Abstract
Pulmonary aspergillosis is classified into invasive, saprophytic, and allergic forms. In this study, we evaluated the usefulness of PCR for differentiating between different forms of aspergillosis or in monitoring disease activity during treatment by detecting DNA specific for Aspergillus species in the serum. Nested PCR was used to detect Aspergillus DNA in the sera of 30 patients with various forms of pulmonary aspergillosis. The results were compared with those of latex agglutination tests for detecting galactomannan antigen. We also examined the serial changes in the results of nested PCR during and after treatment of a subgroup of patients with invasive pulmonary aspergillosis with amphotericin B. The highest proportion of positive nested PCR results were in patients with invasive aspergillosis (10 of 12; 83%), while patients with pulmonary aspergilloma had the lowest frequency of positive tests (1 of 9; 11%). These results suggested that the sensitivity of the nested PCR depends on the extent of invasion by Aspergillus species. Serial assays showed that the results of nested PCR became negative shortly after commencement of antifungal treatment and that such changes did not correlate with clinical responsiveness to treatment. Our results indicate the potential usefulness of nested PCR with serum samples for the diagnosis of invasive aspergillosis and the detection of a shift in the status of infection from a noninvasive type to invasive aspergillosis. However, the results of the nested PCR did not correlate with the response to antifungal treatment.
Pulmonary aspergillosis is classified into three types: invasive, saprophytic, and allergic. The type of disease caused by Aspergillus species is determined by the host immune activity and structural abnormalities of the bronchi and lung (2, 10, 17). Recently, Gefter et al. (8, 9) proposed a new type of semi-invasive pulmonary aspergillosis as an intermediate form between the invasive and saprophytic types. Binder et al. (3) later described a similar condition as chronic necrotizing pulmonary aspergillosis (CNPA). In spite of this classification, it is often difficult to differentiate among these disease forms, and intermediate or borderline cases often exist (1, 15).
In a series of recent studies, we reported the usefulness of nested PCR for the detection of DNA specific for Aspergillus species in serum samples and for the diagnosis of invasive pulmonary aspergillosis (IPA) (11, 22). In the present study, we evaluated the usefulness of nested PCR for differentiating between different forms of pulmonary aspergillosis.
MATERIALS AND METHODS
Patients.
A total of 30 patients with various types of pulmonary aspergillosis were included in the study. They represented consecutive patients admitted to the Second Department of Internal Medicine at Oita Medical University Hospital and its affiliated hospitals between November 1992 and September 1997. Serum samples were obtained from these patients at least twice prior to diagnosis and at different intervals after treatment (mean number of samples per patient, 4.7; range, 2 to 12 samples). The same samples were analyzed by both nested PCR and galactomannan antigen assay. In addition, if the condition of the patient permitted, bronchoalveolar lavage (BAL) was performed by standard techniques and BAL fluid samples were cultured for the detection of Aspergillus species.
Disease types.
The presence or absence of underlying disorders; serial changes in chest X rays, including the preexistence of pulmonary cavities; and the clinical course were reviewed to classify pulmonary aspergillosis into the following categories.
(i) Pulmonary aspergilloma.
Patients with pulmonary aspergilloma showed fungus balls in preexisting pulmonary cavities caused by destructive pulmonary diseases, such as tuberculosis. All patients had positive Aspergillus precipitin tests (10).
(ii) CNPA.
Patients with CNPA showed pulmonary infiltration with progressive cavity formation irrespective of the presence or absence of preexisting pulmonary cavities, indicating chronic pulmonary damage over months to years (3).
(iii) IPA.
Patients with IPA had severe underlying disorders, such as hematologic malignancies or fever resistant to antibiotics, and had chest X-ray findings consistent with this type of disease, such as round pneumonia, cavitation, or extensive infiltration (10). Furthermore, this category was divided into the following subcategories: (a) proven IPA, comprising those patients with microbiologically or histologically proven IPA; and (b) presumptive IPA, comprising the remainder of the patients, for whom a diagnosis of IPA was suggested by chest radiograph and other clinical manifestations.
(iv) Aspergillus empyema.
Patients with Aspergillus empyema had a relatively mild infiltration in the lungs and apparent pleural effusion. Aspergillus cells were isolated by the culture of pus obtained by thoracocentesis (4).
Clinical cultures and identification of fungal pathogens.
Routine culture for fungi was performed after centrifugation of the BAL fluid sample or pleural effusion at 1,200 × g for 10 min. The pellet was plated onto Sabouraud glucose agar and was cultured for 7 days at 30 and 37°C. Aspergillus species were identified by their culture characteristics and the morphologies of their conidiophores and conidia.
Nested PCR.
PCR was performed in our laboratory as described previously (11, 22), with two sets of oligonucleotide primers for detection of DNA specific for Aspergillus species in serum samples. Briefly, DNA was extracted from the serum samples by treatment with proteinase K and was used as a template. Oligonucleotide primers used in the single PCR step were Asp. 5 (5′-GATAACGAACGAGACCTCGG-3′) and Asp. 8 (5′-TGCCAACTCCCCTGAGCCAG-3′). Thirty cycles were performed, including 1 min of DNA denaturation at 94°C, 1 min of primer annealing at 50°C, and 3 min of DNA extension at 72°C. Nested PCR was performed with primers Asp. 1 (5′-CGGCCCTTAAATAGCCCGGTC-3′) and Asp. 7 (5′-CCTGAGCCAGTCCGAAGGCC-3′) in a manner similar to that described above except that primer annealing was performed at 65°C. To avoid possible contamination of the PCR mixtures, all reactions were performed under stringent conditions, as recommended by Kwok and Higuchi (12). Furthermore, the room where the PCR was performed was separate from the room where DNA extraction was performed. Three negative controls, including reagent controls and sera from healthy volunteers, were run along with every five test samples for all reactions. As a positive control, the Aspergillus fumigatus gene was also run along with the test samples. The nested PCR products were electrophoresed on a 2% agarose gel containing ethidium bromide, and the results were photographed.
Detection of galactomannan antigen.
Serum galactomannan antigen was detected with a Pastorex Aspergillus kit (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France) by the protocol recommended by the manufacturer (5, 7, 18, 21).
RESULTS
Classification of aspergillosis.
As shown in Table 1, of the 30 patients investigated, 9 were diagnosed with pulmonary aspergilloma, 6 were diagnosed with CNPA, 3 were diagnosed with Aspergillus empyema, and 12 were diagnosed with IPA (4 with proven IPA and 8 with presumptive IPA). Culture of BAL fluid showed Aspergillus species in five patients with pulmonary aspergilloma and in all patients with CNPA. Culture of pleural effusion fluid showed growth of Aspergillus species in all patients with Aspergillus empyema. In contrast, because BAL could not be performed on patients with severe underlying diseases, Aspergillus organisms were detected in clinical specimens from only four patients with IPA.
TABLE 1.
Results of galactomannan antigen assay and nested PCR with sera of patients with various forms of pulmonary aspergillosis
| Patient no. | Age (yr) | Sexa | Aspergillosis type | Underlying disease(s)b | Galactomannan antigenc | Nested PCRc | Cultured straind |
|---|---|---|---|---|---|---|---|
| 1 | 84 | M | Aspergilloma | Old pulmonary tuberculosis | − | − | A. fumigatus |
| 2 | 67 | M | Aspergilloma | Pneumoconiosis and tuberculosis | − | − | A. fumigatus |
| 3 | 74 | M | Aspergilloma | Pneumoconiosis and tuberculosis | − | − | Aspergillus niger |
| 4 | 70 | M | Aspergilloma | Old pulmonary tuberculosis | − | − | NP |
| 5 | 76 | M | Aspergilloma | Old pulmonary tuberculosis | − | − | NP |
| 6 | 73 | M | Aspergilloma | Old pulmonary tuberculosis | − | − | NP |
| 7 | 55 | M | Aspergilloma | Pulmonary cyst | − | + | A. fumigatus |
| 8 | 78 | M | Aspergilloma | Old pulmonary tuberculosis | − | − | NP |
| 9 | 57 | M | Aspergilloma | Old pulmonary tuberculosis | − | − | A. fumigatus |
| 10 | 65 | M | CNPA | IIP | − | + | A. fumigatus |
| 11 | 68 | M | CNPA | Old pulmonary tuberculosis | + | + | A. fumigatus |
| 12 | 64 | M | CNPA | Diabetes mellitus | + | + | Aspergillus sp. |
| 13 | 73 | M | CNPA | Old pulmonary tuberculosis | − | − | Aspergillus sp. |
| 14 | 67 | M | CNPA | Old pulmonary tuberculosis | − | + | Aspergillus sp. |
| 15 | 69 | M | CNPA | Pulmonary cyst | + | − | A. fumigatus |
| 16 | 42 | M | Proven IPA | AML | + | + | A. fumigatus |
| 17 | 52 | F | Proven IPA | Nephrotic syndrome | + | + | A. fumigatus |
| 18 | 37 | M | Proven IPA | ATL | + | + | A. niger |
| 19 | 18 | M | Proven IPA | CGD | + | + | A. fumigatus |
| 20 | 65 | F | Presumptive IPA | AML | − | + | NP |
| 21 | 48 | F | Presumptive IPA | ATL | − | − | NP |
| 22 | 45 | M | Presumptive IPA | AML | − | + | NP |
| 23 | 60 | M | Presumptive IPA | AML | − | + | NP |
| 24 | 76 | M | Presumptive IPA | Malignant lymphoma | − | + | NP |
| 25 | 68 | F | Presumptive IPA | AML | + | + | NP |
| 26 | 21 | M | Presumptive IPA | CGD | − | − | NP |
| 27 | 68 | M | Presumptive IPA | Malignant lymphoma | + | + | NP |
| 28 | 85 | M | Pyothorax | Unknown | + | + | A. niger |
| 29 | 60 | F | Pyothorax | Wegener’s granulomatosis | − | − | A. fumigatus |
| 30 | 86 | M | Pyothorax | Unknown | + | − | A. fumigatus |
M, male; F, female.
IIP, idiopathic interstitial pneumonia; AML, acute myelogenous leukemia; CGD, chronic granulomatous disease; ATL, adult T-cell leukemia.
+, positive; −, negative.
NP, not performed.
Data from five of six patients with CNPA were analyzed in detail. The clinical features and PCR results for these patients are shown in Table 2. Among four PCR-positive patients, patient 10 was treated with a low dose of corticosteroids for idiopathic interstitial pneumonia while patient 12 had diabetes mellitus. These patients were considered to have a slight systemic immune deficiency. For patients 11 and 12, there were relatively extensive pathological changes in both lungs. In contrast, for patient 13, who had no preexisting immunosuppressive disease, the PCR was negative and the pathological changes were restricted to the lower lobe of the right lung.
TABLE 2.
Clinical findings and results of nested PCR in patients with CNPA
| Patient no. | Age (yr) | Smoking level (BI)a | Underlying diseaseb | Corticosteroidsc | Feverd | Neutrophil count/mm3 | Lymphocyte count/mm3 | CRPe (mg/dl) | Chest X-ray findingsf | Nested PCRg |
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 65 | 1,800 | IIP | + | − | 4,480 | 1,450 | 7.90 | LUL abscess (+) | + |
| 11 | 68 | 900 | Old TB | − | − | 3,490 | 2,300 | 6.88 | RUL + LUL cavity (+) | + |
| 12 | 64 | 1,200 | DM | − | + | 7,360 | 1,150 | 19.7 | RUL + LUL cavity (+) | + |
| 13 | 73 | 1,010 | Old TB | − | − | 7,460 | 1,150 | 3.80 | RLL cavity (+) | − |
| 14 | 67 | 600 | Old TB | − | − | 3,290 | 1,990 | 4.75 | RUL cavity (+) | + |
BI, Brinkman smoking index.
IIP, idiopathic interstitial pneumonia; TB, tuberculosis; DM, diabetes mellitus.
+, used; −, not used.
+, present; −, absent.
CRP, C-reactive protein.
LUL, left upper lobe; RUL, right upper lobe; RLL, right lower lobe. +, present.
+, positive; −, negative.
Comparison of results of nested PCR and galactomannan antigen assay.
The results of nested PCR and the galactomannan antigen assay are shown in Table 1. Twenty-two samples from 9 patients with aspergilloma, 25 samples from 6 patients with CNPA, 86 samples from 12 patients with IPA, and 8 samples from 3 patients with empyema were tested. Among the pulmonary aspergilloma patients, one of nine (11%) was positive by nested PCR but all were negative for the galactomannan antigen. For six patients with CNPA, higher positive rates for nested PCR (four of six; 67%) and galactomannan antigen (three of six; 50%) were noted. Of the 12 patients with IPA, 10 (83%) and 6 (50%) were positive by nested PCR and for galactomannan antigen, respectively. Overall, the results of nested PCR were positive for 16 of 30 patients (53%) with pulmonary aspergillosis, while for the same patients, the galactomannan antigen assay was positive in 11 (37%) cases.
Serial changes in nested PCR and galactomannan antigen assay during antifungal treatment.
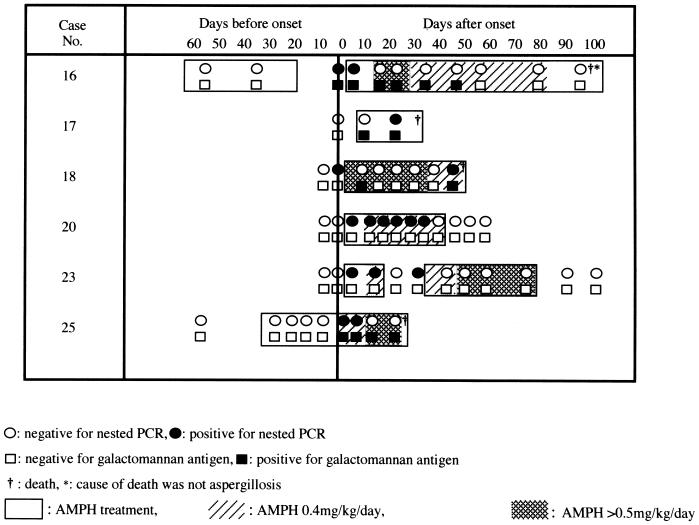
Sera were obtained from six patients with IPA at different intervals during the course of therapy and were tested by nested PCR and the galactomannan antigen assay (Fig. 1). In cases where both tests were positive (patients 16, 17, 18, and 25), there was no significant difference in the time of positivity of DNA detection by PCR compared to the Latek assay.
FIG. 1.
Serial changes in the results of nested PCR and galactomannan assays in patients with IPA treated with AMPH.
In cases of positive nested PCR, five of these patients showed a positive PCR by day 7 after the chest radiographs showed characteristic shadows suggestive of IPA. The clinical manifestations improved in three cases (patients 16, 20, and 23) following treatment with amphotericin B (AMPH), which is associated with negative results of nested PCR. However, deterioration of chest radiographs was noted for two patients (patients 18 and 25) who ultimately died, although the nested PCR became negative during AMPH treatment.
DISCUSSION
Pulmonary aspergillosis can be classified into various types depending on the immune status of the host and the invasiveness of the fungus (4, 17). Gefter et al. (8) described a new type, semi-invasive pulmonary aspergillosis, intermediate between the invasive and saprophytic types. By introducing this definition, they provided the concept of a wide spectrum of pulmonary aspergillosis. Thus, pulmonary aspergilloma is a condition characterized by colonization of Aspergillus cells at preexisting disease loci in the bronchi or lung. On the other hand, semi-invasive aspergillosis or CNPA (3) is a condition in which the fungus attacks the lungs in mildly immunocompromised states (e.g., sarcoidosis, tuberculosis, diabetes mellitus, etc.). Finally, IPA is a condition in which the fungus invades healthy areas of the lung in patients with systemic immune deficiency. Since the nested PCR used in the present experiment detected the presence of DNA specific for Aspergillus species in the serum, the results were positive only when the blood vessels were invaded by the fungus. This conclusion is based on our finding that the highest proportion of positive nested PCR tests were noted in patients with IPA while patients with pulmonary aspergilloma had the lowest rate of positive tests (10 of 12 versus 1 of 9; P = 0.002 by Fisher’s exact test). Thus, these results suggested that the frequency of positive nested PCR depends on the extent of invasiveness of Aspergillus cells.
Our results with a subgroup of patients with CNPA suggested that, within the spectrum of pulmonary aspergillosis, the clinical manifestations in a proportion of these patients were similar to those in patients with IPA. The former group of patients showed a slight systemic immunodeficiency or extensive impairment of pulmonary immune activity associated with invasion of Aspergillus cells into the blood vessels.
Rafferty et al. (15) reported that the disease profile in 5 of their 23 patients with pulmonary aspergilloma shifted to IPA after long-term follow-up. However, we did not see any shift in the disease state from the noninvasive type to the invasive type, probably because of the short period of follow-up in the present study. Invasion of blood vessels by the fungus is considered to be one of the events accompanying a shift in the disease state. Because the results of nested PCR reflected the presence of fungal DNA in the serum, the information derived from this technique might be important for determination of the therapeutic modality or prognosis of the disease.
However, the results of nested PCR in the present study did not show a clear correlation with the outcome of therapy. The nested PCR tended to show a negative result shortly after the commencement of treatment without correlation with the clinical responsiveness to antifungal treatment. This was particularly striking in patients treated with high doses (≥0.5 mg/kg of body weight/day) of AMPH (Fig. 1). It is possible that the nested PCR was inhibited by the presence of residual AMPH. However, Deventer et al. (6) showed positive PCR results for DNA of Candida albicans in blood samples of mice treated with AMPH, suggesting that AMPH does not exhibit inhibitory activity on PCR. Alternatively, treatment with AMPH may cause a marked decrease in the number of Aspergillus cells in the blood. No experimental data are available to explain these aspects of therapy. Further evaluation is necessary for PCR monitoring of pulmonary aspergillosis with animal models.
Several other techniques are available for the diagnosis of aspergillosis. These include the latex agglutination test for the detection of galactomannan antigen, which was also used in the present study; a method for measuring the amount of (1→3)-β-d-glucan (13, 14); and a sandwich enzyme-linked immunosorbent assay (ELISA) for the detection of galactomannan (16, 19, 20). The second method probably has low specificity, since the level of (1→3)-β-d-glucan is known to increase in patients with deep fungal infections other than aspergillosis (14). Although the sensitivity and specificity of the sandwich ELISA are excellent, it can only be performed in a limited number of laboratories. Since these techniques provide a quantitative assessment, the information obtained is usually useful for evaluating the efficacy of treatment. In contrast, since the nested PCR provides a target different from those of the other methods, it will be helpful to combine the methodologies to improve early diagnosis and observation of the disease profile.
In conclusion, we demonstrated in the present study the potential usefulness of nested PCR for the diagnosis of invasive aspergillosis by using serum samples as well as for detecting a shift in the pattern of infection from a noninvasive type to invasive aspergillosis. However, for a better evaluation of nested PCR in the diagnosis of pulmonary aspergillosis, a long-term study with a larger population sample is necessary.
ACKNOWLEDGMENTS
We thank Takako Sato for performing galactomannan antigen assays and Masumi Ikuta for preparing the manuscript. We also thank F. G. Issa (Department of Medicine, University of Sydney, Sydney, Australia) for the careful reading and editing of the manuscript.
This work was supported in part by a grant-in-aid for scientific research (C) (08670670) from the Ministry of Education, Science and Culture, Japan.
REFERENCES
- 1.Anderson C J, Craig S, Bandana E J., Jr Allergic bronchopulmonary aspergillosis and bilateral fungus balls terminating in disseminated aspergillosis. J Allergy Clin Immunol. 1980;65:140–144. doi: 10.1016/0091-6749(80)90199-2. [DOI] [PubMed] [Google Scholar]
- 2.Aslam P A, Eastridge C E, Hughes F A., Jr Aspergillosis of the lung—an eighteen-year experience. Chest. 1971;59:28–32. doi: 10.1378/chest.59.1.28. [DOI] [PubMed] [Google Scholar]
- 3.Binder R E, Faling L J, Pugatch R D, Mahasaen C, Snider G L. Chronic necrotizing pulmonary aspergillosis: a discrete clinical entity. Medicine. 1982;61:109–124. doi: 10.1097/00005792-198203000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Bodey G P, Vartivarian S. Aspergillosis. Eur J Clin Microbiol Infect Dis. 1989;8:413–437. doi: 10.1007/BF01964057. [DOI] [PubMed] [Google Scholar]
- 5.Cutsem J, Meulemans L, Van Gerven F, Stynen D. Detection of circulating galactomannan by Pastorex Aspergillus in experimental invasive aspergillosis. Mycoses. 1990;33:61–69. doi: 10.1111/myc.1990.33.2.61. [DOI] [PubMed] [Google Scholar]
- 6.Deventer A J M, Goessens W H F, Belkum A, Etten E W M, Vliet H J A, Verbrugh H A. PCR monitoring of response to liposomal amphotericin B treatment of systemic candidiasis in neutropenic mice. J Clin Microbiol. 1996;34:25–28. doi: 10.1128/jcm.34.1.25-28.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dupont B, Huber M, Kim S J, Bennett J E. Galactomannan antigenemia and antigenuria in aspergillosis: study in patients and experimentally infected rabbits. J Infect Dis. 1987;155:1–11. doi: 10.1093/infdis/155.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Gefter W B, Weingrad T R, Epstein D M, Ochs R H, Miller W T. “Semi-invasive” pulmonary aspergillosis. Radiology. 1981;140:313–321. doi: 10.1148/radiology.140.2.7255704. [DOI] [PubMed] [Google Scholar]
- 9.Gefter W B. The spectrum of pulmonary aspergillosis. J Thorac Imaging. 1992;7:56–74. doi: 10.1097/00005382-199209000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Greene R. The pulmonary aspergilloses: three distinct entities or spectrum of disease. Radiology. 1981;140:527–530. doi: 10.1148/radiology.140.2.7255737. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto, A., Y. Yamakami, P. Kamberi, E. Yamagata, R. Karashima, H. Nagaoka, and M. Nasu. Comparison of PCR, (1→3)-β-d-glucan and galactomannan assays in sera of rats with experimental invasive aspergillosis. J. Clin. Lab. Anal., in press. [DOI] [PMC free article] [PubMed]
- 12.Kwok S, Higuchi R. Avoiding false positives with PCR. Nature (London) 1989;339:237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- 13.Mitsutake K, Kohno S, Miyazaki T, Yamamoto Y, Yanagihara K, Kakeya H, Hashimoto A, Koga H, Hara K. Detection of (1→3)-β-d-glucan in a rat model of aspergillosis. J Clin Lab Anal. 1995;9:119–122. doi: 10.1002/jcla.1860090208. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki T, Kohno S, Mitsutake K, Maesaki S, Tanaka K, Ishikawa N, Hara K. Plasma (1→3)-β-d-glucan and fungal antigenemia in patients with candidemia, aspergillosis, and cryptococcosis. J Clin Microbiol. 1995;33:3115–3118. doi: 10.1128/jcm.33.12.3115-3118.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rafferty P, Biggs B A, Crompton G K, Grant I W. What happens to patients with pulmonary aspergilloma? Analysis of 23 cases. Thorax. 1983;38:579–583. doi: 10.1136/thx.38.8.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swanink C M A, Meis J F G M, Rijs A J M M, Donnelly J P, Verweij P E. Specificity of a sandwich enzyme-linked immunosorbent assay for detecting Aspergillus galactomannan. J Clin Microbiol. 1997;35:257–260. doi: 10.1128/jcm.35.1.257-260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner-Warwick M. Aspergillus fumigatus and lung disease. Postgrad Med. 1979;55:642–644. doi: 10.1136/pgmj.55.647.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verweij P E, Rijs A J M M, DePauw B E, Horrevorts A M, Hoogkamp-Korstanje J A A, Meis J F G M. Clinical evaluation and reproducibility of Pastorex Aspergillus antigen latex agglutination test for diagnosing invasive aspergillosis. J Clin Pathol. 1995;48:474–476. doi: 10.1136/jcp.48.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verweij P E, Stynen D, Rijs A J M M, de Pauw B E, Hoogkamp-Korstanje J A A, Meis J F G M. Sandwich enzyme-linked immunosorbent assay compared with Pastorex latex agglutination test for diagnosing invasive aspergillosis in immunocompromised patients. J Clin Microbiol. 1995;33:1912–1914. doi: 10.1128/jcm.33.7.1912-1914.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verweij P E, Latgé J-P, Rijs A J M M, Melchers W J G, De Pauw B E, Hoogkamp-Korstanje J A A, Meis J F G M. Comparison of antigen detection and PCR assay using bronchoalveolar lavage fluid for diagnosing invasive pulmonary aspergillosis in patients receiving treatment for hematological malignancies. J Clin Microbiol. 1995;33:3150–3153. doi: 10.1128/jcm.33.12.3150-3153.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warnock D W, Foot A B M, Johnson E M, Mitchell S B, Cornish J M, Oakhill A. Aspergillus antigen latex test for diagnosis of invasive aspergillosis. Lancet. 1991;338:1023–1024. doi: 10.1016/0140-6736(91)91890-7. [DOI] [PubMed] [Google Scholar]
- 22.Yamakami Y, Hashimoto A, Tokimatsu I, Nasu M. PCR detection of DNA specific for Aspergillus species in serum of patients with invasive aspergillosis. J Clin Microbiol. 1996;34:2464–2468. doi: 10.1128/jcm.34.10.2464-2468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]