Abstract
Cell encapsulation has been studied for various applications ranging from cell transplantation to biological production. However, current encapsulation technologies focus on cell protection rather than cell regulation that is essential to most if not all cell-based applications. Here we report a method for cell nanoencapsulation and regulation using an ultrathin biomimetic extracellular matrix as a cell nanocapsule to carry nanoparticles (CN2). This method allows high-capacity nanoparticle retention at the vicinity of cell surface. The encapsulated cells maintain high viability and normal metabolism. When gold nanoparticles (AuNPs) are used as a model to decorate the nanocapsule, light irradiation transiently increases the temperature, leading to the activation of the heat shock protein 70 (HSP70) promoter and the regulation of reporter gene expression. As the biomimetic nanocapsule can be decorated with any or multiple NPs, CN2 is a promising platform for advancing cell-based applications.
Keywords: cell regulation, encapsulation, biomimetic material, nanoparticle, cell surface engineering
GraphicalAbstract
A biomimetic extracellular matrix is synthesized on the surface of live cells as a cell nanocapsule to carry nanoparticles. Nanoparticles at the vicinity of the encapsulated cell surface allow for on-demand regulation of intracellular gene expression. As any or multiple nanoparticles can be applied to decorate the nanocapsule, this work demonstrates a promising technology platform for cell encapsulation and regulation.
Cells are “living factories” that can actively release biomolecules.[1, 2] For example, mesenchymal stem cells (MSCs) can release a multitude of immunomodulatory factors in response to inflammatory mediators.[3] Beta cells in the islets can release insulin in response to glucose.[4] Thus, live cells have been widely used as intelligent tools for both in vivo and ex vivo applications.[5] However, cells are fragile and easy to lose functions in the applications. When cells are delivered in vivo for cell therapies, cells have to face an immune attack if they are not autologous.[6] While the immune attack is not a concern for ex vivo applications, other factors such as shear stress are problematic. Thus, cells need protection to ensure that their normal functions will not be lost.
Encapsulation is currently the main concept developed for cell protection.[7–9] Both microcapsules and nanocapsules have been studied, depending on specific needs or methods to be used. Microcapsules can be formed when cells are suspended in a polymer solution and extruded from a channel into a reaction solution.[10] Nanocapsules can be formed by alternatively treating cells with positively and negatively charged polymers using the layer-by-layer coating method.[11] Both in vivo and ex vivo studies have shown that microcapsules and nanocapsules can protect cells from environmental attacks. In addition, cells in microcapsules or nanocapsules face a minimal hurdle in nutrient and oxygen transport due to the smaller geometry of the capsules compared to those immobilized in bulk biomaterials. Thus, cell encapsulation has been widely studied for applications such as diabetes treatment, cancer therapy, biological production, three-dimensional bioprinting, etc.[12–15]
Cells reside in the extracellular matrix (ECM).[16] The ECM not only allows for cell immobilization and protection, but also dynamically regulates cell functions through biochemical and biophysical stimulation. The ECM and its components have been widely used for cell culture and regulation either in a three-dimensional space or on a two-dimensional surface.[17] Therefore, in principle, microcapsules and nanocapsules can be designed to mimic the functions of the ECM to regulate cellular functions. For example, the Tsukruk group applied layer-by-layer to develop polyelectrolyte nanocapsules and control the cellular functions by reversible charge variation on the cell surface.[18] In addition to the biomimetic ECM, an exoskeleton-like nanocoating was also developed using inorganic NPs.[19] Tannic acid-mediated interparticle binding and NP-cell complexation led to virtually instantaneous coating of cells. This nanocoating could not only protect cells, but also control the replicative states of cells. In this work, we studied the CN2 method for cell nanoencapsulation and regulation with a NP-decorated biomimetic ECM. This method harnesses the merits of both polymeric materials and inorganic NPs. Polymeric nanocapsules are mechanically soft, which is highly similar to the natural living environment of most cells. Moreover, as NPs can be designed with a diverse array of properties and functions, there will be numerous options of regulating cellular functions. Therefore, the decoration of nanocapsules or microcapsules with NPs is expected to become a new technology platform for the regulation of encapsulated cells.[20]
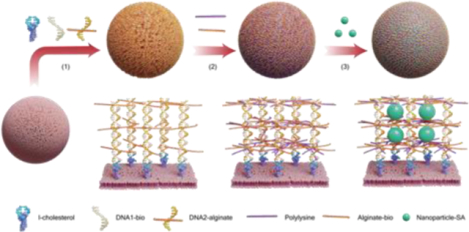
The principle of CN2 is schematically illustrated in Fig. 1a. As cholesterol has been widely used to display molecules (e.g., DNA) on the cell membrane,[21] DNA initiator is anchored on the cell membrane through cholesterol insertion. The initiator triggers the assembly of DNA1 and DNA2 to form a DNA nanostructure (Supplementary Fig. 1, Supplementary Fig. 2, and Supplementary Table 1). A variety of DNA nanostructures with a diverse array of shapes and geometries have been studied.[22, 23] nanostructures in principle can be further functionalized with any biomolecules to acquire new functions.[24] Thus, when DNA2 is conjugated with alginate, the DNA nanostructure can direct alginate display to form a biomimetic nanocapsule around the cell through electrostatic crosslinking. The three-dimensional space of the biomimetic nanocapsule can be further decorated with NPs through conjugation.
Figure 1.

Illustration of the CN2 method. a, Schematic illustration of encapsulating cells with the supramolecular DNA-alginate-polylysine complex and decorating the nanocapsule with NPs. The DNA nanostructure is tethered on the cell membrane through cholesterol insertion and DNA assembly (1). Alginate and polylysine are crosslinked through electrostatic interactions (2). NPs are immobilized in the supramolecular complex through molecular binding or conjugation (3). To illustrate the concept, biotinylated DNA1 (DNA1-bio), biotinylated alginate (Alginate-bio) and streptavid-coated NPs (Nanoparticle-SA) were used in this work. b, Fluorescence imaging of microparticle encapsulation and NP decoration. For fluorescence imaging, DNA was labeled with Cy5 and quantum dots (QD525) were used as the model of NPs. The modified microparticles were analyzed using fluorescence microscopy (middle) and Maestro Imaging (right). c, Flow cytometry analysis of CN2 functionalized microparticles. The concentration of DNA was increased from 0.1 μM to 2.0 μM. Representative histograms for each concentration are shown. Data are presented as mean fluorescence intensity (n = 3).
We first used microparticles as a cell model to establish the CN2 method. Quantum dot (QD) 525 was used as a model of NPs as QD nanocrystals are highly bright and photostable.[25] DNA1 and alginate were biotinylated, and NPs were functionalized with streptavidin (SA) for conjugating NPs to the nanocapsule. For observation under the fluorescence microscope, alginate and polylysine were labeled with Cy5 and Cy3, respectively. The microparticles exhibited the signals of Cy5, Cy3 and QD525, demonstrating the feasibility of using the CN2 method to encapsulate the microparticles and decorate the encapsulated microparticles with NPs (Supplementary Fig. 3,). This observation was confirmed by the scanning electron microscopy images showing the change of the surface morphology of microparticles. We further studied the effects of different conditions on NP decoration. The fluorescence intensity of both the nanocapsule and NPs increased with the DNA concentration (Fig. 1b). The mean fluorescence intensity (MFI) of microparticles increased 4 times from approximately 51 to 205 when the DNA concentration increased from 0.1 to 2.0 μM (Fig. 1c). These data indicate that CN2 would be a promising method for cell nanoencapsulation and NP decoration.
Next, we studied CN2 for CCRF-CEM (a suspension cell line) nanoencapsulation and NP decoration. The confocal microscopy images show the strong fluorescence signals of DNA1-FAM, DNA2-Cy5, and PLL-Cy3 (Supplementary Fig. 4). With sequential coating treatment and NP decoration, the cell exhibited the strong fluorescence signals of Cy5, Cy3, and QD525 (Fig. 2a and Supplementary Fig. 5). To demonstrate the effectiveness of CN2 in cell nanoencapsulation and NP decoration, flow cytometric analysis was used. CN2-NPs were successfully decorated on the cell (Fig. 2b). Scanning transmission electron microscopy images were consistent with the confocal microscopic observation and flow cytometry analysis, confirming that the nanocapsule carried NPs on the surface (Fig. 2c).
Figure 2.

Cell encapsulation and NP decoration. a, Confocal microscopy images of the encapsulated cells with QD525. DNA2 was labeled with Cy5 and polylysine (PLL) was labeled with Cy3. Inset images show the fluorescence signals of a representative cell (scale bar: 5 μm). b, Flow cytometry histograms for the analysis of CN2-NPs for carrying NPs on the cell surface. c, Scanning transmission electron microscopy images of cell surface. d, Kinetics of NP dissociation and internalization when the cell was decorated with CN2-NPs. e, Retention of NPs on the cell surface at different time points. f, Confocal microscopy images of cells acquired at 0 h and 4 h post decoration. The right figure is a 3D fluorescence intensity plot of one confocal plane (i.e., the inset image) captured at 4 h (scale bar: 5 μm).
As we intended to use NPs to regulate the functions of encapsulated cells, we investigated NP behavior on the cell surface. NPs have three states including dissociation from the membrane, internalization into cytoplasm, and retention on the surface.[26] We hypothesized that CN2-NPs would be sustainably retained on the cell surface. To test this hypothesis, we measured the fluorescence intensity of cells at different time points to quantify NP dissociation at 37 °C. We treated the cells at different time points with trypan blue that is a membrane impermeable molecule with a unique function to quench QD525.[27] Thus, we could use this method to determine NP internalization.
The dissociation of NPs from the encapsulated cells exhibits a linear relationship with time (Fig. 2d). After 4 hours, only 12% of CN2-NPs were dissociated from the cell surface. Interestingly, the internalization of NPs also exhibits a linear relationship with time. However, the degree of internalization was less than dissociation. After 4 hours, the percentage of internalized CN2-NPs was 5%. Based on the quantification of NP dissociation and internalization, we calculated NP retention on the cell surface. The percentage of the remaining CN2-NPs on the cell surface was 83.1% after 4 hours (Fig. 2e). We also used confocal microscopy imaging to examine the distribution of NPs (Fig. 2f). Few CN2-NPs were internalized into the cytoplasm. These data collectively show that NPs in the nanocapsules could be sustainably retained on the cell surface. Long-term durability of the nanocapsule and NP retention was further investigated. The flow cytometry showed that 67.4%, 49.7%, 23.2%, and 0.5% of CN2-NPs were retained after 6, 12, 18, and 24 hours post decoration, respectively (Supplementary Fig. 6).
Cell protection by the nanocoating was also analyzed by reiterative cell exposure to centrifugation (Supplementary Fig. 7). Native and CN2-NP decorated cells exhibited 76.4% and 95.9% viability, respectively. The result shows that the nanocapsule can protect the host cells from physical assault. Effects of the nanocapsule on cell-cell interactions were also examined by using CCRF-CEM cells and natural killer (NK) cells as a model system. Surprisingly, NK cells bound native CCRF-CEM cells (4.6%) and nanocoated cells (5.0%) nearly equally (Supplementary Fig. 8). The result indicates that the nanocoating does not affect the contact between NK and CCRF-CEM cells.
CCRF-CEM is a non-adherent cell line. To test the rigor of CN2, we further studied the concept using human umbilical vein endothelial cells (HUVECs) and MSCs that are adherent cells. Consistent with CCRF-CEM, HUVECs were successfully decorated with CN2-NPs (Supplementary Fig. 9). MSCs have been studied for a variety of potential applications. Using MSCs also allowed us to examine whether CN2 can be used to regulate cells for potential biomedical applications. In this work, we investigated gene regulation in MSCs for angiogenesis. The data show that MSCs could be encapsulated and stably decorated with NPs (Supplementary Fig. 10). Thus, with these three cell types, we have demonstrated the universality of this CN2method.
We further evaluated the effects of nanoencapsulation and NP decoration on the functionality of MSCs including cell viability, migration, and differentiation, and protein expressions. The data of oxidoreductase activity show that MSCs with CN2-NPs didn’t show significant cytotoxicity in comparison to native cells (Supplementary Fig. 11). The migration of MSCs with CN2-NPs was slightly slower than native MSCs. As MSCs are multipotent cells, we also examined their differentiation. The cell staining showed that encapsulated MSCs with CN2-NPs maintained their function of differentiation. MSCs after CN2 decoration exhibited a similar pattern of protein expression as native MSCs. For example, the five cytokines that are closely relevant to angiogenic activity had a similar level for encapsulated MSCs with and without CN2-NPs. These data show that nanoencapsulation and CN2-NP decoration did not interfere with the normal functionality of MSCs.
NPs have been developed with a diverse array of functions.[28] Among them, gold nanoparticles (AuNPs) have attracted a significant attention due to their high biocompatibility and high light-to-heat conversion efficiency in response to irradiation.[29] Thus, we used AuNPs as a model to illustrate the proposed concept. When AuNPs are displayed at the vicinity of the cell surface, we expected that light irradiation would transiently increase the intracellular temperature and thereby activate heat shock gene promoters for cell regulation. Heat shock protein 70 (HSP70) promoter is a heat-inducible promoter commonly used for designing gene regulation systems in a broad range of studies.[30] We designed a DNA plasmid with a HSP70 promoter and a functional gene encoding either vascular endothelial growth factor (VEGF) or luciferase. VEGF can promote local blood flow due to its ability to increase vascular permeability and induce angiogenesis. MSCs transfected with VEGF plasmids hold a potential for the treatment of tissue ischemia.[31] Luciferase is a reporter molecule commonly used for the characterization of gene expression. Thus, we transfected MSCs with the luciferase or VEGF plasmids carrying the HSP70 promoter to test the hypothesis (Fig. 3a).
Figure 3.

Examination of cell regulation. a, Illustration of plasmid delivery into cells and heat-responsive gene expression. Plasmids with the HSP70 promoter are delivered into cells before decoration. Transfected cells are encapsulated with the supramolecular DNA-alginate-polylysine complexes and decorated with AuNPs. Under laser irradiation, AuNPs produce heat for the activation of the HSP70 promoter and the expression of reporter genes. The bar graph shows heat-induced luciferase expression of transfected cells incubated in 37 °C water bath for 30 min before treated with luciferin. The luminescence signals were quantified using the Maestro Imaging System. b, Kinetics of luciferase expression after the decorated cells were treated with laser irradiation. The panel (left) shows the images of the four cell samples captured at different time points. The graph (right) shows luciferase expression quantified via the measurement of luminescence (n = 4). c, Quantification of induced VEGF expression. CN2: decorated cells without VEGF plasmid transfection; CN2:VEGF: decorated cells with VEGF plasmid transfection. The level of VEGF was measured using ELISA. d, Experimental design of in vivo study using the chicken chorioallantoic membrane model. The encapsulated cells were transplanted at ED8 when the heart and vascular system of the chicken embryo were mostly developed. The blood flow was analyzed at ED8 and ED11 before the embryo moved to position for hatching. e, Laser speckle contrast imaging of blood flow in chicken embryos at ED8 and ED11. Blank: chicken chorioallantoic membrane without any treatment. Native: chicken chorioallantoic membrane treated with native MSCs. CN2:laser: MSCs treated with laser irradiation after encapsulation and NP decoration. CN2:VEGF: MSCs transfected with VEGF plasmids before encapsulation and NP decoration. CN2:VEGF:Laser: MSCs transfected with VEGF plasmids, encapsulated, decorated with NPs, and treated with laser irradiation. The blood flow was quantified using ImageJ software (n = 4; statistical significance was determined using one-way ANOVA with Dunnett’s multiple comparisons test, **p < 0.01, ***p < 0.001)
We examined the effects of irradiation duration, plasmid delivery, and gene expression duration on the level of gene expression. Irradiation quickly increased the temperature of AuNP solution by more than 10 °C within 10 min but caused negligible cytotoxicity on native MSCs (Supplementary Fig. 12). Irradiation did not significantly affect the necrosis and apoptosis of decorated MSCs within 10 min (Supplementary Fig. 13). However, 15 min of continuous irradiation caused a significant increase in cell necrosis and apoptosis. These data indicate that 10 min of irradiation may be optimal for effective regulation of gene expression. As expected, we detected the highest level of luciferase activity after 10 min of irradiation (Supplementary Fig. 14). We also varied the condition of plasmid delivery by changing the lipofectamine concentration (Supplementary Fig. 15). Irradiation triggered a significant increase of luciferase expression in all groups. The highest level of gene expression was observed in the 2 μL lipofectamine group. We further studied the kinetics of luciferase expression after irradiation. Luciferase expression quickly started one hour post irradiation and gradually reached a plateau from 12 to 24 hours. (Fig. 3b). Taken together, these data clearly demonstrate that CN2-NPs on the cell membrane can sensitively regulate intracellular gene expression.
After the luciferase expression experiments, we transfected MSCs with the HSP70-VEGF plasmid to examine VEGF expression. Consistent with luciferase expression, the level of VEGF expression significantly increased when the transfected MSCs with CN2-NPs (CN2:VEGF) were irradiated (Fig. 3c). Accordingly, the released VEGF stimulated the migration of endothelial cells (Supplementary Fig. 16). The in vivo chicken chorioallantoic membrane model was further used to examine the potential of decorated MSCs in stimulating local blood flow (Fig. 3d). Blood flow increased by over 270% in the transplantation area treated with CN2:VEGF and irradiation (Fig. 3e). It was significantly higher than the controls. Thus, these data suggest that with NP decoration, encapsulated MSCs could be regulated to promote the blood flow in vivo.
As a variety of inorganic and organic NPs have been synthesized, there is no limit in developing NP-decorated nanocapsules with new functions. For example, while we used AuNPs to regulate protein expression, AuNPs can be substituted with any other NPs such as magnetic NPs and drug-loaded polymeric NPs. With these NPs, cells can be regulated by magnetic forces and drugs. Importantly, as the nanocapsule is a three-dimensional material, it has high capacity to carry multiple NPs simultaneously. The functions of encapsulated cells can be regulated by multiple NPs with different properties. Moreover, while we used the supramolecular DNA-alginate-polylysine complex to encapsulate cells and carry NPs, any other nanocapsules or microcapsules can be functionalized with NPs for cell regulation using the CN2 method. However, as living cells are fragile, it is important to ensure that the procedure of cell encapsulation and NP decoration is biocompatible and easy to operate for potential scalability. Otherwise, cells may lose viability. The current CN2 method does not involve harsh conditions such as the use of cytotoxic polymers, low pH, or organic solvent, which is commonly accompanied during cell encapsulation in bulk hydrogels. Importantly, encapsulating cells and carrying NPs only require the incubation of cells in solutions without the need of using any sophisticated equipment. These characteristics are important to the potential scalability of this method. Meanwhile, we want to point out that the CN2 method involves multiple steps and takes a longer time to accomplish than instantaneous NP deposition methods.[19]
In summary, CN2 is a promising method for cell nanoencapsulation and NP decoration. The nanocapsule can sustainably carry NPs at the vicinity of encapsulated cells. The cellular functions such as gene expression can be facilely regulated by NPs. While DNA nanostructures were used to prepare nanocapsules to carry QDs and AuNPs, the same method can be applied to any other polymeric nanocoatings or nanoparticles. In addition, CN2 can in principle be applied to encapsulate any cell and carry any or multiple NPs. Therefore, CN2 is a promising method harnessing the characteristics of both polymeric nanocoating and inorganic NPs, opening a new avenue for advancing cell-based applications such as stem cell delivery for regenerative medicine, immune cell delivery for immunotherapy, and cell-mediated nanoparticle delivery for cancer treatment.
Supplementary Material
Acknowledgements
This work was partly supported by the National Institutes of Health (R01HL122311; R01AR073364), and the U.S. National Science Foundation (1802953). The schematic illustrations were created using the BioRender online tool.
References
- [1].Orive G, Santos E, Poncelet D, Hernández RM, Pedraz JL, Wahlberg LU, Vos PD, Emerich D, Trends Pharmacol. Sci 2015, 36, 537–546. [DOI] [PubMed] [Google Scholar]
- [2].Uludag H, Vos PD, Tresco PA, Adv. Drug Deliv. Rev 2000, 42, 29–64. [DOI] [PubMed] [Google Scholar]
- [3].Planat-Benard V, Varin A, Casteilla L, Front. immunol 2021, 12, 626755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Campbell JE, Newgard CB, Nat. Rev. Mol. Cell Biol 2021, 22, 142–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xia H, Li X, Gao W, Fu X, Fang RH, Zhang L, Zhang K, Nat. Rev. Mater 2018, 3, 174–193. [Google Scholar]
- [6].Madl CM, Heilshorn SC, Blau HM, Nature, 2018, 557, 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mao AS, Özkale B, Shah NJ, Vining KH, Descombes T, Zhang L, Tringides CM, Wong SW, Shin JW, Scadden DT, Weitz DA, Mooney DJ, Proc. Natl. Acad. Sci. U.S.A 2019, 116, 15392–15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yu X, Lin W, Lai Q, Song J, Liu Y, Su R, Niu Q, Yang L, Yang C, Zhang H, Zhu Z, Angew. Chem. Int. Ed 2023, e202301083. [DOI] [PubMed] [Google Scholar]
- [9].Shi P, Zhao N, Coyne J, Wang Y, Nat. Commun 2019, 10, 2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Alkayyali T, Cameron T, Haltli B, Kerr RG, Ahmadi A, Anal. Chim. Acta, 2019, 1053, 1–21. [DOI] [PubMed] [Google Scholar]
- [11].Liu T, Wang Y, Zhong W, Li B, Mequanint K, Luo G, Xing M, Adv. Healthc. Mater 2019, 8, 1800939. [DOI] [PubMed] [Google Scholar]
- [12].Tomei AA, Manzoli V, Fraker CA, Giraldo J, Velluto D, Najjar M, Pileggi A, Molano RD, Ricordi C, Stabler CL, Hubbell JA, Proc. Natl. Acad. Sci. U.S.A 2014, 111, 10514–10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shah K, Biomatter, 2013, 3, e24278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang W, Encapsulation of transgenic cells for gene therapy. Gene Therapy-Principles and Challenges. 2015. [Google Scholar]
- [15].Kim H, Bae C, Kook YM, Koh WG, Lee K, Park MH, Stem Cell Res. Ther 2019, 10, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Frantz C, Stewart KM, Weaver VM, J. Cell Sci 2010, 123, 4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].a) Zhang Y, He Y, Bharadwaj S, Hammam N, Carnagey K, Myers R, Atala A, Van Dyke M, Biomaterials, 2009, 30, 4021–4028.; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hussey GS, Dziki JL, Badylak SF, Nat. Rev. Mater 2018, 3, 159–173.; [Google Scholar]; c) Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, Shenoy VB, Nature, 2020, 584, 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].a) Drachuk I, Gupta MK, Tsukruk VV, Adv. Funct. Mater 2013, 23, 4437–4453.; [Google Scholar]; b) Drachuk I, Shchepelina O, Lisunova M, Harbaugh S, Kelley-Loughnane N, Stone M, Tsukruk VV, ACS nano, 2012, 6, 4266–4278. [DOI] [PubMed] [Google Scholar]
- [19].Zhu W, Guo J, Amini S, Ju Y, Agola JO, Zimpel A, Shang J, Noureddine A, Caruso F, Wuttke S, Croissant JG, Brinker CJ, Adv. Mater 2019, 31, 1900545. [DOI] [PubMed] [Google Scholar]
- [20].a) Youn W, Kim JY, Park J, Kim N, Choi H, Cho H, Choi IS, Adv. Mater 2020, 32, 1907001.; [DOI] [PubMed] [Google Scholar]; b) Bodelon G, Costas C, Perez-Juste J, Pastoriza-Santos I, Liz-Marzan LM, Nano Today, 2017, 13, 40–60.; [DOI] [PubMed] [Google Scholar]; c) Drachuk I, Gupta MK, Tsukruk VV, Adv. Funct. Mater 2013, 23, 4437–4453. [Google Scholar]
- [21].Munro S, Cell, 2003, 115, 377–388. [DOI] [PubMed] [Google Scholar]
- [22].a) Seeman NC, Sleiman HF, Nat. Rev. Mater 2017, 3, 17068.; [Google Scholar]; b) Rothemund PWK, Nature, 2006, 440, 297–302.; [DOI] [PubMed] [Google Scholar]; c) Green LN Subramanian HKK, Mardanlou V, Kim J, Hariadi RF, Franco E Nat. Chem 2019, 11, 510–520. [DOI] [PubMed] [Google Scholar]
- [23].a) Li Y, Tseng YD, Kwon SY, d’Espaux L, Bunch JS, McEuen PL, Luo D, Nat. Mater 2004, 3, 38–42.; [DOI] [PubMed] [Google Scholar]; b) Lee JB, Peng S, Yang D, Roh YH, Funabashi H, Park N, Rice EJ, Chen L, Long R, Wu M, Luo D, Nat. Nanotechnol 2012, 7, 816–820.; [DOI] [PubMed] [Google Scholar]; c) Wei B, Dai M, Yin P, Nature, 2012, 485, 623–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].a) Veneziano R, Moyer TJ, Stone MB, Wamhoff EC, Read BJ, Mukherjee S, Shepherd TR, Das J, Schief WR, Irvine DJ, Bathe M, Nat. Nanotechnol 2020, 15, 716–723.; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu X, Zhang F, Jing X, Pan M, Liu P, Li W, Zhu B, Li J, Chen H, Wang L, Lin J, Liu Y, Zhao D, Yan H, Fan C, Nature, 2018, 559, 593–598.; [DOI] [PubMed] [Google Scholar]; c) Lee JB, Roh YH Um SH, Funabashi H, Cheng W, Cha JJ, Kiatwuthinon P, Muller DA, Luo D, Nat. Nanotechnol 2009, 4, 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jin Y, Gao X, Nat. Nanotechnol 2009, 4, 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].a) Verma A, Stellacci F, Small 2010, 6, 12–21.; [DOI] [PubMed] [Google Scholar]; b) Yameen B, Choi WI, Vilos C, Swami A, Shi J, Farokhzad OC, J. Control. Release, 2014, 190, 485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Loike JD, Silverstein SC, Immunol J. Methods 1983, 57, 373–379. [DOI] [PubMed] [Google Scholar]
- [28].a) Croissant JG, Butler KS, Zink JI, Brinker CJ, Nat. Rev. Mater 2020, 5, 886–909.; [Google Scholar]; b) Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI, Chem. Soc. Rev 2012, 41, 2590–2605.; [DOI] [PubMed] [Google Scholar]; c) Gao J, Gu H, Xu B, Acc. Chem. Res 2009, 42, 1097–1107. [DOI] [PubMed] [Google Scholar]
- [29].Kang S, Bhang SH, Hwang S, Yoon JK, Song J, Jang HK, Kim S, Kim BS, ACS nano, 2015, 9, 9678–9690. [DOI] [PubMed] [Google Scholar]
- [30].a) Zhao L, Li D, Zhang Y, Huang Q, Zhang Z, Chen C, Xu C, Chu X, Zhang Y, Yang X, ACS nano, 2022, 16, 13821–13833.; [DOI] [PubMed] [Google Scholar]; b) Andersson HA, Kim YS, O’Neill BE, Shi ZZ, Serda RE, Vaccines, 2014, 2, 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jiang MH, Li G, Liu J, Liu L, Wu B, Huang W, He W, Deng C, Wang D, Li C, Lahn BT, Shi C, Xiang AP, Biomaterials, 2015, 50, 56–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


