Abstract
BACKGROUND
On-pump cardiac surgery triggers sterile inflammation and postoperative complications such as postoperative atrial fibrillation (POAF). Hematopoietic somatic mosaicism (HSM) is a recently identified risk factor for cardiovascular diseases and results in a shift toward a chronic proinflammatory monocyte transcriptome and phenotype.
OBJECTIVES
The aim of this study was to assess the prevalence, characteristics, and impact of HSM on preoperative blood and myocardial myeloid cells as well as on outcomes after cardiac surgery.
METHODS
Blood DNA from 104 patients referred for surgical aortic valve replacement (AVR) was genotyped using the HemePACT panel (576 genes). Four screening methods were applied to assess HSM, and postoperative outcomes were explored. In-depth blood and myocardial leukocyte phenotyping was performed in selected patients using mass cytometry and preoperative and postoperative RNA sequencing analysis of classical monocytes.
RESULTS
The prevalence of HSM in the patient cohort ranged from 29%, when considering the conventional HSM panel (97 genes) with variant allelic frequencies ≥2%, to 60% when considering the full HemePACT panel and variant allelic frequencies ≥1%. Three of 4 explored HSM definitions were significantly associated with higher risk for POAF. On the basis of the most inclusive definition, HSM carriers exhibited a 3.5-fold higher risk for POAF (age-adjusted OR: 3.5; 95% CI: 1.52–8.03; P= 0.003) and an exaggerated inflammatory response following AVR. HSM carriers presented higher levels of activated CD64+CD14+CD16− circulating monocytes and inflammatory monocyte-derived macrophages in presurgery myocardium.
CONCLUSIONS
HSM is frequent in candidates for AVR, is associated with an enrichment of proinflammatory cardiac monocyte–derived macrophages, and predisposes to a higher incidence of POAF. HSM assessment may be useful in the personalized management of patients in the perioperative period. (Post-Operative Myocardial Incident & Atrial Fibrillation [POMI-AF]; NCT03376165)
Keywords: cardiac surgery, clonal hematopoiesis, inflammation, postoperative atrial fibrillation, somatic mosaicism
Cardiac surgery is among the most common major surgical procedures performed worldwide. Despite the emergence of transcatheter interventions, such as transaortic valve replacement and percutaneous edge-to-edge transcatheter mitral valve repair, the number of patients undergoing cardiac surgery is still significant because of the aging population and the rising prevalence of associated comorbidities.1 These high-risk elderly patients are more prone to perioperative complications such as acute hemodynamic instability, type 5 myocardial infarction (MI), postoperative atrial fibrillation (POAF) and acute kidney injury (AKI), resulting in worse clinical outcomes.2,3
On-pump cardiac surgery triggers sterile systemic and local innate inflammatory responses.4 This inflammatory response, which peaks 1 to 3 days after surgery, stems mainly from blood coming in direct contact with the cardiopulmonary bypass tubing system and from the organ reperfusion injury (eg, the heart), leading to subsequent release of danger-associated molecular pattern molecules, complement activation, and direct surgical trauma.5 Its intensity is highly variable among patients and remains unpredictable. Although evidence suggests that perioperative inflammation may be causally related to POAF, previous attempts to dampen the inflammatory response showed mitigated results on clinical outcomes.6
Aging is associated with accumulating postzygotic mutations due to errors in the repair or replication of damaged DNA, leading to genetic heterogeneity within tissues called somatic mosaicism.7 This age-related somatic mosaicism has been characterized largely in circulating leukocytes, with emphasis on genes previously found to be involved in leukemogenesis. Furthermore, low-frequency mutations in a selection of hematopoietic somatic mosaicism (HSM) genes, termed “clonal hematopoiesis of indeterminate potential” (CHIP) genes, have been recently associated with adverse clinical outcomes such as coronary artery disease, chronic heart failure, incident atrial fibrillation (AF), and mortality following percutaneous aortic valve replacement (AVR).8–13 Importantly, studies associating HSM with clinical outcomes used different screening methods with varying sequencing depths, gene panels, and/or variant allelic frequency (VAF) thresholds, resulting in a significant heterogeneity in HSM definition (ie, gene number encompassed by the panel and VAF threshold). Although not completely understood, the mechanisms linking HSM to adverse cardiovascular outcomes are believed to result from a proinflammatory immune shift, which was characterized mainly in the context of DNMT3A and TET2 mutations.14–16
In this study, we assessed HSM frequency, defined using different criteria, in a population of patients undergoing cardiac surgery. We then determined whether HSM presence increases (preoperative) myeloid cell activation and myocardial recruitment and affects the postoperative inflammatory response and outcomes after surgery.
METHODS
COHORT AND PREOPERATIVE CLINICAL ASSESSMENT.
The clinical cohort is part of the POMI-AF (Post-Operative Myocardial Incident & Atrial Fibrillation; NCT03376165) study, approved by the institutional ethics committee (Comité de Protection des Personnes Ile de France V). Written informed consent was obtained from all patients before inclusion.
Patients (≥18 years of age) were referred to the cardiovascular surgery department at Lille University Hospital for AVR (with or without coronary artery bypass grafting) because of severe aortic valve stenosis. The day before surgery, the medical history of each patient was collected, and an electrocardiogram was recorded to detect potential existing AF at baseline.
A total of 442 candidates for cardiac surgery were included in the POMI-AF study from February 2017 to April 2019. Among 176 patients scheduled for AVR, we excluded patients with histories of AF or who developed postoperative infection (defined as all clinical conditions leading to antibiotic prescription within the first week following surgery) to avoid infection-induced interference with HSM-dependent inflammatory responses, resulting in a total of 133 patients (Supplemental Figure 1). As specified in the statistical power calculation, the study was conducted until 52 patients presenting POAF were enrolled. Because POAF patients were globally older (Supplemental Table 1), we selected 52 patients without POAF with a comparable distribution of predefined variables (age, body mass index [BMI], diabetic status, and gender). Further details regarding the patient selection process are available in the Supplemental Appendix. In these 104 selected patients, a large panel of 576 genes were genotyped for HSM (HemePACT panel) (Supplemental Table 2).
HSM SCREENING STRATEGIES.
To assess the correlation of HSM with age and clinical outcomes, 4 different definitions were adopted. These definitions are based upon different gene sets reported in previous studies, which included: 1) HSM defined according to a list of 97 genes previously screened in studies assessing the association between CHIP and cardiovascular outcomes (conventional CHIP panel) (Supplemental Table 3)9,12,17; and 2) HSM defined according to a large oncogenic panel including 576 genes previously associated with hematological malignancies, clonal hematopoiesis, and solid tumors (HemePACT panel) (Supplemental Table 2).
For each gene set, according to previous studies, 2 VAF thresholds were assessed (2% and 1%) to evaluate the relevance of low VAF and its correlations with age and POAF.
Thus, 4 definitions for HSM were determined on the basis of these 2 combined criteria: 1) conventional CHIP panel, VAF ≥2%; 2) conventional CHIP panel, VAF ≥1%; 3) HemePACT panel, VAF ≥2%; and 4) HemePACT panel, VAF ≥1%.
Prevalence of HSM was reported according to each definition, as well as its association with age and risk for POAF. HSM as defined by the HemePACT with VAF ≥1% was used as the reference for exploring its impact on preoperative and postoperative immunoinflammation.
STATISTICAL ANALYSES OF CLINICAL OUTCOMES.
Continuous variables were tested for normality with the Shapiro test. Continuous variables with Gaussian distributions are expressed as mean ± SD. Continuous variables with non-Gaussian distributions are expressed as median (IQR). Categorical variables are expressed as percentages of individuals. Bivariate comparisons were performed using Student’s t-test for normally distributed continuous variables or the Mann-Whitney U test for variables not normally distributed. Bivariate comparisons of categorical variables were done using the chi-square test.
A backward logistic regression model was used to explore predictors of POAF, AKI, and type 5 MI and included HSM status and prespecified confounders previously associated with these outcomes (age, BMI, diabetes, and extracorporeal circulation duration). For the backward logistic regression model, candidate variables were removed when P > 0.20. A backward multiple regression model was performed for postoperative C-reactive protein (CRP) peak, and postoperative troponin peak analysis and included HSM status, age, BMI, diabetes, extracorporeal circulation duration, and POAF. Normality for residuals of multiple regression models was assessed using the Shapiro-Wilk test. All analyses were done using MedCalc version 16.4 (MedCalc Software).
Extended methods regarding postoperative outcome assessment, sample size calculation, DNA sequencing, mass cytometry, and RNA sequencing analysis are provided in the Supplemental Appendix.
RESULTS
HSM IS HIGHLY PREVALENT IN PATIENTS UNDERGOING CARDIAC SURGERY.
HSM was assessed using deep targeted DNA sequencing of leukocytes from 104 patients (52 with POAF and 52 in sinus rhythm) elected for AVR using a large panel of 576 genes (HemePACT panel) (Supplemental Table 2). The median age of the 104 patients was 70 years (IQR: 66–75 years), 36% were women, and 29% had type 2 diabetes mellitus. As a result of the patient selection process, patients with POAF and those in sinus rhythm had comparable distributions regarding age, BMI, diabetic status, and sex (Supplemental Table 1).
Analysis based on the 4 HSM definitions, differing in the number of included genes and VAF threshold (Figure 1A), resulted in an overall high prevalence of HSM, ranging from 29% to 60% of patients (29% for the conventional CHIP panel with VAF ≥2%, 45% for the conventional CHIP panel with VAF ≥1%, 44% for the HemePACT panel with VAF ≥2%, and 60% for the HemePACT panel with VAF ≥1%) (Figure 1B). Advanced age was positively associated with HSM irrespective of its definition (Figure 1C).
FIGURE 1. Prevalence of HSM According to Screening Strategies and Association With POAF.
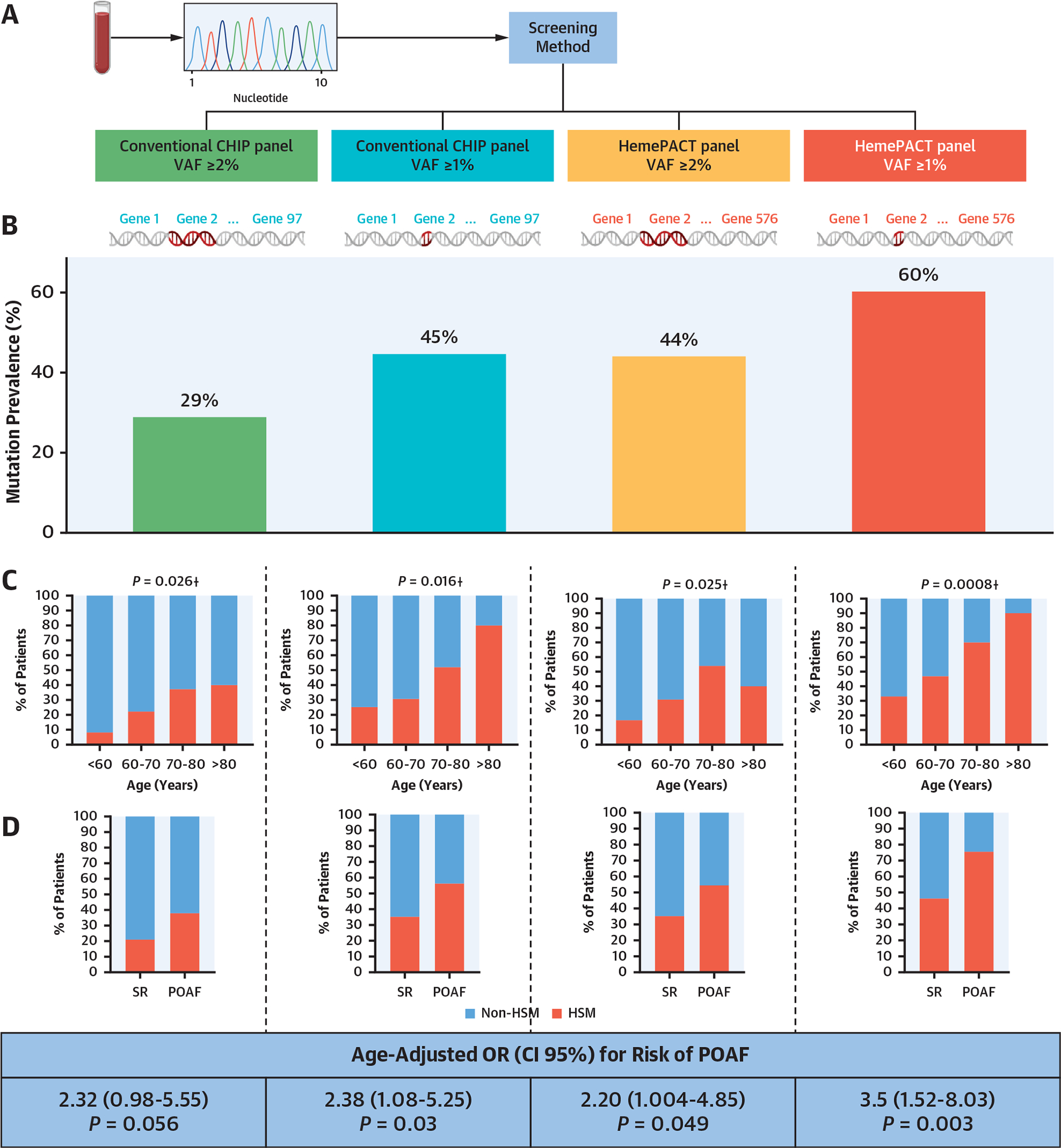
(A) Four screening strategies were applied to detect hematopoietic mosaicism (HSM): conventional clonal hematopoiesis of indeterminate potential (CHIP) panel with variant allelic frequency (VAF) ≥2%, conventional CHIP panel with VAF ≥1%, HemePACT panel with VAF ≥2%, and HemePACT panel with VAF ≥1%. (B) Prevalence of HSM according to each screening method. (C) Prevalence of HSM according to age and screening method. †Chi-square for trend. (D) Association between postoperative atrial fibrillation (POAF) and HSM according to screening method. (E) Proportion of patients with multiple mutation among mutation carriers. (F) Venn diagram showing the mutated genes in patients in sinus rhythm (SR) and those with POAF. Numbers in parentheses indicate the number of patients carrying at least 1 mutation for each gene. For genes mutated in both patients in SR and those with POAF, numbers in parentheses are the number of patients in SR carrying at least 1 mutation and the number of patients with POAF carrying at least 1 mutation for each gene.
Using the HemePACT panel with VAF ≥1%, the most inclusive definition, most mutations consisted of single-nucleotide variants resulting in missense mutations (Supplemental Figure 2A and 2B). As previously reported, the mutations frequently affected genes encoding epigenetic regulators and DNA damage response proteins, such as DNMT3A in 17% of patients (26% of mutations) and TET2 in 11% of patients (13% of mutations) (Supplemental Figure 2C, Supplemental Table 4). Additional mutations were found in several genes associated with the DNA damage response, such as ATM and SF3B1 (in 7% and 3% of patients) (Figure 1F). Thirty-two percent of patients with mutations had multiple mutated genes (Figure 1E). VAFs ranged from 1.1% to 33.9%, with marked intergene heterogeneity (Supplemental Figure 2D). Moreover, as previously shown, the number of mutations per patient increased with age (Supplemental Figure 2E). This indicates that HSM is frequent in patients scheduled for cardiac surgery, which is in line with their elevated age.
HSM IDENTIFIES PATIENTS AT RISK FOR POAF IRRESPECTIVE OF OTHER COMORBIDITIES.
HSM carriers, as defined by the HemePACT panel with VAF ≥1%, were older than non-HSM carriers but did not display other significant differences regarding clinical preoperative features (Supplemental Table 5). Importantly, these HSM carriers had a 3.5-fold higher risk for POAF even after adjustment for age (age-adjusted OR: 3.5; 95% CI: 1.52–8.03; P = 0.003). This association between HSM and POAF was found for all 4 HSM definitions (Figure 1D), although it was only borderline significant with the conventional CHIP panel with VAF ≥2%.
The association of HSM with POAF onset was independent not only of age but also of BMI, diabetes status, and extracorporeal circulation duration (Supplemental Tables 6 and 7). HSM status was not associated with the occurrence of other early complications such as AKI and type 5 MI.
HSM CARRIERS DISPLAY PREOPERATIVE ALTERATIONS IN PERIPHERAL BLOOD MONOCYTES AND MYOCARDIAL MACROPHAGES AND AN EXACERBATED POSTOPERATIVE INFLAMMATORY RESPONSE.
Both HSM and POAF have been previously associated with inflammation; therefore the impact of HSM on postoperative inflammation and leukocytes was assessed.
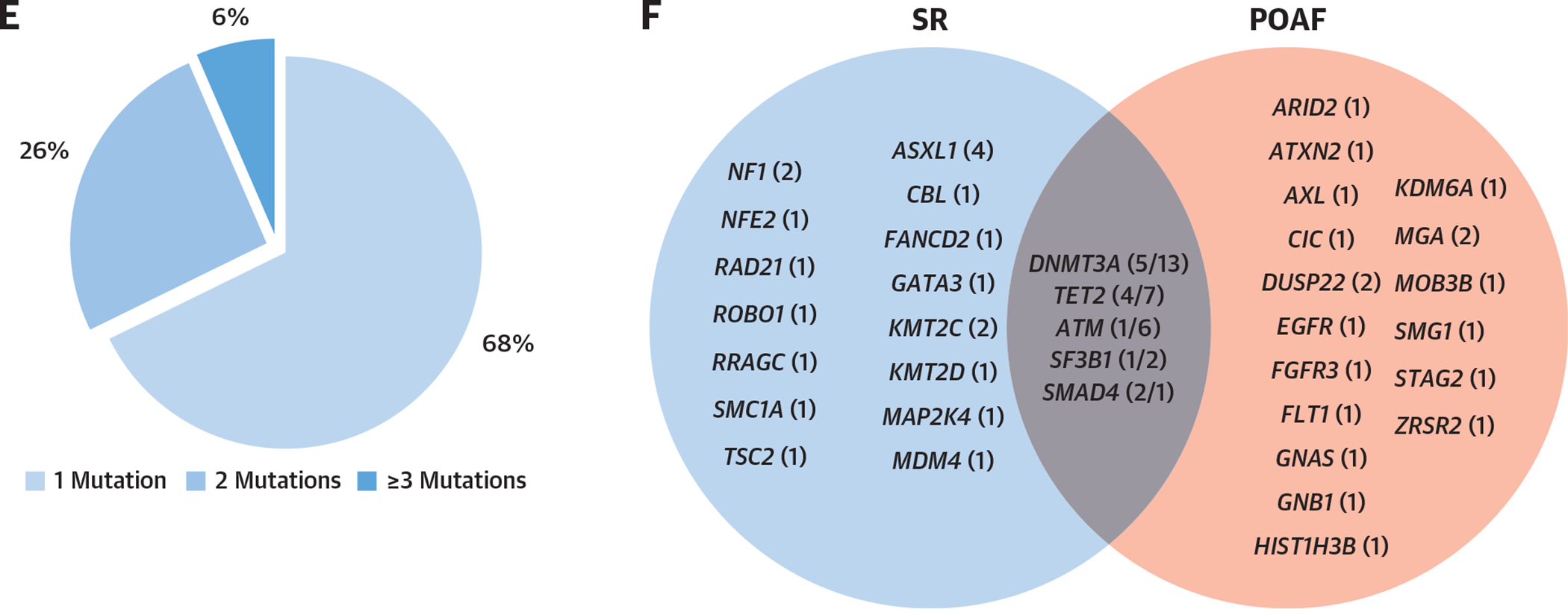
Despite normal preoperative blood CRP levels, HSM carriers had a slightly higher preoperative number of peripheral blood leukocytes because of an increase in monocytes (Supplemental Table 5). Moreover, HSM carriers had a higher inflammatory response following AVR, as measured by the postoperative CRP peak within 72 hours (148 ± 60 mg/L for non-HSM vs 200 ± 88 mg/L for HSM; P = 0.002) (Figure 2A). The association of HSM with higher postoperative inflammation remained significant after adjustment for age, BMI, diabetes, and extracorporeal circulation duration. Interestingly, both POAF and HSM were independently associated with the postoperative CRP peak (Supplemental Table 8).
FIGURE 2. Postoperative Inflammatory Response and Preoperative High-Dimensional Peripheral Blood Mononuclear Cells Immune Phenotype.
(A) Postoperative C-reactive protein (CRP) peak (within 72 hours) according to hematopoietic mosaicism (HSM). (B) Unsupervised clustering map of peripheral blood mononuclear cells with general populations and 37 individual clusters. (C) Heatmap representing characteristics of each identified cluster regarding the intensity of expression of each marker. (D) Volcano plot representing differences in each cluster according to HSM status. (E) Violin plots representing significantly dysregulated clusters according to HSM. Cluster 1: 62,432 (IQR: 30,600–168,688) cells/mL for non-HSM vs 148,535 (IQR: 117,895–211,597) cells/mL for HSM (false discovery rate [FDR] < 0.001); cluster 2: 62,197 (IQR: 54,074–122,329) cells/mL for non-HSM vs 130,048 (IQR: 52,188–259,142) cells/mL for HSM (FDR < 0.001); cluster 11: 39,904 (IQR: 28,766–71,982) cells/mL for non-HSM vs 96,683 (IQR: 63,700–149,464) cells/mL for HSM (FDR = 0.04). HSM was defined according to the HemePACT panel with variant allelic frequency ≥1%.
To gain insight into the exacerbated inflammatory response following surgery and the increased POAF risk of HSM carriers, 2 subgroups of patients were selected for further detailed exploration of the preoperative phenotype of peripheral blood and myocardial leukocytes. The immune phenotype of circulating leukocytes was assessed using in-depth unsupervised mass cytometry analysis of peripheral blood mononuclear cell samples from 17 patients (11 with HSM and 6 non-HSM carriers) with myocardial tissue available (Supplemental Tables 9 to 12). Unsupervised clustering revealed 37 clusters of peripheral blood leukocytes (Figures 2B and 2C), of which 3 were significantly higher in patients with HSM (Figures 2D and 2E). Two clusters (1 and 11) contained CD14+CD16− monocyte subsets expressing CD64/FcγRIα, the high-affinity immunoglobulin G receptor associated with activation and inflammation, and 1 cluster related to a CD4+ T cell subset (cluster 2) (Figure 2C).
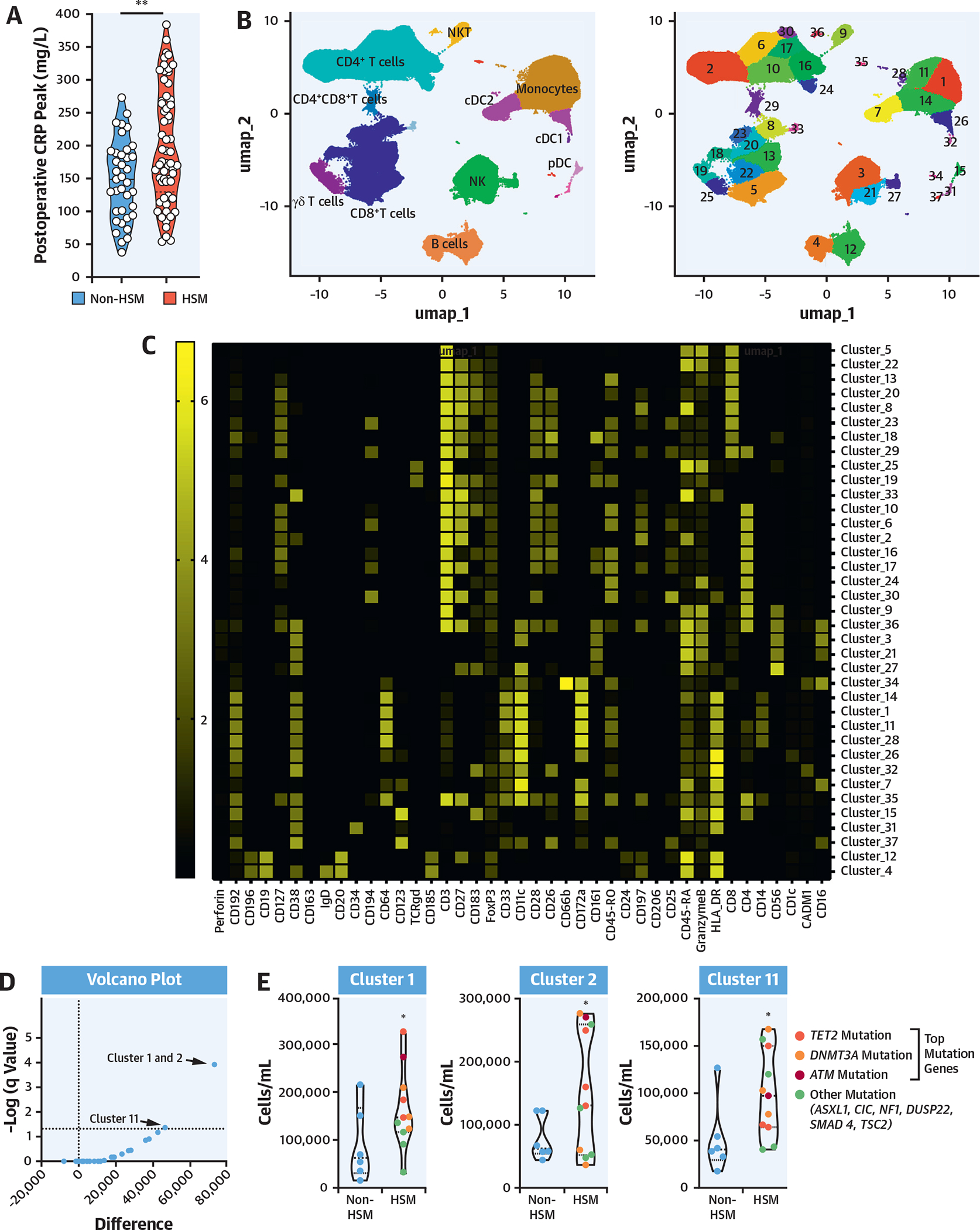
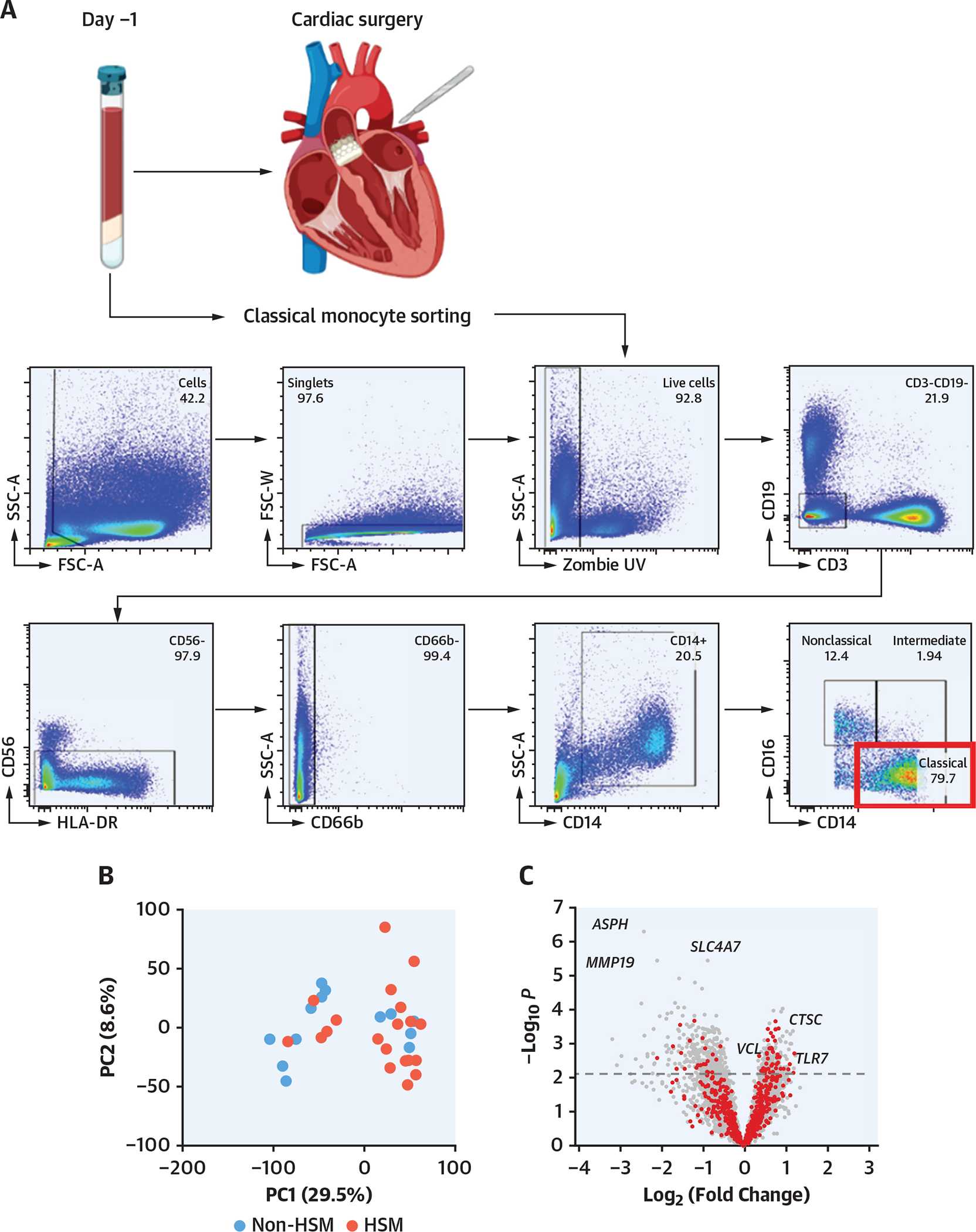
As patients with HSM displayed alterations in preoperative peripheral blood mononuclear cell subsets (Figure 2E), including increased monocytes, as well as an increased postoperative inflammatory response (Figure 2A), and because classical monocytes are known to be associated with surgery-induced inflammation, RNA sequencing transcriptomic analysis was performed on sorted preoperative classical CD14++CD16− monocytes (Figure 3A) from 20 HSM and 13 non-HSM carriers (Supplemental Tables 13 and 14). Principal component analysis of differentially expressed genes identified 2 major clusters segregating most HSM carriers from noncarriers (Figure 3B). Volcano plot analysis showed that a larger proportion of genes were down-regulated in monocytes from HSM carriers (327 vs 185 genes up-regulated, on the basis of false discovery rate <0.1) (Figure 3C). None of the differentially expressed genes was overtly related to classical inflammation, such as IL-1β, IL-6, or TNF. Yet pathway analysis revealed that several pathways were differentially expressed in HSM vs non-HSM carriers. These pathways corresponded to myeloid leukocyte activation, regulation of catabolic processes and autophagy, histone H3K4 demethylation, apoptosis processes, endosomal transport, and TLR7 signaling (Figure 3D). The most significant changes were observed in the “myeloid leukocyte activation” pathway, in which several up-regulated genes showed interaction by gene correlation network analysis (Figure 3E). Interestingly, several of these up-regulated genes have been previously associated with cardiovascular or metabolic pathologies, such as TLR7, fibrinogen-like 2 (FGL2), phospholipase D1 (PLD1), anoctamine 6 (ANO6), vinculin (VCL), complement receptor 1 (CR1), PECAM1, and cathepsin S (CTSS) (Figure 2E). Interestingly, the expression of genes involved in monocyte proliferation and differentiation (CSF1R), recruitment (CCR2), or immunoglobulin G–mediated activation (FCGR1a/CD64) was also up-regulated, whereas the expression of the nuclear receptor PPARγ, involved in M2/anti-inflammatory macrophage polarization, was down-regulated (Figure 3F).
FIGURE 3. Preoperative Transcriptome Phenotype of Peripheral Blood Monocytes.
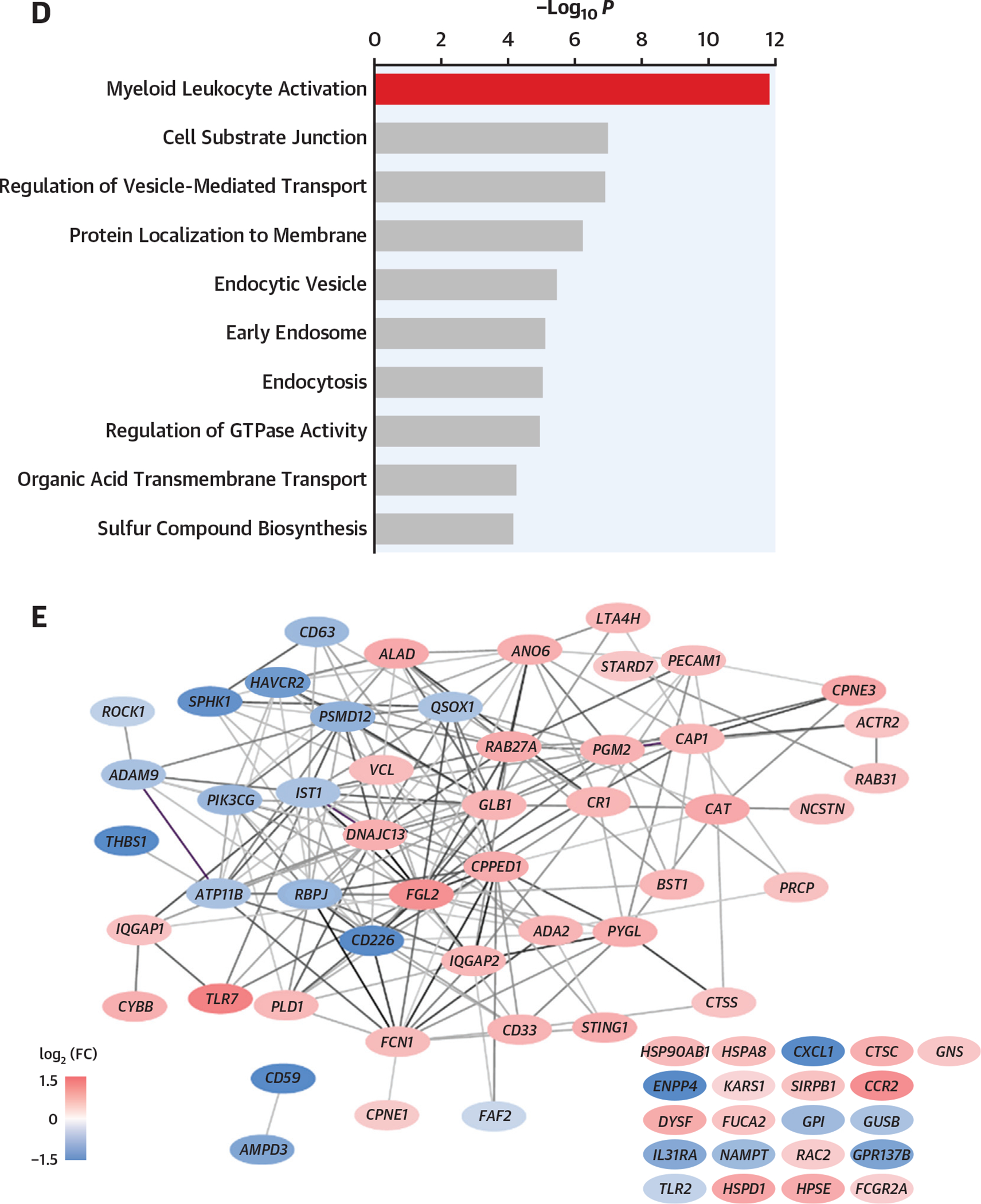
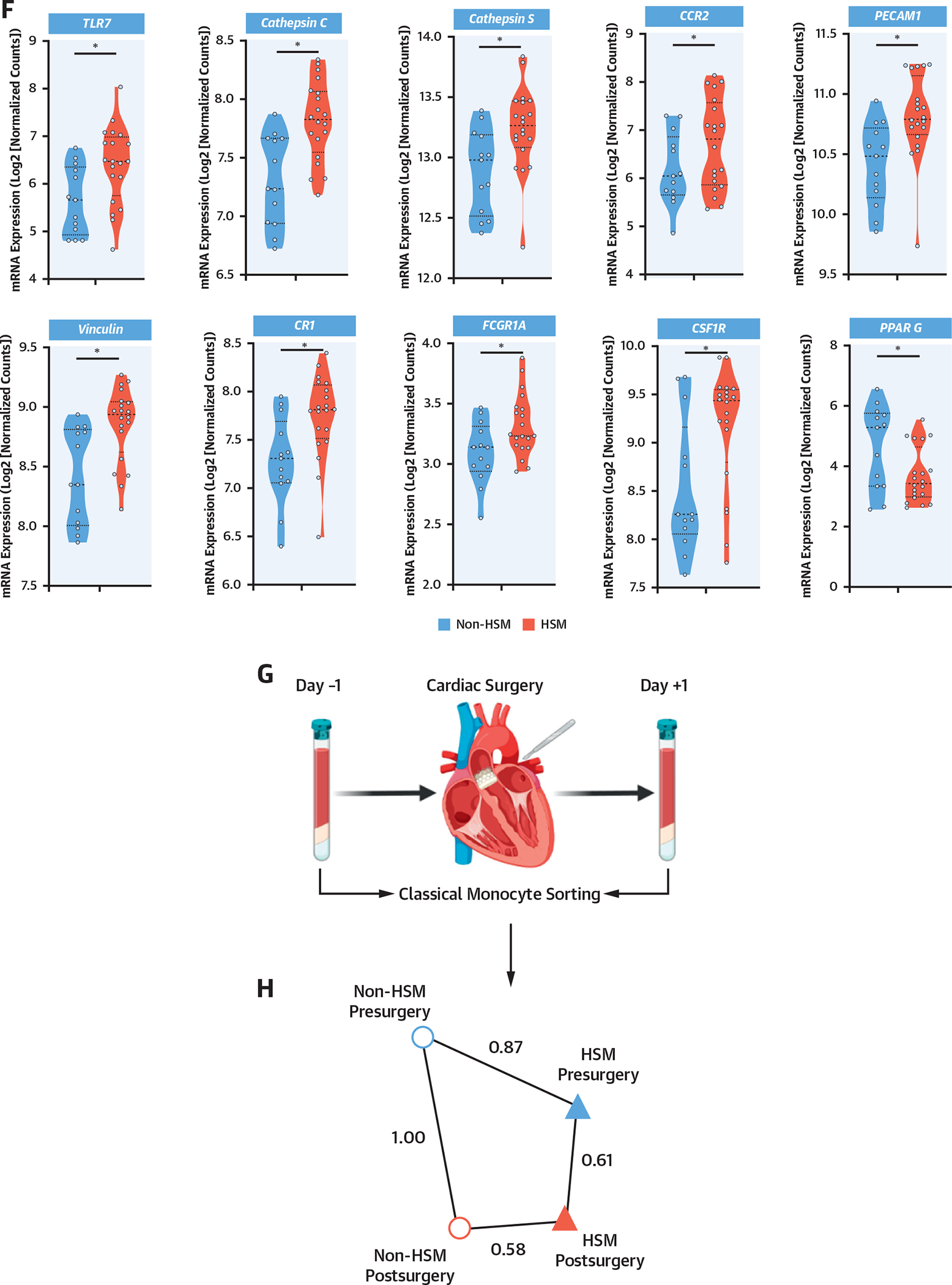
(A) Classical monocyte sorting. (B) Principal component analysis (PCA) plot, using log-normalized expression values of the 10,000 top-expressing genes. (C) Volcano plot of the 10,000 top-expressing genes. “Myeloid leukocyte activation” genes are in red. Dashed line indicates FDR of 0.1. (D) Biological process terms most affected by HSM, as ranked by consensus P value. Terms with more than 75% of member genes in the set of 10,000 most expressed genes were considered. (E) Correlation network of the top 70 significant genes in “myeloid leukocyte activation” term. Edges connect genes whose partial correlation adjusted for HSM has Pearson’s r > 0.8. (F) Violin plots of selected genes: TLR7 (P = 0.002; FDR = 0.07; log2 fold change [FC] = 1.22), cathepsin C (P = 0.0002; FDR = 0.059; log2 FC = 0.77), cathepsin S (P = 0.004; FDR = 0.09; log2 FC = 0.56), CCR2 (P = 0.01; FDR = 0.11; log2 FC = 1.09), PECAM1 (P = 0.001; FDR = 0.07; log2 FC = 0.62), vinculin (P = 0.001, FDR = 0.07, log2 FC = 0.63), CR1 (P = 0.004; FDR = 0.09; log2 FC = 0.70), CSF1R (P = 0.009, FDR = 0.11, log2 FC = 0.85), FCGR1A (P = 0.02, log2 FC = 0.58), PPAR G (P = 0.003, FDR = 0.08; log2 FC = −2.12). *Indicates P value <0.05. (G) Surgery-induced transcriptome explored in peripheral blood mononuclear cells obtained before and 48 hours after surgery. (H) Euclidian distance extrapolated from PCA between transcriptome status of patients with HSM and those without HSM, before and after surgery. FSC-A = forward scatter area; FSC-W = forward scatter width; GTPase = guanosine triphosphatase; mRNA = messenger RNA; PC = principal component; SSC-A = side scatter area; SSC-H = side scatter area; other abbreviations as in Figures 1 and 2.
Taken together, these results indicate that inflammatory monocytes of HSM carriers display transcriptomic changes suggesting an enhanced (preoperative) activation state with up-regulation of genes involved in proliferation, chemotaxis, cellular adhesion, and polarization.
To gain insight into the activation state of the monocytes after surgery, an RNA sequencing transcriptomic analysis was performed in paired samples of sorted classical monocytes taken from 3 patients with HSM and 3 without HSM before and 24 hours after surgery. A significant surgery effect was observed in all patients, which affected the innate immune response, cytokine signaling, regulation of apoptosis, and leukocyte activation pathways (Supplemental Figure 3A). Although patients with HSM and those without HSM shared common surgery-induced pathways (leukocyte cell-cell adhesion, chemical homeostasis, ion homeostasis, IL2-STAT5 signaling, regulation of dendritic cell antigen presentation, and iron ion transmembrane transport pathways), some were specific to patients with HSM, such as the major histocompatibility complex class II protein complex, positive regulation of cell adhesion, antigen processing, and clathrin-coated endocytic vesicle membrane pathways (Supplemental Figure 3B).
Interestingly, comparison of presurgery and postsurgery transcriptomes assessed using principal component analysis revealed a lower Euclidian distance between presurgery and postsurgery states in patients with HSM compared with patients without HSM (Figure 3H, Supplemental Figures 3D and 3E), indicating smaller presurgery to postsurgery differences in monocyte transcriptomes in patients with HSM, indicative of the existence of a monocyte priming prior to surgery in patients with HSM.
As such an activation state might favor monocyte egress from the bloodstream and myocardial recruitment, mass cytometry analysis of the myocardial myeloid leukocytes was performed in the 17 patients in whom the circulating leukocyte phenotype was previously analyzed (Figure 4A). As such, 16 myeloid clusters were identified (Figures 4B and 4C). Although HSM and non-HSM carriers presented similar clinical characteristics, the former displayed a distinct cardiac myeloid leukocyte population with an enrichment in cluster 4, which corresponds to a subset of monocyte-derived macrophages (HLADR+CCR2+CD11c+CD64+CD206+) with high expression of CD64, a hallmark of inflammatory macrophages, and CD206, which has been associated with myocardial fibrosis18 (Figures 4D to 4F). Furthermore, this dysregulated macrophage cluster significantly correlated with 1 dysregulated circulating monocyte cluster (Figures 2C and 2D) identified in the patients with HSM (Figure 4G).
FIGURE 4. Myocardial Immune Phenotype According to HSM Status.
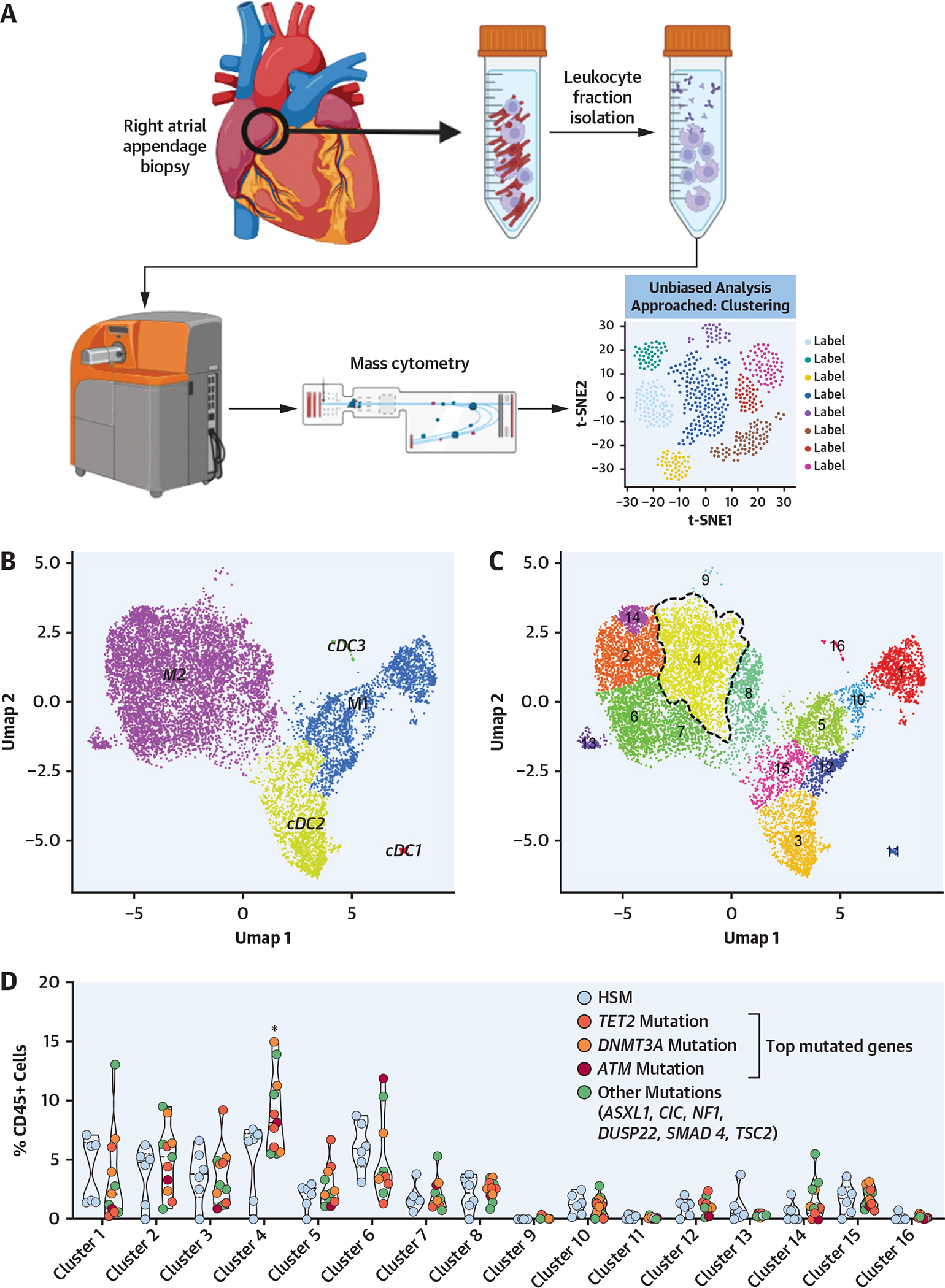
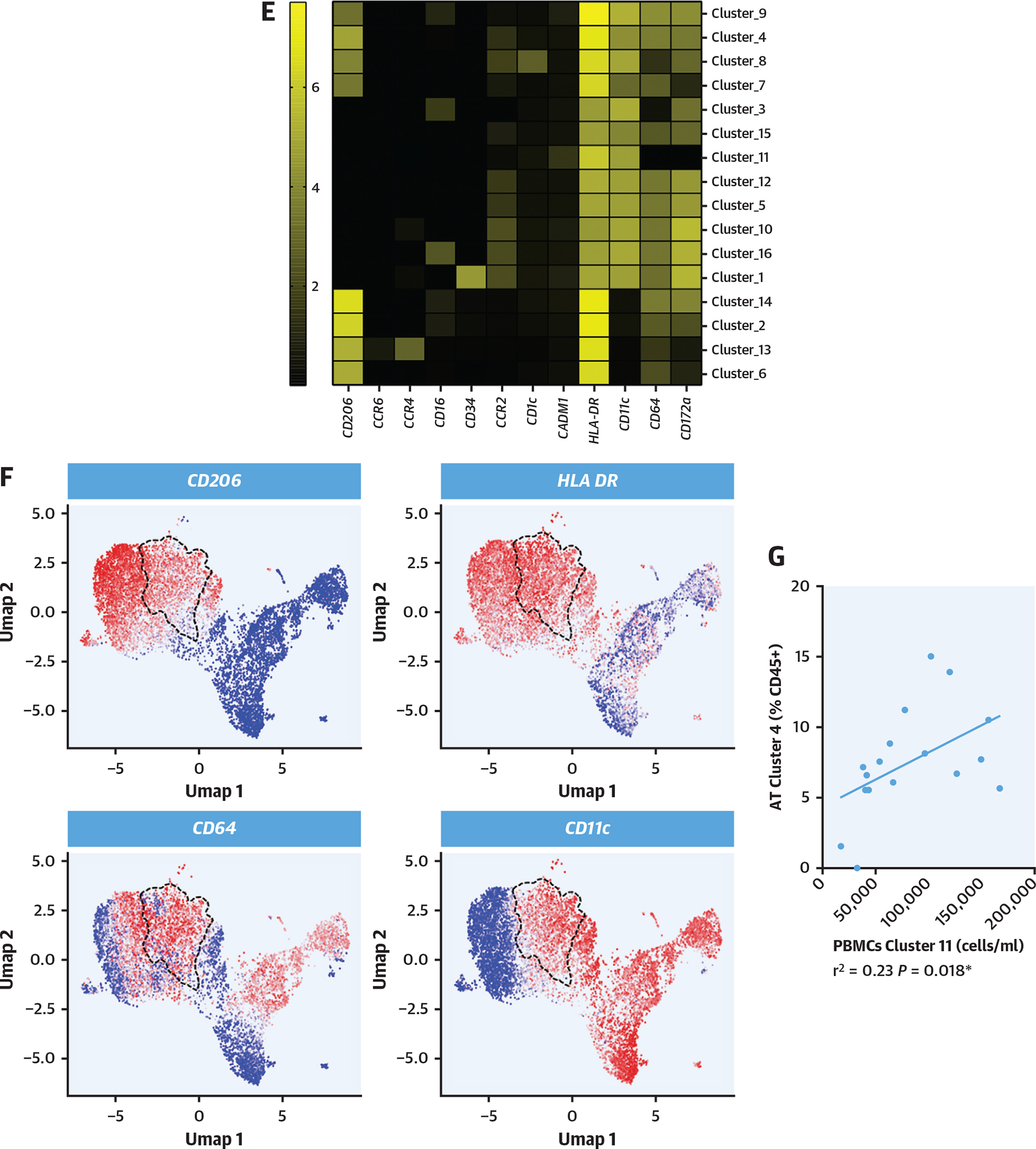
(A) Right appendage biopsies were collected from 17 patients, and leukocyte fraction was isolated and characterized using mass cytometry with unbiased approach on the basis of clustering. (B, C) Unsupervised clustering map of myeloid cells with general populations (top) and16 clusters (bottom) reidentified. (D) Heatmap representing characteristics of each identified cluster regarding the intensity of each marker. (E) Violin plots representing the proportion of each cluster among the total CD45+ cells in myocardium according to HSM status. Computations assumed the same scatter for subsets (*cluster 4: 4.9% ± 3.2% vs 8.5% ± 3.4% of CD45+ cells, FDR < 0.001). (F) Relative expression of markers defining cluster 4 in the map. Dashed line represents cluster 4 on each map. (G) Correlation between atrial tissue (AT) cluster 4 and peripheral blood mononuclear cell (PBMC) cluster 11 (monocytes) up-regulated in patients with HSM. HSM was defined according to the HemePACT panel with VAF ≥1%. t-SNE = t-distributed stochastic neighbor embedding; Umap = uniform manifold approximation and projection; other abbreviations as in Figures 1 and 2.
Altogether, these data indicate that HSM carriers display an increase in a cluster of recruited monocyte-derived macrophages exhibiting an inflammatory phenotype in the myocardium, which correlates with clusters of circulating monocytes displaying similar inflammatory characteristics. These observations suggest a potential link between the circulating and tissue immune cell subsets.
DISCUSSION
Our results show that patients undergoing cardiac surgery display a high prevalence of HSM (as high as 60% with the most inclusive definition), which increases with age. HSM most frequently occurs in 2 genes (DNMT3A and TET2) encoding epigenetic regulators. Moreover, we found that the presence of HSM is associated with: 1) increased circulating monocyte counts, specifically inflammatory CD64+ monocytes; 2) circulating classical inflammatory monocyte gene profiles indicative of activation and chemotaxis; and 3) an increase in monocyte-derived CD64+ inflammatory macrophages in the myocardium. In line, HSM carriers display a more pronounced perioperative inflammatory response with a higher incidence of POAF independently of age and other confounders.
Our study highlights critical variations in HSM prevalence depending on the screening methods used. The presence of HSM was initially identified as a major risk factor for coronary artery disease.9 When screening 74 predefined genes, these investigators reported a prevalence of CHIP mutations of 17% and 10% in a cohort of elderly patients (median age 70 years) with and without coronary artery disease, respectively, with VAFs ranging from 4% to 50%.9 Other studies, using deep-sequencing methodologies, reported a higher prevalence of HSM. For instance, a study among 20 healthy individuals 50 to 60 years of age, assessing a panel of 54 genes with variant detections as low as 0.003% VAF, identified a prevalence of 95% of TET2 and/or DNMT3A mutations.19 Thus, the high proportion of HSM observed in our cohort using the most inclusive definition is likely due to both the depth of the sequencing allowing the identification of variants with >1% VAF, as well as the large number of screened genes. Interestingly, our approach allowed the identification of mutations in genes playing roles in the DNA damage response, such as ataxia telangiectasia mutated (ATM)20 or splicing factor 3B subunit 1 (SF3B1).21 Although not directly involved in epigenetic regulation, mutations in genes related to the DNA damage response might induce a proinflammatory “senescence-associated secretory” phenotype.22
Although a few studies investigated a functional link between cardiovascular phenotypes and specific mutations involving TET2,23 JAK2,16 or DNMT3A24 in animal models, other studies highlighted that HSM might result from chronic inflammation, leading to increased myeloid turnover and subsequent mutations.25 Thus, the link between HSM and cardiac pathologies is probably a complex process involving both non-HSM-related and HSM-related inflammation. Furthermore, the diversity of mutations and mosaicism and their various mechanistic effects (eg, loss of Y chromosome leading to a profibrotic phenotype involving the TGF-β pathway,26 TET2 mutations involving the IL-1β pathway) render this pathophysiological model highly complex. Interestingly, we did not identify transcriptome signatures associated with IL-1β pathway alteration when pooling different mutation subtypes. This lack of association might be related to the fact that IL-1β pathway is not statistically dominant compared with pathways involved in inflammation.
We observed a preoperative increase in specific circulating inflammatory monocyte subsets, which correlated with an increase in myocardial inflammatory macrophages. In line, among the transcriptomic alterations found in inflammatory monocytes, the expression of several genes related to activation pathways, involved in the response of monocytes to proinflammatory or chemotactic stimuli, were elevated. Consistent with an increased number of proinflammatory macrophages in the myocardium, we observed an increased inflammatory response in HSM carriers following surgery, associated with a higher incidence of POAF.
POAF occurs when transient postoperative triggers act on a vulnerable atrial substrate produced by preoperative, surgery-induced, and postoperative remodeling processes.2 Taken together, our data suggest that HSM could help identify patients at risk for: 1) a higher inflammatory response resulting in a higher postoperative trigger driven by monocyte activation; and 2) greater atrial vulnerability resulting from an immune cell population shift within the atrial myocardium. HSM carrier patients may, as such, be good candidates for anti-inflammatory drugs to prevent “disproportionate” postoperative inflammation and POAF occurrence.
STUDY LIMITATIONS.
This clinical study could not formally establish a causal link among the presence of HSM, preoperative alterations of monocyte and macrophage populations, and postoperative inflammation and complications. This exploratory study was designed to be hypothesis generating regarding HSM and POAF and requires additional confirmation and mechanistic studies. Furthermore, our study provides a basis to elaborate further studies and to more accurately estimate the sample size needed to correlate HSM and POAF outcomes using alternative screening methods. Although the selected patients display similar distribution profiles regarding several clinical variables, the selection process did not involve the use of propensity scores. The present study did not allow us to explore specifically the effect of high clone size on POAF, as the number of patients presenting high VAFs was low. Such association should be explored by additional studies.
CONCLUSIONS
HSM is frequent in patients undergoing cardiac surgery. Its prevalence critically depends on the gene number included in the definition, with the broadest definition identifying patients at very high risk for POAF following surgery. This increased risk for POAF could be related to an increased postoperative inflammatory response driven by primed monocytes and increased atrial vulnerability driven by an immune shift in the atrial myocardium (Central Illustration). Assessment of HSM and/or its related alterations in monocytes might thus be valuable indicators to personalize management in the context of cardiac surgery.
CENTRAL ILLUSTRATION. Association Between Hematopoietic Mosaicism and Postoperative Atrial Fibrillation.
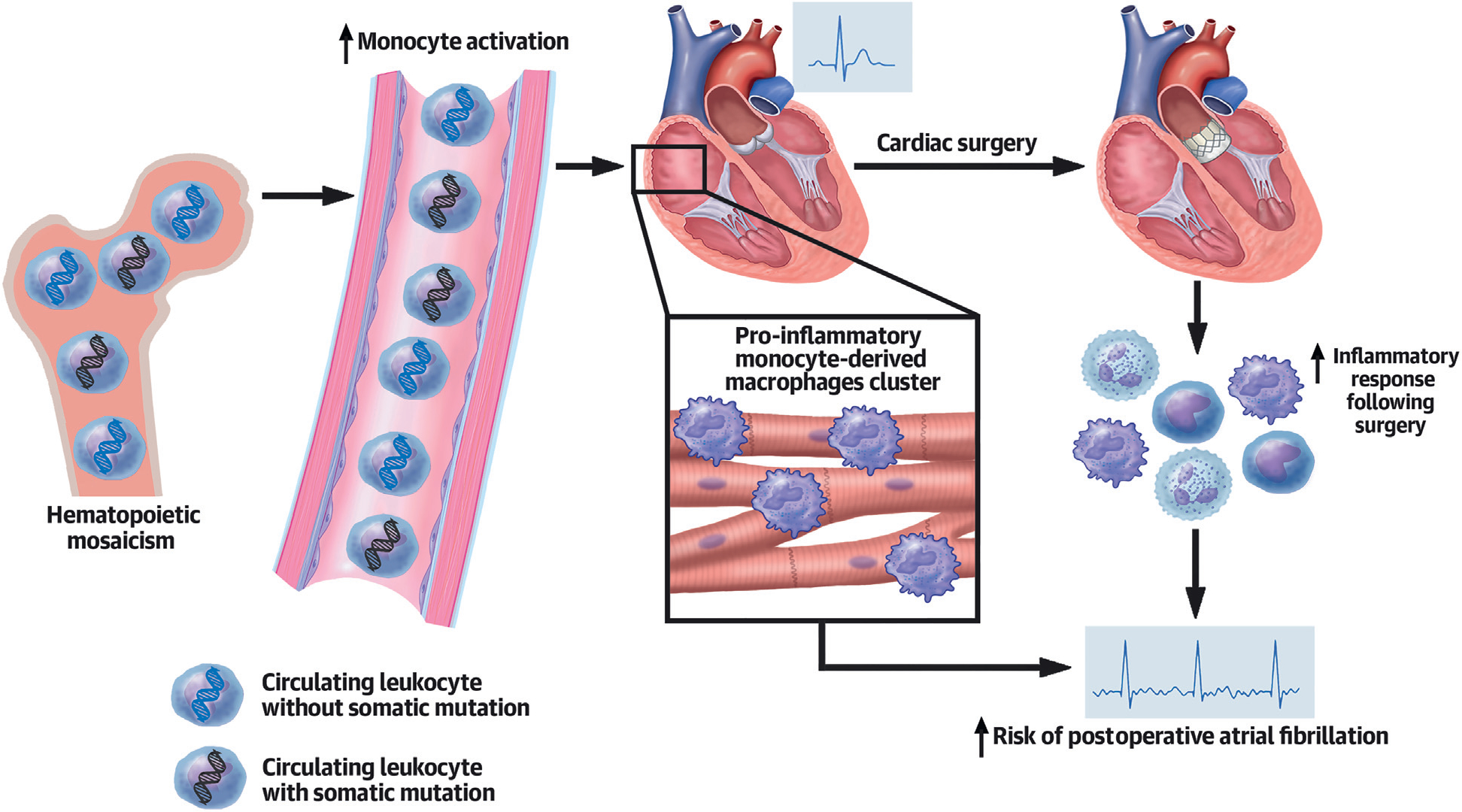
Aging leads to accumulating postzygotic mutations in bone marrow hematopoietic stem cells. These mutations are associated with increased monocyte activation in peripheral blood and monocytes derived macrophages in myocardium. As a result of the surgery, an increased inflammatory response is observed in hematopoietic mosaicism (HSM) carriers. Such an increase in the inflammatory response, combined with myocardial macrophage infiltration, could lead to the increase in postoperative atrial fibrillation incidence observed in HSM carriers.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Carriers of HSMs exhibit a more pronounced inflammatory response to cardiac surgery than noncarriers, and this is associated with a 3.5-fold greater incidence of POAF independent of age and other recognized risk factors.
TRANSLATIONAL OUTLOOK:
Additional research is needed to understand the mechanisms linking HSM to the adverse to cardiac surgery and its relationship to adverse clinical events.
ACKNOWLEDGMENTS
The authors thank Bertrand Accart and the Biological Resources Center of Centre Hospitalier de Lille (BB 0033-00030) for handling and providing biological samples from the POMI-AF study.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This study was supported by grants from Fédération Française de Cardiologie, Fondation Leducq convention 16CVD01 (“Defining and Targeting Epigenetic Pathways in Monocytes and Macrophages That Contribute to Cardiovascular Disease”), the European Genomic Institute for Diabetes (ANR-10-LABX-0046), Fondation Pour la Recherche Médicale (REFERENCE PROJET EQU202203014650), and Agence Nationale de la Recherche (TOMIS leukocytes: ANR-CE14-0003-01). Dr Staels is a recipient of an Advanced European Research Council Grant (694717). Dr Vicario was supported by the 2018 American Association for Cancer Research–Bristol Myers Squibb Fellowship for Young Investigators in Translational Immuno-Oncology. Work at the Memorial Sloan Kettering Cancer Center (MSKCC) is supported by an MSKCC core grant (P30 CA008748), National Institutes of Health grants 1R01NS115715-01, 1 R01 HL138090-01, and 1 R01 AI130345-01, Basic and Translational Immunology Grants from the Ludwig Center for Cancer Immunotherapy to Dr Geissmann. Dr de Winther is funded by grants from the Netherlands Heart Foundation (CVON: GENIUS2) and the Netherlands Heart Foundation and Spark-Holding (2019B016). Dr Neele is a Dekker fellow of the Netherlands Heart Foundation (2020T029). Dr White is founder and owner of Resphera Biosciences. Dr Geissmann has performed consulting for Third Rock Ventures. Dr Fragkogianni is employed by Tempus Labs. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- AF
atrial fibrillation
- AKI
acute kidney injury
- AVR
aortic valve replacement
- BMI
body mass index
- CABG
coronary artery bypass graft
- CHIP
clonal hematopoiesis of indeterminate potential
- CRP
C-reactive protein
- HSM
hematopoietic somatic Mosaicism
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- POAF
postoperative atrial fibrillation
- VAF
variant allelic frequency
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For supplemental methods, references, tables, and figures, please see the online version of this paper.
REFERENCES
- 1.Englum BR, Ganapathi AM, Schechter MA, Harrison JK, Glower DD, Hughes GC. Changes in risk profile and outcomes of patients undergoing surgical aortic valve replacement from the pre- to post-transcatheter aortic valve replacement eras. Ann Thorac Surg. 2016;101:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobrev D, Aguilar M, Heijman J, Guichard J-B, Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. 2019;16:417–436. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13:697–711. [DOI] [PubMed] [Google Scholar]
- 4.Wan S, Marchant A, DeSmet JM, et al. Human cytokine responses to cardiac transplantation and coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1996;111:469–477. [DOI] [PubMed] [Google Scholar]
- 5.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95–112. [DOI] [PubMed] [Google Scholar]
- 6.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: the COPPS-2 randomized clinical trial. JAMA. 2014;312:1016–1023. [DOI] [PubMed] [Google Scholar]
- 7.Vijg J, Dong X. Pathogenic mechanisms of somatic mutation and genome mosaicism in aging. Cell. 2020;182:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaiswal S, Libby P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat Rev Cardiol. 2020;17: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mas-Peiro S, Hoffmann J, Fichtlscherer S, et al. Clonal haematopoiesis in patients with degenerative aortic valve stenosis undergoing transcatheter aortic valve implantation. Eur Heart J. 2020;41:933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu B, Roberts MB, Raffield LM, Zekavat SM, et al. Association of clonal hematopoiesis with incident heart failure. J Am Coll Cardiol. 2021;78: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascual-Figal DA, Bayes-Genis A, Díez-Díez M, et al. Clonal hematopoiesis and risk of progression of heart failure with reduced left ventricular ejection fraction. J Am Coll Cardiol. 2021;77:1747–1759. [DOI] [PubMed] [Google Scholar]
- 13.Kar SP, Quiros PM, Gu M, et al. Genome-wide analyses of 200,453 individuals yield new insights into the causes and consequences of clonal hematopoiesis. Nat Genet. 2022;54:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abplanalp WT, Cremer S, John D, et al. Clonal hematopoiesis-driver DNMT3A mutations alter immune cells in heart failure. Circ Res. 2021;128: 216–228. [DOI] [PubMed] [Google Scholar]
- 15.Abplanalp WT, Mas-Peiro S, Cremer S, John D, Dimmeler S, Zeiher AM. Association of clonal hematopoiesis of indeterminate potential with inflammatory gene expression in patients with severe degenerative aortic valve stenosis or chronic postischemic heart failure. JAMA Cardiol. 2020;5:1170–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fidler TP, Xue C, Yalcinkaya M, et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature. 2021;592(7853):296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Y, Mouton AJ, Lindsey ML. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl Res. 2018;191:15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Comm. 2016;7:12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackford AN, Jackson SP. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol Cell. 2017;66:801–817. [DOI] [PubMed] [Google Scholar]
- 21.Singh S, Ahmed D, Dolatshad H, et al. SF3B1 mutations induce R-loop accumulation and DNA damage in MDS and leukemia cells with therapeutic implications. Leukemia. 2020;34:2525–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang B, Fu D, Xu Q, et al. The senescence-associated secretory phenotype is potentiated by feedforward regulatory mechanisms involving Zscan4 and TAK1. Nat Comm. 2018;9:1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sano S, Oshima K, Wang Y, et al. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J Am Coll Cardiol. 2018;71:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rauch PJ, Silver AJ, Gopakumar J, et al. Loss-of-function mutations in Dnmt3a and Tet2 lead to accelerated atherosclerosis and convergent macrophage phenotypes in mice. Blood. 2018;132:745. [DOI] [PubMed] [Google Scholar]
- 25.Heyde A, Rohde D, McAlpine CS, et al. Increased stem cell proliferation in atherosclerosis accelerates clonal hematopoiesis. Cell. 2021;184:1348–1361.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sano S, Horitani K, Ogawa H, et al. Hematopoietic loss of Y chromosome leads to cardiac fibrosis and heart failure mortality. Science. 2022;377:292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


