Dear editor,
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most common monogenic small vessel disease and presents various clinical manifestations, however, chorea has rarely been manifested. Here, we report the 66‐year‐old woman with genetic‐confirmed CADASIL presenting continuous chorea, and review movement disorders associated with CADASIL.
A 66‐year‐old woman presented with generalized but asymmetric involuntary movement, started 8 years ago. Symptoms started in left toe, then spread into left ankle, orolingual area, and minimally on right toe (Video 1). After six months of progression, symptoms remained stable for the 8 years. The movement was brief, continuous, and irregular features, suggestive of chorea. She denied taking D2 receptor‐blocking agents. We tried antipsychotics (haloperidol, risperidone), but symptom did not improve. Detailed family history is unavailable, as both her parents died, and she is an only child. Specifically, her mother died at 60s of an ischemic stroke. The patient's only son had no cognitive or movement symptoms.
Video 1.
The patient presents involuntary movement mainly on her left toe and ankle, and orolingual area, with minimal involvement on right foot. The movement is characterized by irregular, flowing features, suggestive of chorea. The chorea is continuous during resting and walking. The patient provided written informed consent for publication of this video.
Brain MRI revealed confluent deep white matter hyperintensity (WMH), including bilateral anterior temporal lobe on FLAIR images (Fig. 1A–D). Mini‐mental status examination score was 17/30, and a neuropsychological test revealed mildly impaired executional, visuospatial, and memory function. Laboratory findings were unremarkable including hyper/hypoglycemia, ceruloplasmin, syphilis markers, paraneoplastic antibodies, or antiphospholipid markers. CAG repetition of HTT gene was normal.
Figure 1.
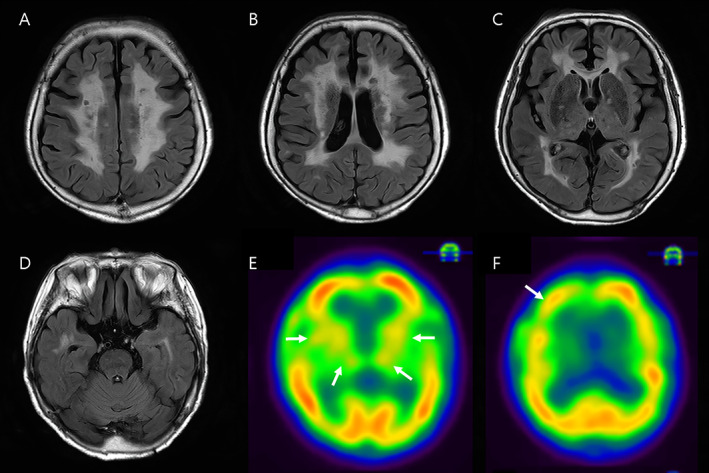
Brain MRI images (A–D) and HMPAO SPECT images (E, F). FLAIR imaging revealed bilateral deep white matter (A, B) and anterior temporal lesions (D). No hyperintense lesions were identified on external capsule. SPECT images revealed reduced perfusion on bilateral striatum, thalamus (E), and asymmetric right frontal cortex (F) (arrows).
Sequence analysis of the NOTCH3 gene (NM_00435.2) identified a heterozygous mutation of c.1630C > T (R544C) in exon 11, which has been one of the most common NOTCH3 mutations in the Asian population, 1 and previously classified as “pathogenic/likely pathogenic”. The hexamethyl propylenamine oxime (HMPAO) single photon emission computed tomography (SPECT) showed the asymmetric perfusion defects in right frontal cortex and diffuse reduction in bilateral basal ganglia and thalamus (Fig. 1E, F). Finally, she was diagnosed with CADASIL, presumed to be the etiology of her chorea.
CADASIL has a wide range of clinical manifestations although movement disorders have rarely been reported. 2 We reviewed the previous literature that presents movement disorders in CADASIL patients, with genetic or pathologic confirmation. To our knowledge, a total of 20 patients have been reported (Table S1.).
Parkinsonism is the most common, with 10 patients of vascular parkinsonism 3 , 4 , 5 , 6 , 7 and two patients with clinical features of progressive supranuclear palsy. 8 , 9 Ragno et al reported five separated patients and a pair of monozygotic twins with levodopa‐unresponsive parkinsonian features. 6 , 7 Ataxia was reported in five cases, without cerebellar infarction. 10 , 11 , 12
Hyperkinetic movement disorders are relatively rare. Two cases of facial dystonia have been reported in a single family. 13 Only one report described the vascular chorea in CADASIL, a 58‐year‐old female with asymmetric chorea on the left hand and lip. MRI showed WMH in the internal and external capsule, and FDG PET showed reduced right striatal uptake. 14 However, the NOTCH3 mutation of this case was not classified as a pathogenic/likely pathogenic, and the skin biopsy was normal, making the diagnosis of hemichorea after ischemic stroke more likely.
There have been no reported movement disorders in CADASIL Type 2 (autosomal dominant form of HTRA1 mutation) and CARASIL (autosomal recessive form of HTRA1 mutation). While one case report described the altered gait or postural tremor in a patient with CARASIL, 15 it is uncertain that these are caused by parkinsonism or ataxia due to many comorbidities (spasticity and spondylosis) in CARASIL.
Our case is the first genetically confirmed CADASIL case presenting vascular chorea without lesion on basal ganglia or thalamus in MRI. Also, in ataxia associated with CADASIL, 10 , 11 , 12 there were no MRI lesions in cerebellum, suggesting that strategic infarct is not always necessary to produce a specific type of movement disorder in CADASIL. Rather, our patient has severe striatal perfusion defect in brain SPECT, usually seen in the neurodegenerative causes of chorea (Huntington's disease, NBIA, chorea‐acanthocytosis), 16 as opposed to striatal hypermetabolism in acquired chorea (Sydenham's chorea, antiphospholipid‐related syndrome, hyperthyroidism). 17 , 18 In CADASIL patients with other movement disorders, five patients (2 parkinsonism, 2 dystonia, and 1 chorea) underwent metabolic imaging and all patients showed hypometabolism on the striatum or cortices (Table S1.). We speculated that vascular endothelial dysfunction in CADASIL may trigger the hypoperfusion that precedes cerebral infarction, and striatal hypometabolism may generate a disconnection of the cortico‐thalamo‐striatal circuits, which leads to chorea.
Another point of interest is the absence of typical CADASIL features, such as stroke or migraine, except for mild cognitive impairment. Although uncertain, genotype–phenotype correlation in CADASIL has been suggested, and previous reports also described atypical features in patients with R544C mutation, such as a low prevalence of migraine or stroke and frequent vascular dementia. 19 Additionally, one patient with the R544C mutation had presented movement disorder (parkinsonism). 5
This case highlights that chorea is a possible manifestation in CADASIL. Clinicians should consider CADASIL as a differential diagnosis of unexplained chorea, particularly if the characteristic WMH is identified in MRI.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Manuscript: A. Writing of the first draft, B. Review and Critique.
M.S.K.: 1B, 1C, 2A
D.G.P.: 1B, 2B
J.H.Y.: 1A, 1B, 2B
Disclosures
Ethical Compliance Statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The institutional review board at Ajou Hospital approved this case report. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. We confirm that the patient and his family provided written informed consent for video acquisition and publication.
Funding Sources and Conflicts of Interest: This study did not receive any industry funding. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months: J.H.Y. reports research support from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (no. NRF‐2022R1F1A1074588). D.G.P. declares that there are no additional disclosures to report. M.S.K. has no disclosures to report.
Supporting information
Supplemental Table S1. Literature review of movement disorders in CADASIL patients. WMH, white matter hyperintensity; BG, basal ganglia; FDG, fluorodeoxyglucose; PET, positron emission tomography; MCI, mild cognitive impairment; PSP, progressive supranuclear palsy; HMPAO, hexamethyl propylenamine oxime; SPECT, single photon emission computed tomography
References
- 1. Hu Y, Sun Q, Zhou Y, et al. NOTCH3 variants and genotype‐phenotype features in Chinese CADASIL patients. Front Genet 2021;12:705284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Donato I, Bianchi S, De Stefano N, et al. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) as a model of small vessel disease: update on clinical, diagnostic, and management aspects. BMC Med 2017;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wegner F, Strecker K, Schwarz J, et al. Vascular parkinsonism in a CADASIL case with an intact nigrostriatal dopaminergic system. J Neurol 2007;254:1743–1745. [DOI] [PubMed] [Google Scholar]
- 4. Valenti R, Bianchi S, Pescini F, D'Eramo C, Inzitari D, Dotti MT, Pantoni L. First report of a pathogenic mutation on exon 24 of the NOTCH3 gene in a CADASIL family. J Neurol 2011;258:1632–1636. [DOI] [PubMed] [Google Scholar]
- 5. Guo W, Xu B, Sun H, et al. Case report: progressive asymmetric parkinsonism secondary to CADASIL without dementia. Front Neurol 2021;12:760164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ragno M, Berbellini A, Cacchio G, et al. Parkinsonism is a late, not rare, feature of CADASIL: a study on Italian patients carrying the R1006C mutation. Stroke 2013;44:1147–1149. [DOI] [PubMed] [Google Scholar]
- 7. Ragno M, Sanguigni S, Manca A, et al. Parkinsonism in a pair of monozygotic CADASIL twins sharing the R1006C mutation: a transcranial sonography study. Neurol Sci 2016;37:875–881. [DOI] [PubMed] [Google Scholar]
- 8. Van Gerpen JA, Ahlskog JE, Petty GW. Progressive supranuclear palsy phenotype secondary to CADASIL. Parkinsonism Relat Disord 2003;9:367–369. [DOI] [PubMed] [Google Scholar]
- 9. Erro R, Lees AJ, Moccia M, et al. Progressive parkinsonism, balance difficulties, and supranuclear gaze palsy. JAMA Neurol 2014;71:104–107. [DOI] [PubMed] [Google Scholar]
- 10. Vedeler C, Bindoff L. A family with atypical CADASIL. J Neurol 2011;258:1888–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sari US, Kisabay A, Batum M, et al. CADASIL with atypical clinical symptoms, magnetic resonance imaging, and novel mutations: two case reports and a review of the literature. J Mol Neurosci 2019;68:529–538. [DOI] [PubMed] [Google Scholar]
- 12. Park DG, Min JH, Sohn SH, Sohn YB, Yoon JH. Ataxia associated with CADASIL: a pathology‐confirmed case report and literature review. Cerebellum 2020;19:907–910. [DOI] [PubMed] [Google Scholar]
- 13. Miranda M, Dichgans M, Slachevsky A, Urbina F, Mena I, Venegas P, Galvez M. CADASIL presenting with a movement disorder: a clinical study of a Chilean kindred. Mov Disord 2006;21:1008–1012. [DOI] [PubMed] [Google Scholar]
- 14. Chung EJ, Kim SJ. A case of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) patient presenting with chorea. Neurol Asia 2012;17:247–249. [Google Scholar]
- 15. Preethish‐Kumar V, Nozaki H, Tiwari S, et al. CARASIL families from India with 3 novel null mutations in the HTRA1 gene. Neurology 2017;89:2392–2394. [DOI] [PubMed] [Google Scholar]
- 16. Oechsner M, Buchert R, Beyer W, Danek A. Reduction of striatal glucose metabolism in McLeod choreoacanthocytosis. J Neurol Neurosurg Psychiatry 2001;70:517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lerjefors L, Andretta S, Bonato G, Mainardi M, Carecchio M, Antonini A. Antiphospholipid‐related chorea: two case reports and role of metabolic imaging. Mov Disord Clin Pract 2022;9:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim M, Lee SM, Yoon JH. Reversible chorea and parkinsonism in a patient with systemic lupus erythematosus. Neurol Sci 2016;37:491–492. [DOI] [PubMed] [Google Scholar]
- 19. Lee JS, Ko K, Oh JH, Park JH, Lee HK. Phenotypic features of cerebral autosomal‐dominant arteriopathy with subcortical infarcts and leukoencephalopathy subjects with R544C mutation. Dement Neurocogn Disord 2016;15:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. Literature review of movement disorders in CADASIL patients. WMH, white matter hyperintensity; BG, basal ganglia; FDG, fluorodeoxyglucose; PET, positron emission tomography; MCI, mild cognitive impairment; PSP, progressive supranuclear palsy; HMPAO, hexamethyl propylenamine oxime; SPECT, single photon emission computed tomography


