Abstract
During embryonic and neonatal life, renin cells contribute to the assembly and branching of the intrarenal arterial tree. During kidney arteriolar development renin cells are widely distributed throughout the renal vasculature. As the arterioles mature, renin cells differentiate into smooth muscle cells, pericytes, and mesangial cells. In adult life, renin cells are confined to the tips of the renal arterioles, thus their name juxtaglomerular cells. Juxtaglomerular cells are sensors that release renin to control blood pressure and fluid-electrolyte homeostasis. Three major mechanisms control renin release: 1) β-adrenergic stimulation, 2) macula densa signaling, and 3) the renin baroreceptor, whereby a decrease in arterial pressure leads to increased renin release whereas an increase in pressure results in decrease renin release. Cells from the renin lineage exhibit plasticity in response to hypotension or hypovolemia, whereas relentless, chronic stimulation induces concentric arterial and arteriolar hypertrophy, leading to focal renal ischemia. The renin cell baroreceptor is a nuclear mechanotransducer within the renin cell that transmits external forces to the chromatin to regulate Ren1 gene expression. In addition to mechanotransduction, the pressure sensor of the renin cell may enlist additional molecules and/or structures including soluble signals and/or membrane proteins such as gap junctions and ion channels. How these various components integrate their actions to deliver the exact amounts of renin to meet the organism needs is unknown. This review describes the nature and origins of renin cells, their role in kidney vascular development and arteriolar diseases, and the current understanding of the blood pressure sensing mechanism.
Keywords: renin, blood pressure, renin baroreceptor, embryonic development, renin-angiotensin system, perfusion, pressure sensing, vascular development, arterial disease
Renin cells and the renin-angiotensin system
The renin-angiotensin aldosterone system (RAAS) is crucial to maintain blood pressure (BP) and fluid-electrolyte homeostasis. Renin, the key regulated enzyme of the RAAS, is synthesized and secreted from a few renin-expressing cells in the kidney (0.01–0,1%)1. In adult mammals, renin-expressing cells are strategically located at the tips of the afferent arterioles at the entrance to the glomeruli and are thus termed juxtaglomerular (JG) cells. JG are sensors that respond to changes in pressure, and the composition and volume of the extracellular fluid2. Renin hydrolyzes angiotensinogen into angiotensin I, which is then converted by the angiotensin-converting enzyme (ACE) into angiotensin II. To maintain homeostasis, angiotensin II increases BP by causing vasoconstriction and induces the adrenal gland to release aldosterone, which enhances tubular reabsorption of sodium chloride (NaCl) in the kidneys, and to a lesser extent in the intestine and skin3. Renin-expressing cells are directly involved in the development of the kidney vasculature by contributing cells to the evolving vessels, by their local generation of angiotensin II and by their expression of angiogenic factors1.
Three distinct mechanisms control renin synthesis and release from JG cells: 1) the renin cells baroreceptor which senses changes in perfusion pressure4. 2) the β-adrenergic receptor (β-AR) bound to G-protein subunit alpha (Gsα) and adenylate-cyclase (AC) is activated by circulating or locally released catecholamines via the sympathetic nervous system; 3) the macula densa mechanism that detects a reduction of NaCl concentration and induces prostaglandin-E2 (PGE2) paracrine signals through cyclooxygenase-2 (COX-2), leading to the activation of the prostaglandin-E2 receptor 4 (EP4R); activation of EP4R also leads to cAMP generation, illustrating the centrality of cAMP in renin cell function. These mechanisms involved in the tight control of renin secretion depend mainly on cyclic AMP (cAMP) generation, ultimately affecting renin synthesis and cell identity5–8. cAMP activates protein kinase A (PKA), which phosphorylates the cAMP-responsive element-binding protein (Creb), which in turn binds the cAMP-responsive element (CRE) in the renin locus and induces renin transcription. The histone acetyltransferase Creb-binding protein (CBP) and p300 acetylate lysine 27 of histone H3 (H3K27ac, characteristic of active enhancers) and may bind CRE to regulate renin expression7,8. Renin cells also express Notch receptors and recombination signal binding protein for immunoglobulin kappa J region (Rbp-j). Rbp-j, the central transcription factor (TF) involved in the Notch/Rbp-j signaling pathway is crucial in regulating basal renin expression and renin cell phenotype9. Furthermore, a number of renin cell-specific super-enhancers regulate TFs and genes essential to the cell’s identity10. Thus, renin synthesis and secretion are tightly regulated at various levels including their intrinsic ability to sense arterial pressure and physiological signals from adjacent structures of the JG apparatus -composed of the afferent and efferent arterioles, the macula densa and the extraglomerular mesangium- circulating hormones, and the renal nerves. The combined action of these effectors conveys the status of the BP and the composition of the extracellular fluid to the JG cell. Increase in intracellular cAMP results in renin release from renin cell granules and unfolding of chromatin at the locus of the renin gene, binding of TFs -including but not limited to Creb, CBP/p300 – with stimulation of renin transcription and consequent renin synthesis (Figure 1). On the other hand, although angiotensin II has been thought to inhibit renin synthesis and release through a negative feedback loop via activation of the type 1 angiotensin II receptor (AT1R) on JG cells, a recent study reported that the deletion of AT1R in renal stromal progenitors and their descendants does not affect renin expression, BP and kidney development, challenging the direct function of AT1R in JG cells11.
Figure 1. Overview of the mechanisms involved in renin synthesis and release and the RAAS cascade.

Three major mechanisms control renin synthesis and release: (1) changes in perfusion pressure activate the renal baroreceptor, (2) β-adrenergic stimulation is triggered by local or circulating catecholamines via sympathetic nervous system activation, and (3) macula densa signaling via prostaglandin-E2 (PGE2) paracrine secretion in response to a reduction in NaCl concentration. Renin generates angiotensin I (Ang I) from angiotensinogen, which is then hydrolyzed by the angiotensin-converting enzyme (ACE) to produce angiotensin II (Ang II). Ang II is a potent direct vasoconstrictor that also stimulates adrenal release of aldosterone, which promotes Na+ tubular reabsorption. These combined actions of the RAAS are essential to maintain BP and fluid-electrolyte homeostasis. Within the renin cell, cAMP activates protein kinase A (PKA), which phosphorylates cAMP-responsive element-binding protein (Creb), which in turn binds the cAMP-responsive element (CRE) in the renin enhancer, and recruits histone acetyltransferases such as P300 resulting in epigenetic changes (H3K27ac) at the regulatory region of the renin gene, opening of chromatin and renin transcription. Activation of the Notch/ recombination signal binding protein for immunoglobulin kappa J region (Rbp-j) pathway also stimulates renin transcription. AC, adenylate cyclase; β-AR indicates beta-adrenergic receptor; COX2, cyclooxygenase-2; EP4R, Prostaglandin-E2 receptor 4; Gsα, activating G-protein–coupled subunit; NHE, Na+/H+ exchanger; NKCC2, Na+-K+−2Cl− cotransporter; PGE2, prostaglandin E2.
Embryonic origin of renin cells and their role in vascular development
In the mammalian embryo, renin cells emerge early during gestation before organogenesis has been initiated, around embryonic day (E) 8.5 in mice in the developing ectoderm. Thereafter, renin cells appear in multiple tissues and organs at different times, including the developing testes, adrenal glands mesonephros, and metanephros. The metanephros, the definitive kidney in mammals, develops in mice around E11.5 from the intermediate mesoderm through the reciprocate interaction between the metanephric mesenchyme and the primitive ureteric bud (UB)12. The metanephric mesenchyme promotes the UB’s growth and division into multiple ureteric tips that will ultimately form the collecting ducts and the ureter. The metanephric mesenchyme in turn, consists of two morphologically distinct compartments: 1) The condensing mesenchyme that will differentiate into all the components of the epithelial nephron (podocytes, proximal tubules, distal tubules, and loops of Henle) and 2) The loose mesenchyme/stromal compartment which contains all the progenitors for the kidney vasculature including: a) endothelial and hematopoietic precursors that express the stem cell leukemia/T-cell acute lymphoblastic leukemia protein (Scl)13, and b) the forkhead box protein D1 (FoxD1) expressing cells, which develop into mural cells of the kidney vasculature, including SMCs, mesangial cells (MCs), interstitial pericytes, and renin-expressing cells, including JG cells (Figure 2)14.
Figure 2. Overview of kidney vascular development, progenitor compartments and fate of renin cells in response to transient or persistent/chronic stimulation of the RAAS.

The ureteric bud gives rise to collecting ducts and ureter, whereas the cap mesenchyme gives rise to all the components of the epithelial nephron. The loose mesenchyme is composed of stem cell leukemia/T-cell acute lymphoblastic leukemia (Scl) expressing endothelial cell (EC) and hematopoietic precursors, and the forkhead box protein D1 (FoxD1) stromal cell precursors, which develop into mural cells of the kidney vasculature [smooth muscle cells (SMC), mesangial cells (MC), interstitial pericytes, and renin-expressing cells]. With maturation, renin cells are restricted to the tips of the renal arterioles. In response to acute threats to homeostasis cells from the renin lineage cells reacquire the renin phenotype resembling the fetal pattern, a phenomenon termed “recruitment,” whereas persistent/chronic stimulation leads to concentric arterial and arteriolar hypertrophy. The histological image shows a longitudinal cut of a hypertrophic arteriolar wall (white arrowhead) from a Ren1c−/− mouse kidney immunostained for α-smooth muscle actin (brown, from42). Scale bar, 75 μm. AA, afferent arteriole; G, glomerulus.
Regarding the origin of the kidney vasculature, whether the first vessels develop by vasculogenesis from intrinsic metanephric precursors or by angiogenesis from branches of the aorta has long been controversial. When prevascular mouse embryonic kidneys are transplanted into the eye’s anterior chamber or under the kidney capsule of adult host mice, the embryonic kidney develops its vasculature from intrinsic precursors present in the transplanted anlagen that connect to the host through chimeric vessels15. In contrast, when embryonic kidneys are transplanted below the kidney capsule of newborn mice when there is still active nephrogenesis, the transplanted kidney develops chimeric glomeruli and vessels with the ECs originating from both the newborn host and the embryonic kidney16, suggesting that angiogenic signals from embryonic glomeruli can attract endothelial precursors from the developing neonatal cortex. However, the mural cells of the arteries and arterioles still originate from the transplanted embryonic kidney. Those studies indicate that the developmental stage and location of vascular progenitors could determine the precise origin of the vasculature. Additionally, kidney organoids developed in vitro from human embryonic stem cells or inducible pluripotent stem (iPS) cells, unconnected to any circulation, exhibit a transient differentiation of endothelial networks with the formation of lumens and some ECs incorporated into the glomeruli, which disappears over time17 Of note, when kidney organoids are exposed to shear stress, the number of ECs increases drastically18, suggesting that the connections to the circulation could promote the development of the kidney vasculature. Recent studies have reported the presence of renin-producing cells in kidney organoids derived from human iPS cells19. Due to the rarity of renin cells and their nature to rapidly stop expressing renin after only a few days in culture20, no in vitro models of human renin cells has been established. Thus, kidney organoids with renin-expressing cells could provide human kidney disease models for the study of the RAAS, such as evaluating the pharmacological effects/toxicity of potential novel RAAS-targeting therapeutics. Furthermore, given the central role of renin cells in the development of the kidney’s arterial tree, organoids with differentiating renin cells may be of use to elucidate how the kidney vasculature develops and ultimately provide insights into how to create vascularized artificial kidneys.
Within the metanephros, renin cells are essential for the formation and branching of the kidney vasculature21. Renin mRNA can be detected in the developing kidney as early as E11–12 in mice and E14 in rats. However, renin protein expression is observed 2 days later in few cells distributed randomly in the stromal-interstitial compartment. Every time that a new arterial blood vessel is formed, a group of renin cells accumulates, elongates, and as maturation proceeds, the cells that compose the maturing vessel differentiate into vascular SMCs, and only the ones located at the tip of the arterioles will continue to express renin. This process repeats in a fractal-like pattern until the whole arteriolar tree is formed1. In fetal mammals, renin cells reside along the afferent arterioles, including larger arterioles, and in the mesangium. Eventually, renin cells are restricted to the JG tip of the renal arterioles at the entrance to the glomeruli22.
Several pathways are crucial for the differentiation of renin-expressing cells, maintenance of the kidney vasculature and overall kidney architecture. The conditional deletion of Gsα causes a significant reduction of renin-expressing cells with the consequent arteriolar abnormalities23,24. Additionally, homozygous conditional deletion of CBP and p300 results in lack of renin-expressing cells and severe nephron-vascular malformations8. As mentioned above, the Notch/Rbp-j pathway is important for renin expression and cell identity. Deletion of Rbp-j in renin cells leads to a decrease in the number of renin-expressing cells and an impaired ability of renin production in response to physiological threats. This occurs by altering the identity of renin cells: the expression of vascular smooth muscle is replaced with the ectopic expression of genes related to hematopoiesis, fibroblasts and immune responses25,26. Furthermore, Rbp-j deletion in Foxd1-positive renin precursor cells causes a reduction in the number of renin-expressing cells accompanied by thin blood vessels and mesangial-deficient glomeruli, resulting in the development of glomerular aneurysms27. Of note, Foxd1 itself is crucial for the normal centrifugal pattern of the renal arterial morphogenesis and renin expression14. As for Notch receptors, a recent study reported that the deletion of Notch3, one of the four Notch isoforms (Notch1–4) essential for the differentiation of vascular SMCs, reduces the contractility of kidney resistance vessels due to deficient calcium entry28. This probably leads to an increase in cAMP in renin cells with the consequent increase in the number of renin-expressing cells along the afferent arterioles and circulating renin as previously observed29. Those studies highlight the interaction and significance of the cAMP and Notch/Rbp-j pathways in sustaining kidney vascular integrity and renin expression.
To identify the TFs that regulate the differentiation pathways of the early progenitors of the mural cells of the kidney vessels, including renin cells, our group recently created a single-cell atlas of gene expression and chromatin accessibility profiles along a comprehensive developmental time course of renin cell differentiation and other FoxD1 descendants. TFs essential for stage-specific differentiation during embryonic and postnatal periods include the Mef2 family and Nfix among others (unpublished data)30. The regulatory landscape of renin lineage cells would provide valuable insight into how renin lineage cells develop and a basis for future research to directly interrogate the individual roles of stage-specific TFs. Understanding the molecular basis underlying the morphogenesis of the kidney vasculature may prove essential for the generation of functioning kidneys and the discovery of novel therapeutic approaches for congenital and acquired renal and vascular diseases and hypertension.
Plasticity of renin lineage cells in response to life-threatening conditions
When homeostasis is threatened by conditions such as severe hypotension, dehydration, hemorrhaging, or chronic RAAS inhibition, JG cells are stimulated to produce more renin. In addition, a subset of vascular SMCs derived from the renin lineage turn on renin expression until homeostasis has been re-established. The ability to produce high levels of renin can restore BP and fluid volume, enabling survival. This process is considered reversible and occurs through reenacting the embryonic pattern of renin expression31–33. This phenomenon has been termed “recruitment,” but it does not entail proliferation or cell migration34. It is caused by dedifferentiation of vascular SMCs along the arterioles, occasionally MCs, and interstitium pericytes which retained the memory to re-express and synthesize renin32–37 This process is mediated by a set of epigenetic marks at the locus of the renin gene and other loci throughout the genome. In fact, in vitro targeted expression of p300 to the renin promoter region on vascular SMCs derived from mouse kidney arteriolar cells turns on renin expression3,10. This memory may be due to imprinting on renin lineage cells during the early development to produce renin in response to threats to homeostasis. The ability to respond to constant threats emerges early in evolution and persists throughout phylogeny, as observed in zebrafish38. The process by which existing cells transform into cells capable of performing precise functions, such as synthesizing specific hormones, in response to a physiological challenge is an important biological homeostatic phenomenon conserved among different cell types and species.
Although the phenomenon of recruitment is reversible, if the challenge to homeostasis persists, the cells will continue to produce renin (Figure 2). Notably, in the presence of unrelenting chronic stimulation, renin cells induce concentric arterial and arteriolar hypertrophy (CAAH) leading to focal kidney ischemia and kidney damage. Mice with deletion of any of the RAAS genes show physiological and histological abnormalities, including CAAH39–43. In adult hypertensive rats, while RAAS inhibitor treatments significantly improve BP and proteinuria, severe kidney damage occurs, including tubulointerstitial fibrosis and CAAH39,44. These changes were more severe in rats receiving dual RAAS inhibitors than a single treatment. In addition, rats treated with ACE inhibitors with a low-sodium diet were reported to display more severe interstitial damage and CAAH than those treated with a control diet45. These results suggest that the excessive effect of RAAS blockade due to multi-drug treatment or salt depletion (and probably a marked decrease in BP) could correlate with the severity of the CAAH.
Furthermore, CAAH by chronic RAAS inhibition is also observed in monkeys and humans46,47. Our group assessed renal biopsy samples from patients with benign nephrosclerosis before and after undergoing long-term RAAS inhibition, and demonstrated an increase in renin cells and CAAH of the preglomerular arterioles in these patients43. Although there are several hypotheses regarding the cause of CAAH, one hypothesis is that high-doses of RAAS blockers could induce renin overproduction by the lack of a negative feedback, leading to accumulation of vascular SMCs through the activation of prorenin/renin receptors47,48. However, CAAH is not observed in animals with ablation of renin cells by diphtheria toxin49 or conditional knockout of integrin-β150, suggesting that CAAH is not induced by the renin protein itself but rather by factors -angiogenic or otherwise-produced by renin cells. In fact, renin cells produce a number of matrix proteins and stimulate the accumulation of SMCs which contribute to the thickening of the vessel wall43. The dynamics and regulatory networks that transform renin cells into cells capable of migrating inside the arterial walls and resulting in the CAAH of nearby cells are still under study. Considering the clear benefits of RAAS inhibitors for reducing mortality and cardiovascular events, identifying the mechanisms underlying CAAH would assist in developing treatments that will allow patients to receive RAAS inhibitors without compromising kidney health.
Nuclear mechanotransduction and the renin cell baroreceptor
The presence of a pressure-sensing system, a renal baroreceptor for the control of renin release, was proposed more than 65 years ago51. The proposed mechanism is that perfusion pressure tightly regulates renin release from renin cells; High-perfusion pressure suppresses and low-perfusion pressure stimulates renin release. In addition, mechanisms underlying the autoregulation of renal blood flow (RBF) and tubuloglomerular feedback (TGF) modulate renin secretion. Changes in renal perfusion pressure are also transduced as alterations in the concentration of NaCl load to the macula densa, leading to changes in afferent arteriolar vasomotor tone52. Interestingly, experiments in dogs indicated that renin secretion remains nearly constant within the autoregulation range of renal blood flow, whereas renin secretion rises sharply once RBF decreases below a certain threshold53. In contrast, using the aglomerular toadfish Opsanus tau, Nishimura et al. showed that an increase in BP suppresses renin while a decrease in BP stimulates renin directly and independently of the influence from the macula densa or the extraglomerular mesangium. They further performed in vitro experiments using superfused renal slices with bicarbonate ringer solution, which also exhibited no interaction with macula densa or extraglomerular mesangium and caused depolarization simulating the activation of stretch receptors, demonstrating membrane depolarization suppressed renin release54,55. Whether repolarization restored the release of renin could not be determined in this setup. Although these findings suggested the presence of a regulatory system for renin synthesis independent of the macula densa/TGF signaling, the baroreceptor’s structure and location, until recently, remained enigmatic.
Our group recently proposed that the renal baroreceptor is a nuclear mechanotransduction mechanism within renin cells that involves sensing and transmitting external forces directly to the nuclear chromatin to control Ren1 gene expression and the overall renin cell phenotype56. Cells are exposed to different types of forces depending on their anatomical position: In blood vessels, SMCs and ECs are constantly subjected to hemodynamic pulsatile pressures and shear stress induced by blood perfusion57. Mechanotransduction is the process whereby cells respond to mechanical changes in their environment by modifying their own structural and biochemical characteristics58, comprising sensors for extracellular forces (i.e., ion channels, membrane-associated receptors, and junction proteins) and subsequent downstream signaling mechanisms59. The mechanosignaling pathways include the extracellular matrix (ECM), integrins, cytoskeleton proteins, and intracellular signaling molecules (Figure 3). The signals outside the nucleus are transmitted to the nuclear lamina through the linker of the nucleoskeleton and cytoskeleton (LINC) complex, influencing the nuclear structure and composition, chromatin organization, and gene expression (Figure 3)60. This physiological process, termed nuclear mechanotransduction, enables cells to adapt their acute behavior, structural stability, and fate to their physical environment61. Below we outline the components of the renin cell baroreceptor.
Figure 3. The renin cell baroreceptor is a nuclear mechanotransducer.
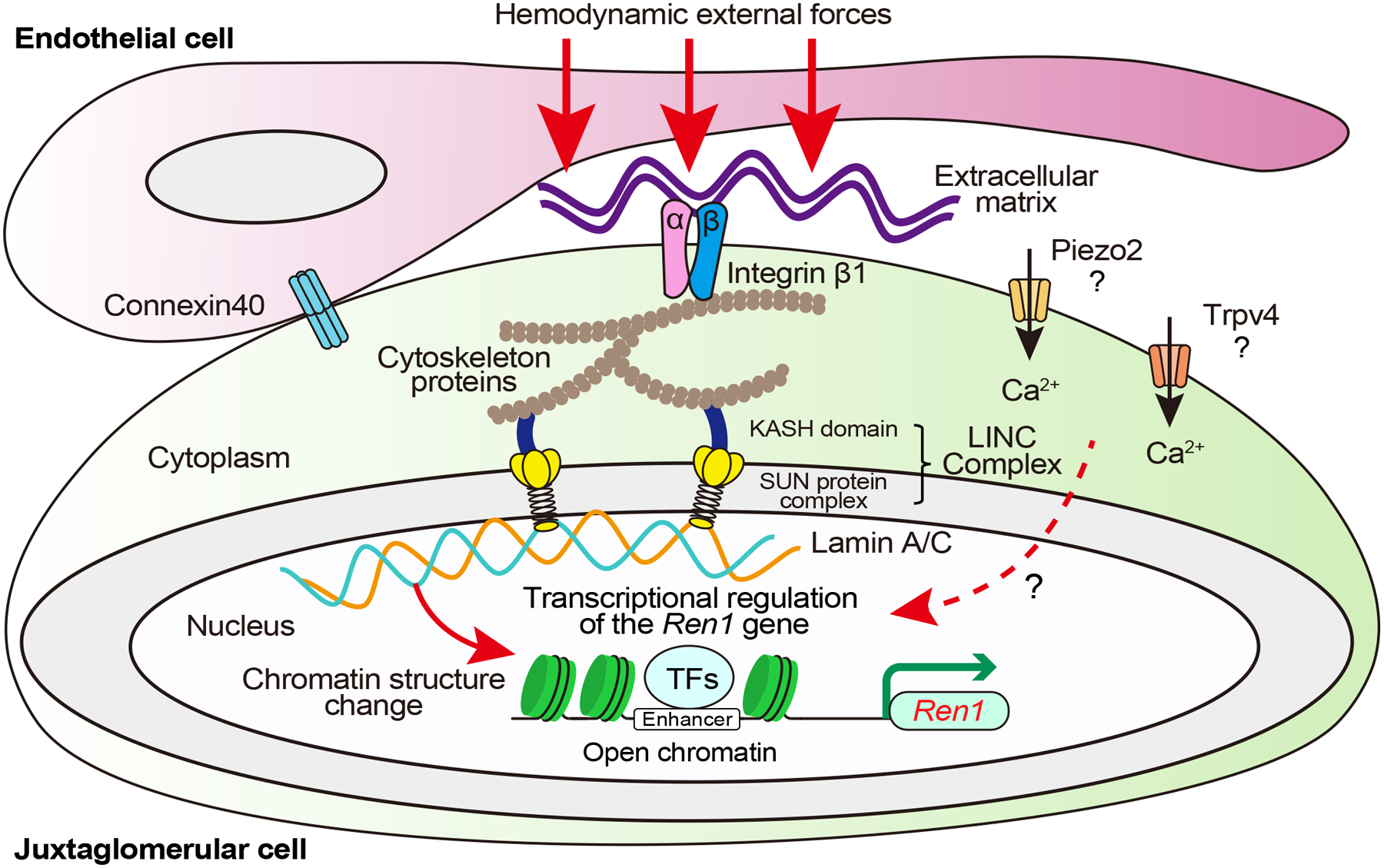
Hemodynamic external forces are sensed by integrins and transmitted through the cytoskeleton likely to the linker of the nucleoskeleton and cytoskeleton (LINC) complex, ultimately impacting lamina-chromatin interactions that change chromatin structure and upregulate Ren1 gene transcription in response to low perfusion pressure and downregulates Ren1 in response to high perfusion pressure. Connexins in renin cells and endothelial cells determine the position around afferent arterioles where the renin cells can adequately sense hemodynamic external forces. Whether mechanically activated non-voltage-gated calcium-permeable ion channels, such as Piezo2 and transient receptor potential vanilloid 4 (Trpv4), regulate intracellular calcium and renin release in juxtaglomerular cells remains to be determined. KASH, Klarsicht/ANC-1/Syne-1 homology; SUN, Sad1/UNC-84 homology.
ECM, integrins, cytoskeleton, and LINC complex
ECM helps maintain the three-dimensional structure of tissues, define their biomechanical properties and determine cell differentiation and fate62. Fibrous proteins (fibronectin, laminin, collagen, etc.) characterize the mechanical properties of the ECM, which provides a dynamic environment for tissue morphogenesis63. For most cells, the ECM stands as their initial exposure to mechanical forces. Integrins are the largest family of ECM proteins. They form heterodimers with distinct alpha and beta subunits and act as transmembrane receptors that maintain the mechanical interconnection between the ECM and cells, transmitting forces from the ECM to the intracellular domains64. The extracellular domain of integrin binds fibrous ECM proteins, whereas the cytoplasmic domain connects to the actin cytoskeleton via various adaptor proteins65. By binding ECM ligands, integrins cluster to form multiprotein focal adhesion complexes, reorganizing the actin cytoskeleton according to mechanical forces66. Thus, the ECM, integrins, and cytoskeleton proteins contribute to mechanotransduction by converting extracellular signals into intracellular ones.
To validate the mechanotransduction, our group tested two different types of mechanical forces on the renin-expressing renal afferent arteriolar SMC line: pneumatic pressure and magnetic beads coated with fibronectin5,56. Fibronectin is an important component of ECM that binds to integrins. The exposure to both mechanical forces reduced Ren1 mRNA expression and increased the intensity of cytoskeletal actin filaments in the cells, indicating that a direct external stimulus shifts the cells to a myocrine phenotype. To investigate the mechanotransduction within renin cells in vivo, our group developed a novel surgical model of aortic coarctation (AoCo) between the base of the left and the right renal arteries, enabling us to compare kidney cells subjected to low and high perfusion pressure within individual mice56. Using this AoCo surgical model, besides changes in Ren1 expression, RNA-seq data of native renin cells revealed distinct variations between renin cell repertoires expressed in the left and right kidneys under low and high perfusion pressure, respectively. Furthermore, mice with conditional knockout of Itgb1 exposed to AoCo surgery showed that their renin cells were unresponsive to changes in perfusion pressure, indicating that integrin-β1 subunit contributes to sensing perfusion pressure changes in renin cells and regulating renin expression56. Although integrin-β1 in renin cells primarily formed heterodimers with integrin αv and α5, their role in mechanotransduction remains to be investigated.
The LINC complex functions as a nuclear envelope-spanning physical bridge connecting the nucleus and cytoskeleton and comprises two protein families: KASH (Klarsicht/ANC-1/Syne-1 homology) domain proteins that interact with the cytoskeleton on the outer nuclear envelope and SUN (Sad1/UNC-84 homology) protein complexes that are bound to the nuclear lamina and chromatin at the inner nuclear envelope67. Of the proteins present in the nuclear membrane, this structure has been shown to modulate the transduction of mechanical forces across the cell surface to the nucleus68. Meanwhile, the relative contributions of the LINC components associated with nuclear mechanotransduction in renin cells have not been defined.
Junction proteins
Several membrane proteins involved in cell-to-cell interactions, which are not directly associated with nuclear mechanotransduction pathways, have been proposed as modulators or components of the renal baroreceptor mechanism. Connexins (Cxs) comprise various transmembrane proteins responsible for intercellular communication and transferring ions or small signaling molecules across cells69. In the renal vasculature, Cx37, Cx40, Cx43, and Cx45 are expressed in the vessel walls, with the prominent expression of Cx40 in the Ecs and Cx45 in the SMCs70. In contrast, JG cells mainly express Cx40 with a smaller amount of Cx37 and Cx4371. In mice with a complete deletion of Cx40 --which forms gap junctions between JG cells and SMCs and Ecs--renin-producing cells are dissociated from Ecs, inhibiting intravascular pressure-mediated negative regulation of renin synthesis and secretion72. Furthermore, selective deletion of Cx40 in renin-expressing cells results in the displacement of renin-expressing cells from the JG apparatus to the surrounding areas of the glomeruli, causing the lack of ability to sense perfusion pressure properly in renin-expressing cells, leading to severe hyperreninaemia and hypertension73. Considering these findings, Cx40 in renin-expressing cells determines the location around afferent arterioles where the cells can properly sense intravascular pressure, and thus Cx40 is indirectly involved in the renal baroreceptor mechanism.
Nuclear lamina, and chromatin architecture
The nuclear lamina underlies the nuclear envelope and is essential for stabilizing the nuclear structure, including nuclear stiffness and nuclear pore distribution. Generally, the nuclear lamina is composed of A-type and B-type lamins, which play crucial roles in DNA replication, gene expression, and genome organization in three dimensions74. In particular, lamin A/C is associated with transcriptionally activated euchromatic regions via lamina-associated domains that control gene expression differently based on the cell type74,75. Studying the epigenomic structure of renin cells10, the locus of the Lmna gene was involved in one of 91 super-enhancers specific for renin cells and epigenetically active, as evidenced by open chromatin signalings in some physiological stages of native renin cells56. Mice with conditional deletion of Lmna in renin-expressing cells exposed to AoCo surgery showed significantly impaired reactivity of Ren1 expression to changes in perfusion pressure56. Furthermore, in vitro experiments with deletion of the Lmna gene using the CRISPR-Cas9 system showed similar effects along with alteration of the nuclear shape of renin-expressing cells subjected to mechanical stimulation and affected the profile of chromatin accessibility. Those findings suggest that lamin A/C inside the nuclei plays a crucial role in regulating Ren1 expression in response to changes in perfusion pressure and that nuclear mechanotransduction induces chromatin architectural changes in renin cells.
Ion channels
Piezo1 and Piezo2 are mechanically activated non-voltage-gated calcium-permeable ion channels that play a critical role in some cellular mechanotransduction processes76. In the vascular system, the Piezo1 channel is mainly expressed in the cardiovascular system and regulates BP and vascular development, whereas the Piezo2 channel is expressed in carotid sinus and aortic arch baroreceptors along with Piezo177–79. Recent studies have suggested an association between piezo channels and renin cells. For instance, Yang et al. reported that Piezo1 colocalized with renin in mouse kidneys and activated Piezo1 downregulated renin in the renin-expressing As4.1 mouse tumoral cell line80. Application of microfluidic mechanical stress to As4.1 cells resulted in intracellular calcium increased in wild-type but not in Piezo1 knockout cells80. Additionally, it has been reported that glomerular MCs and renin-producing cells express Piezo2, which was upregulated with renin overproduction in renin-producing cells in response to dehydration as a mechanical load reduction81. Whether in vivo loss of function of either Piezo1 or 2 affects renin expression remains to be determined. Other mechanosensitive ion channels, such as the Transient receptor potential vanilloid 4 (Trpv4), have been reported to induce pressure-induced calcium transport and inhibit renin secretion82. While it is assumed that these non-voltage-gated ion channels contribute to the nuclear mechanotransduction via intracellular calcium increases in renin cells, their specific contribution to the kidney baroreceptor mechanism remains elusive.
Conclusion
Renin cells play a crucial role in regulating homeostasis, kidney vasculature development, and possess an intrinsic and powerful baroreceptor that responds to changes in perfusion pressure via a nuclear mechanotransduction mechanism. Renin cells are extremely plastic and switch on and off their endocrine properties in response to challenges to homeostasis. They also play a role in vascular development and in adult life if constantly stimulated to synthesize renin, they contribute to the CAAH and kidney fibrosis. Of note, most of the signaling and molecular events that regulate embryonic and fetal development are utilized in adult life in situations of homeostatic stress or disease. A now classical mechanism is the ability of SMCs who descended from renin progenitors to transform back to the renin phenotype when homeostasis is threatened (hypotension, dehydration, blood loss).
Similarly, many of the molecules used during vasculogenesis/angiogenesis of the kidney vasculature are utilized when the kidney regenerates after release of ureteral obstruction. Further, knowledge gained from understanding the contribution of renin cells to kidney nephrovascular development helps explain how exposure to inhibitors of the RAS during fetal life caused massive kidney morphological abnormalities accompanied by fluid-electrolyte losses and renal failure.
There is still a lot to learn about the sensing mechanisms that renin cells use to regulate tissue development and perfusion of vital organs. How mechanical and soluble signals -such as those emanating from β-AR, or EP4R, or the dubious participation of piezo channels, integrate their responses to coordinate the precise release of renin and the exact rate of renin synthesis in response to a variety of stimuli, remains to be investigated.
Sources of Funding
MLSSL is supported by National Institutes of Health grants DK 116196, DK 096373, and HL 148044 and RAG by DK 096373 and DK 11671.
Footnotes
Disclosures
None.
References
- 1.Gomez RA, Sequeira-Lopez MLS. Renin cells in homeostasis, regeneration and immune defence mechanisms. Nat Rev Nephrol. 2018;14:231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez RA, Sequeira-Lopez MLS. Novel Functions of Renin Precursors in Homeostasis and Disease. Physiology. 2016;31:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guessoum O, Martini A de G, Sequeira-Lopez MLS, Gomez RA. Deciphering the Identity of Renin Cells in Health and Disease. Trends Mol Med. 2020;27:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurtz A Control of Renin Synthesis and Secretion. Am J Hypertens. 2012;25:839–847. [DOI] [PubMed] [Google Scholar]
- 5.Pentz ES, Lopez MLSS, Cordaillat M, Gomez RA. Identity of the renin cell is mediated by cAMP and chromatin remodeling: an in vitro model for studying cell recruitment and plasticity. Am J Physiol-heart C. 2008;294:H699–H707. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer B, Machura K, Chen M, Weinstein LS, Oppermann M, Sequeira-Lopez ML, Gomez RA, Schnermann J, Castrop H, Kurtz A, Wagner C. Development of vascular renin expression in the kidney critically depends on the cyclic AMP pathway. Am J Physiol-renal. 2009;296:F1006–F1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez RA, Pentz ES, Jin X, Cordaillat M, Lopez MLSS. CBP and p300 are essential for renin cell identity and morphological integrity of the kidney. Am J Physiol-heart C. 2009;296:H1255–H1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pentz ES, Cordaillat M, Carretero OA, Tucker AE, Lopez MLSS, Gomez RA. Histone acetyl transferases CBP and p300 are necessary for maintenance of renin cell identity and transformation of smooth muscle cells to the renin phenotype. Am J Physiol-heart C. 2012;302:H2545–H2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunskill EW, Sequeira-Lopez MLS, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA. Genes that Confer the Identity of the Renin Cell. J Am Soc Nephrol. 2011;22:2213–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez MF, Medrano S, Brown EA, Tufan T, Shang S, Bertoncello N, Guessoum O, Adli M, Belyea BC, Lopez MLSS, Gomez RA. Super-enhancers maintain renin-expressing cell identity and memory to preserve multisystem homeostasis. J Clin Invest. 2018;128:4787–4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrankl J, Neubauer B, Fuchs M, Gerl K, Wagner C, Kurtz A. Apparently normal kidney development in mice with conditional disruption of ANG II-AT1 receptor genes in FoxD1-positive stroma cell precursors. Am J Physiol-renal. 2019;316:F1191–F1200. [DOI] [PubMed] [Google Scholar]
- 12.Dressler GR. The Cellular Basis of Kidney Development. Cell Dev Biology. 2006;22:509–529. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, Li M, Göthert JR, Gomez RA, Sequeira-Lopez MLS. Hemovascular Progenitors in the Kidney Require Sphingosine-1-Phosphate Receptor 1 for Vascular Development. J Am Soc Nephrol. 2016;27:1984–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sequeira-Lopez MLS, Lin EE, Li M, Hu Y, Sigmund CD, Gomez RA. The earliest metanephric arteriolar progenitors and their role in kidney vascular development. Am J Physiology-regulatory Integr Comp Physiology. 2015;308:R138–R149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez MLSS Pentz ES, Robert B Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol-renal. 2001;281:F345–F356. [DOI] [PubMed] [Google Scholar]
- 16.Robert B, John PLS, Hyink DP, Abrahamson DR. Evidence that embryonic kidney cells expressing flk-1 are intrinsic, vasculogenic angioblasts. Am J Physiol-renal. 1996;271:F744–F753. [DOI] [PubMed] [Google Scholar]
- 17.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Lopes SMC de S, Little MH. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–568. [DOI] [PubMed] [Google Scholar]
- 18.Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, Morizane R. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods. 2019;16:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shankar AS, Du Z, Mora HT, Bosch TPP van den, Korevaar SS, Berg-Garrelds IMV den, Bindels E, Lopez-Iglesias C, Groningen MCC, Gribnau J, Baan CC, Danser AHJ, Hoorn EJ, Hoogduijn MJ. Human kidney organoids produce functional renin. Kidney Int. 2021;99:134–147. [DOI] [PubMed] [Google Scholar]
- 20.Karginova EA, Pentz ES, Kazakova IG, Norwood VF, Carey RM, Gomez RA. Zis: a developmentally regulated gene expressed in juxtaglomerular cells. Am J Physiol-renal. 1997;273:F731–F738. [DOI] [PubMed] [Google Scholar]
- 21.Reddi V, Zaglul A, Pentz ES, Gomez RA. Renin-expressing cells are associated with branching of the developing kidney vasculature. J Am Soc Nephrol. 1998;9:63–71. [DOI] [PubMed] [Google Scholar]
- 22.Gomez RA. Fate of Renin Cells During Development and Disease. Hypertension. 2018;69:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Kim SM, Oppermann M, Faulhaber-Walter R, Huang Y, Mizel D, Chen M, Lopez MLS, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsα in juxtaglomerular cells. Am J Physiol-renal. 2007;292:F27–F37. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Faulhaber-Walter R, Wen Y, Huang Y, Mizel D, Chen M, Lopez MLS, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Renal Failure in Mice with Gsα Deletion in Juxtaglomerular Cells. Am J Nephrol. 2010;32:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castellanos-Rivera RM, Monteagudo MC, Pentz ES, Glenn ST, Gross KW, Carretero O, Sequeira-Lopez MLS, Gomez RA. Transcriptional regulator RBP-J regulates the number and plasticity of renin cells. Physiol Genomics. 2011;43:1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castellanos-Rivera RM, Pentz ES, Lin E, Gross KW, Medrano S, Yu J, Sequeira-Lopez MLS, Gomez RA. Recombination Signal Binding Protein for Ig-κJ Region Regulates Juxtaglomerular Cell Phenotype by Activating the Myo-Endocrine Program and Suppressing Ectopic Gene Expression. J Am Soc Nephrol. 2015;26:67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin EE, Sequeira-Lopez MLS, Gomez RA. RBP-J in FOXD1+ renal stromal progenitors is crucial for the proper development and assembly of the kidney vasculature and glomerular mesangial cells. Am J Physiol-renal. 2014;306:F249–F258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helle F, Hultström M, Kavvadas P, Iversen B, Chadjichristos CE, Chatziantoniou C. Deletion of Notch3 Impairs Contractility of Renal Resistance Vessels Due to Deficient Ca2+ Entry. Int J Mol Sci. 2022;23:16068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castellanos-Rivera RM. RBP-J Regulates the Identity and Plasticity of Renin Cells: a Fundamental Mechanism to Control Homeostasis. PhD thesis, University of Virginia. 2012; [Google Scholar]
- 30.Martini AG, Smith JP, Medrano S, Sheffield NC, Sequeira-Lopez MLS, Gomez RA. Determinants of renin cell differentiation: a single cell epi-transcriptomics approach. Biorxiv. 2023;2023.01.18.524595. [Google Scholar]
- 31.Gomez RA, Chevalier RL, Everett AD, Elwood JP, Peach MJ, Lynch KR, Carey RM. Recruitment of renin gene-expressing cells in adult rat kidneys. Am J Physiol-renal. 1990;259:F660–F665. [DOI] [PubMed] [Google Scholar]
- 32.López MLSS Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin Cells Are Precursors for Multiple Cell Types that Switch to the Renin Phenotype When Homeostasis Is Threatened. Dev Cell. 2004;6:719–728. [DOI] [PubMed] [Google Scholar]
- 33.Berg AC, Chernavvsky-Sequeira C, Lindsey J, Gomez RA, Sequeira-Lopez MLS. Pericytes synthesize renin. World J Nephrol. 2013;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guessoum O, Zainab M, Sequeira‐Lopez MLS, Gomez RA. Proliferation does not contribute to murine models of renin cell recruitment. Acta Physiol. 2020;230:e13532. [Google Scholar]
- 35.Gomez RA, Lopez MLSS. Plasticity of Renin Cells in the Kidney Vasculature. Curr Hypertens Rep. 2017;19:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makhanova N, Sequeira-Lopez MLS, Gomez RA, Kim H-S, Smithies O. Disturbed Homeostasis in Sodium-Restricted Mice Heterozygous and Homozygous for Aldosterone Synthase Gene Disruption. Hypertension. 2006;48:1151–1159. [DOI] [PubMed] [Google Scholar]
- 37.Stefanska A, Briski A, Khan NS, Kenyon C, Péault BM, Mullins JJ. Role of Pericytes in the Development of the Renin/Angiotensin System: Induction of Functional Renin in Cultures of Pericytes. Methods Mol Biology. 2021;2235:169–180. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann S, Mullins L, Rider S, Brown C, Buckley CB, Assmus A, Li Z, Beltran MS, Henderson N, Pozo J del, Martini ADG, Sequeira-Lopez MLS, Gomez RA, Mullins J. Comparative Studies of Renin-Null Zebrafish and Mice Provide New Functional Insights. Hypertension. 2021;79:e56–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tufro-McReddie A, Romano LM, Harris JM, Ferder L, Gomez RA. Angiotensin II regulates nephrogenesis and renal vascular development. Am J Physiol-renal. 1995;269:F110–F115. [DOI] [PubMed] [Google Scholar]
- 40.Hilgers KF, Reddi V, Krege JH, Smithies O, Gomez RA. Aberrant Renal Vascular Morphology and Renin Expression in Mutant Mice Lacking Angiotensin-Converting Enzyme. Hypertension. 1997;29:216–221. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi N, Lopez MLSS, Cowhig JE, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim H-S, Smithies O. Ren1c Homozygous Null Mice Are Hypotensive and Polyuric, but Heterozygotes Are Indistinguishable from Wild-Type. J Am Soc Nephrol. 2005;16:125–132. [DOI] [PubMed] [Google Scholar]
- 42.Oka M, Medrano S, Sequeira-Lόpez MLS, Gómez RA. Chronic Stimulation of Renin Cells Leads to Vascular Pathology. Hypertension. 2018;70:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe H, Martini AG, Brown EA, Liang X, Medrano S, Goto S, Narita I, Arend LJ, Sequeira-Lopez MLS, Gomez RA. Inhibition of the renin-angiotensin system causes concentric hypertrophy of renal arterioles in mice and humans. Jci Insight. 2021;6:e154337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moniwa N, Varagic J, Ahmad S, VonCannon JL, Simington SW, Wang H, Groban L, Brosnihan KB, Nagata S, Kato J, Kitamura K, Gomez RA, Lopez MLS, Ferrario CM. Hemodynamic and Hormonal Changes to Dual Renin–Angiotensin System Inhibition in Experimental Hypertension. Hypertension. 2013;61:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamming I, Navis G, Kocks M, Goor H van. ACE inhibition has adverse renal effects during dietary sodium restriction in proteinuric and healthy rats. J Pathol. 2006;209:129–139. [DOI] [PubMed] [Google Scholar]
- 46.Owen RA, Molon-Noblot S, Hubert M-F, Kindt MV, Keenan KP, Eydelloth RS. The Morphology of Juxtaglomerular Cell Hyperplasia and Hypertrophy in Normotensive Rats and Monkeys Given an Angiotensin II Receptor Antagonist. Toxicol Pathol. 1994;22:606–619. [DOI] [PubMed] [Google Scholar]
- 47.Nagai Y, Yamabe F, Sasaki Y, Ishii T, Nakanishi K, Nakajima K, Shibuya K, Mikami T, Akasaka Y, Urita Y, Yamanaka N. A Study of Morphological Changes in Renal Afferent Arterioles Induced by Angiotensin II Type 1 Receptor Blockers in Hypertensive Patients. Kidney Blood Press Res. 2020;45:194–208. [DOI] [PubMed] [Google Scholar]
- 48.Liu G, Hitomi H, Hosomi N, Shibayama Y, Nakano D, Kiyomoto H, Ma H, Yamaji Y, Kohno M, Ichihara A, Itoh H, Nishiyama A. Prorenin induces vascular smooth muscle cell proliferation and hypertrophy via epidermal growth factor receptor-mediated extracellular signal-regulated kinase and Akt activation pathway. J Hypertens. 2011;29:696–705. [DOI] [PubMed] [Google Scholar]
- 49.Pentz ES, Moyano MA, Thornhill BA, Lopez MLSS, Gomez RA. Ablation of renin-expressing juxtaglomerular cells results in a distinct kidney phenotype. Am J Physiology-regulatory Integr Comp Physiology. 2004;286:R474–R483. [DOI] [PubMed] [Google Scholar]
- 50.Mohamed TH, Watanabe H, Kaur R, Belyea BC, Walker PD, Gomez RA, Sequeira-Lopez MLS. Renin-Expressing Cells Require β1-Integrin for Survival and for Development and Maintenance of the Renal Vasculature. Hypertension. 2020;76:458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tobian L, Tomboulian A, Janecek J. The effect of high perfusion pressures on the granulation of juxtaglomerular cells in an isolated kidney. J Clin Invest. 1959;38:605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carlström M, Wilcox CS, Arendshorst WJ. Renal Autoregulation in Health and Disease. Physiol Rev. 2015;95:405–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirchheim HR, Ehmke H, Hackenthal E, Löwe W, Persson P. Autoregulation of renal blood flow, glomerular filtration rate and renin release in conscious dogs. Pflügers Archiv - European J Physiology. 1987;410:441–449. [DOI] [PubMed] [Google Scholar]
- 54.Nishimura H, Lunde LG, Zucker A. Renin response to hemorrhage and hypotension in the aglomerular toadfish Opsanus tau. Am J Physiol-heart C. 1979;237:H105–H111. [DOI] [PubMed] [Google Scholar]
- 55.Nishimura H, Madey MA. Signals controlling renin release in aglomerular toadfish. Fish Physiol Biochem. 1989;7:323–329. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe H, Belyea BC, Paxton RL, Li M, Dzamba BJ, DeSimone DW, Gomez RA, Sequeira-Lopez MLS. Renin Cell Baroreceptor, a Nuclear Mechanotransducer Central for Homeostasis. Circ Res. 2021;129:262–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamashiro Y, Yanagisawa H. The molecular mechanism of mechanotransduction in vascular homeostasis and disease. Clin Sci. 2020;134:2399–2418. [DOI] [PubMed] [Google Scholar]
- 58.Graham DM, Burridge K. Mechanotransduction and nuclear function. Curr Opin Cell Biol. 2016;40:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis MJ, Earley S, Li Y-S, Chien S. Vascular mechanotransduction. Physiol Rev. 2023;103:1247–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kirby TJ, Lammerding J. Emerging views of the nucleus as a cellular mechanosensor. Nat Cell Biol. 2018;20:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niethammer P Components and Mechanisms of Nuclear Mechanotransduction. Annu Rev Cell Dev Bi. 2021;37:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Bio. 2014;15:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: A dynamic view. Dev Biol. 2010;341:126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bachmann M, Kukkurainen S, Hytönen VP, Wehrle-Haller B. Cell Adhesion by Integrins. Physiol Rev. 2019;99:1655–1699. [DOI] [PubMed] [Google Scholar]
- 65.Jülich D, Cobb G, Melo AM, McMillen P, Lawton AK, Mochrie SGJ, Rhoades E, Holley SA. Cross-Scale Integrin Regulation Organizes ECM and Tissue Topology. Dev Cell. 2015;34:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biology. 2005;171:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Starr DA. KASH and SUN proteins. Curr Biol. 2011;21:R414–R415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tajik A, Zhang Y, Wei F, Sun J, Jia Q, Zhou W, Singh R, Khanna N, Belmont AS, Wang N. Transcription upregulation via force-induced direct stretching of chromatin. Nat Mater. 2016;15:1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dbouk HA, Mroue RM, El-Sabban ME, Talhouk RS. Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun Signal. 2009;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanner F, Sorensen CM, Holstein-Rathlou N-H, Peti-Peterdi J. Connexins and the kidney. Am J Physiology-regulatory Integr Comp Physiology. 2010;298:R1143–R1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurtz A. Connexins, renin cell displacement and hypertension. Curr Opin Pharmacol. 2015;21:1–6. [DOI] [PubMed] [Google Scholar]
- 72.Wagner C, Wit C de, Kurtz L, Gruünberger C, Kurtz A, Schweda F. Connexin40 Is Essential for the Pressure Control of Renin Synthesis and Secretion. Circ Res. 2007;100:556–563. [DOI] [PubMed] [Google Scholar]
- 73.Wagner C, Jobs A, Schweda F, Kurtz L, Kurt B, Lopez MLS, Gomez RA, Veen TAB van, Wit C de, Kurtz A. Selective deletion of Connexin 40 in renin-producing cells impairs renal baroreceptor function and is associated with arterial hypertension. Kidney Int. 2010;78:762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Briand N, Collas P. Laminopathy-causing lamin A mutations reconfigure lamina-associated domains and local spatial chromatin conformation. Nucleus. 2018;9:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gesson K, Rescheneder P, Skoruppa MP, Haeseler A von, Dechat T, Foisner R. A-type lamins bind both hetero- and euchromatin, the latter being regulated by lamina-associated polypeptide 2 alpha. Genome Res. 2016;26:462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M, Patapoutian A. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, Jodar M, Moro C, Offermanns S, Feng Y, Demolombe S, Patel A, Honoré E. Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. Cell Reports. 2015;13:1161–1171. [DOI] [PubMed] [Google Scholar]
- 78.Zeng W-Z, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, Liberles SD, Patapoutian A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science. 2018;362:464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest. 2016;126:4527–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang X, Zeng H, Wang L, Luo S, Zhou Y. Activation of Piezo1 downregulates renin in juxtaglomerular cells and contributes to blood pressure homeostasis. Cell Biosci. 2022;12:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mochida Y, Ochiai K, Nagase T, Nonomura K, Akimoto Y, Fukuhara H, Sakai T, Matsumura G, Yamaguchi Y, Nagase M. Piezo2 expression and its alteration by mechanical forces in mouse mesangial cells and renin-producing cells. Sci Rep-uk. 2022;12:4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seghers F, Yerna X, Zanou N, Devuyst O, Vennekens R, Nilius B, Gailly P. TRPV4 participates in pressure‐induced inhibition of renin secretion by juxtaglomerular cells. J Physiology. 2016;594:7327–7340. [DOI] [PMC free article] [PubMed] [Google Scholar]


