CLTC encodes clathrin heavy chain 1 (CHC1), a major subunit of the clathrin triskelion polymer, combining three heavy and three light chains, 1 which is implied in vesicles trafficking and synaptic neurotransmitters homeostasis through the formation of clathrin‐coated vesicles. 2 CLTC is highly expressed in the developing brain and de novo mutations in this gene underlie a wide spectrum of infantile and childhood neurodevelopmental disorders, including facial dysmorphism, brain malformations, musculoskeletal defects, intellectual disability, and epileptic encephalopathy (MIM 617854). 3 , 4 , 5 , 6 , 7 , 8
Although CLTC deficiency may affect the neurotransmitters vesicle recycle, 6 the precise underlying pathogenic mechanisms remain largely unknown. Here, we report on the positive effect of monoamine oxidase B (MAO‐B) inhibitor selegiline on the movement disorder (MD) of a CLTC‐positive subject.
This 5‐year‐old girl was born at 35 weeks of gestational age for preterm premature rupture of membranes, via C‐section because of a past maternal pelvic fracture. She suffered from severe gastroesophageal reflux and difficulties in chewing and swallowing. Generalized rigidity was noted as early as 2 months of life progressing toward dystonic back arching and choreoathetotic movements during the following months. A severe sight and gaze impairment was early noted. Visual evoked potential at 4 months detected low cortical response, whereas the electroretinogram and brainstem auditory‐evoked potentials were normal. Brain magnetic resonance imaging at 4 and 16 months showed pontocerebellar hypoplasia (Fig. 1).
FIG. 1.
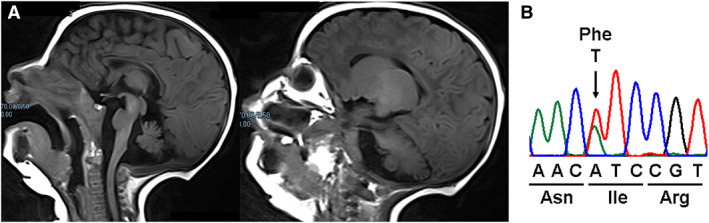
(A) Sagittal magnetic resonance imaging brain scan of the child shows a severe pontocerebellar hypoplasia. (B) Electropherogram shows the transition (c.2023A > T/p.Ile675Phe) in the CLTC gene identified in the patient of present study.
At 4.2 years of age, the girl showed microcephaly (Z‐score, −2.89) and severe developmental delay with no language skills, severe irritability, and poor social participation. Facial mimicry was scarce. Bradykinesia and impaired postural control of the trunk were associated with limb postural and action dystonia (left side more than right). She required constant support to stand up and walked with a gait ataxia. The sitting position was maintained with a kyphotic posture and frequent oscillation of the trunk. Postural reactions were ineffective with some response elicitable only by latero‐lateral imbalance. Besides lower limbs’ brisk reflexes, no other pyramidal signs were detected. She showed erratic eye movements, hyperfixation, and disturbances of gaze pursuit on both horizontal and vertical plans with constant co‐adjuvant nodding head movement as well as trunk rotation (Video 1).
Video 1.
Segment 1, Pre‐treatment. The girl shows bradykinesia, postural instability, lack of postural reactions, and dystonic postures in the attempt to stand. With walking, possible with truncal support, dystonic gait, and upper limbs dystonic posturing becomes evident. Segment 2, Post‐treatment. After 2 months of treatment (selegiline titrated up to 5 mg/day) the child could stand autonomously, and bradykinesia and postural control improved allowing functional voluntary movements of the upper limbs. Walking was possible with frontal hand support with improved speed and reduced lower limb dystonia. Hypomimia is still evident (no blinking can be seen) and gaze pursuit is now possible with less evident nodding head movements. Saccadic latency is still present, but with a shorter delay. Post‐treatment follow‐up: on follow‐up, there is a reduction of limb dysmetria and less trunk oscillation while in a standing position.
Cerebrospinal fluid examination detected normal levels of neurotransmitters. Trio‐based whole‐exome sequencing analysis disclosed a new de novo pathogenetic variant (c.2023A > T/p.Ile675Phe) in CLTC (Fig. 1). Selegiline, a MAO‐B inhibitor, was titrated up to 5 mg/day at 4.2 years of age. After 2 months the girl showed a clear clinical improvement with a longer attention span, markedly reduced bradykinesia, more adequate social participation, and nonverbal communication. Saccadic latency and visual pursuit improved with the reduction of erratic eye movements. Head titubation and trunk oscillations diminished also during voluntary movements that were less affected by action dystonia and bradykinesia. The sitting position was more stable, and the girl was able to reach the standing position autonomously with improved velocity (Video 1).
After a 10‐month treatment, she further improved her postural control. Voluntary movements were even less bradykinetic. Her attention span was longer, showing a great variety of communication initiatives (Video 1).
Finally, suspension of the therapy after a 4‐month treatment was followed by marked neurological regression, which was restored by the reintroduction of selegiline.
The clinical spectrum of CLTC deficiency is heterogeneous, ranging from patients presenting with severe neurodevelopmental disorders, which are variably associated with microcephaly, thin corpus callosum, epilepsy, and MD, to cases where the neurodevelopmental disorder may be milder and isolated. MD spectrum is heterogeneous in CLTC defect and is summarized in Table S1., 3 , 4 , 5 , 6 , 7
Severe cases are associated with in‐frame or missense variants that induce a dominant‐negative effect, whereas nonsense and frameshift variants, resulting in haploinsufficiency, would be associated with a milder phenotype. 6
CLTC deficiency should be suspected in individuals with neurodevelopmental disorders, possibly associated with facial dysmorphisms, early‐onset hyperkinetic MD or parkinsonism, spastic‐ataxic cerebral palsy‐like phenotype, and brain abnormalities (such as thin corpus callosum or cerebellar atrophy/hypoplasia).
Based on our previous observation on the positive effect of selegiline in a CLTC adult patient with levodopa unresponsive parkinsonism, 5 we tested the medicament in the affected child harboring the p.Ile675Phe substitution associated with neurodevelopmental dystonia. The trial resulted in clinically relevant improvement of cognitive and movement disorders.
Irreversible MAO‐B inhibitor selegiline is a firstline treatment for Parkinson's disease. MAO‐B catabolizes different biogenic amines such as norepinephrine, serotonin, and dopamine (DA), and is highly expressed in the central nervous system, especially at the level of basal ganglia. 9 , 10 MAO‐B inhibitors prevent the breakdown of DA and other neurotransmitters, resulting in increased levels of these biologically active biogenic amines at the synaptic cleft. In the presence of an altered machinery of DA recycling and release because of clathrin dysfunction, the secondary depletion of the neurotransmitters cannot be corrected by DA precursor supplementation. Conversely, the inhibition of DA catabolism may be an effective and well‐tolerated therapeutic option for MD treatment if they are part of the disease presentation.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
F.N.: 1A, 1B, 1C, 3A, 3B
R.B.: 1B, 1C, 3A, 3B
M.T.G.: 1C, 3B
F.M.: 1C, 3B
A.D.G.: 1C, 3B
P.P.: 1C, 3B
S.M.: 1C, 3B
V.L.: 1A, 1B, 3B
Disclosures
Ethical Compliance Statement: Written informed consent for offline and online distribution of the video material was obtained from parents and is available on request. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: No specific funding was received for this work. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for Previous 12 Months: The authors declare that there are no additional disclosures to report.
Supporting information
Table S1. Movement disorders in subjects harboring a CLTC pathogenic variant.
Acknowledgments
We thank the patient and the patient's parents for their trust and openness.
Relevant disclosures and conflict of interest are listed at the end of this article.
References
- 1. Robinson MS. Forty years of Clathrin‐coated vesicles. Traffic 2015;16(12):1210–1238. 10.1111/tra.12335 Epub 2015 Nov 6. PMID: 26403691. [DOI] [PubMed] [Google Scholar]
- 2. Kaksonen M, Roux A. Mechanisms of Clathrin‐mediated endocytosis. Nat Rev Mol Cell Biol 2018;19:313–326. [DOI] [PubMed] [Google Scholar]
- 3. DeMari J, Mroske C, Tang S, Nimeh J, Miller R, Lebel RR. CLTC as a clinically novel gene associated with multiple malformations and developmental delay. Am J Med Genet A 2016;170A:958–966. [DOI] [PubMed] [Google Scholar]
- 4. Hamdan FF, Myers CT, Cossette P, et al. High rate of recurrent De novo mutations in developmental and epileptic encephalopathies. Am J Hum Genet 2017;101:664–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manti F, Nardecchia F, Barresi S, et al. Neurotransmitter trafficking defect in a patient with Clathrin (CLTC) variation presenting with intellectual disability and early‐onset parkinsonism. Parkinsonism Relat Disord 2019;61:207–210. [DOI] [PubMed] [Google Scholar]
- 6. Nabais Sa MJ, Venselaar H, Wiel L, Trimouille A, Lasseaux E, Naudion S, et al. De novo CLTC variants are associated with a variable phenotype from mild to severe intellectual disability, microcephaly, hypoplasia of the corpus callosum, and epilepsy. Genet Med 2020;22:797–802. [DOI] [PubMed] [Google Scholar]
- 7. Itai T, Miyatake S, Tsuchida N, et al. Novel CLTC variants cause new brain and kidney phenotypes. J Hum Genet 2022;67(1):1–7. 10.1038/s10038-021-00957-3. [DOI] [PubMed] [Google Scholar]
- 8. Martín Fernández‐Mayoralas D, Muñoz Jareño N, Alba Menéndez A, Fernández‐Jaén A. Periventricular heterotopias: broadening of the clinical spectrum of the Clathrin 1 gene (CLTC) pathogenic variants. Heterotopías periventriculares: ampliación del espectro clínico de las variantes patogénicas del gen de la clatrina 1 (CLTC). Neurologia (Engl Ed) 2021;36(4):327–329. [DOI] [PubMed] [Google Scholar]
- 9. Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 2006;7(4):295–309. 10.1038/nrn1883 PMID: 16552415. [DOI] [PubMed] [Google Scholar]
- 10. Magyar K. The pharmacology of selegiline. Int Rev Neurobiol 2011;100:65–84. 10.1016/B978-0-12-386467-3.00004-2 PMID: 21971003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Movement disorders in subjects harboring a CLTC pathogenic variant.


