Abstract
Previous epidemiological studies of Chlamydia trachomatis frequently have required expansion of isolates in tissue culture. The possibility that C. trachomatis omp1 might undergo mutation during such expansion has not been examined systematically. We found no differences in the omp1 sequences from 10 clinical specimens before and after 20 in vitro passages.
The omp1 gene of Chlamydia trachomatis encodes the major outer membrane protein (MOMP), an immunogenic surface protein containing serotype-specific epitopes (1, 4–6, 10, 20–23). MOMP appears to be the most variable genetic marker for C. trachomatis, making omp1 genotyping a useful epidemiologic tool. Several laboratories have observed omp1 sequence variation within serovars (2, 7, 9, 11, 18). It has been suggested that such variation reflects adaptation in response to host immune selection, allowing organisms to evade host immune defenses (2, 14).
While clinical diagnosis of chlamydial infection no longer requires expansion of organisms in vitro, experimental procedures frequently do, in order to provide sufficient quantities of organisms for neutralization assays, antibiotic susceptibility testing, and examination of growth rates, etc. For these reasons, it is important to know the frequency with which in vitro mutations that could affect phenotype might occur. Moreover, the possibility that some of the previously observed mutations in omp1 might have occurred during in vitro growth has not been examined systematically. There is precedent indicating the need for such an analysis. Differences in the omp1 nucleotide sequences of the reference strains E/Bour (8, 15) and B/TW5/OT (12, 17) have been reported. Since the isolates of origin were presumably the same, the possibility arises that in these cases MOMP might have undergone mutation during unrestricted in vitro growth. To test this hypothesis, we obtained the omp1 sequences from 10 clinical specimens containing C. trachomatis and compared them to the sequences of omp1 from these 10 isolates after 20 in vitro passages.
Two isolates each of the five most prevalent serovars in our population—D, E, F, I, and J—were chosen (Table 1) from clinical urogenital specimens from which C. trachomatis previously had been isolated and immunotyped (19). All specimens consisted of the original transport medium into which a swab specimen had been inoculated and which had been maintained at −70°C. Each specimen was split into two aliquots. One aliquot was used for sequencing, and the other was used for expansion in vitro, as previously described (13). Seventy-two hours postinoculation, one vial from the passage of each isolate was reimmunotyped by fluorescent-antibody staining with serovar-specific monoclonal antibodies (19). The remaining vials were further passaged, as previously described (13). At passages 5 and 10, vials for each isolate were again stained with serovar-specific monoclonal antibodies to verify the integrity of the passage. There was insufficient volume in original specimen 115718 for sequencing and expansion. Therefore, the initial in vitro expansion was used as the “original” sample for sequencing. All isolates were grown for 20 passages with the exception of isolate 115644, which failed to grow after the sixth in vitro passage. DNA extraction was done as previously described (18).
TABLE 1.
Isolates used in this study and consecutively passaged 20 times in vitro
| Isolate | Source | Serovar | Genotypea | GenBank accession no. (reference) |
|---|---|---|---|---|
| 115053 | Urethra | I | Ia | AF063201 (18) |
| 115245 | Cervix | F | F | X52080 (24) |
| 115328 | Cervix | F | F | X52080 (24) |
| 115644b | Urethra | E | E | X52557 (15) |
| 115718c | Cervix | J | Ja | AF063203 (18) |
| 116015 | Cervix | D | D2 | AF086854 |
| 116293 | Cervix | D | D6 | AF086855 |
| 117424 | Cervix | E | E | X52557 (15) |
| 117542 | Urethra | J | J2 | AF086856 |
| 123130 | Cervix | I | Ia | AF063201 (18) |
Genotypes were established by direct DNA sequencing of the omp1 PCR product amplified from each isolate before and after 20 passages in vitro.
This isolate did not expand in vitro. Only the clinical specimen was sequenced.
An aliquot of the first in vitro passage was used as the original specimen for sequencing.
PCR was done with the following primers specific to the C. trachomatis MOMP: MOMP-108 (5′-GGC CAT TAA TTG CTA CAG GAC ATC TTG TC-3′), located 108 bp upstream of the ATG initiator, and RVS-END (5′-AAG YCG AGC CCA GAA AYA CGG ATA GTG-3′), located about 80 bp downstream of the terminal TAA. One negative control reaction was run for every seven experimental reactions. DNA amplification was done in duplicate with PCR Core Kit Plus uracil-DNA-glycosylase (Boehringer Mannheim, Indianapolis, Ind.), as previously described (18). Nested reamplifications were required for the original clinical specimens due to the low yield of chlamydial DNA in these samples (18). Negative controls from the first round of amplification were also used as templates in the nested reamplifications of negative controls. Replicate PCR mixtures were pooled for sequencing. Automated sequencing was performed by Merlin Core Services (Vista, Calif.). The sequence of omp1 was determined for each original specimen and for each isolate after 20 in vitro passages. Sequences were aligned manually by using the program ESEE (3).
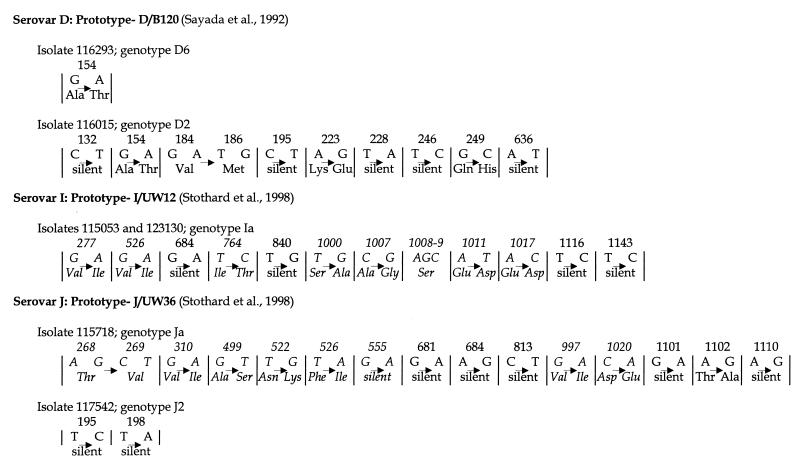
omp1 from all 10 specimens was successfully amplified by nested reamplification with the original specimen as the template. The resulting PCR product was approximately 1,050 bp (18). Full-length PCR products for omp1 were obtained from all isolates after expansion in cell culture with the exception of 115644. Sequencing results are summarized in Fig. 1. Where sequence was obtained for both the original specimen and the expanded isolate, sequences were identical. Several strains exhibited variation compared to published prototypes (Fig. 1). Although isolate 115644 could not be expanded in cell culture, the omp1 sequence of the original specimen and the omp1 sequences of the other serovar E isolate, 117424, were identical to that of E/Bour reported by Peterson et al. (15) and confirmed by us (18). This differs from the E/Bour sequence reported by Dean and Millman (8) by 10 nucleotides and four amino acids.
FIG. 1.
Nucleotide and amino acid changes in the omp1 genes of isolates analyzed in this study which exhibited unique genotypes compared to those of prototype strains. Nucleotide bases and amino acids listed to the left of each arrow are those in the prototype strain. Nucleotide bases and amino acids listed to the right of each arrow are the substitutions found in the study isolate. Nucleotide bases and amino acids listed in italics are residues located in variable regions of the omp1 genes. Numbers above the nucleotide and amino acid changes indicate the base pair positions of the substitutions in the omp1 genes. Genotype designations (D2, D6, J2) are arbitrary but were derived by comparing the omp1 nucleotide sequence of the variant isolate to the most closely related prototype sequence and numbering the new genotype in succession (e.g., D2 is a variant of the D prototype [16] and was the second D variant discovered in the Indianapolis population).
The nucleotide sequences for B/TW5/OT (12, 17) and E/Bour (8, 15) omp1 have been reported twice. The two B/TW5/OT sequences differ by five synonymous substitutions, four of which occur in conserved region 1. The two E/Bour sequences differ from each other in conserved regions of omp1 by 10 nucleotides and four amino acids. We also have sequenced our stock of E/Bour, which we have had since 1993, and found it to be identical to that reported by Peterson et al. (15). Since in each case the strains are purported to be from different stocks of the same isolate, one difference between them may be the time grown in vitro. Therefore, it is legitimate to suggest that prolonged expansion in cell culture could have led to the reported differences in omp1.
Alternatively, as these two strains were identified and classified at a time when immunotyping was the only means of classification, it is possible that they are not from the same stock but represent two different strains of the same serovar. Indeed, the data presented here suggest that serial passage in cell culture does not induce mutations in omp1 with any frequency under normal in vitro conditions. Consequently, it is reasonable to assume that omp1 sequences derived from expanded clinical specimens accurately represent the sequences of the organisms recovered from the infected person. However, this study does not address the extent to which variation might arise during in vivo passage (natural infection). There are multiple factors in natural infections, including the effect of the host immune response on MOMP, which are not duplicated in tissue culture but which could induce changes in the gene coding for this protein.
Acknowledgments
This work was supported by grant AI-31494 to R.B.J. from the National Institute for Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.Baehr W, Zhang Y-X, Joseph T, Su H, Nano F E, Everett K D E, Caldwell H D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci USA. 1988;85:4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunham R, Yang C, Maclean I, Kimani J, Maitha G, Plummer F. Chlamydia trachomatis from individuals in a sexually transmitted disease core group exhibit frequent sequence variation in the major outer membrane protein (omp1) gene. J Clin Investig. 1994;94:458–462. doi: 10.1172/JCI117347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabot E L, Beckenbach A T. Simultaneous editing of multiple nucleic acid and protein sequences with ESEE. Comput Appl Biosci. 1989;5:233–234. doi: 10.1093/bioinformatics/5.3.233. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell H D, Judd R C. Structural analysis of chlamydial major outer membrane proteins. Infect Immun. 1982;38:960–968. doi: 10.1128/iai.38.3.960-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldwell H D, Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982;35:1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean D, Oudens E, Bolan G, Padian N, Schachter J. Major outer membrane protein variants of Chlamydia trachomatis are associated with severe upper genital tract infections and histopathology in San Francisco. J Infect Dis. 1995;172:1013–1022. doi: 10.1093/infdis/172.4.1013. [DOI] [PubMed] [Google Scholar]
- 8.Dean D, Millman K. Molecular and mutation trends analyses of omp1 alleles for serovar E of Chlamydia trachomatis. J Clin Investig. 1997;99:475–483. doi: 10.1172/JCI119182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frost E H, Deslandes S, Gendron D, Bourgaux-Ramoisy D, Bopurgaux P. Variation outside variable segments of the major outer membrane protein distinguishes trachoma from urogenital isolates of the same serovar of Chlamydia trachomatis. Genitourin Med. 1995;71:18–23. doi: 10.1136/sti.71.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatch T P, Vance D W, Al-Hossainy E. Identification of a major envelope protein in Chlamydia spp. J Bacteriol. 1981;146:426–429. doi: 10.1128/jb.146.1.426-429.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes L J, Pecharatana S, Bailey R L, Hampton T J, Pickett M A, Mabey D C W, Watt P J, Ward M E. Extent and kinetics of genetic change in the omp1 gene of Chlamydia trachomatis in two villages with endemic trachoma. J Infect Dis. 1995;172:268–272. doi: 10.1093/infdis/172.1.268. [DOI] [PubMed] [Google Scholar]
- 12.Hayes L J, Pickett M A, Conlan J W, Ferris S, Everson J S, Ward M E, Clark I N. The major outer membrane proteins of Chlamydia trachomatis serovars A and B: intra-serovar amino acid changes do not alter specificities of serovar- and C subspecies-reactive antibody-binding domains. J Gen Microbiol. 1990;136:1559–1566. doi: 10.1099/00221287-136-8-1559. [DOI] [PubMed] [Google Scholar]
- 13.Jones R B, Van Der Pol B, Katz B P. Effect of differences in specimen processing and passage technique on recovery of Chlamydia trachomatis. J Clin Microbiol. 1989;27:894–898. doi: 10.1128/jcm.27.5.894-898.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lampe M F, Wong K G, Kuehl L M, Stamm W E. Chlamydia trachomatis major outer membrane protein variants escape neutralization by both monoclonal antibodies and human immune sera. Infect Immun. 1997;65:317–319. doi: 10.1128/iai.65.1.317-319.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson E M, Markoff B A, de la Maza L M. The major outer membrane protein nucleotide sequence of Chlamydia trachomatis, serovar E. Nucleic Acids Res. 1990;18:3414. doi: 10.1093/nar/18.11.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayada C, Denamur E, Elion J. Complete sequence of the major outer membrane protein-encoding gene of Chlamydia trachomatis serovar Da*. Gene. 1992;120:129–130. doi: 10.1016/0378-1119(92)90022-h. [DOI] [PubMed] [Google Scholar]
- 17.Stephens R S, Sanchez-Pescador R, Wagar E A, Inouye C, Urdea M S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987;169:3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stothard D R, Boguslawski G, Jones R B. Phylogenetic analysis of the Chlamydia trachomatis major outer membrane protein and examination of potential pathogenic determinants. Infect Immun. 1998;66:3618–3625. doi: 10.1128/iai.66.8.3618-3625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Der Pol B, Jones R B. Comparison of immunotyping of Chlamydia trachomatis by indirect fluorescent-antibody staining and radioimmunoassay J. Clin Microbiol. 1992;30:1014–1015. doi: 10.1128/jcm.30.4.1014-1015.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S P, Kuo C-C, Barnes R C, Stephens R S, Grayston J T. Immunotyping of Chlamydia trachomatis with monoclonal antibodies. J Infect Dis. 1985;152:791–800. doi: 10.1093/infdis/152.4.791. [DOI] [PubMed] [Google Scholar]
- 21.Wang S P, Grayston J T. Three new serovars of Chlamydia trachomatis: Da, Ia, and L2a. J Infect Dis. 1991;163:403–405. doi: 10.1093/infdis/163.2.403. [DOI] [PubMed] [Google Scholar]
- 22.Yuan Y, Zhang Y-X, Watkins N G, Caldwell H D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989;57:1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y-X, Stewart S, Joseph T, Taylor H R, Caldwell H D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987;138:575–581. [PubMed] [Google Scholar]
- 24.Zhang Y-X, Morrison S G, Caldwell H D. The nucleotide sequence of the major outer membrane protein gene of Chlamydia trachomatis serovar F. Nucleic Acids Res. 1990;18:1061. doi: 10.1093/nar/18.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]