Rapid development of molecular-targeted and immune-based agents has substantially expanded clinically available therapeutic options in hepatocellular carcinoma (HCC).[1] However, the limited response rate to the agents (only up to 30% of the patients) highlights necessity to characterize underlying biological diversity across the tumors/patients to infer the response and refine indication of each therapeutic regimen. Recent single-cell and/or spatial molecular profiling studies have indeed revealed substantial inter- and intra-tumor heterogeneities in histological and molecular traits across the HCC tumors/patients, which may influence the therapeutic response.[2] Currently available predictive biomarker of drug response remains only alpha-fetoprotein for second-line ramucirumab, highlighting the urgent unmet need for biomarkers to inform therapeutic decision making and ultimately improve the poor HCC prognosis.
Clinically, approximately one-fourth of HCC tumors present massive intra-tumor fibrous tissue and cellular infiltrates, which are accompanied with induction of fibrogenic and inflammatory molecular pathways such as transforming growth factor-beta (TGF-beta) signaling.[2, 3] Recent studies in various cancer types have shown that the intra-tumor fibrous tissue can generate perpetual cancer-permissive tissue microenvironment by serving as a reservoir of pro-inflammatory cytokines and oncogenic growth factors.[4] In addition, the accumulated extracellular matrix (ECM) proteins as well as associated hemodynamic alterations and retention of interstitial fluids can cause physical forces (e.g., stretch, shear stress, compression, and stiffness) that lead to induction of oncogenic cellular signaling, e.g., RET kinase pathway.[5] Such mechano-transduction, sensing and responding to the physical alterations, is increasingly recognized as one of the pathogenic drivers in several chronic liver disease such as non-alcoholic fatty liver disease (NAFLD).[6] Thus, in-depth characterization of the stromal tissue microenvironment may provide clues to new biomarkers and therapeutic targets in the fibrotic HCC tumors.
Recent advances in the proteomic profiling technologies and accumulated datasets have enabled comprehensive characterization of ECM and related components as the “matrisome”, which has pathophysiological implications in various clinical conditions.[7, 8] The matrisome encompasses the full spectrum of ECM proteins (e.g., glycoproteins, collagens, and proteoglycans) and secretory factors that regulate ECM homeostasis, which collectively orchestrate cellular and tissue phenotypes. These emerging resources may shed light on new biological aspects of the fibrotic stroma-rich HCC tumors, which could lead to improved management of the patients.
In this issue of Hepatology, Desert et al. applied a tandem mass spectrometry-based comprehensive matrisomic characterization of clinical HCC patient cohorts and chemically-induced mouse models of hepatic fibrosis and carcinogenesis.[9] Clinical HCC tumors with (n=10) or without (n=10) high-grade intra-tumor fibrous nests (defined as focal intra-tumor ECM deposits) and adjacent non-tumor liver tissues were first subjected to the matrisome profiling. Seventy-four ECM proteins more abundant in the fibrotic HCC tumors included fibrillar and basement membrane collagens, proteoglycans, and growth factors, whereas 20 proteins involved in coagulation and wound healing were more abundant in less fibrotic HCC tumors. Some of the fibrotic HCC-specific proteins were also abundant in diethylnitrosamine-induced HCC and carbon tetrachloride-induced liver fibrosis in mice. Molecular pathway analysis revealed enhanced glycolysis and decreased oxidative phosphorylation in the fibrotic HCC tumors, suggesting presence of a metabolic shift like the Warburg effect. Interestingly, fibrotic HCC tumors were more frequently accompanied with less fibrotic liver compared to less fibrotic tumors, although why such inverse correlation exists is an open question. Integrative analysis with genome-wide transcriptome data of 2,285 clinical samples identified a molecular subgroup of HCC with fibrous nest, which showed similarity to the Hoshida S1 subtype with TGF-beta and canonical Wnt pathway activation.[10] The fibrous nest subtype was characterized by cancer-specific ECM remodeling and more frequent early tumor recurrence and poorer overall survival after surgical HCC treatment compared to the rest of the patients. Finally, an 11-protein multiplex immunohistochemistry signature of the fibrous nest HCC was defined for its clinical application.
The investigators also examined the matrisome genes in transcriptome profiles of 444 HBV-related HCC patients obtained from public databases.[11] The analysis identified 80 matrisome genes involved in immune response, ECM remodeling, and cancer-related pathways as potential drivers of HBV-related HCC. One of the matrisome-based patient subgroups (approximately 15%) was characterized by elevated metabolic activity, inactive cell cycle, immune infiltration, lower tumor purity, and better prognosis compared to the rest. In addition, four liver-specific matrisome genes were identified as correlates with progression and prognosis of HBV-related HCC.
These studies demonstrate clinical relevance and potential utility of matrisome characterization to gain additional layer of biological information to refine our understanding about inter/intra-tumor heterogeneity in fibrotic HCC tumors (Figure 1). Such information may not only refine prediction of treatment response and prognosis, but also provide clues to new molecular targets and strategies to circumvent resistance to existing therapies. Furthermore, given that the fibrous nests inside and outside the tumor serves as the niche to foster cancer-permissive immune microenvironment, in-depth characterization of immune cell types and their functional status warrants further research especially with the current active development of immune-based combination therapies. In addition to the composition and abundance of the matrisome genes/proteins, their spatial organization and architecture, and crosstalk between involved cell types will be the focuses in future research to expand our mechanistic understanding and elucidate druggable molecular and immunologic targets in the fibrotic HCC subtype. Clinically applicable biomarker assays to monitor the functional status of the matrisome will also be important for clinical translation of the findings.
Figure 1. Matrisomic characterization of hepatocellular carcinoma for identification of new therapeutic targets and biomarkers.
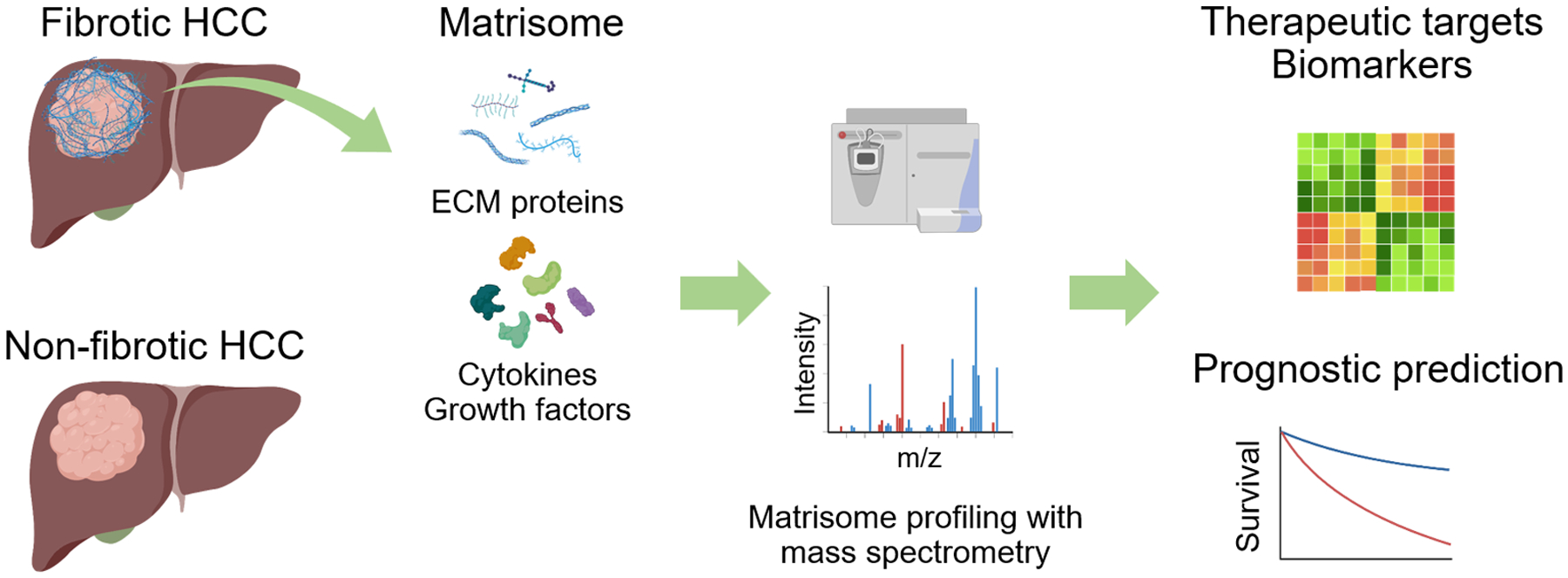
HCC, hepatocellular carcinoma; ECM, extracellular matrix.
Acknowledgements
Figure was created with BioRender.com.
Funding
YH is supported by grants from National Institutes of Health (CA233794, CA222900, CA230694, CA255621), European Commission (ERC-AdG-2020-101021417), Cancer Prevention and Research Institute of Texas (RR180016, RP200554).
Footnotes
Conflict of interest
YH is shareholder for Alentis Therapeutics and Espervita Therapeutics, advisory for Helio Genomics, Espervita Therapeutics, and Roche Diagnostics.
References
- 1.Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet 2022;400:1345–1362. [DOI] [PubMed] [Google Scholar]
- 2.Dhanasekaran R, Suzuki H, Lemaitre L, Kubota N, Hoshida Y. Molecular and immune landscape of hepatocellular carcinoma to guide therapeutic decision making. Hepatology 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan PS, Nakagawa S, Goossens N, Venkatesh A, Huang T, Ward SC, Sun X, et al. Clinicopathological indices to predict hepatocellular carcinoma molecular classification. Liver Int 2016;36:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev 2017;121:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Sanchez ME, Barbier S, Whitehead J, Bealle G, Michel A, Latorre-Ossa H, Rey C, et al. Mechanical induction of the tumorigenic beta-catenin pathway by tumour growth pressure. Nature 2015;523:92–95. [DOI] [PubMed] [Google Scholar]
- 6.Mitten EK, Baffy G. Mechanotransduction in the pathogenesis of non-alcoholic fatty liver disease. J Hepatol 2022;77:1642–1656. [DOI] [PubMed] [Google Scholar]
- 7.Naba A 10 years of extracellular matrix proteomics: Accomplishments, challenges, and future perspectives. Mol Cell Proteomics 2023:100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arteel GE, Naba A. The liver matrisome - looking beyond collagens. JHEP Rep 2020;2:100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desert R, Chen W, Ge X, Viel R, Han H, Athavale D, Das S, et al. Hepatocellular carcinomas, exhibiting intratumor fibrosis, express cancer-specific extracellular matrix remodeling and WNT/TGFB signatures, associated with poor outcome. Hepatology 2023. [DOI] [PubMed] [Google Scholar]
- 10.Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, Villanueva A, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res 2009;69:7385–7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen W, Desert R, Ge X, Han H, Song Z, Das S, Athavale D, et al. The Matrisome Genes From Hepatitis B-Related Hepatocellular Carcinoma Unveiled. Hepatol Commun 2021;5:1571–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]


