Abstract
Growth and health-promoting bacteria can boost crop productivity in a sustainable way. Pseudomonas simiae WCS417 is such a bacterium. It efficiently colonizes roots, modifies the root system architecture increasing the size of the root system, and induces systemic resistance to make plants more resistant to pests and pathogens. Our previous work employing reporter lines for immunity and plant growth hormones suggested that WCS417-induced phenotypes are controlled by root cell type-specific mechanisms. Yet it is unknown how WCS417 affects these mechanisms. Therefore, we transcriptionally profiled five Arabidopsis thaliana root cell types following WCS417 colonization. The cortex and endodermis have the most differentially expressed genes, even though they are not in direct contact with this epiphytic bacterium. Many of these genes are associated with reduced cell wall biogenesis, and mutant analysis suggests that this downregulation facilitates WCS417-driven root architectural changes. Furthermore, we observed elevated expression of suberin biosynthesis genes and increased deposition of suberin in the endodermis of WCS417-colonized roots. Using an endodermal barrier mutant we showed the importance of endodermal barrier integrity for optimal plant-beneficial bacterium association. Comparison of the transcriptome profiles in the two epidermal cell types that are in direct contact with WCS417 – trichoblasts that form root hairs and atrichoblasts that do not – implies a difference in potential for defense gene activation. While both cell types respond to WCS417, trichoblasts displayed both higher basal and WCS417-dependent activation of defense-related genes compared to atrichoblasts. This suggests that root hairs may activate root immunity, a hypothesis which is supported by differential immune responses in root hair mutants. Altogether, we highlight the strength of cell type-specific transcriptional profiling to uncover “masked” biological mechanisms underlying beneficial plant-microbe associations.
Keywords: FACs, cell type-specific transcriptomics, root immunity, beneficial rhizobacteria, suberin, root hair
Short summary
This paper investigates how the epiphytic plant-beneficial bacterium Pseudomonas simiae WCS417 affects different root cell types of Arabidopsis thaliana using cell type-specific transcriptomics. Bioinformatic analysis and experiments demonstrate that WCS417 alters cell wall biogenesis and suberin deposition inside the root, potentially facilitating root development and bacterial colonization. Furthermore, the study highlights the role of root hairs in immune activation.
Introduction
Plants are sessile organisms that cannot move in response to environmental changes. Instead, they adapt to changes by modifying the morphology and exudation of their roots or by activating a range of defense responses. The root system of the model plant Arabidopsis thaliana (Arabidopsis) consists of a primary root with branching lateral roots (Motte et al., 2019; Petricka et al., 2012). Modifications in the spatial configuration of roots, the root system architecture, are especially important for water and nutrient uptake (Koevoets et al., 2016; Li et al., 2016; Rogers and Benfey, 2015; Shahzad and Amtmann, 2017). Root structure is also vital for adaptation to different conditions. Plant roots are organized in concentric cycles consisting of different cell types, with the outer cell types (trichoblasts, atrichoblasts) being in contact with the environment and the inner ones (cortex, endodermis, pericycle, vasculature) being indispensable for nutrient/water transport between below- and aboveground plant tissues (Stassen et al., 2021; Wachsman et al., 2015).
Exudation of specialized plant metabolites and structural fortification of inner cell types such as the endodermis are essential for nutrient uptake from the soil and a balanced interaction with the microbial communities surrounding the roots, known as the microbiome (Kashyap et al., 2021; Pascale et al., 2020). Exudates like coumarins can facilitate iron uptake from the soil but also shape the root microbiome (Harbort et al., 2020; Stringlis et al., 2018b). Fortification of the endodermis includes the coating of endodermal cells by a hydrophobic polymer, suberin, and the deposition of lignin-based structures to form the Casparian strip in the junction between two adjacent endodermal cells (Barberon, 2017; Barberon et al., 2016; Geldner, 2013; Naseer et al., 2012). The amount of suberin deposition around endodermal cells is dynamically regulated during nutrient stresses and by the root microbiome (Barberon, 2017; Barberon et al., 2016; Salas-Gonzalez et al., 2021). An extra level of plant adaptation is achieved via the modification of root system architecture in response to beneficial soil micro-organisms (Vacheron et al., 2013; Verbon and Liberman, 2016). In Arabidopsis, the number and/or length of lateral roots and root hairs increase in response to different rhizobacteria and fungi (Contreras-Cornejo et al., 2009; Lopez-Bucio et al., 2007; Vacheron et al., 2018; Zamioudis et al., 2013). All these chemical, morphological or structural modifications of roots in response to the root microbiome rely on the prompt perception of microbes or their defense-eliciting molecules (Microbe-Associated Molecular Patterns or MAMPs). These changes ultimately allow plants to maintain a beneficial interaction with their microbiome and avoid colonization by potentially harmful microbes (Beck et al., 2014; Colaianni et al., 2021; Hacquard et al., 2017; Millet et al., 2010; Stringlis et al., 2018a; Teixeira et al., 2019; Wyrsch et al., 2015).
Studies on the interaction between Arabidopsis and the beneficial rhizobacterium Pseudomonas simiae WCS417 (WCS417) unearthed different aspects of the interplay between plants and their associated beneficial microbes (Pieterse et al., 2021). WCS417 stimulates Arabidopsis growth (Berendsen et al., 2015; Zamioudis et al., 2013) and induces systemic resistance against many pathogens in Arabidopsis and several crop species (Pieterse et al., 1996; Pieterse et al., 2014). Arabidopsis responds to root colonization by WCS417 by inhibiting primary root growth and increasing the number of lateral roots and root hairs (Stringlis et al., 2018a; Zamioudis et al., 2013). The increased number of lateral roots upon WCS417 colonization is due to an increase in lateral root initiation events, observed as an increased number of lateral root primordia, and increased outgrowth of these primordia (Zamioudis et al., 2013). Lateral roots originate from pericycle cells, a cell layer surrounding the vasculature, and subsequently force their way through the endodermis, cortex, and finally the epidermis, to protrude from the primary root (Du and Scheres, 2018; Malamy and Benfey, 1997; Moller et al., 2017; Otvos and Benkova, 2017). The plant hormone auxin is important for all phases of lateral root development (Du and Scheres, 2018). In line with this, the increase in lateral root number in response to WCS417 is dependent on auxin signaling (Zamioudis et al., 2013). Similarly, the WCS417-mediated increase in root hair number is dependent on auxin signaling (Zamioudis et al., 2013). In Arabidopsis, root hairs are formed by specialized cells in the epidermis: the trichoblasts. Trichoblasts and the atrichoblasts, another epidermal cell type, form the outermost root cell layer (Gilroy and Jones, 2000; Ryan et al., 2001; Vissenberg et al., 2020). The activity of several transcription factors, including TRANSPARENT TESTA GLABRA (TTG), CAPRICE (CPC) and WEREWOLF (WER), and the spatial localization of the cells, with cells located over two cortical cells becoming trichoblasts, regulate whether trichoblasts or atrichoblasts are formed (Vissenberg et al., 2020). In response to WCS417, the increased number of root hairs is due to an increased number of cortical cells and therefore an increased number of cells becoming trichoblasts (Zamioudis et al., 2013). A root system with a greater number of lateral roots and/or root hairs can mine more soil area for nutrients, has a larger surface to facilitate colonization by plant growth-promoting rhizobacteria (Lugtenberg and Kamilova, 2009; Vacheron et al., 2013), and has greater potential to release nutrient-mobilizing exudates (e.g. iron-chelating coumarins) (Robe et al., 2021).
Establishment and maintenance of beneficial plant-microbe interactions requires a fine balance between plant growth and defense. Beneficial microbes, like pathogenic ones, can elicit MAMP-triggered immunity (MTI) which, when left unchecked, inhibits growth (Ma et al., 2021; Teixeira et al., 2021). Previous studies demonstrated that WCS417 can repress part of the root defense responses (Millet et al., 2010; Stringlis et al., 2018a), probably via the production of gluconic acid (Yu et al., 2019b). In recent years, many studies have demonstrated that the different cell types of the root can mount defense responses of varying levels depending on the MAMP and the responsible microbe colonizing the roots (Rich-Griffin et al., 2020a; Salas-Gonzalez et al., 2021; Wyrsch et al., 2015; Zhou et al., 2020). These studies suggest that by compartmentalizing detection of microbes and activation of defense responses, the plant can maintain a proper growth - defense balance, avoiding costly and/or late defense activation (Teixeira et al., 2019; Yu et al., 2019a).
The structure of the Arabidopsis root system is defined by the distinct biological functions of each of its cell types. In parallel, specialized responses activated in each cell type upon microbial colonization allow plants to grow optimally in microbe-rich environments. Our previous studies on whole roots provided us with global information on the interaction between WCS417 and Arabidopsis (Stringlis et al., 2018a; Verhagen et al., 2004; Zamioudis et al., 2014). However, cell type-specific transcriptomics can reveal which cell types respond to WCS417 most strongly or quickly, what responses are activated in each cell type, and how these responses contribute to successful colonization and subsequent effects on root architecture and the establishment of a mutualistic interaction (Rich-Griffin et al., 2020b). Here, we used a set of fluorescent marker lines to isolate trichoblast, atrichoblast, cortical, endodermal and vasculature cells with fluorescence-activated cell sorting (FACS) (Birnbaum et al., 2005; Birnbaum et al., 2003; Brady et al., 2007a). To build a map of gene expression changes in the root, we transcriptionally profiled these cell populations after colonization by WCS417 in comparison with untreated control roots. Our data showed distinct cell type-specific responses to WCS417 exposure. The most dramatic changes were seen in the cortex and endodermis, even though WCS417 strictly colonizes the root surface. In these inner cell layers, we observed an enrichment of genes associated with cell wall reorganization, reflecting the morphological observations of increased lateral root formation. We found evidence for functional specialization of the root epidermal cell types indicating a prominent role for root hair epidermal cells (trichoblasts) in activation of immunity upon bacterial colonization. Additionally, we found that endodermal cells increase their protective barrier in response to WCS417 by locally increasing suberin biosynthesis and deposition.
Results
WCS417 rapidly induces root developmental changes
Plant growth-promoting rhizobacteria can affect plant root system architecture (Vacheron et al., 2013; Verbon and Liberman, 2016). In accordance with previous reports (Stringlis et al., 2018a; Zamioudis et al., 2013), WCS417 inhibited primary root length and increased the total number of lateral roots after seven days of co-inoculation. After only two days of co-inoculation, we observed increased formation of lateral roots following bacteria application (Figure S1). We reasoned that by studying this timepoint using cell type-specific transcriptomics, we would capture the transcriptional events in different cell types underlying early plant modifications in response to the epiphytic bacterium WCS417 and identify processes involved in the establishment of a beneficial plant-microbe interaction.
Cell type-specific transcriptional profiling of the Arabidopsis root
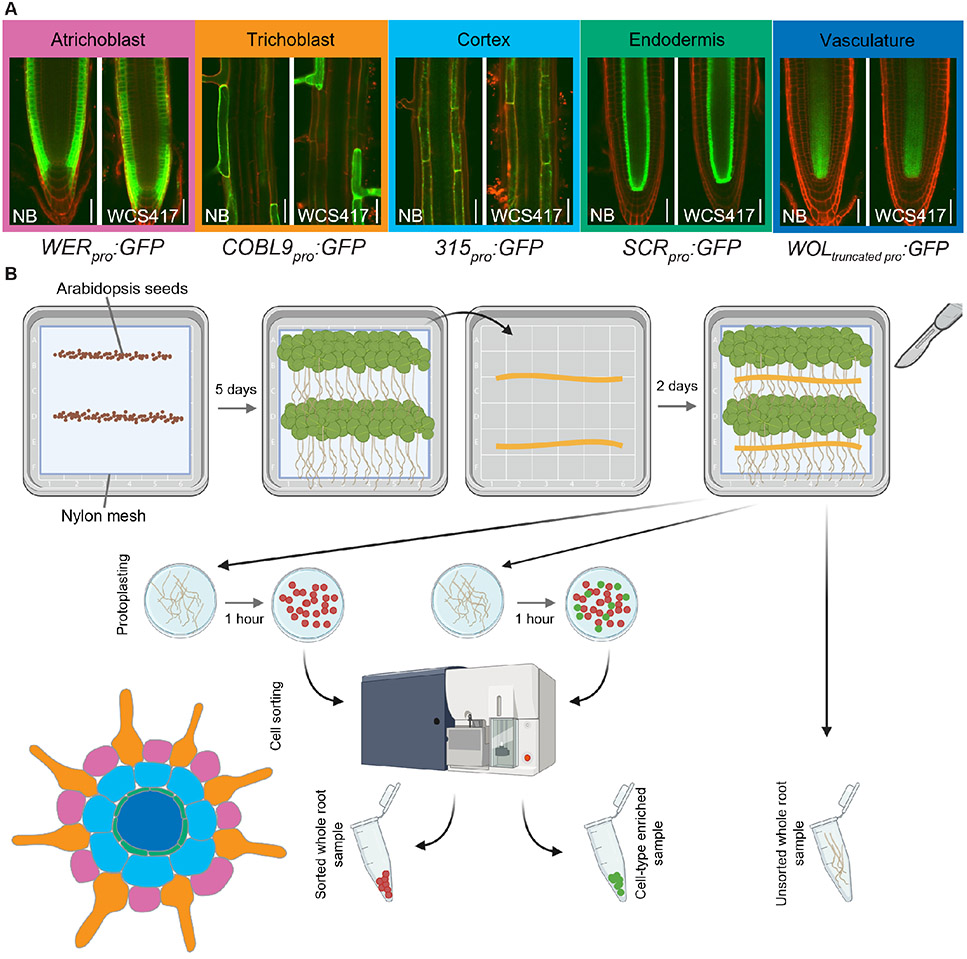
To create a spatial map of root transcriptional changes in response to colonization by WCS417, we isolated several root cell types using FACS. First, we confirmed that WCS417 does not affect the expression pattern of GREEN FLUORESCENT PROTEIN (GFP) when driven by the cell type-specific promoters WEREWOLF (WER; atrichoblast), COBRA-LIKE 9 (COBL9; trichoblast), 315 (cortex), SCARECROW (SCR; endodermis), or truncated WOODENLEG (WOL; vasculature) (Figure 1A). Subsequently, we grew the transgenic lines carrying these promoter-GFP fusions under high-density conditions and exposed them to WCS417 in addition to control treatment. Two days after inoculation, we harvested the roots, performed FACS and isolated RNA (Figure 1B).
Figure 1. Exposure of five transgenic plant lines to WCS417 to obtain cell type-specific samples by performing fluorescence-activated cell sorting (FACS).
A) Sterile and WCS417-exposed plants from the transgenic plant lines WEREWOLFpro:GFP (WER: immature epidermis and atrichoblast), COBRA-LIKE9pro:GFP (COBL9: trichoblast), 315pro:GFP (315: cortex), SCARECROWpro:GFP (SCR: endodermis), and WOODENLEGtruncated_pro:GFP (WOL: vasculature). Pictures of the seedlings were taken all along the root from day five (day of bacterial inoculation) till day seven. GFP settings were kept the same between bacteria-exposed and sterile-grown plants. Representative images are shown, and colored text boxes are used to match with the coloring of cell types in the Arabidopsis root cross section graphic (bottom left of figure). B) Experimental design used to obtain WCS417-treated and control samples enriched for one out of five root cell types. Sterilized and vernalized Arabidopsis seeds were sown on 1 × MS 1% sucrose plates and left to grow in long-day conditions. Five days later, half of the plants of each line were transferred on their mesh onto 1 × MS 1% sucrose plates with WCS417. Plants were left to grow for a further two days of growth before root harvest. Wild-type Col-0 roots were either directly flash-frozen (unsorted control) or protoplasted and put through the cell sorter, collecting non-fluorescent cells (sorted control). Transgenic lines with cell type-specific GFP expression were similarly protoplasted and put through the cell sorter for fluorescence-activated cell sorting (FACS).
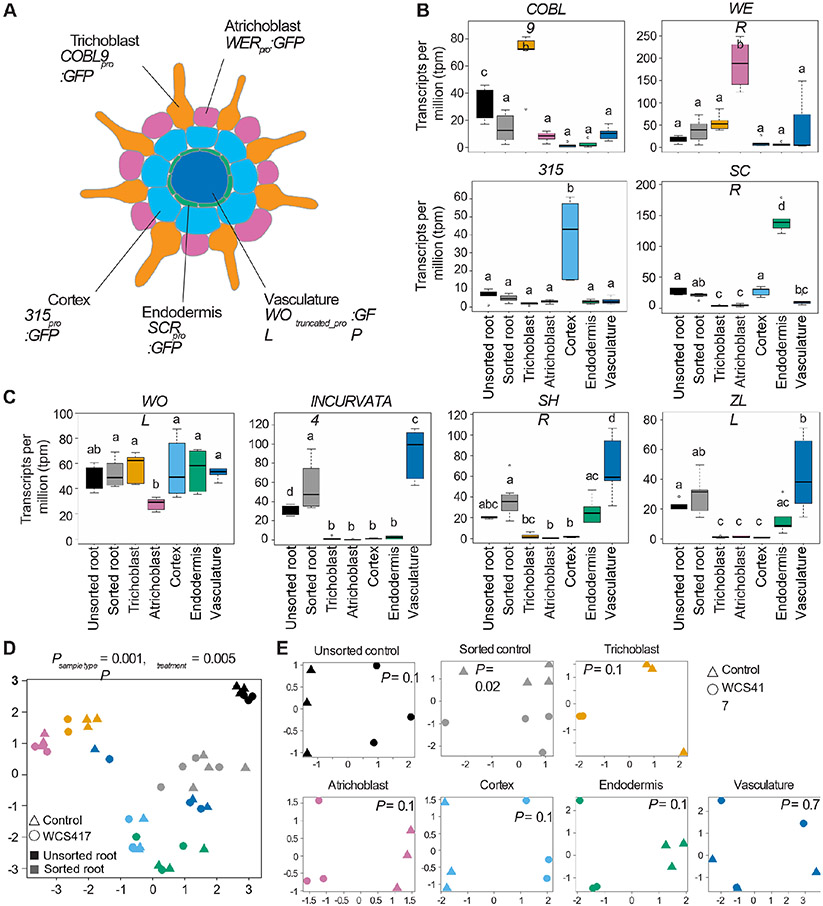
To determine the success of the sorting procedure, we checked the expression of the marker genes WER, COBL9, 315, SCR and WOL in our transcriptomic dataset (Figure 2A). The expression of each of these markers should be highest in the FACS samples obtained from the transgenic plant lines in which the corresponding promoter was used to drive GFP expression. Indeed, the expression of WER, COBL9, 315 and SCR is highest in the samples obtained from their respective lines (Figure 2B). The expression of WOL is not enriched in the vasculature line, yet, we did observe enrichment for the well-established vasculature marker genes, INCURVATA4, SHORTROOT (SHR) and ZWILLE (ZLL) (Figure 2C) suggesting the succesful enrichment of vasculature cells . Notably, this line uses a truncated WOL promoter to drive GFP expression (Mahonen et al., 2000) rather than the full promoter, indicating that only the truncated WOL promoter is in fact vasculature cell type-specific. By analyzing the expression of WOL and these three vasculature marker genes in the recently published single-cell root atlas by Shahan et al. (2022) we could confirm this observation (Figure S2). Notably, WOL expression appears in all cells and cell types whereas the expression of INCURVATA4, SHR and ZLL is restricted to the xylem, phloem, pericycle and procambium cell types (Figure S2B). Similar to our findings shown in Figure 2C, SHR is an exception as it has been found also in endodermal cells. To further evaluate whether the cell type-specific identities were preserved in the experiment and during the sorting procedure, we compared the expression of the top 50 cell type-specific marker genes from the single-cell root atlas for each relevant cell type (Shahan et al., 2022). This analysis corroborated the appropriate identities for the different cell types, i.e., cell type-specific marker genes are expressed highest in FACS samples obtained from the same cell type, and shows that the cellular identities of WCS417-treated cells (WCS) appear similar to the untreated, control ones (NB) (Figure S3A). Interestingly, atrichoblast cells that are sorted using the WEREWOLF (WER) proWER::GFP selective marker appear enriched for lateral root cap (LRC), atrichoblast, and to some extent trichoblast marker gene expression. Finally, we compared the mean gene expression level of all detected genes in sorted versus unsorted cells, with and without treatment. These gene expression profiles are highly correlated (R2 = 0.88 and 0.89 for control (NB) and WCS417-treated roots, respectively) indicating that the sorting procedure itself did not have a major effect on the global gene expression landscape (Figure S3B).
Figure 2. Gene expression differences among the samples reflect Arabidopsis root development patterns.
A) Schematic cross section of the Arabidopsis root, with each cell type labeled with the promoter::GFP fusion that was used to enrich samples for that cell type by FACS. B) mRNA levels of the marker genes COBL9, WER, 315, and SCR. C) mRNA levels of the marker gene WOL, and of the vasculature-specific genes INCURVATA4, SHR, and ZLL. Data was analyzed with an ANOVA test followed by the Tukey post hoc test in R (p-value <0.05). GFP: green fluorescent protein, COBL9: COBRA-LIKE 9, WER: WEREWOLF, SCR: SCARECROW, WOL: WOODENLEG, SHR: SHORTROOT, ZLL: ZWILLE. Multidimensional scaling (MDS) plot of counts (log scale) per million of all samples (D) and per cell type (E). WCS417-exposed samples are represented by circles, control, untreated samples by triangles. Colors in panel B-E correspond to the color scheme of the schematic in panel A. Black samples represent the unsorted wild-type roots, grey represents the sorted wild-type roots. In panel E, ‘P’ represents the p-value of the WCS417-treatment effect.
To study the global similarities and dissimilarities among samples and treatments, we performed multidimensional scaling on gene expression levels. The transcriptional profiles cluster primarily by sample type (Psample type = 0.001; Figure 2D). The cortical and endodermal cells cluster close together, as do the two epidermal cell types. This is in line with the known development of the Arabidopsis root, in which the cortex and endodermis develop from a shared stem cell population, as do the trichoblasts and atrichoblasts (Dolan et al., 1993; Shahan et al., 2022; Van den Berg et al., 1995), and fits with the UMAP (Uniform Manifold Approximation and Projection for Dimension Reduction) projection of the single-cell root atlas by Shahan et al. (2022) (Figure S2A). In addition to the sample-type effect, we find an effect of bacterial treatment on gene expression (Ptreatment = 0.005; Figure 2D). When comparing gene expression patterns of samples within sample types, each cell type except the vasculature clusters primarily based on bacterial treatment (Figure 2E).
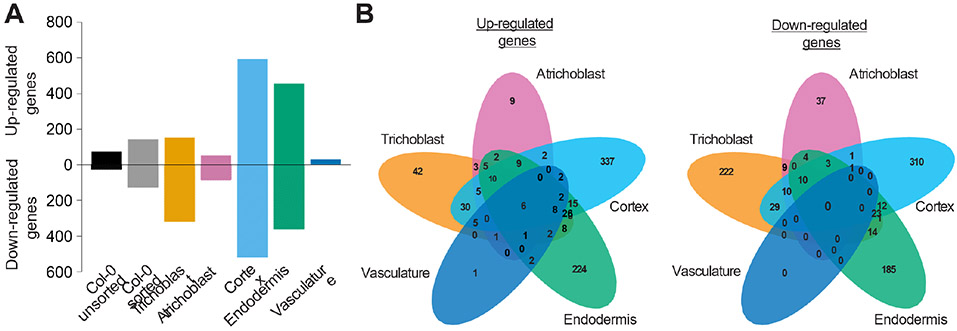
Next, we determined which genes are differentially expressed (DEGs) in response to WCS417 in each of the cell types compared to untreated roots (Table S1A-C). The number of DEGs differs greatly among the cell types, ranging from 30 in the vasculature to 1,109 in the cortex (Figure 3A). Interestingly, the cortical and endodermal cells, which do not interact directly with the strictly epiphytic WCS417 bacterium, displayed the largest number of DEGs (1,109 and 815, respectively), while the trichoblast and atrichoblast cells, which are in direct contact with WCS417 displayed far fewer DEGs (469 and 137, respectively). Apart from a quantitative difference, the response is also qualitatively different between cell types: of the 1,862 DEGs across all five cell types, 72% are affected in only one cell type and only six genes are affected in all cell types (Figure 3B). Notably, the majority of genes affected in only a single cell type are not identified as differentially expressed in the whole root, while most genes affected in four or five cell types are identified as either up- or down-regulated in whole roots (Table S2). In contrast, the majority of genes found to be up- or down-regulated in the sorted or unsorted control were identified as differentially expressed in response to WCS417 in one or more cell types (Table S3). In conclusion, genes affected in only single cell types were often not identified as differentially expressed in the whole root. This explained the higher number of identified DEGs in the cell type-specific data set as compared to the sorted whole root (1,862 genes versus 270 genes; Figure 3A).
Figure 3. Root cell types have unique responses to root colonization by WCS417.
A) Number of differentially expressed genes upon WCS417 application found in the respective samples (false discovery rate (FDR) < 0.1; −2 < log2FC > 2). B) Venn diagrams showing the overlap in genes affected by WCS417 treatment in the five studied cell types.
High responsiveness of trichoblasts, relative to atrichoblasts, when exposed to WCS417
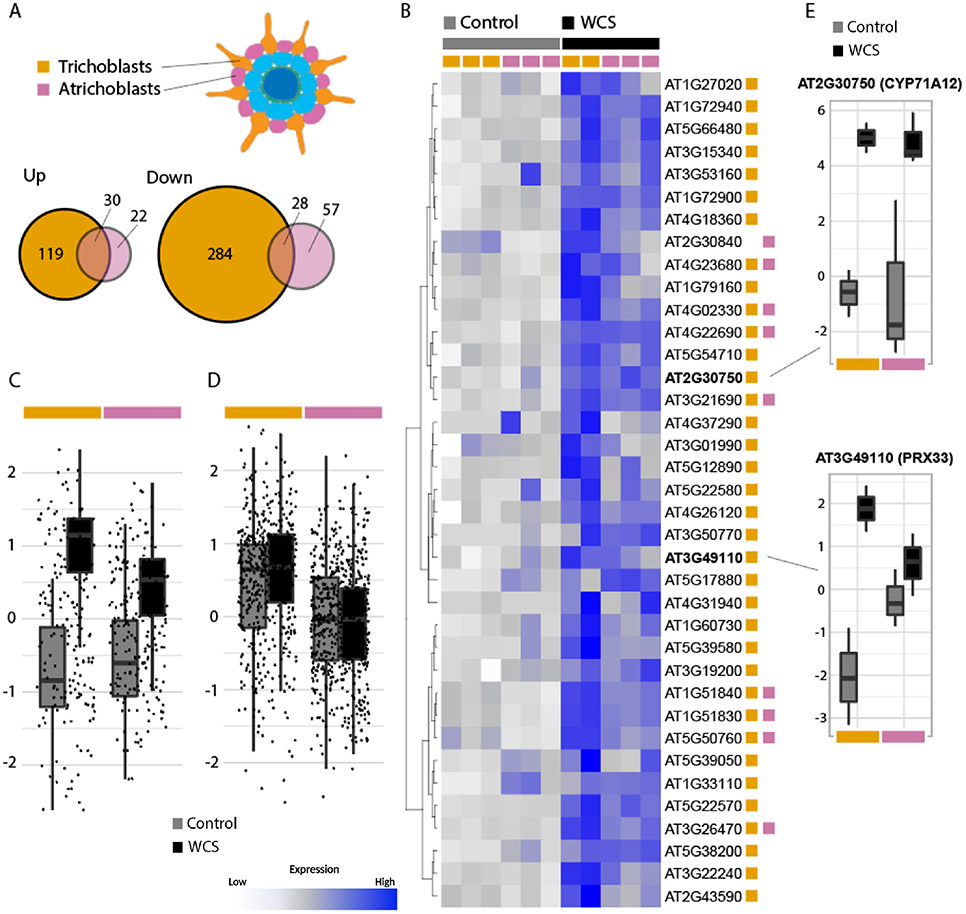
To identify the biological processes affected by WCS417 colonization in the different cell types, we conducted biological process gene ontology (GO) term enrichment analyses on the DEGs (Table S4-S13). Most significant among the up-regulated DEGs in the trichoblasts, cortex, endodermis and vasculature are processes related to defense and immunity (Table S4). Interestingly, defense activation is not prominent in atrichoblasts in response to WCS417 (Table S6). This suggests that the two cell types directly in contact with WCS417 activate distinct biological processes, as could be expected from the limited overlap in DEGs between these cell types (Figure 3B and 4A). To further analyze these differences, we examined the expression of the genes within the GO term defense response (GO:0006952) that are differentially upregulated in one or both epidermal cell types. We identified 37 such genes in total, 28 specifically for trichoblast cells, 1 for atrichoblasts and 8 were found differentially upregulated in both epidermal cell types (Table S14). Notably, most of these genes appear elevated in both WSC417-treated (WCS) epidermal cell types upon comparison of their normalized expression levels (Figure 4B), but the overall relative increase in trichoblast cells is more pronounced following WCS417 inoculation (Figure 4C, E). To further assess the relative importance of trichoblast over atrichoblast cells with regards to the defense response we next examined the normalized expression of all defense response-related genes (GO:0006952) in both cell types, irrespective of differential expression. This analysis confirmed that these genes are expressed at relatively higher levels in trichoblast cells (Figure 4D), but it also indicates that only a part of the defense-related genes respond to WCS417 application as the treatment effect is now largely absent.
Figure 4. High responsiveness of trichoblasts to root colonization by WCS417.
A) Overlap of the DEGs in response to WCS417 in the trichoblasts and atrichoblasts. B) Heatmap of the expression of genes associated with the GO term defense response (GO:0006952) that were differentially expressed (DE) in either or both epidermal cell types in response to WCS417. DE status in either cell type shown by the colored squares (trichoblasts, orange; atrichoblasts, pink). Heatmap is scaled by row and gene expression is shown as the normalized log-counts-per-million, with low gene expression in white, and high expression in dark blue. C) Scaled (per-gene) expression levels of DEGs as shown in B. D) Scaled (per-gene) expression levels of all genes associated with GO:0006952. E) Expression of candidate marker genes CYP71A12 and PRX33 as normalized log-counts-per-million.
Root hairs contribute to microbial perception and affect plant responses to WCS417
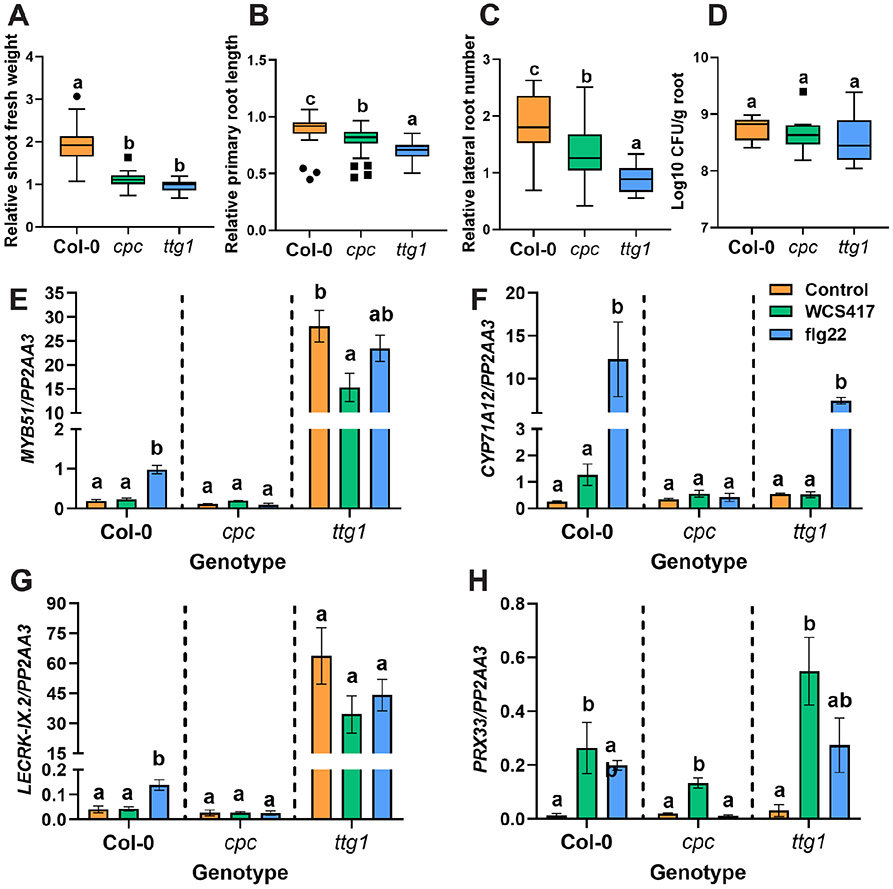
We found that upon exposure to WCS417, trichoblasts, in particular, appear to activate defense-related genes, illustrated by the enrichment for defense gene expression in these cells (Figure 4B and C, Table S4). This could indicate that cell types destined for the formation of root hairs (trichoblasts) might be more sensitive to microbial signals and this sensitivity could affect plant responses to microbes. To test this hypothesis, we assessed the effect of WCS417 on two mutants with contrasting patterns of root hair formation, cpc that forms fewer root hairs compared to wild-type and ttg1 where most epidermal cells produce root hairs . We grew Arabidopsis wild-type Col-0 plants and the two mutants in plates containing 105 colony-forming units (CFU) · ml−1 WCS417 based on a protocol developed by Paredes et al. (2018) and measured growth-promotion traits and levels of root colonization. Interestingly, the beneficial effects of WCS417 were less pronounced on both mutants as compared to wild-type plants, since relative changes in fresh shoot weight, primary root length and number of lateral roots were significantly lower (Figure 5A, B, C and Figure S4). Nevertheless, WCS417 colonization was comparable between wild-type and mutant roots (Figure 5D). We then reasoned that the number of root hairs might also affect defense responses to WCS417 and the bacterial MAMP flg22. For this, we tested the expression of WCS417- and flg22-responsive MTI-related genes MYB51 (MYB DOMAIN PROTEIN 51; AT1G18570), CYP71A12 (CYTOCHROME P450, FAMILY 71, SUBFAMILY A, POLYPEPTIDE 12; AT2G30750), PRX33 (PEROXIDASE 33; AT3G49110) and LECRK-IX.2 (L-TYPE LECTIN RECEPTOR KINASE IX.2; AT5G65600) (Millet et al., 2010; Stringlis et al., 2018a). MYB51 and CYP71A12 have roles in indole glucosinolate and camalexin biosynthesis respectively (Millet et al., 2010), PRX33 is a cell wall peroxidase involved in the generation of reactive oxygen species (ROS) during defense activation (Kaman-Toth et al., 2019), and LECRK-IX.2 is a L-type lectin receptor kinase that acts as a positive regulator of MTI (Luo et al., 2017). We previously found that all four of these genes are induced in young Col-0 seedlings at multiple, consequtive timepoints by both WCS417 and flg22 treatment . In the current dataset, we found that all four genes are induced in one or more cell types, with the exception of LECRK-IX.2. Specifically, CYP71A12 and PRX33 are induced in trichoblast and cortical cells, and in endodermal cells in the case of CYP71A12, while MYB51 is induced in endodermal cells only. WCS417- and flg22-induced expression patterns of MYB51 (Figure 5E), CYP71A12 (Figure 5F), LECRK-IX.2 (Figure 5G) and PRX33 (Figure 5H) are affected in mutants with altered root hair density. In which mutant cpc displayed a reduced response to the elicitors, while ttg1 appeared unresponsive to elicitation but displayed generally elevated expression of MYB51 and LECRK-IX2. Overall, we found that the presence of root hairs affects the expression of MTI-related genes, and the beneficial effects by WCS417 in mutants with altered root hair density were less pronounced.
Figure 5. Arabidopsis root hair mutants display differential responses to WCS417.
Relative A) shoot fresh weight, B) primary root length, C) lateral root number, and D) colonization levels of WCS417 on roots of Col-0, cpc and ttg1 genotypes at 7 days after seedlings were transferred to plates with Hoagland medium (0% sucrose) containing 105 CFU · ml−1 WCS417. Different letters indicate statistically significant differences across genotypes (One-way ANOVA, Tukey’s test; P < 0.05). In the case of growth parameters n= 39–40 and in case of root colonization n= 8. Relative values were calculated by dividing the value of a parameter in each WCS417 inoculated seedling by the average value of all the mock seedlings. (E – H) Relative expression levels of MYB51, CYP71A12, LECRK-IX.2 and PRX33 as quantified by qRT-PCR. The reference gene PP2AA3 (AT1G13320) was used for normalization. Expression was evaluated in roots of 8-day-old seedlings at 6 h after inoculation with WCS417 (OD600equal to 0.1, 108 CFU · ml−1) or treated with 1 μM flg22. Error bars represent SEM. Different letters represent statistically significant differences among control, WCS417 and flg22 in same plant genotype as indicated by the dashed separating lines (One-way ANOVA, Tukey’s test; P < 0.05, n= 3-4).
WCS417 might facilitate lateral root formation by loosening cell walls of cell layers overlaying lateral root primordia
Among the down-regulated DEGs in the cortical and endodermal cell layers upon WCS417 treatment we observed many genes related to processes associated with root and tissue development and cell wall organization or biogenesis. Conversely, cell wall modification (GO:0042545) was enriched among the up-regulated DEGs in the endodermis (Table S9-S12). Cell wall remodeling and cell volume loss in the cortex and endodermis are known to be required to accommodate emerging lateral roots and are possibly even required for the initiation of lateral root primordia (Stoeckle et al., 2018; Vermeer et al., 2014). Many of the processes enriched within the down-regulated genes in the cortex are related and are typically relatively aspecific. However, when considering the odds-ratio of observations we noted xylan metabolism (GO:0045491) as one of the more specific biological processes significantly overrepresented in the down-regulated genes. Upon inspection of the genes within this process we observed significant down-regulation of several genes, i.e., GUX2 (GLUCURONIC ACID SUBSTITUTION OF XYLAN 2; AT4G33330), IRX9 (IRREGULAR XYLEM 9; AT2G37090) and IRX14-L (IRREGULAR XYLEM 14-LIKE; AT5G67230), involved in glucuronoxylan biosynthesis (GO:0045491). To characterize the genes related to this process in more detail we plotted the expression of all genes within this process across all cell types (Figure S5A). Although only three out of the twelve genes annotated in this process in Arabidopsis were significantly down-regulated, many of the other genes were in fact also repressed upon bacterial treatment. Overall we observed the highest expression of these genes in vasculature cells, while they were particularly repressed in the cortical and endodermal cells (Figure S5B). Among the genes upregulated in the endodermis with involvement in cell wall modification were genes which encode polygalacturonases that have been shown to be expressed at the site of lateral root emergence. They have been implicated in cell separation, possibly to accommodate emerging lateral roots (Ogawa et al., 2009). Up-regulation of these and other genes involved in cell wall modification and down-regulation of genes involved in cell wall biogenesis, particularly xylan biosynthesis, in the cortex and endodermis might therefore be an integral part of the molecular and physiological changes that take place in response to WCS417, which lead to the observed increase in the number of lateral roots (Figure S1 and (Zamioudis et al., 2013)). To investigate the predicted effect of cell wall loosening, we obtained mutants for selected genes, IRX9, IRX9L (IRREGULAR XYLEM 9-LIKE; AT1G27600), IRX14 (IRREGULAR XYLEM 14; AT4G35890) and IRX14L, that are repressed by WCS417 and involved in glucuronoxylan metabolism (Figure S5A). All four genes are involved with xylan synthesis and backbone elongation, and accordingly, mutants display irregular xylem (IRX) phenotypes (Wu et al., 2010). We assessed growth promotion, colonization and lateral root emergence on all IRX mutants in comparison with wild-type Col-0 plants. All mutants displayed similar promotion of plant shoot biomass to Col-0 following WCS417 inoculation (Figure S6A), and WCS417 population density was comparable between Col-0 and mutant plants (Figure S6B). Interestingly, upon WCS417 colonization three out of the four tested mutants, i.e., irx14, irx14-L and irx9-L, developed significantly more lateral roots per cm primary root in comparison to Col-0 (+41% - 78%; p < 0.01). These observations are consistent with our hypothesis that bacterial colonization leads to a repression of cell wall fortification supporting accelerated emergence of lateral root primordia.
WCS417 induces suberin biosynthesis in endodermal cells
Defense in general is the most significant process affected in our cell type-specific gene expression analysis. This is, in part, because many genes are known to be part of this GO term. To study biological processes in which fewer genes are involved, like the beforementioned xylan metabolism, we subsequently studied enriched GO terms with the highest odds ratios, i.e., with the largest difference in expected versus actual counts. In this analysis, we found a significant enrichment for suberin biosynthesis (GO:0010345) in the endodermis, specifically, with an odds ratio of 20.9 (Table S10). Suberin is a hydrophobic polymer deposited between the primary cell wall and the plasma membrane of endodermal cells. There, suberin together with the lignin-containing Casparian strip that builds tight junctions between adjacent endodermal cells, they block free movement of water and nutrients into the endodermis and consequently the innermost cell layers of the Arabidopsis root (Barberon, 2017; Geldner, 2013). Like the formation of lateral roots and root hairs, the production of suberin is affected by nutrient availability (Barberon et al., 2016). In addition, recent data suggest that suberin and the Casparian strip are involved in the interaction between plants and root-associated commensals and soil-borne phytopathogens (Froschel et al., 2020; Salas-Gonzalez et al., 2021).
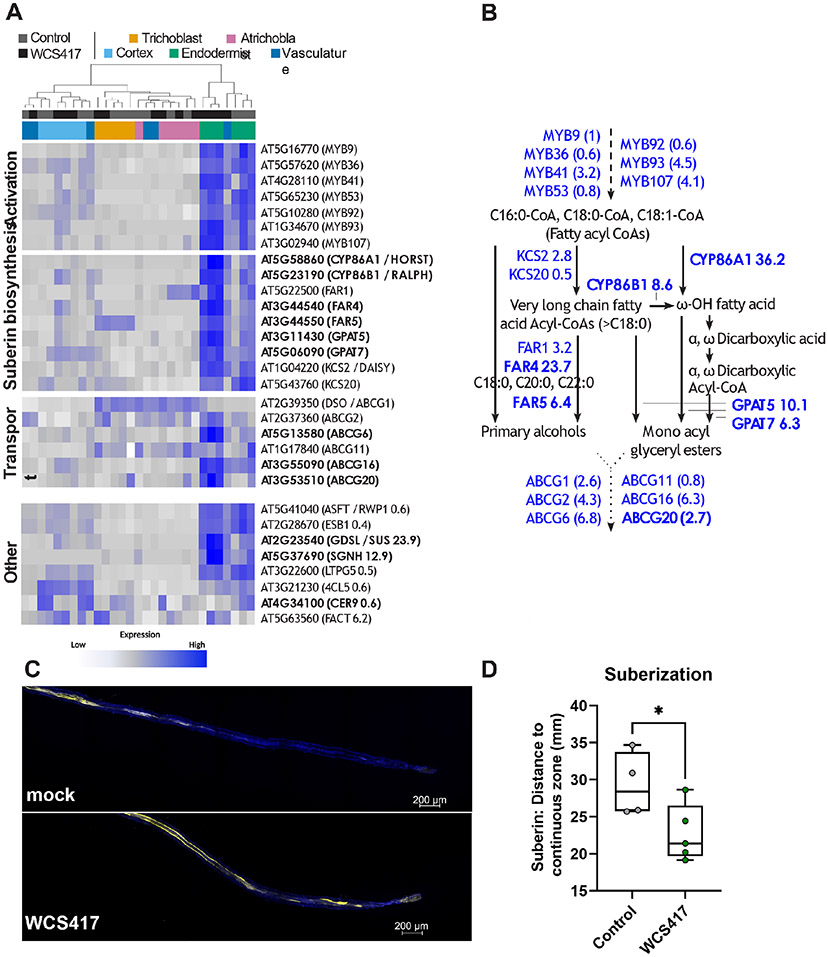
We analyzed the effect of WCS417 on genes related to suberin production, such as MYB transcription factors MYB9 (AT5G16770), MYB36 (AT5G57620), MYB41 (AT4G28110), MYB53 (AT5G65230), MYB92 (AT5G10280), MYB93 (AT1G34670) and MYB107 (AT3G02940) suggested to activate suberin biosynthesis (Kosma et al., 2014; Lashbrooke et al., 2016; Shukla et al., 2021), and enzymes involved in suberin biosynthesis, including β-KETOACYL-CoA-SYNTHASEs KCS2 (AT1G04220) and KCS20 (AT5G43760), fatty acid cytochrome P450 oxidases CYP86A1 (AT5G58860) and CYP86B1 (AT5G23190), FATTY ACUL-CoA REDUCTASEs FAR1 (AT5G22500), FAR4 (AT3G44540) and FAR5 (AT3G44550), GLYCEROL-3-PHOSPHATE SN2-ACYLTRANSFERASEs GPAT5 (AT3911430) and GPAT7 (AT5G06090) as well as transporters such as the ATP-binding cassette transporter proteins ABCG1 (AT2G39350), ABCG2 (AT2G37360), ABCG6 (AT5G13580), ABCG11 (AT1G17840), ABCG16 (AT3G55090), and ABCG20 (AT3G53510) (Barberon, 2017; Panikashvili et al., 2010; Vishwanath et al., 2015; Yadav et al., 2014). As expected, based on the available literature and our GO term analyses, spatial gene expression patterns showed that suberin biosynthesis is primarily restricted to the endodermis and is significantly induced by WCS417 (Figure 6A-B). Next, we assessed biosynthesis and deposition of suberin following bacterial colonization by staining roots with fluorol yellow to visualize suberin (Barberon et al., 2016). We found that WCS417 colonization indeed led to an increase of suberin in the endodermis as quantified by a decreased distance from the root tip to the continuously suberized root zone (Figure 6C-D).
Figure 6. WCS417 induces suberization of the endodermis.
A) Heatmap of the expression of genes known to be involved in suberin biosynthesis (GO:0010345, suberin biosynthetic process; Lashbrooke, 2016; Vishwanath, 2015). Heatmap is scaled per row (gene). Genes that are significantly up-regulated (logFC > 2, FDR < 0.1) by WCS417 in the endodermis are shown in bold. B) Overview of the suberin biosynthesis pathway, its activation and suberin monomer transport out of the cell, adapted from (Vishwanath et al. 2015). Genes known to be involved in these processes are shown in blue, fold changes as found in our dataset in response to WCS417 are shown. Statistical significantly differentially expressed genes (FDR < 0.1) are depicted in bold. Dashed lines show activation, solid lines show compound conversions, dotted lines show transport processes. C-D) Suberization in roots of 7-d-old Arabidopsis at 2 d after they were transferred in Hoagland plates containing WCS417. Suberin was visualized using fluorol yellow staining and quantified as the distance from the root tip to the continuous zone of suberization in roots of Arabidopsis (n = 4-5). Representative confocal images are shown.
Root endodermal barriers have a role in colonization by WCS417 and the subsequent activation of defense responses
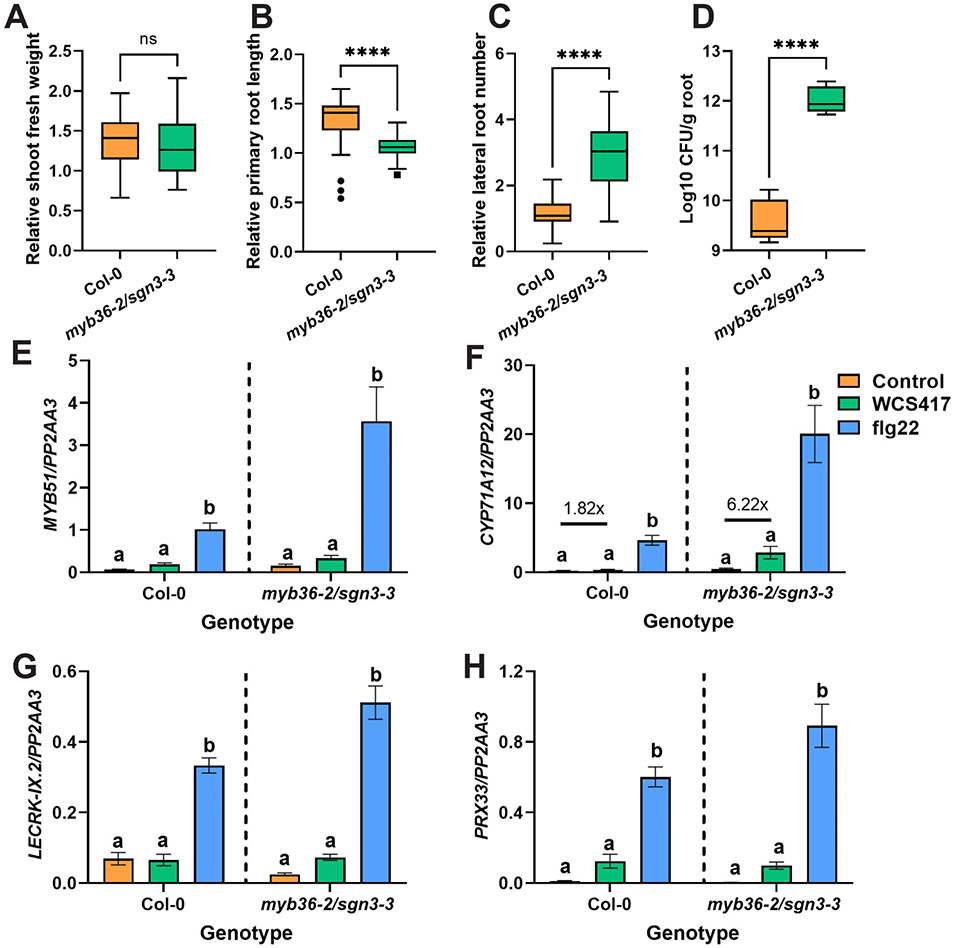
Based on the increased suberization following colonization by WCS417 (Figure 6), we hypothesized that endodermal barriers might play a role in the interaction between Arabidopsis and WCS417. To test this, we grew wild-type plants and myb36-2/sgn3-3 mutants, with developmentally delayed and reduced endodermal barriers (Salas-Gonzalez et al., 2021) and performed experiments with WCS417 similar to those described above for root hair and cell wall assembly mutants. The effects of WCS417 on wild-type and endodermal barrier mutant plants were similar in terms of shoot growth promotion (Figure 7A and Figure S7A). This was not the case for primary root length and lateral root formation, with WCS417 having a more pronounced effect on myb36-2/sgn3-3 plants as compared to wild-type plants (Figure 7B-C and Figure S7B-C). Next, we assessed the colonization levels of WCS417 on roots of wild-type and mutant plants. Remarkably, WCS417 colonization levels were much higher (almost one hundred times) on myb36-2/sgn3-3 roots as compared to the wild-type (Figure 7D) indicating that disruption of endodermal barriers greatly affected the interaction with WCS417. To further confirm this observation, we studied the expression of MTI-related marker genes MYB51, CYP71A12, PRX33 and LECRK-IX.2 in roots of wild-type and myb36-2/sgn3-3 treated with WCS417 and flg22 (Figure 7E-H). For all genes tested, there was a stronger response to flg22 in the endodermal barrier mutant, indicating that increased permeability of the endodermis makes roots more responsive to MAMPs. This is consistent with findings showing that flg22 could reach the inner cell types of plants with dysfunctional endodermal barriers and activate stonger expression of MTI-related marker genes (Zhou et al., 2020). The roots of the endodermal barrier mutant also produced a stronger induction of CYP71A12 following treatment with WCS417 (6.22 times more expression compared to myb36-2/sgn3-3 control and 3.4 times more compared to the respective change in Col-0; Figure 7F), suggesting that the increased colonization of these roots (Figure 7D) can lead to stronger root defense responses, probably via diffusion of WCS417 MAMPs into deeper root layers.
Figure 7. Root endodermal barrier integrity is needed for balanced interaction with WCS417.
Relative A) shoot fresh weight, B) primary root length, C) lateral root number, and D) colonization levels of WCS417 on roots of Col-0 and myb36-2/sgn3-3 at 7 days after seedlings were transferred to plates with Hoagland medium (0% sucrose) containing 105 CFU · ml−1 WCS417. Asterisks indicate significant differences between genotypes following WCS417 inoculation (Student's t-test; * P < 0.05, **P < 0.01, ***P < 0.001,****P < 0.0001; ns, not significant). In the case of growth parameters n= 30 and in case of root colonization n= 6. Relative values were calculated by dividing the value of a parameter in each WCS417 inoculated seedling by the average value of all the mock seedlings. (E – H) Relative expression levels of MYB51, CYP71A12, LECRK-IX.2 and PRX33 as quantified by qRT-PCR. The reference gene PP2AA3 (AT1G13320) was used for normalization. Expression was evaluated in roots of 8-day-old seedlings at 6 h after inoculation with WCS417 (OD600= 0.1, 108 CFU · ml−1) or treated with 1 μM flg22. Error bars represent SEM. Error bars represent SEM. Different letters represent statistically significant differences among control, WCS417 and flg22 in same plant genotype as indicated by the dashed separating lines (One-way ANOVA, Tukey’s test; P < 0.05, n= 3-4).
Discussion
Cell type-specific transcriptomics reveal “hidden” root responses to WCS417
The ‘hidden half’ of plants, the root system, is of crucial importance when breeding for plants that are drought tolerant or better able to grow under nutrient-limiting conditions (Rogers and Benfey, 2015; Koevoets et al., 2016). Additionally, the root surface lies at the interface between plants and beneficial soil micro-organisms, which increase plant growth and health (Lugtenberg and Kamilova, 2009; Pieterse et al., 2014; Bakker et al., 2018). Cell type-specific transcriptomics is an approach to better understand root response to beneficial bacteria at the individual cell type level. Innovative methods have been developed to assess cell type-specific gene expression changes. These include protoplasting-based scRNA-seq, but also TRAP-Seq which uses nuclei rather than protoplasts for RNA extraction and consequently allows for direct snap freezing of samples (Denyer and Timmermans, 2022; Thellmann et al., 2020). Using this approach, one could circumvent potential effects of protoplasting. At the time this experiment was conducted the relevant TRAP-Seq transgenic reporter lines were not available. Therefore, we studied gene expression changes in five Arabidopsis root cell types after colonization with WCS417 using FACS (Figures 2-3). The total number of DEGs identified across the five cell types is approximately ten-fold greater than the number identified in the sorted whole root control. A similar increase in detection power of cell type-specific versus whole root transcriptomic analyses was obtained previously when examining the Arabidopsis root response to salt, iron deficiency, and nitrogen (Dinneny et al., 2008; Gifford et al., 2008).
We showed that the increased sensitivity can be traced to the cell type-specific nature of the root response to WCS417. The five cell types differ in their response to WCS417 both quantitatively, with major differences in the number of DEGs, and qualitatively, with little overlap in DEGs between cell types (Figure 3). This supports previous studies on cell type-specific gene expression changes in response to both abiotic and biotic stresses and refutes the concept of a global stress response (Dinneny et al., 2008; Gifford et al., 2008; Iyer-Pascuzzi et al., 2011; Rich-Griffin et al., 2020a; Walker et al., 2017).
Root hairs, the cortex and the endodermis have roles in the WCS417-Arabidopsis interaction
The number and type of DEGs in our spatial map uncovered two interesting findings: 1) the cortex and endodermis responded most strongly to WCS417 in terms of the number of DEGs, and 2) the responsiveness of the two epidermal cell types was distinct (Figure 4). The strong response of the cortex and endodermis is surprising, as these cell types were likely not in direct contact with WCS417, that preferentially colonizes the root surface . Previous studies, however, demonstrated the ability of MAMPs to reach the cortex and the endodermis (Zhou et al., 2020), and mount MTI responses (Wyrsch et al., 2015). Also timing likely plays a role, as cell type-specific transcriptional profiling of the Arabidopsis root response to flg22 showed that the epidermis responded as strongly as the cortex at two hours post inoculation (Rich-Griffin et al., 2020a). Possibly, the epidermis responds strongly at first and down-regulates its response by two days after inoculation, while the cortex and endodermis maintain or increase their response over that period, to restrict continuous and unwanted activation of the outer cell types exposed to the microbe-rich environment. We observed enrichment of processes related to decreased cell wall biogenesis in these inner cell types specifically. This might have allowed these cells to lose volume which is required for lateral root initiation and outgrowth (Vermeer et al., 2014; Stoeckle et al., 2018). Indeed, we observed that cell wall mutants display increased emergence of lateral roots (Figure S6) suggesting that repression of cell wall strengthening might explain the observed increase in lateral root formation in WCS417-exposed roots (Stringlis et al., 2018a; Zamioudis et al., 2013). A time-series experiment could further elucidate the timing and magnitude of these spatially-separated responses, while future studies on the responsiveness of younger and older parts of the root to different stimuli could provide further evidence as to how roots contribute to plant homeostasis in microbe-rich and stress-abundant environments.
In addition to decreased cell wall biogenesis, we showed increased expression of genes involved in suberin biosynthesis in the endodermis (Figure 6). This was further confirmed by suberin staining, which demonstrated increased suberization of the endodermis (Figure 6). On the one hand, manipulation of suberin deposition (desuberization and resuberization) might be part of the increased lateral root emergence triggered by WCS417, since emerging lateral roots need to cross the endodermis and suberin degradation is needed for correct lateral root emergence (Ursache et al., 2021). On the other side, suberin and the Casparian strip are essential for protecting the inner root tissues from the surrounding soil environment (Barberon, 2017). Suberin displays plasticity to nutrient stresses such as iron deficiency (Barberon et al., 2016), but it can also be modulated in response to beneficial and pathogenic members of the microbiome (Froschel et al., 2020; Kashyap et al., 2022; Salas-Gonzalez et al., 2021). It is probable that both beneficial and pathogenic microorganisms manipulate the functioning and deposition of endodermal barriers to achieve sufficient colonization of the root and access to root-derived sugars. Indeed, previous research has shown that Arabidopsis activates the iron deficiency response upon root colonization by WCS417 (Verhagen et al., 2004; Zamioudis et al., 2014; Zamioudis et al., 2015). This response is normally activated when plants experience a shortage of iron and results in a decreased deposition of suberin to facilitate iron uptake (Barberon et al., 2016), suggesting that WCS417 modulates nutrient availability or use within the plant. Our data further suggested that this might be a transient response during colonization, since at 48 h after colonization we observed increased, rather than decreased, suberization in the roots, suggesting that plants adapt to the interaction with WCS417 and re-seal the endodermis to avoid unwanted effects. This hypothesis is supported by our experiment with the myb36-2/sgn3-3 double mutant with dysfunctional endodermal barriers. This mutant is colonized to a higher degree by WCS417 and has elevated expression of the MTI-related marker gene CYP71A12 compared to wild-type plants (Figure 7). Additionally, in this mutant three of four MTI-related marker genes showed increased expression in response to flg22, further confirming the role of this barrier in MTI sensitivity and in fine-tuning growth and defense. Therefore, for an optimal interaction with WCS417, Arabidopsis needs a functional endodermal barrier to prevent limitless bacterial proliferation on the root.
Next our findings indicated that root hairs could be acting as first responders to environmental signals and inform plants to adapt their growth and development to an upcoming interaction. Literature supports this, since in other plant species root hairs are colonization hotspots for rhizobia (Poole et al., 2018), the formation and pattern of root hairs are responsive to nutrient stresses , root hairs mediate exudation of iron-mobilizing coumarins (Robe et al., 2021), and barley mutant plants with contrasting root hair characteristics accommodate distinct root-associated microbial communities (Robertson-Albertyn et al., 2017). Interestingly, cpc and ttg1 mutants used here, despite their contrasting pattern in root hair formation, seemed unaffected in terms of WCS417 colonization, but displayed less growth promotion compared to wild-type plants. It is possible that root hairs might have a prominent role in colonization by endophytes like rhizobia or microbes affected by exudates released by root hairs such as coumarins. As WCS417 can colonize the whole root system and remains on the root surface root hairs might not be critical for its establishment on the root, yet this might also be a consequence of the simple, binary setup in which plant roots were solely colonized by one bacterium. We did observe that cpc and ttg1 respond in a different transcriptional manner when colonized by WCS417. Thus, detailed transcriptomic analysis of root hair mutants and assessment of said mutants in natural, soil conditions are pertinent questions that require our attention in the future to address their role in plant-beneficial microbe interactions.
Concluding remarks
We created a spatial map of gene expression changes induced in the Arabidopsis root in response to colonization by the beneficial bacterium WCS417. Our dataset uncovered localized, cell type-specific gene expression patterns that otherwise remain hidden in global analyses of gene expression and that correspond to observed root architectural changes. We reveal a role for root hairs and endodermal barriers in the interaction between roots, WCS417 and MAMPs. In addition, further mining of our dataset will enable other researchers to determine the spatial pattern of microbe-induced expression of genes of interest.
Methods
Plant material and growth conditions.
FACS experiment
Arabidopsis accession Columbia-0 (Col-0) and transgenic Col-0 with the COBRA-LIKE9pro:GFP (Brady et al., 2007a; Brady et al., 2007b), WEREWOLFpro:GFP (Lee and Schiefelbein, 1999), 315pro:GFP (AT1G09750) (Lee et al., 2006), SCARECROWpro:GFP (Wysocka-Diller et al., 2000), or WOODENLEGtruncated_pro:GFP construct (Mahonen et al., 2000) were grown as described previously (Dinneny et al., 2008). Briefly, seeds were liquid sterilized in 50% bleach and stratified by incubation at 4°C for 2 d. Sterilized seeds were plated in two dense lines of three seeds thick each on nylon mesh (Nitex Cat 03-100/44, Sefar) on sterile 1 × MS (Murashige and Skoog (1962)) 1% sucrose plates. Plates were sealed with Parafilm and placed vertically in long day conditions (22°C; 16 h light, 8 h dark) for a total of 7 d.
Microscopy for suberin localization
Col-0 seeds were surface sterilized (Van Wees et al., 2013) and sown on plates containing agar-solidified Hoagland medium with 1% sucrose and pH was adjusted to 5.5 . After 2 d of stratification at 4°C, the plates were positioned vertically and transferred to a growth chamber (22°C; 10 h light, 14 h dark; light intensity 100 μmol · m−2 · s−1). When 5-d-old, seedlings were transferred to agar-solidified Hoagland plates without sucrose (0.75% agar) where Pseudomonas simiae WCS417 (WCS417) was mixed in the medium based on the protocol developed by Paredes et al. (2018). After 2 d of Arabidopsis-WCS417 interaction, Fluorol yellow (FY) staining of roots was performed as described before (Kajala et al., 2021; Lux et al., 2005).
Colonization and growth promotion experiments with root hair, cell wall integrity and endodermal barrier mutants
Col-0 seeds and mutants in Col-0 background: myb36-2/sgn3-3 (Reyt et al., 2021), cpc-1 and ttg1 (Wada et al., 1997; Walker et al., 1999) and irx9, irx9L, irx14 and irx14L (Wu et al., 2010) were surface sterilized and sown on agar-solidified Hoagland plates (as before). When 7-d-old, seedlings, except irx mutants, were transferred to agar-solidified Hoagland plates with 0% sucrose where WCS417 was mixed in the medium (as before). In the case of irx mutants, 7-day-old seedlings were transferred to Hoagland plates with 1% sucrose, and subsequently the plants are inoculated with either WCS417 bacteria by applying 10 μl of a bacterial suspension on the root-shoot junction or a mock solution containing 10 mM MgSO4. Seven days later the shoots of the seedlings were weighed using an analytical scale, photos were taken to analyze root growth and development (via Image J) and colonization of WCS417 on roots was assessed (Paredes et al., 2018).
Analysis of gene expression in endodermal barrier and root hair mutants
For testing root transcriptional responses to WCS417 and flg22, plants were grown and treated based on a protocol developed by Stringlis et al. (2018a). Briefly, uniform 9-day-old seedlings were transferred from MS agar plates to six-well plates (ø 35 mm per well) containing liquid 1 × MS with 0.5% sucrose, after which they were cultured for 7 more days under the same growth conditions. One day before treatment with either WCS417 or flg22, the medium of each well was replaced with fresh 1 × MS medium with 0.5% sucrose. At 6 h after treatment with WCS417 or 1 μM flg22 (GenScript), roots were flash frozen in liquid nitrogen for downstream gene expression analysis.
WCS417 treatment.
FACS experiment
Plants were inoculated with bacteria 5 d after being placed in long-day conditions using a slightly adapted version of a previously published protocol (Zamioudis et al., 2015). Briefly, rifampicin-resistant WCS417 was streaked from a frozen glycerol stock onto solid King’s medium B (KB) (King et al., 1954) containing 50 μg · ml−1 rifampicin and grown at 30°C overnight. One day before plant treatment, a single colony from the plate was put in liquid KB with rifampicin and grown in a shaking incubator at 30°C overnight. The following morning, the bacterial suspension was diluted in fresh KB with rifampicin and grown in a shaker until the suspension reached an OD600 value between 0.6 and 1.0 (OD600 of 1.00 is equal to 109 colony-forming units (CFU) · ml−1), after which the bacteria were washed twice with 10 mM MgCl2.
To decide which bacterial concentration to add to the plants, the washed bacteria were resuspended in 10 mM MgCl2 to a final density ranging from 101 to 108 CFU · μl−1. Two horizontal lines of either 10 μl of 10 mM MgCl2 or 10 μl of one of the bacterial suspensions were applied per 1 × MS 1% sucrose plate. Five-day-old Col-0 seedlings were transferred on their mesh onto these plates. Seedlings were transferred such that the roots of the seedlings were on top of the bacteria. Finally, all plates were resealed with Parafilm and left to grow in long-day conditions. At 2 and 7 d after treatment ten plants from each treatment were randomly picked and removed from the plate. By this time, the bacteria had formed an extensive biofilm covering the entire root, visible by the naked eye. The total number of emerged lateral roots was counted under a stereo microscope. ImageJ was used to determine primary root length per plant from images made with a scanner.
Based on the results of this trial, we chose a density of 106 CFU · μl−1, amounting to 107 CFU per row of plants, for the sorting experiment (see below). Wild-type Col-0 plants and plants of each of the five transgenic lines were exposed to this bacterial density after 5 d of plant growth as described above and incubated in long-day growth conditions for an additional 2 d.
Suberin staining experiment and colonization, assessment of growth and gene expression of endodermal and root hair mutants
For the rest of experiments, WCS417 was prepared and applied based on previously established protocols (Paredes et al., 2018; Stringlis et al., 2018a). WCS417 was cultured at 28°C on KB agar plates supplemented with 50 μg · ml−1 of rifampicin. After 24 h of growth, cells were collected in 10 mM MgSO4, washed twice with 10 mM MgSO4 by centrifugation for 5 min at 5000 g, and finally resuspended in 10 mM MgSO4. For suberin staining and growth/colonization experiments of Col-0 and mutant seedlings, WCS417 was mixed in Hoagland agar plates without sucrose in a concentration of 105 CFU · ml−1. This mix was then poured in plates and the seedlings were transferred in the plate once solidified.
For qRT-PCR gene expression analysis of Col-0 and respective mutants, WCS417 bacteria were added in each well to a final OD of 0.1 at 600 nm (108 CFU · ml−1).
Fluorescence-activated cell sorting (FACS).
After a total of 7 d of growth, roots were cut from the shoot with a carbon steel surgical blade. Whole roots of Col-0 destined for the unsorted control were immediately frozen in liquid nitrogen in an Eppendorf tube. For the other samples, all to be put through a cell sorter, roots were cut twice more and the root pieces from 4 - 6 plates were collected and protoplasted as described previously (Birnbaum et al., 2003, 2005). Briefly, they were placed in a 70-μm cell strainer submerged in enzyme solution (600 mM mannitol, 2 mM MgCl2, 0.1% BSA, 2 mM CaCl2, 2 mM MES, 10 mM KCl, pH 5.5 with 0.75 g cellulysin and 0.05 g pectolyase per 50 ml). Roots were mixed in the strainer at room temperature (RT) on an orbital shaker to dissociate protoplasts. After one hour, the suspension surrounding the strainer, containing the protoplasts, plus a few roots to pull the protoplasts down, were pipetted into a 15-ml conical tube and spun at 200 g for 6 min at RT. The top of the supernatant was pipetted off and the remaining solution resuspended in 700 μl of the protoplasting solution without enzymes (600 mM mannitol, 2 mM MgCl2, 0.1% BSA, 2 mM CaCl2, 2 mM MES, 10 mM KCl, pH 5.5). This suspension was filtered successively through a 70-μm cell strainer and a 40-μm strainer. The filtrate was finally collected in a cell sorting tube and taken to the cell sorter (Astrios, Beckman Coulter) at RT.
Protoplasts sorted by the machine were collected into RLT buffer (Qiagen) with β-mercaptoethanol. The samples were immediately placed on dry ice to inhibit RNA degradation. Samples were stored at −80°C until RNA isolation.
RNA isolation and sequencing.
FACS experiment
Whole root tissue for the unsorted control was lysed by grinding with a liquid-nitrogen-cooled mortar and pestle. RNA was isolated with the RNeasy Plant Mini Kit (Qiagen) for the six unsorted whole root samples and for six out of eight sorted whole root samples. RNA from the remaining two sorted whole root samples and all cell type-enriched samples were isolated with the Micro Kit (Qiagen). RNA concentration was checked with a Qubit Fluorometer (Thermo Scientific), and RNA integrity was assessed with a Bioanalyzer (Agilent Technologies). Subsequently, RNA libraries were made from samples with RNA integrity number (RIN) values above six. All libraries were made with the NEBNext Ultra RNA Library Prep Kit for Illumina (NEB). RNA for the six control unsorted (whole root) and the first six control sorted samples were poly-A selected using Dynal Oligo-dT beads. These 12 libraries were generated using 100 ng of total RNA. The remaining libraries were generated from total RNA selected using NEBNext Oligo-dT beads. Because of limited RNA yields from some of the sorted cell populations, 50 ng of total RNA was used as starting material for all sorted library preparations. Libraries were sequenced on an Illumina HiSeq 2500 using 50 base pair Single-Read (Duke University Sequencing Core). Three biological replicates were performed for each sample type and condition, except for the sorted control, for which we performed four biological replicates.
qRT-PCR experiment of Col-0 and mutants
For the qRT-PCR experiment, roots of Col-0 and mutants were collected in four replicates at six h after treatment with live WCS417 cells or flg22. Roots of untreated Col-0 and mutant seedlings were collected at the same time point (as controls). Each of the four biological replicates per treatment consisted of 10-12 pooled root systems. After harvest, root samples were snap-frozen in liquid nitrogen and stored at −80°C. Arabidopsis roots were homogenized using a mixer mill (Retsch) set to 30 Hz for 45 s. RNA extraction was performed with the RNeasy Plant Mini Kit (Qiagen). RNA concentration was checked with a Qubit Fluorometer (Thermo Scientific). For qRT-PCR analysis, DNase treatment, cDNA synthesis and PCR reactions and subsequent analysis were performed as described by Stringlis et al. (2018b). Primer sequences for the reference gene PP2AA3 and the MTI marker genes MYB51, CYP71A12, LECRK-IX.2 and PRX33 are listed in Table S15.
Data analysis.
FACS analysis
The reads generated by Illumina sequencing were pseudoaligned to the TAIR10 cDNA database (Lamesch et al., 2012) using Kallisto (v0.43.0) with 100 bootstraps and default settings (Bray et al., 2016). The percentage of aligned reads is lower for the 12 samples that were poly-A selected using Dynal Oligo-dT beads because of a high number of rRNA sequences. This is probably due to differences in the bead-selection procedure and the greater amount of RNA used as starting material. We do not expect this to interfere with our analyses, as the number of expressed genes in these samples is in the same range as previously published data in this species. The resulting transcript counts were subsequently summarized to the gene level with tximport (v1.2.0) (Soneson et al., 2015). We then assessed the number of detected expressed genes by counting the number of genes with count above 0, above 10 or with count per million (cpm) above 2. One bacteria-exposed sample enriched for trichoblasts was excluded from further analyses because of low coverage for all three of these criteria. Only genes with cpm greater than two in all samples were kept for the remaining analysis. The counts per gene of the remaining samples and genes were used to generate a digital gene expression list (DGE list) in EdgeR (v3.16.5) (Robinson et al., 2010). A generalized linear model (glm) was fit using a negative binomial model and quasi-likelihood (QL) dispersion estimated from the deviance with the glmQLFit function in EdgeR. DEGs were then determined by comparing the bacteria-exposed and the non-exposed samples with the glmQLFTest (FDR < 0.1; −2 < log2FC > 2). GO term analysis was performed in R based on the genome-wide annotation for Arabidopsis within org.At.tair.db (Carlson M, 2018) with the program GOstats (Falcon and Gentleman, 2007).
Analysis of cell type specificity
To investigate and confirm the cell type-specific expression of WOL (AT2G01830) and selected vasculature-specific genes INCURVATA4 (AT1G52150), SHR (AT4G37650) and ZLL (AT5G43810) we used the Arabidopsis single-cell root atlas and associated procedures described in Shahan et al. (2022). To support the cell type specificity of the here-described FACS dataset, we also took the top-50 marker genes per cell type from Shahan et al. (2022). Specifically, we obtained the top-50 marker genes for trichoblasts, atrichoblasts, cortical cells, endodermal cells, and vasculature cells from Shahan et al., 2022 (Supplementary Dataset 2K), and derived the corresponding TPM value for each sample in our FACS dataset. Next, we scaled each gene using the base R function scale across all samples and plotted the scaled TPM values for each Shahan et al. (2022) marker-based, cell type-top-50 gene for each FACS sample type in a boxplot using the ggplot2 R geom_boxplot function.
Fluorescence microscopy.
Approximately 20 sterilized and vernalized seeds of the COBL9pro:GFP, WERpro:GFP, 315pro:GFP, SCRpro:GFP, and WOLtruncated_pro:GFP transgenic lines were sown on a 1 × MS 1% sucrose plate and placed in long-day conditions. After 5 d either 105 WCS417 cells in 10 μl MgCl2 or sterile 10 μl MgCl2 was added to each root. GFP localization was observed once per day in 5, 6 and 7-day-old seedlings with a 510 upright confocal microscope with a 20x objective (Zeiss).
For FY staining of suberin, Col-0 seeds were sown on Hoagland plates 1% sucrose and placed in short-day conditions. When 5-days-old, seedlings were transferred in Hoagland medium without sucrose mixed with 105 CFU · ml−1 WCS417. After 2 d, roots were washed in MQ, separated from the leaves and 5 roots were added in each well of 6-well plates and incubated in FY088 (0.01% w/v, dissolved in lactic acid) for 1 h at RT in darkness, rinsed three times with water (5 mins per wash), and counterstained with aniline blue (0.5% w/v, dissolved in water) for 1 h at RT in darkness. Roots were mounted with 50% glycerol on glass slides and kept in dark until observation. Confocal Laser Scanning microscopy was performed on a Zeiss LSM 700 laser scanning confocal microscope with the 20x objective and GFP filter (488nm excitation, 500-550nm emission). To quantify the suberization pattern, the distance from the root tip to the start of continuous suberization was determined with ImageJ (v1.53g).
Supplementary Material
Acknowledgements
The authors want to thank Niko Geldner for the myb36-2/sgn3-3 lines, Christian Dubos for cpc and ttg1 lines, Cara M. Winter for help with FACS lines microscopy, Hao Zhang for helping with the irx mutants experiments and Rosa Toonen for the experiment involving suberin staining and confocal microscopy. There is no conflict of interest to declare.
Funding
This research was funded in part by the Netherlands Organization of Scientific Research through ALW Topsector Grant no. 831.14.001 (E.H.V.), by a postdoctoral fellowship from the Jane Coffin Childs Memorial Fund for Medical Research (L.M.L.), by the NIH (5R01-GM-043778), the NSF (MCB-06-18304), the Gordon and Betty Moore Foundation and the Howard Hughes Medical Institute (P.N.B.), by a postdoctoral fellowship from the Research Foundation Flanders (FWO 12B8116N) (R.d.J.), by the NWO Green II Grant no. ALWGR.2017.002 (R.d.J.), the Novo Nordisk Foundation Grant no. NNF19SA0059362 (R.d.J.), the China Scholarship Council (CSC) scholarship no. 201908320054 (JZ), scholarship no 202006990074 (JY), the Technology Foundation Perspective Program “Back2Roots” Grant no. 14219 (C M. J.P.), the ERC Advanced Grant no. 269072 of the European Research Council (C M. J.P.), and the NWO Gravitation Grant no. 024.004.014 (I.A.S and C.M.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability
The raw RNA-Seq read data are deposited with links to BioProject accession number PRJNA836026 in the NCBI BioProject database.
References
- Barberon M (2017). The endodermis as a checkpoint for nutrients. New Phytol. 213:1604–1610. [DOI] [PubMed] [Google Scholar]
- Barberon M, Vermeer JE, De Bellis D, Wang P, Naseer S, Andersen TG, Humbel BM, Nawrath C, Takano J, Salt DE, et al. (2016). Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164:447–459. [DOI] [PubMed] [Google Scholar]
- Beck M, Wyrsch I, Strutt J, Wimalasekera R, Webb A, Boller T, and Robatzek S (2014). Expression patterns of FLAGELLIN SENSING 2 map to bacterial entry sites in plant shoots and roots. J. Exp. Bot 65:6487–6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen RL, Van Verk MC, Stringlis IA, Zamioudis C, Tommassen J, Pieterse CMJ, and Bakker PAHM (2015). Unearthing the genomes of plant-beneficial Pseudomonas model strains WCS358, WCS374 and WCS417. BMC Genomics 16:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum K, Jung JW, Wang JY, Lambert GM, Hirst JA, Galbraith DW, and Benfey PN (2005). Cell type-specific expression profiling in plants via cell sorting of protoplasts from fluorescent reporter lines. Nat. Methods 2:615–619. [DOI] [PubMed] [Google Scholar]
- Birnbaum K, Shasha DE, Wang JY, Jung JW, Lambert GM, Galbraith DW, and Benfey PN (2003). A gene expression map of the Arabidopsis root. Science 302:1956–1960. [DOI] [PubMed] [Google Scholar]
- Brady SM, Orlando DA, Lee JY, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, and Benfey PN (2007a). A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318:801–806. [DOI] [PubMed] [Google Scholar]
- Brady SM, Song S, Dhugga KS, Rafalski JA, and Benfey PN (2007b). Combining expression and comparative evolutionary analysis. The COBRA gene family. Plant. Physiol 143:172–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaianni NR, Parys K, Lee HS, Conway JM, Kim NH, Edelbacher N, Mucyn TS, Madalinski M, Law TF, Jones CD, et al. (2021). A complex immune response to flagellin epitope variation in commensal communities. Cell Host & Microbe 29:635–649. [DOI] [PubMed] [Google Scholar]
- Contreras-Cornejo HA, Macias-Rodriguez L, Cortes-Penagos C, and Lopez-Bucio J (2009). Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant. Physiol 149:1579–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer T, and Timmermans MCP (2022). Crafting a blueprint for single-cell RNA sequencing. Trends. Plant. Sci 27:92–103. [DOI] [PubMed] [Google Scholar]
- Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, and Benfey PN (2008). Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320:942–945. [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, and Scheres B (1993). Cellular organisation of the Arabidopsis thaliana root. Development 119:71–84. [DOI] [PubMed] [Google Scholar]
- Du Y, and Scheres B (2018). Lateral root formation and the multiple roles of auxin. J. Exp. Bot 69:155–167. [DOI] [PubMed] [Google Scholar]
- Froschel C, Komorek J, Attard A, Marsell A, Lopez-Arboleda WA, Le Berre J, Wolf E, Geldner N, Waller F, Korte A, et al. (2020). Plant roots employ cell-layer-specific programs to respond to pathogenic and beneficial microbes. Cell Host & Microbe 29:299–310. [DOI] [PubMed] [Google Scholar]
- Geldner N (2013). The endodermis. Annu. Rev. Plant Biol 64:531–558. [DOI] [PubMed] [Google Scholar]
- Gifford ML, Dean A, Gutierrez RA, Coruzzi GM, and Birnbaum KD (2008). Cell-specific nitrogen responses mediate developmental plasticity. Proc. Natl. Acad. Sci. U S A 105:803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, and Jones DL (2000). Through form to function: root hair development and nutrient uptake. Trends. Plant. Sci 5:56–60. [DOI] [PubMed] [Google Scholar]
- Hacquard S, Spaepen S, Garrido-Oter R, and Schulze-Lefert P (2017). Interplay between innate immunity and the plant microbiota. Annu. Rev. Phytopathol 55:565–589. [DOI] [PubMed] [Google Scholar]
- Harbort CJ, Hashimoto M, Inoue H, Niu Y, Guan R, Rombolà AD, Kopriva S, Voges MJEEE, Sattely ES, Garrido-Oter R, et al. (2020). Root-secreted coumarins and the microbiota interact to improve iron nutrition in Arabidopsis. Cell Host & Microbe 28:825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer-Pascuzzi AS, Jackson T, Cui H, Petricka JJ, Busch W, Tsukagoshi H, and Benfey PN (2011). Cell identity regulators link development and stress responses in the Arabidopsis root. Dev. Cell 21:770–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajala K, Gouran M, Shaar-Moshe L, Mason GA, Rodriguez-Medina J, Kawa D, Pauluzzi G, Reynoso M, Canto-Pastor A, Manzano C, et al. (2021). Innovation, conservation, and repurposing of gene function in root cell type development. Cell 184:5070. [DOI] [PubMed] [Google Scholar]
- Kaman-Toth E, Danko T, Gullner G, Bozso Z, Palkovics L, and Pogany M (2019). Contribution of cell wall peroxidase- and NADPH oxidase-derived reactive oxygen species to Alternaria brassicicola-induced oxidative burst in Arabidopsis. Mol. Plant Pathol 20:485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap A, Jimenez-Jimenez AL, Zhang W, Capellades M, Srinivasan S, Laromaine A, Serra O, Figueras M, Rencoret J, Gutierrez A, et al. (2022). Induced ligno-suberin vascular coating and tyramine-derived hydroxycinnamic acid amides restrict Ralstonia solanacearum colonization in resistant tomato. New Phytol.: 1411–1429. [DOI] [PubMed] [Google Scholar]
- Kashyap A, Planas-Marques M, Capellades M, Valls M, and Coll NS (2021). Blocking intruders: inducible physico-chemical barriers against plant vascular wilt pathogens. J. Exp. Bot 72:184–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koevoets IT, Venema JH, Elzenga JT, and Testerink C (2016). Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front. Plant Sci 7:1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosma DK, Murmu J, Razeq FM, Santos P, Bourgault R, Molina I, and Rowland O (2014). AtMYB41 activates ectopic suberin synthesis and assembly in multiple plant species and cell types. The Plant Journal 80:216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashbrooke J, Cohen H, Levy-Samocha D, Tzfadia O, Panizel I, Zeisler V, Massalha H, Stern A, Trainotti L, Schreiber L, et al. (2016). MYB107 and MYB9 homologs regulate suberin deposition in angiosperms. Plant Cell 28:2097–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Colinas J, Wang JY, Mace D, Ohler U, and Benfey PN (2006). Transcriptional and posttranscriptional regulation of transcription factor expression in Arabidopsis roots. Proc. Natl. Acad. Sci. U S A 103:6055–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MM, and Schiefelbein J (1999). WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal cell patterning. Cell 99:473–483. [DOI] [PubMed] [Google Scholar]
- Li XX, Zeng RS, and Liao H (2016). Improving crop nutrient efficiency through root architecture modifications. J. Integr. Plant Biol 58:193–202. [DOI] [PubMed] [Google Scholar]
- Lopez-Bucio J, Campos-Cuevas JC, Hernandez-Calderon E, Velasquez-Becerra C, Farias-Rodriguez R, Macias-Rodriguez LI, and Valencia-Cantero E (2007). Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol. Plant. Microbe. Interact 20:207–217. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B, and Kamilova F (2009). Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol 63:541–556. [DOI] [PubMed] [Google Scholar]
- Luo X, Xu N, Huang J, Gao F, Zou H, Boudsocq M, Coaker G, and Liu J (2017). A lectin receptor-like kinase mediates pattern-triggered salicylic acid signaling. Plant. Physiol 174:2501–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux A, Morita S, Abe J, and Ito K (2005). An improved method for clearing and staining free-hand sections and whole-mount samples. Ann. Bot 96:989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma KW, Niu Y, Jia Y, Ordon J, Copeland C, Emonet A, Geldner N, Guan R, Stolze SC, Nakagami H, et al. (2021). Coordination of microbe-host homeostasis by crosstalk with plant innate immunity. Nat. Plants 7:814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahonen AP, Bonke M, Kauppinen L, Riikonen M, Benfey PN, and Helariutta Y (2000). A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes Dev. 14:2938–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, and Benfey PN (1997). Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124:33–44. [DOI] [PubMed] [Google Scholar]
- Millet YA, Danna CH, Clay NK, Songnuan W, Simon MD, Werck-Reichhart D, and Ausubel FM (2010). Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell 22:973–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller BK, Xuan W, and Beeckman T (2017). Dynamic control of lateral root positioning. Curr. Opin. Plant Biol 35:1–7. [DOI] [PubMed] [Google Scholar]
- Motte H, Vanneste S, and Beeckman T (2019). Molecular and environmental regulation of root development. Annual Review of Plant Biology 70:465–488. [DOI] [PubMed] [Google Scholar]
- Murashige T, and Skoog F (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15:473–497. [Google Scholar]
- Naseer S, Lee Y, Lapierre C, Franke R, Nawrath C, and Geldner N (2012). Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proceedings of the National Academy of Sciences USA 109:10101–10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Kay P, Wilson S, and Swain SM (2009). ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 21:216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos K, and Benkova E (2017). Spatiotemporal mechanisms of root branching. Curr. Opin. Genet. Dev 45:82–89. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Shi JX, Bocobza S, Franke RB, Schreiber L, and Aharoni A (2010). The Arabidopsis DSO/ABCG11 transporter affects cutin metabolism in reproductive organs and suberin in roots. Mol. Plant 3:563–575. [DOI] [PubMed] [Google Scholar]
- Paredes SH, Gao TX, Law TF, Finkel OM, Mucyn T, Teixeira PJPL, Gonzalez IS, Feltcher ME, Powers MJ, Shank EA, et al. (2018). Design of synthetic bacterial communities for predictable plant phenotypes. PLoS Biol. 16:e2003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale A, Proietti S, Pantelides IS, and Stringlis IA (2020). Modulation of the root microbiome by plant molecules: The basis for targeted disease suppression and plant growth promotion. Front. Plant Sci 10:1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petricka JJ, Winter CM, and Benfey PN (2012). Control of Arabidopsis root development. Annual Review of Plant Biology 63:563–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Berendsen RL, de Jonge R, Stringlis IA, Van Dijken AJH, Van Pelt JA, Van Wees SCM, Yu K, Zamioudis C, and Bakker PAHM (2021). Pseudomonas simiae WCS417: star track of a model beneficial rhizobacterium. Plant and Soil 461:245–263. [Google Scholar]
- Pieterse CMJ, Van Wees SCM, Hoffland E, Van Pelt JA, and Van Loon LC (1996). Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell 8:1225–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, Van Wees SCM, and Bakker PAHM (2014). Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol 52:347–375. [DOI] [PubMed] [Google Scholar]
- Poole P, Ramachandran V, and Terpolilli J (2018). Rhizobia: from saprophytes to endosymbionts. Nat. Rev. Microbiol 16:291–303. [DOI] [PubMed] [Google Scholar]
- Reyt G, Ramakrishna P, Salas-Gonzalez I, Fujita S, Love A, Tiemessen D, Lapierre C, Morreel K, Calvo-Polanco M, Flis P, et al. (2021). Two chemically distinct root lignin barriers control solute and water balance. Nat. Commun 12:2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Griffin C, Eichmann R, Reitz MU, Hermann S, Woolley-Allen K, Brown PE, Wiwatdirekkul K, Esteban E, Pasha A, Kogel KH, et al. (2020a). Regulation of cell type-specific immunity networks in Arabidopsis roots. The Plant Cell 32:2742–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Griffin C, Stechemesser A, Finch J, Lucas E, Ott S, and Schafer P (2020b). Single-cell transcriptomics: A high-resolution avenue for plant functional genomics. Trends. Plant. Sci 25:186–197. [DOI] [PubMed] [Google Scholar]
- Robe K, Conejero G, Gao F, Lefebvre-Legendre L, Sylvestre-Gonon E, Rofidal V, Hem S, Rouhier N, Barberon M, Hecker A, et al. (2021). Coumarin accumulation and trafficking in Arabidopsis thaliana: a complex and dynamic process. New Phytol. 229:2062–2079. [DOI] [PubMed] [Google Scholar]
- Robertson-Albertyn S, Alegria Terrazas R, Balbirnie K, Blank M, Janiak A, Szarejko I, Chmielewska B, Karcz J, Morris J, Hedley PE, et al. (2017). Root hair mutations displace the barley rhizosphere microbiota. Front. Plant Sci 8:1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ED, and Benfey PN (2015). Regulation of plant root system architecture: implications for crop advancement. Curr Opin Biotechnol 32:93–98. [DOI] [PubMed] [Google Scholar]
- Ryan E, Steer M, and Dolan L (2001). Cell biology and genetics of root hair formation in Arabidopsis thaliana. Protoplasma 215:140–149. [DOI] [PubMed] [Google Scholar]
- Salas-Gonzalez I, Reyt G, Flis P, Custodio V, Gopaulchan D, Bakhoum N, Dew TP, Suresh K, Franke RB, Dangl JL, et al. (2021). Coordination between microbiota and root endodermis supports plant mineral nutrient homeostasis. Science 371:eabd0695. [DOI] [PubMed] [Google Scholar]
- Shahan R, Hsu CW, Nolan TM, Cole BJ, Taylor IW, Greenstreet L, Zhang S, Afanassiev A, Vlot AHC, Schiebinger G, et al. (2022). A single-cell Arabidopsis root atlas reveals developmental trajectories in wild-type and cell identity mutants. Dev. Cell 57:543–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad Z, and Amtmann A (2017). Food for thought: how nutrients regulate root system architecture. Curr. Opin. Plant Biol 39:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla V, Han JP, Cleard F, Lefebvre-Legendre L, Gully K, Flis P, Berhin A, Andersen TG, Salt DE, Nawrath C, et al. (2021). Suberin plasticity to developmental and exogenous cues is regulated by a set of MYB transcription factors. Proc. Natl. Acad. Sci. U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassen MJJ, Hsu S-H, Pieterse CMJ, and Stringlis IA (2021). Coumarin communication along the microbiome–root–shoot axis. Trends. Plant. Sci 26:169–183. [DOI] [PubMed] [Google Scholar]
- Stoeckle D, Thellmann M, and Vermeer JE (2018). Breakout-lateral root emergence in Arabidopsis thaliana. Curr. Opin. Plant Biol 41:67–72. [DOI] [PubMed] [Google Scholar]
- Stringlis IA, Proietti S, Hickman R, Van Verk MC, Zamioudis C, and Pieterse CMJ (2018a). Root transcriptional dynamics induced by beneficial rhizobacteria and microbial immune elicitors reveal signatures of adaptation to mutualists. Plant J. 93:166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringlis IA, Yu K, Feussner K, de Jonge R, Van Bentum S, Van Verk MC, Berendsen RL, Bakker PAHM, Feussner I, and Pieterse CMJ (2018b). MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proceedings of the National Academy of Sciences USA 115:E5213–E5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira P, Colaianni NR, Law TF, Conway JM, Gilbert S, Li H, Salas-Gonzalez I, Panda D, Del Risco NM, Finkel OM, et al. (2021). Specific modulation of the root immune system by a community of commensal bacteria. Proceedings of the National Academy of Sciences USA 118:e2100678118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira PJP, Colaianni NR, Fitzpatrick CR, and Dangl JL (2019). Beyond pathogens: microbiota interactions with the plant immune system. Curr. Opin. Microbiol 49:7–17. [DOI] [PubMed] [Google Scholar]
- Thellmann M, Andersen TG, and Vermeer JE (2020). Translating ribosome affinity purification (TRAP) to investigate Arabidopsis thaliana root development at a cell type-specific scale. J. Vis. Exp [DOI] [PubMed] [Google Scholar]
- Ursache R, De Jesus Vieira Teixeira C, Denervaud Tendon V, Gully K, De Bellis D, Schmid-Siegert E, Grube Andersen T, Shekhar V, Calderon S, Pradervand S, et al. (2021). GDSL-domain proteins have key roles in suberin polymerization and degradation. Nat. Plants 7:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moenne-Loccoz Y, Muller D, Legendre L, Wisniewski-Dye F, and Prigent-Combaret C (2013). Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci 4:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacheron J, Desbrosses G, Renoud S, Padilla R, Walker V, Muller D, and Prigent-Combaret C (2018). Differential contribution of plant-beneficial functions from Pseudomonas kilonensis F113 to root system architecture alterations in Arabidopsis thaliana and Zea mays. Mol. Plant. Microbe. Interact 31:212–223. [DOI] [PubMed] [Google Scholar]
- Van den Berg C, Willemsen V, Hage W, Weisbeek P, and Scheres B (1995). Cell fate in the Arabidopsis root meristem determined by directional signalling. Nature 378:62–65. [DOI] [PubMed] [Google Scholar]
- Van Wees SCM, Van Pelt JA, Bakker PAHM, and Pieterse CMJ (2013). Bioassays for assessing jasmonate-dependent defenses triggered by pathogens, herbivorous insects, or beneficial rhizobacteria. Methods Mol. Biol 1011:35–49. [DOI] [PubMed] [Google Scholar]
- Verbon EH, and Liberman LM (2016). Beneficial microbes affect endogenous mechanisms controlling root development. Trends. Plant. Sci 21:218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen BW, Glazebrook J, Zhu T, Chang HS, Van Loon LC, and Pieterse CMJ (2004). The transcriptome of rhizobacteria-induced systemic resistance in Arabidopsis. Mol. Plant-Microbe Interact 17:895–908. [DOI] [PubMed] [Google Scholar]
- Vermeer JE, von Wangenheim D, Barberon M, Lee Y, Stelzer EH, Maizel A, and Geldner N (2014). A spatial accommodation by neighboring cells is required for organ initiation in Arabidopsis. Science 343:178–183. [DOI] [PubMed] [Google Scholar]
- Vishwanath SJ, Delude C, Domergue F, and Rowland O (2015). Suberin: biosynthesis, regulation, and polymer assembly of a protective extracellular barrier. Plant Cell Rep. 34:573–586. [DOI] [PubMed] [Google Scholar]
- Vissenberg K, Claeijs N, Balcerowicz D, and Schoenaers S (2020). Hormonal regulation of root hair growth and responses to the environment in Arabidopsis. J. Exp. Bot 71:2412–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsman G, Sparks EE, and Benfey PN (2015). Genes and networks regulating root anatomy and architecture. New Phytol. 208:26–38. [DOI] [PubMed] [Google Scholar]
- Wada T, Tachibana T, Shimura Y, and Okada K (1997). Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science 277:1113–1116. [DOI] [PubMed] [Google Scholar]
- Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, Blundell TL, Esch JJ, Marks MD, and Gray JC (1999). The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. The Plant Cell 11:1337–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L, Boddington C, Jenkins D, Wang Y, Gronlund JT, Hulsmans J, Kumar S, Patel D, Moore JD, Carter A, et al. (2017). Root architecture shaping by the environment is orchestrated by dynamic gene expression in space and time. The Plant Cell 29:2393–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AM, Hornblad E, Voxeur A, Gerber L, Rihouey C, Lerouge P, and Marchant A (2010). Analysis of the Arabidopsis IRX9 IRX9-L and IRX14/IRX14-L pairs of glycosyltransferase genes reveals critical contributions to biosynthesis of the hemicellulose glucuronoxylan. Plant. Physiol 153:542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrsch I, Dominguez-Ferreras A, Geldner N, and Boller T (2015). Tissue-specific FLAGELLIN-SENSING 2 (FLS2) expression in roots restores immune responses in Arabidopsis fls2 mutants. New Phytol. 206:774–784. [DOI] [PubMed] [Google Scholar]
- Wysocka-Diller JW, Helariutta Y, Fukaki H, Malamy JE, and Benfey PN (2000). Molecular analysis of SCARECROW function reveals a radial patterning mechanism common to root and shoot. Development 127:595–603. [DOI] [PubMed] [Google Scholar]
- Yadav V, Molina I, Ranathunge K, Castillo IQ, Rothstein SJ, and Reed JW (2014). ABCG transporters are required for suberin and pollen wall extracellular barriers in Arabidopsis. Plant Cell 26:3569–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Pieterse CMJ, Bakker PAHM, and Berendsen RL (2019a). Beneficial microbes going underground of root immunity. Plant Cell Environ 42:2860–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Tichelaar R, Liu Y, Savant N, Lagendijk E, Van Kuijk S, Stringlis IA, Van Dijken AJ, Haney CH, Pieterse CMJ, et al. (2019b). Rhizosphere-associated Pseudomonas suppress local root immune responses by gluconic acid-mediated lowering of environmental pH. Curr. Biol 29:3913–3920. [DOI] [PubMed] [Google Scholar]
- Zamioudis C, Hanson J, and Pieterse CMJ (2014). β-Glucosidase BGLU42 is a MYB72-dependent key regulator of rhizobacteria-induced systemic resistance and modulates iron deficiency responses in Arabidopsis roots. New Phytol. 204:368–379. [DOI] [PubMed] [Google Scholar]
- Zamioudis C, Korteland J, Van Pelt JA, Van Hamersveld M, Dombrowski N, Bai Y, Hanson J, Van Verk MC, Ling HQ, Schulze-Lefert P, et al. (2015). Rhizobacterial volatiles and photosynthesis-related signals coordinate MYB72 expression in Arabidopsis roots during onset of induced systemic resistance and iron-deficiency responses. The Plant Journal 84:309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamioudis C, Mastranesti P, Dhonukshe P, Blilou I, and Pieterse CMJ (2013). Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant. Physiol 162:304–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Emonet A, Denervaud Tendon V, Marhavy P, Wu D, Lahaye T, and Geldner N (2020). Co-incidence of damage and microbial patterns controls localized immune responses in roots. Cell 180:440–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw RNA-Seq read data are deposited with links to BioProject accession number PRJNA836026 in the NCBI BioProject database.