Abstract
The efficacy mepolizumab in severe asthmatic patients is proven in the literature. Primarily to study the effect of mepolizumab on exacerbations, steroid dependence, and the continuation of efficacy in the long term. Secondarily to evaluate the effect of the drug on nasal polyps. Analyzing data from SANI (Severe Asthma Network Italy) clinics, we observed severe asthmatic patients treated with mepolizumab 100 mg/4 weeks, for a period of 3 years. 157 patients were observed. Exacerbations were reduced from the first year (−84.6%) and progressively to 90 and 95% in the second and third ones. Steroid-dependent patients decreased from 54% to 21% and subsequently to 11% in the second year and 6% in the third year. Patients with concomitant nasal polyps, assessed by SNOT-22, showed a 49% reduction in value from baseline to the third year. The study demonstrated the long-term efficacy of mepolizumab in a real-life setting.
Keywords: severe asthma, eosinophils, mepolizumab, CRSwNP, IL-5, real life, registry
1. Introduction
Asthma is a chronic airway disease characterized by bronchial obstruction, usually reversible either spontaneously or with therapy. It affects over 300 million people worldwide, representing one of the highest prevalence diseases in the field of respiratory diseases [1]. The severe form of this disease affects 5–10% of all patients and is characterized by poor symptom control despite maximal inhaled therapy dose, recurrent exacerbations and frequent use of systemic oral corticosteroids (OCS) to gain symptom control [2]. To perform real precision and personalized medicine, it started with a phenotyping of patients and continued with an endotyping linked to inflammation characteristics and cellularity [1]. The more detailed understanding of the mechanisms of inflammation facilitated and promoted the process of patients’ endotyping, allowing for the identification of identify new biological therapeutic options for patients with severe forms of disease. Beginning with the role of immunoglobulin (Ig) E, then continuing with the one of eosinophils and fractional exhaled nitric oxide (FeNO), and finally with the deepest knowledge of cytokines and proteins, like interleukin (IL) 4, 5, 13, 23, 33, and thymic stromal lymphoid protein (TSLP), cells like Innate lymphoid (ILC) type 2, and T helper lymphocyte (TH) 2, a deepest knowledge of disease inflammation mechanisms was allowed [3,4]. In relation to the type of inflammation, two different mechanisms were identified: the first one related to type 2 (T2) inflammation, and the second, not associated with T2 cells and cytokines, that still remains a challenge [5,6], and is actually not easily treatable with marketed biologics. T2 inflammation generally shows a good response to corticosteroids, the use of which, however, implies short, and long-term side effects [7,8].
With the aim of searching for a control of disease, limiting systemic corticosteroid use, and reducing exacerbations, several biologic drugs were developed [9]. One of the main targets of these drugs are eosinophils, which are observed to be cells that are usually increased in the blood of severe asthmatic patients. Among biologics, mepolizumab (MEP) was the first to be marketed for patients with an eosinophilic endotype of the disease [10]. MEP is a monoclonal antibody against IL-5 that controls eosinophils’ proliferation, maturation, and activity [11,12]. The link between IL-5 and eosinophils is well known in fact, this cytokine is necessary for the maturation of their precursors located in the bone marrow and subsequently for the release of mature cells into the blood [13]. The production and stimulation of IL-5 are prompted by TH2 and ILC2 cells, the latter being in turn principally activated by signals from epithelial cytokines like IL-25, IL-33, and TSLP [14,15].
The effects of MEP were studied first in randomized controlled trials (RCT) [16,17,18] and then in real-life studies [19,20,21], confirming its efficacy and providing useful information on some aspects such as its pharmacoeconomics and the effect on comorbidities [22,23,24,25]. The effect of MEP was clearly demonstrated in patients with eosinophilic severe asthma with a cell count greater than 300/μL in the 12 months prior to the drug’s administration and at least 150 at the time of the first dose. Clinical trials focused on the efficacy of the medication in a sample of patients generally treated for 24–52 weeks, and recent extension studies demonstrated the efficacy after several years. A recent real-life (RL) study demonstrated the drug’s efficacy in a sample of 51 patients observed for 36 months [19].
RL studies can provide additional and complementary information to RCTs [26], especially on long-term effects and safety. This is essential in the case of biological drugs, where the populations of regulatory trials and RL often differ. Long-term efficacy of MEP was addressed only in clinical trials and in a maximum of 2 years of real-life observations; with this manuscript, we want to describe data from 3 years of analysis on asthmatic patients treated with MEP. Considering that chronic rhinosinusitis with nasal polyposis (CRSwNP) is the more frequent comorbidity in severe asthma, we also evaluated the effects of MEP in such selected patients, from clinic afferents to the national Italian severe asthma registry (SANI).
2. Methods
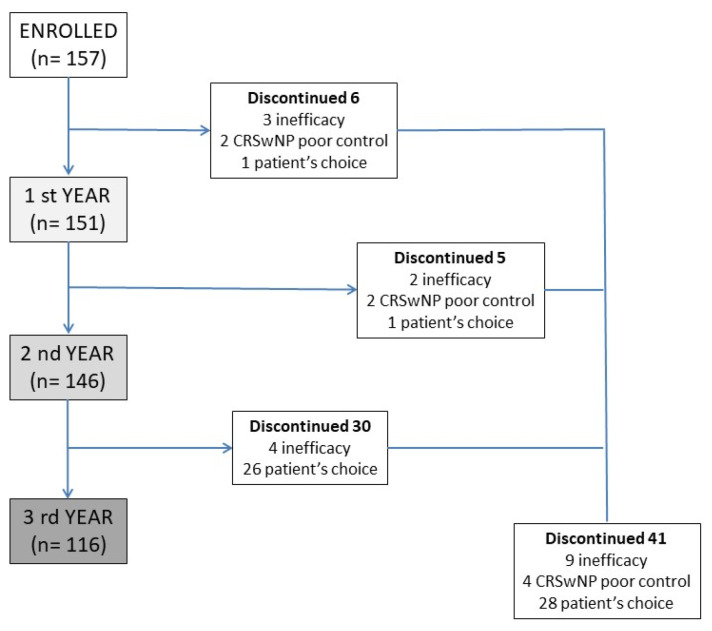
A prospective multicenter observational study was developed, involving several severe asthma centers in the SANI (Severe Asthma Network Italy) registry [27], with the aim of analyzing the data from patients treated, for severe asthma with MEP 100 mcg subcutaneously/4 weeks for at least 3 years. No restriction about the age of patients was chosen. Patients analyzed were all affected by T2 inflammation, confirmed by blood eosinophil counts. All patients were eligible for treatment with MEP according to the prescribing criteria of the Italian regulatory agency (uncontrolled severe asthma, eosinophils > 150 cells/mcL at the time of first administration and >300 in the previous 12 months, at least 2 exacerbations in the previous 12 months, systemic steroid therapy lasting for more than 6 months). The diagnosis of asthma was done according to the reversibility of obstruction or methacholine test. The first administration was given between June 2017 and January 2019, and this warranted 3 years of observation. The reasons for which some patients discontinued the MEP treatment were carefully recorded and analyzed. All patients were evaluated for exacerbations and use of OCS (converted to prednisone equivalent). Lung function tests were performed at baseline and every 12 months (inhaled therapy was discontinued one half-life before the test), as well as the Asthma Control Test (ACT) and Fractional exhaled nitric oxide (FeNO). All patients were evaluated for chronic rhinosinusitis with endoscopic tests and/or CT-scan imaging, and the clinical impact of nasal polyps was assessed with the Sinonasal Outcome Test (SNOT-22), which was performed at baseline and every 12 months. The disposition of patients is summarized in Figure 1. This study used the data from patients included in the SANI registry; therefore, all of them provided signed consent to use their data for medical research, previously approved by Genoa’s ethics committee (year 2017, ID 3663). All data were analyzed with descriptive statistics. An indirect comparison was made with the so-called super responder patient cohort [28].
Figure 1.
Patients’ disposition during the 3 years of observation, with discontinuation rates and the reason of drug suspension. CRSwNP—Chronic rhinosinusitis with nasal polyps.
The appropriate statistical analysis was applied according to the characteristics of the variables in the exam. Fisher’s exact test, χ2, one-way ANOVA, and Student t test were used when necessary.
3. Results
At the beginning of the study were enrolled 157 patients, 51% male, with a mean age of 59 (range 21–84). Of them, 99 patients (63%) also had CRSwNP. Eosinophils mean level before starting treatment was 718 (±579) cells/μL; 85 (54%) of patients were steroid-dependent with a mean dose of administered prednisone of 15 (±11) mg/day and 5.8 g/year. Mean exacerbations rate at baseline was 3.9 ± 2.8 with 1.4 (±0.5) exacerbations. The mean value of FEV1 was 2.21 ± 1.0 L corresponding to 70 ± 33% of the predicted value. The control of the disease was evaluated with ACT (17 ± 4 at baseline). Patients with CRSwNP had a SNOT-22 score of 51 ± 15 (Table 1).
Table 1.
Characteristics of patients at each year of observation and respective comparisons.
| Baseline (n = 157) |
1° Year (n = 151) |
p-Value Baseline vs. 1° y | 2° Year (n = 146) |
p-Value 1° vs. 2° y | 3° Year (n = 116) |
p-Value 2° vs. 3° y |
p-Value Baseline vs. 3° |
|
|---|---|---|---|---|---|---|---|---|
| Male (%) | 80 (51) | 76 (50) | 0.864 | 77 (53) | 0.706 | 59 (51) | 0.833 | 0.937 |
| Age mean (range) | 59 (21–84) | 59 (22–85) | 0.945 | 59 (23–81) | 0.899 | 60 (24–82) | 0.910 | 0.904 |
| Age onset | 41 (15.7) | n.a. | - | n.a. | - | n.a. | - | - |
| BMI | 25.8 (8.8) | 26.1 (7.6) | 0.894 | 25.9 (6.4) | 0.903 | 26.0 (6.1) | 0.945 | 0.849 |
| CRSwNP (%) | 99 (63) | 95 (63) | 0.901 | 88 (60) | 0.811 | 70 (60) | >0.999 | 0.705 |
| Blood Eosinophils + | 718 (579) | 88 (43) | <0.0001 | 91 (23) | >0.999 | 90 (31) | >0.999 | <0.0001 |
| OCS dependent (%) | 85 (54) | 31 (21) | <0.0001 | 16 (11) | 0.02 | 7 (6) | 0.19 | <0.001 |
| OCS daily dose ° | 15.0 (11) | 9.8 (10) | 0.022 | 7.6 (9) | 0.891 | 6.3 (4) | 0.933 | 0.046 |
| OCS cumulative yearly dose (g) | 5.8 (4.0) | 3.6 (3.11) | 0.039 | 2.7 (2.7) | 0.867 | 2.2 (1.3) | 0.822 | 0.049 |
| Exacerbations | 3.9 (2.8) | 0.6 (1.2) | <0.0001 | 0.4 (0.9) | 0.656 | 0.2 (0.5) | 0.842 | <0.0001 |
| Hospitalizated patients (%) | 35 (22) | 2 (1.3) | <0.0001 | 1 (0.7) | >0.999 | 1 (0.8) | >0.999 | <0.0001 |
| Hospitalization § | 1.4 (0.5) | 0.02 (0.18) | <0.0001 | 0.006 (0.08) | >0.999 | 0.03 (0.2) | >0.999 | <0.0001 |
| FEV1 % | 70 (33) | 83 (24) | 0.158 | 82 (22) | 0.981 | 84 (20) | 0.933 | 0.206 |
| FEV1 L | 2.21 (1.0) | 2.38 ¥ (1.0) | 0.044 | 2.33 (0.86) | >0.999 | 2.39 (0.9) | >0.999 | 0.078 |
| FeNO | 58 (42) | 34 (18) | <0.0001 | 38 (14) | 0.443 | 35 (18) | 0.718 | <0.0001 |
| ACT | 17 (4) | 23 (2) ¥ | <0.0001 | 23 (2) | 0.898 | 23 (2) | 0.691 | <0.0001 |
| SNOT-22 | 51 (15) | 37 (15) ¥ | 0.0002 | 34 (16) | 0.857 | 26 (14) | 0.05 | <0.0001 |
All data are expressed as mean and SD, where not otherwise specified. + Eosinophils are expressed in cells/mcl. § mean of hospitalized, due to asthma, patients; ° in OCS-dependent patients, expressed as mg of prednisone equivalent. ¥ MCID (Minimal clinically important difference) value exceeded. BMI—Body Mass Index; CRSwNP—Chronic rhinosinusitis with nasal polyps; OCS—Oral corticosteroids; FeNO—Fractioned exhaled nitric oxide; ACT—Asthma Control Test; SNOT-22—Sino nasal outcome test.
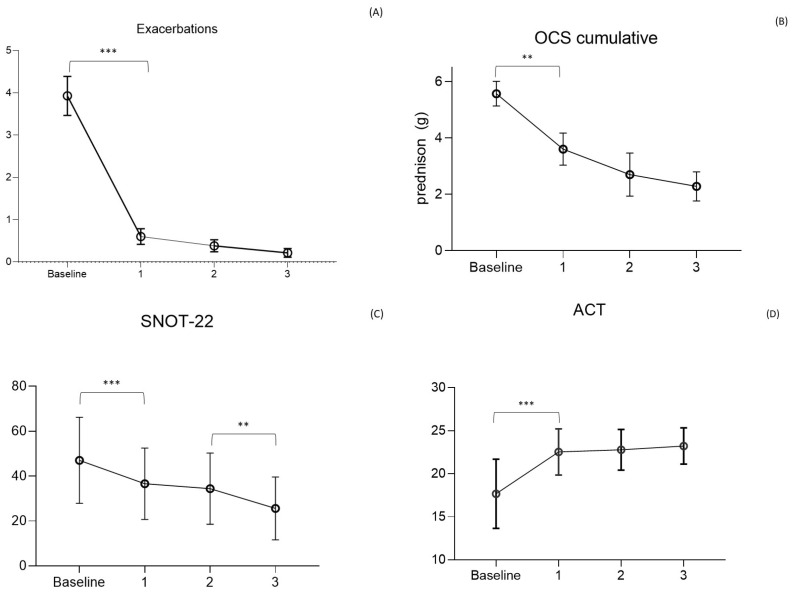
MEP reduced exacerbations as early as the first year, with a decrease from 3.9 ± 2.8/year to 0.6 ± 1.2/year (F = 58.8; p < 0.0001). The reduction continued in subsequent years, with a mean of 0.4 ± 0.9/year and 0.2 ± 0.5/year in the second and third years of observation. No statistical difference was observed between the reduction of exacerbations in the years following the first, highlighting the maintained and prolonged efficacy of the drug over time.
OCS-dependent patients decreased between the beginning of the study and the first year, going from 54% to 21% (p < 0.0001), with a progressive reduction as low as 11% (p = 0.02) and 6%, respectively, at the second and third years. The mean daily dose of OCS in dependent patients decreased from 15 ± 11 mg of prednisone at baseline to 9.8 ± 10 mg at the first year (F = 0.57; p = 0.022), 7.6 ± 9.0 mg at the second, and 6.3 mg/day at the third year. The calculated cumulative dose decreased from 5.8 ± 4.0 g of prednisone-equivalent per year at baseline to 3.6 ± 3.1 g the first year and 2.7 ± 2.7 g and 2.1 ± 1.3 g, respectively, in the second and third years. Over 3 years of observation, the drug allowed the complete discontinuation of steroids in 87% of dependent patients (summing those who discontinued the drug and those who maintained MEP administration), being able to reduce the average daily dose to 6.3 ± 4 mg (Figure 2) in the remaining OCS dependent patients. Lung function tests, measured using FEV1, demonstrate an increase in the value of 170 mL in the first year, overcoming the minimal clinically important difference (MCID) value of 100 mL [29] and keeping it stable in the three years of observation.
Figure 2.
Main outcomes of the study. (A) Asthma exacerbations rate per year (Mean ± SD); (B) Oral corticosteroids (OCS) cumulative dose/y (g prednisolone) in steroid dependent patients; (C) Asthma Control Test (ACT) score; (D) Sinonasal Outcome Test (SNOT)-22 score (mean ± SD). *** p < 0.01; ** p < 0.05.
Asthma control, as per ACT, showed an increase from 17 ± 4 at baseline to 23 ± 2 at the first year of observation (F = 27.16; p < 0.0001) when the value plateaued for the following two years. As for asthma, the control of nasal symptoms was also evaluated, with the SNOT-22 test showing a reduction from a baseline level of 51 ± 15 to 37 ± 15 (F = 0.32; p = 0.0002) in the first year and 34 ± 16 (p = 0.856) and 26 ± 14 (p = 0.05) respectively in the second and third years. The global main results, about asthma exacerbations, OCS cumulative dose, ACT, and SNOT-22 values, are condensed in Figure 2.
Patients who discontinued the therapy over the three-year observation period were 41 (26%), none of them for drug-related adverse events. Discontinuation due to drug inefficacy was recorded in 9 patients (6%), of whom only 4 required systemic steroid therapy with a mean of 5 mg per day. Of the remaining 32 patients, 4 interrupted therapy for inefficacy in nasal symptoms and frequent CRSwNP acute exacerbations, and 28 patients independently decided to suspend the therapy (Figure 1). The patients who requested discontinuation of the drug were all found to be controlled; the main reason for the request for discontinuation was their desire to suspend monthly biologic drug administration given full disease control.
The comparison of our patients with the cohort of the so-called “super responders” described by Kavanagh showed a statistically significant difference in BMI, where RL asthmatics were found to be thinner (25.8 ± 8.8 vs. 8.2 ± 4.5; p = 0.0008) but did not differ in exacerbations at baseline (3.9 ± 2.8 vs. 3.57 ± 2.2; p = 0.142), FEV1 measured in liters (2.21 ± 1.0 vs. 2.10 ± 0.65; p = 0.171), or in the concomitant presence of CRSwNP (63% vs. 67.9%; p = 0.188) (Table 2).
Table 2.
Comparison of the principal super responders characteristics, according to Kavanagh, between the RL cohort, Cosmos and Kavanagh patients.
| RL vs. COSMEX/COSMOS | RL Cohort | Cosmos 651 |
p-Value | Kavanagh 28 |
p-Value (Cosmos vs. Kavanagh) | p-Value (RL vs. Kavanagh) |
|---|---|---|---|---|---|---|
| Exacerbations * | 3.9 (2.8) | 3.67 | 0.305 | 3.57 (2.2) | 0.811 | 0.142 |
| OCS dose ° | 15 (11) | 12.5 | <0.005 | 10 | 0.013 | <0.0001 |
| BMI | 25.8 (8.8) | 28.1 (6.1) | 0.001 | 28.2 (4.5) | 0.907 | 0.0008 |
| FEV1 (L) | 2.21 (1.0) | 1.99 (0.7) | 0.008 | 2.10 (0.65) | 0.378 | 0.171 |
| CRSwNP § | 99 (63%) | 24 (7%) | <0.0001 | 19 (67.9) | <0.001 | 0.188 |
* t-test one sample, ° patients from Sirius; § z-test one proportion. BMI—Body Mass Index; CRSwNP—Chronic rhinosinusitis with nasal polyps; OCS—Oral corticosteroids; ACT—Asthma Control Test.
4. Discussion
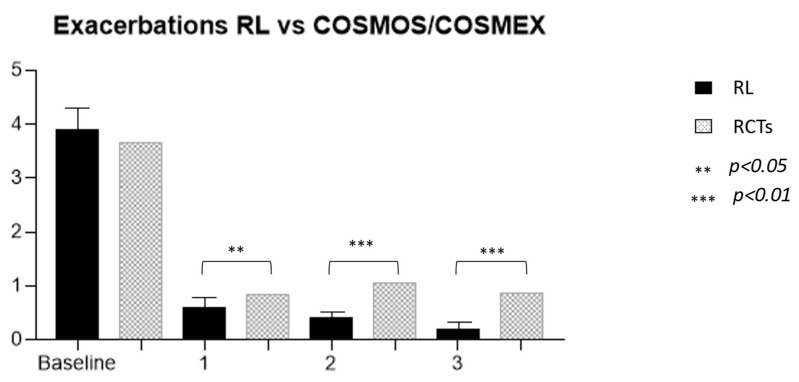
The efficacy of MEP was clearly demonstrated both in RCTs [16,17,30,31] and in RL settings [32]. The prolonged administration of this drug allowed for the observation of its effects in patients treated for longer periods than the limited timeframe of RCTs, which was generally 24–52 weeks. The first observation that can be made from the present data analysis is that not only is the effect of the drug maintained over time but also that the reduction in exacerbation occurrence is progressive with time (Figure 2). These RL data confirm the one found in the extension trials of MEP (COSMEX), which showed a progressive and then maintained reduction of exacerbation rate in treated patients, as well as in those who were previously treated with placebo in the double-blind phase and after that with MEP in the unblinded one [33]. Even in real life, therefore, prolonged disease control is confirmed, which does not vary over time, providing the clinician with a prolonged expectation of efficacy in patients being treated with MEP for asthma. In addition, as already shown in other observational trials, real-life data results are more encouraging than in RCTs [34]. In fact, the cohort of treated patients we observed had a higher reduction in exacerbation rate over years in comparison to the COSMEX/COSMOS trials [33,35] (Figure 3). It is interesting to point out that, although the starting population of the two samples did not have a statistically different mean number of exacerbations (3.9 RL vs. 3.67 RCTs), in later years the reduction was more pronounced and continued to be progressively more pronounced in patients in RL (0.6 vs. 0.85, p = 0.012; 0.4 vs. 1.05, p < 0.0001; 0.2 vs. 0.86, p < 0.0001). The difference found between drug efficacy in real life and in RCTs, in this case COSMOS and COSMEX, could be related to the characteristics of the patients treated with MEP in RL. One of the first aims of biological therapies in asthma is certainly to reduce, or better yet, to discontinue, systemic steroid therapy. If we compare the average prednisone dose in OCS-dependent subjects of our cohort with the one reported by other works [17,36,37,38,39], our patients result in receiving a higher dose of systemic steroids at baseline. The first important observation regards the reduction of steroid-dependent patients, decreasing from 54% at baseline to 6% after 3 years of treatment. Secondarily, the mean daily intake of prednisone equivalent by each patient was reduced by 15 ± 11 to 6.3 ± 4 mg after 3 years. A more precise observation about long-term steroid side effects regards cumulative doses, expressed in grams, allowing to more specifically count not only the intake of dependent patients but also the one of people who use steroids only during exacerbations [8]. In our study, the mean dose of steroids corresponds to a cumulative dose of 5.8 ± 4 g per year. In the study, the dose could be reduced by 38% during the first year in steroid-dependent subjects (21% of the entire cohort compared to baseline), and then continued to be reduced in subsequent years up to a cumulative dose of 2.2 ± 1.3 g/year, in 6% of the cohort. The response to therapy remains constant over time with regard to the effect on exacerbations and respiratory function, already visible in the first year, while the effect on steroid dependence, already significantly reduced after 12 months of therapy, not only persists but progressively improves over the years, reaching discontinuation of OCS in 87% of patients after 3 years. The response to the drug in the observed cohort seems to be even better, compared with that described in randomized clinical trials. In particular, there was a significantly greater reduction in exacerbations since the first year of treatment, and this persisted throughout the duration of the study.
Figure 3.
Comparison of exacerbation rates between our real-life cohort and Cosmos/Cosmex long-term trials.
The efficacy of the drug on the RL cohort, particularly in exacerbation, OCS sparing effect, and disease control, appears to be similar to what is observed in super-responder patients, as described by Kavanagh, and better than what is described in the COSMOS study. Analyzing the characteristics of patients in RL, those in clinical trials, and those defined as super responders, focusing on differences and similarities, we found that a simultaneous presence of CRSwNP and a baseline better respiratory function were present in patients in whom we observed a better response to the drug. Both characteristics are present in our cohort and in the one described by Kavanagh. The presence of both factors, CRSwNP and more conserved respiratory function, seems to be factors able to influence a better response to the drug. Furthermore, the percentage of patients affected by asthma and CRSwNP too turns out to be higher than past publications; in the current analysis, it is 63% vs. 38–40% [40,41].
In addition to being an indicative marker of good response to therapy, CRSwNP also turns out to be a possible target of the drug. Thus, we described what happens to nasal symptoms reported by patients using the SNOT-22 questionnaire in those who have asthma and CRSwNP. What emerged is in line with data from dedicated registry clinical trials for patients with only CRSwNP. The reduction of the mean SNOT-22 values after one year of administration was higher than the minimal clinically important difference for the test, which is set to be 9 points by several authors [42] and 8.9 by others [43]. The impact of the drug not only on asthma but also on CRSwNP turns out to be successful, both from a clinical and pharmacoeconomic point of view. Patients with both of these conditions are known to be most frequently burdened by exacerbations, higher systemic corticosteroid use [32], and consequently higher acute and chronic OCS damage, as well as higher health care expenditures [44].
Our observation in real life would suggest that the definition of super-responder overestimates what happens in real life.
Lastly, the data on respiratory function, although not reaching statistical significance but far exceeding the MCID value, is not only important for the reported outcome of the patient, but in agreement with what other authors have described [45], a marked improvement in FEV1 can be considered an indirect sign of airway remodeling, adding an important feature to this drug.
In conclusion, MEP has been shown to be effective, also in RL and over a long observation period of 3 years, in reducing exacerbations and controlling asthma. The efficacy of MEP was also demonstrated in patients with CRSwNP comorbidity, which is far more prevalent in RL than in RCTs. The effect of the drug also appeared significant on CRSwNP itself, as evidenced by a progressive reduction in SNOT-22 values over the years. According to the recent literature, it can be hypothesized that the treatment, although effective, should be maintained for life [46].
Abbreviations
| ACT | Asthma Control Test |
| BMI | Body mass index |
| CRSwNP | Chronic Rhinosinusitis with nasal polyps |
| FeNO | Fractional exhaled nitric oxide |
| FEV1 | Forced expiratory volume 1 s |
| Ig | Immunoglobulin |
| IL | Interleukin |
| MEP | Mepolizumab |
| OCS | Oral Corticosteroids |
| MCID | Minimal clinically important difference |
| RCT | Randomized controlled trials |
| RL | Real Life |
| SANI | Severe Asthma Network Italy |
| SNOT-22 | Sinonasal Outcome Test |
| T2 | Type 2 |
Appendix A
SANI: C Allegrini, A Antonelli, A Aruanno, E Bacci, B Beghè, M Bonini, M F Caiaffa, C Calabrese, G Camiciottoli, M Cavallini, F Chiecobianchi, M E Conte, A G Corsico, L Cosmi, M T Costantino, G Costanzo, M Crivellaro, S D’Alò, M D’Amato, A Detoraki, M C Di Proietto, N C Facciolongo, S Ferri, V Fierro, M P Foschino, M Latorre, L Macchia, M Montagni, E M Parazzini, R Parente, V Patella, G Pelaia, F Puggioni, L Ricciardi, E Ridolo, J Rolo, N Scichilone, G Scioscia, P Solidoro, G Varricchi, A Vianello, M R Yacoub, B Yang.
Author Contributions
Conceptualization writing—original draft and methodology, D.B.; Original draft and revision, D.B., S.N. and E.T.; Writing, D.B., S.N., E.T. and G.P. (Giovanni Passalacqua); Review and editing, G.P. (Giovanni Passalacqua); Data Curation, L.B., L.P., M.C., F.P., R.F.C., L.M., A.I., L.G., C.L., M.M., F.L., M.R., L.D.F., A.M.R., G.G., M.B., D.F., F.B. (Francesco Balbi), C.C. and G.P. (Giovanni Paoletti); Validation, P.P., F.B. (Francesco Blasi), E.H., G.W.C. and G.S.; Resources, SANI. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) Genoa (year 2017, ID 3663).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are unavailable due to privacy.
Conflicts of Interest
Authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chiu C.-J., Huang M.-T. Asthma in the Precision Medicine Era: Biologics and Probiotics. Int. J. Mol. Sci. 2021;22:4528. doi: 10.3390/ijms22094528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung K.F., Wenzel S.E., Brozek J.L., Bush A., Castro M., Sterk P.J., Adcock I.M., Bateman E.D., Bel E.H., Bleecker E.R., et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2013;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 3.Kyriakopoulos C., Gogali A., Bartziokas K., Kostikas K. Identification and treatment of T2-low asthma in the era of biologics. ERJ Open Res. 2021;7 doi: 10.1183/23120541.00309-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonato M., Bazzan E., Snijders D., Turato G., Turrin M., Cosio M.G., Barbato A., Saetta M., Baraldo S. Innate Lymphocytes -ILC2—Might Be the Drivers of T2-High Nonatopic Asthma in Children. Eur. Respir. J. 2021;58:PA861. doi: 10.1183/13993003.CONGRESS-2021.PA861. [DOI] [Google Scholar]
- 5.Fahy J.V. Type 2 Inflammation in Asthma-Present in Most, Absent in Many. Nat. Rev. Immunol. 2015;15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodruff P.G., Modrek B., Choy D.F., Jia G., Abbas A.R., Ellwanger A., Arron J.R., Koth L.L., Fahy J.V. T-helper Type 2–driven Inflammation Defines Major Subphenotypes of Asthma. Am. J. Respir. Crit. Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voorham J., Xu X., Price D.B., Golam S., Davis J., Ling J.Z.J., Kerkhof M., Ow M., Tran T.N. Healthcare Resource Utilization and Costs Associated with Incremental Systemic Corticosteroid Exposure in Asthma. Allergy. 2019;74:273–283. doi: 10.1111/all.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price D.B., Trudo F., Voorham J., Xu X., Kerkhof M., Jie J.L.Z., Tran T.N. Adverse Outcomes from Initiation of Systemic Corticosteroids for Asthma: Long-Term Observational Study. J. Asthma Allergy. 2018;11:193–204. doi: 10.2147/JAA.S176026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGregor M.C., Krings J.G., Nair P., Castro M. Role of Biologics in Asthma. Am. J. Respir. Crit. Care Med. 2019;199:433–445. doi: 10.1164/rccm.201810-1944CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agache I., Beltran J., Akdis C., Akdis M., Canelo-Aybar C., Canonica G.W., Casale T., Chivato T., Corren J., del Giacco S., et al. Efficacy and Safety of Treatment with Biologicals (Benralizumab, Dupilumab, Mepolizumab, Omalizumab and Reslizumab) for Severe Eosinophilic Asthma. A Systematic Review for the EAACI Guidelines—Recommendations on the Use of Biologicals in Severe Asthma. Allergy. 2020;75:1023–1042. doi: 10.1111/all.14221. [DOI] [PubMed] [Google Scholar]
- 11.Menzella F., Lusuardi M., Galeone C., Taddei S., Zucchi L. Profile of Anti-IL-5 MAb Mepolizumab in the Treatment of Severe Refractory Asthma and Hypereosinophilic Diseases. J. Asthma Allergy. 2015;8:105–114. doi: 10.2147/JAA.S40244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azim A., Pini L., Khakwani Z., Kumar S., Howarth P. Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Those on Mepolizumab Therapy. Ann. Allergy Asthma Immunol. 2021;126:438–440. doi: 10.1016/j.anai.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenfeder S., Umland S.P., Cuss F.M., Chapman R.W., Egan R.W. Th2 Cytokines and Asthma—The Role of Interleukin-5 in Allergic Eosinophilic Disease. Respir. Res. 2001;2:71. doi: 10.1186/rr41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potaczek D.P., Miethe S., Schindler V., Alhamdan F., Garn H. Role of Airway Epithelial Cells in the Development of Different Asthma Phenotypes. Cell. Signal. 2020;69:109523. doi: 10.1016/j.cellsig.2019.109523. [DOI] [PubMed] [Google Scholar]
- 15.Potaczek D.P., Harb H., Michel S., Alhamwe B.A., Renz H., Tost J. Epigenetics and Allergy: From Basic Mechanisms to Clinical Applications. Epigenomics. 2017;9:539–571. doi: 10.2217/epi-2016-0162. [DOI] [PubMed] [Google Scholar]
- 16.Ortega H.G., Liu M.C., Pavord I.D., Brusselle G.G., FitzGerald J.M., Chetta A., Humbert M., Katz L.E., Keene O.N., Yancey S.W., et al. Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma. N. Engl. J. Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 17.Bel E.H., Wenzel S.E., Thompson P.J., Prazma C.M., Keene O.N., Yancey S.W., Ortega H.G., Pavord I.D. Oral Glucocorticoid-Sparing Effect of Mepolizumab in Eosinophilic Asthma. N. Engl. J. Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 18.Pavord I.D., Korn S., Howarth P., Bleecker E.R., Buhl R., Keene O.N., Ortega H., Chanez P. Mepolizumab for Severe Eosinophilic Asthma (DREAM): A Multicentre, Double-Blind, Placebo-Controlled Trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 19.Matucci A., Vivarelli E., Bormioli S., Francesca N., Chiccoli F., Valentina M., Francesca G., Oliviero R., Parronchi P., Vultaggio A. Long-term retention rate of mepolizumab treatment in severe asthma: A 36-months real-life experience. J. Asthma. 2022;60:158–166. doi: 10.1080/02770903.2022.2036754. [DOI] [PubMed] [Google Scholar]
- 20.Bagnasco D., Massolo A., Bonavia M., Brussino L., Bucca C., Caminati M., Canonica G.W., Caruso C., D’Amato M., de Ferrari L., et al. The Importance of Being Not Significant: Blood Eosinophils and Clinical Responses Do Not Correlate in Severe Asthma Patients Treated with Mepolizumab in Real Life. Allergy. 2019;75:1460–1463. doi: 10.1111/all.14135. [DOI] [PubMed] [Google Scholar]
- 21.Bagnasco D., Menzella F., Caminati M., Caruso C., Guida G., Bonavia M., Riccio A., Milanese M., Manfredi A., Senna G., et al. Efficacy of Mepolizumab in Patients with Previous Omalizumab Treatment Failure: Real-Life Observation. Allergy Eur. J. Allergy Clin. Immunol. 2019;74:2539–2541. doi: 10.1111/all.13937. [DOI] [PubMed] [Google Scholar]
- 22.Bagnasco D., Povero M., Pradelli L., Brussino L., Rolla G., Caminati M., Menzella F., Heffler E., Canonica G.W., Paggiaro P., et al. Economic Impact of Mepolizumab in Uncontrolled Severe Eosinophilic Asthma, in Real Life. World Allergy Organ. J. 2021;14:100509. doi: 10.1016/j.waojou.2021.100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pharmacoeconomic Review Report—Mepolizumab (Nucala)—NCBI Bookshelf. [(accessed on 21 April 2022)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK409872/
- 24.Detoraki A., Tremante E., D’Amato M., Calabrese C., Casella C., Maniscalco M., Poto R., Brancaccio R., Boccia M., Martino M., et al. Mepolizumab Improves Sino-Nasal Symptoms and Asthma Control in Severe Eosinophilic Asthma Patients with Chronic Rhinosinusitis and Nasal Polyps: A 12-Month Real-Life Study. Ther. Adv. Respir. Dis. 2021;15:17534666211009398. doi: 10.1177/17534666211009398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kassem F., Cohen-Confino R., Meir-Shafrir K., Lachover-Roth I., Cohen-Engler A., Nageris B., Rosman Y. Mepolizumab for Eosinophilic Chronic Sinusitis with Nasal Polyposis: Real-Life Experience. Rhinology. 2021;59:110–112. doi: 10.4193/Rhin20.416. [DOI] [PubMed] [Google Scholar]
- 26.Blonde L., Khunti K., Harris S.B., Meizinger C., Skolnik N.S. Interpretation and Impact of Real-World Clinical Data for the Practicing Clinician. Adv. Ther. 2018;35:1763–1774. doi: 10.1007/s12325-018-0805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heffler E., Blasi F., Latorre M., Menzella F., Paggiaro P., Pelaia G., Senna G., Canonica G.W., Barbuto S., Bradicich M., et al. The Severe Asthma Network in Italy: Findings and Perspectives. J. Allergy Clin. Immunol. Pract. 2019;7:1462–1468. doi: 10.1016/j.jaip.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Kavanagh J.E., d’Ancona G., Elstad M., Green L., Fernandes M., Thomson L., Roxas C., Dhariwal J., Nanzer A.M., Kent B.D., et al. Real-World Effectiveness and the Characteristics of a “Super-Responder” to Mepolizumab in Severe Eosinophilic Asthma. Chest. 2020;158:491–500. doi: 10.1016/j.chest.2020.03.042. [DOI] [PubMed] [Google Scholar]
- 29.Bonini M., di Paolo M., Bagnasco D., Baiardini I., Braido F., Caminati M., Carpagnano E., Contoli M., Corsico A., del Giacco S., et al. Minimal Clinically Important Difference for Asthma Endpoints: An Expert Consensus Report. Eur. Respir. Rev. 2020;29:190137. doi: 10.1183/16000617.0137-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haldar P., Brightling C.E., Hargadon B., Gupta S., Monteiro W., Sousa A., Marshall R.P., Bradding P., Green R.H., WardIaw A.J., et al. Mepolizumab and Exacerbations of Refractory Eosinophilic Asthma. N. Engl. J. Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nair P., Pizzichini M.M.M., Kjarsgaard M., Inman M.D., Efthimiadis A., Pizzichini E., Hargreave F.E., O’Byrne P.M. Mepolizumab for Prednisone-Dependent Asthma with Sputum Eosinophilia. N. Engl. J. Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 32.Israel E., Canonica G.W., Brusselle G., Yang S., Howarth P.H., Martin A.L., Koufopoulou M., Smith S.G., Alfonso-Cristancho R. Real-Life Effectiveness of Mepolizumab in Severe Asthma: A Systematic Literature Review. J. Asthma. 2022;59:2201–2217. doi: 10.1080/02770903.2021.2008431. [DOI] [PubMed] [Google Scholar]
- 33.Khurana S., Brusselle G.G., Bel E.H., FitzGerald J.M., Masoli M., Korn S., Kato M., Albers F.C., Bradford E.S., Gilson M.J., et al. Long-Term Safety and Clinical Benefit of Mepolizumab in Patients with the Most Severe Eosinophilic Asthma: The COSMEX Study. Clin. Ther. 2019;41:2041–2056.e5. doi: 10.1016/j.clinthera.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Bagnasco D., Milanese M., Rolla G., Lombardi C., Bucca C., Heffler E., Canonica G.W., Passalacqua G. The North-Western Italian Experience with Anti IL-5 Therapy Amd Comparison with Regulatory Trials. World Allergy Organ. J. 2018;11:34. doi: 10.1186/s40413-018-0210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lugogo N., Domingo C., Chanez P., Leigh R., Gilson M.J., Price R.G., Yancey S.W., Ortega H.G. Long-Term Efficacy and Safety of Mepolizumab in Patients with Severe Eosinophilic Asthma: A Multi-Center, Open-Label, Phase IIIb Study. Clin. Ther. 2016;38:2058–2070.e1. doi: 10.1016/j.clinthera.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Bagnasco D., Caminati M., Menzella F., Milanese M., Rolla G., Lombardi C., Bucca C., Heffler E., Paoletti G., Testino E., et al. One Year of Mepolizumab. Efficacy and Safety in Real-Life in Italy. Pulm. Pharmacol. Ther. 2019;58:101836. doi: 10.1016/j.pupt.2019.101836. [DOI] [PubMed] [Google Scholar]
- 37.Pini L., Caruso C., Colantuono S., Bagnasco D., Maxwell A., Price R.G., Howarth P., Canonica G.W. Prospective Italian Real-World Study of Mepolizumab in Severe Eosinophilic Asthma Validates Retrospective Outcome Reports. Clin. Transl. Allergy. 2021;11:e12067. doi: 10.1002/clt2.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelaia C., Busceti M.T., Solinas S., Terracciano R., Pelaia G. Real-Life Evaluation of the Clinical, Functional, and Hematological Effects of Mepolizumab in Patients with Severe Eosinophilic Asthma: Results of a Single-Centre Observational Study. Pulm. Pharmacol. Ther. 2018;53:1–5. doi: 10.1016/j.pupt.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Pilette C., Canonica G.W., Chaudhuri R., Chupp G., Lee F.E.-H., Lee J.K., Almonacid C., Welte T., Alfonso-Cristancho R., Jakes R.W., et al. REALITI-A Study: Real-World Oral Corticosteroid-Sparing Effect of Mepolizumab in Severe Asthma. J. Allergy Clin. Immunol. Pract. 2022;10:2646–2656. doi: 10.1016/j.jaip.2022.05.042. [DOI] [PubMed] [Google Scholar]
- 40.Canonica G.W., Malvezzi L., Blasi F., Paggiaro P., Mantero M., Senna G., Heffler E., Bonavia M., Caiaffa P., Calabrese C., et al. Chronic Rhinosinusitis with Nasal Polyps Impact in Severe Asthma Patients: Evidences from the Severe Asthma Network Italy (SANI) Registry. Respir. Med. 2020;166:105947. doi: 10.1016/j.rmed.2020.105947. [DOI] [PubMed] [Google Scholar]
- 41.Harrison T., Canonica G.W., Chupp G., Lee J., Schleich F., Welte T., Valero A., Gemzoe K., Maxwell A., Joksaite S., et al. Real-World Mepolizumab in the Prospective Severe Asthma REALITI-A Study: Initial Analysis. Eur. Respir. J. 2020;56:2000151. doi: 10.1183/13993003.00151-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chowdhury N.I., Mace J.C., Bodner T.E., Alt J.A., Deconde A.S., Levy J.M., Smith T.L. Investigating the Minimal Clinically Important Difference for SNOT-22 Symptom Domains in Surgically Managed Chronic Rhinosinusitis. Int. Forum Allergy Rhinol. 2017;7:1149–1155. doi: 10.1002/alr.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hopkins C., Gillett S., Slack R., Lund V.J., Browne J.P. Psychometric Validity of the 22-Item Sinonasal Outcome Test. Clin. Otolaryngol. 2009;34:447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 44.Canonica G.W., Colombo G.L., Bruno G.M., di Matteo S., Martinotti C., Blasi F., Bucca C., Crimi N., Paggiaro P., Pelaia G., et al. Shadow Cost of Oral Corticosteroids-Related Adverse Events: A Pharmacoeconomic Evaluation Applied to Real-Life Data from the Severe Asthma Network in Italy (SANI) Registry. World Allergy Organ. J. 2019;12:100007. doi: 10.1016/j.waojou.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varricchi G., Ferri S., Pepys J., Poto R., Spadaro G., Nappi E., Paoletti G., Virchow J.C., Heffler E., Canonica W.G. Biologics and Airway Remodeling in Severe Asthma. Allergy. 2022;77:3538–3552. doi: 10.1111/all.15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore W.C., Kornmann O., Humbert M., Poirier C., Bel E.H., Kaneko N., Smith S.G., Martin N., Gilson M.J., Price R.G., et al. Stopping versus Continuing Long-Term Mepolizumab Treatment in Severe Eosinophilic Asthma (COMET Study) Eur. Respir. J. 2022;59:2100396. doi: 10.1183/13993003.00396-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are unavailable due to privacy.