Abstract
To determine the benefit of a 4-week incubation for mycology cultures, we evaluated all positive cultures during the fourth week of incubation in a 1-year period. Of 3,855 positive mycology cultures (yeast, 82%; molds, 18%), 62 (1.6%) were positive during the fourth week (yeast, 42%; molds, 58%). Only 15 of the 62 cultures (24%) were considered clinically relevant (2 isolates from invasive fungal infection and 13 isolates from cutaneous mycosis). With the exception of those from skin samples, isolates recovered during the fourth week are rarely important for patient care.
With the increasing number of patients who are critically ill or have immunosuppressive diseases, the mycology laboratory has become a very important part of the clinical microbiology laboratory (1–4). Several aspects of routine fungal cultures should be evaluated in order to implement appropriate necessary changes. There is a universal recommendation that mycology cultures be incubated for 4 weeks (5, 6). However, there is no recent evidence supporting this practice (7). Most of the decisions in infectious diseases are empiric or based on rapid test results; therefore, positive cultures obtained after 3 weeks of incubation may only confirm previous clinical decisions or be nonrelevant for patient care.
(This work was partially presented at the 98th General Meeting of the American Society for Microbiology, 17 to 21 May 1998, Atlanta, Ga. [5a].)
We conducted a retrospective study to evaluate the clinical relevance of positive cultures obtained during the fourth week of incubation and the cost-effectiveness of the observation during that week. During a 1-year period, we evaluated the incubation time for all positive mycology cultures. Mycology specimens were plated on Sabouraud glucose and brain heart infusion agar (SABHI; Becton Dickinson Microbiology Systems, Cockeysville, Md.) and inhibitory mold agar with gentamicin (Becton Dickinson Microbiology Systems) and incubated at 30°C for 4 weeks. Isolates recovered from cutaneous and deep fungal infections during the fourth week were considered clinically relevant. Isolates were considered clinically nonrelevant if they were recovered from patients who had the same organism isolated within the first 3 weeks of incubation from the same specimen source, if the isolates were considered to be colonizers or contaminants, or if the isolates were from environmental sources or proficiency test samples. Contamination or colonization was assumed if the patient did not receive specific antifungal treatment, if it was stated in the patient’s chart that the isolate was considered a contaminant, or if there was no mention of the culture result in the chart after 3 months of follow-up. In all of the above situations, the patient should not have clinical symptoms of fungal infection in order for the positive culture to be considered a contaminant or colonizer.
From 1 June 1996 through 30 May 1997 a total of 21,813 mycology cultures were submitted. Of these, 3,855 (17.67%) cultures were positive (3,178 [82%] for yeasts and 677 [18%] for molds). A total of 138 (3.6%) isolates were recovered during the third week (46 [33.3%] yeasts and 92 [66.6%] molds), and 62 (1.6%) isolates were recovered during the fourth week (26 [42%] yeasts and 36 [58%] molds) (Tables 1 and 2). The proportion of molds recovered during the third and fourth weeks was significantly higher than those recovered during the first two weeks (P ≤ 0.001, χ2 test). Fungal species recovery during the fourth week is shown in Table 3.
TABLE 1.
Yeast recovered during the third and fourth weeks of incubation, by sourcea
| Specimen type | Total no. of positive specimens | Specimens recovered in 15–21 days
|
Specimens recovered in >21 days
|
||
|---|---|---|---|---|---|
| n | %b | n | %b | ||
| Blood | 22 | 0 | 0.0 | 0 | 0.0 |
| Blood from intravenous line | 47 | 2 | 4.2 | 0 | 0.0 |
| Bronchoalveolar lavage fluid | 120 | 1 | 0.8 | 1 | 0.8 |
| Bronchoalveolar wash fluid | 133 | 2 | 1.5 | 0 | 0.0 |
| Fluid | 55 | 1 | 1.8 | 3 | 5.4 |
| Miscellaneousc | 191 | 6 | 3.1 | 4 | 2.1 |
| Respiratory | 52 | 0 | 0.0 | 1 | 1.9 |
| Skin | 162 | 3 | 1.8 | 2 | 1.2 |
| Sputum | 891 | 12 | 1.3 | 5 | 0.6 |
| Stool | 61 | 0 | 0.0 | 0 | 0.0 |
| Throat | 549 | 8 | 1.4 | 4 | 0.7 |
| Tissue | 38 | 1 | 2.6 | 2 | 5.0 |
| Urine | 577 | 3 | 0.5 | 2 | 0.3 |
| Wounds | 52 | 0 | 0.0 | 1 | 2.0 |
| Othersd | 228 | 4 | 1.8 | 1 | 0.7 |
| Total | 3,178 | 46 | 1.4 | 26 | 0.8 |
Data were collected from 1 June 1996 through 30 May 1997.
Percentage of all positive cultures by source.
Specimen types without a computer code.
Specimen types that occurred in small numbers.
TABLE 2.
Molds recovered during the third and fourth weeks of incubation, by sourcea
| Specimen type | Total no. of positive specimens | Specimens recovered in 15–21 days
|
Specimens recovered in >21 days
|
||
|---|---|---|---|---|---|
| n | %b | n | %b | ||
| Blood | 2 | 1 | 50.0 | 0 | 0.0 |
| Blood from intravenous line | 1 | 0 | 0.0 | 0 | 0.0 |
| Bronchoalveolar lavage fluid | 62 | 8 | 13.0 | 1 | 1.5 |
| Bronchoalveolar wash fluid | 67 | 9 | 13.0 | 3 | 4.4 |
| Fluid | 0 | 0 | 0.0 | 0 | 0.0 |
| Miscellaneousc | 227 | 36 | 15.8 | 6 | 2.6 |
| Respiratory | 2 | 1 | 50.0 | 0 | 0.0 |
| Skin | 58 | 14 | 24.0 | 11 | 19.0 |
| Sputum | 169 | 13 | 7.6 | 10 | 5.9 |
| Stool | 5 | 1 | 20.0 | 0 | 0.0 |
| Throat | 20 | 3 | 15.0 | 1 | 5.0 |
| Tissue | 18 | 3 | 16.0 | 3 | 16.0 |
| Urine | 2 | 0 | 0.0 | 0 | 0.0 |
| Wounds | 8 | 0 | 0.0 | 0 | 0.0 |
| Othersd | 36 | 3 | 8.3 | 1 | 2.9 |
| Total | 677 | 92 | 13.5 | 36 | 5.5 |
Data were collected from 1 June 1996 through 30 May 1997.
Percentage of all positive cultures by source.
Specimen types without a computer code.
Specimen types that occurred in small numbers.
TABLE 3.
Fungus species isolated during the fourth week of incubation
| Species | No. (%) of isolates |
|---|---|
| Candida albicans | 15 (24.2) |
| Candida glabrata | 6 (9.6) |
| Aspergillus fumigatus | 4 (6.5) |
| Trichophyton tonsurans | 4 (6.5) |
| Aspergillus nidulans | 3 (4.8) |
| Penicillium sp. | 3 (4.8) |
| Trichophyton rubrum | 3 (4.8) |
| Candida parapsilosis | 2 (3.2) |
| Candida tropicalis | 2 (3.2) |
| Saccharomyces cerevisiae | 2 (3.2) |
| Aspergillus niger | 2 (3.2) |
| Scopulariopsis sp. | 2 (3.2) |
| Other | 14 (22.6) |
| Total | 62 (100.0) |
Only 15 of the 62 (24%) isolates recovered during the fourth week were considered clinically relevant. These included isolates from two patients with invasive fungal infections confirmed by biopsy (one immunocompetent patient with pulmonary histoplasmosis and one neutropenic patient with an invasive sinus infection) and 13 isolates from skin or nails. In two patients, the fungal isolate had an uncertain significance (one patient with pneumonia and a sputum culture positive for Candida albicans and one patient with pneumonia and a bronchoalveolar lavage fluid culture positive for Aspergillus niger). Nonrelevant positive cultures obtained during the fourth week included samples from 21 patients who had the same organism isolated within the first 3 weeks from duplicate specimens, four samples from environmental sources or a proficiency test, and 20 isolates considered to be contaminants or colonizers.
Very few isolates were recovered from urine cultures during the third and fourth weeks. Of the 577 yeast isolates recovered from urine cultures, only 3 (0.5%) were detected during the third week and 2 (0.3%) were detected during the fourth week. The last 2 positive urine cultures (Candida glabrata and Saccharomyces cerevisiae) were from patients with multiple previous positive urine cultures for the same organism within the first 2 weeks of incubation. During the fourth week, skin samples had the highest proportion of molds recovered (19% of the total positive skin samples). Skin and nail specimens represented 29% (18 of 62 samples) of cultures grown during the fourth week. Most of them were from outpatient clinics and represented fungi commonly isolated from cutaneous mycoses.
A total of 18,020 cultures were observed twice during the fourth week. Two plates were observed for each culture. A technologist can observe approximately six plates per minute; therefore, a total of 200.2 h was used for plate observation during 1 year. Using a standard cost of $20.00 per hour, a total of $4,004 was expended in plate observation through the year. In addition, the cost for identifying the 45 nonrelevant isolates grown during the fourth week should also be considered.
Our results agree with a previous study, which evaluated the length of incubation for mycology cultures and supported the possibility of reducing the incubation time (7). Slow growth of isolates could be the result of unfavorable growth conditions, such as residual antifungal drugs in the specimens. Most of the slow-growing organisms, collected from patients with duplicate positive isolates, were recovered from patients receiving systemic antifungal treatment on the specimen collection date (16 of 21 isolates [76%]).
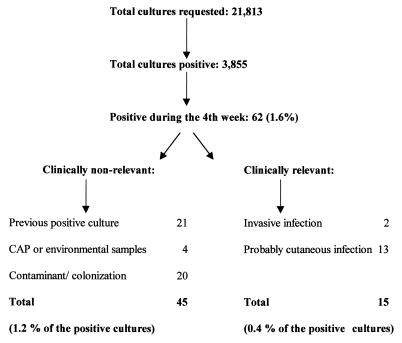
Of the 21,813 mycology cultures requested, only 62 grew during the fourth week. Of these 62, with the exception of cultures from skin or nails, only 2 isolates were considered clinically relevant (Fig. 1). However, no change in the therapeutic approach was implemented in either of these two patients because specific antifungal treatment was started earlier, based on the histological findings.
FIG. 1.
Summary of the culture results and the clinical relevance of growth that occurred during the fourth week of incubation. For the cultures positive during the fourth week, there were two isolates with uncertain significance. Data were obtained at the UCLA Clinical Microbiology Laboratory for samples submitted between 1 June 1996 and 30 May 1997.
Our study provides information from one tertiary-care hospital, where a large number of surveillance cultures were performed. This was in part due to the implementation of antifungal prophylaxis protocols for transplant patients. Validation of these results in a different setting should be done, especially in fungal reference laboratories and in hospitals with large numbers of oncology patients. We have concluded that cultures positive during the fourth week are rarely relevant for patient care, and in most cases the incubation time could be reduced to 3 weeks, saving money and time. Exceptions, which must be considered, include samples from skin or nails and samples in which dimorphic fungi are suspected. The possibility of reducing incubation time to 2 weeks for urine and throat specimens should also be explored.
REFERENCES
- 1.Beck-Saguè C, Jarvis W R. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. National Nosocomial Infections Surveillance System. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 2.Emory T G, Gaynes R P. An overview of nosocomial infections, including the role of the clinical microbiology laboratory. Clin Microbiol Rev. 1993;6:428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarvis W R. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis. 1995;20:1526–1530. doi: 10.1093/clinids/20.6.1526. [DOI] [PubMed] [Google Scholar]
- 4.Jarvis W R, Martone W J. Predominant pathogens in hospital infections. J Antimicrob Chemother. 1991;28:15–19. doi: 10.1093/jac/29.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- 5.Kwon-Chung K J, Bennett J E. Medical mycology. 3rd ed. Philadelphia, Pa: Lea & Febiger; 1992. p. 51. [Google Scholar]
- 5a.Labarca J A, Wagar E A, Grasmick A E, Kokkinos H M, Bruckner D A. Abstracts of the 98th General Meeting of the American Society of Microbiology. Washington, D.C: American Society for Microbiology; 1998. Critical evaluation of the four-week incubation for fungal cultures: is the fourth week useful? abstr. F-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merz W G, Roberts G D. Detection and recovery of fungi from clinical specimens. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 709–722. [Google Scholar]
- 7.Morris A J, Byrne T C, Madden J F, Reller L B. Duration of incubation of fungal cultures. J Clin Microbiol. 1996;34:1583–1585. doi: 10.1128/jcm.34.6.1583-1585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]