Abstract
Surgical site infections (SSI) due to Staphylococcus aureus among 256 male and 158 female patients (mean age, 28 years) undergoing elective surgery at the Soba University Hospital (Khartoum, Sudan) were studied. During an 11-month study period all patients were analyzed for nasal carriage of S. aureus at the time of admission. Follow-up of the development of SSI proceeded until 4 weeks after the operations. In addition, nasal swabs were obtained periodically during the same period from 82 members of the staff. In order to discriminate autoinfection from cross infection, bacterial isolates were typed by random amplification of polymorphic DNA (RAPD), pulsed-field gel electrophoresis (PFGE) of DNA macrorestriction fragments, and restriction fragment length polymorphism analysis of the protein A and coagulase genes. Preoperative cultures revealed the presence of S. aureus in the noses of 98 patients (24%). The overall number of postsurgical wound infections in the entire group was 57 (14%), 24 of which were due to S. aureus. Only 6 of the 98 nasal S. aureus carriers suffered from wound infections by the same species. In these six cases the infecting strain could not be genetically discriminated from the nasal inhabitant, substantiating autoinfection. However, nasal carriage of S. aureus is not a significant risk factor for the development of SSI in this setting (6 of 98 patients with autoinfection versus 18 of 316 patients [414 − 98 patients] with cross infection; P = 0.81), most probably due to the fact that noncarriers are at a significant and relatively large risk for acquiring an independent S. aureus SSI. The other S. aureus strains causing SSI showed a high degree of genetic heterogeneity, demonstrating that it is not an epidemic strain that is causing the SSI. Among the staff personnel screened, 47.4% did not carry S. aureus in the nose at any time during the study period, whereas 13.2% persistently carried a single strain in the nose. Another 39.5% could be classified as intermittent carriers. When strains derived from staff personnel were genetically typed, it was demonstrated that most of the strains represented genetic variants clearly differing from the isolates causing SSI. On the other hand, possible cross colonization among staff personnel and even cross infection from staff personnel to patients or from patient to patient were demonstrated in some cases, but epidemic spread of a single strain or a few clonally related strains of S. aureus could be excluded.
Major waves of infectious agents have to be battled on the African continent. One of these agents is the bacterium Staphylococcus aureus. It has been documented, for instance, that 3 to 4% of all surgical admissions in tropical countries concern drainage of pyomyositic infections (2). On the order of 50% of these abscesses, generating 30% mortality if untreated, are caused by a staphylococcal infection (17). Advanced stages of AIDS seem to predispose patients to the development of intramuscular abscesses caused by S. aureus as well (2). S. aureus has also been implicated as a causal organism in various other diseases in the tropics. High percentages of neonatal sepsis are due to S. aureus (6), and strains that are isolated from blood in rural Africa are generally penicillin resistant and often resistant to other antibiotics as well (21). A recent study performed in hospitals in Mogadishu, the capital of Somalia, demonstrated the presence of multidrug-resistant S. aureus strains; a surprisingly high level of methicillin resistance due to overproduction of β-lactamases was documented (20). Multiple studies have demonstrated that on the order of 40% of all clinically evident cases of persistent middle ear effusion in otitis media are due to S. aureus (5, 19). A recent Nigerian study demonstrated that S. aureus can coexist in a clinically manifest fashion with paramyxoviruses causing acute bronchiolitis (13). Thus, it is obvious that S. aureus has a major clinical impact on the African continent. The recent emergence of relatively high percentages of methicillin-resistant strains (10) and the poorly controlled distribution and inappropriate use of antibiotics may aggravate these problems in the coming years.
In contrast to the African situation, in developed countries S. aureus is mainly encountered as an opportunistic and nosocomial pathogen. Various reports confirmed that nosocomial infection with S. aureus is an important cause of morbidity and mortality among hospitalized patients in western European countries and the North American continent (7, 12). Surgical site infections (SSI) caused by S. aureus are an important complication of surgery. A considerable amount of medical literature showed that S. aureus appears to be the major pathogen involved in SSI, and a main risk factor for the development of SSI was proven to be S. aureus nasal carriage (4, 15). SSI is a common problem in the Sudan as well, although only a limited number of studies to unravel the local microbial epidemiology have been undertaken up to now (1, 8). The aim of the present study was to assess the role of nasal S. aureus carriage among patients and staff personnel in the development of SSI in the surgery department of a large teaching hospital in Khartoum, the capital city of Sudan.
MATERIALS AND METHODS
Study setting.
The present study was carried out at Soba University Hospital, Khartoum, Sudan, between July 1996 and May 1997. All patients (n = 414) who underwent elective surgery during the study period were enrolled. Each of them had a nasal swab for S. aureus taken on the first day of admission. After surgery, patients were monitored for 4 weeks for the development of SSI (defined according to Centers for Disease Control and Prevention criteria [12]). In addition, 82 people on the surgical staff, which is a large majority of the people working in this department, were screened for nasal S. aureus carriage every 2 weeks during the same period or when available if regular sampling was impossible.
Cultivation and identification of S. aureus.
Sterile dry cotton swabs (Transswab; Medical Wire & Equipment Co. Ltd., Corsham, Wiltshire, United Kingdom) were used to collect staphylococci from the nostrils. The swab was circled through both nostrils consecutively while applying an even pressure. The swabs were inoculated directly onto 5% blood agar and phenol red mannitol salt agar (Difco Laboratories) and incubated at 37°C for 24 to 48 h. S. aureus colonies were selected on the basis of their morphological characteristics and identified directly with Staphaurex Plus (Murex Diagnostics, Dartford, United Kingdom), which is a rapid agglutination test. This screening method was documented to be highly sensitive and has been extensively validated by other authors (22). S. aureus isolates were transported at room temperature as monocultures in brain heart infusion agar to the Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Center Rotterdam (Rotterdam, The Netherlands), for further identification and genotyping.
Antibiotic susceptibility testing.
Antibiotic susceptibilities for 85 of the S. aureus isolates were determined with the Microscan WalkAway-96 (Dade International, Maurepas, France), an automated system for bacterial identification and antimicrobial susceptibility testing. The following antibiotics were tested: penicillin, oxacillin, tetracycline, erythromycin, gentamicin, rifampin, clindamycin, cotrimoxazole, vancomycin, fusidic acid, and ciprofloxacin. Methicillin susceptibility was determined by the disk diffusion method according to National Committee for Clinical Laboratory Standards guidelines (18). The 85 strains were derived from both patients and staff personnel and were selected at random with the single restriction that one isolate per individual was included.
DNA isolation and RAPD analysis.
DNA was isolated by the combined action of lysostaphin, guanidinium isothiocyanate, and subsequent Celite affinity chromatography (Janssen Pharmaceuticals, Beerse, Belgium) as described previously (13). Random amplification of polymorphic DNA (RAPD) analysis employed 0.2 U of Taq polymerase (SuperTaq; HT Biotechnology, Cambridge, United Kingdom) and 5 ng of template DNA per reaction. Cycling was performed in a model 60 thermocycler (Biomed, Theres, Germany) and consisted of the following steps: predenaturation at 94°C for 4 min and 40 cycles of 45 s at 94°C, 45 s at 25°C, and 2 min at 72°C. Primers used to discriminate S. aureus strains were RAPD1 (5′-GGTTGGGTGAGAATTCACG-3′), RAPD7 (5′-GTAGGATGCGA-3′), and ERIC2 (5′-AAGTAAGTGACTGGGGTGAGGCG-3′), and the experimental protocol has been described in detail previously (24–26, 29). PCR fingerprints were identified by visual inspection of the banding patterns and given a numerical index for the identification of separate types.
Protein A and coagulase gene PCR.
Restriction fragment length polymorphism (RFLP) in the staphylococcal protein A gene was determined by PCR essentially as described before (9, 24). The repetitive region within the gene was amplified (primers: 5′-TGTAAAACGACGGCCAGTGCTAAAAAGCTAAACGATGA-3′ and 5′-CAGGAAACAGCTATGACCCCACCAAATACAGTTGTTACC-3′) with the restriction endonuclease RsaI (Boehringer GmbH, Mannheim, Germany). RFLP was determined by electrophoresis in Nusieve GTG agarose 3% gel (Biozym, Leek, The Netherlands). The number of repetitive units present was estimated by comparison with molecular weight markers (100-bp ladder; Pharmacia, Gouda, The Netherlands). RFLP types were identified by capital letters. Amplification of the coagulase gene was done with primers COAG2 and COAG3 (5′-CGAGACCAAGATTCAACAAG-3′ and 5′-AAAGAAAACCACTCACATCA-3′, respectively) (11, 24). The amplification product was digested with RsaI (Boehringer GmbH) and analyzed as described above.
PFGE.
Pulsed-field gel electrophoresis (PFGE) was performed essentially as described previously (27, 28). Bacteria were suspended in 0.8% low-melting-point Incert agarose (FMC Bioproducts, Rockland, Fla.) and lysed with lysostaphin–proteinase K–1% sodium dodecyl sulfate at 37°C overnight. DNA was digested with SmaI (Boehringer GmbH), and the resultant DNA fragments were separated in 1% SeaKem agarose (FMC Bioproducts and Biozym) in a contour-clamped homogeneous electric field machine (Chef-Mapper; Bio-Rad, Veenendaal, The Netherlands). Banding patterns were interpreted according to the guidelines brought forward by Tenover et al. (23). This means that types are identified by capital letters and subtypes are identified by numbers. Types and subtypes differ in 1 to 3 band positions.
RESULTS AND DISCUSSION
Screening for S. aureus carriage and surveillance of SSI in patients.
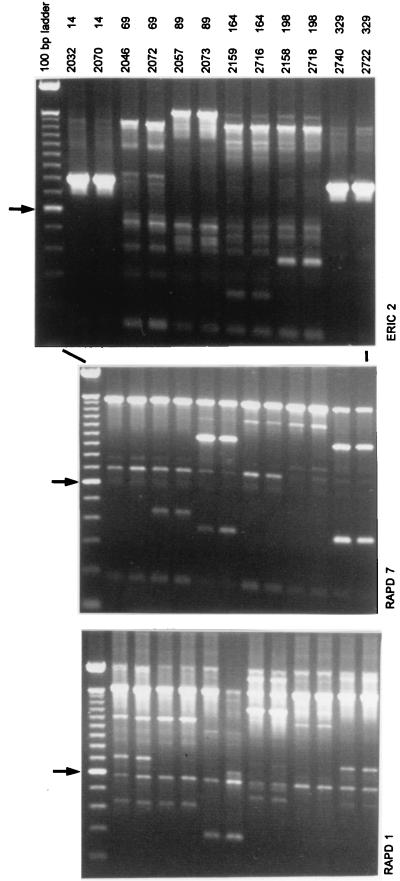
The 414 patients who underwent elective surgery included 256 males (61.7%) and 158 females (38.3%). Their mean age was 28.3 years (range, 1 month to 85 years). Ninety-eight patients (23.9%) had S. aureus-positive nasal cultures preoperatively. Fifty-seven patients (13.8%) developed SSI; in 24 (5.8%) S. aureus was the primary pathogen. The incidence of SSI was not significantly different for nasal S. aureus carriers (6 of 98 patients; 6.0%) compared to noncarriers (18 of 316 patients; 5.7%) (relative risk, 1.08; 95% confidence interval, 0.41 to 2.82). Six nasal carriers developed SSI, and, based on genotyping data, all of them had identical strains in their wounds and noses (Fig. 1 and Table 1). Among the pairs of nose and wound isolates, combined application of three RAPD tests, PFGE, and RFLP analysis for two separate genes did not reveal a single difference. The RFLP data did not significantly contribute to the observed levels of genetic heterogeneity, nor did these data define carriage characteristics (data not shown). Among the patients no identical strains were observed. S. aureus strains isolated from SSI in the noncarrier group showed a similarly high degree of genetic heterogeneity based on the combination of a single RAPD assay (primer RAPD1) and PFGE (Table 2). Overall, the frequency of S. aureus SSI is somewhat higher than those established for other centers in the Western Hemisphere (see the recent review by Kluytmans et al. [14]). Since the densities of the populations inhabiting the nostrils of the different individuals were not quantified, the putative association between bacterial load and the risk for developing an SSI cannot be assessed for the present group of volunteers.
FIG. 1.
Survey of RAPD data obtained for paired S. aureus strains. Each pair was derived from the nose and surgical wound of the same patient. Above the lanes, strain and patient numbers are given. The different panels display experimental data obtained by the application of different RAPD primers (ERIC2, RAPD7, and RAPD1). Data are summarized in Table 2. The arrow on the left identifies the molecular length marker that is 600 bp long in the 100-bp ladder. Adjacent markers differ by 100 bp.
TABLE 1.
Genotyping of S. aureus strains derived from the noses and infected wounds of nasal S. aureus carriers
| Patient | Strain (sitea) | Type by:
|
Typec from RFLP analysis of gene of:
|
||
|---|---|---|---|---|---|
| RAPDb | PFGE | Protein A | Coagulase | ||
| 14 | 2032 (n) | 16, 1, 1 | H1 | A | A |
| 2070 (w) | 16, 1, 1 | H1 | A | A | |
| 69 | 2046 (n) | 18, 2, 2 | S | B | B |
| 2072 (w) | 18, 2, 2 | S | B | B | |
| 89 | 2057 (n) | 3, 3, 3 | BB | C | C |
| 2073 (w) | 3, 3, 3 | BB | C | C | |
| 164 | 2159 (n) | 29, 4, 4 | O | B | B |
| 2716 (w) | 29, 4, 4 | O | B | B | |
| 198 | 2158 (n) | 25, 5, 5 | X1 | D | D |
| 2718 (w) | 25, 5, 5 | X1 | D | D | |
| 329 | 2740 (n) | 30, 3, 2 | CC | E | C |
| 2722 (w) | 30, 3, 2 | CC | E | C | |
n, nose; w, wound.
The numbers are, from left to right, the indices derived from PCR analysis with primers RAPD1, RAPD7, and ERIC2.
The resolution of the coagulase and protein A gene RFLP analyses does not allow for discrimination between the strains obtained from patients 69 and 164. A similar lack of resolution can be observed in the coagulase gene data for patients 89 and 329.
TABLE 2.
Genotyping results for strains of S. aureus isolated from patients suffering from wound infections who did not carry S. aureus in their noses
| Strain | Warda | Type by:
|
|
|---|---|---|---|
| PFGE | RAPD | ||
| 2721 | 1 | AA | 23 |
| 2724 | 1 | X1 | 25 |
| 2727b | 1 | D | 4 |
| 2071 | 2 | B | 14 |
| 2075 | 2 | W | 24 |
| 2717 | 2 | X1 | 25 |
| 2076c | 2 | U1 | 25 |
| 2720c | 2 | U1 | 25 |
| 2723 | 2 | X2 | 25 |
| 2725 | 2 | D1 | 27 |
| 2078 | 4 | X | 26 |
| 2714 | 4 | Y | 27 |
| 2715 | 4 | E | 28 |
| 2719 | 4 | Z | 16 |
| 2726 | 4 | J | 20 |
1, General Surgery (Male); 2, General Surgery (Female); 3, Urology; 4, Pediatrics.
The type associated with this strain is frequently encountered among staff personnel.
This strain may represent a departmental cross infection.
Screening for S. aureus carriage in staff personnel.
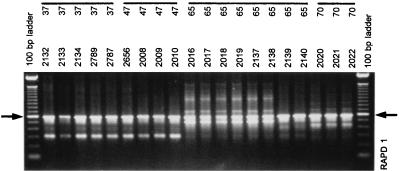
Several members of the surgical staff were screened for S. aureus nasal carriage every 2 weeks, and the nasal carriage rate was 26.8% overall. Thirteen percent of them were persistent carriers (always culture positive), 40% were intermittent carriers (at least 80% of samples were culture positive), and 47% were noncarriers. Genotyping of serial S. aureus isolates from both persistent and intermittent carriers showed that 60% of the carriers kept the same strain over time while 40% had different S. aureus strains during the 10-month screening period (see Fig. 2 for some illustrations and Table 3 for a review).
FIG. 2.
Survey of RAPD data obtained for S. aureus strains derived from the noses of staff personnel in a longitudinal fashion. Data shown were collected with primer RAPD1 and represent samples from four different persons (highlighted by lines). Note that for person 65 a clear shift in the nature of the colonizing strain can be observed: strain 2138 is clearly different from strain 2139. Data are summarized in Tables 3 and 4. The arrows on the left and on the right identify the molecular length marker that is 600 bp long in the 100-bp ladder. Adjacent markers differ by 100 bp.
TABLE 3.
RAPD genotyping results for persistently and intermittently S. aureus-colonized staff members in the surgery department of Soba University Hospital
| Carrier group | Person identification no. | Strain | PCR typea |
|---|---|---|---|
| Persistent | |||
| S-37 | 2005 | 4 | |
| S-37 | 2081 | 4 | |
| S-37 | 2080/2788 | 4 | |
| S-37 | 2129 | 4 | |
| S-37 | 2130 | 4 | |
| S-37 | 2131 | 4 | |
| S-37 | 2132 | 4 | |
| S-37 | 2133 | 4 | |
| S-37 | 2134 | 4 | |
| S-37 | 2789 | 4 | |
| S-37 | 2787 | 4 | |
| S-47 | 2656 | 4 | |
| S-47 | 2008 | 4 | |
| S-47 | 2009 | 4 | |
| S-47 | 2010 | 4 | |
| S-72 | 2024 | 18 | |
| S-72 | 2025 | 18 | |
| S-72 | 2144 | 18 | |
| S-72 | 2145 | 18 | |
| S-72 | 2146 | 18 | |
| S-72 | 2147 | 18 | |
| S-72 | 2148 | 18 | |
| S-72 | 2149 | 18 | |
| S-72 | 2790 | 18 | |
| S-72 | 2793 | 18 | |
| S-72 | 2791 | 18 | |
| Intermittent | |||
| S-65 | 2016 | 15 | |
| S-65 | 2017 | 15 | |
| S-65 | 2018 | 15 | |
| S-65 | 2019 | 15 | |
| S-65 | 2137 | 15 | |
| S-65 | 2138 | 15 | |
| S-65 | −b | NAc | |
| S-65 | 2139 | 10 | |
| S-65 | 2140 | 10 | |
| S-65 | − | NA | |
| S-65 | − | NA | |
| S-70 | 2020 | 16 | |
| S-70 | 2021 | 16 | |
| S-70 | 2022 | 16 | |
| S-70 | 2141 | 16 | |
| S-70 | 2142 | 16 | |
| S-70 | − | NA | |
| S-70 | 2792 | 16 | |
| S-74 | 1969 | 4 | |
| S-74 | − | NA | |
| S-74 | 2026 | 4 |
A clear strain shift can be observed for person S-65 (from RAPD type 15 to 10). See Fig. 2 for some additional detail.
−, negative.
NA, not applicable.
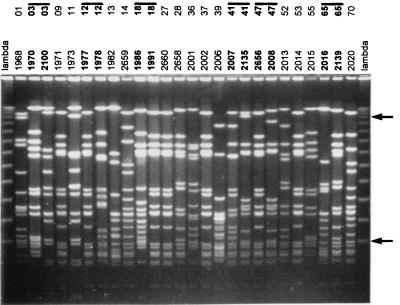
The RAPD typing of 33 randomly selected strains obtained from the surgical staff gave 22 different profiles. Further typing by PFGE for the same group of isolates revealed limited additional heterogeneity (22 PFGE types and 1 subtype) (Fig. 3 and Table 4). When the single-primer RAPD1 and PFGE genocodes were combined, 26 different overall types could be identified. A single strain (overall type IV, D4) was found in seven individuals and represents a nosocomially prevalent strain. This strain was also encountered among the isolates recovered from wounds (Table 2, strain 2727). This observation supports the notion that cross infection occurs in this setting.
FIG. 3.
PFGE of DNA macrorestriction fragments of S. aureus strains that were isolated from nasal carriers among the staff personnel in the surgical ward of Soba Hospital. Numbers for individuals and strains are above the lanes. The lane marked lambda contains lambda concatemers that differ in size by multiples of 50 kbp. The fragments of 50 and 500 kbp are highlighted by arrows on the right. For several of the staff members (indicated by lines and boldface) multiple strains are included. All of the pairs are different, and this is reminiscent of strain shifts in intermittent carriers. Data are summarized in Table 4. Note that the patterns for strains 1971, 1977, 1991, 2660, 2002, 2656, and 2014 are identical. Also, strains 2658 and 2139 cannot be discriminated on the basis of PFGE.
TABLE 4.
Results of PFGE and RAPD analysis for S. aureus strains isolated from the noses of members of the surgical staff
| Strain | Professiona | Wardb | Type by:
|
Overall genotyped | |
|---|---|---|---|---|---|
| PFGEc | RAPD | ||||
| 1968 | Dr | 4 | A | 1 | I |
| 1970 | S | 3 | B | 2 | II |
| 2100 | S | 3 | C | 3 | III |
| 1971 | S | 1, 2 | D | 4 | IV |
| 1973 | N | 4 | E | 5 | V |
| 1977 | N | 4 | D | 4 | IV |
| 1978 | N | 4 | F | 6 | VI |
| 1982 | N | 1 | G | 7 | VII |
| 2659 | N | 4 | H | 8 | VIII |
| 1986 | N | 4 | I | 9 | IX |
| 1991 | N | 4 | D | 4 | IV |
| 2660 | N | 4 | D | 4 | IV |
| 2658 | N | 4 | J | 10 | X |
| 2001 | N | 1 | K | 11 | XI |
| 2002 | N | 1 | D | 4 | IV |
| 2006 | Dr | 1, 2 | L | 12 | XII |
| 2007 | N | 1 | M | 10 | XIII |
| 2135 | N | 1 | N | 13 | XIV |
| 2656 | N | 2 | D | 4 | IV |
| 2008 | N | 2 | O | 3 | XV |
| 2013 | N | 5 | P | 6 | XVI |
| 2014 | N | 5 | D | 4 | IV |
| 2015 | N | 1 | B | 14 | XII |
| 2016 | Dr | 1, 2 | Q | 15 | XIII |
| 2139 | Dr | 1, 2 | J | 10 | X |
| 2020 | Dr | 1, 2 | H1 | 16 | XIX |
| 2023 | N | 4 | R | 17 | XX |
| 2024 | N | 4 | S | 18 | XXI |
| 2150 | N | 1 | T | 19 | XXII |
| 1969 | Dr | 1, 2 | U | 20 | XXIII |
| 2027 | N | 4 | U | 21 | XXIV |
| 2795 | N | 1 | V | 22 | XXV |
| 1962 | Dr | 1, 2 | N | 3 | XXVI |
Antibiotic susceptibilities.
All strains were fully susceptible to oxacillin, gentamicin, vancomycin, and rifampin but were resistant to penicillin. A large proportion of the strains was resistant to cotrimoxazole (24 of 85; 28%) or tetracycline (53 of 85; 62%). Fewer strains were documented to be resistant for ciprofloxacin (5 of 85). In addition, a large fraction of the strains appeared to be intermediately resistant to this antibiotic (32 of 85; 38%). For erythromycin and clindamycin a similar trend was observed: for erythromycin four strains (5%) were resistant and two strains (2%) appeared to be intermediately resistant; for clindamycin the corresponding percentages were 6 and 7%, respectively. One strain was resistant to fusidic acid. Multidrug resistance was documented on several occasions. The observed pattern of resistances adequately reflects the frequency at which antibiotics prescribed in Soba University Hospital and available over the counter in Sudan as a whole are used. No differences in antibiotic susceptibilities were observed when isolates from staff personnel were compared to the isolates from patients.
Concluding remarks.
It was analyzed whether nasal S. aureus carriage was a risk factor for the development of SSI caused by S. aureus in the surgical ward of a teaching hospital in Khartoum, Sudan. The incidence of S. aureus SSI appeared to be somewhat higher than those in Western hospitals. However, the number of autoinfections represented only 25% of all cases, indicating a relatively large degree of independent cross infection. Although we identified a strain that occurred more frequently than others among staff personnel (type IV, D4), this did not explain the occurrence of cross infection. Although type IV was encountered as the SSI-causing organism on one occasion, the staff personnel do not seem to serve as a major reservoir from which multiple SSI develop. Additional reservoirs may be the family members who take care of the sick persons. Other sources remain unidentified, but the data of the present study indicate that clearance of S. aureus nasal colonization by the application of mupirocin, which was demonstrated to be successful in several intervention studies in Western hospitals (14, 16), is unlikely to help diminish the frequency of S. aureus SSI in this Sudanese university hospital. Further studies are needed to evaluate the role of general infection control measures in reducing the incidence of SSI in hospitals in the tropics.
REFERENCES
- 1.Abugroun E A S M. Ph.D. thesis. London, United Kingdom: University of London; 1991. [Google Scholar]
- 2.Ansaloni L. Tropical pyomyositis. World J Surg. 1996;20:613–617. doi: 10.1007/s002689900094. [DOI] [PubMed] [Google Scholar]
- 3.Boom R, Sol C J A, Salimans M M M, Jansen C L, Wertheim van Dillen P M E, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention, Hospital Infections Program. National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986–April 1996, issued May 1996: a report from the NNIS system. Am J Infect Control. 1996;24:380–388. [PubMed] [Google Scholar]
- 5.Cisse M F, Sow A I, Adjovi D R, Samb A. Bacteriological study of purulent otitis media in children in CHU in the tropical zone. Arch Pediatr. 1995;2:29–33. doi: 10.1016/0929-693x(96)89805-8. [DOI] [PubMed] [Google Scholar]
- 6.Dawodu A H, Alausa O K. Neonatal septicaemia in the tropics. Afr J Med Sci. 1985;9:1–6. [PubMed] [Google Scholar]
- 7.Dixon R E. Cost of nosocomial infections and benefits of infection control programs. In: Wenzel R P, editor. Prevention and control of nosocomial infections. Baltimore, Md: The Williams and Wilkins Co.; 1995. pp. 19–25. [Google Scholar]
- 8.El Raba’a S, Ibrahim A. Early post operative wound infection in clean surgery at Khartoum. East Afr Med J. 1987;64:183–189. [PubMed] [Google Scholar]
- 9.Frenay H M, Theelen L M, Schouls L M, Vandenbroucke-Grauls C M J E, Verhoef J, van Leeuwen W J, Mooi F R. Discrimination of epidemic and nonepidemic methicillin-resistant Staphylococcus aureus strains on the basis of protein A gene polymorphisms. J Clin Microbiol. 1994;32:846–847. doi: 10.1128/jcm.32.3.846-847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gakuu L N. Review of methicillin resistant Staphylococcus aureus with special reference to handling of surgical patients. East Afr Med J. 1997;74:198–202. [PubMed] [Google Scholar]
- 11.Goh S-H, Byrne S K, Zhang J L, Chow A W. Molecular typing of Staphylococcus aureus on the basis of coagulase gene polymorphism. J Med Microbiol. 1992;30:1642–1645. doi: 10.1128/jcm.30.7.1642-1645.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horan T C, Gaynes R P, Martone W J, Jarvis W R, Emori T G. CDC definitions of nosocomial surgical site infections 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;17:780–785. [PubMed] [Google Scholar]
- 13.Johnson A W, Aderele W I, Osinusi K, Gbadero D A, Fagbami A H, Rotowa N A. Acute bronchiolitis in tropical Africa: a hospital-based perspective in Ibadan, Nigeria. Pediatr Pulmonol. 1996;22:236–247. doi: 10.1002/(SICI)1099-0496(199610)22:4<236::AID-PPUL3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 14.Kluytmans J A J W, van Belkum A, Verbrugh H A. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kluytmans J A J W, Mouton J W, Ijzerman E P F, Vandenbroucke-Grauls C M J E, Maat A W P M, Wagenvoort J H T, Verbrugh H A. Nasal carriage of S. aureus as a major risk factor for wound infection after cardiac surgery. J Infect Dis. 1995;171:216–219. doi: 10.1093/infdis/171.1.216. [DOI] [PubMed] [Google Scholar]
- 16.Kluytmans J A J W, Mouton J W, VandenBergh M F Q, Manders M A A J, Maat A W P M, Wagenvoort J H T, Micheland M F, Verbrugh H A. Reduction of surgical site infections in cardiothoracic surgery by elimination of nasal carriage of S. aureus. Infect Control Hosp Epidemiol. 1996;17:780–785. doi: 10.1086/647236. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-de Jesus F R, Mendiola-Segura I. Clinical stage, age and treatment in tropical pyomyositis: a retrospective study including forty cases. Arch Med Res. 1996;27:165–170. [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. 6th ed. Approved standard M2-A6. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 19.Ndip R N, Obi M C, Obi C L, Nwawolo C, Igumbor E O, Obi A A. Antibiogram of bacterial isolates from cases of otitis media and lower respiratory tract infections. Afr J Med Med Sci. 1995;24:353–357. [PubMed] [Google Scholar]
- 20.Nur Y A, Vandenbergh M F Q, Yusuf M A, van Belkum A, Verbrugh H A. Nasal carriage of multiresistant Staphylococcus aureus among health care workers and pediatric patients in two hospitals in Mogadishu, Somalia. Int J Infect Dis. 1996;1:186–191. [Google Scholar]
- 21.Petit P L, Schneeberger P, Lidala V, Butter M, Wamola I A. Bacteriology of infections in a rural tropical area of Kenya: isolates and antibacterial susceptibility. East Afr Med J. 1991;68:500–506. [PubMed] [Google Scholar]
- 22.Riewerts Eriksen N, Espersen F, Thamdrup Rosdahl V, Jensen K. Evaluation of methods for the detection of nasal carriage of Staphylococcus aureus. APMIS. 1994;102:407–412. doi: 10.1111/j.1699-0463.1994.tb04891.x. [DOI] [PubMed] [Google Scholar]
- 23.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Belkum A, Riewerts Eriksen N, Sijmons M, van Leeuwen W, van den Bergh M, Kluytmans J, Espersen F, Verbrugh H. Coagulase and protein A polymorphisms do not contribute to persistence of nasal colonization by S. aureus. J Med Microbiol. 1997;46:222–232. doi: 10.1099/00222615-46-3-222. [DOI] [PubMed] [Google Scholar]
- 25.van Belkum A, Bax R, Prevost G. Comparison of four genotyping assays for epidemiological study of methicillin resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1994;13:420–424. doi: 10.1007/BF01972002. [DOI] [PubMed] [Google Scholar]
- 26.van Belkum A, Bax R, Peerbooms P, Goessens W H F, van Leeuwen N, Quint W G V. Comparison of phage typing and DNA fingerprinting by polymerase chain reaction for discrimination of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1993;31:798–803. doi: 10.1128/jcm.31.4.798-803.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Belkum A, van Leeuwen W, Kaufmann M E, Cookson B, Forey F, Etienne J, Goering R, Tenover F, Steward C, O’Brien F, Grubb W, Tassios P, Legakis N, Morvan A, El Solh N, de Ryck R, Struelens M, Salmenlinna S, Vuopio-Varkila J, Kooistra M, Talens A, Witte W, Verbrugh H. Assessment of resolution and intercenter reproducibility of results of genotyping Staphylococcus aureus by pulsed-field gel electrophoresis of SmaI macrorestriction fragments: a multicenter study. J Clin Microbiol. 1998;36:1653–1659. doi: 10.1128/jcm.36.6.1653-1659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Belkum A, van Leeuwen W, Verkooyen R, Can Sacilik S, Cokmus C, Verbrugh H A. Dissemination of a single clone of methicillin-resistant Staphylococcus aureus among Turkish hospitals. J Clin Microbiol. 1997;35:978–981. doi: 10.1128/jcm.35.4.978-981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Versalovic J, Koeuth T, Lupski J R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]