Abstract
Objective:
To determine whether a multiple-dose series of an inactivated whole cell mycobacterial vaccine, Mycobacterium vaccae, can prevent HIV-associated tuberculosis.
Design and methods:
The DarDar trial was a randomized, placebo-controlled, double-blind trial. The study was carried in an outpatient facility in Dar es Salaam, Tanzania. HIV-infected patients with CD4 cell counts of at least 200cells/μl and a Bacille Calmette–Guérin scar were chosen for the study. The intervention was carried out by random 1:1 assignment to five intradermal doses of M.vaccae or placebo. Tuberculin skin tests were performed, and patients with reactions of at least 5mm were administered isoniazid for 6 months. The main outcome measures were disseminated (primary endpoint), definite, and probable tuberculosis (secondary endpoints).
Results:
Two thousand thirteen individuals were randomized (1006 to M. vaccae, 1007 to placebo) and followed every 3 months for a median of 3.3 years. The trial was terminated early because of slow accrual of cases of disseminated tuberculosis and significant protection against definite tuberculosis. Hazard ratios were disseminated tuberculosis 0.52 (95% confidence interval 0.21–1.34; seven cases in M. vaccae, 13 cases in placebo; log-rank P=0.16), definite tuberculosis 0.61 (95% confidence interval 0.39–0.96; 33cases in M. vaccae, 52cases in placebo; P=0.03), and probable tuberculosis 1.17 (95% confidence interval 0.76–1.80; 48 cases in M. vaccae, 40 cases in placebo; P=0.46). Immunization was well tolerated, with no adverse effect on CD4 cell count or HIV viral load, and no increase in the rate of serious adverse events.
Conclusion:
Administration of a multiple-dose series of M. vaccae to HIV-infected adults with childhood Bacille Calmette–Guérin immunization is safe and is associated with significant protection against definite tuberculosis. These results provide evidence that immunization with a whole cell mycobacterial vaccine is a viable strategy for the prevention of HIV-associated tuberculosis.
Keywords: Bacille Calmette–Guérin, HIV, prevention, tuberculosis, vaccine
Introduction
The global tuberculosis (TB) epidemic continues to grow with an estimated 9.3 million new cases and 1.7 million deaths in 2007 [1]. HIV infection is a major contributor to the epidemic, and neither isoniazid (INH) preventive therapy (IPT) nor antiretroviral therapy (ART) is completely effective in reducing this risk [2–5]. Consequently, TB remains the major cause of death in most regions where TB and HIV coexist [4–7]. Disease due to multiple-drug resistant (MDR) TB and extensively-drug resistant (XDR) TB has also fueled the global epidemic [8–10]. Despite the wider application of ART and the development of newer and more potent antimicrobials, the current multifaceted TB epidemic continues to grow at a rate of 1% per year [7].
An effective preventive TB vaccine would have the potential to impact each of the components of the global epidemic [11]. The existing TB vaccine, Mycobacterium bovis, Bacille Calmette–Guérin (BCG), is more than 80% effective in childhood when given to mycobacteria-naive newborns but is generally considered to offer minimal or no protective effect when administered to previously unvaccinated adults [12,13]. BCG is also ineffective for boosting immunity in those who received the vaccine in childhood [14–16].
Before the widespread acceptance of BCG, inactivated whole-cell mycobacterial vaccines, derived from both Mycobacterium tuberculosis and nontuberculous mycobacteria, had been shown to be effective in preventing TB in experimental models and in clinical trials but were not widely adopted, presumably because of the need for multiple-dose immunization [17–19]. We considered that an inactivated whole-cell mycobacterial vaccine could be used in a prime-boost immunization strategy after childhood BCG and would be expected to be well tolerated in HIV-infected persons. Our international epidemiologic studies [20–24] of disseminated mycobacterial infections in AIDS suggested that although African AIDS patients were exposed to organisms of the Mycobacterium avium complex (MAC), they were generally protected against disseminated MAC, presumably from immunity induced by prior TB and/or prior BCG immunization. This conclusion is consistent with extensive epidemiologic and experimental studies demonstrating that infection with one species of mycobacteria provides cross-protection against other (heterologous) species (e.g., infection with nontuberculous mycobacteria protects against TB) [25–27]. Further, recent evidence suggests that antigens of the DosR regulon, present in most nontuberculous mycobacteria, but not in BCG, are associated with the control of latent TB [28].
Our hypothesis was that in patients with HIV infection, the mycobacterial immunity that had already been primed by childhood BCG immunization might be boosted to provide protection against TB. We posited that a successful prime-boost strategy against TB in HIV infection would need to meet the following several requirements. First, it would need to be given early in HIV infection for an optimal immune response. Second, it would need to present multiple antigens because of the reduced T-cell diversity in HIV infection. Third, to be well tolerated in HIV, it could not include live mycobacteria. An inactivated whole-cell mycobacterial reagent would satisfy all these requirements.
Such a vaccine was developed by Drs John Stanford and Graham Rook using a strain of Mycobacterium vaccae [29]. Single-dose studies [30,31] of M. vaccae for the treatment of drug-sensitive TB were unsuccessful. We realized that an inactivated vaccine would need to be administered in a multiple-dose series to induce the immune response we sought for the prevention of TB. We, therefore, conducted phase I studies [32–35] to demonstrate the safety of a multiple-dose series of M. vaccae in healthy adults and in HIV-infected adults and children. A phase II study [36] in Zambia indicated that five doses of M. vaccae could induce T-cell responses against mycobacteria in HIV-infected patients, and that responses were maximal in recipients primed with childhood BCG immunization. A subsequent, randomized, controlled, phase II trial [37] in Finland demonstrated that five doses of M. vaccae were well tolerated in HIV-infected patients and boosted T-cell mycobacterial responses in recipients primed with BCG. The present phase III trial was designed to test the efficacy of a five-dose series of M. vaccae in the prevention of HIV-associated TB among BCG-primed recipients in a TB-endemic country.
Methods
Participants
Eligible participants were ambulatory HIV-positive residents of Dar es Salaam, Tanzania, with age at least 18 years with CD4 cell count of at least 200 cells/μl, a visible BCG scar from childhood immunization (sensitivity >90%[38]), a negative pregnancy test, and no evidence of active TB. Participants were recruited from HIV testing centers. At baseline, all patients were interviewed, examined, and had the following tests performed: HIV ELISA, CD4 cell count, tuberculin skin test (TST; RT-23; State Serum Institute, Copenhagen, Denmark), single posterior–anterior chest radiograph, and collection of three sputum samples for acid fast bacillus (AFB) stain and mycobacterial culture. Patients with a TST result of at least 5mm and no history of prior treatment for TB were given 6 months of IPT, 300 mg daily for 6 months [39].
Immunization protocol
A 1:1 randomization list was generated at Dartmouth and assigned sequential participants in Dar es Salaam to one of the 20 numbered but otherwise unidentified boxes of M. vaccae or placebo (10 each) for their entire treatment course. Treatment assignments were generated in randomly permuted blocks of eight, 10, 12, 14, and 16 participants. Thus, all patients and study personnel, including the technical nurse who administered the vaccine, were blinded to treatment assignment; no evaluations for vaccine site reactions or suspect TB were performed by the technical nurse. Patients received a five-dose series of 0.1ml intradermal M. vaccae (M. vaccae, SRL 172, ~1 mg, 109 colony-forming units; Immodulon, London, UK) or placebo (borate buffered isotonic saline, same appearance, identical vial) over the deltoid at 0, 2, 4, 6, and 12 months.
Follow-up
All patients were seen routinely every 3 months and were instructed to return to the study site whenever they experienced new symptoms or clinical events and to report any hospitalizations. CD4 cell counts and HIV viral loads were performed annually on all patients through 22 August 2003. Patients with CD4 cell count of less than 200 cells/μl, new active TB, or who met other Ministry of Health (MOH) guidelines for ART were referred to an MOH clinic for HIV treatment. Evaluation for active TB was conducted by study staff and included a physical examination, chest radiograph, three expectorated sputum samples for AFB stain and culture (one spot sample and two first morning samples; standard techniques including culture on Lowenstein Jensen agar), and an automated mycobacterial blood culture (a single 5-ml sample until March 2004, then two 5-ml samples; MB BacT; bioMérieux, Durham, North Carolina, USA). Patients with suspect TB were referred to the MOH for TB treatment. Patients with suspect or treated TB were subsequently categorized as disseminated, definite, or probable TB based on the consensus of three blinded experts who reviewed all clinical and laboratory data, interpreted all chest radiographs, and applied definitions for study endpoints (Table 1).
Table 1.
Tuberculosis endpoints.
| Endpoint | Subcategory | n |
|---|---|---|
|
| ||
| Definite | A1 – ≥1 positive blood culture for M. tuberculosis (disseminated) | 20 |
| A2 – ≥1 positive sputum culture(s) with ≥10 CFU | 51 | |
| A3 – ≥2 positive sputum cultures with 1–9 CFU | 6 | |
| A4 – ≥2 sputum smears with ≥2 AFB/100 oil immersion fields | 7 | |
| A5 – ≥1 positive culture, or positive AFB smear and caseous necrosis from a sterile site (other than blood) | 1 | |
| Probable | B1 – caseous necrosis on a biopsy | 2 |
| Pulmonary | ||
| B2 – a positive chest radiographa along with either one sputum AFB smear with ≥2 AFB/100 oil immersion fields or one sputum culture with 1–9 CFU | 5 | |
| B3 – clinical symptoms/signsb along with either one positive sputum AFB smear, ≥2 AFB/100 oil immersion fields or one sputum culture with 1–9 CFU | 11 | |
| B4 – clinical symptoms/signs and a positive radiograph and a response to antituberculous therapyc | 62 | |
| Extrapulmonary | ||
| B5 – clinical symptoms/signs and a positive imaging study and a response to antituberculous therapy | 5 | |
| B6 – one positive AFB smear from a sterile site along with clinical symptoms/signs of tuberculosis | 1 | |
AFB, acid fast bacillus;CFU, colony-forming units; M. tuberculosis, Mycobacterium tuberculosis.
A positive chest radiograph, compared with a baseline film, is defined as exhibiting any of the following new findings: pulmonary infiltrates (with or without cavitation), pleural effusion, pulmonary fibrosis, or intrathoracic lymphadenopathy. These findings would be expected to persist after 10 days of treatment for routine community-acquired pneumonia.
Symptoms are defined as any of the following: cough for at least 2 weeks, fever for at least 2 weeks, and weight loss of at least 5 kg/year. Signs include any of the following findings on physical examination: rales, dullness to percussion, egophony or bronchial breath sounds (consistent with consolidation), hepatosplenomegaly, lymphadenopathy of at least 1 cm at at least two sites, pericardial friction rub, and focal bone tenderness.
Response to therapy is defined as at least two of the following: gain of more than 3 kg since start of treatment, clearing of fever, reduction of at least 50% in cough frequency, reduction of at least 50% in pleural effusions, infiltrates or intrathoracic or extrathoracic adenopathy, reduction of at least 50% by imaging of other disease due to TB (e.g., pericardial effusion, osteomyelitis, and so on), conversion of AFB smear from positive to negative thrice, conversion of sputum culture from at least 1CFU to negative thrice.
Statistical analysis
Study endpoints defined prospectively by protocol included disseminated, definite, and probable TB (Table 1). The primary endpoint was the time to disseminated TB (defined by a positive blood culture) in the intention-to-treat (ITT) population. The three secondary endpoints were time to first episode of definite, probable, or definite along with probable TB (‘all tuberculosis’). For patients with more than one endpoint, only the first endpoint was included in the analysis.
The ITT population was defined as all randomized patients. The per-protocol population was defined as all patients who received at least three doses of vaccine. Time until TB outcome was calculated on the basis of the study population and the outcome. The starting point for ITT population analyses was the date of randomization, and for the per-protocol population, it was the date of the third dose of vaccine. Each episode of TB was assigned a diagnosis date. For cases with a positive smear or culture, this was the date when the first positive specimen was obtained; otherwise, it was the date when treatment was started. Participants with no TB found during follow-up were censored at the date of the last examination.
The planned sample size was selected to have 80% power to detect a 50% reduction in disseminated TB hazard at the 0.05 (two-sided) level of significance. The required enrollment was a total 2274 patients expected to experience 71 cases of disseminated TB (1.60% per person-year) over a median follow-up of 48 months. One interim efficacy analysis, planned to occur at the midpoint of follow-up, was based on an O’Brien–Fleming stopping boundary [40]. Baseline characteristics of the randomized participants were compared using descriptive statistics, Student’s t-test, and Fisher’s exact test as appropriate. The probability of TB over time was estimated using the Kaplan–Meier product limit method; treatment arms were compared using the logrank test, and hazard ratios were calculated using proportional hazards regression (M. vaccae relative to placebo). To evaluate the effect of M. vaccae in the presence of ART, we performed a proportional hazards regression analysis, which included a time-dependent covariate reflecting ART status. Prespecified subgroup analyses evaluated the vaccine effect according to age, sex, baseline TST, and baseline CD4 cell count. Effect modification by these factors was assessed using proportional hazards regression with appropriate interaction terms. The time-to-event was measured in days; repeat analyses using interval-censored follow-up design with 3-month intervals yielded indistinguishable results. Efron’s method of handling ties was used. Serious adverse events were compared using Fisher’s exact test. All P values are two-sided, and a P value of less than 0.05 was considered statistically significant.
Study design
The study protocol was approved by the Dartmouth Committee for the Protection of Human Subjects, by the Muhimbili University of Health and Allied Sciences (MUHAS) Research Ethics Committee, and by the Division of AIDS Clinical Science Review Committee, National Institutes of Health (NIH). All study participants gave written informed consent. Regulatory oversight of the trial was conducted by the Tanzanian Food and Drug Authority. The study results (including blinded safety and efficacy data) were reviewed every 6 months by an independent Data Safety and Monitoring Board (DSMB) comprising six members from the United States and Tanzania, with expertise in vaccines, HIV, TB, and statistics. The DSMB could request unblinded data and make nonbinding recommendations regarding the completion of enrollment and continuation of the study.
Results
The first patient was enrolled in September 2001 and the last patient in September 2005. A total of 4973 patients were screened to identify 2013 eligible, randomized patients before expiration of the vaccine lot. The study was terminated early on 31 January 2008 based on a recommendation from a closed session of the DSMB after their independent consultation with the sponsor [National Institute of Allergy and Infectious Diseases (NIAID), Division of AIDS, NIH]. The DSMB cited two reasons for termination. First, cases of the primary endpoint, disseminated TB, although showing a trend toward protection, were not accruing at a sufficient rate to provide adequate power for detecting a difference between vaccine and placebo arms. Second, the rate of definite TB indicated a significant level of protection by the vaccine.
Figure 1 shows the recruitment and follow-up of all patients. Of the 2013 patients randomized, 1006 were allocated to M. vaccae and 1007 were allocated to placebo and form the ITT population. Baseline characteristics of the two treatment groups in the ITT population were similar (Table 2). The per-protocol population who received at least three doses was composed of 1853 (92%) patients. The median duration of follow-up from enrollment, with interquartile values, was 3.3 years for M. vaccae (2.5–4.2) and 3.3 years for placebo(2.4–4.3). Loss to follow-up occurred in 184 (18.3%) of M. vaccae recipients and 182 (18.1%) of placebo recipients.
Fig. 1. Consort diagram.
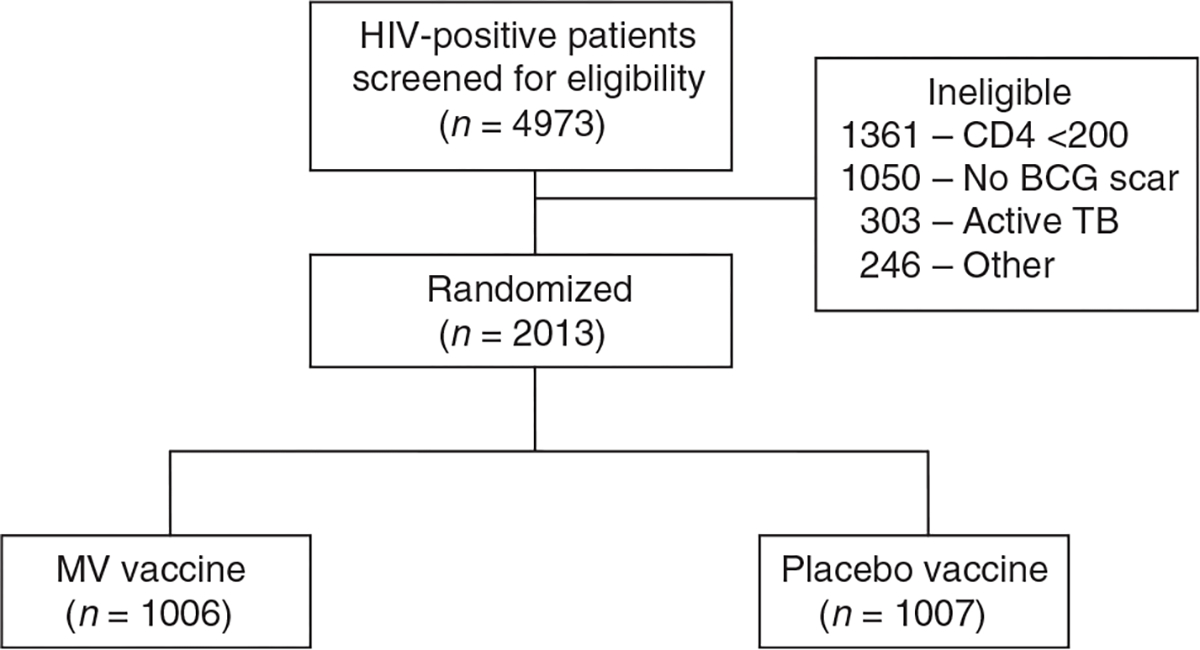
BCG, Bacille Calmette–Guérin; MV, Mycobacterium vaccae; TB, tuberculosis.
Table 2.
Baseline characteristics of study participants.
| Characteristic | MV (n = 1006) | Placebo (n = 1007) | P |
|---|---|---|---|
|
| |||
| Age (years), mean (SD) | 33.2 (8.0) | 33.0 (7.8) | 0.57 |
| Female sex | 774 (77%) | 766 (76%) | 0.68 |
| BMI (kg/m2), median (range) | 23.7 (15.3–50.8) | 23.6 (16.1–47.4) | 0.45 |
| CD4 cell count (cells/μl), median (minimum, 25th–75th percentile, maximum) | 428 (200, 317–573, 1645) | 404 (200, 298–580, 2000) | 0.50 |
| Log HIV viral load (copies/ml), median (range) | 4.12 (1.70–5.70) | 4.08 (1.70–5.70) | 0.26 |
| HIV antiviral therapy | 26 (2.6%) | 34 (3.4%) | 0.36 |
| Prior treatment for TB | 88 (9%) | 82 (8%) | 0.68 |
| Tuberculin skin test ≥5mma | 315 (31%) | 324 (32%) | 0.71 |
MV, Mycobacterium vaccae; TB, tuberculosis.
Two hundred forty-six MV recipients and 247 placebo recipients completed isoniazid preventive therapy.
Table 3 summarizes cases of TB endpoints and hazard ratios. The number of disseminated TB endpoints was insufficient to determine whether M. vaccae was effective against the primary endpoint of disseminated TB; protection was significant for the secondary endpoint of definite TB but not for probable TB (or the combination of definite and probable, Appendix). Prespecified subgroup analyses showed no significant modification of vaccine efficacy for definite TB by age, sex, baseline TST, INH treatment, or baseline CD4 cell count. For patients with a history of prior TB (n=169), the hazard ratio for definite TB was 0.31 [95% confidence interval (CI) 0.10–0.98] compared with 0.70 (95% CI 0.43–1.13) among recipients without such a history (interaction P=0.18).
Table 3.
Study endpoints and protection against tuberculosis.
| Endpoint | Intention-to-treat (n = 2013) |
Per protocola (n = 1853) |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of endpoints MV | Placebo | Hazard ratio (95% CI) | P | No. of endpoints MV | Placebo | Hazard ratio (95% CI) | P | |
|
| ||||||||
| Disseminated tuberculosis | 7 | 13 | 0.52 (0.21–1.34) | 0.16 | 7 | 13 | 0.54 (0.21–1.37) | 0.17 |
| Definite tuberculosis | 33 | 52 | 0.61 (0.39–0.96) | 0.03 | 33 | 51 | 0.64 (0.41–1.00) | 0.04 |
| Probable tuberculosis | 48 | 40 | 1.17 (0.76–1.80) | 0.46 | 47 | 39 | 1.20 (0.78–1.85) | 0.39 |
CI, confidence interval; MV, Mycobacterium vaccae.
Per protocol, at least three doses of MV or placebo.
The time-to-event analyses for the ITT population are shown in Fig. 2 (a and b). We examined the effect of ART, although this was not a prespecified analysis, as ART use was uncommon at the start of the study. Overall, 566 (28%) patients received ARTat some point during the study (292 vs. 274 at M. vaccae and placebo, respectively). There were 857 person-years of follow-up of patients on ART and 5639 person-years of follow-up of patients not on ART. Using a proportional hazards regression model with ART status as a time-dependent covariate, there was no significant interaction between vaccine efficacy and ART (interaction P=0.37). However, the estimated hazard ratio for definite TB in the absence of ART (0.56; 95% CI 0.34–0.93) was numerically lower than in the presence of ART (0.94; 95% CI 0.14–6.17).
Fig. 2. Tuberculosis study endpoints (Kaplan–Meier, intention-to-treat).

(a) Disseminated TB. (b) Definite TB. ITT, intention-to-treat; M. vaccae, Mycobacterium vaccae; TB, tuberculosis.
A total of 4616 doses of M. vaccae and 4603 of placebo were administered. For the first 165 patients, the vaccine site was assessed at days 7 and 28 after each of the five doses. Among M. vaccae recipients, the median vaccine site induration after each dose was 5–6mm (range 0–30 mm) at both 7 and 28 days; among placebo recipients, all median values were 0 mm (range 0–8 mm). At 7 days after each dose, skin desquamation was observed in 35–61% of M. vaccae recipients (0–3% placebo) and local drainage in 9–35% (0–3% placebo). At 28 days, these changes were seen in 18–23% and 7–19% of M. vaccae recipients (0–1% and 0% placebo). Local reactions in M. vaccae recipients were maximal after third dose and typically resolved within several weeks. The proportion of M. vaccae and placebo recipients who reported systemic symptoms including fever, malaise, or headache, ranged from 4 to 23% across all assessments and was similar for both treatment groups (Table A in Appendix).
Immunization was discontinued by the on-site investigator for 12 (1.2%) M. vaccae recipients (and one placebo recipient) because of adverse reactions considered related to vaccine. These events included three vaccine site abscesses, five other local reactions, and four generalized rashes. Vaccine site abscesses occurred after the first dose in one patient and after the third dose in two patients (both with a history of prior TB); all abscesses drained spontaneously and resolved with routine wound care and oral antibiotic therapy. An additional 65 patients (35 M. vaccae, 30 placebo) withdrew themselves from the study before completing immunization, typically citing a switch to alternative medicine, perceived vaccine side effects, or inconvenience.
There were 94 deaths in each treatment group (hazard ratio 0.97, 95% CI 0.73–1.30, P=0.86). The causes of death as reported by relatives or confidantes did not differ between the two treatment groups, including deaths attributed to TB, AIDS, and other febrile illnesses. The proportion of patients with one or more serious adverse events was similar in both groups (Table 4); events attributed to malaria (including some clinical diagnoses and some false-positive smears, thus some ‘malaria’ cases were possibly due to other causes of fever such as TB) were significantly lower in M. vaccae recipients. No adverse effects were noted on CD4 cell count or HIV viral load (data not shown). Repeat TST data on 215 patients showed that among those with a TST of less than 10 mm at baseline, the frequency of a TST of at least 10 mm following the fifth dose was similar in both treatment groups [8/81 (9.9%) M. vaccae vs. 8/71 (11.3%) placebo; P=0.92]. No patients were observed to have a Koch reaction.
Table 4.
Serious adverse events in the intention-to-treat population.
| Category | MV | Placebo | P |
|---|---|---|---|
|
| |||
| Hospitalization, tuberculosis | 2 | 6 | 0.29 |
| Hospitalization, malaria | 76 | 103 | 0.04 |
| Hospitalization, other | 128 | 159 | 0.06 |
| Infection, other | 41 | 44 | 0.82 |
| Obstetric | 8 | 6 | 0.61 |
| All other | 57 | 40 | 0.08 |
| Total patients with ≥1 SAE | 209 | 232 | 0.24 |
MV, Mycobacterium vaccae; SAE, serious adverse event.
Discussion
The present study demonstrates that a multiple-dose series of inactivated M. vaccae given to BCG-primed recipients with HIV infection and baseline CD4 cell count of at least 200 cells/μl is well tolerated and provides significant protection against definite TB with rigorous microbiologic confirmation. It was underpowered to determine the efficacy of M. vaccae in preventing the terminal HIV complication of disseminated TB. To our knowledge, this is the first placebo-controlled trial to demonstrate effective vaccine-based prevention of an opportunistic infection in HIV-infected adults. Vaccine-based prevention of pneumococcal disease has been demonstrated in a placebo-controlled trial in HIV-infected children [41].
We chose disseminated or bacteremic TB as the primary study endpoint based on our prior studies [24,42–44], indicating that this form of disease is common in advanced HIV infection (CD4 cell count <75 cells/μl). Further, it is strictly defined, more readily classified than pulmonary TB, and might represent the most sensitive indicator of a biologic effect of immunization (e.g., BCG is most effective in preventing miliary and meningeal TB, which are likely mediated via bacteremia [13]). At the time of termination, only 20 cases of disseminated TB had been detected compared with the 71 expected cases. Factors that may have directly reduced the rate of disseminated TB include prevention of dissemination of TB by aggressive surveillance for and treatment of the earlier complication of pulmonary TB, the increasing use of ART over time, administration of INH to patients with positive TSTs, and the return of patients with advanced AIDS to their home villages, making follow-up less complete for this endpoint. The observed reduction in disseminated TB cases (seven M. vaccae vs. 13 placebo) paralleled the statistically significant reduction in definite TB cases.
Definite TB, defined here as a secondary endpoint, is the usual primary endpoint in trials for the prevention of TB [3,45], and protection was significant against this rigorously defined and clinically meaningful endpoint. The lack of M. vaccae-induced protection against the other secondary endpoint, clinically defined or ‘probable’ TB, is parallel to the finding in the recent update by Aronson et al. [46] of the 3287 persons BCG trial conducted in the western United States in the 1930s. BCG was found to offer significant protection against culture-confirmed TB for as long as 50–60 years but no protection against clinical/probable TB (nine probable TB cases in BCG recipients and three probable cases in the placebo group) [46]. In the one, other successful BCG trial from the meta-analysis by Colditz et al. [12] of prospective trials, which included definitions for both culture-confirmed definite TB and clinically categorized probable TB, vaccine efficacy was lower for probable than definite TB [47]. There are two possible explanations for this repeated observation in TB vaccine trials: case misclassification or a fundamental and as yet unidentified characteristic of ‘clinical’ or probable TB. In either case, future trials of new vaccines against TB should carefully distinguish definite vs. probable cases of TB.
Local and systemic reactions to M. vaccae were milder and less frequent than generally observed with BCG [13]. We observed three vaccine site abscesses among the 1006 M. vaccae recipients (0.3%); these resolved with simple treatment. Overall, 94% of enrolled patients had a preexisting humoral or cellular immune response to one or more of the five mycobacterial antigens [48] and 8% had a history of prior TB. Nevertheless, we did not observe any Koch reactions or other pathologic local or systemic responses to repeated doses of M. vaccae [49]. Immunization with M. vaccae was not associated with ‘conversion’ of the TST and would not be expected to affect newer interferon gamma release assays, as the M. tuberculosis RD1 antigens used in these tests are absent from M. vaccae [50].
These data conclusively establish the safety of this inactivated whole-cell mycobacterial vaccine in persons with HIVand are consistent with those reported in other trials of M. vaccae in persons without HIV [30,31]. However, the major differences in both the regimens and objectives for our M. vaccae trial compared with previous ones are important to note. We examined the ability of multiple-dose M. vaccae to boost immunity and prevent TB in persons primed by BCG. Previous trials studied single-dose M. vaccae as an adjunct to treatment of drugsusceptible TB [30,31]. Inactivated whole-cell vaccines must be given in a multiple-dose schedule to obtain an optimal preventive immune response [51].
Although morbidity due to TB was reduced by immunization, we did not observe an effect on mortality. In our research setting, all patients were followed closely, had TB diagnosed promptly, sometimes before the onset of clinical symptoms or radiologic abnormalities [52,53], and were referred for ART. It is possible that a mortality benefit might accrue among patients with more limited access to medical care, with delayed diagnosis and treatment of TB. Only a limited number of patients in this trial were receiving ART. Although there was a trend toward lower vaccine efficacy in those receiving ART, the difference was not statistically significant.
Notable strengths of the trial include that it was randomized, placebo-controlled, double-blinded, and well executed. We were able to achieve high rates of compliance with a five-dose schedule, a regimen that might encourage closer follow-up of patients with HIV infection. However, we have shown that a three-dose schedule is immunogenic [37], and further study of this more practical schedule should also be considered.
Visible acute vaccine site reactions in M. vaccae recipients, such as those in the early phases of the immunization schedule, could have led to unblinding by on-site staff, an issue also identified as a potential problem in BCG trials. However, as the trial proceeded, only small scars remained in M. vaccae recipients, all of whom also had BCG scars and some of whom had smallpox scars. Further, decisions to obtain sputum samples and chest radiographs or refer patients for treatment were never made by the technical nurse who administered the immunizations. If a referral bias existed for TB treatment, it might have favored patients with no local reactions who may have been felt to be at a greater risk of TB. Finally, all study endpoints were adjudicated by a blinded panel that did not have access to information regarding vaccine site reactions. Enrollment in this study was restricted to patients with a CD4 cell count of at least 200 cells/μl, a BCG scar, and no active TB; consequently, the results are only applicable to this population.
Conclusion
This study indicates that boosting childhood BCG with a five-dose series of M. vaccae provides 39% protection against definite TB in HIV-infected adults with a CD4 cell count of at least 200 cells/μl in Tanzania. The reduction in risk observed in this study supports further development of a prime-boost immunization strategy using BCG and a whole-cell inactivated mycobacterial vaccine for preventing the most important opportunistic infection affecting HIV-infected persons in the developing world. As the risk of TB in HIV begins before the current CD4 cell threshold for initiation of ART, booster immunization could be considered at the time of initial diagnosis of HIV in areas where TB is endemic.
Results
Among 246 ineligibles whose reason was ‘other’ (consort diagram) subcategories were: failure to return – 79, HIV negative – 43, no CD4 count – 52, serious underlying disease – 26, refused to continue – 18, moved – 8, screening incomplete when enrollment stopped – 20. Subjects who were lost to follow-up compared to subjects who remained in the study were less often female (69% vs. 77%, respectively, P<0.0001), less likely to have had prior tuberculosis (4.4% vs. 9.3%, P <0.001), and less likely to have a positive TST (27% vs. 33%, P=0.02); age, sex, and baseline CD4 counts did not differ between the two groups.
The following number of subjects received the following minimum number of doses: one dose 1975 (988 MV, 987 P); two doses 1902 (950,952); three doses 1853 (928, 925); four doses 1804 (903, 901); five doses 1687 (848, 839).
The HR for definite plus probable tuberculosis in the ITT analysis was 0.83 (95% CI: 0.61–1.13; 79 MV, 92 P; P=0.22) and in the PP analysis was 0.86 (95% CI: 0.63–1.17; 78 MV, 90 P, P=0.31). Two MV subjects had episodes of both definite and probable tuberculosis; only one episode was included for each of these subjects in the definite plus probable calculation.
M. tuberculosis was isolated from 81 subjects; 71 study isolates and 168 concurrent community control isolates were available for IS6110 typing. Five clusters (four with identical IS6110 results and one with similar IS6110 results) were identified and reviewed by the expert panel. One cluster was determined to represent a false positive result and resulted in the change of one tuberculosis case classification from definite to unlikely.
Acknowledgements
We thank Drs Neal Halsey, Clyde Crumpacker, Kenneth McIntosh, Andrew Swai, Paige Williams, Mary Wilson (members of the DSMB); Drs Paul Fine, Daniel Hoft, Marja-Leena Katila, John F. Modlin, Christopher Whalen (consultants), Dr Barry Kreiswirth (molecular typing), Nancy Wray (administrative support), Louise Grant (MB/BacT, bioMerieux), David Kolesar (Isolator, Inverness), Laura Rose Brunet (Silence Therapeutics), Betty Mchaki, Outi Rautio, other laboratory and clinical staff and the many Tanzanian participants who volunteered for the study. We thank Dr Jerald Sadoff of the Aeras Global Tuberculosis Vaccine Foundation for helpful comments.
This study is supported by grants from the NIH, DAIDS, AI 45407 and the Fogarty International Center, D43-TW006807. Vaccine for the study was provided by SR Pharma (London). The study is registered at ClinicalTrials.gov as NCT00052195.
Appendix
Participants
HIV viral load was performed at baseline until August 2003. Blood was obtained at baseline and after dose 5 for immunologic assays including lymphocyte proliferation assays and interferon gamma release assays in response to four mycobacterial antigens (these studies are the subject of a separate report). Persons with tuberculin skin test reactions (TST)≥5 mm at baseline and no history of prior treatment for tuberculosis were given isoniazid 300mg daily for 6 months and followed monthly during this interval [54]. TSTs were repeated 2 months after dose 5.
Subjects were randomized 1:1 in blocks of 6–14 to receive a 5-dose series of 0.1mL intradermal Mycobacterium vaccae (MV; SRL 172; batch MV 017 until July 31, 2004 then batch S00631; Silence Therapeutics, London) or placebo (P; borate buffered saline in an identical vial) on the forearm at 0, 2, 4, 6, and 12 months.
Substudies
In Substudy A (safety), the first 165 subjects were scheduled to return at one and four weeks after each dose of vaccine for detailed examination of vaccine site reactions and for interviews on local and systemic side effects (Table A). Subjects also had HIV viral load and CD4 count determined 2 months after each dose of vaccine. In Substudy B (immunogenicity), the next 150 subjects had vaccine immunology performed 2 months after dose 3 and 5, and annually. Subjects in both Substudy A and Substudy B had repeat tuberculin skin testing performed 2 months after dose 3 and 2 months after dose 5.
Follow-up
Patients with suspect tuberculosis were referred to the National Tuberculosis and Leprosy Control Program (NTLP) with results of these evaluations for decisions about treatment. Standard tuberculosis treatment at the inception of the study was directly observed isoniazid, rifampin, ethambutol and pyrazinamide for 2 months followed by isoniazid and ethambutol for another 6 months. On February 1, 2006 NTLP treatment guidelines were changed to directly observed isoniazid, rifampin, ethambutol and pyrazinamide for 2 months followed by isoniazid and rifampin for another 4 months. Follow up data on treatment outcome were obtained by interview and examination of patients at 2, 5 and 8 months of treatment (and after the change in treatment policy in 2006 at 2, 4 and 6 months).
All subjects were treated for intercurrent infections at the study site or by referral to district hospitals for inpatient care. At each visit, subjects were evaluated to determine if they met current national criteria for initiation of antiretroviral therapy (ART). ART-eligible subjects were counseled and referred to a Tanzanian Ministry of Health Care and Treatment Center for ART. Dates and putative causes of death were obtained from interviews with a relative or confidante named at the time of study entry, and, less commonly, from treating medical facilities and personnel.
Laboratory studies
Sputum AFB stains were performed using the Ziehl-Neelsen method. Mycobacterial sputum cultures were performed at the study laboratory in Tanzania using standard methods: 1% NaOH decontamination followed by culture on Lowenstein-Jensen agar slants with 10 weeks of air incubation. Blood cultures were processed by inoculation of 5mL blood into an automated broth system with a 6 week incubation period (MB/BacT, bio-Merieux, Lyon, France) with back-up use of the Isolator mycobacterial blood system (Inverness Medical Professional Diagnostics, Princeton, NJ) at times when the automated system was not operational [55]. Beginning November 19, 2004 a 6 week terminal subculture to Lowenstein-Jensen medium was performed on all MB/BacT negative blood cultures. AFB positive isolates were shipped to Dartmouth Medical School and tested for the M. tuberculosis complex (MTBC) using DNA probes (Accuprobe, Gen-Probe, San Diego, CA).
IS6110 typing was performed on study isolates and concurrent community isolates obtained from the NTLP laboratory by Dr. Barry Kreiswirth using standard methods [56]. Isolates with IS6110 patterns that were identical or differed by 1 band were examined for date of processing to determine possible cross contamination [57]. The date processed (or date collected if former was unavailable) was reviewed for matching patterns to identify clusters that may have represented false positive results due to either laboratory cross contamination or mislabeling. Date parameters used to define clusters were ±4 days from date processed and ±10 days from date of specimen collection.
CD4 counts were performed by flow-cytometry (FACS-Count, Becton Dickinson, Franklin Lakes, New Jersey). Viral loads were performed using Versant HIV-1 RNA 3.0–—bDNA (Bayer Diagnostics, Emeryville, CA) or Versant 340 bDNA (Siemens Diagnostics, Norwood, MA).
Table A.
Systemic reactions 7 days after immunization.
| Reaction | MV n = 367 | Placebo n = 380 | P value |
|---|---|---|---|
|
| |||
| Measured temperature ≥38°C | 2/367 (1%) | 5/380 (1%) | 0.45 |
| After Dose A | 0 | 3/82 (4%) | |
| Dose B | 2/81 (2%) | 1/79 (1%) | |
| Dose C | 0 | 1/76 (1%) | |
| Dose D | 0 | 0 | |
| Dose E | 0 | 0 | |
| Self-reported fever | 40/367 (11%) | 31/380 (8%) | 0.21 |
| After Dose A | 9/81 (11%) | 9/82 (11%) | |
| Dose B | 11/77 (14%) | 8/79 (10%) | |
| Dose C | 10/77 (13%) | 7/76 (9%) | |
| Dose D | 5/72 (7 %) | 3/72 (4%) | |
| Dose E | 5/60 (8%) | 4/71 (6%) | |
| Headache | 50/367 (14%) | 37/380 (10%) | 0.11 |
| After Dose A | 14/81 (17%) | 11/82 (13%) | |
| Dose B | 14/77 (18%) | 10/79 (13%) | |
| Dose C | 4/77 (5%) | 8/76 (11%) | |
| Dose D | 12/72 (17%) | 3/72 (4%) | |
| Dose E | 6/60 (10%) | 5/71 (7%) | |
| Malaise | 64/367 (17%) | 53/380 (14%) | 0.19 |
| After Dose A | 19/81 (23%) | 15/82 (18%) | |
| Dose B | 18/77 (23%) | 10/79 (13%) | |
| Dose C | 8/77 (10%) | 9/76 (12%) | |
| Dose D | 11/72 (15%) | 11/72 (15%) | |
| Dose E | 8/60 (13%) | 8/71 (11%) | |
Statistical analysis
The trial was designed to detect with 80% power a 50% decrease in the risk of disseminated tuberculosis at the 0.05 (2-sided) statistical significance level. An annual disseminated tuberculosis rate of 1.60% and an annual death rate of 9.16% were expected in the placebo group. Follow-up loss was expected to be 27% over the entire study duration. Based on these assumptions, a total of 71 events was needed and was expected to be observed among 2274 participants followed over a median of four years.
One interim efficacy analysis for the primary endpoint of disseminated tuberculosis was conducted in November 2005 when 9 cases had been observed. This resulted in an information time of 0.13 with a corresponding critical value using an O-Brien Fleming boundary of 6.206. The DSMB reviewed the log rank tests and compared their P values to the nominal P value at the interim analysis, which did not indicate a significant difference warranting stopping (or considering to stop) the trial.
Footnotes
There are no conflicts of interest reported. Dr Vuola is now with GlaxoSmithKline, Europe.
References
- 1.World Health Organization. Global tuberculosis control: epidemiology, strategy, financing: WHO report 2009.WHO;2009. [Google Scholar]
- 2.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS 2006; 20:1605–1612. [DOI] [PubMed] [Google Scholar]
- 3.Whalen CC, Johnson JL, Okwera A, Hom DL, Huebner R, Mugyeni P, et al. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. N Engl J Med 1997; 337:801–808. [DOI] [PubMed] [Google Scholar]
- 4.Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, King BS, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS 2007;21:1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havlir DV, Getahun H, Sanne I, Nunn P. Opportunities and challenges for HIV care in overlapping HIV and TB epidemics. JAMA 2008; 300:423–430. [DOI] [PubMed] [Google Scholar]
- 6.Ansari NA, Kombe AH, Kenyon TA, Hone NM, Tappero JW, Nyirenda ST, et al. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997–8. Int J Tuberc Lung Dis 2001; 6:55–63. [PubMed] [Google Scholar]
- 7.World Health Organization. 2006 tuberculosis facts. WHO; 2006. [Google Scholar]
- 8.Hamilton CD, Sterling TR, Blumberg HM, Leonard M, McAuley J, Schlossberg D, et al. Extensively drug resistant tuberculosis: are we learning from history or repeating it? Clin Infect Dis 2007; 45:338–342. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006;368:1575–1580. [DOI] [PubMed] [Google Scholar]
- 10.Zignol M, Hosseini S, Wright A, Lambregts-van Weezenbeek C, Nunn P, Watt CJ, et al. Global incidence of multi-drug resistant tuberculosis. J Infect Dis 2006; 194:479–485. [DOI] [PubMed] [Google Scholar]
- 11.Hoft DF. Tuberculosis vaccine development: goals, immunological design and evaluation. Lancet 2008; 372:164–175. [DOI] [PubMed] [Google Scholar]
- 12.Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, Fineberg HV. The efficacy of bacillus Calmette-Guérin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 1995; 96:29–35. [PubMed] [Google Scholar]
- 13.von Reyn CF, Vuola J. New vaccines for the prevention of tuberculosis. Clin Infect Dis 2002; 35:465–474. [DOI] [PubMed] [Google Scholar]
- 14.Karonga Trial Prevention Group. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet 1996; 348:17–24. [PubMed] [Google Scholar]
- 15.Rodrigues LC, Pereira SM, Cunha SS, Genser B, Ichihara MY, de Brito SC, et al. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster randomized trial. Lancet 2005; 366:1290–1295. [DOI] [PubMed] [Google Scholar]
- 16.Brandt L, Cunha JF, Olsen AW, Chilima B, Hirsch P, Appleberg R, Andersen P. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect Immun 2002; 70:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Opie EL, Flahiff EW, Smith HH. Protective inoculation against human tuberculosis with heat-killed tubercle bacilli. Am J Hyg 1939; 29:155–164. [Google Scholar]
- 18.Weiss DW. Vaccination against tuberculosis with non-living vaccines. I. The problem and its historical background. Am Rev Respir Dis 1959; 80:676–688. [DOI] [PubMed] [Google Scholar]
- 19.Freund J, Opie EL. Sensitization and antibody formation with increased resistance to tuberculous infection induced by heat killed tubercle bacilli. J Exp Med 1938; 68:273–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Reyn CF, Arbeit RD, Tosteson ANA, Ristola MA, Barber TW, Waddell R, et al. The international epidemiology of disseminated Mycobacterium avium complex infection in AIDS. AIDS 1996; 10:1025–1032. [DOI] [PubMed] [Google Scholar]
- 21.von Reyn CF, Arbeit RD, Horsburgh CR, Ristola M,Waddell RD, Tvaroha SM, et al. Sources of disseminated Mycobacterium avium infection in AIDS. J Infect 2002; 44:166–170. [DOI] [PubMed] [Google Scholar]
- 22.von Reyn CF, Waddell RD, Eaton T, Arbeit RD, Maslow JN, Barber TW, et al. Isolation of Mycobacterium avium complex from water in the United States, Finland, Zaire, and Kenya. J Clin Micro 1993; 31:3227–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Reyn CF, Barber TW, Arbeit RD, Sox CD, O’Connor GT, Brindle RJ, et al. Evidence of previous infection with Mycobacterium avium-Mycobacterium intracellulare complex among healthy subjects: an international study of dominant mycobacterial skin test reactions. J Infect Dis 1993; 168:1553–1558. [DOI] [PubMed] [Google Scholar]
- 24.Gilks CF, Brindle RJ, Mwachari C, Batchelor B, Bwayo J, Kimari J, et al. Disseminated Mycobacterium avium infection among HIV-infected patients in Kenya. J AIDS 1995; 8:195–198. [PubMed] [Google Scholar]
- 25.Edwards ML, Goodrich JM, Muller D, Pollack A, Ziegler JE, Smith DW. Infection with Mycobacterium avium-intracellulare and the protective effects of Bacille Calmette-Guerin. J Infect Dis 1982; 145:733–741. [DOI] [PubMed] [Google Scholar]
- 26.Edwards L, Acquaviva F, Livesay V. Identification of tuberculous infected: dual tests and density of reaction. Am Rev Respir Dis 1973; 108:1334–1339. [DOI] [PubMed] [Google Scholar]
- 27.Fine PEM. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 1995; 346:1339–1345. [DOI] [PubMed] [Google Scholar]
- 28.Lin MY, Reddy TB, Arend SM, Friggen AH, Franken KL, van Meijgaarden KE, et al. Cross-reactive immunity to Mycobacterium tuberculosis DosR-regulon encoded antigens inindividuals infected with environmental, non-tuberculous mycobacteria. Infect Immun 2009; 77:5071–5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanford JL, Bahr GM, Rook GA, Shaaban MA, Chugh TD, Gabriel M, et al. Immunotherapy with Mycobacterium vaccae as an adjunct to chemotherapy in the treatment of pulmonary tuberculosis. Tubercle 1990; 71:87–93. [DOI] [PubMed] [Google Scholar]
- 30.Durban Immunotherapy Trial Group. Immunotherapy with Mycobacterium vaccae in patients with newly diagnosed pulmonary tuberculosis: a randomized controlled trial. Lancet 1999; 354:116–119. [PubMed] [Google Scholar]
- 31.Johnson JL, Kamya RM, Okwera A, Loughlin AM, Nyole S, Hom DL, et al. Randomized controlled trial of Mycobacterium vaccae immunotherapy in non-human immunodeficiency virus-infected Ugandan adults with newly diagnosed pulmonary tuberculosis. J Infect Dis 2000; 181:1304–1312. [DOI] [PubMed] [Google Scholar]
- 32.von Reyn CF, Arbeit RD, Yeaman G, Waddell RD, Marsh BJ, Morin P, et al. Immunization of healthy adult subjects in the United States with inactivated Mycobacterium vaccae administered in a three-dose series. Clin Infect Dis 1997; 24:843–848. [DOI] [PubMed] [Google Scholar]
- 33.von Reyn CF, Marsh BJ, Waddell R, Lein AD, Tvaroha S, Morin P, Modlin JF. Cellular immune responses to mycobacteria in healthy and human immunodeficiency virus positive subjects in the United States after a five dose schedule of Mycobacterium vaccae vaccine. Clin Infect Dis 1998; 27:1517–1520. [DOI] [PubMed] [Google Scholar]
- 34.Marsh BJ, von Reyn CF, Arbeit RD, Morin P. Immunization of HIV-infected adults with a three-dose series of inactivated Mycobacterium vaccae. Am J Med Sci 1997; 313:377–383. [DOI] [PubMed] [Google Scholar]
- 35.Johnson D, Waddell RD, Pelton SI, Jaeger AS, Modlin JF, Yogev R, et al. Randomized trial of intradermal Mycobacterium vaccae or intradermal hepatitis B immunisation in children with HIV infection. Vaccine 1999; 17:2583–2587. [DOI] [PubMed] [Google Scholar]
- 36.Waddell RD, Chintu C, Lein D, Zumla A, Karagas M, Baboo KS, et al. Safety and immunogenicity of a 5 dose series of inactivated Mycobacterium vaccae vaccination for the prevention of HIV-associated tuberculosis. Clin Infect Dis 2000; 30 (Suppl 3):S309–S315. [DOI] [PubMed] [Google Scholar]
- 37.Vuola J, Ristola M, Cole B, Järviluoma A, Tvaroha SM, Rönkkö T, et al. Immunogenicity of an inactivated mycobacterial vaccine for the prevention of HIV-associated tuberculosis: a randomized, controlled trial. AIDS 2003; 17:2351–2355. [DOI] [PubMed] [Google Scholar]
- 38.Floyd S, Ponnighaus JM, Bliss L, Warndorff DK, Kasunga A, Mogha P, Fine P. BCG scars in northern Malawi: sensitivity and repeatability of scar reading, and factors affecting scar size. Int J Tuberc Lung Dis 2000; 12:1133–1142. [PubMed] [Google Scholar]
- 39.Munseri P, Talbot E, Mtei L, von Reyn CF. Completion of isoniazid preventive therapy among HIV-infected patients in Tanzania. Int J Tuberc Lung Dis 2008; 12:1037–1041. [PubMed] [Google Scholar]
- 40.Niyongabo T, Henzel D, Idi M, Nimubona S, Gikoro E, Melchior JC, et al. Tuberculosis, human immunodeficiency virus infection, and malnutrition in Burundi. Nutrition 1999;15:289–293. [DOI] [PubMed] [Google Scholar]
- 41.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med 2003; 349:1341–1348. [DOI] [PubMed] [Google Scholar]
- 42.Waddell RD, Lishimpi K, von Reyn CF, Chintu C, Baboo KS, Kreiswirth B, et al. Bacteremia due to Mycobacterium tuberculosis or M. bovis, Bacille Calmette-Guerin (BCG) among HIV-positive children and adults in children. AIDS 2001; 15:55–60. [DOI] [PubMed] [Google Scholar]
- 43.Archibald LK, den Dulk MO, Pallangyo KJ, Reller LB. Fatal Mycobacterium tuberculosis bloodstream infections in febrile hospitalized adults in Dar es Salaam, Tanzania. Clin Infect Dis 1998; 26:290–296. [DOI] [PubMed] [Google Scholar]
- 44.von Reyn CF. The significance of bacteremic tuberculosis among persons with HIV infection in developing countries. AIDS 1999; 13:2193–2195. [DOI] [PubMed] [Google Scholar]
- 45.Hawkridge A, Hatherill M, Little F, Goetz MA, Barker L, Mahomed H, et al. Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South African infants: randomised trial [abstract]. BMJ 2008; 337:a2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aronson NE, Santosham M, Comstock GW, Howard RS, Moulton LH, Rhoades ER, Harrison LH. Long-term efficacy of BCG vaccine in American Indians and Alaska natives: a 60-year follow-up study. JAMA 2004; 291:2086–2091. [DOI] [PubMed] [Google Scholar]
- 47.Vandiviere HM, Dworski M, Melvin IG, Watson HA, Begley J. Efficacy of bacillus Calmette-Guerin and isoniazid-resistant bacillus Calmette Guerin with and without isoniazid chemoprophylaxis from the day of vaccination. II: Field trial in man. Am Rev Respir Dis 1973; 108:301–313. [DOI] [PubMed] [Google Scholar]
- 48.Matee M, Lahey T, Vuola JM, Mtei L, Cole BF, Bakari M, et al. Baseline mycobacterial immune responses in HIV-infected adults primed with bacille Calmette-Guerin during childhood and entering a tuberculosis booster vaccine trial. J Infect Dis 2007; 195:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rook GA, Stanford JL. The Koch phenomenon and the immunopathology of tuberculosis. Curr Top Microbiol Immunol 1996; 215:239–262. [DOI] [PubMed] [Google Scholar]
- 50.Demangel C, Garnier T, Rosenkrands I, Cole ST. Differential effects of prior exposure to environmental mycobacteria on vaccination with Mycobacterium bovis BCG or a recombinant BCG strain expressing RD1 antigens. Infect Immun 2005; 73:2190–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tapa S, Cvjetanovic B. Controlled field trial on the effectiveness of one and two doses of acetone-inactivated and dried typhoid vaccine. Bull World Health Organ 1975; 52:75–80. [PMC free article] [PubMed] [Google Scholar]
- 52.Mtei L, Matee M, Herfort O, Bakari M, Horsburgh CR, Waddell RD, et al. High rates of clinical and subclinical tuberculosis among ambulatory HIV-positive subjects in Tanzania. Clin Infect Dis 2005; 40:1500–1507. [DOI] [PubMed] [Google Scholar]
- 53.Bakari M, Arbeit RD, Mtei L, Lyimo J, Waddell R, Matee M, et al. The role of chest x-ray and sputum culture in the decision to treat for active tuberculosis in HIV infection: the role of chest x-ray and sputum culture [abstract]. BMC Infect Dis 2008; 8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Munseri P, Talbot E, Mtei L, von Reyn CF. Completion of isoniazid preventive therapy among HIV-infected patients in Tanzania. Int J Tuberc Lung Dis 2008; 12:1037–1041. [PubMed] [Google Scholar]
- 55.Crump JA, Tanner DC, Mirrett S, McKnight CM, Reller LB. Controlled comparison of BACTEC 13 A, MYCO/F LYTIC, BacT/ALERT MB, and ISOLATOR 10 systems for detection of mycobacteremia. J Clin Micro 2003; 41:1987–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bifani PJ, Mathema B, Liu Z, Moghazeh SL, Shopsin B, Tempalski B, et al. Identification of a W variant outbreak of Mycobacterium tuberculosis via population based molecular epidemiology. JAMA 1999; 24:2321–2327. [DOI] [PubMed] [Google Scholar]
- 57.Braden CR, Templeton GL, Stead WW, Bates JH, Cave MD, Valway SE. Retrospective detection of laboratory cross-contamination of Mycobacterium tuberculosis cultures with use of DNA fingerprint analysis. Clin Infect Dis 1997; 24:35–40. [DOI] [PubMed] [Google Scholar]


