Abstract
Bacteria are single-celled organisms, but the survival of microbial communities relies on complex dynamics at the molecular, cellular, and ecosystem scales. Antibiotic resistance, in particular, is not just a property of individual bacteria or even single-strain populations, but depends heavily on the community context. Collective community dynamics can lead to counterintuitive eco-evolutionary effects like survival of less resistant bacterial populations, slowing of resistance evolution, or population collapse, yet these surprising behaviors are often captured by simple mathematical models. In this review, we highlight recent progress — in many cases, advances driven by elegant combinations of quantitative experiments and theoretical models — in understanding how interactions between bacteria and with the environment affect antibiotic resistance, from single-species populations to multispecies communities embedded in an ecosystem.
Introduction
Antibiotic resistance is a growing threat to public health, as the rise of resistance outpaces the development of new antimicrobial drugs. Decades of progress in molecular biology, microbiology, and biophysics has driven us deep within the bacterial cell, where we increasingly understand the molecular events — the enzymatic reactions that hydrolyze drugs, or the molecular machines shuttling toxic molecules out of the cell — that separate bacterial survival from death. Yet bacteria do not live as isolated cells, but as ever-evolving members of complex communities with potentially billions of neighbors. Within these communities, physical and chemical interactions between cells impart complex spatial and temporal dynamics to the miniature bacterial ‘civilizations’ that comprise human infections [1–4]. These collective effects may have a profound impact on strategies to eradicate or control bacterial growth. While antibiotics directly target intracellular functions, such as protein synthesis, that are required for survival of a single cell, these drugs frequently originated as tools of molecular warfare pioneered by microorganisms [5], and are thus themselves a byproduct of community interactions. A deeper understanding of the interactions that shape the microbial communities may therefore hold clues for slowing, and even reversing, antibiotic resistance [6].
Predicting how bacterial communities and antibiotic treatments will interplay in a specific environment is a significant challenge, in part because the complexity of multispecies communities grows exponentially as the number of microbial and chemical players increases [7]. Yet a growing body of research implicates collective behaviors of bacterial populations (single species) and communities (multiple species) in the emergence of antibiotic resistance. We are far from a comprehensive, mechanistic model of these complex communities, but recent advances in both experiment and theory are highlighting ecological and evolutionary principles that shape community composition (Figure 1a) and may eventually be leveraged to prolong the effectiveness of currently available antibiotics. These breakthroughs reveal interconnected dynamics across length scales, tying physical interactions between single cells to the fate of communities on evolutionary horizons. In this review, we highlight a subset of recent advances — with particular emphasis on those inspired by mathematical and physical models — with the potential to inspire new (bacterial) community-based strategies for slowing resistance.
Figure 1.
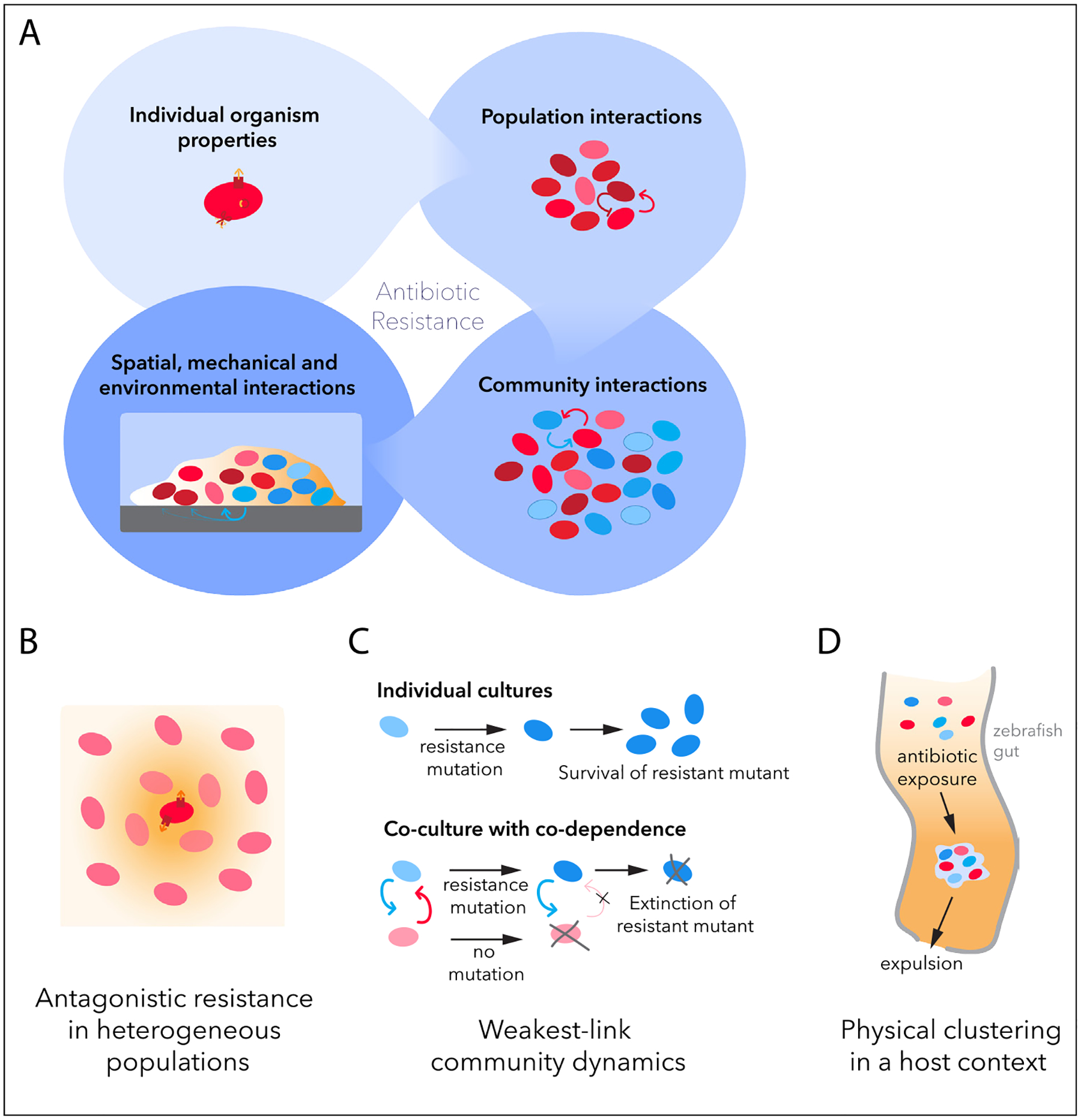
Antibiotic resistance integrates properties across different scales of bacterial life. (a) Molecular mechanisms at the single-organism level (e.g. enzymes or efflux pumps) are an important component of antibiotic resistance (top left). Antibiotic resistance is, however, also influenced by interactions in both homogeneous and physiologically or genetically heterogeneous single-species populations, such as competition and cooperation (top right). Multispecies communities of bacteria can contain manifold interspecies interactions, such as cross-feeding, antibiotic protection or active mutual inhibition, whose balance significantly determines community composition and response to antibiotic (bottom right). In addition, how communities aggregate and organize in space, as well as how they interact with other components of their ecosystem, are important contributing factors to observed antibiotic resistance (bottom left). (b–d) Examples of community effects include antagonistic resistance in heterogeneous populations [18] (b), weakest-link dynamics in cross-feeding communities [30] (c), and antibiotic-induced aggregation and expulsion of bacterial aggregates from the host gut [46] (d).
Collective effects in single-species populations
Antibiotic resistance occurs across different scales of bacterial life. At the single-cell level, a suite of molecular defense mechanisms — for example, drug degrading enzymes or efflux pumps — protect bacteria from antibiotic [8]. But as microbiologists have long recognized, environmental context can change the bacterial response to antibiotic. Perhaps the simplest example is the well-known inoculum effect: The minimum inhibitory concentration of an antibiotic depends on the initial (inoculating) density of bacteria in media [9–11]. Although typically cell density increases antibiotic resistance, it decreases resistance to some antibiotics [10]. The mechanisms for this phenotype are diverse — including enzymatic degradation of drug [12], growth bistability driven by the heat-shock response [11], or modulation of environmental pH [10], an effect potentially exacerbated in multispecies communities [13,14].
The density dependence of antibiotic activity may also impact ecological and evolutionary dynamics among subpopulations with varying levels of resistance. Antibiotic-resistant cells can either reduce [15–17] or accentuate [18] the direct effects of antibiotics on nearby susceptible cells. Interestingly, these effects do not always require the secretion of diffusible molecules into the extracellular media; for example, β-lactamase producing Streptococcus pneumoniae [13] and Enterococcus faecalis [19] function as local reaction sinks for diffusing antibiotic, even when the enzyme remains associated to the producing cells. Similarly, drug efflux from individual Escherichia coli can increase the local drug concentration for neighboring cells [18] (Figure 1b).
‘Cooperative’ resistant cells can also raise the antibiotic threshold for the spread of resistance-gene carrying plasmids to susceptible cells [20], and subtle feedback loops between cooperating subpopulations can trigger counterintuitive dynamics such as population collapse [21]. Conversely, resource-consuming susceptible cells are able to indirectly slow the growth of resistant populations, and time-dependent drug dosing schemes can, in principle, exploit this competition. For example, recent experiments in computer-controlled bioreactors showed that doxycycline-resistant E. coli populations can be held in check by doxycycline when dosing is designed to maintain a competing subpopulation of sensitive cells [22]. Mathematical models suggest this competitive suppression may apply beyond the laboratory, and it serves as the basis for adaptive therapies targeting infectious diseases and cancer [23,24].
Multispecies community interactions
Interactions between susceptible and resistant bacteria can also modulate the dynamics of more complex communities, ranging from small, well-defined communities with a few species to highly complex microbiome assemblies. Cooperative resistance can pose a particular problem for antibiotic treatment when endogenous bacterial species protect susceptible pathogens [25]. For example, Stenotrophomonas maltophilia have been shown to offer enzyme-mediated protection from the antibiotic imipenem to pathogenic Pseudomonas aeruginosa in a multispecies model of cystic fibrosis [26]. This effect is dependent on a drug-specific trade-off between killing efficacy and the rate of enzymatic degradation of antibiotic. Exchanging imipenem for a closely related carbapenem alters this trade-off, leading to a strikingly different outcome where the susceptible species is no longer protected [26]. In contrast, mathematical models indicate that antibiotic resistance in commensals can be beneficial for control of a more susceptible pathogen during coinfection when the interaction between commensals and pathogens is competitive or exploitative [28].
In some cases, interactions between cohabiting species can lead to counterintuitive outcomes such as the more susceptible species taking over the community. For example, short-term exposure to a range of antibiotics (chloramphenicol, gentamycin, or ampicillin) is able to drive a bistable community of mutually inhibiting species (Corynebacterium ammoniagenes and Lactobacillus plantarum) to recover to a state dominated by the more drug susceptible, but faster growing species (C. ammoniagenes) [27]. A similar effect can also be driven by antibiotic tolerance (characterized by growth after lag time) rather than genetic resistance. In mixed cultures of Bacillus subtilis and E. coli, only the latter proliferates in the presence of β-lactams; by contrast, in mono-cultures B. subtilis is tolerant while E. coli is susceptible [29]. These ‘antithetic’ responses can be predicted from a kinetic model that captures binding of β-lactam to its intracellular targets (penicillin binding proteins) in the two species, which exhibit differential rates of antibiotic deactivation. In mixed cultures, the common pool of drug molecules harms the species that inactivates antibiotic faster (B. subtilis) and benefits the slow inactivator (E. coli). As a whole, these results indicate that the susceptibility profiles of individual species alone do not determine community-level dynamics, and illustrate the power of simple ecological and biophysical models for predicting these emergent community behaviors.
In addition to ecological dynamics, community-level interactions can directly affect how antibiotic resistance evolves. Microbial communities with mutualistic cross-feeding interactions can exhibit ‘weakest link’ dynamics, in which the most antibiotic susceptible community member defines the antibiotic susceptibility of the whole community [30]. In a cross-feeding community of E. coli and Salmonella enterica, this ecological property of mutualism changed the evolutionary emergence of resistance. Under two different antibiotics, ampicillin and rifampin, cocultures displayed a slower increase in resistance than monocultures of each species. Under ampicillin, both the speed and molecular mechanisms of adaptation differed in co- versus mono-culture. A mathematical model suggests that the slowing of resistance evolution by mutualism may be a general mechanism, because rare large-effect mutations are less advantageous in coculture, when susceptibility is still dependent on a more susceptible partner species [31] (Figure 1c).
Translating results from simple communities with a handful of species — where the dynamics, and increasingly, even the underlying molecular or mathematical mechanisms can be teased apart — to complex natural communities is an enormous challenge. However, a number of recent breakthroughs are pointing a way forward by uncovering surprisingly simple principles — based, for example, on pairwise approximations, geometric constraints, or stochasticity — that drive population-level outcomes, including composition [32–36], multicellularity [37], and complex metabolic inter-dependencies [38,39]. Whether similar simplifying principles describe the response of complex communities to antibiotics is an open question.
Innovative top-down approaches have started to decipher how pathogen interactions and antibiotic resistance behave within natural microbiome communities, which are notable not only for their immense complexity, but also for the fact that many species cannot even be cultured individually in the lab. For example, multiple studies have found that microbiomes can reduce antibiotic resistance evolution or selection to different antibiotics in E. coli [40,41], and community influences can uncouple selection for resistance from antibiotic concentrations above a certain threshold [42]. Suggested mechanisms included a higher cost of resistance, but also protection of susceptible bacteria, which reduces selective pressure. Understanding these complex communities will require continued efforts that draw on the success of both bottom-up and top-down approaches to ecological and evolutionary dynamics.
Spatial, mechanical, and environmental effects on resistance
Bacterial populations exist within larger, complex ecosystems, and thus the populations’ compositional complexity is shaped by the physical environment in which they are embedded. Classical ecological interactions — such as cross-feeding or enzymatic protection — are governed, in part, by diffusion of intercellular signaling molecules, which can be heavily influenced by spatial and temporal heterogeneity.
Further, most bacterial life exists in biofilms, dense surface-attached populations. The biofilm lifestyle has long been known to influence how cells respond to antibiotics [43], in part because of physical limitations imposed on diffusive signaling; antibiotic exposure can also affect biofilm structure [44,45]. Recent work in zebrafish has shown that antibiotics can modulate aggregation of gut bacteria, which in turn increases their expulsion from the gut [46] (Figure 1d). Mathematical models suggest that statistical properties of these gut aggregates arise from a set of simple, and common, biological ‘ingredients’: the growth of bacteria within clusters, the escape of cells from the clusters, and a multicluster aggregation process that drives the system to a single large cluster, making these populations fascinating examples of living gels [47]. Aggregation has also been observed to occur as a community interaction between Staphylococcus aureus and P. aeruginosa in co-culture model of cystic fibrosis, which alters the community’s antibiotic resistance [48].
Spatial segregation between species of a community is considered an important evolutionary source of cooperation — allowing producers of public goods, such as antibiotic-degrading enzymes, to preferentially benefit [49,50]. The spatial arrangement of resistant cells may also provide strong synergistic ecological benefit [51], allowing mixed communities to exhibit properties of purely resistant colonies when only a small fraction of the colony surface includes resistant cells [19]. A wide range of physical interactions, including mechanical crowding [52], cell motility [53], and electrical signaling [54], are increasingly recognized as drivers of ecological and evolutionary dynamics. It remains to be seen whether, and how, these interactions shape the response of cells to antibiotics.
Conclusions and future directions
In this review, we aimed to highlight recent advancements in our understanding of the eco-evolutionary dynamics of bacterial communities. We focused specifically on the interactions of these communities with antibiotics, and on studies that used experiments and modeling to extract quantitative and predictive insights. The work, as a whole, underscores the importance of interrogating antimicrobial resistance at a systems level, where interactions across scales give rise to complex, and often counterintuitive, dynamics. While simple mathematical models have proven remarkably successful at describing and predicting microbial dynamics, particularly in simple populations, these coarse-grained models are largely agnostic to the species-specific properties that are undoubtedly important as we look on finer scales. Connecting the molecular and biophysical events that occur within the cell to emergent community level properties will continue to be an important challenge, and one that, in the long-run, may inspire new therapeutic strategies — for example, personalized antibiotic regimens based on machine learning [55] or control theoretic principles [56] — aimed at eradicating or controlling microbial communities.
Acknowledgements
This work was supported by the Jane Coffin Childs Fellowship, USA (to MDL) and by NIH R35GM124875, USA (to KW).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
No data were used for the research described in the article.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
•of special interest
••of outstanding interest
- 1.Short FL, Murdoch SL, Ryan RP: Polybacterial human disease: the ills of social networking. Trends Microbiol 2014, 22:508–516, 10.1016/j.tim.2014.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan J, Bassler BL: Surviving as a community: antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe 2019, 26:15–21, 10.1016/j.chom.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vos MGJ, Zagorski M, McNally A, Bollenbach T: Interaction networks, ecological stability, and collective antibiotic tolerance in polymicrobial infections. Proc Natl Acad Sci USA 2017, 114:10666–10671, 10.1073/pnas.1713372114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z, van Kleunen M, Becks L, Thakur MP: Towards a general understanding of bacterial interactions. Trends Microbiol 2020, 28:783–785, 10.1016/j.tim.2020.05.010 [DOI] [PubMed] [Google Scholar]
- 5.Niehus R, Oliveira NM, Li A, Fletcher AG, Foster KR: The Evolution of Strategy in Bacterial Warfare via the Regulation of Bacteriocins and Antibiotics. ELife 2021, 10:e69756, 10.7554/elife.69756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bottery MJ, Pitchford JW, Friman VP: Ecology and evolution of antimicrobial resistance in bacterial communities. ISME J 2021, 15:939–948, 10.1038/s41396-020-00832-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopatkin AJ, Collins JJ: Predictive biology: modelling, understanding and harnessing microbial complexity. Nat Rev Microbiol 2020, 18:507–520, 10.1038/s41579-020-0372-5 [DOI] [PubMed] [Google Scholar]
- 8.Darby EM, Trampari E, Siasat P, Gaya MS, Alav I, Webber MA, Blair JMA: Molecular mechanisms of antibiotic resistance revisited. Nature Reviews Microbiology 2022,1–16, 10.1038/s41579-022-00820-y [DOI] [PubMed] [Google Scholar]
- 9.Udekwu KI, Parrish N, Ankomah P, Baquero F, Levin BR: Functional relationship between bacterial cell density and the efficacy of antibiotics. J Antimicrob Chemother 2009, 63:745–757, 10.1093/jac/dkn554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karslake J, Maltas J, Brumm P, Wood KB: Population density modulates drug inhibition and gives rise to potential bistability of treatment outcomes for bacterial infections. PLoS Comput Biol 2016, 12:e1005098, 10.1371/JOURNAL.PCBI.1005098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan C, et al. : The inoculum effect and band-pass bacterial response to periodic antibiotic treatment. Mol Syst Biol 2012, 8:617, 10.1038/msb.2012.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabath LD, Garner C, Wilcox C, Finland M: Effect of inoculum and of beta lactamase on the anti staphylococcal activity of thirteen penicillins and cephalosporins. Antimicrob Agents Chemother 1975, 8:344–349, 10.1128/AAC.8.3.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aranda-Díaz A, et al. : Bacterial interspecies interactions modulate pH-mediated antibiotic tolerance. Elife 2020, 9:e51493, 10.7554/eLife.51493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghuneim LAJ, et al. : Complex and unexpected outcomes of antibiotic therapy against a polymicrobial infection. ISME J 2022, 16:2065–2075, 10.1038/s41396-022-01252-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorg RA, et al. : Collective resistance in microbial communities by intracellular antibiotic deactivation. PLoS Biol 2016, 14:e2000631, 10.1371/journal.pbio.2000631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicoloff H, Andersson DI: Indirect resistance to several classes of antibiotics in cocultures with resistant bacteria expressing antibiotic-modifying or -degrading enzymes. J Antimicrob Chemother 2016, 71:100–110, 10.1093/jac/dkv312 [DOI] [PubMed] [Google Scholar]
- 17.Yurtsev EA, Chao HX, Datta MS, Artemova T, Gore J: Bacterial cheating drives the population dynamics of cooperative antibiotic resistance plasmids. Mol Syst Biol 2013, 9:683, 10.1038/msb.2013.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.•.Wen X, Langevin AM, Dunlop MJ: Antibiotic export by efflux pumps affects growth of neighboring bacteria. Sci Rep 2018, 8:1–9, 10.1038/s41598-018-33275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes an excellent example of spatially heterogeneous community interactions in the context of antibiotic exposure response.
- 19.Sharma A, Wood KB: Spatial segregation and cooperation in radially expanding microbial colonies under antibiotic stress. ISME J 2021, 15:3019–3033, 10.1038/s41396-021-00982-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bottery MJ, Wood AJ, Brockhurst MA: Selective conditions for a multidrug resistance plasmid depend on the sociality of antibiotic resistance. Antimicrob Agents Chemother 2016, 60:2524–2527, 10.1128/AAC.02441-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallinen KM, Karslake J, Wood KB: Delayed antibiotic exposure induces population collapse in enterococcal communities with drug-resistant subpopulations. Elife 2020, 9:e52813, 10.7554/eLife.52813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen E, Karslake J, Woods RJ, Read AF, Wood KB: Antibiotics can be used to contain drug-resistant bacteria by maintaining sufficiently large sensitive populations. PLoS Biol 2020, 18:e3000713, 10.1371/journal.pbio.3000713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gatenby RA, Silva AS, Gillies RJ, Frieden BR: Adaptive therapy. Cancer Res 2009, 69:4894–4903, 10.1158/0008-5472.CAN-08-3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen E, Read AF: Modifying adaptive therapy to enhance competitive suppression. Cancers 2020, 12:3556, 10.3390/cancers12123556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perlin MH, et al. : Protection of Salmonella by ampicillin-resistant Escherichia coli in the presence of otherwise lethal drug concentrations. Proc R Soc B: Biol Sci 2009, 276:3759–3768, 10.1098/rspb.2009.0997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.••.Bottery MJ, Matthews JL, Wood AJ, Johansen HK, Pitchford JW, Friman VP: Inter-species interactions alter antibiotic efficacy in bacterial communities. ISME J 2022, 16:812–821, 10.1038/s41396-021-01130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper nicely shows how the quantitative balance of interactions between species and antibiotic can lead to different community outcomes even for closely related antibiotics.
- 27.••.Amor DR, Gore J: Fast growth can counteract antibiotic susceptibility in shaping microbial community resilience to antibiotics. Proc Natl Acad Sci USA 2022, 119:e2116954119, 10.1073/pnas.2116954119. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates the effect of even transient antibiotic exposure on community composition, and how species properties such as growth and resistance interplay with community interactions to shape antibiotic response.
- 28.Wollein Waldetoft K, Sundius S, Kuske R, Brown SP: Defining the benefits of antibiotic resistance in commensals and the scope for resistance optimization. mBio 2022, 14:e01349–22, 10.1128/mbio.01349-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.••.Galera-Laporta L, Garcia-Ojalvo J: Antithetic Population Response to Antibiotics in a Polybacterial Community. Science Advances (10) 2020, 6, 10.1126/sciadv.aaz5108. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper expertly employs a simple model to explain how the behavior of multispecies communities can be opposite of what is expected from single-species antibiotic susceptibility.
- 30.••.Adamowicz EM, Flynn J, Hunter RC, Harcombe WR: Cross-feeding modulates antibiotic tolerance in bacterial communities. ISME J 2018, 12:2723–2735, 10.1038/s41396-018-0212-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows how mutualistic community interactions and resulting ‘weakest link’ dynamics can shape the evolution of de novo antibiotic resistance.
- 31.Adamowicz EM, Muza M, Chacón JM, Harcombe WR: Cross-feeding modulates the rate and mechanism of antibiotic resistance evolution in a model microbial community of Escherichia coli and Salmonella enterica. PLoS Pathog 2020, 16:e1008700, 10.1371/journal.ppat.1008700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz-Colunga J, et al. : Top-down and bottom-up cohesiveness in microbial community coalescence. Proc Natl Acad Sci USA (6) 2022, 119:e2111261119, 10.1073/pnas.2111261119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman J, Higgins LM, Gore J: Community structure follows simple assembly rules in microbial microcosms. Nat Ecol Evol 2017, 1:1–7, 10.1038/s41559-017-0109 [DOI] [PubMed] [Google Scholar]
- 34.Weiss AS, et al. : In vitro interaction network of a synthetic gut bacterial community. ISME J 2022, 16:1095–1109, 10.1038/s41396-021-01153-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratzke C, Barrere J, Gore J: Strength of species interactions determines biodiversity and stability in microbial communities. Nat Ecol Evol 2020, 4:376–383, 10.1038/s41559-020-1099-4 [DOI] [PubMed] [Google Scholar]
- 36.Moran J, Tikhonov M: Defining coarse-grainability in a model of structured microbial ecosystems. Phys Rev X 2022, 12:021038, 10.1103/PhysRevX.12.021038 [DOI] [Google Scholar]
- 37.Yanni D, Jacobeen S, Márquez-Zacarías P, Weitz JS, Ratcliff WC, Yunker PJ: Topological constraints in early multicellularity favor reproductive division of labor. Elife 2020, 9:1–17, 10.7554/ELIFE.54348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo X, Boedicker JQ: The contribution of high-order metabolic interactions to the global activity of a four-species microbial community. PLoS Comput Biol 2016, 12:e1005079, 10.1371/journal.pcbi.1005079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez JG, Wingreen NS: Noisy metabolism can promote microbial cross-feeding. Elife 2022, 11:e70694, 10.7554/eLife.70694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klümper U, et al. : Selection for antimicrobial resistance is reduced when embedded in a natural microbial community. ISME J 2019, 13:2927–2937, 10.1038/s41396-019-0483-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baumgartner M, Bayer F, Pfrunder-Cardozo KR, Buckling A, Hall AR: Resident microbial communities inhibit growth and antibiotic-resistance evolution of Escherichia coli in human gut microbiome samples. PLoS Biol 2020, 18:e3000465, 10.1371/journal.pbio.3000465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray AK, et al. : Novel insights into selection for antibiotic resistance in complex microbial communities. mBio (4) 2018, 9:e00969–18, 10.1128/mBio.00969-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall CW, Mah TF: Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev 2017, 41:276–301, 10.1093/femsre/fux010 [DOI] [PubMed] [Google Scholar]
- 44.Díaz-Pascual F, et al. : Breakdown of Vibrio cholerae biofilm architecture induced by antibiotics disrupts community barrier function. Nat Microbiol 2019, 4:2136–2145, 10.1038/s41564-019-0579-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dale JL, Nilson JL, Barnes AMT, Dunny GM: Restructuring of Enterococcus faecalis biofilm architecture in response to antibiotic-induced stress. npj Biofilms Micro 2017, 3:1–9, 10.1038/s41522-017-0023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.•.Schlomann BH, Wiles TJ, Wall ES, Guillemin K, Parthasarathy R: Sublethal antibiotics collapse gut bacterial populations by enhancing aggregation and expulsion. Proc Natl Acad Sci USA 2019, 116:21392–21400, 10.1073/pnas.1907567116. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper connects the effects of antibiotic exposure on physical community aggregation and host intestinal movement, showing how physical properties and environment affect antibiotic resistance and community survival.
- 47.Schlomann BH, Parthasarathy R: Gut bacterial aggregates as living gels. Elife 2021, 10:e71105, 10.7554/eLife.71105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beaudoin T, et al. : Staphylococcus aureus interaction with Pseudomonas aeruginosa biofilm enhances tobramycin resistance. NPJ Biofilms Micro 2017, 3:25, 10.1038/s41522-017-0035-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Estrela S, Brown SP: Community interactions and spatial structure shape selection on antibiotic resistant lineages. PLoS Comput Biol (6) 2018, 14:e1006179, 10.1371/journal.pcbi.1006179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nadell CD, Drescher K, Foster KR: Spatial structure, cooperation and competition in biofilms. Nat Rev Microbiol 2016, 14:589–600, 10.1038/nrmicro.2016.84 [DOI] [PubMed] [Google Scholar]
- 51.Ibberson CB, Barraza JP, Holmes AL, Cao P, Whiteley M: Precise spatial structure impacts antimicrobial susceptibility of S. aureus in polymicrobial wound infections. Proc Natl Acad Sci USA (51) 2022, 119:e2212340119, 10.1073/pnas.2212340119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kayser J, Schreck CF, Gralka M, Fusco D, Hallatschek O: Collective motion conceals fitness differences in crowded cellular populations. Nat Ecol Evol 2019, 3:125–134, 10.1038/s41559-018-0734-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gude S, Pinçe E, Taute KM, Seinen AB, Shimizu TS, Tans SJ: Bacterial coexistence driven by motility and spatial competition. Nature 2020, 578:588–592, 10.1038/s41586-020-2033-2 [DOI] [PubMed] [Google Scholar]
- 54.Prindle A, Liu J, Asally M, Ly S, Garcia-Ojalvo J, Süel GM: Ion channels enable electrical communication in bacterial communities. Nature 2015, 527:59–63, 10.1038/nature15709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stracy M, Snitser O, Yelin I, Amer Y, Parizade M, Katz R, Rimler G, Wolf T, Herzel E, Koren G, Kuint J, Foxman B, Chodick G, Shalev V, Kishony R: Minimizing treatment-induced emergence of antibiotic resistance in bacterial infections. Science (6583) 2022, 375:889–894, 10.1126/science.abg9868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iram S, Dolson E, Chiel J, et al. : Controlling the speed and trajectory of evolution with counterdiabatic driving. Nat Phys 2021, 17:135–142, 10.1038/s41567-020-0989-3 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used for the research described in the article.


