Abstract
Background
Functional movement disorders (FMD) are associated with a high prevalence of psychiatric comorbidities.
Objective
To assess the frequency of obsessive‐compulsive symptoms (OCS) in FMD.
Methods
A total of 167 consecutive patients with clinically definite FMD (mean age = 44.4 years, standard deviation [SD] = 12.0, 119 females) and 145 healthy controls (mean age = 43.2 years, SD = 11.8, 103 females) completed the Obsessive‐Compulsive Inventory‐Revised (OCI‐R), which is a widely used tool for assessing OCS. The cutoff score ≥21 is indicative of clinically significant obsessive‐compulsive disorder (OCD). Motor symptom severity was assessed using the Simplified FMD Rating Scale (S‐FMDRS). All subjects completed questionnaires for depression, anxiety, pain, fatigue, cognitive complaints, health‐related quality of life, and childhood trauma. Personality traits were assessed using the Big Five questionnaire.
Results
FMD patients had higher mean OCI‐R score and higher proportion of individuals with OCI‐R ≥ 21 42%, 95% confidence interval (CI) = (30.2, 54.6) versus 16%, 95% CI = (8.2, 28.2) in controls, P < 0.001. Patients had higher scores in three domains: checking, ordering, and obsessing (P < 0.001). FMD patients with OCI‐R score ≥21 had higher depression, anxiety, cognitive complaints, and lower quality of life compared to those with score <21 (P < 0.001). No correlation between OCI‐R and S‐FMDRS scores was found.
Conclusions
FMD patients reported higher rates of OCS compared to controls, along with higher rates of non‐motor symptoms and lower quality of life. This finding may have clinical implications and raises the possibility of shared risk factors and common pathophysiological mechanisms in FMD and OCD.
Keywords: functional movement disorder, motor functional neurological disorder, conversion disorder, obsessive‐compulsive disorder
Functional movement disorders (FMD) are highly prevalent disorders, which are associated with significant disability and high health care costs. 1 , 2 FMD are clinically characterized by variability and inconsistency of symptoms that are incongruent with movement disorders known to be caused by an organic neurological disease. 3 The clinical presentation is heterogeneous. Patients often present with multiple motor as well as non‐motor symptoms such as anxiety, depression, cognitive symptoms, fatigue, pain, and sensory symptoms. 1 , 4
FMD is characterized by neurophysiological and behavioral abnormalities such as impaired inhibitory function at the cortical and subcortical level 5 , 6 and impaired inhibition control. 7 , 8 These abnormalities are not specific to FMD as they have also been found in other neuropsychiatric disorders (eg, dystonia, schizophrenia, Tourette syndrome, and obsessive‐compulsive disorder [OCD]). 9 Dysfunction of inhibitory control of thoughts and motor actions is a central feature of OCD, which has been found in higher frequencies in numerous conditions including anxiety disorders, mood disorders, impulse‐control disorders, substance use disorders, and Tourette syndrome. 10 Despite the overlap between the OCD spectrum and other neuropsychiatric disorders and shared neurophysiological abnormalities, no attempt has been made to characterize obsessive‐compulsive symptoms (OCS) in FMD.
OCD is a complex disorder characterized by repetitive and intrusive thoughts, urges, images, or fears (obsessions) and repetitive behaviors or mental acts (compulsions). 10 Regardless of cultural background, common symptoms include fears of contamination and excessive hand washing, hoarding (failing to discard items), preoccupation with symmetry and ordering, intrusive and distressing unacceptable or taboo thoughts (aggressive, sexual, religious obsessions) with mental rituals or praying and concerns about harm with repetitive checking. 11
We hypothesized there was a higher occurrence of OCS in FMD. We also hypothesized that higher OCS scores were associated with a higher frequency of non‐motor symptoms in FMD, specifically anxiety, depression, cognitive complaints, and lower health‐related quality of life (HRQoL).
Because OCD is a heterogeneous disorder, we decided to leverage a dimensional approach to symptomatology. 12 Rather than determining the prevalence of fully expressed OCD in FMD, we aimed to establish differences in OCS dimensions between FMD patients and healthy controls (HC) that would provide more fine‐grained characterization of OCS in FMD. In a case–control study design, we used a validated tool for assessment of self‐reported OCS in prototypical domains of OCD symptoms: washing, checking, ordering, neutralizing, obsessing, and hoarding. We analyzed the association of higher OCS scores indicative of OCD with clinical characteristics of FMD and with risk factors common to both OCD and FMD. We also assessed the impact of OCS on HRQoL.
Materials and Methods
We recruited 200 consecutive patients with FMD (mean age = 44.9 years, SD = 12.1, 145 females, mean disease duration = 6.41 years, SD = 6.44) and 145 sex‐ and age‐matched healthy controls (mean age = 43.2 years, SD = 11.8, 103 females) between January 2019 and November 2021. The study was approved by the local ethics committee (approval no. 37/19) and all participants gave their written consent to take part in the study.
Patients were diagnosed with clinically definite FMD according to Gupta and Lang 3 criteria at the specialized outpatient service for FMD at the Neurology Department. The diagnosis was based on detailed clinical interviews and on an examination by an experienced movement disorders specialist (T.S.) based on positive signs of functional weakness or abnormal movements inconsistent and incongruent with known movement disorders.
Control subjects were recruited from a database of healthy subjects willing to participate in clinical studies. All controls received financial compensation. In all subjects, a complete medical history was obtained, and a full neurological examination was performed. None of the controls had any sensorimotor symptoms and/or objective signs of a neurological disorder. Exclusion criteria for both groups of subjects included age <18 years, inability to complete questionnaires because of language difficulties, severe learning disabilities or cognitive impairment, the presence of a significant illness that could be associated with non‐motor symptoms, substance dependence or psychosis, and history of an organic neurological disorder of the brain.
Motor symptoms found in FMD patients were classified as functional weakness, tremor, dystonia, myoclonus, gait disorder, or speech disorder.
Motor disorder severity was assessed using The Simplified FMD Rating Scale (S‐FMDRS). 13 Abnormal movements were recorded in each of the seven body regions and rated according to severity and duration of symptoms (maximum score, 54). 13
The patients’ use of antidepressants was recorded.
Obsessive‐Compulsive Inventory‐Revised (OCI‐R) is an 18 item self‐report questionnaire evaluating OCS severity in the past month, which assesses six domains of OCD symptoms: washing, checking, ordering, neutralizing, obsessing, and hoarding. Items include description of symptoms rated on a scale from 0 to 4 based on the degree of associated distress. The total score ranges from 0 to 72. 14 A cutoff score of 21 points or more indicates the presence of OCD with 65.6% sensitivity and 63.9% specificity. 14 , 15 Subjects with high (≥21) and low (<21) OCI‐R scores were grouped separately for analysis of differences in clinical characteristics and risk factors.
The Beck Depression Inventory (BDI‐II) was used to measure depressive symptoms. A total of 21 items survey is scored on a scale 0 to 3. Total score is 0 to 63. 16
To measure levels of anxiety, we used the State–Trait Anxiety Inventory (STAI) questionnaire as a measure of state (20 item STAI X‐1) and trait anxiety (20 items STAI X‐2) with the range of 20 to 80 for each part. 17
The Fatigue Severity Scale (FSS) was used to assess fatigue. A nine‐item scale with range 1 to 7 focuses on functional impact and severity of physical and mental fatigue. The total score range is 1 to 7. 18
Visual analogue scales from the PainDetect tool were used to describe pain, assessing current pain intensity, average pain and maximum pain over the last 4 weeks with the range of 0 to 10 for each subscale (VAS, 0 = no pain, 10 = maximum pain). The average of these values provided a composite pain score (range of 0–10) for each subject, which was used for analyses. 19
Cognitive difficulties in the last 6 months were assessed using the Czech validated version of the Cognitive Complaints Questionnaire (Le questionnaire de plainte cognitive [QPC]) of the original French questionnaire. 20 , 21 Ten‐items identify difficulties in general memory abilities, spatial orientation, language, instrumental activities, and personality change. The total score range is 0 to 10. 20
HRQoL was assessed using the 12‐Item Short Form Health Survey (SF‐12) reflecting physical functioning, role physical and emotional limitations (both physical and emotional), social functioning, pain, mental health, vitality, and general health. The total score range is 12 to 44. 22
History of childhood trauma was assessed using the Childhood Trauma Questionnaire (CTQ). CTQ is a 28‐ item questionnaire quantifying self‐reported physical, sexual, and emotional abuse as well as physical and emotional neglect using a five‐point Likert scales to rate each item. The total score range for each subscale is 5 to 125. 23
Personality traits were quantified using the short version of the Big Five Inventory (BFI‐44), a validated 44‐item personality inventory encompassing five factors of personality including neuroticism, extraversion, openness, agreeableness, and conscientiousness with the range 0 to 4 for each subscale. 24
Statistical Analysis
The statistical analysis was performed using the R: A language and environment for statistical computing. 25 Continuous variables such as age, clinical scales or self‐report questionnaires were compared by Student's t test, whereas proportions such as the proportion of subjects with OCI‐R ≥21 were compared by the two‐proportion z‐test. For correlation analysis between OCI‐R scores and other clinical variables, the significance of the Pearson correlation coefficient was assessed using the Student's t‐distribution.
To examine the relationship between the OCI‐R score and the clinical and self‐reported variables, a multiple regression analysis was separately preformed in FMD patients and healthy controls. (For details see Table S3).
The resulting P‐values were adjusted using the Bonferroni method to maintain the family‐wise error rate 0.05; the analysis included 42 comparisons, which means that the adjusted significance level is ~0.00012.
Results
All 200 consecutive patients with motor FMD who met the inclusion criteria underwent a full clinical assessment and agreed to complete the questionnaires. However, in 33 patients (26 females, mean age = 47.5 years, SD = 12.9, mean disease duration = 6 years, SD = 6.1) data from questionnaires were missing (14 patients did not return the questionnaires and 19 did not complete the questionnaires assessing OCS and non‐motor symptoms). All subjects with missing data were excluded from the analysis. Data on risk factors were collected separately and were available for consecutive 155 patients and 144 controls (CTQ and BFI). The CTQ was available for 159 patients and all 145 controls.
Demographic and clinical characteristics of both groups are presented in Table 1.
TABLE 1.
Demographic and clinical characteristics
| FMD patients (n = 167) | Controls (n = 145) | P value | |
|---|---|---|---|
| Subjects N | 167 | 145 | |
| Age (years) (SD) | 44.6 (12) | 43.2 (11.8) | 0.400 |
| Females | 119 | 103 | 1 |
| FMD duration (years) (SD) | 6.5 (6.5) | – | |
| FMDRS | 12.2 (7.5) | – | |
| Motor phenotype (present as dominant/present) | n (%)/n (%) | ||
| Weakness | 40 (24.0)/78 (46.7) | – | |
| Gait disorder | 55 (32.9)/65 (38.2) | – | |
| Tremor | 36 (21.5)/53 (31.7) | – | |
| Dystonia | 24 (14.3)/27 (16.2) | – | |
| Myoclonus | 11 (6.6)/10 (6) | – | |
| Speech disturbance | 1 (0.6)/21 (12.6) | – |
Note: Mean values (SD) are presented; P‐values are nominal values uncorrected.
Abbreviations: FMD, functional movement disorder; SD, standard deviation; FMDRS, The Simplified FMD Rating Scale.
The proportion of individuals with OCI‐R ≥21 was significantly higher in the patient group compared to healthy controls (42%, 95% CI = [30.2, 54.6] vs. 16%, 95% CI = [8.2, 28.2], P < 0.001). The mean OCI‐R score was 19.33 (SD = 12.4) in the patient group and 12.2 (SD = 9.7) (P < 0.001) in the control group. (Fig. 1). Patients had significantly higher mean scores in three domains: checking, ordering, and obsessing (P < 0.001) (Fig. 2). Patients also had higher scores of depression, anxiety, cognitive complaints, pain, and fatigue and subjectively reported lower quality of life. In personality trait assessment, patients had higher scores in extraversion and openness and neuroticism. See Table 2 for details.
FIG. 1.

Comparison of the total Obsessive‐Compulsive Inventory‐Revised scores in the patient and in the control group. The results are displayed as the mean ± standard deviation. ***P < 0.001.
FIG. 2.
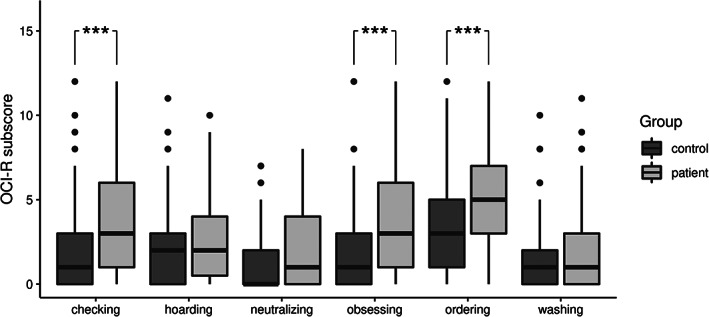
Comparison of the Obsessive‐Compulsive Inventory‐Revised domain subscores in the patient and in the control group. The results are displayed as the mean ± standard deviation. ***P < 0.001.
TABLE 2.
Between‐group comparison of self‐reported measures in FMD patients and control subjects
| Controls (n = 145) | Patients (n = 167) | P value | |
|---|---|---|---|
| OCI‐R | 12.2 (9.7) | 19.3 (1.4) | <0.0001*** |
| BDI‐II | 6.2 (6.6) | 19.6 (13) | <0.0001*** |
| STAI X‐1 | 36.9 (9) | 47.7 (13.5) | <0.0001*** |
| STAI X‐2 | 38 (10) | 48.9 (11.9) | <0.0001*** |
| FSS | 3.5 (1.2) | 5.5 (1.3) | <0.0001*** |
| Pain | 2.1 (2) | 6 (2.3) | <0.0001*** |
| QPC | 1.4 (1.8) | 4.6 (3) | <0.0001*** |
| SF‐12 | 36.6 (4.3) | 23.7 (6.1) | <0.0001*** |
| CTQ | 7.3 (2.3) | 8.5 (3.3) | 0.00175 |
| BFI | |||
| Extraversion | 2.3 (0.7) | 1.85 (1) | <0.0001*** |
| Agreeableness | 2.7 (0,5) | 2.7 (0.6) | 0.59702 |
| Conscientiousness | 2.5 (0.7) | 2.5 (0.6) | 0.87159 |
| Neuroticism | 1.66 (0.8) | 2.3 (0.9) | <0.0001*** |
| Openness | 2.63 (0.7) | 2.2 (0.7) | <0.0001*** |
Note: Mean values (SD) are presented; P‐values are nominal values uncorrected. *P values significant after correction for multiple testing (***P < 0.001).
Abbreviations: FMD, functional movement disorder; OCI‐R, Obsessive‐Compulsive Inventory Revised; BDI‐II, The Beck Depression Inventory II; STAI X‐1/STAI X‐2, The State/Trait Anxiety Inventory; FSS, The Fatigue Severity Scale; Pain scores; QPC, Cognitive Complaints Questionnaire; SF‐12, 12‐Item Short Form Health Survey; CTQ, Childhood Trauma Questionnaire; BFI, Big Five Inventory; SD, standard deviation.
FMD patients with OCI‐R score ≥21 had higher depression, anxiety, cognitive complains, and lower quality of life compared to FMD patients with score <21. See Table 3 for details. FMD patients with OCI‐R scores ≥21 presented with higher scores in all measures of non‐motor symptoms severity and lower HRQoL scores compared to controls with OCI‐R scores ≥21. See Table S4 for details. In the subgroup of patients with OCI‐R scores ≥21, a significantly higher proportion of subjects were taking antidepressants than in the subgroup with scores <21 (58.6% vs. 36%, P < 0.01). No difference in OCI‐R scores was found across different motor phenotypes (represented as dominant or additional) (See Table S1).
TABLE 3.
Within‐group comparison of self‐reported measures between FMD patients with OCI‐R scores ≥21 and <21
| OCI‐R ≥21 (n = 70) | OCI‐R <21 (n = 127) | P value | |
|---|---|---|---|
| Female | 49 | 70 | 0.89520 |
| Age | 46.5 (11.6) | 43.5 (14.1) | 0.08000 |
| OCI‐R | 31.6 (7.8) | 10.4 (5.9) | |
| BDI‐II | 27.2 (13) | 14.1 (9.8) | <0.0001*** |
| STAI X‐1 | 54.8 (12) | 42.5 (12) | <0.0001*** |
| STAI X‐2 | 55.5 (9.9) | 44.2 (11.9) | <0.0001*** |
| FSS | 5.9 (1.2) | 5.2 (2.5) | 0.00104 |
| Pain | 6.7 (1.9) | 5.5 (2.5) | 0.00140 |
| QPC | 5.7 (3.9) | 3.9 (2.9) | <0.0001*** |
| SF‐12 | 21.2 (25.5) | 25.5 (6.2) | <0.0001*** |
| CTQ | 9.2 (3.4) | 8 (3.1) | 0.00864 |
| BFI | |||
| Extraversion | 1.72 (1.2) | 1.9 (0.7) | 0.39205 |
| Agreeableness | 2.6 (0.5) | 2.8 (0.6) | 0.02692 |
| Conscientiousness | 2.4 (0.6) | 2.6 (0.7) | 0.95833 |
| Neuroticism | 2.6 (0.8) | 2 (0.9) | 0.01682 |
| Openness | 2.5 (0.8) | 2.2 (0.7) | 0.31208 |
Note: Mean values (SD) are presented; P‐values are nominal values uncorrected. *P values significant after correction for multiple testing (***P < 0.001).
Abbreviations: FMD, functional movement disorder; OCI‐R, Obsessive‐Compulsive Inventory Revised; BDI‐II, Beck Depression Inventory II; STAI X‐1/STAI X‐2, State/Trait Anxiety Inventory; FSS, Fatigue Severity Scale; Pain scores; QPC, Cognitive Complaints Questionnaire; SF‐12, 12‐Item Short Form Health Survey; CTQ, Childhood Trauma Questionnaire; BFI, Big Five Inventory; SD, standard deviation.
Correlation analysis results are presented in Table S2. In HCs, the total OCI‐R score correlated positively with depression, anxiety, fatigue, cognitive complaints, and negatively with HRQoL scores and agreeableness. In FMD patients, the total OCI‐R score correlated positively with all self‐reported non‐motor measures, total score of childhood trauma and neuroticism. Negative correlation was found between total OCI‐R score agreeableness and HRQoL scores. No significant correlation was found in age, mean duration of FMD, and severity of symptoms assessed with S‐FMDRS.
In healthy subjects, multiple regression analysis identified cognitive complaints (QPC) as the most important predictor of OCI‐R score (b = 1.168, P < 0.0001). In FMD patients, the BDI‐II score (b = 0.624, P < 0.0001), the STAI X‐1 score (b = 0.558, P < 0.0001), and the BFI openness score (b = 4.227, P = 0.00055) were the most important OCI‐R score predictors. The complete results of regression analysis are presented in Table S3.
Discussion
In this study, using a widely used screening tool for OCD, we found an increased frequency of self‐reported OCS in a consecutive sample of patients with FMD compared to healthy controls. Patients reported significantly higher prevalence/frequency of checking, ordering, and obsessing. In both groups, OCS scores above the cutoff value for OCD were associated with higher self‐reported depression, anxiety, cognitive complaints, and in patients only, with poorer quality of life suggesting a clinically relevant problem. No relation was found between OCS frequency and age, mean duration of FMD, and severity of symptoms as assessed using S‐FMDRS.
Our results suggest an increased prevalence of OCD in patients with FMD. In control subjects, the frequency of OCI‐R scores above the cutoff for OCD was higher than the lifetime prevalence of OCD in general population estimated at 1% to 3%. 10 However, the mean scores of OCI‐R were similar to those previously reported in healthy controls. 26 , 27 A relatively low sensitivity and specificity of the OCI‐R to detect OCD highlights the use of this questionnaire as a mere screening tool. 14 Diagnosis of OCD should be based on clinical evaluation using a structured interview by a trained psychiatrist. 28
Only few studies reported presence of comorbid OCD based on structured clinical interview in their FMD cohorts. In a relatively large sample of patients with functional weakness, the occurrence of clinically diagnosed OCD did not significantly differ from control group. 29 In one study, 4 of 27 patients with functional tremor and none of the essential tremor patients had OCD. 30 One recent study found that obsessive‐compulsive personality disorder is the most common type of personality disorder in FMD, which also is often comorbid with OCD. 31 , 32 , 33
In our study, patients reported an increased frequency of obsessing and two types of compulsive behaviors, ordering associated with symmetry concerns and checking, which is associated with concerns about harming oneself or others. No differences were found in mental neutralizing (mental rituals or praying associated with harmful or intrusive thoughts), washing, and also hoarding, which are considered as separate diagnostic entity in the Diagnostic and Statistical Manual of Mental Disorders (DSM)‐5. 28
In accordance with previous studies, we found a significant association between OCS severity and severity of depression, anxiety, fatigue, cognitive complaints, pain, and an impaired HRQoL in both groups of subjects. 11 In FMD patients, agreeableness could be a protective factor, whereas neuroticism could be a predisposing factor in this clinical population. 34
In line with some previous evidence, 35 cognitive complaints were the only predictor of OCS severity in healthy controls. In FMD patients, OCS severity was predicted by depression and anxiety and by higher openness scores. The association between OCD and a high openness to experience has previously described in community subjects. 36
The co‐occurrence of OCS and FMD suggests the two conditions might share common risk factors, predisposing vulnerabilities, and mechanisms. Childhood trauma seems to represent a vulnerability factor in both OCD and functional neurological disorders, although studies of early life adversities have provided less consistent results for FMD than for dissociative seizures. 37 , 38 We found no significant differences in the total score of childhood trauma between FMD patients and controls, nor within‐group differences (high vs. low OCI‐R scores). Nevertheless, patients with higher scores of childhood trauma, but not the controls, reported higher OCS frequency suggesting possible interaction between these environmental factors and other FMD related/specific factors, which have yet to be clarified. Indeed, previous neuroimaging studies in both FMD and OCD showed structural and resting‐state magnetic resonance abnormalities associated with childhood trauma. 39 , 40 , 41
Future studies are needed to clarify possible interactions between genetic and environmental factors that may predispose individuals to OCD, FMD, and other movement disorders. Importantly, increased frequency of OCS/OCD is not specific to FMD. It has also been consistently reported in various other movement disorders such as choreas and Tourette syndrome, suggesting that these disorders share common neural basis possibly with the involvement of frontal‐striatal circuitry. 42 , 43 Interestingly, the evidence has varied across dystonia subgroups (eg, focal, generalized, and different genetically defined dystonias), 42 whereas an increased frequency of OCS/OCD was not found in Parkinson's disease nor movement disorders of peripheral origin such as hemifacial spasm. 43 , 44 An assessment of the potential link between OCS and neurological symptoms comparing different clinical groups with movement disorders would be needed.
OCD and FMD also share common neurophysiological and behavioral abnormalities such as impaired cortical inhibition and silent period, 5 , 45 , 46 subcortical inhibition, 6 , 47 and poor performance in the Go‐No go task or anti‐saccade task. 7 , 8 , 48 , 49 However, these abnormalities in inhibitory mechanism have also been reported in other movement disorders (eg, dystonia, Tourette syndrome). 9
A recent large analysis of the genome‐wide association data from consortia of 25 brain disorders (including 2936 OCD patients, but not conversion disorder/FMD patients) found that psychiatric disorders broadly share a considerable portion of their common variant genetic risk. It provided evidence that current clinical boundaries do not reflect distinct underlying pathogenic processes, at least on the genetic level. 50
Imaging and neurophysiological studies should explore differences and commonalities in pathophysiological mechanisms involved in OCD/OCS and various movement disorders including FMD at the level of neural circuits. Different aspects of OCD such as imbalance between goal‐directed and habitual action, inhibition, impaired cognitive control, cognitive flexibility, and their neural correlates should be systematically investigated in FMD and other movement disorders. 9 , 11
This study has some limitations. While we used a validated screening questionnaire to assess the presence of OCS, to determine the prevalence of OCD in FMD, the diagnosis would need to be established by clinical interview in accordance with current diagnostic criteria. Future studies should further focus on the association of OCD, obsessive‐compulsive personality disorder, and alexithymia in FMD patients. 31
When managing FMD, treatable psychiatric comorbidities should be identified as they have been correlated with negative effect on outcomes in some studies. 1 , 51 In a significant proportion of FMD, OCD may be a common treatable comorbidity, which could be misdiagnosed for anxiety or depression as documented in general population. 52 A higher proportion of patients on antidepressants in the subgroup with suggestive OCD further highlights the need for appropriate treatment, which often requires higher doses of medication than anxiety or mood disorders and/or specific psychotherapy. 10
Conclusions
A large proportion of FMD patients reported higher rates of OCS as indicative of OCD compared to controls. Higher rates of OCS were associated with higher anxiety and depression and lower quality of life. Establishing clinical diagnosis of OCD using a structured interview and providing appropriate treatment of comorbid OCD may, therefore, be necessary for FMD patients. Moreover, the association of FMD and OCS raises the possibility of common pathophysiological mechanisms and genetic risk factors, which should be further addressed.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution. (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique. (3) Manuscript: A. Writing of the First Draft, B. Review and Critique. (4) Funding: A. Obtaining Funding.
L.N.: 1B, 1C, 3A, 3B.
J.A.: 2A, 2B, 2C, 3A.
Z.F.: 1C, 3B.
T.R.: 1C, 3B.
G.V.: 1C, 3B.
P.S.: 3B.
E.R.: 3B, 4A.
T.S.: 1A, 1B, 1C, 2A, 2C, 3A, 3B, 4A.
Disclosures
Ethical Compliance Statement: The study was approved by the local ethics committee (approval no. 37/19) and all participants gave their written consent to take part in the study. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: This work was supported by the Czech Ministry of Health Project AZV NU20‐04‐0332, the project National Institute for Neurological Research (Programme EXCELES, ID Project no. LX22NPO5107)—funded by the European Union‐Next Generation EU; Charles University: Cooperation Program in Neuroscience; and General University Hospital in Prague project MH CZ‐DRO‐VFN64165. The authors declare that there are no conflicts of interest relevant to this work. The authors declare that there are no additional disclosures to report.
Financial Disclosures of for the Previous 12 Months: L.N. is employed by the Department of Neurology and Centre of Clinical Neuroscience, Charles University in Prague, 1st Faculty of Medicine and General University Hospital in Prague, Czech Republic. L.N. received grants from the Ministry of Health of the Czech Republic. J.A. is employed by the Department of Cybernetics, Faculty of Electrical Engineering, Czech Technical University in Prague, Czech Republic. Z.F. is employed by the Department of Neurology and Centre of Clinical Neuroscience, Charles University in Prague, 1st Faculty of Medicine and General University Hospital in Prague, Czech Republic. Z.F. received grants from the Ministry of Health of the Czech Republic. T.R. is employed by the Department of Neurology and Centre of Clinical Neuroscience, Charles University in Prague, 1st Faculty of Medicine and General University Hospital in Prague, Czech Republic. G.V. is employed by First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic; National Institute of Mental Health, Klecany, Czech Republic. G.V. has contracts with Independent Contractor Agreement (independent rater in clinical trial) and ProPhase. G.V. received grants from Ministry of Health of the Czech Republic. P.S. is employed by the Department of Neurology and Centre of Clinical Neuroscience, Charles University in Prague, 1st Faculty of Medicine and General University Hospital in Prague, Czech Republic. P.S. received grants from Ministry of Health of the Czech Republic. E.R. is employed by the Department of Neurology and Centre of Clinical Neuroscience, Charles University in Prague, 1st Faculty of Medicine and General University Hospital in Prague, Czech Republic. E.R. received grants from Ministry of Health of the Czech Republic. T.S. is employed by the Department of Neurology and Centre of Clinical Neuroscience, Charles University in Prague, 1st Faculty of Medicine and General University Hospital in Prague, Czech Republic. T.S. received grants from Ministry of Health of the Czech Republic.
Supporting information
Table S1. Comparison of proportion of subjects with each phenotype in group of FMD patients with OCI‐R <21 and ≥21.
Table S2. Correlations between total score of OCI‐R and clinical characteristics.
Table S3. Multiple regression model estimating OCI‐R in healthy controls and FMD patients.
Table S4. Between‐group comparison of self‐reported measures in FMD patients and control subjects with severe obsessive‐compulsive symptoms defined as OCI‐R ≥21.
Table S5. Within‐group comparison of self‐reported measures between control subjects with OCI‐R scores ≥21 and <21.
Relevant disclosures and conflict of interest are listed at the end of this article.
Data Availability Statement
Datasets analyzed during the current study are available on reasonable request. All data will be anonymized.
References
- 1. Gelauff J, Stone J, Edwards M, Carson A. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry 2014;85(2):220–226. [DOI] [PubMed] [Google Scholar]
- 2. Stephen CD, Fung V, Lungu CI, Espay AJ. Assessment of emergency department and inpatient use and costs in adult and pediatric functional neurological disorders. JAMA Neurol 2021;78(1):88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta A, Lang AE. Psychogenic movement disorders. Curr Opin Neurol 2009;22(4):430–436. [DOI] [PubMed] [Google Scholar]
- 4. Vechetova G, Slovak M, Kemlink D, et al. The impact of non‐motor symptoms on the health‐related quality of life in patients with functional movement disorders. J Psychosom Res 2018;115:32–37. [DOI] [PubMed] [Google Scholar]
- 5. Espay AJ, Morgante F, Purzner J, Gunraj CA, Lang AE, Chen R. Cortical and spinal abnormalities in psychogenic dystonia. Ann Neurol 2006;59(5):825–834. [DOI] [PubMed] [Google Scholar]
- 6. Hanzlikova Z, Kofler M, Slovak M, et al. Prepulse inhibition of the blink reflex is abnormal in functional movement disorders. Mov Disord 2019;34:1022–1030. [DOI] [PubMed] [Google Scholar]
- 7. Voon V, Ekanayake V, Wiggs E, Kranick S, Ameli R, Harrison NA, Hallett M. Response inhibition in motor conversion disorder. Mov Disord 2013;28(5):612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Slovak M, Sieger T, Bonnet C, et al. Antisaccades and vergence abnormalities in functional movement disorders: a video‐oculographic study. Mov Disord 2016;31(7):1072–1073. [DOI] [PubMed] [Google Scholar]
- 9. Jahanshahi M, Rothwell JC. Inhibitory dysfunction contributes to some of the motor and non‐motor symptoms of movement disorders and psychiatric disorders. Philos T R Soc B 2017;372(1718):20160198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stein DJ, Costa DLC, Lochner C, et al. Obsessive–compulsive disorder. Nat Rev Dis Primers 2019;5(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robbins TW, Vaghi MM, Banca P. Obsessive‐compulsive disorder: puzzles and prospects. Neuron 2019;102(1):27–47. [DOI] [PubMed] [Google Scholar]
- 12. Spagnolo PA, Garvey M, Hallett M. A dimensional approach to functional movement disorders: heresy or opportunity. Neurosci Biobehav Rev 2021;127:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nielsen G, Ricciardi L, Meppelink AM, Holt K, Teodoro T, Edwards M. A simplified version of the psychogenic movement disorders rating scale: the simplified functional movement disorders rating scale (S‐FMDRS). Mov Disord Clin Pract 2017;4(5):710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foa EB, Huppert JD, Leiberg S, Langner R, Kichic R, Hajcak G, Salkovskis PM. The obsessive‐compulsive inventory: development and validation of a short version. Psychol Assess 2002;14(4):485–496. [PubMed] [Google Scholar]
- 15. Abramovitch A, Abramowitz JS, McKay D. The OCI‐12: a syndromally valid modification of the obsessive‐compulsive inventory‐revised. Psychiatry Res 2021;298:113808. [DOI] [PubMed] [Google Scholar]
- 16. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 17. Spielberger CD. STAI: Manual for the Stait‐Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 18. Krupp LB, LaRocca NG, Muir‐Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46(10):1121–1123. [DOI] [PubMed] [Google Scholar]
- 19. Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22(10):1911–1920. [DOI] [PubMed] [Google Scholar]
- 20. Dujardin K, Duhamel A, Delliaux M, Thomas‐Anterion C, Destee A, Defebvre L. Cognitive complaints in Parkinson's disease: its relationship with objective cognitive decline. J Neurol 2010;257(1):79–84. [DOI] [PubMed] [Google Scholar]
- 21. Markova H, Andel R, Stepankova H, et al. Subjective cognitive complaints in cognitively healthy older adults and their relationship to cognitive performance and depressive symptoms. J Alzheimers Dis 2017;59(3):871–881. [DOI] [PubMed] [Google Scholar]
- 22. Ware J Jr, Kosinski M, Keller SD. A 12‐item short‐form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 23. Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 1994;151(8):1132–1136. [DOI] [PubMed] [Google Scholar]
- 24. John OP, Srivastava S. The big‐five Trait Taxonomy: history, Measurement, and Theoretical Perspectives. New York: Guilford Press; 1999. [Google Scholar]
- 25. R Core Team . R: A Language and Environment for Statistical Computing. Viena: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 26. Taberner J, Fullana MA, Caseras X, et al. Are obsessive‐compulsive symptom dimensions familial in nonclinical individuals? Depress Anxiety 2009;26(10):902–908. [DOI] [PubMed] [Google Scholar]
- 27. Cadman T, Spain D, Johnston P, et al. Obsessive‐compulsive disorder in adults with high‐functioning autism spectrum disorder: what does self‐report with the OCI‐R tell us? Autism Res 2015;8(5):477–485. [DOI] [PubMed] [Google Scholar]
- 28. APA . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 29. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain 2010;133(Pt 5):1537–1551. [DOI] [PubMed] [Google Scholar]
- 30. Espay AJ, Maloney T, Vannest J, et al. Impaired emotion processing in functional (psychogenic) tremor: a functional magnetic resonance imaging study. Neuroimage Clin 2018;17:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Demartini B, Petrochilos P, Ricciardi L, Price G, Edwards MJ, Joyce E. The role of alexithymia in the development of functional motor symptoms (conversion disorder). J Neurol Neurosurg Psychiatry 2014;85(10):1132–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pinto A, Liebowitz MR, Foa EB, Simpson HB. Obsessive compulsive personality disorder as a predictor of exposure and ritual prevention outcome for obsessive compulsive disorder. Behav Res Ther 2011;49(8):453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fineberg NA, Reghunandanan S, Kolli S, Atmaca M. Obsessive‐compulsive (anankastic) personality disorder: toward the ICD‐11 classification. Braz J Psychiatry 2014;36:40–50. [DOI] [PubMed] [Google Scholar]
- 34. Samuels J, Bienvenu OJ, Krasnow J, et al. General personality dimensions, impairment and treatment response in obsessive–compulsive disorder. Personal Ment Health 2020;14(2):186–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abramovitch A, McCormack B, Brunner D, Johnson M, Wofford N. The impact of symptom severity on cognitive function in obsessive‐compulsive disorder: a meta‐analysis. Clin Psychol Rev 2019;67:36–44. [DOI] [PubMed] [Google Scholar]
- 36. Bienvenu OJ, Samuels JF, Costa PT, Reti IM, Eaton WW, Nestadt G. Anxiety and depressive disorders and the five‐factor model of personality: a higher‐ and lower‐order personality trait investigation in a community sample. Depress Anxiety 2004;20(2):92–97. [DOI] [PubMed] [Google Scholar]
- 37. Ludwig L, Pasman JA, Nicholson T, et al. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta‐analysis of case‐control studies. Lancet Psychiatry 2018;5(4):307–320. [DOI] [PubMed] [Google Scholar]
- 38. Destree L, Brierley ME, Albertella L, Jobson L, Fontenelle LF. The effect of childhood trauma on the severity of obsessive‐compulsive symptoms: a systematic review. J Psychiatr Res 2021;142:345–360. [DOI] [PubMed] [Google Scholar]
- 39. Perez DL, Matin N, Barsky A, et al. Cingulo‐insular structural alterations associated with psychogenic symptoms, childhood abuse and PTSD in functional neurological disorders. J Neurol Neurosurg Psychiatry 2017;88(6):491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chu M, Xu T, Wang Y, et al. The impact of childhood trauma on thalamic functional connectivity in patients with obsessive‐compulsive disorder. Psychol Med 2020;52(13):1–10. [DOI] [PubMed] [Google Scholar]
- 41. Spagnolo PA, Norato G, Maurer CW, Goldman D, Hodgkinson C, Horovitz S, Hallett M. Effects of TPH2 gene variation and childhood trauma on the clinical and circuit‐level phenotype of functional movement disorders. J Neurol Neurosurg Psychiatry 2020;91(8):814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fibbe LA, Cath DC, van den Heuvel OA, Veltman DJ, Tijssen MAJ, van Balkom AJLM. Relationship between movement disorders and obsessive–compulsive disorder: beyond the obsessive–compulsive–tic phenotype. A systematic review. J Neurol Neurosurg Psychiatry 2012;83(6):646–654. [DOI] [PubMed] [Google Scholar]
- 43. Berardelli I, Pasquini M, Conte A, Bologna M, Berardelli A, Fabbrini G. Treatment of psychiatric disturbances in common hyperkinetic movement disorders. Expert Rev Neurother 2019;19(1):55–65. [DOI] [PubMed] [Google Scholar]
- 44. Harbishettar V, Pal PK, Janardhan Reddy YC, Thennarasu K. Is there a relationship between Parkinson's disease and obsessive‐compulsive disorder? Parkinsonism Relat Disord 2005;11(2):85–88. [DOI] [PubMed] [Google Scholar]
- 45. Khedr EM, Elbeh KA, Elserogy Y, et al. Motor cortical excitability in obsessive‐compulsive disorder: transcranial magnetic stimulation study. Neurophysiol Clin 2016;46(2):135–143. [DOI] [PubMed] [Google Scholar]
- 46. Kang JI, Kim DY, Lee CI, Kim CH, Kim SJ. Changes of motor cortical excitability and response inhibition in patients with obsessive‐compulsive disorder. J Psychiatr Neurosci 2019;44(4):261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Impaired sensorimotor gating in unmedicated adults with obsessive‐compulsive disorder. Neuropsychopharmacology 2012;37(5):1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Penades R, Catalan R, Rubia K, Andres S, Salamero M, Gasto C. Impaired response inhibition in obsessive compulsive disorder. Eur Psychiatry 2007;22(6):404–410. [DOI] [PubMed] [Google Scholar]
- 49. Bey K, Lennertz L, Grutzmann R, et al. Impaired antisaccades in obsessive‐compulsive disorder: evidence from meta‐analysis and a large empirical study. Front Psych 2018;9:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brainstorm C, Anttila V, Bulik‐Sullivan B, et al. Analysis of shared heritability in common disorders of the brain. Science 2018;360(6395):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perez DL, Aybek S, Popkirov S, et al. A review and expert opinion on the neuropsychiatric assessment of motor functional neurological disorders. J Neuropsychiatry Clin Neurosci 2021;33(1):14–26. [DOI] [PubMed] [Google Scholar]
- 52. Blanco C, Olfson M, Stein DJ, Simpson HB, Gameroff MJ, Narrow WH. Treatment of obsessive‐compulsive disorder by U.S. psychiatrists. J Clin Psychiatry 2006;67(6):946–951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of proportion of subjects with each phenotype in group of FMD patients with OCI‐R <21 and ≥21.
Table S2. Correlations between total score of OCI‐R and clinical characteristics.
Table S3. Multiple regression model estimating OCI‐R in healthy controls and FMD patients.
Table S4. Between‐group comparison of self‐reported measures in FMD patients and control subjects with severe obsessive‐compulsive symptoms defined as OCI‐R ≥21.
Table S5. Within‐group comparison of self‐reported measures between control subjects with OCI‐R scores ≥21 and <21.
Data Availability Statement
Datasets analyzed during the current study are available on reasonable request. All data will be anonymized.


