Summary
Recent literature has significantly advanced our knowledge and understanding of the cholangiocarcinoma tumor immune microenvironment. Detailed characterization of the immune landscape has defined new patient subtypes. While not utilized in clinical practice yet, these novel classifications will help inform decisions regarding immunotherapeutic approaches. Suppressive immune cells, such as tumor-associated macrophages and myeloid derived suppressor cells, form a barrier that shields tumor cells from immune surveillance. The presence of this immunosuppressive barrier in combination with a variety of immune escape mechanisms employed by tumor cells lead to poor tumor immunogenicity. Broad strategies to re-equip the immune system include blockade of suppressive immune cell recruitment to priming cytotoxic effector cells against tumor antigens. While immunotherapeutic strategies are gaining traction in the treatment of cholangiocarcinoma, there is a long road of discovery ahead in order to make meaningful contributions to patient therapy and survival.
Keywords: Immunosuppressive myeloid cells, immune evasion, immunogenic, preclinical models
Introduction
Cholangiocarcinoma (CCA) is a highly lethal, heterogeneous, primary hepatic malignancy that arises from the biliary epithelium. It is classified into three major subtypes based on site of anatomic origin: intrahepatic (iCCA), perihilar (pCCA), and distal (dCCA).1 While it is a rare malignancy, the incidence of CCA, particularly iCCA, has been increasing over the past four decades.1 Despite recent advances in the understanding of CCA tumor biology and therapeutic target identification, patient prognosis has not improved significantly.2 Median overall survival is 11.7-13 months with an estimated 5-year survival of 20%.3,4 Due to a lack of early symptoms and reliable diagnostic markers, patients frequently present at an advanced stage which results in very limited treatment options.1–3 Surgery remains the only potentially curative treatment for patients with CCA.2 Thus, current chemotherapeutic options and treatment adjuncts primarily prolong survival in patients with inoperable disease.5 Therefore, a critical need exists to discover novel systemic therapeutic strategies for CCA.
The knowledge and ability to reengineer the immune system for antitumor defense is a promising strategy in the armamentarium of cancer treatment. Multiple immune cell types are present in the tumor immune microenvironment (TIME) and play a significant role in cancer biology.6 Chemotherapy has demonstrated immunomodulatory effects in multiple cancer types and immune checkpoint inhibition (ICI) has been an effective therapeutic in several tumors, including a subset of biliary tract cancers.7–9 Durvalumab was recently approved in combination with standard of care cytotoxic chemotherapy (gemcitabine and cisplatin) for advanced biliary tract cancer by the FDA based on the results of the TOPAZ-1 phase III trial.7 The combination of pembrolizumab and gemcitabine/cisplatin is currently being evaluated in the KEYNOTE-966 trial, a randomized phase III trial in patients with advanced and/or unresectable biliary tract cancer. Pending peer-reviewed publication, the study’s sponsor communicated in a press release that the combination demonstrated a statistically significant improvement in overall survival compared to chemotherapy alone.10 While these results of ICI in combination with cytotoxic therapy are encouraging, historically response to ICI monotherapy in CCA has been poor.11 However, cancer types have unique and specific immunological environments which either lend themselves to effective immunotherapy or serve as barriers to immunotherapeutics. CCAs are postulated to be immunologically “cold” tumors with a non-T cell infiltrated TIME.12 Ongoing research continues to report the presence of an immunosuppressive barrier around CCA tumors.13,14 This barrier includes the presence of immune checkpoint molecules, increased expression of which is associated with worse prognosis.15 Therefore, modulation of TIME components to overcome the immunosuppressive barrier remains an area of untapped potential in the treatment of CCA.
Herein, we will review the current understanding of the CCA TIME and discuss the mechanisms employed for immune surveillance evasion and immunosuppression. We will then discuss emerging preclinical research that can potentially reengineer the CCA TIME for antitumor defense. Finally, we will coalesce the current insights of CCA immunobiology and point to new areas of potential research.
Immune Classification of CCA
CCA is classically divided into three subtypes based on anatomic site of origin.1 In addition, a number of molecular subgroups have been identified with targetable alterations. The emergence of immunotherapy as an effective treatment option in a subset of cancer patients has emphasized the need for a more comprehensive understanding of the TIME and development of immune-based classifications. Indeed, retrospective analyses of immunotherapy treated patients have uncovered different TIME subtypes that are associated with varying levels of immunotherapy responsiveness.6
TIME-based classifications of CCA have identified CCA subtypes or subclasses that are characterized by differing mechanisms of immune escape and patient outcomes (Table 1). An immune classification of iCCA derived from bulk transcriptomic data of 198 resected iCCA patients described immune-desert, immunogenic, myeloid and mesenchymal subclasses.16 Tumors from the inflamed subtype were characterized by a high TIL population which has immunotherapeutic implications. Consistent with emerging knowledge that CCAs are overall immunosuppressive, the largest percentage of CCAs were identified as immune desert. Likewise, a TIME classification based on bulk transcriptomic data stratified 961 iCCA patients in 5 classes with the majority (65%) encompassing a non-inflamed profile.17
Table 1 – Immune based classifications of iCCA.
Subtypes of iCCA identified include immune-infiltrated, immune altered, or immune excluded/immunosuppressive.
| Immune-infiltrated | Immune-altered, Immunosuppressive infiltrate | Immune-excluded | |
|---|---|---|---|
| TME-Based iCCA Classification16 | |||
| Immune Desert | 46-48% | ||
| Immunogenic | 9-13% | ||
| Myeloid-rich | 13-19% | ||
| Mesenchymal | 22-28% | ||
| Total % of iCCAs | 31-41% | 13-19% | 46-48% |
| iCCA Stroma, Tumor, and Immune Microenvironment Classification17 | |||
| Immune Classical | 10% | ||
| Inflammatory Stroma | 25% | ||
| Hepatic Stem-Like | 35% | ||
| Tumor Classical | 10% | ||
| Desert-like | 20% | ||
| Total % of iCCAs | 10% | 60% | 30% |
| TLS-Based iCCA Classification20 | |||
| Class I – TLS predominantly in peri-tumoral region | 28.1% | ||
| Class II – Heterogeneous distribution of TLS, majority in peritumor region | 31.1% | ||
| Class III – Heterogeneous distribution of TLS, majority in intra-tumor region | 28.4% | ||
| Class IV – TLS predominantly in intra-tumoral region | 12.5% | ||
| Total % of iCCAs | 12.5% | 59.5% | 28.1 % |
iCCA, intrahepatic cholangiocarcinoma; TLS, tertiary lymphoid structures; TME, tumor microenvironment
Single cell transcriptomic analysis of 14 pairs of human iCCA tumor and adjacent liver identified novel markers for differentiation of the two main histological subtypes of iCCA, perihilar large duct and peripheral small duct.18 SSP1 and S100P were observed as the optimal biomarkers for iCCA peripheral small duct type and perihilar large duct type, respectively. Accurate subtype differentiation subsequently allowed improved TIME characterization.
Tertiary lymphoid structures (TLSs) resemble lymph nodes in terms of composition, are often located in or near tumors, and appear to play a significant role in dictating the TIME.19 A comprehensive assessment of the spatial distribution, abundance, and cellular composition of TLS in iCCA characterized 4 immune subclasses with prognostic implications.20 The 5-year survival of patients stratified to the immune-excluded class was significantly worse than those in the immune-active class. In aggregate, these analyses indicate that CCAs are generally immunosuppressive and lend an explanation for the subpar clinical response to ICI monotherapy in CCA patients.
Immunosuppressive Barrier in the CCA TIME
CCAs are characterized by a dense desmoplastic microenvironment composed of stromal, endothelial, and, immune cells.21,22 Existing within an extensively remodelled extracellular matrix, these cells work in concert to form an intricate web of molecular crosstalk that ultimately promotes CCA progression, immunosuppression, and therapeutic resistance.12,21,22 Emerging evidence suggests that there is wide-ranging, immunosuppressive crosstalk within the CCA TIME highlighting the integral role of the suppressive cell type.13,14,23
Tumor-associated macrophages (TAMs) are a dominant suppressive cell type in the CCA TIME and heavily implicated in tumor immune escape mechanisms.13,14,24 TAMs are phagocytic, innate immune cells that are mainly derived from either circulating monocytes or liver-resident Kupffer cells.25,26 TAMs perform an essential role in CCA development and progression through various mechanisms including immune escape and angiogenesis.24 Higher TAM infiltration has been associated with worse outcomes in CCA.27–29 Recent literature has provided insight into mechanisms underlying TAM recruitment, infiltration, and polarization. The majority of TAMs in established tumors are recruited peripheral monocytes.13 Once infiltrated, TAMs often deviate into a tumor-promoting, immunosuppressive phenotype.13,14,30 The interactions between immunosuppressive TAMs, cancer cells, and other suppressive elements in the TIME have been observed to induce many of the hallmarks of cancer.31 This in turn creates a delicate balance that can pose a challenge for immunotherapeutic approaches and suggests that depletion of a single suppressive population will not be sufficient as an antitumor strategy. Indeed, TAM depletion in mice did not improve murine survival due to a compensatory emergence of myeloid-derived suppressor cells (MDSCs).13 Dual TAM and G-MDSC blockade was employed to successfully sensitize preclinical CCA models to ICI therapy with subsequent prolongation of murine survival.
MDSCs are a population of immature myeloid cells that embody potent immunosuppressive functionality.32,33 Accordingly, higher levels of MDSCs correlate with worse disease prognosis and MDSCs have been implicated in limiting the success of ICI.32,34,35 MDSCs are divided into two main groups: monocytic - MDSCs and granulocytic(G-MDSCs)/polymorphonuclear (PMN-MDSCs).32 Our understanding of MDSC recruitment and functionality in hepatopancreaticobiliary cancers is primarily based on studies of hepatocellular carcinoma or pancreatic ductal adenocarcinoma. MDSC biology remains relatively unexplored in CCA leading to sometimes conflicting results. There is some evidence of a positive correlation between CCA tumor burden and MDSC levels, although this was not confirmed in a subsequent study.36,37 Further studies are needed to elucidate the biological mechanisms underlying MDSCs in CCA given they likely play a critical role in tumor immune evasion.13,14,23
Tumor-associated neutrophils (TANs) are another key suppressive population in the CCA TIME and play a much larger role than previously understood.38 There is increasing evidence that TANs promote carcinogenesis by supporting angiogenesis, modulating tumor immune escape, and stimulating tumor cell migration.38–40 However, TANs retain some molecular plasticity and take functional cues from the surrounding TIME, similar to TAMs. Thus, their functionality can be phenotypically multifaceted and sometimes opposing.38,41 Neutrophil infiltration is mediated by various chemokines and cytokines.38 Elevated numbers of infiltrated TANs have been associated with increased tumor aggression, unfavorable prognosis, and poor overall survival in patients with CCA.40,42 Additionally, elevated neutrophil-to-lymphocyte ratio is associated with worse overall survival in patients with CCA.43,44 Single-cell RNA sequencing (scRNA-seq) analysis has demonstrated significant overlap between mouse and human neutrophil subsets providing a widely accessible platform for further investigative studies to uncover the mechanisms underlying TAN activation and phenotypic control.40
Finally, regulatory T cells (Tregs) confer immune tolerance by variable mechanisms including the utilization of interleukin (IL)-IO and transforming growth factor- β1 (TGF-β1) to induce immunosuppressive responses against antigen presenting, natural killer (NK), and cytotoxic T cells.45 An altered network of transcription factors was identified by scRNA-seq between tumor-infiltrating and peritumoral T cells and suggested augmented immunosuppressive function of intratumoral Tregs.46 Both inhibitory myeloid and activating Treg pathways were identified and implicate specific targets for future work. Increased overall Tregs in the CCA TIME by IHC of resected human CCA specimens are associated with poor survival in patients.42,47 Thus, there are various suppressive elements in the CCA TIME that form layers of resistance or “walls of defense” within the TIME that protect the tumor and allow it to hide from the immune system.
Mechanisms of immune evasion
Poorly immunogenic tumors such as CCAs leverage multiple mechanisms to escape the anti-tumor immune response. Suppressive immune cells in the CCA TIME secrete immunosuppressive elements and modulate oncogenic signaling pathways in order to promote attenuated immune response and tumor growth. Another compelling area of research is the exploration of the gut-liver axis and how CCA cells manipulate the gut microbiome to mediate host immune defense. Finally, CCA cells modulate genetic and epigenetic expression to evade immune surveillance and immunogenic cell death.
Oncogenic Signaling and Immunosuppressive Cytokines and Molecules
Suppressive immune cells in the CCA TIME promote oncogenic signaling which leads to reduced cytotoxic T lymphocyte (CTL) infiltration and a subsequent protumor TIME. Signal transducer and activator of transcription 3 (STAT3) signaling is implicated in CTL suppression as STAT3 knockdown in TAMs and TANs resulted in the loss of their protumor effects.39 MicroRNA (miR)-183-5p is upregulated in iCCA-derived exosomes.48 Exosomal miR-183-5p resulted in increased programmed cell death ligand-1 (PD-L1) expression on TAMs and suppressed CTL activity through a phosphatase and tensin homolog/AKT/PDL-1 pathway. The effects of miR-183-5p were reversible with TAM PD-L1 blockade.
Cluster of differentiation 47 (CD47) is a transmembrane, antiphagocytic glycoprotein with ubiquitous presence on the surface of human cells.49 Increased expression of CD47 has been observed on CCA cells as a means to prevent tumor cell phagocytosis and avoid immune-mediated cell death. Anti-CD47 treatment in preclinical CCA models resulted in increased macrophage phagocytosis.50 Fas/Fas ligand (FasL) has also been implicated as a tumor immune escape strategy utilized by iCCA cells as increased expression led to apoptosis of CTLs and NK cells.51
Secretion of immunosuppressive elements functions as the primary mechanism through which tumors evade the immune system. Tumor cells secrete immunosuppressive cytokines, IL-10 and TGF-β, as a means of inducing Tregs and inhibiting dendritic cell (DC) antigen presentation.52 These cytokines result in repression of CD4+ and CD8+ T cell activity. Treatment of monocyte-derived DCs with IL-10 and TGF-β receptor neutralizing antibodies resulted in increased IFN-γ production and improved DC activated-CTL effectivity against CCA cells in vitro,53 In a subsequent study, self-differentiated (SD)-DCs were treated with lentiviral short-hairpin RNAs to knockdown IL-10 and TGF-β receptors. SD-DCs were subsequently pulsed with CCA tumor antigen and cocultured with cytotoxic effector cells. Suppression of the IL-10 and TGF-β receptors resulted in activated SD-DCs, increased IFN-γ production, and enhanced cytotoxicity of T-cells against CCA.54 TGF-β and IL-6 promote the differentiation of naive T cells into Tregs or IL-17 producing T helper cells (Th17).55 TGF-β1 expression by CCA cells resulted in the accumulation of Tregs at the tumor center, while IL-6 expression resulted in Th17 accumulation at the invasion front.56 Thus, CCA cells, through specific cytokine release, can engender a tumor escape mechanism by allowing infiltration of suppressive elements while restricting anti-tumor cells to the periphery.
An essential and thematic mechanism of tumor immune evasion is increased expression of immune checkpoint molecules in the TIME.8,15,57 PD-L1 interacts with programmed cell death protein 1 (PD-1) and results in inhibition of effector T cell function.57 PD-L1+ TAMs have increased expression in both murine and human CCA and are likely the predominant source of PD-L1 in CCA.13,14,58,59 As such, TAMs have been implicated in initiating and propagating an immunosuppressive barrier around CCA tumors and consequent attenuation of cytotoxic immune cell functionality. Single-cell RNA-sequencing analysis of 8 human iCCAs and adjacent tissues found that tumor-infiltrating Tregs exhibit high immunosuppression markers including cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and T cell immunoreceptor with Ig and ITIM domains.60 CTLA-4 is another immune checkpoint molecule that binds CD80 on antigen-presenting cells and inhibits cytotoxic T cell activation. Low CTLA-4 expression in the peri-tumoral region was associated with improved relapse-free survival in a study profiling the immune-related transcriptome of 53 resected biliary tract cancers.61
Gut-Liver Axis
The gut microbiome has been previously implicated in the promotion of carcinogenesis and mediation of host immune response through dysbiosis or metabolic processes. In the CCA paradigm, knowledge and characterization of the gut microbiome has been explored as a biomarker for CCA detection or to predict ICI response.62,63 Our understanding of the gut-liver axis, however, is evolving and recent discoveries have demonstrated that manipulation of the gut-liver axis could provide a novel therapeutic approach in CCA. Tumors may co-opt commensal gut bacteria to evade immunosurveillance. Accordingly, the gut microbiome may impact response to immunotherapy.62 Preclinical data in mice supports this notion as commensal gut bacteria have been demonstrated to control the accumulation of C-X-C motif chemokine receptor (CXCR)-2+ PMN-MDSCs through a toll-like receptor 4/CXCL1/CXCR2-dependent mechanism. Increased CXCR-2+ PMN-MDSCs led to accelerated CCA growth in preclinical murine models of colitis, primary sclerosing cholangitis, and CCA. Blocking CXCL1, either by antibiotic treatment or antibody neutralization, resulted in PMN-MDSC depletion and inhibited CCA growth.23
Genetic and Epigenetic Alterations and Immune Cells
CCA cells also promote genetic and epigenetic alterations to evade immune surveillance (Figure 1). Human NK cell express inhibitory NK immunoglobulin-like receptors (KIRs) which recognize specific HLA class I molecules and regulate NK cell response.64 Alterations in the KIR and HLA gene loci were more predominant in CCA specimens compared to controls resulting in diminished NK cell tumor surveillance and immune escape.65 Manipulation of an activating NK cell receptor, natural killer group 2D, by genetic variation has also led to tumor immune escape and variants have been associated with development of CCA in patients with primary sclerosing cholangitis.66
Figure 1 – Genetic and epigenetic alterations employed by CCA to promote immune evasion.
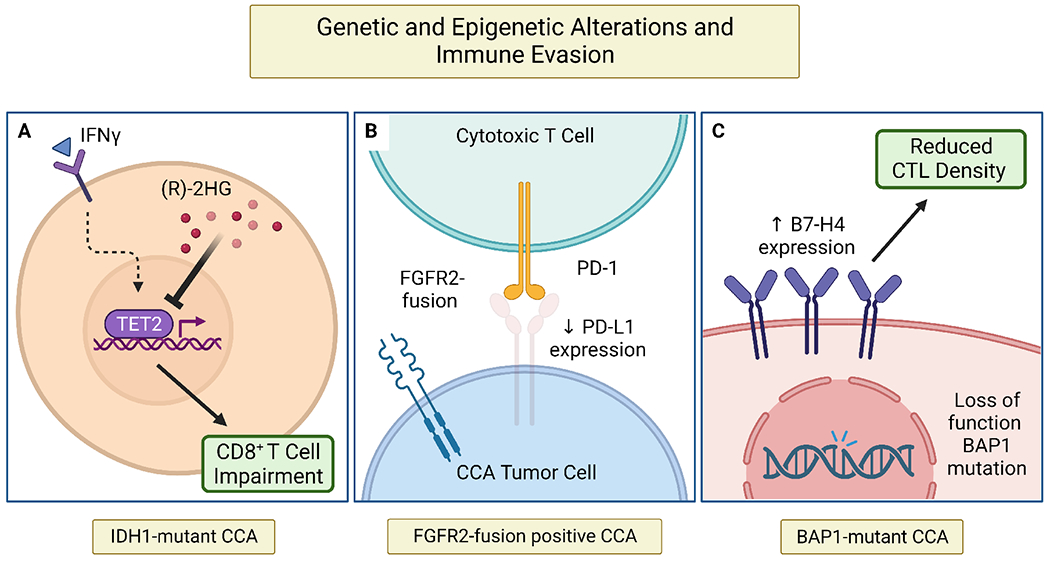
Genetic aberrations frequently identified in the CCA landscape have been linked to mechanisms of immune escape. (A) In IDH1-mutant CCA, oncometabolite (R)-2-hydroxyglutaratesuppresses CD8+ T cell functionality and inactivates TET2, a DNA demethylase, leading to repression of IFNγ response by tumor cells. (B) FGFR2-fusion positive CCAs are associated with reduced PD-L1 expression on tumor cells (C) Increased expression of immune checkpoint molecule, B7-H4, has been associated with loss of function BAP1 mutations resulting in decreased CTL density in the CCA TIME.
BAP1, BRCA1 associated protein-1; CCA, cholangiocarcinoma; CTL, cytotoxic T lymphocyte; FGFR2, fibroblast growth factor receptor 2; IDH1, isocitrate dehydrogenase 1; IFNγ, interferon gamma; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand; (R)-2HG, (R)-2-hydroxyglutarate; TET2, ten-eleven translocation-2; TIME, tumor immune microenvironment
Isocitrate dehydrogenase 1 (IDH1) is commonly mutated in CCA cells and promotes tumor maintenance via CD8+ T cell suppression and TET2 inactivation (Figure 1A).67 Inhibition of mutant IDH1 (mIDH1) in a genetically engineered mouse model resulted in depression of tumor immune evasion. Accordingly, mIDH1 inhibition in combination with CTLA-4 blockade demonstrated synergistic effects that lead to complete and durable responses. Fibroblast growth factor receptor 2 (FGFR2) fusions are another frequent genetic aberration found almost exclusively in iCCAs.2 An analysis of 94 resected CCA specimens demonstrated a paucity of PD-L1 on tumor cells of patients with FGFR2-fusion positive CCAs (Figure 1B).68 Further characterization of the CCA TIME in patients harboring actionable alterations is needed prior to consideration of dual targeted and ICI therapy.
B7-H4 is an immune checkpoint molecule that negatively regulates CTL-mediated antitumor response. Increased B7-H4 expression by CCA cells has been associated with loss of function BRCA1-associated protein 1 (BAP1) mutations and reduced CTL density in the TIME utilizing multiplex immunohistochemistry on resected CCA specimens (Figure 1C).69 Thus, broad genetic profiling of the CCA TIME may provide insight into immune escape mechanisms and assist in predicting patient response to ICI.
Reengineering the CCA TIME
Given that CCAs are largely immune-excluded tumors by either the presence of immune suppressive elements or application of immune escape mechanisms, the success of immunotherapeutic approaches in CCA is reliant on reengineering the CCA TIME. Strategies to re-equip the immune system include inhibition of suppressive immune cell oncogenic signaling, blockade of suppressive immune cell recruitment, and priming the inherent, inflammatory immune system elements for ICI therapy (Figure 2).
Figure 2 – Reengineering the CCA TIME.
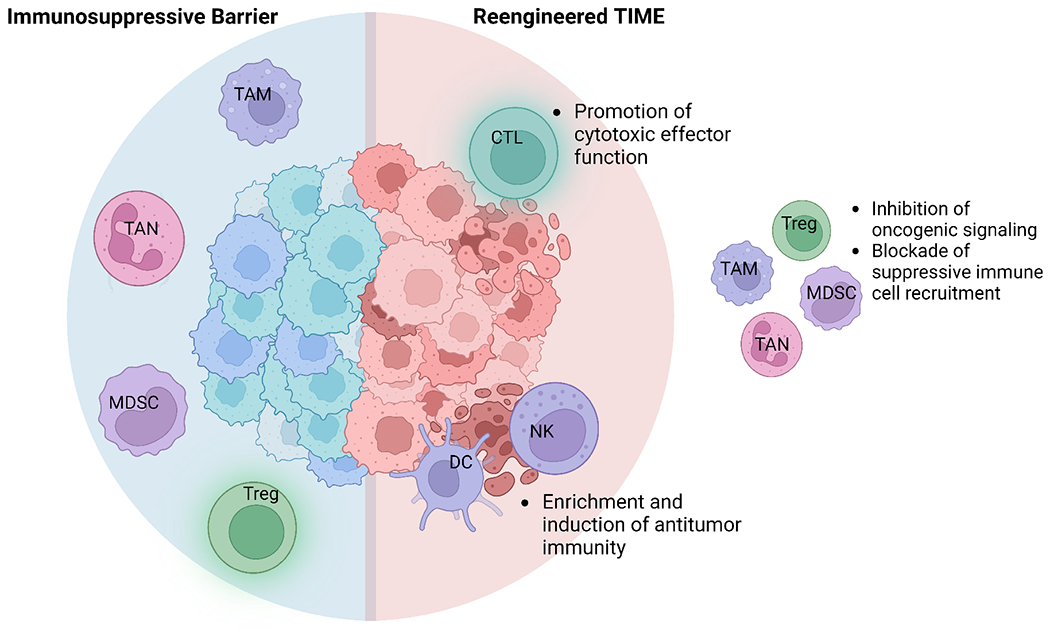
Successful application of ICI in CCA relies on reengineering the CCA TIME by promoting the activity of inherent, inflammatory immunogenic elements. CCA, cholangiocarcinoma; CTL, cytotoxic T lymphocyte; DC, dendritic cell; ICI, immune checkpoint inhibition; MDSC, myeloid-derived suppressor cell; NK, natural killer cell; TAM, tumor-associated macrophage; TAN, tumor-associated neutrophil; TIME, tumor immune microenvironment; Treg, regulatory T cell
Targeting Oncogenic Signaling by Suppressive Immune Cells
Suppressive immune cells employ a varied and dexterous approach to modulate cholangiocarcinogenesis and tumor progression; consequently, they represent a prime target for anti-cancer therapy.24 TAMs promote biliary proliferation and oncogenic transformation through Jun N-terminal kinase signaling.70 Targeting of the JNK axis and/or depletion of TAMs demonstrated attenuation of CCA development.70 The protumor effects of WNT signaling in the CCA TIME are also predominantly induced by TAMs. Activation of canonical WNT/β catenin signaling results in tumorigenesis and progression across several preclinical models of CCA. Consequently, TAM depletion or WNT inhibition was found to reduce CCA tumor burden, suggesting that therapeutic targeting of WNT signaling may have potential benefit in CCA.71,72 MDSCs promote cancer sternness in iCCA via 5-lipoxygenase/leukotriene B4/leukotriene B4 receptor type 2 (BLT2) axis.73 BLT2 blockade reduced MDSC-driven stemness effects and sensitized iCCA patient-derived xenografts to cytotoxic chemotherapy.
Tumor cells and suppressive immune cells can release pro-tumor exosomes, membrane-bound nanovesicles that carry various cargo including non-coding RNAs, protein, and DNA. TAMs can produce exosomal circular RNAs (circRNAs) Circ_0020256 induces CCA proliferation, migration, and invasion.74 Blockade of circ_0020256 by small interference RNA in preclinical models resulted in attenuated CCA progression. Thus, exosomes and their cargo are another potential molecular therapeutic target.
Blocking Suppressive Immune Cell Recruitment
Another approach to reengineering the CCA TIME is by blocking the recruitment and differentiation of immunosuppressive elements to the CCA TIME. The complex and intricate crosstalk amongst immunosuppressive elements of the TIME can contribute to resistance to immunotherapeutics due to compensatory infiltration of other suppressive cell types. Genetic or pharmacologic blockade of TAMs using anticolony stimulating factor 1 did not lead to an attenuation of tumor burden in murine CCA due to a compensatory emergence of G-MDSCs.13 However, blockade of both populations enhanced the efficacy of anti-PD-1 and led to a significant increase in murine survival.
GM-CSF plays a critical role in myelopoiesis and could be a driving factor behind the high incidence of myeloid suppressive cells observed in the CCA TIME. In a murine CCA model, GM-CSF blockade prevented recruitment of bone marrow derived monocytes, inhibited M2-like protumor macrophage polarization, and reduced TAM viability.14 Moreover, GM-CSF blockade repolarized TAMs and MDSCs with resultant augmentation of CTL infiltration and activation. Interestingly, GM-CSF blockade did not result in compensatory immunosuppression by MDSCs or other immune elements in the TIME.
TNF-like weak inducer of apoptosis (TWEAK)/fibroblast growth factor-inducible 14 (Fn14) is upregulated in CCA and induces CCA cell expression of monocyte chemoattractant protein-1 (MCP-1).75 MCP-1 activates and recruits TAMs into the CCA TIME. Accordingly, blockade of TWEAK/Fn14 or treatment with anti-MCP-1 antibody reduced TAM recruitment and resulted in smaller tumors in a CCA xenograft model.
Bispecific antibodies (bsAbs) and bispecific T cell engagers (BiTE) are an evolving class of novel immune therapeutics. In a recent study, a recombinant PD-L1xCD3 BiTE successfully bound to both CD3+ T lymphocytes and PD-L1+ CCA cells in coculture.76 The resulting effect was enhanced CTL function and increased cytotoxic mediated tumor cell death. Prior gemcitabine treatment of tumor cells resulted in increased PD-L1 expression which further sensitized cells to PD-L1xCD3 BiTE treatment. Further preclinical studies in this arena are warranted along with exploration of optimal antigen target combinations.
Priming the TIME
Other therapeutic approaches employed have primed the CCA TIME for ICI therapy or inherent CTL activation. Glucocorticoid-induced TNF receptor-related protein (GITR) is a co-stimulatory molecule that when activated attenuates Treg-driven immunosuppression and promotes CTL effector function.77 Agonistic ex vivo stimulation of GITR using soluble GITR-ligand promoted CTL activation.57 Systemic therapies can promote immunomodulation within the TIME and potentiate response to immunotherapy. ICCA tumor-bearing mice were treated with trametinib, a mitogen-activated kinase (MEK) inhibitor, anti-PD-1, or a combination of both therapeutics.78 Trametinib monotherapy resulted in increased expression of major histocompatibility complex and PD-L1 on tumor cells thus priming the TIME for immunotherapy. Accordingly, trametinib in combination with anti-PD-1 had increased anti-tumor efficacy compared to either agent alone.
Globo H-ceramide (Globo-H) is the predominant tumor-associated carbohydrate antigen expressed on epithelial cancers, including iCCA 79 Globo-H vaccination in breast cancer patients resulted in the formation of anti-Globo H antibodies that promoted complement-dependent and antibody-dependent cellular cytotoxicity in cancer patients.80 Tumor growth in a rat thioacetamide-induced CCA model was suppressed using a monoclonal antibody targeting Globo-H. Enrichment of NK cells was observed following treatment and proposed as a contributing mechanism to the anti-tumor activity of Globo-H targeted therapy.79 Utilizing mucin 1 (MUC1) as an antigen target, a chimeric antigen receptor (CAR) T cell containing an anti-MUC1-single-chain variable fragment produced higher levels of TNF-α, IFN-γ and granzyme B when exposed to MUC1-expressing CCA cells compared to conventional T cells.81 Anti-MUC1-CAR4 T cells also demonstrated enhanced cytolytic killing of CCA cells and spheroids.
Induction or loading of DCs has proven to be an effective method of reequipping the suppressed anti-tumor immune cells in the CCA TIME. CD40, a member of the TNF receptor superfamily, is expressed on antigen presenting cells.82 Activation of CD40, through interaction with its ligand, CD40L, promotes DC-mediated CTL activation and re-polarization of macrophages to an anti-tumor, inflammatory phenotype. Thus, CD40 agonism is an attractive strategy to shift the TIME balance from immunosuppression to antitumor immunity. In several iCCA preclinical models, CD40 agonism in combination with anti-PD-1 therapy significantly reduced murine tumor burden compared to IgG control or either monotherapy alone.78 Furthermore, combination of anti-CD40/anti-PD-1 enhanced the efficacy of first line gemcitabine/cisplatin therapy as exhibited by increased murine survival compared to gemcitabine/cisplatin alone. Increased number and activity of cytotoxic effector cells was observed in tumor-bearing mice treated with anti-CD40/PD-1.78 Transduction of DCs with adenovirus encoding CD40L resulted in DC activation and promotion of DC-mediated effector cell cytotoxicity.83 Aspartate-β-hydroxylase (ASPH) is a tumour-associated cell surface protein present in a number of malignancies, including CCA.84 Treatment of an orthotopic rat model of iCCA with ASPH loaded DCs induced suppression of tumour growth and metastasis and increased CD3+ lymphocyte infiltration.
Future perspectives
While steady progress has been made in uncovering the components, mechanisms, and targets in the CCA TIME, these discoveries are just beginning to have meaningful contributions to patient therapy and survival. Immunotherapy will likely have an increasing presence in the future CCA treatment armamentarium given the recent positive phase III studies (TOPAZ-1 and KEYNOTE-966). While only modest improvements in patient outcomes have been observed thus far, there is a clear cohort of patients that derive a robust and durable benefit from immunotherapy. Further investigation is required, particularly in the domain of high-throughput clinically applicable biomarkers, to accurately define which patient populations are appropriate for immediate immunotherapeutics or which require prior biologic modification to render them immune-responsive. Tumor biopsy before and after treatment or at time of progression to assess changes in the CCA TIME would greatly benefit translational research programs and should be considered by clinical investigators. Moving forward, investment in technologies including multiplex immunohistochemistry, single-cell omics, spatial transcriptomics and proteomics will be essential to probe deeper into the many imbricated layers of the CCA TIME. Defining and delineating immune cell types and their function will be critical for resolving the immunosuppressive tumor barrier as we currently understand it. Given the highly heterogeneous nature of CCAs, combinatorial therapeutics will continue to prove their merit over single arm therapies; cytotoxic, targeted, and immunomodulating drugs all have roles to play. Finally, the importance of developing new immunocompetent, preclinical models of CCA that recapitulate the human disease and reflect defined patient cohorts will be critical for the advancement of our understanding of CCA biology. While the studies included in this review illuminate the substantial advancements in our understanding of CCA immunobiology made over the recent years, they also highlight the long road of discovery ahead.
Key Points:
Novel molecular tumor immune microenvironment-based subtypes of cholangiocarcinoma with differing mechanisms of immune escape and patient outcomes have been characterized.
A barrier of suppressive immune cells around cholangiocarcinoma tumors permits tumor immune evasion.
Poorly immunogenic cholangiocarcinoma tumors leverage multiple mechanisms to escape the anti-tumor immune response.
Reengineering the immunosuppressive barrier has the potential to enhance efficacy of immune checkpoint inhibition.
Preclinical models of cholangiocarcinoma are crucial for further characterization of the tumor immune microenvironment and investigation of immunotherapeutics.
Grant Support:
S.I. Ilyas was supported by the NIH/NCI (1K08CA236874), Mayo Center for Cell Signaling in Gastroenterology (Pilot & Feasibility Award P30DK084567), the Mayo Hepatobiliary Cancer SPORE (P50 CA210964) Career Enhancement Program, the Satter Family Liver Cancer Award, and the Mayo Foundation.
Abbreviations:
- ASPH
Aspartate-β-hydroxylase
- BAP1
BRCA1-associated protein 1
- BLT2
leukotriene B4 receptor type 2
- CAR
chimeric antigen receptor
- CCA
cholangiocarcinoma
- CD47
cluster of differentiation 47
- CircRNA
circular RNA
- CTLA-4
cytotoxic T-lymphocyte associated protein 4
- CTL
cytotoxic lymphocyte
- CXCR
C-X-C motif chemokine receptor
- DC
dendritic cell
- dCCA
distal cholangiocarcinoma
- FasL
Fas ligand
- FGFR2
fibroblast growth factor receptor 2
- Fn14
fibroblast growth factor-inducible 14
- GITR
glucocorticoid-induced TNF receptor-related protein
- Globo-H
Globo H-ceramide
- G-MDSC
granulocytic myeloid-derived suppressor cells
- IDH1
isocitrate dehydrogenase 1
- ICI
immune checkpoint inhibition
- IL
interleukin
- iCCA
intrahepatic cholangiocarcinoma
- KIR
inhibitory NK immunoglobulin-like receptor
- LTB4
- mIDH1
mutant isocitrate dehydrogenase 1
- MCP-1
monocyte chemoattractant protein-1
- MEK
mitogen-activated kinase
- miR
microRNA
- M-MDSC
monocytic myeloid-derived suppressor cells
- MDSC
myeloid-derived suppressor cells
- MUC1
mucin 1
- NK
natural killer cell
- pCCA
perihilar cholangiocarcinoma
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death ligand 1
- PMN-MDSC
polymorphonuclear myeloid-derived suppressor cells
- scRNA-seq
single-cell RNA sequencing
- SD-DC
self-differentiated dendritic cell
- STAT3
signal transducer and activator or transcription 3
- TAM
tumor-associated macrophage
- TAN
tumor-associated neutrophil
- TIME
tumor immune microenvironment
- TGF-β1
transforming growth factor – β1
- Th17
IL-17 producing T helper cell
- TLS
tertiary lymphoid structures
- Treg
regulatory T cells
- TWEAK
TNF-like weak inducer of apoptosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: JWV declares Speakers’ Bureau and honoraria for AstraZeneca and Merck. SII declares consulting for AstraZeneca. JLT declares no conflicts of interest.
References
- 1.Rizvi S, Khan SA, Hallemeier CL, Kelley RK & Gores GJ Cholangiocarcinoma — evolving concepts and therapeutic strategies. Nature Reviews Clinical Oncology 15, 95–111, doi: 10.1038/nrclinonc.2017.157 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nature Reviews Gastroenterology & Hepatology 17, 557–588, doi: 10.1038/s41575-020-0310-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A et al. Cholangiocarcinoma. Nat Rev Dis Primers 7, 65, doi: 10.1038/s41572-021-00300-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valle JW, Vogel A, Denlinger CS, He AR, Bai L-Y et al. Addition of ramucirumab or merestinib to standard first-line chemotherapy for locally advanced or metastatic biliary tract cancer: a randomised, double-blind, multicentre, phase 2 study. The Lancet Oncology 22, 1468–1482, doi: 10.1016/S1470-2045(21)00409-5 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Oh DY, Lee KH, Lee DW, Yoon J, Kim TY et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol 7, 522–532, doi: 10.1016/S2468-1253(22)00043-7 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature Medicine 24, 541–550, doi: 10.1038/s41591-018-0014-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh D-Y, He AR, Qin S, Chen L-T, Okusaka T et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evidence 1, EVIDoa2200015, doi:doi: 10.1056/EVIDoa2200015 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Huang AC & Zappasodi R A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nature Immunology 23, 660–670, doi: 10.1038/s41590-022-01141-1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. New England Journal of Medicine 378, 2078–2092, doi: 10.1056/NEJMoal801005 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Merck. (Rahway, NJ, USA, 2023). [Google Scholar]
- 11.Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer 147, 2190–2198, doi: 10.1002/ijc.33013 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Loeuillard E, Conboy CB, Gores GJ & Rizvi S Immunobiology of cholangiocarcinoma. JHEP Rep 1, 297–311, doi: 10.1016/j.jhepr.2019.06.003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loeuillard E, Yang J, Buckarma E, Wang J, Liu Y et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J Clin Invest 130, 5380–5396, doi: 10.1172/jci137110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruffolo LI, Jackson KM, Kuhlers PC, Dale BS, Figueroa Guilliani NM et al. GM-CSF drives myelopoiesis, recruitment and polarisation of tumour-associated macrophages in cholangiocarcinoma and systemic blockade facilitates antitumour immunity. Gut 71, 1386–1398, doi: 10.1136/gutjnl-2021-324109 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A et al. Genomic spectra of biliary tract cancer. Nature Genetics 47, 1003–1010, doi: 10.1038/ng.3375 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Job S, Rapoud D, Dos Santos A, Gonzalez P, Desterke C et al. Identification of Four Immune Subtypes Characterized by Distinct Composition and Functions of Tumor Microenvironment in Intrahepatic Cholangiocarcinoma. Hepatology 72, 965–981, doi: 10.1002/hep.31092 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Serrano MA, Kepecs B, Torres-Martin M, Bramel ER, Haber PK et al. Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications. Gut, gutjnl-2021-326514, doi: 10.1136/gutjnl-2021-326514 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song G, Shi Y, Meng L, Ma J, Huang S et al. Single-cell transcriptomic analysis suggests two molecularly distinct subtypes of intrahepatic cholangiocarcinoma. Nature Communications 13, 1642, doi: 10.1038/s41467-022-29164-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelhard VH, Rodriguez AB, Mauldin IS, Woods AN, Peske JD et al. Immune Cell Infiltration and Tertiary Lymphoid Structures as Determinants of Antitumor Immunity. The Journal of Immunology 200, 432–442, doi: 10.4049/jimmunol.1701269 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding G-Y, Ma J-Q, Yun J-P, Chen X, Ling Y et al. Distribution and density of tertiary lymphoid structures predict clinical outcome in intrahepatic cholangiocarcinoma. Journal of Hepatology 76, 608–618, doi: 10.1016/j.jhep.2021.10.030 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Loeuillard E, Gores GJ & Ilyas SI Cholangiocarcinoma: what are the most valuable therapeutic targets - cancer-associated fibroblasts, immune cells, or beyond T cells? Expert Opin Ther Targets 25, 835–845, doi: 10.1080/14728222.2021.2010046 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabris L, Sato K, Alpini G & Strazzabosco M The Tumor Microenvironment in Cholangiocarcinoma Progression. Hepatology 73 Suppl 1, 75–85, doi: 10.1002/hep.31410 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Ma C, Duan Y, Heinrich B, Rosato U et al. Gut Microbiome Directs Hepatocytes to Recruit MDSCs and Promote Cholangiocarcinoma. Cancer Discovery 11, 1248–1267, doi: 10.1158/2159-8290.Cd-20-0304 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantovani A, Marchesi F, Malesci A, Laghi L & Allavena P Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14, 399–416, doi: 10.1038/nrclinonc.2016.217 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sierro F, Evrard M, Rizzetto S, Melino M, Mitchell AJ et al. A Liver Capsular Network of Monocyte-Derived Macrophages Restricts Hepatic Dissemination of Intraperitoneal Bacteria by Neutrophil Recruitment. Immunity 47, 374–388.e376, doi: 10.1016/j.immuni.2017.07.018 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Wang J & Kubes P A Reservoir of Mature Cavity Macrophages that Can Rapidly Invade Visceral Organs to Affect Tissue Repair. Cell 165, 668–678, doi: 10.1016/j.cell.2016.03.009 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Hasita H, Komohara Y, Okabe H, Masuda T, Ohnishi K et al. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Science 101, 1913–1919, doi: 10.1111/j.1349-7006.2010.01614.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun D, Luo T, Dong P, Zhang N, Chen J et al. CD86+/CD206+ tumor-associated macrophages predict prognosis of patients with intrahepatic cholangiocarcinoma. PeerJ 8, e8458, doi: 10.7717/peerj.8458 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunk PR, Dougherty SC, Lynch K, Whitehair R, Meneveau M et al. Myeloid Cell Infiltration Correlates With Prognosis in Cholangiocarcinoma and Varies Based on Tumor Location. J Immunother 44, 254–263, doi: 10.1097/cji.0000000000000378 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou M, Wang C, Lu S, Xu Y, Li Z et al. Tumor-associated macrophages in cholangiocarcinoma: complex interplay and potential therapeutic target. eBioMedicine 67, doi: 10.1016/j.ebiom.2021.103375 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pittet MJ, Michielin O & Migliorini D Clinical relevance of tumour-associated macrophages. Nat Rev Clin Oncol 19, 402–421, doi: 10.1038/s41571-022-00620-6 (2022). [DOI] [PubMed] [Google Scholar]
- 32.Gabrilovich DI Myeloid-Derived Suppressor Cells. Cancer Immunol Res 5, 3–8, doi: 10.1158/2326-6066.Cir-16-0297 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med 6, 237ra267, doi: 10.1126/scitranslmed.3007974 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother 63, 247–257, doi: 10.1007/s00262-013-1508-5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Fu X, Li T & Yan H The prognostic value of myeloid derived suppressor cell level in hepatocellular carcinoma: A systematic review and meta-analysis. PLOS ONE 14, e0225327, doi: 10.1371/journal.pone.0225327 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X-D, Hu J, Wang M, Peng F, Tian R et al. Circulating myeloid-derived suppressor cells in patients with pancreatic cancer. Hepatobiliary & Pancreatic Diseases International 15, 099–105, doi: 10.1016/S1499-3872(15)60413-1 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Bayik D, Lauko AJ, Roversi GA, Serbinowski E, Acevedo-Moreno L-A et al. Hepatobiliary malignancies have distinct peripheral myeloid-derived suppressor cell signatures and tumor myeloid cell profiles. Scientific Reports 10, 18848, doi: 10.1038/s41598-020-75881-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coffelt SB, Wellenstein MD & de Visser KE Neutrophils in cancer: neutral no more. Nat Rev Cancer 16, 431–446, doi: 10.1038/nrc.2016.52 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Zhou Z, Wang P, Sun R, Li J, Hu Z et al. Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3. Journal for ImmunoTherapy of Cancer 9, e001946, doi: 10.1136/jitc-2020-001946 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue R, Zhang Q, Cao Q, Kong R, Xiang X et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature 612, 141–147, doi: 10.1038/s41586-022-05400-x (2022). [DOI] [PubMed] [Google Scholar]
- 41.Granot Z & Fridlender ZG Plasticity beyond cancer cells and the “immunosuppressive switch”. Cancer Res 75, 4441–4445, doi: 10.1158/0008-5472.Can-15-1502 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Kitano Y, Okabe H, Yamashita Y.-i., Nakagawa S, Saito Y et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. British Journal of Cancer 118, 171–180, doi: 10.1038/bjc.2017.401 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buettner S, Spolverato G, Kimbrough CW, Alexandrescu S, Marques HP et al. The impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio among patients with intrahepatic cholangiocarcinoma. Surgery 164, 411–418, doi: 10.1016/j.surg.2018.05.002 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Kitano Y, Yamashita YI, Yamamura K, Arima K, Kaida T et al. Effects of Preoperative Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios on Survival in Patients with Extrahepatic Cholangiocarcinoma. Anticancer Res 37, 3229–3237, doi: 10.21873/anticanres.11685 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Raffin C, Vo LT & Bluestone JA Treg cell-based therapies: challenges and perspectives. Nature Reviews Immunology 20, 158–172, doi: 10.1038/s41577-019-0232-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvisi G, Termanini A, Soldani C, Portale F, Carriero R et al. Multimodal single-cell profiling of intrahepatic cholangiocarcinoma defines hyperactivated Tregs as a potential therapeutic target. J Hepatol 77, 1359–1372, doi: 10.1016/j.jhep.2022.05.043 (2022). [DOI] [PubMed] [Google Scholar]
- 47.Vigano L, Soldani C, Franceschini B, Cimino M, Lleo A et al. Tumor-Infiltrating Lymphocytes and Macrophages in Intrahepatic Cholangiocellular Carcinoma. Impact on Prognosis after Complete Surgery. J Gastrointest Surg 23, 2216–2224, doi: 10.1007/sl1605-019-04111-5 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Luo C, Xin H, Zhou Z, Hu Z, Sun R et al. Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression. Hepatology 76, 982–999, doi: 10.1002/hep.32387 (2022). [DOI] [PubMed] [Google Scholar]
- 49.Yanagita T, Murata Y, Tanaka D, Motegi SI, Arai E et al. Anti-SIRPα antibodies as a potential new tool for cancer immunotherapy. JCI Insight 2, e89140, doi: 10.1172/jci.insight.89140 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaeteewoottacharn K, Kariya R, Pothipan P, Fujikawa S, Pairojkul C et al. Attenuation of CD47-SIRPα Signal in Cholangiocarcinoma Potentiates Tumor-Associated Macrophage-Mediated Phagocytosis and Suppresses Intrahepatic Metastasis. Transl Oncol 12, 217–225, doi: 10.1016/j.tranon.2018.10.007 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carnevale G, Carpino G, Cardinale V, Pisciotta A, Riccio M et al. Activation of Fas/FasL pathway and the role of c-FLIP in primary culture of human cholangiocarcinoma cells. Scientific Reports 7, 14419, doi: 10.1038/s41598-017-14838-3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wallet MA, Sen P & Tisch R Immunoregulation of dendritic cells. Clin Med Res 3, 166–175, doi: 10.3121/cmr.3.3.166 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thepmalee C, Panya A, Junking M, Chieochansin T & Yenchitsomanus PT Inhibition of IL-10 and TGF-β receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells. Hum Vaccin Immunother 14, 1423–1431, doi: 10.1080/21645515.2018.1431598 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thepmalee C, Panya A, Sujjitjoon J, Sawasdee N, Poungvarin N et al. Suppression of TGF-β and IL-10 receptors on self-differentiated dendritic cells by short-hairpin RNAs enhanced activation of effector T-cells against cholangiocarcinoma cells. Hum Vaccin Immunother 16, 2318–2327, doi: 10.1080/21645515.2019.1701913 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238, doi: 10.1038/nature04753 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Kinoshita M, Kobayashi S, Gotoh K, Kubo M, Hayashi K et al. Heterogeneity of Treg/Th17 According to Cancer Progression and Modification in Biliary Tract Cancers via Self-Producing Cytokines. Dig Dis Sci 65, 2937–2948, doi: 10.1007/s10620-019-06011-9 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Zhou G, Sprengers D, Mancham S, Erkens R, Boor PPC et al. Reduction of immunosuppressive tumor microenvironment in cholangiocarcinoma by ex vivo targeting immune checkpoint molecules. J Hepatol 71, 753–762, doi: 10.1016/j.jhep.2019.05.026 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Kitano Y, Yamashita YI, Nakao Y, Itoyama R, Yusa T et al. Clinical Significance of PD-L1 Expression in Both Cancer and Stroma Cells of Cholangiocarcinoma Patients. Ann Surg Oncol 27, 599–607, doi: 10.1245/s10434-019-07701-4 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Gani F, Nagarajan N, Kim Y, Zhu Q, Luan L et al. Program Death 1 Immune Checkpoint and Tumor Microenvironment: Implications for Patients With Intrahepatic Cholangiocarcinoma. Annals of Surgical Oncology 23, 2610–2617, doi: 10.1245/sl0434-016-5101-y (2016). [DOI] [PubMed] [Google Scholar]
- 60.Zhang M, Yang H, Wan L, Wang Z, Wang H et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J Hepatol 73, 1118–1130, doi: 10.1016/j.jhep.2020.05.039 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Ghidini M, Cascione L, Carotenuto P, Lampis A, Trevisani F et al. Characterisation of the immune-related transcriptome in resected biliary tract cancers. European Journal of Cancer 86, 158–165, doi: 10.1016/j.ejca.2017.09.005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mao J, Wang D, Long J, Yang X, Lin J et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J Immunother Cancer 9, doi: 10.1136/jitc-2021-003334 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jia X, Lu S, Zeng Z, Liu Q, Dong Z et al. Characterization of Gut Microbiota, Bile Acid Metabolism, and Cytokines in Intrahepatic Cholangiocarcinoma. Hepatology 71, 893–906, doi: 10.1002/hep.30852 (2020). [DOI] [PubMed] [Google Scholar]
- 64.Wolf NK, Kissiov DU & Raulet DH Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nature Reviews Immunology, doi: 10.1038/s41577-022-00732-1 (2022). [DOI] [PubMed] [Google Scholar]
- 65.Cornillet M, Jansson H, Schaffer M, Hertwig L, Berglin L et al. Imbalance of Genes Encoding Natural Killer Immunoglobulin-Like Receptors and Human Leukocyte Antigen in Patients With Biliary Cancer. Gastroenterology 157, 1067–1080.e1069, doi: 10.1053/j.gastro.2019.06.023 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Melum E, Karlsen TH, Schrumpf E, Bergquist A, Thorsby E et al. Cholangiocarcinoma in primary sclerosing cholangitis is associated with NKG2D polymorphisms. Hepatology 47, 90–96, doi: 10.1002/hep.21964 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Wu MJ, Shi L, Dubrot J, Merritt J, Vijay V et al. Mutant IDH Inhibits IFNγ-TET2 Signaling to Promote Immunoevasion and Tumor Maintenance in Cholangiocarcinoma. Cancer Discov 12, 812–835, doi: 10.1158/2159-8290.Cd-21-1077 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sridharan V, Neyaz A, … Deshpande V, Goyal L FGFR mRNA Expression in Cholangiocarcinoma and Its Correlation with FGFR2 Fusion Status and Immune Signatures. Clin Cancer Res 28, 5431–5439, doi: 10.1158/1078-0432.Ccr-22-1244 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carapeto F, Bozorgui B, Shroff RT, Chagani S, Solis Soto L et al. The immunogenomic landscape of resected intrahepatic cholangiocarcinoma. Hepatology 75, 297–308, doi: 10.1002/hep.32150 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuan D, Huang S, Berger E, Liu L, Gross N et al. Kupffer Cell-Derived Tnf Triggers Cholangiocellular Tumorigenesis through JNK due to Chronic Mitochondrial Dysfunction and ROS. Cancer Cell 31, 771–789.e776, doi: 10.1016/j.ccell.2017.05.006 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boulter L, Guest RV, Kendall TJ, Wilson DH, Wojtacha D et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest 125, 1269–1285, doi: 10.1172/jci76452 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loilome W, Bungkanjana P, Techasen A, Namwat N, Yongvanit P et al. Activated macrophages promote Wnt/β-catenin signaling in cholangiocarcinoma cells. Tumour Biol 35, 5357–5367, doi: 10.1007/s13277-014-1698-2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin Y, Cai Q, Chen Y, Shi T, Liu W et al. CAFs shape myeloid-derived suppressor cells to promote stemness of intrahepatic cholangiocarcinoma through 5-lipoxygenase. Hepatology 75, 28–42, doi: 10.1002/hep.32099 (2022). [DOI] [PubMed] [Google Scholar]
- 74.Chen S, Chen Z, Li Z, Li S, Wen Z et al. Tumor-associated macrophages promote cholangiocarcinoma progression via exosomal Circ_0020256. Cell Death & Disease 13, 94, doi: 10.1038/s41419-022-04534-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dwyer BJ, Jarman EJ, Gogoi-Tiwari J, Ferreira-Gonzalez S, Boulter L et al. TWEAK/Fn14 signalling promotes cholangiocarcinoma niche formation and progression. Journal of Hepatology 74, 860–872, doi: 10.1016/j.jhep.2020.11.018 (2021). [DOI] [PubMed] [Google Scholar]
- 76.Wathikthinnakon M, Luangwattananun P, Sawasdee N, Chiawpanit C, Lee VS et al. Combination gemcitabine and PD-L1xCD3 bispecific T cell engager (BiTE) enhances T lymphocyte cytotoxicity against cholangiocarcinoma cells. Scientific Reports 12, 6154, doi: 10.1038/s41598-022-09964-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zappasodi R, Sirard C, Li Y, Budhu S, Abu-Akeel M et al. Rational design of anti-GITR-based combination immunotherapy. Nat Med 25, 759–766, doi: 10.1038/s41591-019-0420-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Diggs LP, Ruf B, Ma C, Heinrich B, Cui L et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J Hepatol 74, 1145–1154, doi: 10.1016/j.jhep.2020.11.037 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hung T-H, Hung J-T, Wu C-E, Huang Y, Lee C-W et al. Globo H Is a Promising Theranostic Marker for Intrahepatic Cholangiocarcinoma. Hepatology Communications 6, 194–208, doi: 10.1002/hep4.1800 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gilewski T, Ragupathi G, Bhuta S, Williams LJ, Musselli C et al. Immunization of metastatic breast cancer patients with a fully synthetic globo H conjugate: a phase I trial. Proc Natl Acad Sci U S A 98, 3270–3275, doi: 10.1073/pnas.051626298 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Supimon K, Sangsuwannukul T, Sujjitjoon J, Phanthaphol N, Chieochansin T et al. Anti-mucin 1 chimeric antigen receptor T cells for adoptive T cell therapy of cholangiocarcinoma. Sci Rep 11, 6276, doi: 10.1038/s41598-021-85747-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vonderheide RH CD40 Agonist Antibodies in Cancer Immunotherapy. Annu Rev Med 71, 47–58, doi: 10.1146/annurev-med-062518-045435 (2020). [DOI] [PubMed] [Google Scholar]
- 83.Sadeghlar F, Vogt A, Mohr RU, Mahn R, van Beekum K et al. Induction of cytotoxic effector cells towards cholangiocellular, pancreatic, and colorectal tumor cells by activation of the immune checkpoint CD40/CD40L on dendritic cells. Cancer Immunol Immunother 70, 1451–1464, doi: 10.1007/s00262-020-02746-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Noda T, Shimoda M, Ortiz V, Sirica AE & Wands JR Immunization with aspartate-β-hydroxylase-loaded dendritic cells produces antitumor effects in a rat model of intrahepatic cholangiocarcinoma. Hepatology 55, 86–97, doi: 10.1002/hep.24629 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]


