Abstract
Background:
Comparisons of lobectomy versus total thyroidectomy for papillary thyroid cancer have not addressed significant threats to valid inference from observational data. The purpose of this study was to compare survival following lobectomy versus total thyroidectomy for papillary thyroid cancer, while addressing bias from unmeasured confounding.
Methods:
This retrospective cohort study included 84,300 patients treated with lobectomy or total thyroidectomy for papillary thyroid cancer in the National Cancer Database from 2004–2017. The primary outcome was overall survival evaluated by flexible parametric survival models and inverse probability weighting on the propensity score. Bias from unobserved confounding was assessed using two-way deterministic sensitivity analysis and two-stage least squares regression.
Results:
The median age of treated patients was 48 years (IQR 37–59), 78% were women, and 76% were white. We found no statistically significant differences in overall survival or 5- and 10-year survival between patients treated with lobectomy or total thyroidectomy. Additionally, we found no statistically significant difference in survival by subgroups, including tumor size (<4cm or ≥4cm), age (<65 or ≥65), or estimated risk of mortality. Sensitivity analyses suggested that an unmeasured confounder would need to have an extremely large effect to change the primary finding.
Conclusions:
This is the first study to compare outcomes of lobectomy and total thyroidectomy while adjusting for and quantifying the potential effects of unmeasured confounding variables on observational data. The findings suggest that total thyroidectomy is unlikely to offer a survival advantage over lobectomy regardless of tumor size, patient age, or overall risk of death.
Article Summary:
We found that total thyroidectomy for papillary thyroid cancer is not associated with improved survival when compared to lobectomy regardless of patient age, tumor size, or overall pre-treatment risk of mortality. Our findings suggest that current guidelines for surgical treatment of papillary thyroid cancer may lead to overtreatment with total thyroidectomy.
INTRODUCTION
There is an urgent need to reassess guidelines for the treatment of papillary thyroid cancer because current guidelines may lead to overtreatment, unnecessarily increasing complications and costs.1,2 Papillary thyroid cancer is the most common endocrine malignancy, with 40–60,000 new cases annually in the U.S., and can typically be treated by removing half of the thyroid (lobectomy) or the entire gland (total thyroidectomy).2 Since lobectomy is associated with fewer complications and greater quality-of-life, total thyroidectomy should be reserved for patients whose tumors justify a more aggressive surgery due to increased risk of death or recurrence.1,3,4
Current guidelines recommend that patients with tumors ≥4 cm should undergo total thyroidectomy, while lobectomy should be reserved for tumors <4cm. These guidelines are based entirely on observational studies because the excellent prognosis for patients with thyroid cancer makes it impractical to design an adequately powered randomized trial evaluating mortality. However, conclusions from observational studies are potentially subject to bias from unmeasured confounding factors that influence both treatment and outcomes.
This study attempts to overcome threats to valid causal inference from observational data that have been inadequately addressed in previous studies, including failure to account for bias from unmeasured confounding, and the absence of sensitivity analysis to test the strength of conclusions and susceptibility to bias.5 Addressing unmeasured confounding is critical because comparisons of lobectomy and total thyroidectomy have typically used large datasets that are missing key patient and tumor variables. This means that any observed outcomes are subject to considerable bias that could change study results. Additionally, our study attempts to provide risk-adjusted information to decision makers in a more intuitive fashion, by describing survival outcomes in terms that are easier to understand and use for comparison of surgical strategies (i.e., survival time differences rather than hazard ratios).
We hypothesized that after accounting for bias from both observed and unobserved confounding variables, overall survival would be similar for patients treated with lobectomy or total thyroidectomy. We also hypothesized that similar survival would be seen in older patients, those with larger tumors, or those at higher risk of death.
METHODS
Study design
We conducted a retrospective cohort study of patients with papillary thyroid cancer captured by the National Cancer Database (NCDB) from 2004–2017. The study is presented according to STROBE guidelines and was considered exempt by the University of Texas Southwestern institutional review board due to use of a publicly available and de-identified dataset.5
Eligibility criteria
We included patients with papillary thyroid cancer, based on International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes, who were treated with lobectomy or total thyroidectomy (Figure 1). Exclusion criteria are also shown in Figure 1. We only evaluated patients who did not receive additional treatments beyond lobectomy or total thyroidectomy (radioactive iodine or neck dissection) because this allows a fair comparison of outcomes based solely on the choice of surgery, and because patients with known nodal disease are unlikely to be reasonable candidates for lobectomy.
Figure 1.
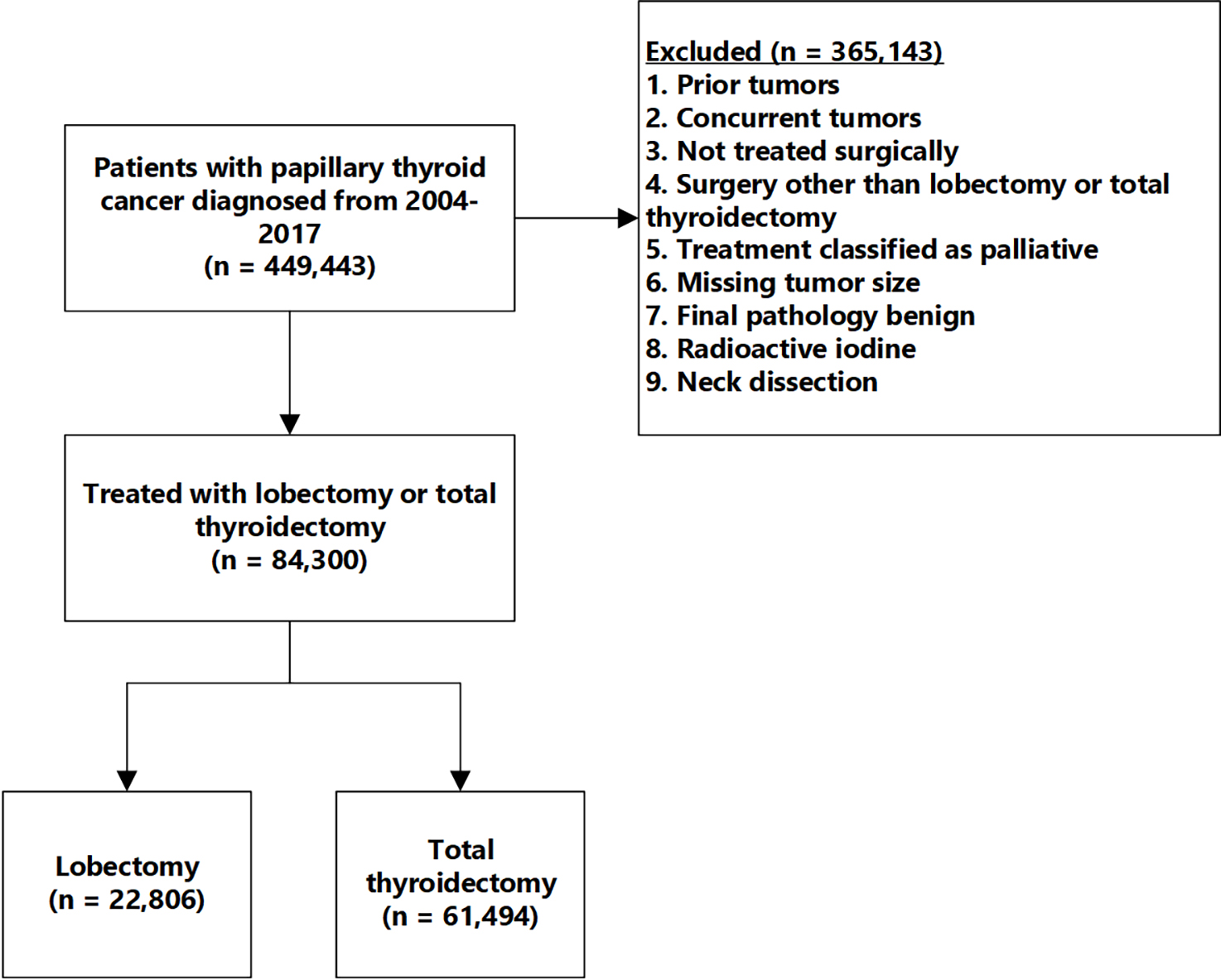
The cohort consists of patients with papillary thyroid cancer who were treated with either lobectomy or total thyroidectomy without neck dissection or radioactive iodine.
Outcomes
Our primary outcome was overall survival measured from the time of surgery until death, with censoring occurring at the date of last follow-up for patients who were not known to be dead at the end of the study.
Exposures
The exposure of interest was surgical treatment: total thyroidectomy or lobectomy.
Effect modifiers
We assessed heterogeneity of treatment effects by testing for interactions between surgery and variables in Table 1 with p<0.05 representing significant interactions. Since this did not identify any significant interactions, results are presented in terms of main effects.
Table 1.
Characteristics of patients treated with lobectomy or total thyroidectomy
| Lobectomy | Total Thyroidectomy | Total | p-value | ||
|---|---|---|---|---|---|
| N=22,806 | N=61,494 | N=84,300 | |||
|
| |||||
| Age, median (IQR) | 51 (41–62) | 51 (41–62) | 51 (41–62) | 0.22 | |
| Sex | Male | 5,042 (22.1) | 11,510 (18.7) | 16,552 (19.6) | <0.001 |
| Female | 17,764 (77.9) | 49,984 (81.3) | 67,748 (80.4) | ||
| Race | White | 17,477 (76.6) | 45,041 (73.2) | 62,518 (74.2) | <0.001 |
| Black | 1,949 (8.5) | 7,061 (11.5) | 9,010 (10.7) | ||
| Hispanic | 1,523 (6.7) | 4,887 (7.9) | 6,410 (7.6) | ||
| Asian | 1,138 (5.0) | 2,630 (4.3) | 3,768 (4.5) | ||
| Other | 344 (1.5) | 955 (1.6) | 1,299 (1.5) | ||
| Unknown | 375 (1.6) | 920 (1.5) | 1,295 (1.5) | ||
| Education Quantile | 1 | 3,426 (15.0) | 10,162 (16.5) | 13,588 (16.1) | <0.001 |
| 2 | 4,745 (20.8) | 13,292 (21.6) | 18,037 (21.4) | ||
| 3 | 6,040 (26.5) | 15,967 (26.0) | 22,007 (26.1) | ||
| 4 | 6,329 (27.8) | 15,908 (25.9) | 22,237 (26.4) | ||
| Income quantile | 1 | 2,786 (12.2) | 8,158 (13.3) | 10,944 (13.0) | <0.001 |
| 2 | 3,818 (16.7) | 10,599 (17.2) | 14,417 (17.1) | ||
| 3 | 5,169 (22.7) | 13,615 (22.1) | 18,784 (22.3) | ||
| 4 | 8,745 (38.3) | 22,916 (37.3) | 31,661 (37.6) | ||
| Urban/Rural Classification | Counties in metro areas of 1 million population or more | 12,349 (54.1) | 33,989 (55.3) | 46,338 (55.0) | <0.001 |
| Counties in metro areas of 250,000 to 1 million population | 4,639 (20.3) | 12,350 (20.1) | 16,989 (20.2) | ||
| Counties in metro areas of fewer than 250,000 population | 2,222 (9.7) | 5,404 (8.8) | 7,626 (9.0) | ||
| Urban population of 20,000 or more adjacent to a metro area | 951 (4.2) | 2,493 (4.1) | 3,444 (4.1) | ||
| Urban population of 20,000 or more not adjacent to a metro area | 337 (1.5) | 738 (1.2) | 1,075 (1.3) | ||
| Urban population of 2,500 to 19,999, adjacent to a metro area | 957 (4.2) | 2,737 (4.5) | 3,694 (4.4) | ||
| Urban population of 2,500 to 19,999, not adjacent to a metro area | 436 (1.9) | 1,177 (1.9) | 1,613 (1.9) | ||
| Completely rural or less than 2,500 urban population, adjacent to a metro area | 162 (0.7) | 479 (0.8) | 641 (0.8) | ||
| Completely rural or less than 2,500 urban population, not adjacent to a metro area | 158 (0.7) | 441 (0.7) | 599 (0.7) | ||
| Unknown/missing | 595 (2.6) | 1,686 (2.7) | 2,281 (2.7) | ||
| Insurance status | Not Insured | 531 (2.3) | 1,684 (2.7) | 2,215 (2.6) | <0.001 |
| Private Insurance/Managed Care | 15,359 (67.3) | 40,750 (66.3) | 56,109 (66.6) | ||
| Medicaid | 1,668 (7.3) | 4,699 (7.6) | 6,367 (7.6) | ||
| Medicare | 4,721 (20.7) | 12,734 (20.7) | 17,455 (20.7) | ||
| Other Government | 222 (1.0) | 747 (1.2) | 969 (1.1) | ||
| Insurance Status Unknown | 305 (1.3) | 880 (1.4) | 1,185 (1.4) | ||
| Year of diagnosis | 2004–2008 | 5,730 (25.1) | 13,863 (22.5) | 19,593 (23.2) | <0.001 |
| 2009–2013 | 8,070 (35.4) | 23,993 (39.0) | 32,063 (38.0) | ||
| 2014–2017 | 9,006 (39.5) | 23,638 (38.4) | 32,644 (38.7) | ||
| Charlson Comorbidities | 0 | 18,924 (83.0) | 50,127 (81.5) | 69,051 (81.9) | <0.001 |
| 1 | 3,067 (13.4) | 9,085 (14.8) | 12,152 (14.4) | ||
| 2 | 579 (2.5) | 1,696 (2.8) | 2,275 (2.7) | ||
| ≥3 | 236 (1.0) | 586 (1.0) | 822 (1.0) | ||
| Surgical approach | Robotic / Laparoscopic | 364 (1.6) | 898 (1.5) | 1,262 (1.5) | <0.001 |
| Open | 14,991 (65.7) | 41,598 (67.6) | 56,589 (67.1) | ||
| Unknown | 7,451 (32.7) | 18,998 (30.9) | 26,449 (31.4) | ||
| Multifocality | Solitary tumor | 18,738 (82.2) | 42,188 (68.6) | 60,926 (72.3) | <0.001 |
| Multifocal tumor | 3,811 (16.7) | 18,522 (30.1) | 22,333 (26.5) | ||
| Unknown | 257 (1.1) | 784 (1.3) | 1,041 (1.2) | ||
| Lymph node status | All nodes examined are negative | 486 (2.1) | 1,708 (2.8) | 2,194 (2.6) | <0.001 |
| One or more positive nodes | 15 (0.1) | 114 (0.2) | 129 (0.2) | ||
| Not applicable/Unknown | 22,305 (97.8) | 59,672 (97.0) | 81,977 (97.2) | ||
| Tumor Size | |||||
| <4cm | 21,200 (97) | 56,224 (96.4) | <0.001 | ||
| ≥4 cm | 660 (3) | 2,086 (3.6) | |||
| Lymphovascular Invasion | |||||
| Present | 340 (1.5) | 1,335 (2.2) | 1,675 (2.0) | ||
| Tumor Grade | Well differentiated | 3,205 (14.1) | 9,167 (14.9) | 12,372 (14.7) | <0.001 |
| Moderately differentiated | 284 (1.2) | 1,227 (2.0) | 1,511 (1.8) | ||
| Poorly/undifferentiated | 69 (0.3) | 236 (0.4) | 305 (0.4) | ||
| Cell type not determined / Unknown | 19,248 (84.4%) | 50,864 (82.7%) | 70,112 (83.2%) | ||
| Surgical margins | No residual tumor | 21,425 (93.9) | 57,582 (93.6) | 79,007 (93.7) | <0.001 |
| Residual tumor | 658 (2.9) | 2,651 (4.3) | 3,309 (3.9) | ||
| Indeterminate | 258 (1.1) | 442 (0.7) | 700 (0.8) | ||
| Unknown or not applicable | 465 (2.0) | 819 (1.3) | 1,284 (1.5) | ||
| Facility Type | Community Cancer Program | 2,013 (8.8) | 4,423 (7.2) | 6,436 (7.6) | <0.001 |
| Comprehensive Community Cancer Program | 8,552 (37.5) | 23,658 (38.5) | 32,210 (38.2) | ||
| Academic/Research Program | 8,968 (39.3) | 24,464 (39.8) | 33,432 (39.7) | ||
| Integrated Network Cancer Program | 3,224 (14.1) | 8,865 (14.4) | 12,089 (14.3) | ||
| Unknown/missing | 49 (0.2) | 84 (0.1) | 133 (0.2) | ||
| Facility Location | New England | 1,611 (7.1) | 3,885 (6.3) | 5,496 (6.5) | <0.001 |
| Middle Atlantic | 5,195 (22.8) | 11,472 (18.7) | 16,667 (19.8) | ||
| South Atlantic | 4,070 (17.8) | 14,278 (23.2) | 18,348 (21.8) | ||
| East North Central | 3,811 (16.7) | 9,851 (16.0) | 13,662 (16.2) | ||
| East South Central | 1,307 (5.7) | 3,991 (6.5) | 5,298 (6.3) | ||
| West North Central | 1,594 (7.0) | 3,643 (5.9) | 5,237 (6.2) | ||
| West South Central | 1,633 (7.2) | 5,157 (8.4) | 6,790 (8.1) | ||
| Mountain | 1,095 (4.8) | 2,883 (4.7) | 3,978 (4.7) | ||
| Pacific | 2,441 (10.7) | 6,250 (10.2) | 8,691 (10.3) | ||
| Unknown/missing | 49 (0.2) | 84 (0.1) | 133 (0.2) | ||
All values are written as n(%) unless otherwise specified.
Potential bias
Our analysis sought to account for several key sources of bias, with primary consideration given to unmeasured confounders.
Justification for sample size
We conducted an a priori estimate of the necessary sample size to detect a 2% difference in 10-year overall survival. Assuming that 10-year survival for total thyroidectomy patients approximates 95%, that no more than 30% of patients undergo lobectomy, and that loss to follow-up is similar between groups, a sample size of 10,000 patients would be sufficient to detect a 2% difference in survival with alpha <0.05 and beta 0.8.
Handling of continuous variables
Converting continuous variables such as age and tumor size into categories is inefficient and introduces bias into statistical modeling.6–8 Consequently, we employed fractional polynomial transformations of age and modeled tumor size using the negative exponential transformation suggested by Royston and Parmar. 7,9–11
Statistical Analysis
Methods used
We employed two complementary approaches to create balanced comparisons of survival following lobectomy and total thyroidectomy, incorporating patient, hospital, and tumor factors (Table 1).
Flexible parametric survival model
Flexible parametric survival models estimate the underlying hazard function as a series of restricted cubic splines.10,11 The model facilitates estimation of the marginal survival effect of surgery and allows computation of differences in the probability of surviving to particular time points. We followed best practices outlined by Royston and Parmar to develop the model using the variables in Table 1 and assessed final model fit using Royston’s R2, Harrell’s C-statistic, and Martingale residuals.10,11
We also used the model to divide patients into five groups based on their predicted risk of death: 1st-20th centile (lowest risk), 20th-40th centile, 40th-60th centile, 60th-80th centile, and 80th-100th centile (highest risk). This allowed comparison of total thyroidectomy versus lobectomy in higher versus lower predicted mortality groups.
Propensity weighting
We applied inverse probability of treatment weights based on the propensity score to calculate the average treatment effect for the treated.12 We estimated the propensity score with the variables listed in Table 1 using an iterative process that incorporated consideration of interactions and higher order polynomials.13,14 The final propensity score model was selected based on optimization of the Bayesian Information Criteria and was considered acceptable if the standardized difference for all variables was less than 10% and the variance approximated 1.14All comparisons were conducted within the common overlap region.
Pre-specified subgroup analyses
Tumor size: Since current guidelines recommend total thyroidectomy for tumors ≥4 cm, we conducted two analyses based on tumor size. First, we compared overall survival between lobectomy and total thyroidectomy for patients with tumors <4 cm and ≥4 cm. Second, we compared survival based on increasing tumor size from 1 cm to 8 cm, in 1 cm increments. This second analysis represented the average difference in survival at each tumor size, treating size as a continuous rather than categorical variable.
Age: We analyzed age-related differences in a similar fashion by comparing overall survival for patients <65 years old and for patients ≥65 years old, and by analyzing the average survival in 10-year increments ranging from 20 years to 90 years.
Risk categories: We used the parametric survival model to categorize each patient’s overall risk of death and divided them into 5 quintiles ranging from low to high risk. We compared overall survival for lobectomy versus total thyroidectomy within each risk category.
Sensitivity analysis
We employed two techniques to assess effects of bias from unmeasured confounders: (1) two-way deterministic sensitivity analysis to quantify bias, and (2) a two-stage least squares model that accounts for the effects of unmeasured confounders.
Two-way deterministic sensitivity analysis
It is important to quantify how much bias would be necessary to change results of observational studies because if it requires large or unrealistic amounts of bias to change a conclusion, then more faith can be placed in those estimates.15 For this study, it is possible that survival for lobectomy patients is biased upward toward longer survival because lobectomy is preferentially offered to patients with less extensive or aggressive disease. We conducted a deterministic sensitivity analysis to quantify how much of this excess survival bias would have to be removed before lobectomy would be associated with worse survival than total thyroidectomy. To accomplish this, we randomly selected different proportions of patients who underwent lobectomy (ranging from 0.1% to 100%) and simulated a decrease in their survival that ranged from 0.1% to 50%. We then re-ran our models to determine whether the simulated change resulted in a significant difference in survival between lobectomy and total thyroidectomy.
Two-stage least squares
To further evaluate whether unmeasured confounding could potentially change our results, we employed instrumental variables in a two-stage least squares model that accounts for unmeasured confounding when estimating treatment effects.16 Details of this approach are provided in Supplemental File 1.
Results
Participants
The cohort included 84,300 patients with papillary thyroid cancer, with 22,806 (27%) lobectomy patients and 61,494 (73%) treated with total thyroidectomy (Figure 1). Mean follow up time was 73 months, with 4% experiencing mortality and 96% censored.
Table 1 shows no significant difference in age between the two groups (median 51 year, IQR 41 years - 62 years for both, p<0.22), but patients having total thyroidectomy were more likely to be women (81% vs 78%, p<0.001), less likely to be white (73% vs 77%, p<0.001), more likely to have multi-focal disease (30% vs 17%, p<0.001), and had larger median tumor size (0.7cm vs 0.5cm, p<0.001). Other differences are shown in Table 1.
Primary outcome: overall survival
Flexible parametric survival models did not demonstrate any significant differences in 5- or 10- year overall survival between patients treated with lobectomy versus total thyroidectomy (Table 2). The estimated risk-adjusted 5-year overall survival for patients treated with lobectomy was 96% (95% CI 95% - 97%) and was similar for total thyroidectomy (96%, 95% CI 95% - 97%). Ten-year overall survival was also similar (Table 2).
Table 2.
There is no significant difference in the risk-adjusted probability of 5- or 10-year overall survival between patients treated with lobectomy or total thyroidectomy
| Probability of 5-year overall survival | Probability of 10-year overall survival | |||
|---|---|---|---|---|
| Lobectomy | Total Thyroidectomy | Lobectomy | Total Thyroidectomy | |
|
| ||||
| Entire cohort | 0.961 (0.952–0.968) | 0.961 (0.953–0.968) | 0.912 (0.896–0.926) | 0.913 (0.897–0.927) |
| Age | ||||
| 20 Years | 0.992 (0.991–0.993) | 0.992 (0.991–0.993) | 0.973 (0.970–0.975) | 0.973 (0.971–0.975) |
| 30 Years | 0.991 (0.990–0.992) | 0.991 (0.990–0.992) | 0.970 (0.967–0.972) | 0.970 (0.968–0.972) |
| 40 Years | 0.989 (0.988–0.990) | 0.989 (0.988–0.990) | 0.963 (0.960–0.966) | 0.964 (0.961–0.966) |
| 50 Years | 0.984 (0.983–0.986) | 0.985 (0.984–0.986) | 0.949 (0.945–0.953) | 0.950 (0.047–0.953) |
| 60 Years | 0.974 (0.972–0.976) | 0.975 (0.973–0.976) | 0.919 (0.913–0.925) | 0.920 (0.916–0.924) |
| 70 Years | 0.949 (0.945–0.953) | 0.950 (0.947–0.953) | 0.852 (0.842–0.862) | 0.854 (0.846–0.861) |
| 80 Years | 0.883 (0.872–0.893) | 0.884 (0.875–0.893) | 0.705 (0.683–0.726) | 0.708 (0.688–0.727) |
| 90 Years | 0.707 (0.677–0.737) | 0.710 (0.681–0.739) | 0.438 (0.399–0.477) | 0.442 (0.404–0.478) |
| <65 Years | 0.982 (0.977–0.985) | 0.982 (0.977–0.986) | 0.949 (0.937–0.959) | 0.949 (0.938–0.959) |
| ≥65 Years | 0.872 (0.848–0.893) | 0.874 (0.851–0.893) | 0.719 (0.679–0.756) | 0.721 (0.682–0.757) |
| Tumor Size | ||||
| 1 cm | 0.968 (0.966–0.970) | 0.969 (0.967–0.970) | 0.912 (0.907–0.917) | 0.913(0.909–0.917) |
| 2 cm | 0.964 (0.962–0.967) | 0.965 (0.963–0.967) | 0.903 (0.897–0.909) | 0.904 (0.900–0.909) |
| 3 cm | 0.961 (0.958–0.964) | 0.961 (0.959–0.963) | 0.895 (0.887–0.902) | 0.896 (0.890–0.901) |
| 4 cm | 0.957 (0.953–0.961) | 0.958 (0.955–0.961) | 0.887 (0.878–0.896) | 0.888 (0.881–0.895) |
| 5 cm | 0.954 (0.949–0.958) | 0.955 (0.951–0.958) | 0.880 (0.869–0.872) | 0.881 (0.872–0.890) |
| 6 cm | 0.951 (0.945–0.956) | 0.952 (0.947–0.956) | 0.873 (0.860–0.885) | 0.874 (0.863–0.884) |
| 7 cm | 0.948 (0.942–0.954) | 0.949 (0.943–0.954) | 0.866 (0.852–0.880) | 0.868 (0.856–0.880) |
| 8 cm | 0.945 (0.938–0.952) | 0.946 (0.940–0.952) | 0.861 (0.845–0.876) | 0.862 (0.848–0.876) |
| <4 cm | 0.962 (0.954–0.97) | 0.963 (0.955–0.97) | 0.916 (0.9–0.929) | 0.916 (0.929–0.901) |
| ≥4 cm | 0.922 (0.904–0.937) | 0.923 (0.905–0.937) | 0.845 (0.816–0.871) | 0.847 (0.819–0.871) |
| Risk Quintile | ||||
| 1 (lowest risk) | 0.998 (0.996–0.999) | 0.996 (0.995–0.997) | 0.993 (0.988–0.995) | 0.988 (0.985–0.991) |
| 2 | 0.993 (0.991–0.995) | 0.993 (0.991–0.994) | 0.981 (0.974–0.986) | 0.979 (0.975–0.982) |
| 3 | 0.986 (0.983–0.989) | 0.989 (0.987–0.991) | 0.979 (0.975–0.982) | 0.967 (0.962–0.971) |
| 4 | 0.975 (0.970–0.979) | 0.972 (0.969–0.975) | 0.926 (0.914–0.936) | 0.918 (0.910–0.925) |
| 5 (highest risk) | 0.880 (0.870–0.889) | 0.886 (0.880–0.892) | 0.685 (0.666–0.703) | 0.697 (0.685–0.709) |
Propensity score analysis also found no significant difference in risk-adjusted average survival between patients treated with lobectomy versus total thyroidectomy (Table 3, absolute survival difference 1.8 months, 95% CI -6 months to 2.5 months, p <0.4).
Table 3.
There is no significant difference in risk-adjusted mean survival between patients treated with lobectomy versus total thyroidectomy
| Average Difference in Survival After Propensity Weighting (months) | |
|---|---|
|
| |
| Entire cohort | −1.8 (−6.0 – 2.5) |
| Age <65 years | −5.5 (−12.1 – 1.0) |
| Age ≥65 years | 0.01 (−5.6 – 5.6) |
| Size <4cm | −2.2 (−6.7 – 2.2) |
| Size ≥4cm | 2.8 (−11.1 – 16.6) |
Subgroup analysis
Tumor size
We compared the probability of 5- and 10-year risk-adjusted survival for lobectomy versus total thyroidectomy as tumor size increased from 1 cm to 8 cm, in 1 cm increments (Table 2). Greater tumor size was associated with lower probability of 5- and 10-year overall survival, but the surgical approach was not associated with significant differences in survival at any level of tumor size (Table 2). There was also no obvious linear trend in the survival differences between lobectomy and total thyroidectomy to indicate that the treatment effect changed as tumor size increased. Additionally, we analyzed survival based on the guideline cutoff of 4 cm and found no statistically significant difference in overall survival for tumors <4 cm or for those ≥4 cm. Comparison of average survival with propensity scores also showed so significant difference for lobectomy versus total thyroidectomy (Table 3).
Age
Since overall mortality increases with age, we evaluated whether different age groups experienced differential benefit from lobectomy. As shown in Table 2, we compared risk-adjusted survival estimates for patients in 10-year increments from age 20 to 90 years old. Although survival for both lobectomy and total thyroidectomy slowly declined with increasing age, at no age was there a significant difference in 5- or 10-year overall survival when comparing lobectomy versus total thyroidectomy. We did not detect any trend towards differential survival as age increased, suggesting that the effects of lobectomy and total thyroidectomy are consistent across the age spectrum. We also compared overall survival for patients <65 and ≥65 years using flexible parametric models and propensity scores but did not find any significant difference in predicted survival (Tables 2 and 3).
Predicted risk category
Patients were divided into 5 quintiles of estimated mortality risk using a flexible parametric model. Table 2 shows that there is no significant difference in overall survival between lobectomy and total thyroidectomy among any of the 5 quintiles, indicating patients at high and low risk of mortality experience similar overall survival regardless of the surgical strategy.
Sensitivity analysis
Two-way deterministic sensitivity analysis
As a check on the potential influence of omitted variables and selection bias, we conducted a sensitivity analysis to quantify the amount of bias necessary to change model results. We simulated how the models would be affected by decreasing survival for a 0.1% to 100% random subset of lobectomy patients by an amount ranging from 0.1% to 50%, representing excess survival due to bias (Figure 2). We found that for total thyroidectomy to be associated with increased survival relative to lobectomy, it would require a significant proportion of lobectomy patients to have substantially worse survival than was actually observed. For example, as shown in Figure 2, 50% of lobectomy patients would need to have their survival reduced by ≥15% before total thyroidectomy would be associated with a significant improvement in survival. Similarly, 75% of lobectomy patients would need to have their survival reduced by ≥10% for total thyroidectomy to be associated with improved survival. The large changes in survival that would be necessary to change our findings strongly suggest that total thyroidectomy is unlikely to be associated with a survival benefit even accounting for significant bias from observational data.
Figure 2.
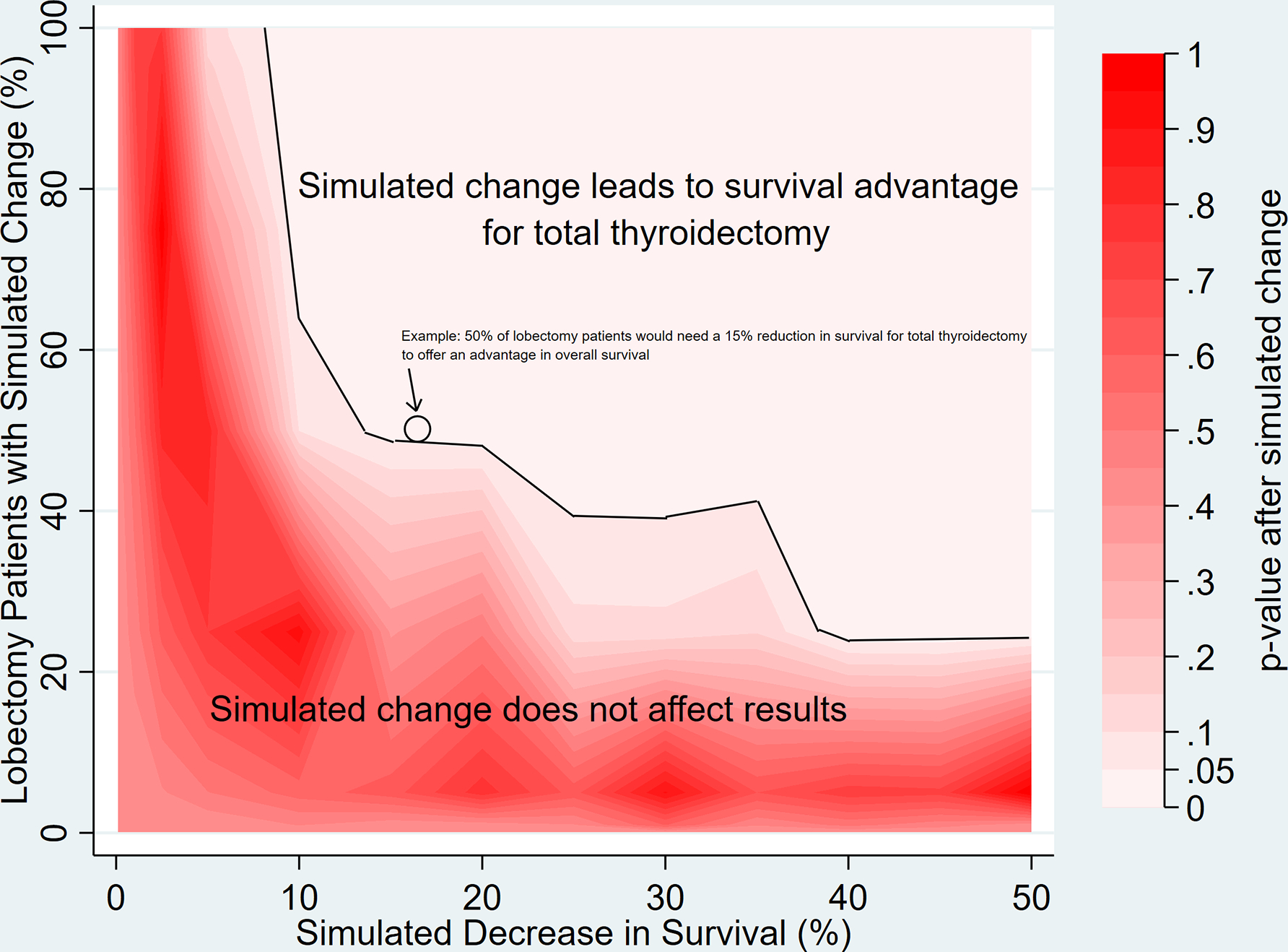
Two-way deterministic sensitivity analysis indicates that the study findings are robust to large potential changes due to bias. The area above the black line indicates the extent of change necessary to alter the main finding that total thyroidectomy is not associated with a significant survival advantage over lobectomy. The x-axis shows the simulated percentage reduction in survival and the y-axis shows the proportion of lobectomy patients affected by the simulated change. This demonstrates the proportion of patients and the extent of change necessary to generate a statistically significant risk-adjusted difference in overall survival that would differ from our main analysis showing no difference in survival. An example case is presented (arrows) demonstrating that 50% of lobectomy patients would need to have their survival reduced by 15% (excess/biased survival time) for total thyroidectomy to be associated with significantly better survival than lobectomy.
Two-stage least squares
As a final check on potential effects of unmeasured confounding variables, we repeated the analysis of the entire cohort and pre-specified subgroups using a two-stage least squares model. This approach also found no significant differences in survival based on choice of surgery for all patients (HR 0.78, 95% CI 0.58–1.06) and each subgroup (data not shown), although the 95% CI for the estimate was fairly wide and the majority of the interval was <1, so these results should be interpreted cautiously.
Discussion
Our primary finding is that for appropriately selected patients, total thyroidectomy was not associated with any detectable survival benefit when compared to lobectomy, regardless of age, tumor size, or overall mortality risk. Additionally, sensitivity analysis indicated that our findings are robust to the potential effects of unmeasured confounding because the amount of bias necessary to change the results is large and a model that accounts for unmeasured confounding also showed no differences in survival. Our study suggests that rather than imposing a hard cutoff where patients with tumors ≥4 cm should only be offered total thyroidectomy, decisions about surgical treatment should be more nuanced and based on informed conversations between patients and their surgeons. If the primary outcome of interest to a particular patient is overall survival or life-expectancy following treatment, then lobectomy appears to be a reasonable option even in larger tumors when patients are appropriately selected. A nuanced consideration of surgical treatment options is especially important because of the growing literature suggesting that total thyroidectomy is associated with diminished quality of life relative to lobectomy.1,17 However, since we were not able to compare the risk of recurrence between lobectomy and total thyroidectomy, it may be reasonable to favor total thyroidectomy if a patient is more concerned about recurrence or reintervention than other factors.
Our study expands the existing literature on outcomes following treatment of papillary thyroid cancer. Prior studies using the National Cancer Database or Surveillance, Epidemiology, and End Results data have generally shown no difference in survival between patients treated with lobectomy or total thyroidectomy.18–22 However, these studies did not account for bias from unmeasured confounding and, despite STROBE recommendations, did not conduct sensitivity analyses to quantify effects of bias. This is particularly important because the datasets lack key patient and tumor variables likely to influence survival. Consequently, findings could be substantially biased by unmeasured confounders. Nixon et al. attempted to analyze a more comprehensive and granular dataset from their cancer center and did not see any differences in survival or recurrence between lobectomy and total thyroidectomy.23 However, their small sample size limited power to detect differences and only included outcomes from one center.
Our study differs from the above work in several key ways: (1) our two-stage least squares models represents the first effort to estimate treatment effects accounting for potential bias from unobserved confounders, (2) we present risk-adjusted comparisons between lobectomy and total thyroidectomy in a format that is simpler for patients and surgeons to use when discussing surgical options. Rather than relying on hazard ratios, we provide quantitative differences in risk-adjusted survival and estimates of surviving for 5 or 10 years after treatment, (3) we used a more complicated but substantially more robust approach to continuous variables such as age and tumor size by treating them as continuous rather than categorical variables. The statistical literature has clearly established the superiority of this approach and the ability to generate more accurate models6, and (4) our use of two-way sensitivity analysis quantifies the extent of bias or other issues that would be needed to invalidate the primary result. In summary, our study provides a more robust estimate of treatment effects and offers greater confidence in the validity of results.
Although we employed robust methods to balance both known and unknown confounding variables and tested assumptions via rigorous sensitivity analysis, there are still limitations to acknowledge. The two-stage least squares model estimates a different outcome than our other models: the local average treatment effect. This represents potential differences in survival among patients and surgeons whose treatment decisions are at least somewhat susceptible to knowledge, beliefs, and practices of other surgeons and patients (i.e., they are more likely to choose or offer lobectomy if more of their peers select lobectomy). If there were surgeons in our cohort who would never offer lobectomy regardless of patient preference or tumor characteristics, then the two-stage model would not accurately reflect differences in outcomes. It seems reasonable, however, that patients and surgeons are at least somewhat amenable to choosing either treatment option even if the actual probability of one option is low. Additionally, when several different methods yield similar conclusions that are robust to large changes in outcomes via sensitivity analysis, it increases confidence in the legitimacy of the findings. It is possible that some cases categorized as lobectomy were completion thyroidectomies and this would bias results toward the null. There is no simple method to address this problem, but we did make a reasonable effort to exclude patients with prior cancer diagnoses and the magnitude of change needed to affect our results makes it unlikely that this limitation would fundamentally change our findings. Finally, we were unable to compare recurrence or reoperation in this dataset. It is conceivable that long-term survival is similar, but recurrence or reintervention might be more common with lobectomy. This difference could have important implications for patients’ quality of life. Although several studies have suggested that lobectomy is associated with improved quality of life relative to total thyroidectomy, it is less clear if this benefit is maintained when reintervention occurs after an initial lobectomy.1,24,25 We are currently designing a study to test how reintervention after lobectomy might affect the risk of recurrence, long-term quality of life, and patient satisfaction with their initial surgical treatment decision.
In summary, our study gives strong observational evidence to motivate endocrine surgeons to reconsider how they discuss surgical treatment options with patients who have papillary thyroid cancer. Rather than using size as a hard cutoff for offering total thyroidectomy, our data suggest that the choice of surgery is unlikely to affect long-term survival. Consequently, surgeons would be better served by discussing both lobectomy and total thyroidectomy and making treatment decisions that align with patient goals, values, and preferences. If patients are comfortable with the possibility of reintervention, are interested in potentially avoiding thyroid hormone supplementation, and are concerned about complications such as permanent hypoparathyroidism, then lobectomy may be the more reasonable option. By contrast, if patients are at higher risk for recurrence, are less willing to tolerate uncertainty about the likelihood of reintervention, or derive greater peace of mind from having the entire thyroid out to eliminate any contralateral cancers, then it becomes much more reasonable to offer total thyroidectomy.
Supplementary Material
Funding/Support
The authors received no funding for this work.
Footnotes
COI/Disclosure
The authors have no relevant financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nickel B, Tan T, Cvejic E, et al. Health-Related Quality of Life After Diagnosis and Treatment of Differentiated Thyroid Cancer and Association With Type of Surgical Treatment. JAMA otolaryngology-- head & neck surgery. 2019;145(3):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. [DOI] [PubMed] [Google Scholar]
- 3.Hauch A, Al-Qurayshi Z, Randolph G, Kandil E. Total thyroidectomy is associated with increased risk of complications for low- and high-volume surgeons. Ann Surg Oncol. 2014;21(12):3844–3852. [DOI] [PubMed] [Google Scholar]
- 4.Al-Qurayshi Z, Farag M, Shama MA, Ibraheem K, Randolph GW, Kandil E. Total Thyroidectomy Versus Lobectomy in Small Nodules Suspicious for Papillary Thyroid Cancer: Cost-Effectiveness Analysis. Laryngoscope. 2020;130(12):2922–2926. [DOI] [PubMed] [Google Scholar]
- 5.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. [DOI] [PubMed] [Google Scholar]
- 6.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25(1):127–141. [DOI] [PubMed] [Google Scholar]
- 7.Royston P, Sauerbrei W. Multivariable Model-Building: A Pragmatic Approach to Regression Analysis Based on Fractional Polynomials for Modelling Continuous Variables. West Sussex, England: John Wiley & Sons, Ltd; 2008. [Google Scholar]
- 8.Harrell FE. Regression Modeling Strategies With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. New York, NY: Springer. [Google Scholar]
- 9.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. New York: Wiley-Interscience; 2000. [Google Scholar]
- 10.Royston P, Lambert PC. Flexible Parametric Survival Analysis Using Stata: Beyond the Cox Model. College Station, TX: Stata Press; 2011. [Google Scholar]
- 11.Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175–2197. [DOI] [PubMed] [Google Scholar]
- 12.Rotnitzky A, Robins JM. Inverse Probability Weighting in Survival Analysis. In: Wiley StatsRef: Statistics Reference Online. [Google Scholar]
- 13.Guo S, Fraser MW. Propensity Score Analysis. Los Angeles: SAGE; 2010. [Google Scholar]
- 14.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Communications in Statistics Simulation and Computation. 2009;38:1228–1234. [Google Scholar]
- 15.Ding P, VanderWeele TJ. Sensitivity Analysis Without Assumptions. Epidemiology. 2016;27(3):368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cameron ACTPK. Microeconometrics using Stata. College Station, Tex.: Stata Press; 2009. [Google Scholar]
- 17.Chen W, Li J, Peng S, et al. Association of Total Thyroidectomy or Thyroid Lobectomy With the Quality of Life in Patients With Differentiated Thyroid Cancer With Low to Intermediate Risk of Recurrence. JAMA Surgery. 2022;157(3):200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246(3):375–381; discussion 381–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adam MA, Pura J, Gu L, et al. Extent of surgery for papillary thyroid cancer is not associated with survival: an analysis of 61,775 patients. Ann Surg. 2014;260(4):601–605; discussion 605–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barney BM, Hitchcock YJ, Sharma P, Shrieve DC, Tward JD. Overall and cause-specific survival for patients undergoing lobectomy, near-total, or total thyroidectomy for differentiated thyroid cancer. Head Neck. 2011;33(5):645–649. [DOI] [PubMed] [Google Scholar]
- 21.Haigh PI, Urbach DR, Rotstein LE. Extent of thyroidectomy is not a major determinant of survival in low- or high-risk papillary thyroid cancer. Ann Surg Oncol. 2005;12(1):81–89. [DOI] [PubMed] [Google Scholar]
- 22.Mendelsohn AH, Elashoff DA, Abemayor E, St John MA. Surgery for papillary thyroid carcinoma: is lobectomy enough? Arch Otolaryngol Head Neck Surg. 2010;136(11):1055–1061. [DOI] [PubMed] [Google Scholar]
- 23.Nixon IJ, Ganly I, Patel SG, et al. Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery. 2012;151(4):571–579. [DOI] [PubMed] [Google Scholar]
- 24.Bongers PJ, Greenberg CA, Hsiao R, et al. Differences in long-term quality of life between hemithyroidectomy and total thyroidectomy in patients treated for low-risk differentiated thyroid carcinoma. Surgery. 2020;167(1):94–101. [DOI] [PubMed] [Google Scholar]
- 25.Lee JI, Kim SH, Tan AH, et al. Decreased health-related quality of life in disease-free survivors of differentiated thyroid cancer in Korea. Health and quality of life outcomes. 2010;8:101–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


