Abstract
Ventricular arrhythmias (VAs) represent a major cause of sudden cardiac death and afflict patients with heart failure from both ischaemic and non-ischaemic origins, and inherited cardiomyopathies. Current VA management, including anti-arrhythmic medications, autonomic modulation, implantable cardioverter–defibrillator implantation, and catheter ablation, remains suboptimal. Catheter ablation may even cause significant cardiomyocyte loss. Cell-based therapies and exosome treatment have been proposed as promising strategies to lessen cardiomyocyte death, modulate immune reaction, and reduce myocardial scarring, and, therefore, are potentially beneficial in treating VAs. In this review, we summarise the current cornerstones of VA management. We also discuss recent advances and ongoing evidence regarding cell-based and exosome therapy, with special attention to VA treatment.
Keywords: Exosomes, Stem cells, Cardiosphere-derived cells, Catheter ablation, Ventricular arrhythmias
Introduction
Ventricular arrhythmias (VAs) continue to be a major contributor to cardiac morbidity and mortality worldwide, despite early coronary interventions and continuous progress in pharmacological therapies for heart failure [1]. Myocardial ischaemic insults [2,3], genetic defects [4,5], and acute/chronic inflammation [6,7] can individually or jointly contribute to arrhythmogenic substrates, facilitating VAs. Current management strategies for VAs include anti-arrhythmic medications, autonomic modulation, implantable cardioverter–defibrillator (ICD) implantation, cardiac stereotactic body radiotherapy (SBRT), and catheter ablation [1,8,9]. Although ICD placement is dramatically effective for terminating VAs and improving survival, ICD shocks can damage myocardial cells and elicit sympathetic responses [10].
Multiple shocks ensue in patients with frequent or incessant VAs, leading to electrical storms and myocardial stunning [10,11]. Superior to the escalation of anti-arrhythmic agents, substrate-based catheter ablation is associated with a higher rate of VA suppression [12,13]; however, it causes some collateral viable myocardium loss [14,15]. The resulting myocardial damage may be pro-arrhythmic, which could contribute to subsequent VAs and worsening cardiac function [15]. Notably, the arrhythmogenic substrates in certain cardiomyopathies remain active and evolve over time. Even though a single ablation procedure is initially effective, patients still encounter recurrent VA events [16,17]. Repeated ablation or “destruction” of heart tissue diminishes more “contractile units” and can exacerbate heart failure [15,16,18]. Alternative approaches to modify VA substrates without further affecting myocardial viability are clearly desired.
Cell-based and cell-derived therapies have been proposed as promising strategies to lessen cardiomyocyte death, modulate immune reaction, and reduce myocardial scarring, leading to improved cardiac function [19–21]. These salutary effects of cells and exosomes merit consideration as potential therapeutic strategies for VAs. Therefore, in this review, we discuss the conventional VA management and current approaches to treating VAs with cell-based and cell-derived therapies. We further discuss the potential mechanisms, including immune modulation and anti-fibrotic effects.
Current Cornerstones of VA Management
The aetiology and pathophysiological mechanisms of VA are complex, thus making the current VA therapeutic effects inconsistent for some patients. In addition to patient- and disease-related factors, beta-blockers and other anti-arrhythmic drugs have limited effectiveness and can sometimes cause undesired adverse drug effects [8]. Many of these drugs may even lead to unintended pro-arrhythmic consequences [8,22]. In patients with refractory VAs, autonomic modulation, such as stellate ganglion block or surgical sympathetic denervation, could reduce VA burden, but temporarily, and thus are usually used for bridging to advanced therapeutic interventions [23]. Ironically, ICDs are dramatically effective for terminating VAs and prolonging survival, yet they do not prevent VA recurrence [10]. VAs recurrence is associated with increased mortality and heart failure hospitalisations in ICD patients, despite effective termination of the arrhythmias [24]. Life-saving shocks themselves have been shown to be independently associated with myocardial tissue damage and an increased risk of heart failure exacerbation, recurrent VAs, and mortality [25,26]. This highlights the importance of blocking VA development rather than terminating VAs. Thus, targeting arrhythmogenic substrates with catheter ablation has emerged as an effective mechanistic-based approach.
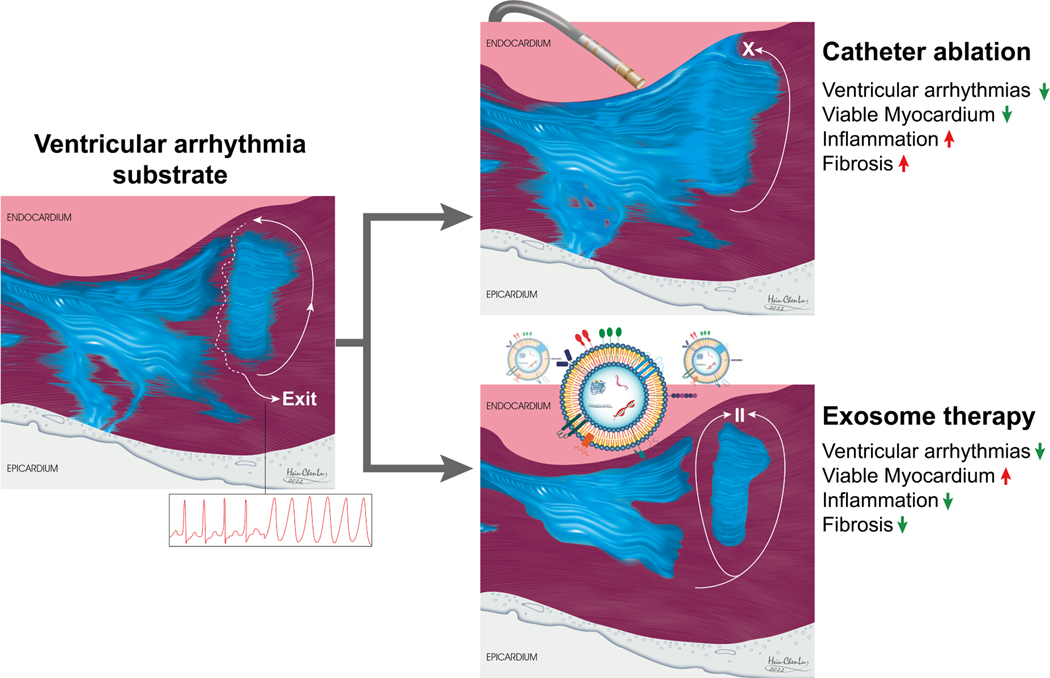
Advances in three-dimensional electroanatomical mapping systems (3D EAM) have greatly facilitated catheter ablation of complex VAs. The multicentre randomised PARTITA trial recently demonstrated that early catheter ablation after the first ICD shock diminished the composite endpoint of worsening heart failure hospitalisations or death compared with standard therapy [27]. Common catheter ablation strategies include scar de-channelling, core isolation, endocardial/epicardial scar homogenisation, and eradication of late potentials or local abnormal activity [28]. In some instances, simultaneous endocardial and epicardial delineation technique addresses arrhythmogenic tissue harbored in the mid-myocardium and subepicardium and enhances the precision of ablation therapy [29]. Although catheter ablation effectively destroys the myocardial tissue underlying critical arrhythmia circuits, patients may lose more heart tissue and have extensive dense scarring at the same time (Figure 1). Electrical isolation of arrhythmogenic substrates results in regional non-contractile myocardium [30], which dampens ventricular systolic and diastolic functions. Another therapeutic strategy that has gained attention is the SBRT. Although it was first developed to treat solid tumours, SBRT has shown promising results by ablating arrhythmogenic sites with highly precise action [9]. Similar to radiofrequency ablation, radioablation induces double-strand deoxyribonucleic acid (DNA) breaks and programmed cell death, which leads to late myocardial fibrosis and electrical inert tissue [31]. In many patients with VA, the arrhythmogenic substrates remain active and evolve over time [16].
Figure 1.
Summary of the effects of catheter ablation and exosome-based therapies on the ventricular arrhythmogenic substrate.
In an arrhythmogenic cardiomyopathy (ACM) cohort, 3D EAM showed progressive scarring and right ventricular dilation during each VA recurrence [16]. Despite aggressive catheter ablation with simultaneous endocardial and epicardial approach, recurrent VAs were still observed in more than a quarter of patients [17]. Sixty (60%) to 100% of patients who underwent cardiac SBRT also suffered from recurrent VAs. Inhomogeneity of radioablation effects on targeted cardiac tissue created new diseased paths, thus altering arrhythmic circuits and limiting therapeutic efficacy [32,33]. An alternative therapeutic concept for healing/repairing arrhythmogenic myocardial tissue has been proposed to alleviate the VA burden to compensate for the loss of more cardiac fibres with each catheter ablation.
Cell-Based Therapy in VA Management: Anti-Arrhythmic or Pro-Arrhythmic?
The delivery of different cell types, which has been shown to halt abnormal cardiac remodelling and restore ventricular contractility, is considered beneficial in the management of VAs. By direct remuscularisation [34,35], indirect remuscularisation [36,37], or non-myogenic paracrine effects [38,39], depending on the type of cells delivered, cell-based cardiac repair seeks to modify arrhythmogenic substrates and thus reduce arrhythmogenicity.
A pioneering phase I trial using autologous skeletal muscle myoblast transplantation in severe ischaemic cardiomyopathy patients reduced the New York Heart Association (NYHA) score and improved systolic function [40]. However, 44% of the patients presented episodes of sustained ventricular tachycardia. Although it elicited arrhythmias, this study showed the feasibility of using cell therapy in patients and opened the possibilities to improve it.
Pluripotent stem cells (PSC), including embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC), are believed to differentiate into de novo cardiomyocytes (CMs) [41], and are therefore able to repair cardiac conduction and eliminate re-entry circuits. Several studies have shown that human (h)ESC-CM grafts transplanted into injured hearts contracted synchronously with host muscle and demonstrated 1:1 host-graft electromechanical coupling [42]. Interestingly, although injured hearts treated with hESC-CM grafts show improved mechanical function and a significantly reduced incidence of both spontaneous and induced VAs, the most concerning obstacle to achieving the clinical translation of the PSC-CM is still arrhythmogenicity [43].
VAs have also been identified post-PSC transplantation in many large animal studies, which unequivocally refute the anti-arrhythmic effects of PSC-CM [43]. Electrophysiological studies performed in large animals (swine and non-human primates) with sustained engraftment have shown increased ventricular arrhythmias at the site of engraftment [34,44,45]. A recent computer simulation study of PSC-CM patches in the human post-myocardial infarction model showed arrhythmogenicity of remuscularisation, which was closely linked to re-entry mechanism [46]. Nonetheless, preclinical studies have suggested that automaticity is the main cause of these VAs [45,47]. Addressing the cellular heterogeneity of current differentiation protocols, improving maturity and gap junctions of engrafted cardiomyocytes, conducting dose escalation experiments, and optimising pharmacological therapy are all paramount in balancing pro-arrhythmic and anti-arrhythmic effects after cell transplantation [43]. To date, barriers remain to cell-based therapies targeting remuscularisation for treating VAs, and an effective solution is yet to be defined.
Transplantation of non-PSC cells, including bone-marrow-derived stem cells (BMSCs), mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs), and cardiosphere-derived cells (CDCs), can exert its salutary effects through non-myogenic paracrine and indirect remuscularisation mechanisms, without raising the concern of developing VAs [41]. Specifically, transplanted cells secrete exosomes that contain bioactive factors promoting angiogenesis, preventing fibrosis, and apoptosis, which results in reduced CM loss and increased native CM proliferation [48]. Furthermore, in a rodent model of heart failure with preserved ejection fraction, CDC therapy decreased VAs by shortening action potential duration (APD), improving APD homogeneity, and decreasing fibrosis [49], suggesting that other non-myogenic mechanisms are beneficial in VA treatment, such as anti-fibrosis and anti-inflammation (Figure 1). The effects of CDC-secreted exosomes on VAs will be further discussed in the following section.
Role of Inflammation and Fibrosis in VA Substrates
Cardiomyocyte death, hyperinflammation, and fibrosis embrace a vicious triangle that underlies most cardiomyopathies, directly or indirectly, responsible for forming arrhythmogenic substrates [50]. Inflammation can exacerbate cell loss and increase cardiac fibrosis, which is associated with altered cardiac electrical properties, namely conduction slowing or block, favouring re-entrant tachyarrhythmias [51]. Specifically, inflammatory cytokines (interleukin [IL]-17, tumour necrosis factor-α, and IL-6) have been suggested to cause gap junction dislocation and downregulation [52,53]. IL-1β decreases inward potassium current, prolongs repolarisation, and increases diastolic SR Ca2+ leak, making cardiomyocytes susceptible to early and late afterdepolarisation [54]. Moreover, infiltrating macrophages can couple to cardiomyocytes via gap junctions and change APD [55]. The increased APD heterogeneity thus serves as a non-fibrotic, “functional” arrhythmogenic substrate. Consistent with this notion, a recent study compared the secretome of human MSCs from failing and nonfailing hearts. MSCs from heart failure patients prolonged APD, increased Ca2+ alternans, and promoted spontaneous calcium release activity [56]. Failing MSCs exhibited increased secretion of IL-1β and IL-6, and anti-cytokine therapies rescued the arrhythmia substrates [56]. Together, inflammation and fibrosis are also critical in arrhythmogenesis. This evidence highlights the potential of immune modulation and antifibrosis therapies to pave new avenues for VA management.
Towards an Exosome Therapy for VAs
Current literature has shown that cell-based therapy stimulates cardiac regeneration largely via indirect non-myogenic paracrine effects, but not through direct remuscularisation [21,48]. Among the biological products secreted by transplanted cells, the role of exosomes is still considered the most important. Exosomes are small extracellular vesicles that contain a variety of bioactive molecules, including nucleic acid, proteins, lipids, and some metabolites [21,57]. Exosomes secreted by progenitor and stromal cells deliver a “regenerative cargo” to the damaged myocardium and exert anti-apoptotic, anti-inflammatory, and anti-fibrotic effects [57,58]. The administration of CDC-derived exosomes has been shown to recapitulate the regenerative and functional effects produced by CDC transplantation; however, inhibition of exosome production by CDCs blocks these benefits [48]. In an acute myocardial infarction model, exosome injection into the border zone improves cardiac function and decreases the level of proinflammatory cytokines. Reduction in scar mass and increased viable myocardium was seen after exosome therapy [48]. The favourable biological effects suggest that exosome therapy can potentially modify the microenvironment and remodel arrhythmogenic substrates with a consequent reduction in arrhythmias. Pro-arrhythmic effects after exosome therapies remain an important concern but have not been shown in animal studies [48,59,60].
Exosomes Attenuate ACM
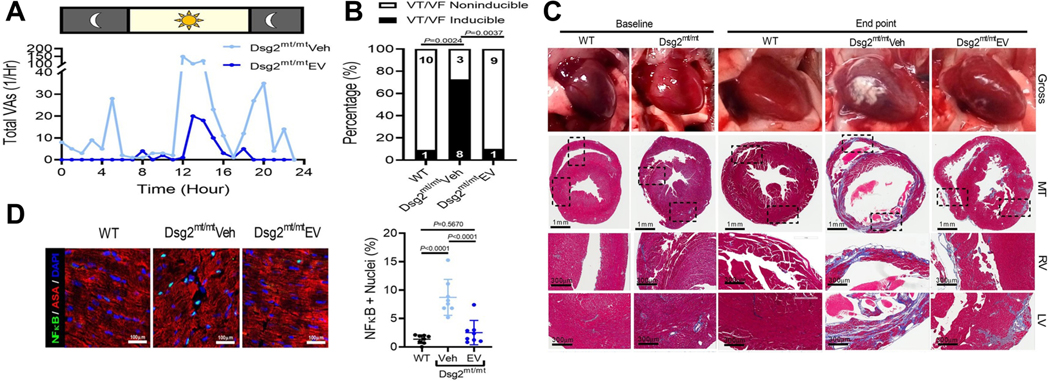
ACM is a hereditary disease characterised by progressive myocyte loss, hyperinflammation, and fibrofatty replacement, which disposes to VAs and sudden cardiac death [50,61]. In a mouse ACM model, weekly injection of exosomes secreted by immortalised CDCs with high β-catenin expression prevented abnormal biventricular remodelling. It is noteworthy that ambulatory telemetry and programmed electrical stimulation showed that spontaneous and inducible VA were reduced after exosomes treatment (Figure 2) [59]. Ex vivo electrophysiology studies using high-resolution optical mapping showed that exosomes restore cardiac conduction, shorten APD and reduce APD dispersion. Immunohistochemistry and transcriptome analyses of the exosome-treated hearts exhibited decreased cell death, tempered inflammation, and reduced fibrosis. Exosomes from engineered CDCs contain a distinct content of microRNAs, with enrichment of miR-4488. Antagonising miR-4488 caused increased inflammation and partially reversed the salutary properties of exosomes, including the anti-arrhythmic effects [59]. Together, this study demonstrated that inflammation plays a vital role in ACM arrhythmogenic substrate, while exosomes can modulate the inflammation, thus alleviating the VA burden. This knowledge may be valuable to other inherited diseases with inflammation as the main driver for arrhythmogenesis. As far as we know, there is no report of exosome-based therapy use for other genetic conditions, which brings the need for more studies in the field.
Figure 2.
Effects of exosome-based therapy on arrhythmogenic cardiomyopathy. (A) Quantification of ventricular arrhythmias over twenty-four hours. (B) Incidence of ventricular tachycardia and fibrillation induced by programmed electrical stimulation. (C) Representative gross pathology (top) and Masson’s trichrome-stained micrographs. (D) Representative images from immunohistochemical staining for nuclear factor-kB and α-sarcomeric actinin (ASA) and quantification of nuclei positive for nuclear factor-kB.
Reproduced with permission from (Lin et al., 2021) [59]. Copyright © 2023, Oxford University Press. Abbreviations: VAs, ventricular arrhythmias; VT, ; VF, ; Dsg2, ; WT, ; Veh, ; EV, ; DSG2; MT, ; RV, LV,
Exosomes Suppress VAs in Ischaemic Cardiomyopathy
Ischaemic heart disease is still the main cardiovascular disease and the primary cause of death in the USA [62]. Most of these deaths are due to sudden cardiac death [63], which is attributed to lethal ventricular arrhythmias [64]. The potential therapeutic effects of exosomes have been tested in a rodent model of acute myocardial infarction [65]. For this purpose, iPSC-derived exosomes were packaged into hydrogel patches and implanted after infarct induction. The treatment with exosomes led to reduced cardiac dilation and improved function two weeks after infarction. Also, hearts treated with exosome-based therapy showed reduced infarct size, apoptosis, hypertrophy, and VA. Interestingly, in contrast to what is observed after iPSC-CM implantation, this cell-free approach was not arrhythmogenic [65].
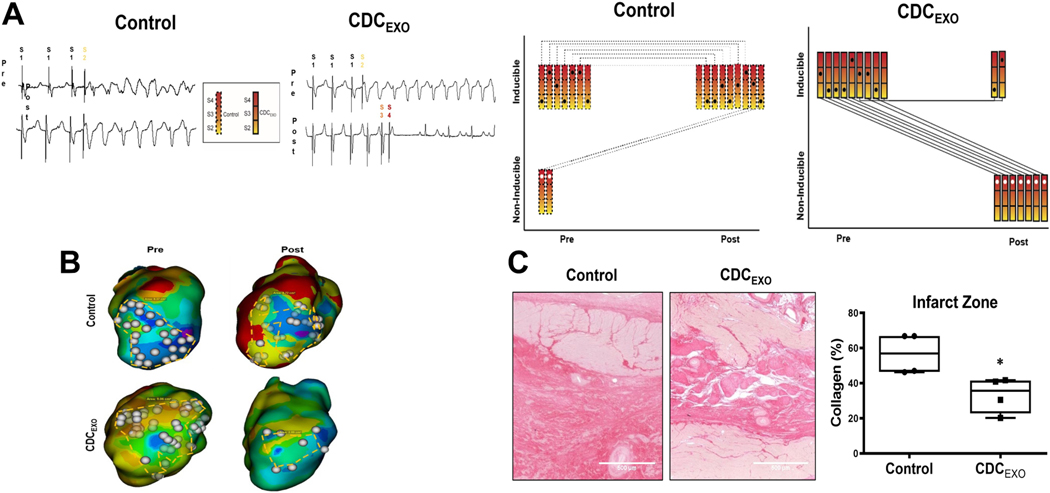
Exosome-based therapy has also been tested in a porcine model of chronic ischaemic cardiomyopathy and has shown anti-arrhythmic benefits (Figure 3) [60]. In this study, 3D EAM was performed at baseline and after exosome injection, and arrhythmogenic substrates were characterised, identifying areas with abundant isolated late potentials (a surrogate of delayed conduction). Exosome treatment reduced the amount of fibrosis, thus altering wavefront propagation, resulting in lack of sustained reentry [60]. Moreover, animals that received exosomes and were initially susceptible to inducible arrhythmias became non-inducible, whereby the arrhythmogenic state of the control animals remained the same at the endpoint [60]. Proteomic analysis of exosome-treated myocardial tissue showed major pattern differences in pathways related to cell proliferation, inflammation, and fibrosis. Sequencing the RNA content of exosomes identified several miRs involved in fibrosis regulation and immunomodulation [60]. In summary, exosomes derived from CDCs not only promote cardiomyocyte proliferation, but also antagonise inflammation and fibrosis. Such changes could synergistically decrease the arrhythmogenic substrate.
Figure 3.
Effects of exosome-based therapy on ischemia cardiomyopathy. (A) Representative traces of programmed electrical stimulation in a porcine model of myocardial infarction. (B) Representative isochronal maps of areas of late activation in myocardial infarction. (C) Picrosirius red-stained sections of the infarct zone of infarcted pigs.
Reproduced with permission from (Dawkins et al., 2022) [60]. Copyright © 2023, Oxford University Press.
Abbreviations: CDCEXO,.
Conclusion
Despite decades of effort, the management of VA remains suboptimal and challenging. The current scenario shows that substrate modification by catheter ablation achieves superior outcomes compared to medical management alone, but VA recurrence rates are still high, and the ablation lesions may beget new arrhythmogenic substrates. In fact, most VA critical circuits are not mappable in haemodynamically unstable patients after induction of VAs. Successful ablation is usually achieved by extensive ablation at the expense of massive cardiomyocyte loss. Rather than destroying viable myocardium, a regenerative and anti-fibrotic approach provides an alternative for VA management. Moving from cell-based therapy to cell-derived therapies, growing evidence has shown the potential of exosomes for modifying arrhythmogenic substrates and exerting anti-arrhythmic effects in animal models of disease. From a future perspective, we believe substrate modifications with catheter ablation, SBRT, and cell-derived therapies are complementary. Cell-derived therapies could benefit patients with substrates and the risk of VAs, while those with incessant or sustained VAs could receive ablation or radiotherapy to eliminate culprit circuits (Table 1). Although additional studies are needed to assess the long-term efficacy and safety of exosomes, including direct comparison with ablation and radiotherapy prior to translating this approach to humans, “cell-free” exosomes-based therapies may open new avenues for the management of arrhythmias.
Table 1.
Comparisons of current and novel methods of arrhythmia substrate modification.
| Catheter Ablation | SBRT | Cell-Derived Therapy | |
|---|---|---|---|
| Mechanism of action | Thermal injury of the arrhythmia substrates with resisted heat and conduction heat | Photon damaging the DNA of myocardial cells and cause subsequent mitotic catastrophe | Reduce inflammation and fibrosis and induce indirect remuscularisation within the arrhythmia substrates |
| Response to therapy | Immediate | In days to weeks | In weeks |
| Advantages | Modify the arrhythmia substrates directly and effectively | Target the arrhythmia substrates regardless of the vascular access, cardiac anatomy | Repair the arrhythmia substrates in a regeneration way without causing cardiomyocyte loss |
| Role of clinical application | Patients with incessant or sustained VAs | Patients with incessant or sustained VAs | Patients with substrate and risks of VAs |
Abbreviations: SBRT, cardiac stereotactic body radiotherapy; DNA, deoxyribonucleic acid; VAs, ventricular arrhythmias.
Sources of Funding
Y.N.L. was supported by DMR-110-247, C1100820020, and DMR-111-021. R.M.S. was supported by the California Institute for Regenerative Medicine (#EDUC4-12751). Research in Dr. Cingolani’s lab is supported by the National Institutes of Health (RO1 HL135866, R01 HL147570).
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri N, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm. 2020;17:e2–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amoni M, Dries E, Ingelaere S, Vermoortele D, Roderick HL, Claus P, et al. Ventricular arrhythmias in ischemic cardiomyopathy—new avenues for mechanism-guided treatment. Cells. 2021;10:2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gräni C, Benz DC, Gupta S, Windecker S, Kwong RY. Sudden cardiac death in ischemic heart disease: from imaging arrhythmogenic substrate to guiding therapies. JACC: Cardiovasc Imaging. 2020;13:2223–38. [DOI] [PubMed] [Google Scholar]
- [4].Kirubakaran S, Bisceglia C, Silberbauer J, Oloriz T, Santagostino G, Yamase M, et al. Characterization of the arrhythmogenic substrate in patients with arrhythmogenic right ventricular cardiomyopathy undergoing ventricular tachycardia ablation. EP Europace. 2017;19:1049–62. [DOI] [PubMed] [Google Scholar]
- [5].Pappone C, Ciconte G, Manguso F, Vicedomini G, Mecarocci V, Conti M, et al. Assessing the malignant ventricular arrhythmic substrate in patients with Brugada syndrome. J Am Coll Cardiol. 2018;71:1631–46. [DOI] [PubMed] [Google Scholar]
- [6].Peretto G, Sala S, Rizzo S, Palmisano A, Esposito A, De Cobelli F, et al. Ventricular arrhythmias in myocarditis: characterization and relationships with myocardial inflammation. J Am Coll Cardiol. 2020;75:1046–57. [DOI] [PubMed] [Google Scholar]
- [7].Tse G, Yeo JM, Chan YW, Lai ETHL, Yan BP. What Is the arrhythmic substrate in viral myocarditis? Insights from clinical and animal studies. Front Physiol. 2016;7:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018;138:e272–391. [DOI] [PubMed] [Google Scholar]
- [9].Krug D, Blanck O, Andratschke N, Guckenberger M, Jumeau R, Mehrhof F, et al. Recommendations regarding cardiac stereotactic body radiotherapy for treatment refractory ventricular tachycardia. Heart Rhythm. 2021;18:2137–45. [DOI] [PubMed] [Google Scholar]
- [10].Stevenson WG, John RM. Ventricular arrhythmias in patients with implanted defibrillators. Circulation. 2011;124:e411–4. [DOI] [PubMed] [Google Scholar]
- [11].Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Arenal Á, Ávila P, Jiménez-Candil J, Tercedor L, Calvo D, Arribas F, et al. Substrate ablation vs anti-arrhythmic drug therapy for symptomatic ventricular tachycardia. J Am Coll Cardiol. 2022;79:1441–53. [DOI] [PubMed] [Google Scholar]
- [13].Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin K, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357:2657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Haegeli LM, Kotschet E, Byrne J, Adam DC, Lockwood EE, Leather RA, et al. Cardiac injury after percutaneous catheter ablation for atrial fibrillation. EP Europace. 2008;10:273–5. [DOI] [PubMed] [Google Scholar]
- [15].Peichl P, Wichterle D, Pavlu L, Cihak R, Aldhoon B, Kautzner J. Complications of catheter ablation of ventricular tachycardia. Circ Arrhyth Electrophysiol.2014;7:684–90. [DOI] [PubMed] [Google Scholar]
- [16].Lin C-Y, Chung F-P, Kuo L, Lin Y-J, Chang S-L, Lo L-W, et al. Characteristics of recurrent ventricular tachyarrhythmia after catheter ablation in patients with arrhythmogenic right ventricular cardiomyopathy. J Cardiovasc Electrophysiol. 2019;30:582–92. [DOI] [PubMed] [Google Scholar]
- [17].Romero J, Patel K, Briceno D, Alviz I, Gabr M, Diaz JC, et al. Endo-epicardial ablation vs endocardial ablation for the management of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2020;31:2022–31. [DOI] [PubMed] [Google Scholar]
- [18].Yokoyama K, Nakagawa H, Wittkampf FHM, Pitha JV, Lazzara R, Jackman WM. Comparison of electrode cooling between internal and open irrigation in radiofrequency ablation lesion depth and incidence of thrombus and steam pop. Circulation. 2006;113:11–9. [DOI] [PubMed] [Google Scholar]
- [19].Guo Y, Yu Y, Hu S, Chen Y, Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020;11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marbán E. The secret life of exosomes: what bees can teach us about next-generation therapeutics. J Am Coll Cardiol. 2018;71:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dan G-A, Martinez-Rubio A, Agewall S, Boriani G, Borggrefe M, Gaita F, et al. Anti-arrhythmic drugs–clinical use and clinical decision making: a consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP). EP Europace. 2018;20:731–732an. [DOI] [PubMed] [Google Scholar]
- [23].Fudim M, Qadri YJ, Waldron NH, Boortz -Marx RL., Ganesh A, Patel CB, et al. Stellate ganglion blockade for the treatment of refractory ventricular arrhythmias. JACC Clin Electrophysiol. 2020;6:562–71. [DOI] [PubMed] [Google Scholar]
- [24].Dorian P, Hohnloser SH, Thorpe KE, Roberts RS, Kuck K-H, Gent M, et al. Mechanisms underlying the lack of effect of implantable cardioverter-defibrillator therapy on mortality in high-risk patients with recent myocardial infarction: insights from the Defibrillation in Acute Myocardial Infarction Trial (DINAMIT). Circulation. 2010;122:2645–52. [DOI] [PubMed] [Google Scholar]
- [25].Bodin A, Labas V, Bisson A, Teixeira-Gomes A-P, Blasco H, Tomas D, et al. Acute pathophysiological myocardial changes following intra-cardiac electrical shocks using a proteomic approach in a sheep model. Sci Rep. 2020;10:20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hulsmans M, Aguirre AD, Bonner MD, Bapat A, Cremer S, Iwamoto Y, et al. A miniaturized, programmable pacemaker for long-term studies in the mouse. Circ Res. 2018;123:1208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Della Bella P, Baratto F, Vergara P, Bertocchi P, Santamaria M, Notarstefano P, et al. Does timing of ventricular tachycardia ablation affect prognosis in patients with an implantable cardioverter defibrillator? Results from the multicenter randomized PARTITA trial. Circulation. 2022;145:1829–38. 10.1161/CIRCULATIONAHA.122.059598. [DOI] [PubMed] [Google Scholar]
- [28].Pandozi C, Lavalle C, Russo M, Galeazzi M, Ficili S, Malacrida M, et al. Mapping of ventricular tachycardia in patients with ischemic cardiomyopathy: current approaches and future perspectives. Clin Cardiol. 2019;42:1041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tung R, Raiman M, Liao H, Zhan X, Chung FP, Nagel R, et al. Simultaneous endocardial and epicardial delineation of 3D reentrant ventricular tachycardia. J Am Coll Cardiol. 2020;75:884–97. [DOI] [PubMed] [Google Scholar]
- [30].Di Biase L, Mohanty S, Trivedi C, Romero J, Natale V, Briceno D, et al. Stroke risk in patients with atrial fibrillation undergoing electrical isolation of the left atrial appendage. J Am Coll Cardiol 2019;74:1019–28. [DOI] [PubMed] [Google Scholar]
- [31].Cuculich PS, Schill MR, Kashani R, Mutic S, Lang A, Cooper D, et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med 2017;377:2325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ninni S, Gallot-Lavallée T, Klein C, Longère B, Brigadeau F, Potelle C, et al. Stereotactic radioablation for ventricular tachycardia in the setting of electrical storm. Circ Arrhythm Electrophysiol. 2022;15:e010955. [DOI] [PubMed] [Google Scholar]
- [33].Gianni C, Rivera D, Burkhardt JD, Pollard B, Gardner E, Maguire P, et al. Stereotactic arrhythmia radioablation for refractory scar-related ventricular tachycardia. Heart Rhythm. 2020;17:1241–8. [DOI] [PubMed] [Google Scholar]
- [34].Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388–91.. [DOI] [PubMed] [Google Scholar]
- [35].Ye L, Chang Y-H, Xiong Q, Zhang P, Zhang L, Somasundaram P, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Huang W, Feng Y, Liang J, Yu H, Wang C, Wang B, et al. Loss of microRNA-128 promotes cardiomyocyte proliferation and heart regeneration. Nat Commun. 2018;9:700. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang LL, Liu Y, Chung JJ, Wang T, Gaffey AC, Lu M, et al. Local and sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischemic injury. Nat Biomed Eng. 2017;1:983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38:201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tachibana A, Santoso MR, Mahmoudi M, Shukla P, Wang L, Bennett M, et al. Paracrine effects of the pluripotent stem cell-derived cardiac myocytes salvage the injured myocardium. Circ Res. 2017;121:e22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Menasché P, Hagège AA, Vilquin J-T, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–83. [DOI] [PubMed] [Google Scholar]
- [41].Yu JK, Franceschi W, Huang Q, Pashakhanloo F, Boyle PM, Trayanova NA. A comprehensive, multiscale framework for evaluation of arrhythmias arising from cell therapy in the whole post-myocardial infarcted heart. Sci Rep. 2019;9:9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shiba Y, Fernandes S, Zhu W-Z, Filice D, Muskheli V, Kim J, et al. hESC-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Selvakumar D, Reyes L, Chong JJH. Cardiac cell therapy with pluripotent stem cell-derived cardiomyocytes: what has been done and what remains to do? Curr Cardiol Rep. 2022;24:445–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu Y-W, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, et al. Human embryonic stem cell–derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol. 2018;36:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Romagnuolo R, Masoudpour H, Porta-Sánchez A, Qiang B, Barry J, Laskary A, et al. Human embryonic stem cell-derived cardiomyocytes regenerate the infarcted pig heart but induce ventricular tachyarrhythmias. Stem Cell Reports. 2019;12:967–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yu JK, Liang JA, Franceschi WH, Huang Q, Pashakhanloo F, Sung E, et al. Assessment of arrhythmia mechanism and burden of the infarcted ventricles following remuscularization with pluripotent stem cell-derived cardiomyocyte patches using patient-derived models. Cardiovasc Res. 2022;118:1247–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chong JJH, Yang X, Don CW, Minami E, Liu Y-W, Weyers JJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ibrahim AG- E, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cho JH, Kilfoil PJ, Zhang R, Solymani RE, Bresee C, Kang EM, et al. Reverse electrical remodeling in rats with heart failure and preserved ejection fraction. JCI Insight. 2018;3:121123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [50].Lin Y-N, Ibrahim A, Marbán E, Cingolani E. Pathogenesis of arrhythmogenic cardiomyopathy: role of inflammation. Basic Res Cardiol. 2021;116:39. [DOI] [PubMed] [Google Scholar]
- [51].Morita N, Mandel WJ, Kobayashi Y, Karagueuzian HS. Cardiac fibrosis as a determinant of ventricular tachyarrhythmias. J Arrhythm. 2014;30:389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Panagopoulou P, Davos CH, Milner DJ, Varela E, Cameron J, Mann DL, et al. Desmin mediates TNF-alpha-induced aggregate formation and intercalated disk reorganization in heart failure. J Cell Biol. 2008;181:761–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Xue J, Yan X, Yang Y, Chen M, Wu L, Gou Z, et al. Connexin 43 dephosphorylation contributes to arrhythmias and cardiomyocyte apoptosis in ischemia/reperfusion hearts. Basic Res Cardiol. 2019;114:40. [DOI] [PubMed] [Google Scholar]
- [54].Monnerat G, Alarcón ML, Vasconcellos LR, Hochman-Mendez C, Brasil G, Bassani RA, et al. Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. Nat Commun. 2016;7:13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fei Y-D, Wang Q, Hou J-W, Li W, Cai X-X, Yang Y-L, et al. Macrophages facilitate post myocardial infarction arrhythmias: roles of gap junction and KCa3.1. Theranostics. 2019;9:6396–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sattayaprasert P, Vasireddi SK, Bektik E, Jeon O, Hajjiri M, Mackall JA, et al. Human cardiac mesenchymal stem cells remodel in disease and can regulate arrhythmia substrates. Circ Arrhythm Electrophysiol. 2020;13:e008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yellon DM, Davidson SM. Exosomes: nanoparticles involved in cardioprotection? Circ Res. 2014;114:325–32. [DOI] [PubMed] [Google Scholar]
- [58].Adamiak M, Sahoo S. Exosomes in myocardial repair: advances and challenges in the development of next-generation therapeutics. Mol Ther. 2018;26:1635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lin Y-N, Mesquita T, Sanchez L, Chen Y-H, Liu W, Li C, et al. Extracellular vesicles from immortalized cardiosphere-derived cells attenuate arrhythmogenic cardiomyopathy in desmoglein-2 mutant mice. Eur Heart J. 2021;42:3558–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dawkins JF, Ehdaie A, Rogers R, Soetkamp D, Valle J, Holm K, et al. Biological substrate modification suppresses ventricular arrhythmias in a porcine model of chronic ischaemic cardiomyopathy. Eur Heart J. 2022;43:2139–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Corrado D, Basso C, Judge DP. Arrhythmogenic cardiomyopathy. Circ Res. 2017;121:784–802. [DOI] [PubMed] [Google Scholar]
- [62].Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–596. [DOI] [PubMed] [Google Scholar]
- [63].Berg DD, Wiviott SD, Braunwald E, Guo J, Im K, Kashani A, et al. Modes and timing of death in 66 252 patients with non-ST-segment elevation acute coronary syndromes enrolled in 14 TIMI trials. Eur Heart J. 2018;39:3810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Solomon SD, Zelenkofske S, McMurray JJV, Finn PV, Velazquez E, Ertl G, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med 2005;352:2581–8. [DOI] [PubMed] [Google Scholar]
- [65].Liu B, Lee BW, Nakanishi K, Villasante A, Williamson R, Metz J, et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat Biomed Eng. 2018;2:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]