Abstract
Objective:
Unawareness of a deficit, anosognosia, can occur for visual or motor deficits and lends insight into awareness itself; however, lesions associated with anosognosia occur in many different brain locations.
Methods:
We analyzed 267 lesion locations associated with either vision loss (with and without awareness) or weakness (with and without awareness). The network of brain regions connected to each lesion location was computed using resting-state functional connectivity from 1000 healthy subjects. Both domain specific and cross-modal associations with awareness were identified.
Results:
The domain-specific network for visual anosognosia demonstrated connectivity to visual association cortex and posterior cingulate while motor anosognosia was defined by insula, supplementary motor area and anterior cingulate connectivity. A cross-modal anosognosia network was defined by connectivity to the hippocampus and precuneus (false discovery rate p<0.05).
Interpretation:
Our results identify distinct network connections associated with visual and motor anosognosia and a shared, cross-modal network for awareness of deficits centered on memory-related brain structures.
Keywords: vision, anosognosia, awareness, lesion network mapping, Anton syndrome
Introduction
Anosognosia, or unawareness of a specific deficit, has been described for many brain functions including motor and visual abilities.1–3 The most commonly reported cause of motor anosognosia, unawareness of weakness, is a right hemispheric stroke, often of the right middle cerebral artery.4, 5 Some studies suggest that motor anosognosia is primarily a disconnection syndrome of premotor regions (e.g. supplementary motor area) from attention/body-monitoring regions (insula, ventral prefrontal cortex and cingulate),6 while other studies suggest an additional, crucial role of memory structures, with disrupted activity in the hippocampus.1, 4, 7, 8
Much less is known about visual anosognosia, also called Anton syndrome, where there is complete cortical blindness and unawareness of vision loss.9 This disorder is almost always associated with bilateral injury to the visual cortex with the most commonly reported cause being bilateral occipital lobe infarcts, but lesion locations vary.2 Despite being described more than 100 years ago, visual anosognosia has had little formal analysis. 2, 10
Here we study lesion locations associated with weakness (with and without awareness) and vision loss (with and without awareness). As these two forms of anosognosia are in different locations, most often due to strokes in different vascular territories, lesions causing motor or visual anosognosia are less likely to directly overlap so the cross-modal correlates are ideally examined at the network level. For this reason, we used a recently validated technique termed lesion network mapping to test whether these lesion-induced deficits map to specific brain networks.11–14 We sought to identify 1) brain network connections associated with domain specific anosognosia and 2) brain network connections associated with anosognosia in general, independent of the specific deficit.
Methods
Patient inclusion and lesion mapping
We performed a systematic literature search to identify cases of visual anosognosia, including cases where brain injury was associated with: 1) complete visual loss, 2) lack of awareness of vision loss,9 3) a published image of sufficient quality and completeness for mapping which identified 24 unique cases of visual anosognosia. These lesions were mapped from the literature (KG) and reviewed for accuracy by a board-certified neurologist (IK). (Figure 1 and Supplementary Tables 1-3.) As controls for this group we included 69 individuals who had brain lesions that caused visual field deficits but were aware of the impairment, mapped in a recent study.11 To determine non-modality specific correlates of anosognosia, we also included previously analyzed, publicly available data from 174 individuals with hemiplegia, 95 of whom had anosognosia for hemiplegia and 79 of whom had awareness.1 To allow for comparison with the 2D vision loss lesions, these hemiplegia lesions were bisected along the axial, sagittal and coronal planes to produce 2D lesion slices. We analyzed previously published, publicly available images with patient consent per individual journal requirements. The study was approved by Mass General Brigham/Partners Institutional Review Board Protocol 2020P002987.
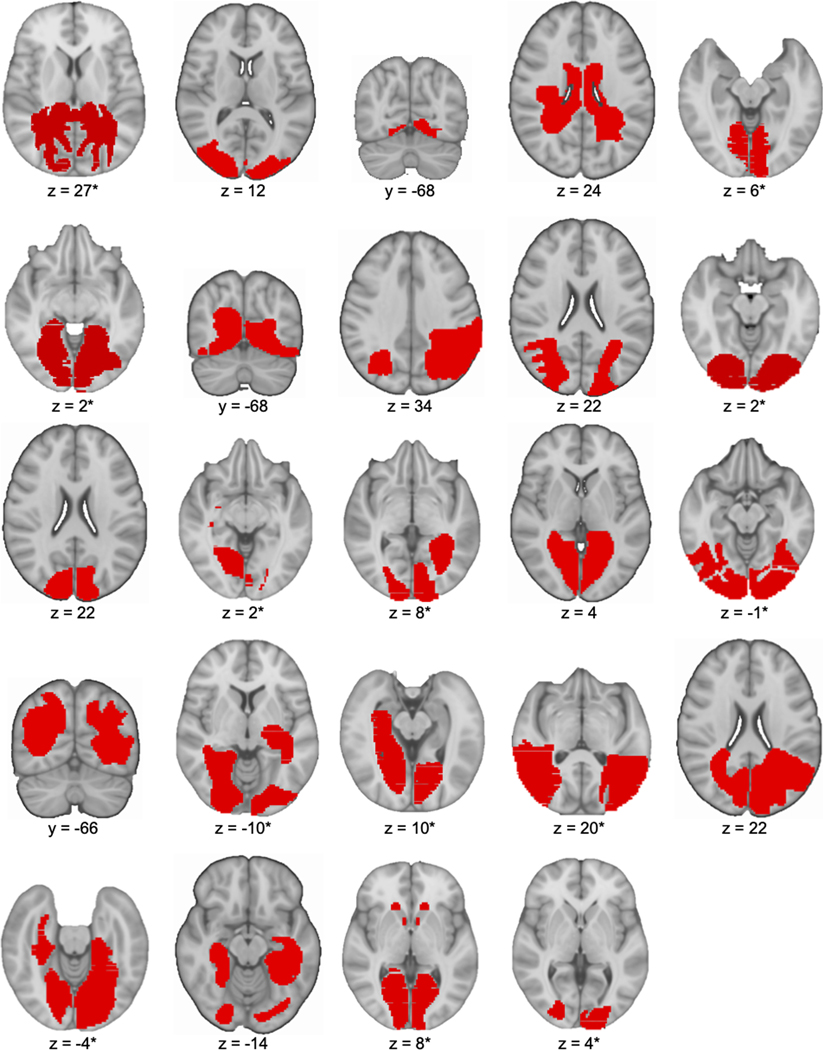
Figure 1.
Lesion maps for 24 patients with visual anosognosia. Outlined are lesions from 24 cases of visual anosognosia where brain injury was associated with: 1) vision loss, 2) lack of awareness of vision loss and 3) a published image of sufficient quality and completeness for mapping. *Brain atlas is re-sliced to a non-conventional orientation to match the non-conventional orientation of the original lesion image and coordinate provided is at the center of the lesion.
Lesion network preparation
All lesions were mapped into standard space and single-subject lesion connectivity maps were produced using a validated approach termed lesion network mapping.12 Specifically, we computed the resting-state functional connectivity between each lesion location and all other brain voxels using a large, publicly available functional connectome (n=1000, mean age 21.3, 42.7% male, 2×2×2mm)15 to produce individual lesion network maps; these unthresholded individual subject lesion network maps were then employed in the voxelwise analysis below.
Modality-specific and cross-modal anosognosia networks
To determine the modality-specific and cross-modal correlates of anosognosia we performed a voxelwise permutation-based ANOVA using Permutation Analysis of Linear Models employing four lesion groups and three key contrasts. The four lesion groups were: 1) visual anosognosia, 2) vision deficit with awareness, 3) motor anosognosia and 4) motor deficit with awareness. The three key contrasts were: 1) vision deficit versus motor deficit (regardless of awareness), 2) visual anosognosia versus motor anosognosia through the interaction effect, controlling for modality-specific deficits. Specifically, we computed visual anosognosia and motor deficit with awareness versus motor anosognosia and vision deficit with awareness, and 3) cross-modal anosognosia, combining visual anosognosia and motor anosognosia versus vision deficit with awareness and motor deficit with awareness. (Figure 2.) False-discovery rate (FDR) p<0.05 was set for significance on voxelwise testing. We elected to use the more liberal FDR rather than family-wise error (FWE) correction for multiple comparisons due to our use of a 4-way ANOVA to identify a general anosognosia network. To our knowledge, this is the first time this statistic has been used in lesion network mapping, which results in less statistical power for detection of true positives compared to a simple two-sample t-test. As such, we believe FDR correction provides a better balance between false positives and false negatives. Our use of FDR is consistent with existing recommendations for non-parametric analyses employing a general linear model in Permutation Analysis of Linear Models16 and prior lesion network mapping studies from our group and others.13, 17 We should also note that prior studies warning that FDR correction risks false positives focused on smaller sample sizes (n=30–60) and parametric models,18 while our analysis uses a large sample size (n=267) and non-parametric permutation-based statistics that are known to mitigate this risk.19, 20
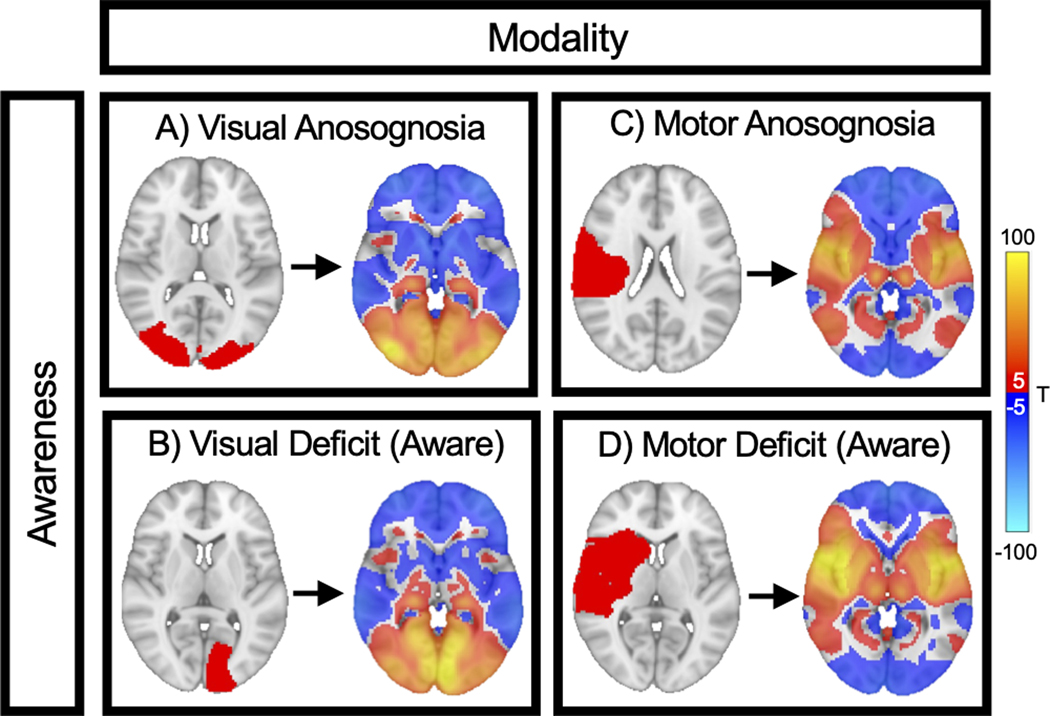
Figure 2. Method for identifying domain specific and cross-modal networks for awareness:
We analyzed four groups of lesions associated with A) visual anosognosia (n=24), B) visual deficit with awareness (n=69), C) motor anosognosia (n=95) and D) motor deficit with awareness (n=79). Each lesion location (red) was mapped to a common brain atlas (left image). Functional connectivity between each lesion location and all other brain voxels was computed using a large functional connectome, generating a lesion network map for each case (right image). Positive connections are shown in warm colors, negative connections are shown in cool colors. These 267 lesion network maps were entered into a single voxelwise ANOVA (modality x awareness) to generate 3 brain maps: 1) A “modality map” comparing visual deficit versus motor deficit (regardless of awareness), 2) a “modality-specific anosognosia map” based on the interaction between modality and awareness, and 3) a cross-modal “awareness map” comparing anosognosia versus awareness (regardless of the deficit).
To further validate our results we completed several secondary analyses. We ran the same ANOVA noted above with the following additional conditions: 1) we controlled for lesion volume, 2) controlled for patient age and lesion volume, 3) we removed visual controls with only a quadrantanopia and included only visual controls (aware of vision loss) with a hemianopia (n=57), and 4) we created bilateral masks of the unilateral vision loss control lesions by flipping across the midline to produce a mirrored mask and then derived lesion network maps for use in the same ANOVA. Note: While it is not known whether these artificially generated, bilateral vision control lesion masks would induce visual anosognosia, this analysis may help correct for some of the differences between visual anosognosia cases (bilateral lesions with complete vision loss) and the visual controls (field deficits with unilateral lesions).
Results
We identified 24 cases of visual deficits without awareness2, 21–41 (visual anosognosia, Figure 1, Supplementary Tables 1 & 2), 69 cases of visual deficits with awareness,11 95 cases of motor deficits without awareness (motor anosognosia), and 79 cases of motor deficits with awareness1 from several sources. The network of voxels connected to each lesion location was computed and the resulting 267 lesion networks were entered into a single voxelwise ANOVA. (Figure 2.)
Vision versus motor networks (main effect of modality):
Contrasting motor versus visual deficits (independent of awareness), showed the expected results: lesions associated with motor deficits are more connected to motor regions while lesions associated with visual deficits are more connected to visual regions. (Figure 3A)
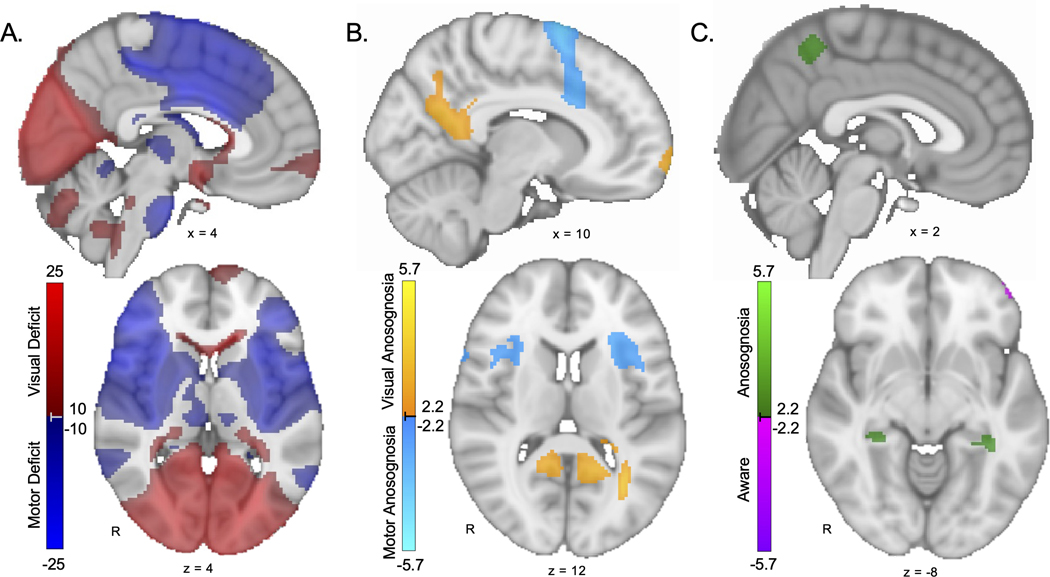
Figure 3. Modality-specific and cross-modal anosognosia networks.
A. Modality map showing lesion connections associated with vision deficits (red) versus motor deficits (blue) regardless of awareness. As expected, this map highlights domain-specific brain regions such as the calcarine sulcus and pre-central gyrus. B. Modality-specific anosognosia map showing lesion connections associated with visual anosognosia (warm colors) versus motor anosognosia (cool colors). Regions more specific for visual anosognosia include the posterior cingulate / inferior precuneus. Regions more specific for motor anosognosia include the anterior insula / frontal operculum and supplementary motor area / anterior cingulate. C. Cross-modal anosognosia map showing lesion connections associated with anosognosia (green) versus awareness of deficits (purple) across both vision and motor domains. Regions in the general anosognosia network include the bilateral hippocampi and bilateral superior precuneus. All voxels shown are false discovery rate p<0.05 (Note: Panel A is shown at a higher threshold of T>10 and T<−10 to better highlight the most significant results.)
Domain-specific anosognosia networks (interaction of modality x awareness):
Lesions associated with visual anosognosia were more connected to V2, posterior cingulate, retrosplenial cortex, inferior parietal lobe, medial frontal pole, corpus callosum and lateral occipital lobe/angular gyrus. Lesions associated with motor anosognosia were more connected to supplementary motor area (SMA), superior frontal gyrus, anterior cingulate, temporo-parietal junction, anterior insula, inferior frontal gyrus and temporal pole. (Figure 3B, Supplementary Table 4)
Cross-modal anosognosia network (main effect of awareness):
Lesions associated with anosognosia (independent of modality) were more connected to the precuneus/superior parietal lobule, posterior cingulate and hippocampus (Figure 3C, Supplementary Table 4). Connectivity to the hippocampi and superior precuneus was unique to this general anosognosia map suggesting no preference for visual versus motor anosognosia.
Secondary analyses
To ensure our findings were robust to methodological variation, we completed several additional analyses: 1) we controlled for lesion volume, 2) we controlled for patient age and lesion volume 3) we included only visual controls with a hemianopia (n=57), and 4) we made visual control lesions artificially bilateral to better match the bilateral visual anosognosia lesions. These analyses all led to similar results. (See Figure 4.)
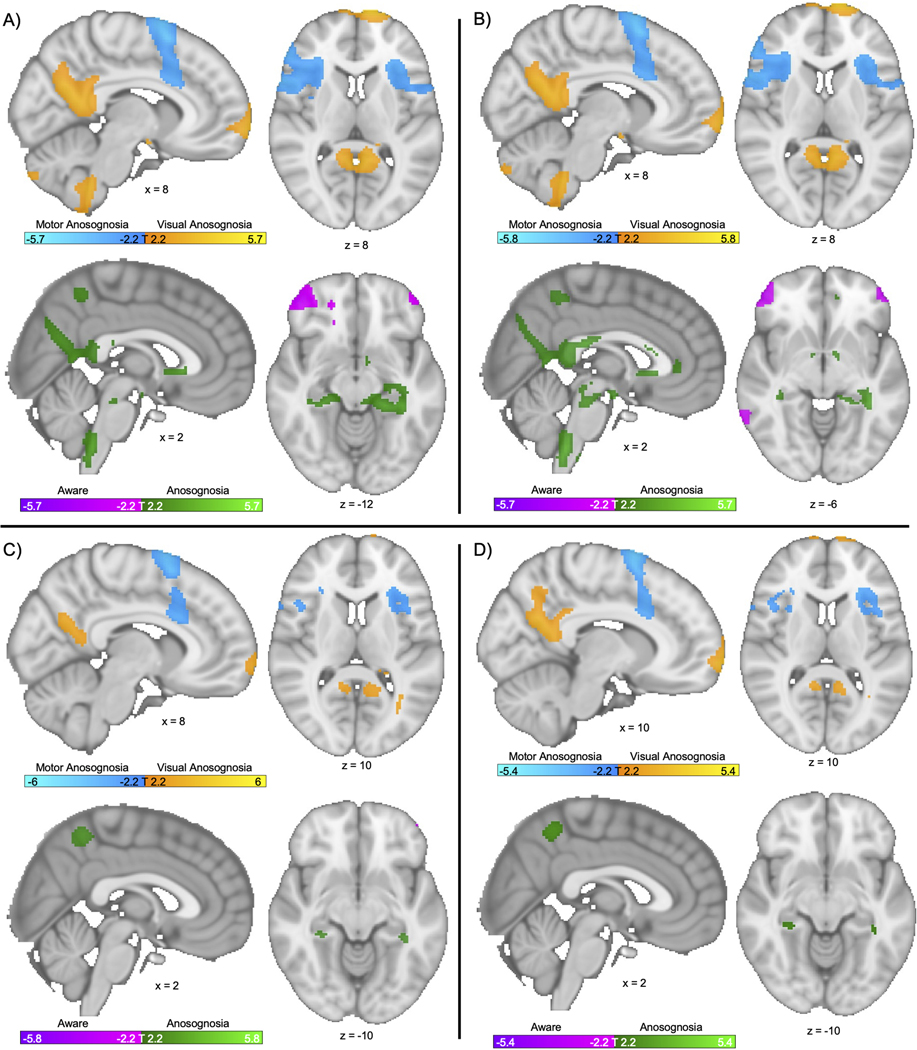
Figure 4. Findings are robust to methodological variation:
We repeated our primary analyses computing a modality-specific anosognosia map (see Figure 3B) and cross-modal anosognosia network (see Figure 3C) using different covariates (A, B) and different variations on our control lesions (C, D). A) Voxelwise ANOVA repeated controlling for lesion volume. Top: Modality specific network. Bottom: Cross-modal network. B) Voxelwise ANOVA repeated controlling for age and lesion volume. Top: Modality specific network. Bottom: Cross-modal network. C) Voxelwise ANOVA repeated with visual controls with hemianopia (n = 57) Top: Modality specific network. Bottom: Cross-modal network. D) Voxelwise ANOVA repeated with all visual controls made artificially bilateral to better mirror the bilateral nature of visual anosognosia subjects. Top: Modality specific network. Bottom: Cross-modal network. Note: All voxels shown are significant on false discovery rate p<0.05.
Discussion
In this study we analyzed the connectivity patterns of lesions associated with vision and motor deficits (with and without awareness of deficits) to identify network connections associated with anosognosia. We identified modality-specific connections for visual and motor anosognosia as well as cross-modal connections associated with anosognosia in general.
Modality-specific anosognosia networks
Our findings specific to motor anosognosia are concordant with prior work which has consistently found dysfunction of the insula, premotor regions, temporo-parietal junction and anterior cingulate to play a role in motor anosognosia.1, 4, 42 While there has been no previous, large formal analysis of visual anosognosia with complete cortical blindness (Anton’s syndrome), our findings of an association of lesion connectivity to V2 and posterior cingulate fits findings from a previous voxel-based morphometry study of the related but more limited condition of anosognosia for a partial visual field deficit.42 While the individual modality-specific regions of motor and visual anosognosia are different, their modality-specific functions share some similarities since the anterior insula/SMA in motor and V2 in vision both maintain internal representations of sensory information.43 Similarly, there seems to be modality-specific associations with different parts of the cingulate, a key region involved in metacognitive abilities,44 with the motor anosognosia network converging on the anterior cingulate and the visual anosognosia network on the posterior cingulate.
Cross-modal anosognosia network and memory
Our cross-modal anosognosia network converged on the precuneus and hippocampus, key structures associated with episodic memory and the default network.45, 46 Several crucial studies have highlighted the role of the hippocampus in motor anosognosia,1, 4, 8 However, our results are the first to identify the role of the hippocampus in a systematic analysis of visual anosognosia which is consistent with prior studies that noted the involvement of episodic memory dysfunction in visual anosognosia.47, 48 As to why only some previous studies on anosognosia converge on the hippocampus, it is likely that in studies focused on a single modality, the effects of modality-specific correlates predominate given their proximal connectivity to injuries. Studies that have used methods sensitive to more distant effects1, 4 converged on the hippocampus.
The precuneus, another key finding in our general anosognosia network, is not surprising given its role in awareness, metacognition and memory.7, 44, 45 Though the precuneus has not been consistently noted in studies of motor anosognosia, it has been associated with other forms of anosognosia, including anosognosia for a visual field deficit42 and cognitive anosognosia.44
Our results may align with the broader cognitive-awareness model of anosognosia44, 45 that hypothesizes that sensory or motor inputs must be compared to prior expectations stored in memory to recognize a new deficit. The cognitive-awareness model relates to prior theories that hypothesized that anosognosia could be caused by disconnection of modality specific regions or injury to a more general awareness system.48, 49 Our findings suggest that at the modality-specific level, anosognosia occurs from lesions functionally connected to both modality-specific representations of information (visual or motor) and metacognitive processing regions in the cingulate. This may disrupt appraisal of motor/visual function by cognitive comparator mechanisms. At the more general, cross-modal network level, both of these forms of anosognosia converge on the hippocampus and precuneus. Memory-associated structures are necessary to recognize a deficit by comparing present inputs to priors stored in memory while updating self-knowledge about perception/motor performance compared to previous abilities.44
Limitations
Our sample size was limited by the rarity of anosognosia for complete visual loss. More importantly we lack an ideal visual control group. It was challenging to identify the ideal set of control lesions for visual anosognosia, as we lacked a cohort of patients with complete cortical blindness who were aware of their deficit (the ideal control). For our main analysis, we used data from patients with partial visual field loss who were aware of their deficit. Results were similar if we restricted this control group to patients with hemianopia (i.e. complete loss of vision on one side) or if we created artificial bilateral lesions. However, none of these controls are ideal, and future work would benefit from studying patients with complete cortical blindness and dedicated testing of awareness. Another limitation is that our visual anosognosia and visual controls are derived from the literature and lack formal testing for awareness. While all patients in the motor cohort and most patients in the visual cohort suffered a stroke as the mechanism of injury, 25% of visual anosognosia patients and 22% of the visual controls11 had a different mechanism of injury. In addition, the timing between injury and visual testing was different in patients with visual anosognosia, where most were tested within one month of injury, and our visual controls where nearly all were tested more than one month from injury.11 Awareness of deficits after stroke is a dynamic phenomenon8, 47 which can improve with time; which lesion locations are more likely to recover awareness cannot be better addressed within our visual anosognosia cohort which relies on prior case reports for this rare condition. Further, we lacked 3-dimensional images for visual anosognosia and visual controls. This, unfortunately, limited our ability to statistically analyze the role of lesion location in anosognosia via techniques such as voxel-lesion symptom mapping. However, in regards to the validity of lesion network maps derived from 2-dimensonal lesion images, previous analyses have demonstrated that 2-dimensional slices can appropriately approximate the connectivity patterns of a whole lesion.12 An additional limitation is that we relied on false discovery rate rather than the more conservative family-wise error correction. There was some overlap between the modality-specific anosognosia network and the general anosognosia network as there can be connections more associated with one modality or the other that are also significant as part of a general anosognosia network.
Conclusion
By analyzing the connectivity patterns of lesions that cause different forms of anosognosia we identify modality-specific networks for visual and motor anosognosia as well as a cross-modal anosognosia network centered on the hippocampus and precuneus.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Valentina Pacella and co-authors for making their lesion dataset publicly available. We also thank Dr. Michael P. H. Stanley and William Drew for their advice and technical assistance. AC was funded by NIH/NIMH K23MH120510, the Child Neurology Foundation, and the Simons Foundation Autism Research Initiative. M.D.F. was supported by the Nancy Lurie Marks Foundation, the Mather’s Foundation, the Ellison / Baszucki Foundation, the Kaye Family Research Endowment and National Institutes of Health grants R21 MH126271, R56 AG069086, R01 MH113929, R01 MH115949, and R01 AG060987.
Footnotes
Potential Conflicts of interest
MDF is a consultant for Magnus Medical and Soterix and holds intellectual property on using connectivity imaging to guide brain stimulation. The other authors report no competing interests.
Data availability
The functional connectivity data employed in this study is available online through the Harvard Dataverse at: https://doi.org/10.7910/DVN/ILXIKS and the pipeline used to prepare the functional connectivity data is available at: https://github.com/bchcohenlab/BIDS_to_CBIG_fMRI_Preproc2016.Lesion data used in this study is publicly available and obtained from published medical literature. (See Supplementary Table 2, Kletenik et.al. 202211 and Pacella et.al. eLife 2019.1) Statistical neuroimaging analyses, specifically the voxelwise ANOVA was performed in MatLab (version 2019b) and FSL (version 5.0.10). The maps for our primary analyses can be accessed on NeuroVault at the following link: https://identifiers.org/neurovault.collection:13792
References
- 1.Pacella V, Foulon C, Jenkinson PM, et al. Anosognosia for hemiplegia as a tripartite disconnection syndrome. Elife. 2019. Aug 6;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim N, Anbarasan D, Howard J. Anton syndrome as a result of MS exacerbation. Neurol Clin Pract. 2017. Apr;7(2):e19–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cocchini G, Beschin N, Cameron A, Fotopoulou A, Della Sala S. Anosognosia for motor impairment following left brain damage. Neuropsychology. 2009. Mar;23(2):223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klingbeil J, Wawrzyniak M, Stockert A, Karnath HO, Saur D. Hippocampal diaschisis contributes to anosognosia for hemiplegia: Evidence from lesion network-symptom-mapping. Neuroimage. 2020. Mar;208:116485. [DOI] [PubMed] [Google Scholar]
- 5.Kortte KB, McWhorter JW, Pawlak MA, Slentz J, Sur S, Hillis AE. Anosognosia for hemiplegia: The contributory role of right inferior frontal gyrus. Neuropsychology. 2015. May;29(3):421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karnath HO, Baier B, Nagele T. Awareness of the functioning of one’s own limbs mediated by the insular cortex? J Neurosci. 2005. Aug 3;25(31):7134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrotin A, Desgranges B, Landeau B, et al. Anosognosia in Alzheimer disease: Disconnection between memory and self-related brain networks. Ann Neurol. 2015. Sep;78(3):477–86. [DOI] [PubMed] [Google Scholar]
- 8.Vocat R, Staub F, Stroppini T, Vuilleumier P. Anosognosia for hemiplegia: a clinical-anatomical prospective study. Brain. 2010. Dec;133(Pt 12):3578–97. [DOI] [PubMed] [Google Scholar]
- 9.Prigatano GP, Wolf TR. The Study of Anosognosia. In: Prigatano GP, editor. New York, USA: Oxford University Press, Incorporated; 2010. [Google Scholar]
- 10.Gaudet K, Fox M, Kletenik I. Network Localization of Visual Anosognosia from 28 Cases of Anton Syndrome (P3–6.001). Neurology. 2022;98(18 Supplement):861. [Google Scholar]
- 11.Kletenik I, Ferguson MA, Bateman JR, et al. Network Localization of Unconscious Visual Perception in Blindsight. Ann Neurol. 2021. Dec 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boes AD, Prasad S, Liu H, et al. Network localization of neurological symptoms from focal brain lesions. Brain. 2015. Oct;138(Pt 10):3061–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen AL, Soussand L, Corrow SL, Martinaud O, Barton JJS, Fox MD. Looking beyond the face area: lesion network mapping of prosopagnosia. Brain. 2019. Dec 1;142(12):3975–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqi SH, Kletenik I, Anderson MC, Cavallari M, Chitnis T, Glanz BI, Khalil S, Palotai M, Bakshi R, Guttmann CRG, Fox MD. Lesion network localization of depression in multiple sclerosis. Nature Mental Health. 2023;1(1). [Google Scholar]
- 15.Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011. Sep;106(3):1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alberton BAV, Nichols TE, Gamba HR, Winkler AM. Multiple testing correction over contrasts for brain imaging. Neuroimage. 2020. Aug 1;216:116760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mithani K, Boutet A, Germann J, et al. Lesion Network Localization of Seizure Freedom following MR-guided Laser Interstitial Thermal Ablation. Sci Rep. 2019. Dec 9;9(1):18598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirman D, Landrigan JF, Kokolis S, Verillo S, Ferrara C, Pustina D. Corrections for multiple comparisons in voxel-based lesion-symptom mapping. Neuropsychologia. 2018. Jul 1;115:112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002. Apr;15(4):870–8. [DOI] [PubMed] [Google Scholar]
- 20.Lindquist MA, Mejia A. Zen and the art of multiple comparisons. Psychosom Med. 2015. Feb-Mar;77(2):114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo Buono V, De Salvo S, Paladina G, et al. Anton’s Syndrome associated with autotopagnosia. Appl Neuropsychol Adult. 2020. May-Jun;27(3):294–8. [DOI] [PubMed] [Google Scholar]
- 22.Martín Juan A, Madrigal R, Porta Etessam J, Sáenz-Francés San Baldomero F, Santos Bueso E. Anton-Babinski syndrome, case report. Arch Soc Esp Oftalmol (Engl Ed). 2018. Nov;93(11):555–7. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhry FB, Raza S, Ahmad U. Anton’s syndrome: a rare and unusual form of blindness. BMJ Case Rep. 2019. Dec 3;12(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zukić S, Sinanović O, Zonić L, Hodžić R, Mujagić S, Smajlović E. Anton’s Syndrome due to Bilateral Ischemic Occipital Lobe Strokes. Case Rep Neurol Med. 2014;2014:474952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwong Yew K, Abdul Halim S, Liza-Sharmini AT, Tharakan J. Recurrent bilateral occipital infarct with cortical blindness and anton syndrome. Case Rep Ophthalmol Med. 2014;2014:795837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollard KC, Brown Bissonnette GA, Norberg SR. Bilateral cortical visual impairment resulting in Anton’s syndrome. Clin Exp Optom. 2020. Nov;103(6):927–8. [DOI] [PubMed] [Google Scholar]
- 27.Zago S, Corti S, Bersano A, et al. A cortically blind patient with preserved visual imagery. Cogn Behav Neurol. 2010. Mar;23(1):44–8. [DOI] [PubMed] [Google Scholar]
- 28.Galetović D, Karlica D, Bojić L, Znaor L. Bilateral cortical blindness--Anton syndrome: case report. Coll Antropol. 2005;29 Suppl 1:145–7. [PubMed] [Google Scholar]
- 29.Elhassan M, Saidahmed O, Adebayo A, Archibald N. Persistent Cortical Blindness Following Posterior Reversible Encephalopathy Syndrome (PRES) as a Complication of COVID-19 Pneumonia. Cureus. 2021. Jan 19;13(1):e12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godasi R, Rupareliya C, Bollu PC. Bilateral Occipital Lobe Hemorrhages Presenting as Denial of Blindness in Posterior Reversible Encephalopathy Syndrome- A Rare Combination of Anton Syndrome and Encephalopathy. Cureus. 2017. Oct 4;9(10):e1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trifiletti RR, Syed EH, Hayes-Rosen C, Parano E, Pavone P. Anton-Babinski syndrome in a child with early-stage adrenoleukodystrophy. Eur J Neurol. 2007. Feb;14(2):e11–2. [DOI] [PubMed] [Google Scholar]
- 32.Saeed N, Khoo CS, Remli R, et al. First Reported Case of Neuroleptospirosis Complicated With Anton’s Syndrome. Front Neurol. 2018;9:966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srikant RG, Deepa D, Murthy PR, Dhar R. Anton’s Syndrome and Cortical Blindness. Indian Journal of Clinical Practice. 2012;23(2). [Google Scholar]
- 34.Kondziella D, Frahm-Falkenberg S. Anton’s syndrome and eugenics. J Clin Neurol. 2011. Jun;7(2):96–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao S, Zhu X, Zhang W, Xia M. Anton’s syndrome as a presentation of Trousseau syndrome involving the bilateral optic radiation. J Int Med Res. 2020. Nov;48(11):300060520972907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maddula M, Lutton S, Keegan B. Anton’s syndrome due to cerebrovascular disease: a case report. J Med Case Rep. 2009. Sep 9;3:9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kartsounis LD, James-Galton M, Plant GT. Anton syndrome, with vivid visual hallucinations, associated with radiation induced leucoencephalopathy. J Neurol Neurosurg Psychiatry. 2009. Aug;80(8):937–8. [DOI] [PubMed] [Google Scholar]
- 38.Carvajal JJR, Cárdenas AAA, Pazmiño GZ, Herrera PA. Visual anosognosia (Anton-Babinski Syndrome): report of two cases associated with ischemic cerebrovascular disease. Journal of Behavioral and Brain Science. 2012;2(03):394–8. [Google Scholar]
- 39.Espinoza-López DA, Soto-Hernández JL, Cárdenas G. Cortical blindness (Anton-Babinski Syndrome), an Unusual Manifestation of Central Nervous System Tuberculosis. Journal of Neurology and Stroke. 2016;4. [Google Scholar]
- 40.Eby SA, Buchner EJ, Bryant MG, Mak HK. The rehabilitation of Anton syndrome. Pm r. 2012. May;4(5):385–7. [DOI] [PubMed] [Google Scholar]
- 41.Chen JJ, Chang HF, Hsu YC, Chen DL. Anton-Babinski syndrome in an old patient: a case report and literature review. Psychogeriatrics. 2015. Mar;15(1):58–61. [DOI] [PubMed] [Google Scholar]
- 42.Baier B, Geber C, Muller-Forell W, Muller N, Dieterich M, Karnath HO. Anosognosia for obvious visual field defects in stroke patients. Brain Struct Funct. 2015;220(3):1855–60. [DOI] [PubMed] [Google Scholar]
- 43.Fotopoulou A, Pernigo S, Maeda R, Rudd A, Kopelman MA. Implicit awareness in anosognosia for hemiplegia: unconscious interference without conscious re-representation. Brain. 2010. Dec;133(Pt 12):3564–77. [DOI] [PubMed] [Google Scholar]
- 44.Morris RG, Mograbi DC. Anosognosia, autobiographical memory and self knowledge in Alzheimer’s disease. Cortex. 2013. Jun;49(6):1553–65. [DOI] [PubMed] [Google Scholar]
- 45.Antoine N, Bahri MA, Bastin C, et al. Anosognosia and default mode subnetwork dysfunction in Alzheimer’s disease. Hum Brain Mapp. 2019. Dec 15;40(18):5330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Therriault J, Ng KP, Pascoal TA, et al. Anosognosia predicts default mode network hypometabolism and clinical progression to dementia. Neurology. 2018. Mar 13;90(11):e932–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redlich FC, Dorsey JF. Denial Of Blindness By Patients With Cerebral Disease. Archives of neurology and psychiatry (Chicago). 1945;53(6):407–17. [Google Scholar]
- 48.McGlynn SM, Schacter DL. Unawareness of deficits in neuropsychological syndromes. J Clin Exp Neuropsychol. 1989. Mar;11(2):143–205. [DOI] [PubMed] [Google Scholar]
- 49.Schacter DL. Toward a cognitive neuropsychology of awareness: implicit knowledge and anosognosia. J Clin Exp Neuropsychol. 1990. Jan;12(1):155–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The functional connectivity data employed in this study is available online through the Harvard Dataverse at: https://doi.org/10.7910/DVN/ILXIKS and the pipeline used to prepare the functional connectivity data is available at: https://github.com/bchcohenlab/BIDS_to_CBIG_fMRI_Preproc2016.Lesion data used in this study is publicly available and obtained from published medical literature. (See Supplementary Table 2, Kletenik et.al. 202211 and Pacella et.al. eLife 2019.1) Statistical neuroimaging analyses, specifically the voxelwise ANOVA was performed in MatLab (version 2019b) and FSL (version 5.0.10). The maps for our primary analyses can be accessed on NeuroVault at the following link: https://identifiers.org/neurovault.collection:13792