto Rico and among Hispanics, non-Hispanic whites, and non-Hispanic blacks in the United States, 1998–2002. Cancer 2009, 115 (13), 3016–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to the confidentiality policy of the Puerto Rico Central Cancer Registry but are available from the corresponding author on reasonable request.