Abstract
Sea anemones (Cnidaria, Actiniaria) are a successful group of marine invertebrates found in a diverse range of environments globally. In spite of their ubiquity, identities for many sea anemones remain unverified, especially those from the Indo-West Pacific region. Here, we clarify the taxonomy of the poorly known Macrodactyla aspera, a shallow-water species first described from the Torres Straits in northern Australia. We re-describe M. aspera based on new morphological and molecular data gathered from the type specimen, other museum vouchers, and from fresh material collected from Singapore. We tested the monophyly of Macrodactyla using three mitochondrial (12S, 16S and cox3) and one nuclear (28S) marker based on three congeners, recovering this genus to be polyphyletic. As a consequence, we transferred M. doreensis to the genus Heteractis, and describe a new species, Macrodactyla fautinae sp. nov. While both M. aspera and M. fautinae sp. nov. share the same arrangement and number of complete mesenteries, a similar distribution of cnidae, and are not symbiotically associated with any other biota, M. fautinae sp. nov. has perforated, lobe-like verrucae on its column, and lacks nematocyst batteries on its tentacles, unlike M. aspera. These two species also occur in similar habitats in Singapore. Finally, because M. aspera strongly resembles Dofleinia armata, the latter species flagged as a danger to public health due to its ability to inflict painful stings, we tested the relationship between these species and found them not to be closely related. However, tentacles of M. aspera, like D. armata, are densely covered with nematocyst batteries and harbour large nematocysts; we infer that M. aspera may also be capable of delivering stings that endanger public health. This study builds upon a growing number of studies that aim to ascertain identities and systematics of sea anemones historically reported from the Indo-West Pacific. Our findings will facilitate accurate species identification, which is crucial for advancing research, formulating conservation measures, and protecting public health.
Keywords: Actinioidea, Anthozoa, Intertidal, Integrative taxonomy, Southeast Asia
BACKGROUND
Sea anemones (Cnidaria, Actiniaria) are a cosmopolitan group of marine invertebrates, occupying a diverse range of habitats globally, extending from the tropical shallow seas to deep oceanic trenches (Gomes et al. 2016). These animals are engaged in important marine ecological processes, including symbiotic relationships with micro-and macro-organisms (e.g., fishes; crustaceans, and dinoflagellates, etc.; see: Dunn 1981, Fransen 1989; Sunagawa et al. 2009, respectively). Sea anemones are also sought after in the marine aquarium trade, and in some cultures, these animals are consumed as food (Dunn 1981; Scott et al. 2014). Some are also feared by humans because of their ability to inflict painful stings upon contact. Like all other cnidarians, sea anemones manufacture intracellular stinging capsules (known as nematocysts) containing venom (Fautin 1988 2009; Mitchell et al. 2020). Stings of some tropical shallow-water species from the Indo-West Pacific region may leave severe, long-lasting scars on their human victims, or in some instances, cause death (Erhardt and Knop 2005). Amongst species from the region that harbour potent stings, and are a threat to public health, four are well-known: Dofleinia armata Wassilief, 1908, Phyllodiscus semoni Kwietniewski, 1897, and members of the family Actinodendridae Haddon, 1898 such as Actinodendrum arboreum (Quoy and Gaimard, 1833) and Actinostephanus haeckeli Kwietniewski, 1897 (see: Coleman 1999; Hoeksema and Crowther 2011; Mizuno et al. 2007; Rowlett 2020).
Despite their diversity, commercial popularity, and the possible threats they may impose on human health, identities for many sea anemone species remain poorly verified. This is especially so for those that occur within the Indo-West Pacific region (den Hartog 1997; Fautin et al. 2009; Yap et al. 2019). This taxonomic inadequacy is a result of these invertebrates being notoriously difficult to identify accurately with confidence. Sea anemones are skeleton-less and among species, they have a similar, uniform body-plan and an apparent “dearth of features” to distinguish them (Fautin 1988: 497). Many morphological characters that were historically used to delimit species boundaries are still poorly defined, leading to taxonomic confusion (see Daly 2003; Daly et al. 2017). Furthermore, emerging molecular evidence has revealed that many distantly related species may exhibit a great degree of morphological convergence among them (Rodríguez et al. 2014; Daly et al. 2017; Titus et al. 2019). Apart from these aforementioned challenges, many species originally described from the Indo-Pacific region have not been verified, due to a lack of taxonomic detail in their original descriptions (Dunn 1974; Fautin et al. 2009; Yap et al. 2019 2021). This greatly impedes further research on these animals. Here, we focused on clarifying the taxonomy of one Indo-West Pacific species: Macrodactyla aspera (Haddon and Shackleton, 1893). This species is of particular interest as it bears a strong resemblance to D. armata, and occurs in similar, sandy and silty habitats (Erhardt and Knop 2005; Rowlett 2020). Like D. armata, M. aspera also bears tentacles that are densely covered with visible batteries that are packed with nematocysts (i.e., nematocyst batteries) which may pose
a threat to public health.
Macrodactyla aspera was first described from
northern Australia by Haddon and Shackleton (1893) as Condylactis aspera (it is also the type species for the genus Macrodactyla; see Carlgren 1949; Fautin 2016). Since then, little taxonomic work has been conducted to confirm its identity. Presently, only two published descriptions have dealt directly with the taxonomy of this species: i) Haddon and Shackleton (1893), and ii) Haddon (1898). By contemporary standards, these two taxonomic publications lacked crucial taxonomic details to accurately identify the animal (e.g., absence of cnidom data). This species was most recently referred to in an annotated checklist of littoral biota from the Gulf of Aqaba (Fishelson 1970). A half-tone photograph of the living animal with its tentacles extended accompanied this checklist (see Fishelson 1970: 100, Fig. 9), but no taxonomic description was given. Much of the animal remained obscured in this half-tone photograph –it is unclear what M. aspera truly looks like when alive. With the exception of Fishelson’s (1970) account, it has been more than a century since the species boundaries of M. aspera have been evaluated and tested; this warrants an investigation of the diagnostic features that were previously reported to define this species.
Other taxonomic works referring to M. aspera were dedicated to revising the original diagnosis of the genus Macrodactyla Haddon, 1898 (i.e., Stephenson 1921 1922; Carlgren 1949; Dunn 1981). This genus was established by Haddon (1898: 431) to accommodate C. aspera, as he recognised that it was different from known members of Condylactis, although he was not explicit in stating his reasons for transferring this species to Macrodactyla. While Haddon and Shackleton (1893) placed this species in Actiniidae Rafinesque, 1815 –a taxonomic decision retained in Haddon (1898) and thereafter –the diagnosis of Macrodactyla by Haddon (1898) contradicted accepted diagnoses of Actiniidae during his time. Haddon (1898) had erroneously reported that all mesenteries present in C. aspera were ‘imperfect’ (i.e., incomplete). Stephenson (1922: 263) subsequently flagged Haddon’s account as an ‘ambiguity’, since members of Actiniidae were accepted at that time to have more than six complete mesenteries (e.g., Hertwig 1882; Carlgren 1893; McMurrich 1889; see: Haddon 1898: 414). After examining the type specimen of C. aspera, Stephenson (1921 1922) corrected Haddon’s (1898) diagnosis for Macrodactyla, confirming the presence of six pairs of complete mesenteries in the type material. However, because Stephenson (1922) had considered actiniids to possess more than six complete pairs of mesenteries, he moved Macrodactyla to a new family Myonanthidae, whose members strictly possessed only six complete pairs. While Carlgren’s (1949) monograph that followed retained much of Stephenson’s (1921 1922) revised diagnosis, he returned Macrodactyla to Actiniidae, as he posited that actiniids have “...Perfect [= complete] pairs of mesenteries rarely six, as a rule more than six,” (Carlgren 1949: 47; additions in square parentheses, our own).
Present perspectives on the systematics of Macrodactyla stem from Dunn’s (1981) [= DG Fautin] expanded definition of the genus. This genus had remained monotypic since its publication until Dunn’s (1981) inclusion of a congener: Macrodactyla doreensis (Quoy and Gaimard, 1833). This congener’s name was a new combination that Dunn (1981) had created, to apply to a number of sea anemones she had previously synonymised into one species. Among those synonymised were Actinia doreensis Quoy and Gaimard, 1833 and Condylactis gelam Haddon and Shackleton, 1893 (see Dunn 1981: 29, for the full synonymy list). While Dunn (1981) was at first uncertain in finding an appropriate genus to classify these synonymised species, she eventually settled on Macrodactyla, rationalizing that they were (Dunn 1981: 29), “... no doubt closely related [witness Haddon and Shackleton’s (1893) original description of both as members of Condylactis]...”. However, Dunn (1981: 35) also noted that name-bearing specimens of these synonymised species (e.g., syntypes of C. gelam), and the type specimen of the type species for Macrodactyla (i.e., C. aspera) were morphologically different in the “...distribution and size of cnidae, character of mesenterial retractor muscles... and tentacle number and form,” casting doubt on her own decision. At present, it is unclear if M. aspera and M. doreensis are congeneric. Recent phylogenetic analyses using only M. doreensis samples have repeatedly recovered this species to be nested within Heteractis Milne Edwards, 1857 (Titus et al. 2019; Yap et al. 2021). Prior to this study, phylogenetic interpretation using M. aspera have not been conducted, and the monophyly of Macrodactyla remains untested.
Apart from these systematic problems, Fautin (2016) also highlighted a nomenclatural issue relating to the use of the name Macrodactyla. This genus name was reported to be a junior homonym to a genus of a beetle described in Hitchcock (1833) (see: Neave 1940; Fautin 2016). Following Neave’s (1940) listing and strict stipulations outlined by the International Commission on Zoological Nomenclature (i.e., Article 23.3.5; the “Code”, henceforth), Fautin (2016) returned both species, presently placed in Macrodactyla, to Condylactis (therefore, as C. aspera and C. doreensis, respectively). Despite her nomenclatural actions, Fautin (2016: 105) also conceded that she had not consulted the original publication where the senior homonym had appeared (i.e., Hitchcock 1833), stating, “... not seen: “Rep. Geo. Min. Zool. Massach., 575...” (emphasis in bold, our own). Subsequent publications after Fautin (2016) have retained the use of Macrodactyla when referring to both M. aspera and M. doreensis (e.g., Titus et al. 2019; Yap et al. 2021), because their morphology was not congruent with Condylactis (e.g., presence of a sphincter muscle; see: González-Muñoz et al. 2012). The nomenclatural status for the genus name Macrodactyla needs to be resolved; Neave’s (1940) assertions and Fautin’s (2016) resultant nomenclatural actions must be verified for the unambiguous usage of this genus name for sea anemones.
In this study, we re-described M. aspera based on new data gathered from its type specimen, accompanied by fresh material collected from Singapore, and from vouchers kept in natural history museums worldwide. Nomenclatural issues relating to the use of the genus name Macrodactyla, were resolved to support its continued usage for sea anemones. We also tested the phylogeny of the two presently accepted species in Macrodactyla (i.e., M. aspera and M. doreensis) and recovered them as polyphyletic. Considering this new evidence, we revised the definition of Macrodactyla, and classified M. doreensis in Heteractis as Heteractis doreensis (Quoy and Gaimard, 1833) comb. nov. Additionally, we tested the relationship between M. aspera and D. armata, due to the strong morphological resemblance between these two species. We found them to be distantly related, with D. armata being recovered with Actinodendridae Haddon, 1898, its members well-known to inflict painful stings.
During field surveys conducted for this study in Singapore, we encountered another species of sea anemone that co-occurs with M. aspera. While this second species has been photographed and is known by local citizen scientists for many years, being nicknamed as the ‘Purple-lip Sand Anemone’ and ‘Tiger Anemone’ in both printed and web-based biodiversity guides (e.g., Rowlett 2020: 323; WildSingapore Fact Sheets: http://www.wildsingapore.com/wildfacts/, respectively), its identity has remained undetermined until now. This second species is new to science, which we formally described as Macrodactyla fautinae sp. nov., and is thus far only known from Singapore. In our phylogenetic analyses, we recovered this new species and M. aspera as a clade. Macrodactyla fautinae sp. nov. differs from M. aspera in bearing adhesive and extensible, perforated lobe-like verrucae, and a lack of visible nematocyst batteries on its tentacles.
Information from this study builds on efforts to accurately identify sea anemones from the Indo-West Pacific region (e.g., England 1987; Fautin et al. 2009; Yap et al. 2019). This is necessary to prime future research involving these marine invertebrates from the region as so little is known about their ecology (e.g., reproductive biology, see Yong et al. 2021). Moreover, given that sea anemones can impact humans due to their ability to deliver harmful stings, precise identification is key to constructive management and mitigative efforts in safeguarding public health.
MATERIALS AND METHODS
Sea anemone collection and processing
Fresh material examined in this study were collected from intertidal habitats along Singapore’s coastlines and offshore islands (Fig. S1). Sea anemones were observed and photographed in situ prior to removing individuals (M. aspera: N = 12: M. fautinae sp. nov.: N = 29) from the soft substratum using a hand-trowel. In the laboratory, tissue was sampled from the animal’s pedal disc, column and tentacles, and preserved in 100% molecular-grade ethanol for further molecular analyses (see below). The animals were subsequently relaxed in 7.5% magnesium chloride and then fixed in 10% formalin. Identities of other sea anemone species, for which their tissues were used for the molecular analyses in this study, were verified using evidence published in primary scientific literature (e.g., Fautin et al. 2009 2015; Fautin and Tan 2016).
Morphological observations
All fixed specimens were dissected so that the internal morphology could be examined. Histological sections, 8 μm thick, were prepared from these materials so that mesentery arrangements and musculature of the animal could be observed. All sections were stained with haematoxylin and counterstained with eosin (Humason 1967).
Cnidae present in the formalin-fixed tissues of the sea anemone’s tentacle tip, marginal projections, mid-column, actinopharynx and mesenterial filaments, were viewed at 1000x magnification under a light microscope. Only intact capsules (i.e., undischarged capsules) were measured using conventions presented in Dunn (1981) and Yap et al. (2021). Cnidae taxonomy followed Mariscal (1974).
Translations of original taxonomic descriptions written in languages other than English (e.g., Wassilieff 1908) were carried out using Google Translate (Google Translate: https://translate.google.ca). For specialised scientific terms that could not be translated suitably, we followed definitions listed in Stachowitsch (1992).
To redefine the species boundaries of M. aspera, syntypes of C. aspera housed at both University Museum of Zoology, Cambridge, United Kingdom (MZC), and Museum of Zoology, Lund University, Sweden (MZL), were examined (for type material listing, see: Fautin 2016). Furthermore, we located and examined museum voucher material that closely resembled M. aspera, or those that were identified as D. armata on their labels. These vouchers originated from Australia, New Caledonia, Singapore and Israel (Fig. S2), in the collections of Muséum National d’Histoire Naturelle, Paris, France (MNHN), Steinhardt Museum of Natural History, Tel Aviv, Israel (SMNHTAU), and Western Australian Museum, Perth, Australia (WAM).
All fresh material collected from Singapore for this study were deposited into the Zoological Reference Collection (ZRC), Lee Kong Chian Natural History Museum, National University of Singapore.
Molecular analyses
Genomic DNA from ethanol preserved tissues were isolated via standard CTAB extraction (see Rodríguez et al. 2014, and references therein). We utilised four published molecular markers in this study: three mitochondrial (i.e., partial 12S rDNA: Chen et al. 2002; 16S rDNA and cox3: Geller and Walton 2001) and one nuclear (i.e., partial 28S rDNA: Chen and Yu 2000). Loci for these markers were amplified with polymerase chain reaction (PCR), following published cycling profiles (i.e., Lauretta et al. 2013; Rodríguez et al. 2014 and references therein). All PCR products were purified with SureClean Plus (Bioline, Singapore). Thereafter cycle sequencing was performed using BigDye Terminator v3.1 (Applied Biosystems, Foster City, California) following the manufacturer’s protocol. All amplicons obtained were sequenced on an ABI 3130 XL Genetic Analyzer (Thermo Scientific).
New sequences acquired in this study were assembled in Geneious v11.1.3 (Invitrogen Corporation) using default parameters. All contigs were searched against GenBank sequences via BLASTn; this was to affirm that nucleotide sequences we had obtained and successfully amplified were only sea anemones. Selected sequences previously published were also used in our analyses (i.e., Rodríguez et al. 2014; Daly et al. 2017; Titus et al. 2019; Yap et al. 2021). Gene sequences were aligned separately using MAFFT v.7.313 under --auto setting. These aligned sequences were then concatenated into a combined matrix. In all, separate analyses for alignments were conducted over the five resultant datasets (i.e., 12S, 16S, cox3, 28S and concatenated matrix).
Maximum likelihood (ML) analyses were conducted in RAxML-NG v0.8.1 (Stamatakis 2014; Kozlov et al. 2019) using 50 random and 50 parsimonious starting trees, with 1000 bootstrap pseudo-replicates. The best model of DNA evolution was determined using ModelTestNG (Darriba et al. 2020) and specified for all datasets in both ML analyses and Bayesian inference (BI) described below. Uncorrected pairwise distances, for each alignment, was calculated using MEGA X (Kumar et al. 2018).
Bayesian inference was carried out only on the concatenated matrix using MrBayes v3.2.6 (Ronquist et al. 2012). Four Markov Chain Monte Carlo (MCMC) runs were carried out for 12 million generations and sampled at every 100th tree. To determine if the runs converged, Tracer v1.7 (Rambaut et al. 2018) was used to visualise the posteriors. Convergence was achieved after discarding the first 60,001 trees as burn-in (average standard deviation of split frequencies = 0.0322, ESSLnL = 1152, ESSLnPr = 2860).
All new sequences obtained in this study are deposited in GenBank (Table S1).
RESULTS
Phylogeny reconstruction
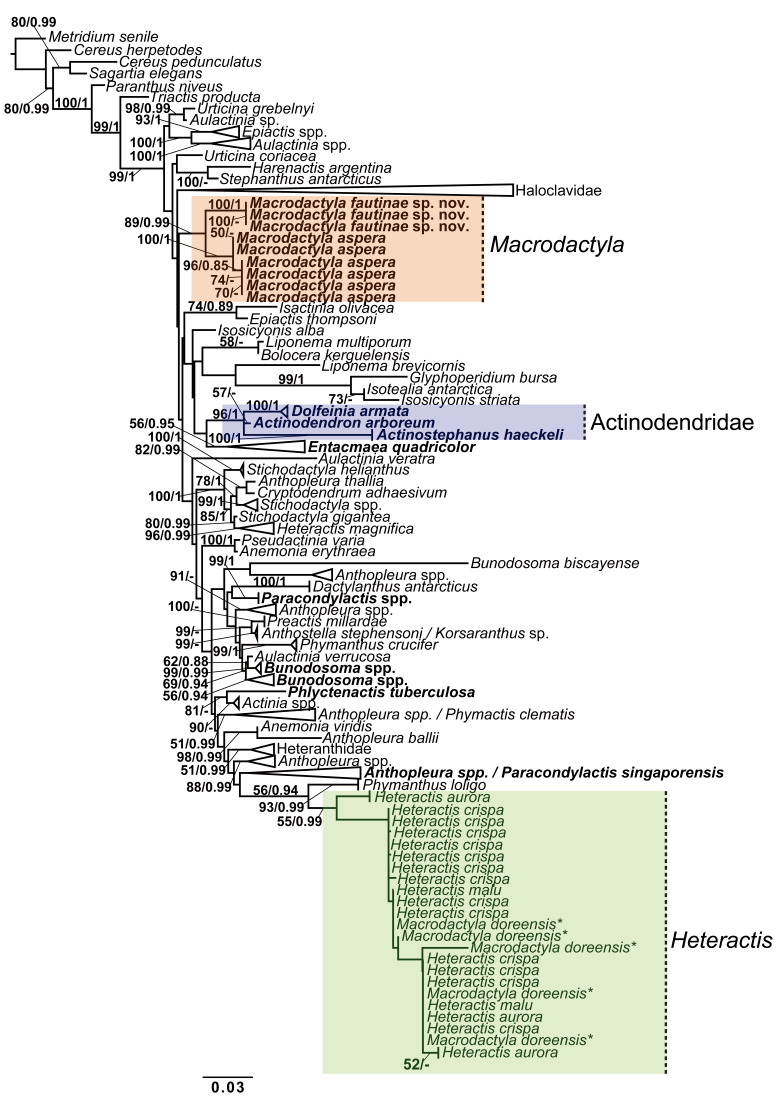
Overall, 198 taxa were present in the concatenated dataset, with 6,195 sites used for phylogeny reconstruction. Broadly, while trees inferred from both ML and BI analyses of the concatenated dataset and four individual molecular markers were not fully concordant, topologies of phylogenetic relationships inferred across all trees remained similar at genus level (Fig. 1; Figs. S3–S7).
Fig. 1.
Phylogeny reconstruction of Macrodactyla aspera and Macrodactyla fautinae sp. nov. among actiniid sea anemones. Tree topology a result of maximum likelihood (ML) analysis, using a concatenated dataset (i.e., cox3, 12S, 16S, 28S). At the branches, bootstrap ML resampling values, and Bayesian inference (BI) posterior probability values, are presented as ML/BI. Only bootstrap values > 50, and posterior probability > 0.8 are indicated here; those less than 50 and 0.8 are denoted by (-). Taxa indicated in bold denotes the presence of new sequences obtained for this study. Indicated by an asterisk (*), we retain the name Macrodactyla doreensis in this figure to aid with the discussion within the main-text. For sequences used and the full tree, refer to table S1 and figure S3, respectively.
In all inferred trees, Macrodactyla was found to be polyphyletic, with M. aspera and M. doreensis failing to form a clade. Instead, M. doreensis was nested among most members of Heteractis, with strong support. Macrodactyla aspera was recovered to be a sister to M. fautinae sp. nov., forming a distinct clade (Fig. 1; Table 1). This relationship is well-supported, with bootstrap values being > 50 at inclusive nodes (Fig. 1). Despite their similarity in appearance, M. aspera and D. armata were not recovered as a clade. The latter was recovered in a clade consisting of members of Actinodendridae (i.e., A. arborerum and Ac. haeckeli, with strong support (i.e., bootstrap > 80) (Fig. 1).
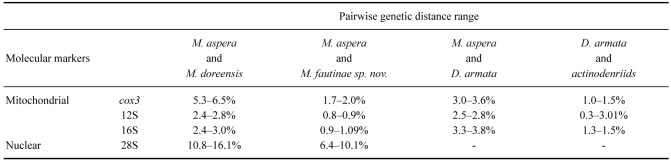
Table 1.
Pairwise genetic distances among Macrodactyla, Dofleinia, and members of family Actinodendridae (i.e., Actinodendron and Actinostephanus) for the four molecular markers targeted in this study. Dashes indicate that no comparison were made, as we failed to obtained 28S sequences from D. armata (see Table S1)
Pairwise genetic distances between congenerics M. aspera and M. doreensis ranged from 2.4% to 16.1%; dependent on the molecular marker examined (Table 1). Between M. aspera and M. fautinae sp. nov., these genetic distances were smaller among the mitochondrial markers (i.e., cox3, 12S and 16S: 0.8% to 2.0%). In contrast, genetic distances were larger in nuclear markers (i.e., 28S: 6.4% to 10.1%; Table 1). While M. aspera may superficially resemble D. armata, pairwise differences of the mitochondrial markers between these two species were larger than between members of Macrodactyla, ranging between 3.0% and 3.8% (Table 1). Dofleinia armata was recovered as a sister to the actinodendrids (Fig. 1), and this finding is further supported here by the small pairwise genetic differences (Table 1).
TAXONOMY
Order Actiniaria Hertwig, 1882
Suborder Enthemonae Rodríguez and Daly, 2014 in Rodríguez et al. 2014
Superfamily Actinioidea Rafinesque, 1815
Family Actiniidae Rafinesque, 1815
Genus Macrodactyla Haddon, 1898
Condylactis [pro parte] –Haddon and Shackleton 1893: 123; Fautin 2016: 105.
Macrodactyla [pro parte] –Haddon 1898: 431 [original description]; Stephenson 1921: 524, 531, 545; Stephenson 1922: 263, 265; Carlgren 1949: 63; Dunn 1981: 28, 35; Fautin et al. 2007: 213; Fautin et al. 2008: 49; Fautin 2016: 104, 105.
Etymology gender: Feminine.
Type species: Condylactis aspera Haddon and Shackleton, 1893, by monotypy, in agreement with Dunn (1981), Fautin et al. (2007) and Fautin (2016).
Definition of genus modified after Dunn (1981) and Fautin et al. (2008). Substantial changes from our research are indicated in bold; all minor additions are underlined.
Diagnosis: Pedal disc distinct, circular to oval in outline, as wide as oral disc. Column elongated, marginal projections present. Adhesive verrucae present on distal end, often with foreign materials often attached, may be lobed and perforated. Fosse very shallow. Tentacles long and stout, covered with nematocyst batteries or entirely smooth; inner tentacles considerably longer than the outer. Actinopharynx often everted when alive, with a pair of diametric siphonoglyphs. Symmetrically-arrayed mesenteries; always 12 pairs complete, all remainder incomplete. All fertile. Retractor muscles band-like, diffuse-circumscribed. Endodermal marginal sphincter muscle conspicuous and restricted. Parietobasilar muscles well-developed, with an extended pennon present for lower order mesenteries. Cnidom: spirocysts, basitrichs, microbasic p-mastigophores.
Nomenclatural considerations: Neave (1940) reports that the Macrodactyla, as a name for a sea anemone genus, is a junior homonym to a beetle genus described by TW Harris in Hitchcock (1833). As mentioned at the outset, Fautin (2016) had returned both Macrodactyla species (i.e., M. aspera and M. doreensis) to the next oldest available generic name applied to them: Condylactis.
We consulted Harris’ contribution in Hitchcock (1833: 575) and found the name Macrodactyla, confirming its appearance in the publication. This listed name is further accompanied by a species epithet, a letter that denotes its taxonomic authority of the species (i.e., ‘F’ = JC Fabricius), and a common name –all of which we render here exactly as (including the way it was stylised; see Hitchcock 1833: 575): (Macrodactyla) subspinosa, F. Rose-Bug.
Harris’ entry in Hitchcock (1833) was meant simply to be part of an inventory of the insect biota of Massachusetts, and it lacks an accompanying description to Macrodactyla.
No beetle species is known by the name listed by Harris in Hitchcock (1833). The closest match is the species Macrodactylus subspinosus (Fabricius, 1775), which also occurs in Massachusetts, and is known by the common names, ‘Rose-bug’ and ‘Rose-chafer’ (see: Blatchely 1910: 953; Schoolmeesters 2021). Originally described by Fabricius (1775) as a member of the genus Melolontha Fabricius, 1775, this species was later placed in a new genus, Macrodactylus, created by Latreille (1825: 372) (Schoolmeesters 2021).
We posit that Harris’ entry in Hitchcock (1833) was a misspelling of the coleopteran genus name Macrodactylus. Nomenclatural information we have found on Ma. subspinosus corroborated with much of those present in Harris’ entry (i.e., taxonomic authority, species epithet, common name, etc.), as with this species’ geographical occurrence. Moreover, Harris’ revised checklist in Hitchcock’s (1835: 565) latter work strongly supports our assertion: in this updated listing, Harris attributed the taxonomic authority of the misspelled beetle genus name (still listed as Macrodactyla) to PA Latreille. No works of Latreille prior to Harris’ entry in Hitchcock (1833) had mentioned Macrodactyla, only the name Macrodactylus was ever used in Latreille’s (1825) publication.
The name Macrodactyla in Hitchcock (1833) is thus not a senior homonym to the genus name that Haddon (1898) had used for the sea anemones; Harris’ entry is an incorrect spelling of the coleopteran genus name Macrodactylus, and therefore cannot enter homonymy (Article 54.3, the Code). As First Revisers, we retain the name Macrodactyla as a genus name for sea anemones; it is valid and available for use.
Taxonomic considerations for the genus Macrodactyla: Historically, sea anemone systematists have considered Haddon’s (1898) report of mesentery number and its arrangement in Macrodactyla to be ambiguous. Based only on the type species, C. aspera, Haddon defined Macrodactyla to have “six pairs of imperfect [= incomplete] mesenteries, and a second and third cycle of imperfect mesenteries” (Haddon 1898: 431; additions in square parentheses, our own). Stephenson (1921) later revised this feature to denote the presence of only six complete mesenteries, elaborating further that Haddon (1898) may been mistaken on this feature (Stephenson 1922: 263). Later definitions of Macrodactyla retained Stephenson’s assertions (e.g., Carlgren 1949). By including a second species to the genus, M. doreensis, Dunn (1981: 28) had amended its definition to include, “... six or more pairs of mesenteries perfect,... ” because this second species had more than six complete pairs of mesenteries. However, in Dunn’s [as Fautin] later publication of this genus, its diagnosis was changed, indicating that only six pairs of complete mesenteries were present in Macrodactyla (see Fautin et al. 2008: 49). In studying the type specimen of the type species for Macrodactyla (i.e., C. aspera; MZC.I.33665), we found that it has 12 pairs of complete mesenteries instead of six, a feature that is consistent across all voucher and fresh material. Here, we have revised the definition of the genus to reflect this.
No data concerning the cnidom for the type species of Macrodactyla (i.e., C. aspera) have ever been reported, it being absent in earlier descriptions of the genus (i.e., Haddon and Shackleton 1893; Haddon 1898; Stephenson 1921 1922). In recent definitions of Macrodactyla, which had included M. doreensis, Fautin et al. (2008: 49) simply reported the types of cnidae present in sea anemones of the genus, stating the presence of, “spirocysts, basitrichs, microbasic p-mastigophores”. We confirmed that these cnidae were present in the name-bearing type specimen of C. aspera.
We deemed Macrodactyla to be the most appropriate genus to place the new species, M. fautinae sp. nov., in the light of both morphological, ecological and genetic evidence gathered from this study. Both M. fautinae sp. nov. and M. aspera share the same number and arrangement of complete mesenteries. Both species also have visible adhesive verrucae on their column, and harbour the same types of cnidae in their tissues (see below, in their respective species description). Like M. aspera, we did not find any symbiotic micro/macro-organisms associated with M. fautinae sp. nov. Our placement of this new species in Macrodactyla is further supported by genetic evidence we have gathered: we had repeatedly recovered both M. fautinae sp. nov. and M. aspera to be sister to each other, within a clade, with strong support (bootstrap > 80; Fig. 1). Our revised diagnosis of Macrodactyla now includes traits of this second species, thereby expanding on its definition.
We failed to recover M. doreensis and M. aspera as a monophyletic clade (Fig. 1). Genetic evidence from this study, as with observations from recent studies, have repeatedly recovered M. doreensis to be nested among clownfish and dinoflagellate-associated sea anemones of the genus Heteractis (Titus et al. 2019; Yap et al. 2021; Fig. 1). Apart from genetic evidence, both M. aspera and M. doreensis also do not share many morphological and ecological traits that would characterise them to be classified in the same genus. While both have verrucae on the distal end, those of M. aspera are always adhesive, whereas verrucae of M. doreensis are not (Dunn 1981; Fautin et al. 2008). Mesentery number and arrangement between them also differ; those in M. aspera are arranged in up to three cycles while those of M. doreensis may go up to seven (Dunn 1981). Furthermore, we found microcnemes to be present in M. doreensis, consistent to its depiction in Dunn (1981: Fig. 13), a trait that is absent in M. aspera. Ecologically, M. doreensis is a host to photosynthetic dinoflagellates and macro-fauna such as clownfishes (Dunn 1981; Fautin and Allen 1992). In contrast, we have not encountered nor observed M. aspera to be involved in any symbiotic relationships with other micro/macro-organisms.
Given the genetic, morphological and ecological evidence presented, we place M. doreensis in the genus Heteractis, under a new combination: Heteractis doreensis (Quoy and Gaimard, 1833). In revising the diagnosis of Macrodactyla, we removed all characters that had extended to M. doreensis (e.g., presence of deep fosse).
Macrodactyla aspera (Haddon and Shackleton, 1893)
(Figs. 2–6)
Condylactis aspera –Haddon and Shackleton, 1893: 117, 124–125 [original description]; Dunn 1981: 29; Fautin 2016: 77, 105, 164.
Macrodactyla aspera –Haddon 1898: 398, 415, 431–432; Stephenson 1922: 263; Carlgren 1949: 63; Fishelson 1970: 109, 110; Stephenson 1922: 263; Dunn 1981: 35.
Dofleinia armata –Rowlett 2020: 292, 293.
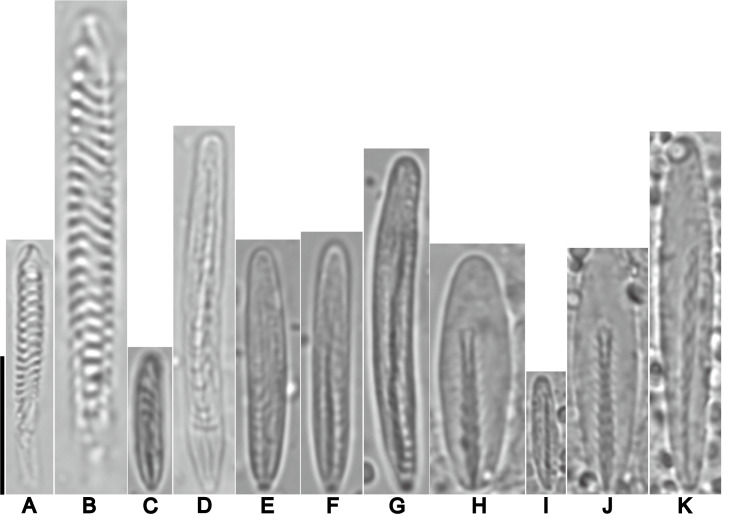
Nomenclature considerations and type material examined: Two lots of the syntypes of C. aspera that was listed in Fautin (2016), were of sea anemones collected from Murray Islands in the Torres Strait, Australia: i) a nearly complete specimen kept at the Cambridge, UK (MZC I.33665, previously without a catalogue number assigned) (Fig. 2A), and ii) three microscope slides comprising of histological sections kept in Lund, Sweden (MZL, no catalogue number) (Fig. 2B). We confirmed Fautin’s (2016) listing of these specimens; the labels present on these slides indicated that they were prepared from the material collected by Haddon and Shackleton (1893) (Fig. 2B). Among these three microscope slides, only histological sections of one closely corresponded to Haddon’s depiction of the musculature, while those present on the other two slides did not agree to his remaining illustrations (see: Haddon 1898: Plate 28, Figs. 3, 4 and 5). Despite this, sections on these slides agreed with the shape of pieces that were missing from the syntype which we had studied at the MZC. These other histological sections from the syntype were likely prepared by O Carlgren, who then kept them at the MZL where he had worked, a practice which he also carried out on other sea anemone species (Fautin 2016: 13; Yap et al. 2021). Pertaining to the original histological sections that were prepared by Haddon and used for his illustrations, we were unable to locate it at the museums we have visited.
Fig. 2.
Type material of Condylactis aspera Haddon and Shackleton, 1893. A, lectotype of C. aspera (MZC I.33665), in three pieces, top view. Note the gastro-cavity of specimen is filled densely with gametogenic tissue. B, Paralectotypes of C. aspera, as three histological slides at MZL (no catalogue number). Abbreviations: gt, gametogenic tissue; m, mesenteries; o, oral disc; s, siphonoglyph; t, tentacle. Scale bar = 10 mm.
To further provide nomenclatural objectivity to the name Condylactis aspera, we hereby designate MZC.I.3365 and the three MZL slides as the lectotype, as the latter slides were prepared from the specimen at MZC. While there was no mention of the number of individuals that were collected and examined in the original description for the species, the account of the species’ tentacle arrangement alludes to more than one individual being examined, as Haddon and Shackleton (1893: 124) stated, “... large tentacles in three or four cycles...” [underlined emphasis, our own]. This observation of a variation is only possible if more than one individual was examined. In this study, NWL Yap attempted to locate other syntypes in other museums where they may be possibly held (e.g., NHM, etc), but failed to find any. Should these other materials be found, they are the paralectotypes of C. aspera (Article 74.1.3, the Code).
Lectotype: Condylactis aspera (MZC I.3365), collected by Haddon and Shackleton from the Torres Straits (Fig. 2A). A single specimen cut into three pieces. Two pieces already removed prior to being examined by the first author: i) a wedge of the oral disc, and ii) a quarter section of the base. Column diameter about 5 mm. Entire specimen greyish, with a dark brown cast (Fig. 2A). Gastro-cavity of specimen choked with gametogenic tissue.
Lectotype: MZL (no catalogue number), three microscope slides prepared from MZC.I.3365 (Fig. 2B). One labelled as “Macrodactyla aspera Torres Str AMS 1893, consists of three transverse sections of the distal most end of the column and tentacle. Second slide, with two labels. The first wider label bears the words, “Macrodactyla aspera sph”, the second slimmer label has “AMS 187” written on it. The slide bears eight sections, all being the distal most end of the specimen. The third slide has a single label with the following printed on it: “Macrodactyla aspera Torres Str, AMS 1893”. This slide also bears three histological sections of the distal most end of the animal. All sections present on these slides are too faded to infer any useful details from them.
Material examined from Singapore (Fig. S1): (*-observed alive): Beting Bronok (ZRC.CNI.1015 x3), Changi beach (ZRC.CNI.1055 x1; ZRC.CNI.1099 x1*; ZRC.CNI.1217 x2*), Changi East beach (ZRC. CNI.1090 x1*), Cyrene Reef (ZRC.CNI.1080 x1; Photographed but not collected), Pulau Sekudu (ZRC. CNI.1222 x3*).
Materials examined from elsewhere (Fig. S2): Israel (Eilat): Gulf of Aqaba (SMNHTAU-Co.7813 x1; SMNHTAU-Co.12133 x1). Australia (Perth): North Fremantle (WAM Z99100 x1), Cockburn Sound (WAM Z50023 x1); Rowley Shoals (WAM Z50026 x2). New Caledonia: Noumea (MNHN 1321 x1); Unspecific location (MNHN 1571 x1).
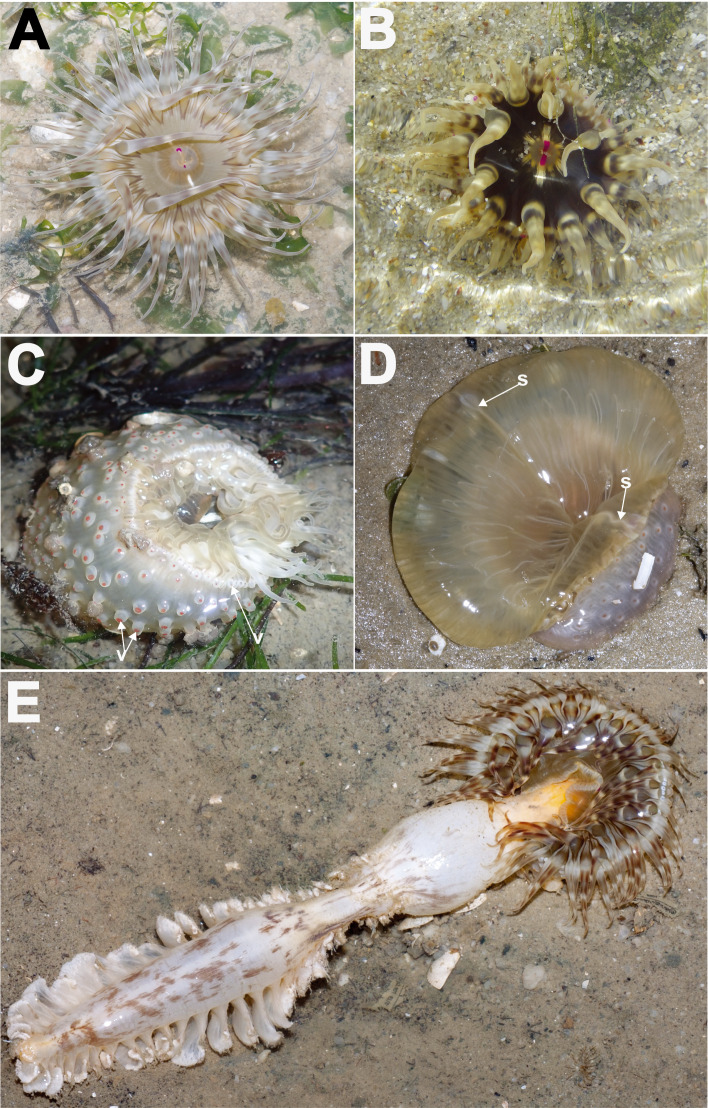
Description: Natural history: Usually solitary, at the middle intertidal of soft silty sand, sandy areas, and among seagrasses (Fig. 3A–B). Distal end of animal exposed during low spring tides, when encountered during the early morning or dusk. Animal partially buried in substratum (Fig. 3A–B). Tentacles extended and oral disc expanded, flat against substratum (Fig. 3A). Often encountered with actinopharynx everted outwards; if everted partially, animal may appear to have luscious lips (Fig. 3B). Small shell pieces, coral rubble, sand and/or small stones adhere to exposed verrucae of distal most column end (Fig. 3C–D). When disturbed, animal does not retract completely, with tentacles and/or its tips remaining partially exposed (Fig. 3D). Cells of symbiotic dinoflagellates not observed in tissue.
Fig. 3.
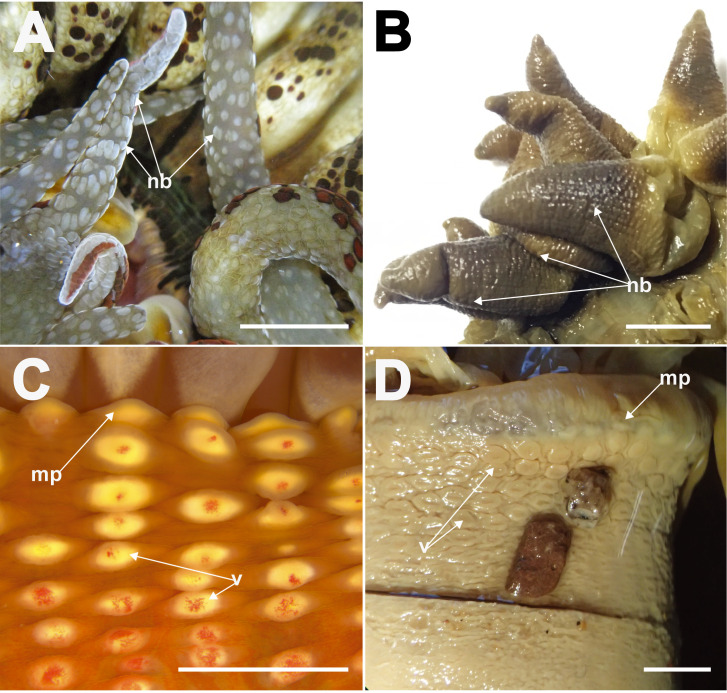
Living individuals of Macrodactyla aspera, external morphology. A, B, expanded individuals extending from the substratum, in situ, top view. Note splotchy brown patterns on oral disc and tentacles in both A and B, and partially everted actinopharynx resembling thick lips in B. Photographs by KS Loh. C, a live collected specimen (ZRC.CNI.1099), side view. Note reddish-orange column and adhesive, conspicuous verrucae, with grains of substratum attached to it. D, a partially retracted specimen (ZRC.CNI.1099), top view. Note that individual does not completely retract its tentacles; note also the presence of marginal projections. Abbreviations: mp, marginal projections; v, verrucae. Scale bars = 10 mm.
Tentacles: 48. Arranged in four cycles, one per endo-/exocoel. Outermost exocoelic; innermost cycles: endocoelic. Innermost cycle longer, greater or equal to length of oral disc radius; outermost shortest, 1/3 length of innermost (Fig. 3A). Covered entirely with visible nematocyst batteries (Figs. 3A–D, 4A, 4B). Tapering in life; may appear conical in fixed specimens due to contraction by fixative (Fig. 4B). Wide at base, narrows to point. Tip pointed-blunt, not perforated. In life, cream-colour with dark-brown splotches on oral side, batteries white or brown (Figs. 3A–D, 4A). Sticky; readily adheres to surfaces of containers. Does not abscise readily when disturbed. Preserved specimens coloured grey, brown to mauve, with intense brown (or dark mauve) cast near the tip (Fig. 4B).
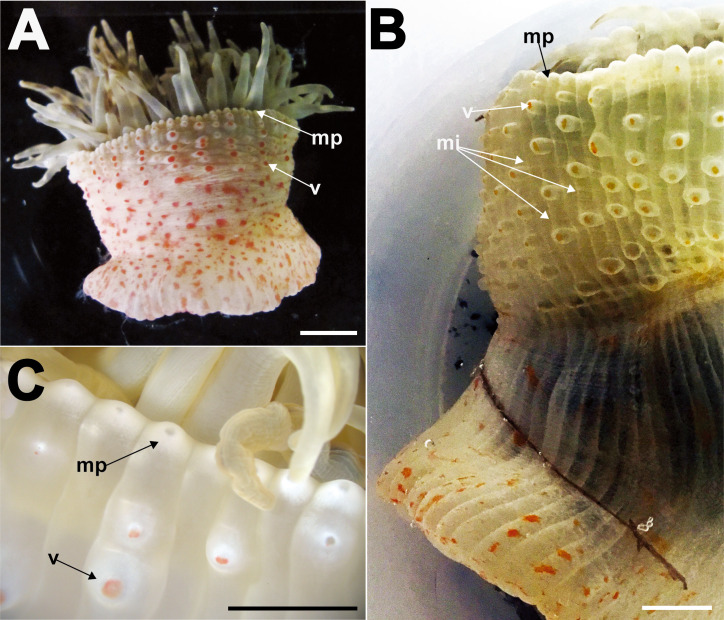
Fig. 4.
Close-up appearance of tentacles and distal most column of Macrodactyla aspera, external view. A, extended tentacles of a live specimen (ZRC. CNI.1080), covered densely with nematocyst batteries. B, tentacles of a fixed specimen (i.e., lectotype, MZC.I.33665). Note the overall stout, conical shape of tentacles once fixed, and the bumpy texture for each of them, indicating the presence of nematocyst batteries. C, marginal projections and adhesive verrucae of a live individual (ZRC.CNI.1080). D, marginal projections and adhesive verrucae of a fixed specimen (SMNHTAU-Co.7813). Note how the shallow fosse had inflated. Also note how the grains of substratum remained attached to the specimen, when it was collected by L Fishelson in 1968. Abbreviations: f, fosse; mp, marginal projections; nb, nematocyst batteries; v, verrucae. Scale bars = 10 mm.
Oral disc: Outline round, flat when fully expanded (Fig. 3A). Diameter up to 45 mm. Nematocyst batteries of tentacles may extend across disc, in some specimens all the way to mouth. Thin-walled; mesenterial insertions as radial lines seen through disc, extending from mouth to tentacles, in life these appear as faint brown lines (Fig. 3A, B), in preserved specimens as white lines. Oval central-mouth, flat, may be slightly raised in some. Actinopharynx often everted (Fig. 3B), sometimes obscuring entire animal. When alive, edge of mouth typically white in colour, with thick brown splotches radiating outwards, translucent when expanded. In preserved materials, edge of mouth with a brown cast.
Column: Thin-walled. Fosse present, very shallow in vivo; in preserved specimens fosse not distinct. When expanded, distal end flared outwards, overhangs slightly. Distal end with marginal projections, endocoelic (Fig. 3C, D). Longitudinal rows of conspicuous verrucae present, extending from distal end to mid-column, 6 to 13 per row; endocoelic (Fig. 3C, D). Verrucae oval, edges slightly raised and thickened, middle thin and translucent; in life, verrucae cream-white with a pink dot in the middle (Fig. 4C), colour is lost in preserved specimens (Fig. 4D). Verrucae adhesive, with small shell fragments, sand, small stones, and/or coral rubble often attached (Fig. 3C, D) even after preservation (Fig. 4D). Mesenterial insertions seen as light lines extending from the distal to proximal end. Remainder of column smooth, no other structures present (e.g., cinclides). In life, distal end brown, region from mid-column to proximal end bright orange; once preserved, specimen may be entirely cream-white, dark brown or grey.
Pedal disc: Overall oval in outline, flat; thin-walled. Mesenterial insertions seen through as white lines. Limbus slightly scalloped. In life, orange with splotches; once preserved entirely cream-coloured or greyish, with a dark-brown cast.
Internal morphology: 24 pairs of mesenteries regularly arrayed in three cycles (6 + 6 + 12), with two pairs of directives symmetrically opposite each other, each pair attached to a siphonoglyph. Mesenteries in primary and secondary cycles complete (i.e., 6 + 6 pairs); those in third cycle, incomplete (i.e., 12 pairs). All mesenteries extended through entire length of column. Mesenterial retractor muscles diffuse-circumscribed, well-developed (Fig. 5A). Parietobasilar muscles of first two cycles well-developed, with pennon (Fig. 5A). Marginal sphincter muscle conspicuous, diffuse-circumscribed. Oral and marginal stomata present (Fig. 5B), but in some specimens may be difficult to view (e.g., SMNHTAU-Co.12133). Parietobasilar muscle present but not easily discernible. Actinopharynx extends proximally slightly past mid-column, longitudinally pleated, pinkish translucent in live, cream-white when preserved (Fig. 5C).
Fig. 5.
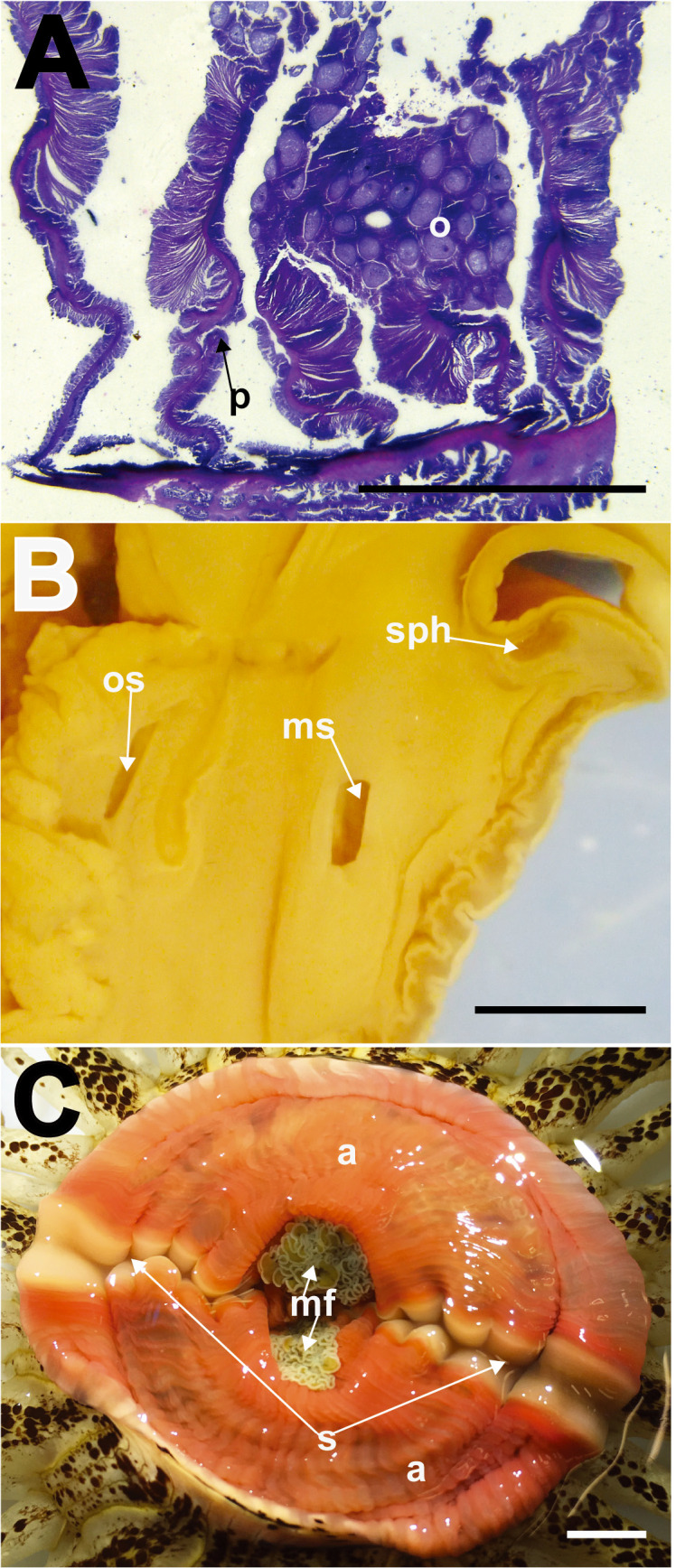
Macrodactyla aspera, internal morphology. A, mesenteries of the lectotype (MZC.I.3365), cross section at mid-column. Note the diffuse circumscribed appearance of the retractor muscles, and presence of the retractor pennon. B, transverse section of the distal most end of column (SMNHTAU-Co.7813). Note the presence of a conspicuous, restricted marginal sphincter muscle and the presence of both oral and marginal stomata. C, everted actinopharynx of a live specimen (ZRC.CNI.1090). Note its pinkish appearance, and the presence of a diametric pair of siphonoglyphs. Abbreviations: a, actinopharynx; mf, mesenterial filaments; ms, marginal stomata; o, oocytes; os, oral stomata; p, pennon; s, siphonoglyph; sph, marginal sphincter muscle. Scale bars = 5 mm.
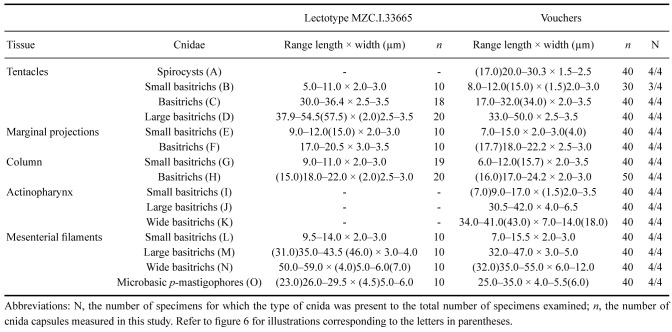
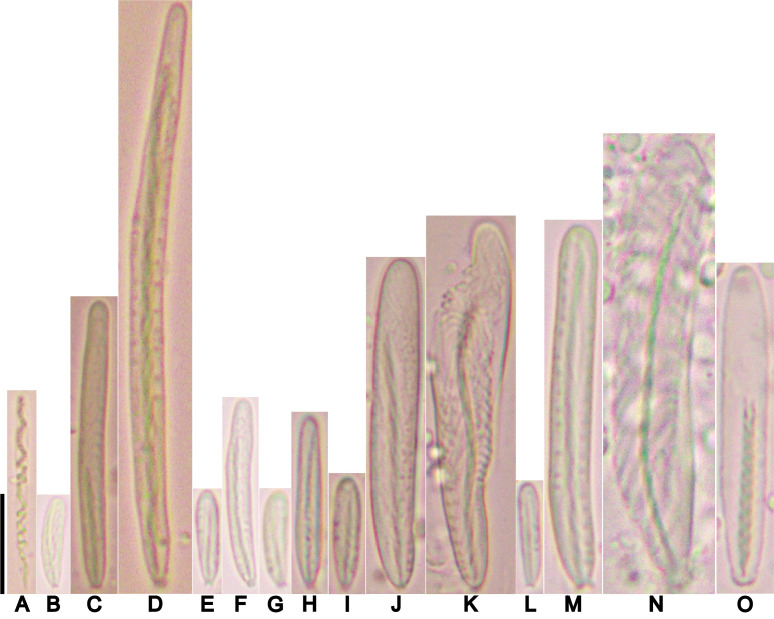
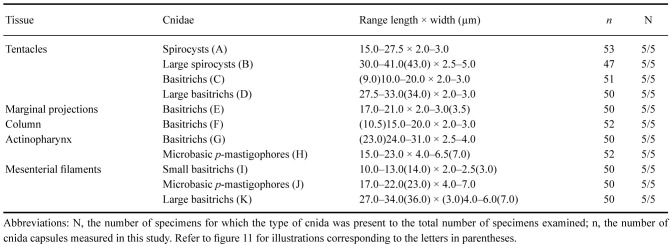
Cnidom: Spirocysts, basitrichs, and microbasic p-mastigophores. Sizes and distribution are shown in table 2; illustrations in figure 6.
Table 2.
Cnidom of Macrodactyla aspera (Haddon and Shackleton, 1893). Sizes of single capsules that fell out of the size range for the cnida type measured are indicated as numerical values in parentheses
Abbreviations: N, the number of specimens for which the type of cnida was present to the total number of specimens examined; n, the cnida capsules measured in this study. Refer to figure 6 for illustrations corresponding to the letters in parentheses.
Fig. 6.
Cnidae of Macrodactyla aspera. A, spirocyst. B, small basitrich. C, basitrich. D, large basitrich. E, small basitrich. F, basitrich. G, small basitrich. H, basitrich. I, small basitrich. J, large basitrich. K, wide basitrich. L, small basitrich. M, large basitrich. N, wide basitrich. O, microbasic p-mastigophore. Scale bar = 10 μm. Refer to table 2 for key to letters.
Distribution (Fig. S2): Type locality: Australia, Torres Straits, Mabuiag Reef (Haddon and Shackleton 1893; Haddon 1898). Published records: Israel, Red Sea, Gulf of Aqaba, Eilat (Fishelson 1970). New records: Australia, Indian Ocean, Perth (i.e., Cockburn Sound, North Fremantle, and Rowley Shoals); New Caledonia, Coral Sea, Noumea; Singapore, in the Straits of Johor (i.e., Beting Bronok, Changi region beaches, Pulau Sekudu) and Singapore Strait (i.e., Cyrene Reef).
Comparisons with other similar sea anemones: In having nematocyst batteries densely covering its tentacles, other discerning taxonomic features (e.g., adhesive verrucae) hidden in the sand, and in sharing a similar habitat, M. aspera have been misidentified as D. armata (e.g., WAM Z99100), or assumed to be a different morphotype of the latter (i.e., Rowlett 2020: 292, 293). However, the presence of longitudinal, adhesive verrucae rows is the obvious feature that distinguishes these two species. These rows are only present on the distal end of M. aspera, while they are absent in D. armata; the absence of verrucae is consistent with taxonomic accounts of D. armata and its holotype (Wassilieff 1908; Carlgren 1949). When alive, another trait that distinguishes D. armata from M. aspera is that the former readily autotomises its tentacles when disturbed, but those of the latter will remain intact.
Remarks: The animal that we have found closely matches the species and its type specimen that was collected and described by Haddon and Shackleton (1893). In all these specimens studied, we found that: i) their tentacles were densely covered with nematocyst batteries, ii) visible, adhesive verrucae were present at the distal end of the column, iii) cnidom data agreed between these specimens, and iv) as stated in our revised definition of the genus, the mesentery arrangement and number were also consistent.
Although the distinct tentacles of M. aspera (i.e., it being densely covered by nematocyst batteries) were not mentioned in its Haddon and Shackleton’s (1893) original description of the species, nor in the main text of Haddon’s (1898) expanded account, they were illustrated in the latter (see: Haddon 1898: Plate 22, Figs. 10 and 11). His depiction also provided a close-up view of a studded tentacle, clearly showing the nematocyst batteries. In examining the lectotype, Dunn (1981: 35) also found this feature to be obvious. The presence of nematocyst batteries densely covering each tentacle was also observed in fresh material collected, and in museum voucher specimens we have found and studied. Because the tentacles of M. aspera strongly resemble those of D. armata, many museum vouchers of the former species were identified and labelled as the latter (e.g., MNHN 1321, WAM 67). We have also examined specimens that Fishelson (1970) had collected (i.e., SMNHTAU-Co.7813 and SMNHTAU-Co.12133), and verified them to be M. aspera.
Like Haddon and Shackleton (1893), we found large visible, adhesive verrucae present on the distal end of the anemone, with shell fragments and small rocks often attached to them. While Haddon and Shackleton (1893: 124) also mentioned that the column of the anemone was also “covered with small, very adhesive suckers,” we failed to find any structural indication of this feature in both the lectotype and the voucher specimens.
The few published accounts pertaining to this species and its lectotype provided no detailed measurements of cnidom data (e.g., Haddon 1898; Fishelson 1970; Dunn 1981). We report these here for the first time. Broadly, measurements made on undischarged capsules extracted from tissues of the voucher material fell within the range of those obtained from the lectotype (Table 2). We were unable to assess cnidae of the lectotype’s actinopharynx as we failed to obtain a piece of tissue for it. Pertaining to the tentacle spirocysts of the lectotype, we found these to be degraded in the tissue, hindering measurements. Despite these two caveats, cnidae measurements of the lectotype and voucher materials agreed well.
Macrodactyla fautinae, sp. nov. Yap, Mitchell, Quek, and Huang
(Figs. 7–11)
urn:lsid:zoobank.org:act:DC99CC5F-0956-47A9-B0E9-A0B3206F5ADE
Material examined: Holotype: ZRC.CNI.1300 (Fig. 7), collected from the beach off Changi Beach (near Carpark 7), Singapore, on 5 July 2016 by the staff of LKCNHM. An individual of unknown gender, cut longitudinally into two equal pieces. Tissue from the pedal disc sampled for genetic work (Table S1).
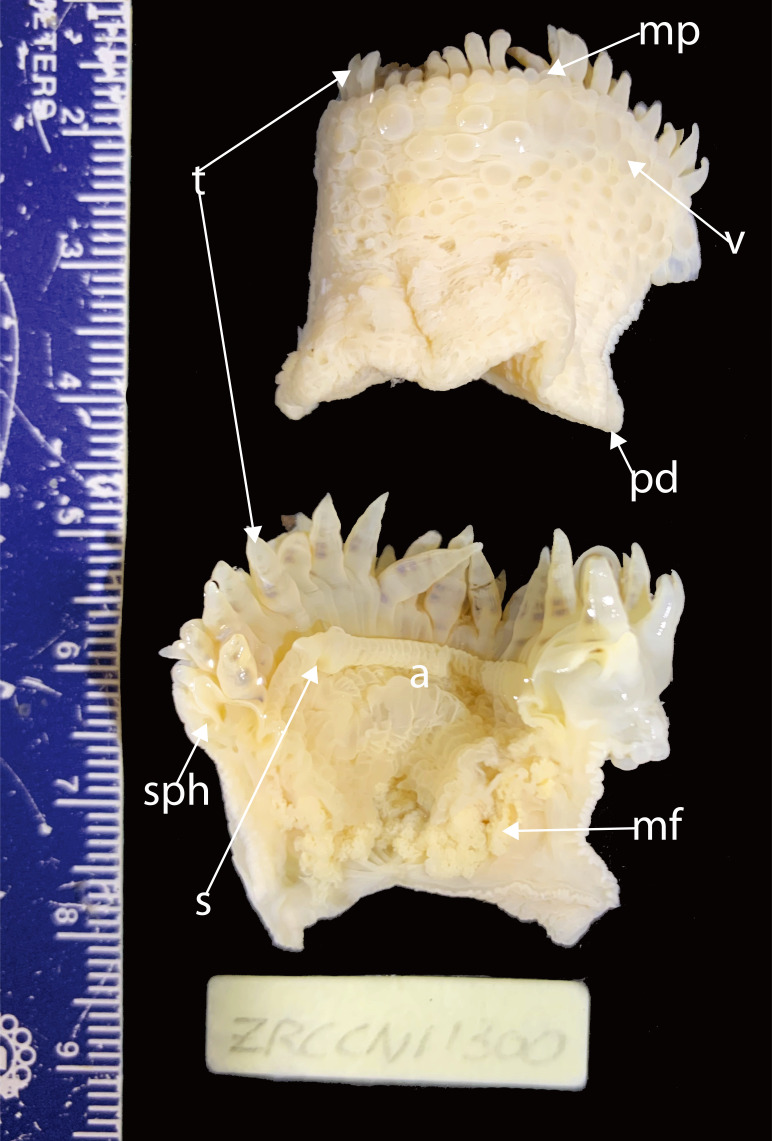
Fig. 7.
Holotype of Macrodactyla fautinae sp. nov. (ZRC.CNI.1300). Abbreviations: a, actinopharynx; m, mesenterial filaments; mp, marginal projections; pd, pedal disc; s, siphonoglyph; sph, sphincter muscle; t, tentacles; v, verrucae.
Paratypes: ZRC.CNI.1156, collected from Changi Beach (near Carpark 4) (Fig. S1), Singapore, on 4 August 2016 by NWL Yap. Tissue sampled from its pedal disc for genetic work (Table S1). ZRC.CNI.0296, collected from Changi Point Beach on 22 July 2009 by DG Fautin et al. A dissected female from which 10 microscope slides with histological sections of the animal were prepared.
Material examined from Singapore (Fig. S1) (*-observed alive): Changi Beach (ZRC.CNI.1020 x5*; ZRC.CNI.1156 x1*; ZRC.CNI.1159 x1*; ZRC. CNI.1300 x1*), Changi Point beaches (ZRC.CNI.0022 x1; ZRC.CNI.0025 x1; ZRC.CNI.00296 x1; ZRC. CNI.00297 x1), Changi Point SAF Chalet beach (ZRC. CNI.0558 x1*; ZRC.CNI.0559 x1*; ZRC.CNI.0566 x1*; ZRC.CNI.0598 x1*; ZRC.CNI.0648 x3*), Changi Point Ferry Terminal beach (ZRC.CNI.0023 x1; ZRC. CNI.0024 x1), Chek Jawa (ZRC.CNI.1370 x1*), Pasir Ris (ZRC.CNI.00254 x1; ZRC.CNI.00255 x1), Pulau Ubin, OBS Camp beach (ZRC.CNI.0788 x1*), Pulau Sekudu (ZRC.CNI.0026 x1).
Etymology: The name Macrodactyla fautinae sp. nov. honours the late Emeritus Professor Daphne Gail Fautin. Throughout her career, she had worked tirelessly to advance the knowledge of sea anemones.
Description: Natural history: Occurs as solitary individuals, at the middle intertidal in soft silty, sandy areas, and also found in seagrass meadows (Fig. 8A–E). Animal partially buried in substratum, distal end of animal exposed during the low tides, with small shell pieces, sand and/or rocks adhering to verrucae (Fig. 8C). Oral disc and tentacles typically expanded, with actinopharynx often everted and inflated outwards, in many instances obscuring the animal (Fig. 8D). This species has also been observed to swallow its prey whole (Fig. 8E). When disturbed, animal does not retract completely, tips of tentacles remaining partially exposed (Fig. 8C). In the process of contraction, water may also be expelled from verrucae-like structures, akin to a watering can.
Fig. 8.
Living individuals of Macrodactyla fautinae sp. nov., external morphology, in situ, top view. A, an expanded individual with a pale oral disc. B, another expanded colour morph with a dark-brown oral disc. Note that in both A and B, a pair of diametric bright pink dots marks the position of the siphonoglyphs. C, a contracted individual with adhesive, papillae-like verrucae at its distal end. Note that shell fragments are attached to the verrucae. D, an individual with much of its actinopharynx everted, obscuring the animal. E, a sea pen (Pteroeides sp.) being swallowed whole by a M. fautinae sp. nov. individual. Abbreviation: s, siphonoglyph; v, verrucae. Photographs by R Tan.
Tentacles: 96. Arranged in five cycles, one per endo-/exocoel. Outermost exocoelic; innermost cycles, endocoelic. Innermost cycles longer and of similar length, greater or equal to length of oral disc radius; outermost shortest, 1⁄2 length of innermost cycles (Fig. 8A, B). All alike in appearance; simple, smooth without nematocyst batteries, tapering to a blunt end in life; in preservative all appeared similarly sized, short, conical with a blunt end, possibly due to contraction in fixative. Colour in life translucent with horizontal bands of dark brown and white bands (Fig. 8A, B); in fixative, colours retained in freshly preserved specimens (i.e., < two years) but are expected to be lost over time, to become translucent cream in colour.
Oral disc: Outline round, flat when expanded (Fig. 8A, B). Diameter up to 80 mm. Smooth and thin-walled, mesenterial insertions may be seen as dark radial lines extending from mouth to disc margin, more prominent in individuals with colourless disc or in fixed specimens (e.g., Fig. 8A). In life, colour and pattern variable among individuals: may be completely colourless, greenish-grey, entirely dark-brown, or brown with white markings or vice-versa, sometimes accompanied by markings of cross bands being V-or W-shaped (seen typically larger individuals; see also Fig. 8A, B); disc in fixative all appear translucent cream-coloured. Central mouth lipless and oval in outline, with actinopharynx everted to some extent (Fig. 8C, D). Two symmetric siphonoglyphs, each marked by bright pink dot in live individuals (Fig. 8A, B); pink dot may be visible in freshly preserved specimens but fades over time.
Column: In life, translucent cream-coloured, with reddish-orange splotches present on lower end (Fig. 9A); once fixed, colour may be retained in specimens, but fades over time. Fosse present, shallow to deep. Thin-walled, mesenterial insertion visible through wall as longitudinal light lines, extending from distal most to proximal end (Fig. 9B); visibility most prominent when animal is expanded and/or relaxed, may be obscured when animal is slightly contracted (Fig. 9A). Distal end with marginal projections, perforated, both endo-/exocoelic (Fig. 9C). Conspicuous adhesive, verrucae present, endocoelic, arranged in vertical rows that extend proximally from the distal end to mid-column, five to seven per row. Verrucae raised, outline round, with a pink dot in the middle (Fig. 9B, C), perforated. When extended, verrucae papillae-like (Fig. 9B). In fixed specimens, verrucae contracted but still prominent. From mid-column to proximal end, without any obvious structures (e.g., cinclides, etc.); smooth.
Fig. 9.
Column appearance of Macrodactyla fautinae sp. nov., external morphology, side view. A, a freshly collected individual that is slightly contracted (ZRC.CNI.0648). Note the pale pinkish column and the presence of splotchy dark pink patches from mid-column to the proximal end. Note also that the mesenterial insertions are not visible when the anemone is in a contracted stated. B, a live individual (ZRC.CNI.1159) with a column that is expanded, with mesenterial insertions that extend the entire column visible as light lines. Note also the extended papillae-like verrucae. C, close-up of the marginal projections and verrucae at the distal end of a live individual (ZRC.CNI.1159). Note that marginal projections are perforated, and a pink dot marks the middle of each verruca. Abbreviations: mi, mesenterial insertions; mp, marginal projections; v, verrucae. Scale bars = 10 mm.
Pedal disc: Outline irregular oval in outline, following contours of surface it clings onto. Thin-walled; mesenterial insertions visible through it as radiating while lines. Scattered reddish-orange splotches present on base. Adheres readily to surfaces.
Internal morphology: 48 pairs of regularly arrayed mesenteries arranged in four cycles (6 + 6 + 12 + 24); all extend the entire length of column. Two pairs of diametric directives, each pair attached to a siphonoglyph. Mesenteries in first two cycles complete, those in third and fourth incomplete. Gametogenic tissues on all mesenteries, if present. Sexes separate. Mesenterial retractor muscles well-developed, diffuse-circumscript (Fig. 10A); mesenteries of lower orders more circumscribed than those higher. Parietobasilar well-developed; free pennon associated with mesenteries of lower orders (Fig. 10A). Oral and marginal stomata present, however former may be difficult to visualise in some individuals (e.g., ZRC.CNI.0566). Marginal sphincter muscle conspicuous and circumscribed, close to fosse (Fig. 10B). Parietobasilar muscle present but not easily discernible. Actinopharynx longitudinally pleated and thin-walled, translucent-white in life, cream-coloured in preserved specimens; extends proximally towards pedal end, slightly pass mid-column.
Fig. 10.
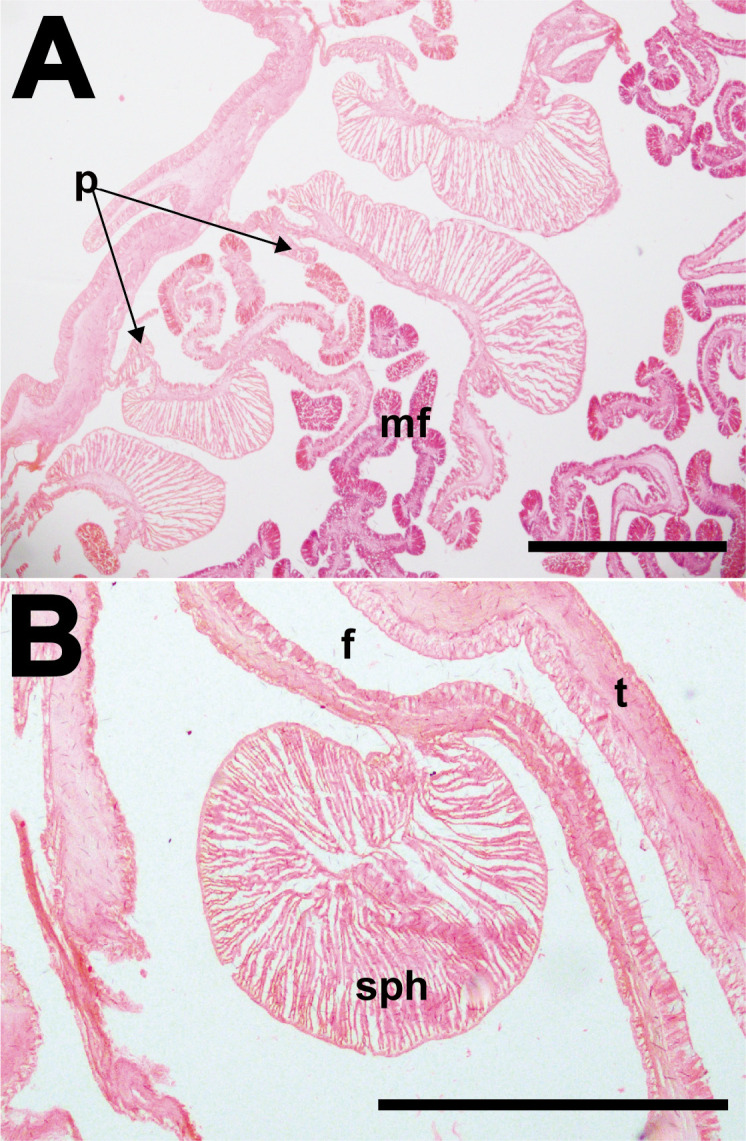
Macrodactyla fautinae sp. nov., internal morphology. A, diffuse-circumscript mesenteries of an individual (ZRC.CNI.0296), cross section at mid-column. Note the presence of the retractor pennon. B, a conspicuous, circumscribed marginal sphincter muscle of another individual (ZRC.CNI.0255). Abbreviations: f, fosse; mf, mesenterial filaments; p, pennon; sph, marginal sphincter muscle; t, tentacles. Scale bars = 1 mm.
Cnidom: Spirocysts, basitrichs, and microbasic p-mastigophores. Sizes and distribution are shown in table 3; illustrations in figure 11.
Table 3.
Cnidom of Macrodactyla fautinae sp. nov. Sizes of single capsules that fell out of the size range for the cnida type measured are denoted as numerical values in parentheses
Abbreviations: N, the number of specimens for which the type of cnida was present to the total number of specimens examined; n, the number of cnida capsules measured in this study. Refer to figure 11 for illustrations corresponding to the letters in parentheses.
Fig. 11.
Cnidae of Macrodactyla fautinae sp. nov. A, spirocyst. B, large spirocyst. C, basitrich. D, large basitrich. E, basitrich. F, basitrich. G, basitrich. H, microbasic p-mastigophore. I, small basitrich. J, microbasic p-mastigophore. K, large basitrich. Scale bar = 10 μm. Refer to table 3 for key to letters.
Distribution: This species has so far been encountered only along the northern shoreline of Singapore, in the Straits of Johor (Fig. S1).
Comparisons with M. aspera: The most obvious feature that distinguishes these two species is the texture of the tentacles of their type species. Those of M. aspera appear rough to the eye, as they are densely covered with nematocyst batteries, while the tentacles of M. fautinae sp. nov. are smooth. We have not encountered any individuals of M. fautinae sp. nov. that bear nematocyst batteries on their tentacles.
Oral disc appearance also differed between the two type species. As with the tentacles, nematocyst batteries are often on the oral disc of M. aspera, while M. fautinae sp. nov. lacks this feature. In many of the examined live M. aspera individuals, the oral disc is often marked by dark brown splotches, whereas those of M. fautinae sp. nov. may vary in appearance, and markings –if present –are typically cross bands that are V-or W-shaped. Consistently in all individuals we have encountered and collected, bright pink dots demarcate the siphonoglyphs of M. fautinae sp. nov., a feature not observed on the oral disc of live M. aspera individuals examined (see Figs. 3A, B; 8A, B).
The appearance of the column also differed consistently between the two species. In general, colour may not be a useful feature when distinguishing between sea anemone species (Stephenson 1928: 69), however, we found this feature to be consistent as a whole in all individuals of both species studied. Macrodactyla aspera has a dark-coloured distal-end and the remainder of its column being bright orange, whereas that of M. fautinae sp. nov. is entirely cream-white, with pink splotches. Columnar structures also differ between both species. While verrucae found in these two species are adhesive, those of M. fautinae sp. nov. are lobe-like when extended, and expels jets of water when the animal contracts, a feature not observed in M. aspera.
Ecological remarks: Both M. fautinae sp. nov. and M. aspera are found to occur in similar habitats along Singapore’s northern coastline, in the Straits of Johor. However, while we have found the latter to also occur in intertidal areas of Singapore’s southern shoreline (i.e., in the Singapore Strait), we have only encountered M. fautinae sp. nov. on the northern shoreline and in no other southern locations that bear a similar habitat (Fig. S1). Apart from sharing similar habitat types, both species also exhibit a curious behaviour of everting an extensive part of the actinopharynx when exposed during the low tide, obscuring most of the animal. The exact rationale of this behaviour is unclear. Inferring from the presence of nematocysts present in the tissue of the actinopharynx and the extent of the eversion, we hypothesize that this behaviour aids in the capture of larger prey, as we have observed (Fig. 8E). To test this hypothesis, further experimental studies involving prey size and measuring the extent of actinopharynx eversion may be conducted.
Similar species: Macrodactyla fautinae sp. nov. may be confused with three other similar looking species that also occur at its type locality, Singapore: Anthopleura buddemeieri Fautin, 2005, An. handi Dunn, 1978, and Paracondylactis singaporensis (England 1987).
While both M. fautinae sp. nov. and An. buddemeieri have a column patterned with splotches of red, with both species having adhesive verrucae on its distal most end, these two species occupy different habitats. Macrodactyla fautinae sp. nov. tends to occur in silty, sandy areas, among seagrasses meadows of the middle intertidal zones, while An. buddemeieri occurs underneath rocks in the upper intertidal zone (Fautin 2005; Fautin et al. 2009). While the size of a sea anemone may not be a good character to distinguish species (Stephenson 1922; Fautin et al. 2008), we have consistently found that adult individuals of M. fautinae sp. nov. are much larger than An. buddemereri (e.g., oral disc diameter: 80 mm versus 7 mm, respectively; see Fautin (2005) for size of An. buddemeieri).
In occupying the same habitat types (i.e., silty and sandy substratum), and having translucent, patterned tentacles, M. fautinae sp. nov. may be mistaken for either An. handi, or P. singaporensis in the field. However, M. fautinae sp. nov. can be immediately distinguished from these other two species by using the following features: 1) a pair of conspicuous pink dots, each marking a siphonoglyph (Fig. 8A, B) –a feature that is absent in both An. handi and P. singaporensis; 2) a light coloured column with bright pink splotches, which differs from the consistently dull and darkly-coloured columns of An. handi and P. singaporensis (England 1987; Fautin et al. 2009; Fautin and Tan 2016); and 3) differing ecology, as M. fautinae sp. nov. does not burrow deeply into the substratum with its column completely buried as P. singaporensis does (England 1987; Fautin and Tan 2016); nor, does it occur in dense clusters like An. handi, (Dunn 1978; Fautin et al. 2009) but rather often occurs singly. For these reasons, M. fautinae sp. nov. cannot be confused for the other two species.
DISCUSSION
Phylogeny of Macrodactyla and D. armata
In this study, we tested the relationship between M. aspera and D. armata, due to their strong resemblance to each other in having tentacles densely covered with visible nematocyst batteries. Our phylogeny reconstruction did not recover M. aspera and D. armata as a clade. Instead, both formed two distinct groups: M. aspera and the new species, M. fautinae sp. nov., were recovered as a clade, while D. armata was closely related to the actinodendrids (i.e., A. arboreum and Ac. haeckeli; Fig. 1).
Morphological and ecological evidence supported our findings that Macrodactyla species and D. armata belong to two distinct groups. Anatomically, D. armata lacks adhesive verrucae on its column, while these are present on M. aspera and M. fautinae sp. nov. In both Macrodactyla species, the sphincter muscle is circumscribed in form, while this musculature is diffused in D. armata (Carlgren 1949). Evidence supporting the close phylogenetic relationship of both macrodactylid species (i.e., M. aspera and M. fautinae sp. nov.) have been discussed earlier in this study (see genus entry of Macrodactyla, above).
Together with our molecular findings and some evidence present in published taxonomic accounts of the Actinodendridae species targeted here, these strongly suggest that D. armata may be actually more closely related to actinodendrids than to Actiniidae, the family in which Dofleinia is currently placed. Our proposition here agrees with Rowlett (2020: 292), who also suggested the close relatedness of Dofleinia to actinodendrids, based on similarities in the ecology and morphology of these animals.
Ecologically, like D. armata, actinodendrids are also well-known to be able to inflict painful stings upon contact; they are popularly referred to as ‘Hell’s Fire Anemones’ (Ardelean and Fautin 2004; Erhardt and Knop 2005; Fautin et al. 2009). Both D. armata and actinodendrids are hosts to anemone shrimps (Bruce 1969 2005; Fransen 1989). Additionally, Rowlett (2020: 292) notes that these anemones exhibit, “burrowing behaviour.”
Broad morphological similarities are shared between D. armata and these actinodendrids, particularly: i) in having a smooth column without any visible structures (e.g., adhesive verrucae, etc.) (Carlgren 1949), and ii) in bearing regions of dense nematocyst clusters along their tentacles (for a brief list of other morphological similarities, see Rowlett 2020: 292). Pertaining to i), while assuming M. aspera to be a morphological variant of D. armata, Rowlett (2020: 292) posited that the presence of verrucae is a diagnostic trait of Dofleinia, despite conceding that this trait was not found on the holotype of its type species (“Dofleinia... having... adhesive verrucae... strangely absent from many specimens (including the type)”). In light of our molecular findings, and the possibility of Dofleinia being related to actinodendrid anemones, we propose that the presence of adhesive verrucae is not a diagnostic trait of Dofleinia. Regarding ii), we note that this feature does differ in appearance between D. armata and the actinodendrids (for an example of acrospheres of an actinodendrid, see Ardelean and Fautin 2004: 192, Fig. 3). Rowlett (2020: 292) suggested that this feature may be homologous between these anemones. At present, the taxonomy of Actiniidae is in chaos and taxa within this family have been found to be polyphyletic (Daly et al. 2017). To determine if D. armata is truly closely related or belongs to the family Actinodendridae will require further thorough taxonomic and genetic work that is beyond the scope of this present study.
Macrodactyla aspera as a potential threat to public safety
In bearing visible nematocyst batteries, and having large nematocysts in its tentacles (i.e., undischarged capsules of length > 50 μm), M. aspera may be capable of delivering a painful sting on contact. Dolfeinia armata is another, similar example: it bears visible nematocyst batteries on its tentacles, and have basitrichs that are > 50 μm (e.g., up to 76 μm; see Carlgren 1945). Other sea anemones that have been reported to be a threat to public health, such as Phyllodiscus semoni and members of Actinodendridae also bear specialised structures along their tentacles that harbour a dense reservoir of nematocysts (e.g., nematocyst batteries) and cnidae larger in size (for sizes, see Carlgren 1945; Fautin et al. 2009; Hoeksema and Crowther 2011). Following this line of logic, we suggest that sea anemone species that may inflict painful stings are ones that usually bear specialised structures on their tentacles, and/or have large nematocysts; M. aspera may also be a potential threat to humans. This requires further investigation and comparisons with other species that are not deemed to be a threat but also bear large nematocysts and specialised structures on their tentacles (e.g., Haloclava hercules Izumi, 2021).
Importance of re-examining name-bearing type specimens
In this study, we re-described M. aspera using new morphological data (i.e., living appearance, cnidom) gathered not only from fresh and voucher specimens, but also from the name-bearing specimen of M. aspera [= C. aspera]. This was necessary to ascertain that established diagnostic characters reported for Macrodactyla (e.g., Stephenson 1921 1922; Carlgren 1949; Dunn 1981) were truly definitive of the genus. Here, we found that the complete mesentery numbers and arrangement present in the lectotype differ from previous accounts that were published. Our findings were not unexpected; long-accepted diagnostic characters of a taxon are often disputed when new observations arise from a careful re-examination of name-bearing types. Thorough treatment involving a comprehensive study of name-bearing types will be needed to resolve the taxonomic challenges for many groups of sea anemones. Members of the superfamily Actinioidea are taxonomically unresolved (Rodríguez et al. 2014; Titus et al. 2019) and will benefit from this detailed approach. Recent efforts have demonstrated such an approach is feasible and fruitful (e.g., Fautin and Tan 2016; Yap et al. 2019 2021).
CONCLUSIONS
In this study, morphological and molecular evidence were integrated to re-diagnose the genus Macrodactyla and redescribe M. aspera. Furthermore, we provided historical, nomenclatural evidence to support the continued usage of the genus name. Since its first description by Haddon and Shackleton (1893), there have been very few published accounts of M. aspera, despite its wide geographical range across the Indo-West Pacific. The reason for this, we suspect, is that this species may have been misidentified as D. armata, due to its strong superficial resemblance. Museum vouchers we have examined support this assertion; some were indeed misidentified as D. armata. Herein, we provided molecular evidence to demonstrate that these two species are distantly related.
Findings from this study add to a growing number of sea anemone species recorded from Singapore. Previous studies have already documented 37 species to occur on the shores and islands of this city-state (e.g., England 1987; Fautin et al. 2009; Yap et al. 2021). Affirming the identities of these sea anemones primes new avenues where more Indo-West Pacific species may be included in future research.
Supplementary materials
Sea anemones species included in our phylogenetic analyses. Information on voucher specimens and associated museum catalogue numbers are provided here, if available. Dashes denote that such information is absent. Accession numbers in bold denotes new sequences to this study. Abbreviations: AMNH, American Museum of Natural History, New York; CAS, California Academy of Sciences, San Francisco; Cat. nos., Catalogue numbers; KUNHM, University of Kansas Natural History Museum; ZRC, Zoological Records Collection, Lee Kong Chian Natural History Museum, Singapore.
Map of Singapore where specimens of both Macrodactyla aspera (Haddon & Shackleton, 1893) and Macrodactyla fautinae sp. nov. were collected for this study: 1, Pulau Ubin OBS Camp (1°25'07.7"N, 103°55'44.3"E); 2, Pasir Ris (1°22'56.45"N, 103°57'3.88"E); 3, Changi Region beaches [this area encompass the following localities that are in very close proximity to each other: Changi beach, Changi beach near Carpark 4; Changi Point beaches; Changi Point SAF Chalet beach; Changi Point Ferry Terminal beach] (1°23'33.34"N, 103°59'20.94"E); 4, Pulau Sekudu (1°24'19"N, 103°59'17"E); 5, Chek Jawa (1°24'25"N, 103°59'23"E); 6, Beting Bronok (1°26'13.00"N, 104°02'58.00"E); 7, Changi beach near Carpark 7 (1°22'26.03"N, 104°0'24.79"E); 8, Changi East next to Changi water Reclamation Plant (1°18'45"N, 104°00'31"E); 9, Cyrene Reef (Terumbu Pandan) (1°15'28"N, 103°45'19"E). White circles represent M. aspera, while stars (both black and white) denote the occurrences of M. fautinae sp. nov., with the single black star indicating the type locality of this new species. Numbers not accompanied by any symbols indicate the presence of both species occurring at the same site. See main-text for full details.
Distribution of Macrodactyla aspera (Haddon & Shackleton, 1893) and Macrodactyla fautinae sp. nov. 1. Murray Islands, Torres Straits. 2. Noumea, New Caledonia. 3. Perth, Western Australia. 4. Singapore. 5. Eilat, Israel. Symbols beside each number represent the occurrence of the sea anemones: circles being M. aspera, stars represents M. fautinae. Symbols that are coloured black denote the type locality of the species they represent. See main-text for full details.
Maximum likelihood phylogram (RAxML) of concatenated dataset (i.e., 12S, 16S, 28S and cox3). Bootstrap resampling values under ML, and posterior probability values of Bayesian inference (BI), are indicated at the branches as ML/BI. Only bootstrap values > 50 and posterior probability > 0.8 are shown, values less than these limits are denoted by a dash (-).
Maximum likelihood phylogram (RAxML) of 12S sequences. Bootstrap values (≥ 50) are indicated on branches.
Maximum likelihood phylogram (RAxML) of 16S sequences. Bootstrap values (≥ 50) are indicated on branches.
Maximum likelihood phylogram (RAxML) of 28S sequences. Bootstrap values (≥ 50) are indicated on branches.
Maximum likelihood phylogram (RAxML) of cox3 sequences. Bootstrap values (≥ 50) are indicated on branches.
Acknowledgments
This work and the new species name were registered with ZooBank under urn:lsid:zoobank.org:pub:560079AC-51E0-448F-8AAE-449058A9CBF8. We thank the efforts of Singapore’s ‘Anemone Army’; many of the specimens featured in this study were found or collected by these citizen scientists. Collections of sea anemones were also made during the Comprehensive Marine Biodiversity Survey (CMBS, 2010–2015) and associated workshops led by the National Parks Board Singapore (NParks) and National University of Singapore (NUS). The CMBS workshops were supported by generous contributions from Asia Pacific Breweries Singapore, Care-for-Nature Trust Fund, Keppel Care Foundation, Shell Companies in Singapore and The Air Liquide Group. We also thank the following institutions that have granted us permission to examine type material and voucher specimens: Lee Kong Chian Natural History Museum, National University of Singapore, Singapore (Singapore); Muséum National d’Histoire Naturelle, Paris (France); University Museum of Zoology, Cambridge University, Cambridge (UK); Museum of Zoology, Lund University, Lund (Sweden); Steinhardt Museum of Natural History, Tel Aviv University, Tel Aviv (Israel); and Western Australian Museum, Perth (Australia). We are grateful to Mr Lok Kok Sheng for permission to use his photograph in this publication. We thank Professor Peter Ng Kee Lin for his advice on nomenclatural matters on the new species. We also thank both reviewers of this manuscript for providing constructive feedback and spotting typographical errors. Computational work for this article was partially performed on resources of the National Supercomputing Centre, Singapore (https://www.nscc.sg). The St John’s Island National Marine Laboratory provided the facilities necessary for conducting this study. This Laboratory is a National Research Infrastructure under the National Research Foundation (NRF), Prime Minister’s Office, Singapore. This research was also supported by the NRF, under its Marine Science R&D Programme (MSRDP-P03 and MSRDP-P38).
Footnotes
Authors’ contributions: NWLY collected fresh specimens, performed morphological comparisons on museum types and vouchers, extracted, sequenced and analysed genetic data, drafted and formatted this manuscript. MLM examined additional WAM specimens, provided 28S sequences for the initial phylogeny analyses and drafted the manuscript. ZBRQ sequenced and analysed the genetic data and had drafted the manuscript. RT collected fresh specimens, photographed them and provided habitat data on these animals. KST had guided NWLY on this study, providing inputs to descriptions and nomenclature matters, and had drafted the manuscript. DH had guided NWLY on this study, analysed the genetic data and drafted the manuscript.
Competing interests: The authors declare that they have no conflict of interest.
Availability of data and materials: Specimens documented are kept in natural history museums as stated in the paper, genetic sequences and supplementary materials were deposited into GenBank and Zenodo respectively.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Ardelean A, Fautin DG. 2004. Variability in nematocysts from a single individual of the sea anemone Actinodendron arboreum (Cnidaria: Anthozoa: Actiniaria). Hydrobiologia 530/531:189–197. doi:10.1007/s10750-004-2662-8.
- Blatchely WS. 1910. An illustrated descriptive catalogue of the Coleoptera or Beetles (exclusive of the Rhynchophora) known to occur in Indiana. With bibliography and descriptions of new species. The Nature Publishing Company, Indianapolis, Indiana. doi:10.5962/bhl.title.56580.
- Bruce AJ. 1969. Preliminary descriptions of sixteen new species of the genus Periclimenes Costa, 1844 (Crustacea, Decapoda Natantia, Pontoniinae). Zool Med Leiden 43(20):253–278.
- Bruce AJ. 2005. Pontoniine shrimps from Papua New Guinea, with designation of two new genera, Cainonia and Colemonia (Crustacea: Decapoda: Palaemonidae). Mem Queensl Mus 51(2):333–383.
- Carlgren O. 1893. Studien über nordische Actinien. K Svenska Vet Akad Hand 25:1–148. doi:10.5962/bhl.title.11612.
- Carlgren O. 1945. Further contributions to a revision of the Actiniaria and Corallimorpharia. Ark Zool 17:1–17.
- Carlgren O. 1949. A survey of the Ptychodactiaria, Corallimorpharia and Actiniaria. K Svenska Vet Akad Hand 1:1–121.
- Chen CA, Wallace CC, Wolstenholme JA. 2002. Analysis of the mitochondrial 12S rRNA gene supports a two-clade hypothesis of the evolutionary history of scleractinian corals. Mol Phylogenet Evol 23:137–149. doi:10.1016/S1055-7903(02)00008-8. [DOI] [PubMed]
- Chen CA, Yu JK. 2000. Universal primers for amplification of mitochondrial small subunit ribosomal RNA-encoding gene in the scleractinian corals. Mar Biotechnol 2:146–153. doi:10.1007/s101269900018. [DOI] [PubMed]
- Coleman N. 1999. Dangerous sea creatures: Aquatic survival guide. Neville Coleman’s Underwater Geographic Pte Ltd, Springwood, Queensland, Australia.
- Darriba D, Posada D, Kozlov AM, Stamatakis A, Morel B, Flouri T. 2020. ModelTest-NG: a new and scalable tool for the selection of DNA and protein evolutionary models. Mol Biol Evol 37(1):291–294. doi:10.1093/molbev/msz189. [DOI] [PMC free article] [PubMed]
- Daly M. 2003. The anatomy, terminology, and homology of acrorhagi and pseudoacrorhagi in sea anemones. Zool Verh Leiden 345:89–101.
- Daly M, Crowley LM, Larson P, Rodríguez E, Heestand Saucier E, Fautin DG. 2017. Anthopleura and the phylogeny of Actinioidea (Cnidaria: Anthozoa: Actiniaria). Org Divers Evol 17:545–564. doi:10.1007/s13127-017-0326-6.
- Dunn DF. 1974. Radianthus paillosa (Coelenterata, Actiniaria) redescribed from Hawaii. Pac Sci 28:171–179.
- Dunn DF. 1978. Anthopleura handi n. sp. (Coelenterata, Actiniaria), an internally brooding, intertidal sea anemone from Malaysia. Wasmann J Biol 35:54–64.
- Dunn DF. 1981. The clownfish sea anemones: Stichodactylidae (Coelenterata: Actiniaria) and other sea anemones symbiotic with pomacentrid fishes. Trans Am Philos Soc 71:1–115. doi:10.2307/1006382.
- England KW. 1987. Certain Actiniaria (Cnidaria, Anthozoa) from the Red Sea and tropical Indo-Pacific Ocean. Bull Brit Mus Nat Hist 53:205–292.
- Erhardt H, Knop D. 2005. Corals. Indo-Pacific field guide. IKAN Unterwasserarchiv, Frankfurt.
- Fautin DG. 1988. Importance of nematocysts to actinian taxonomy. In: Hessinger DA, Lenhoff HM (eds) The Biology of Nematocysts. Academic Press Inc, San Diego. doi:10.1016/B978-0-12-345320-4.50030-4.
- Fautin DG. 2005. Three species of intertidal sea anemones (Anthozoa: Actiniidae) from the tropical Pacific: Description of Anthopleura buddemeirei, n. sp., with remarks on Anthopleura asiatica and Gyractis sesere. Pac Sci 59:379–391. doi:10.1353/psc.2005.0035.
- Fautin DG. 2009. Structural diversity, systematics, and evolution of cnidae. Toxicon 54:1054–1064. doi:10.1016/j.toxicon.2009.02. 024. [DOI] [PubMed]
- Fautin DG, Allen GR. 1992. Anemone fishes and their host sea anemones: a guide for aquarists and divers. Western Australian Museum, Francis Street, Perth.
- Fautin DG, Crowther AL, Wallace CC. 2008. Sea anemones (Cnidaria: Anthozoa: Actiniaria) of Moreton Bay. [Proceedings of the Thirteenth International Marine Biological Workshop: The Marine Fauna and Flora of Moreton Bay, Queensland, P. J. F Davie and J. A. Phillips, editors] Memoirs of the Queensland Museum -Nature 54(1):35–64.
- Fautin DG. 2016. Catalog to families, genera, and species of orders Actiniaria and Corallimorpharia (Cnidaria: Anthozoa). Zootaxa 4145:1–449. doi:10.11646/zootaxa.4145.1.1. [DOI] [PubMed]
- Fautin DG, Tan R. 2016. Sea anemones (Cnidaria: Actiniaria) of Singapore: redescriptions of Paracondylactis singaporensis (England, 1987) and P. hertwigi (Wassilieff, 1908). Raffles Bull Zool Suppl 34:170–177.
- Fautin DG, Tan R, Yap NWL, Tan SH, Crowther A, Goodwill R, Sanpanich K, Tay YC. 2015. Sea anemones (Cnidaria, Actiniaria) of Singapore: shallow-water species known also from the Indian subcontinent. Raffles Bull Zool Suppl 31:44–59.
- Fautin DG, Tan SH, Tan R. 2009. Sea anemones (Cnidaria: Actiniaria) of Singapore: abundant and well-known shallow-water species. Raffles Bull Zool Suppl 22:121–143.
- Fautin DG, Zelechuk T, Raveendran D. 2007. Genera of orders Actiniaria and Corallimorpharia (Cnidaria, Anthozoa, Hexacorallia), and their type species. Zootaxa 1668:183–244. doi:10.11646/zootaxa.1668.1.12.
- Fabricius JC. 1775. Systema Entomologiae, sistens Insectorum Classes, Ordines, Genera, Species, adiectis Synonymis, Locis, Descriptionibus. Kortii, Flenshurgi [Flensburg] & Lipsiae [Leipzig]. doi:10.5962/bhl.title.36510.
- Fishelson L. 1970. Littoral fauna of the Red Sea: the population of non scleractinian anthozoans of shallow waters of the Red Sea (Eilat). Mar Biol 6:106–116. doi:10.1007/BF00347239.
- Fransen CHJM. 1989. Notes on Caridean shrimps collected during the Snellius-II Expedition. 1. Associates of Anthozoa. Neth J Sea Res 23(2):131–147. doi:10.1016/0077-7579(89)90008-2.
- Geller JB, Walton ED. 2001. Breaking up and getting back together: evolution of symbiosis and cloning in sea anemones (genus Anthopleura) inferred from a molecular phylogeny. Evolution 55:1781–1794. doi:10.1111/j.0014-3820.2001.tb00827.x. [DOI] [PubMed]
- Gomes PB, Targino AG, Brandão A, Pérez D. 2016. Diversity and distribution of Actiniaria. In: Goffredo S, Dubinsky Z (eds) The Cnidaria Past, Present and Future. Springer Nature, Switzerland. doi:10.1007/978-3-319-31305-4_9.
- González-Muñoz R, Simões N, Sanchez-Rodríguez J, Rodríguez E, Segura Puertas L. 2012. First inventory of sea anemones (Cnidaria: Actiniaria) of the Mexican Caribbean. Zootaxa 3556:1–38. doi:10.11646/zootaxa.3556.1.1.
- Haddon AC, Shackleton AM. 1893. Description of some new species of Actiniaria from Torres Straits. Sci Proc R Dublin Soc 8:116–131.
- Haddon AC. 1898. The Actiniaria of Torres Straits. Sci Proc R Dublin Soc Series 2(6):393–520.
- den Hartog JC. 1997. The sea anemone fauna of Indonesian coral reefs. In: Tomascik T, Mah AJ, Nontji A, Moosa MK (eds) The Ecology of the Indonesian Seas Part 1. Periplus Editions, Republic of Singapore.
- Hertwig R. 1882. Die Actinien der Challenger Expedition. Gustav Fisher, Jena.
- Hitchcock E. 1833. Report on the Geology, Mineralogy, Botany, and Zoology of Massachusetts. Amherst: Press of J.S and C. Adams, Massachusetts. doi:10.5962/bhl.title.35851.
- Hitchcock E. 1835. Report on the Geology, Mineralogy, Botany, and Zoology of Massachusetts. Second edition. Amherst: Press of J.S and C. Adams, Massachusetts. doi:10.5962/bhl.title.69848.
- Hoeksema BW, Crowther AL. 2011. Masquerade, mimicry and crypsis of the polymorphic sea anemone Phyllodiscus semoni and its aggregations in South Sulawesi. Contrib Zool 80(4):251–268. doi:10.1163/18759866-08004003.
- Humason GL. 1967. Animal tissue techniques. Second edition. WH Freeman & Company, San Francisco, California, USA.
- International Commission on Zoological Nomenclature. 1999. International Code of Zoological Nomenclature, 4th Edition. International Trust for Zoological Nomenclature, London, United Kingdom.
- Izumi T. 2021. The largest cnidae among the sea anemones; description of a new haloclavid species from Japan, Haloclava hercules (Cnidaria: Actiniaria: Enthemonae: Haloclavidae). Species Divers 26:241–247. doi:10.12782/specdiv.26.241.
- Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A. 2019. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35(21):4453–4455. doi:10.1093/bioinformatics/btz305. [DOI] [PMC free article] [PubMed]
- Kwietniewski CR. 1897. Ein Beitrag zur Anatomie und Systematik der Actiniarien. Universitaet Jena, Jena.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi:10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed]
- Lauretta D, Häussermann V, Brugler M, Rodríguez E. 2013. Ispoaractis fionae sp. nov. (Cnidaria: Anthozoa: Actiniaria) from Southern Patagonia, with a discussion of the family Isanthidae. Org Divers Evol 14:31–42. doi:10.1007/s13127-013-0149-z.
- Latreille PA. 1825. Encyclopédie Méthodique. Histoire Naturelle. Entomologie, ou Histoire naturelle des Crustacés, des Arachnides et des Insectes [Volume 10 (Part 2: 345–832)]. Agasse, Paris. doi:10.5962/bhl.title.82248.
- Mariscal RN. 1974. Nematocysts. In: Muscatine L, Lenhoff HM (eds) Coelenterate Biology: Reviews and New Perspectives. Academic Press Inc, New York, pp. 129–178.
- McMurrich JP. 1889. The Actiniaria of the Bahama Islands, W.I. J Morphol 3:1–80. doi:10.1002/jmor.1050030102.
- Milne Edwards H. 1857. Histoire Naturelle des Coralliaires ou Polypes Proprement Dits. Vol. 1. Librairie Encylopédique de Roret, Paris. doi:10.5962/bhl.title.11574.
- Mitchell ML, Tonkin-Hill GQ, Morales RAV, Purcell AW, Papenfuss AT, Norton RS. 2020. Tentacle transcriptomes of the speckled anemone (Actiniaria: Actiniidae: Oulactis sp.): venom-related components and their domain structure. Mar Biotechnol 22:207–219. doi:10.1007/s10126-020-09945-8. [DOI] [PubMed]
- Mizuno M, Nozaki M, Morine N, Suzuki N, Nishikawa K, Morgan BP, Matsuo S. 2007. A protein toxin from the sea anemone Phyllodiscus semoni targets the kidney and causes a severe renal injury with predominant glomerular endothelial damage. Am J Pathol 17(2):402–414. doi:10.2353/ajpath.2007.060984. [DOI] [PMC free article] [PubMed]
- Neave SA. 1940. Nomenclator Zoologicus Vol. IV. Zoological Society of London, London, UK.
- Quoy JRC, Gaimard P. 1833. Voyage de Découvertes de l’Astrolabe Exécuté par Ordre du Roi, Pendant les Années 1826-1827-1828-1829, sous le Commandement de M.J. Dumont D’Urville. J. Tastu, Paris. doi:10.5962/bhl.title.2132.
- Rafinesque CS. 1815. Analyse de la Nature ou Tableau de l’Univers et des Corp Organisés. CS Rafinesque, Palerme. doi:10.5962/bhl. title.106607.
- Rambaut, A, Suchard MA, Xie D, Drummond AJ. 2018. Tracer: MCMC trace analysis tool. Version 1.6. Available at: http://www. marinespecies.org. Accessed 25 Sept. 2021.
- Rodríguez E, Barbeitos MS, Brugler MR, Crowley LM, Grajales A, Gusmão L, Häussermann V, Reft A, Daly M. 2014. Hidden among sea anemones: the first comprehensive phylogenetic reconstruction of the order Actiniaria (Cnidaria, Anthozoa, Hexacorallia) reveals a novel group of hexacorals. PLoS ONE 9(5):1–7. doi:10.1371/journal.pone.0096998. [DOI] [PMC free article] [PubMed]
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. doi:10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed]
- Rowlett J. 2020. Indo-Pacific corals. Independently published.
- Schoolmeesters P. 2021. World Scarabaeidae Database. In: Bánki O, Roskov Y, Vandepitte L, DeWalt RE, Remsen D, Schalk P, Orrell T, Keping M, Miller J, Aalbu R, Adlard R, Adriaenssens E, Aedo C, Aescht E, Akkari N, Alonso-Zarazaga MA, Alvarez B, Alvarez F, Anderson G, et al., Catalogue of Life Checklist (Version 2021-10-04). doi:10.48580/d4sz-38g. Accessed 23 Sept. 2021.
- Scott A, Hardefeldt JM, Hall KC. 2014. Asexual propagation of sea anemones that host anemonefishes: Implications for the marine ornamental aquarium trade and restocking programs. PLoS ONE 9(10):e109566. doi:10.1371/journal.pone.0109566. [DOI] [PMC free article] [PubMed]
- Stachowitsch M. 1992. The Invertebrates: An Illustrated Glossary. Wiley-Liss Inc, New York, USA.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post analysis of large phylogenies. Bioinformatics 30:1312–1313. doi:10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed]
- Stephenson TA. 1921. On the classification of Actiniaria. Part II –Consideration of the whole group and its relationships, with special reference to forms not treated in Part I. Q J Microsc Sci 65:493–576. doi:10.1242/jcs.s2-65.260.493.
- Stephenson TA. 1922. On the classification of Actiniaria. Part III -Definitions connected with forms dealt with in Part II. Q J Microsc Sci 66:247–319. doi:10.1242/jcs.s2-66.262.247.
- Stephenson TA. 1928. The British sea anemones Volume I. The Ray Society, London, UK.
- Sunagawa S, Wilson EC, Thaler M, Smith ML, Caruso C, Pringle JR, Weis VM, Medina M, Schwarz JA. 2009. Generation and analysis of transcriptomic resources for a model system on the rise: the sea anemone Aiptasia pallida and its dinoflagellate endosymbiont. BMC Genom 10:258. doi:10.1186/1471-2164-10-258. [DOI] [PMC free article] [PubMed]
- Titus BM, Benedict C, Laroche R, Gusmāo LC, Deusen VV, Chiodo T, Meyer CP, Berumen ML, Bartholomew A, Yanagi K, Reimer JD, Fujii T, Daly M, Rodríguez E. 2019. Phylogenetic relationships among the clownfish-hosting sea anemones. Mol Phylogenet Evol 139:106526. doi:10.1016/j.ympev.2019.106526. [DOI] [PubMed]
- Wassilieff A. 1908. Japanische Actinien. Abh Math-Phys Inst K Bayer Akad Wiss Suppl 1:1–49.
- Yap NWL, Quek ZBR, Tan R, Nugroho DA, Lee JN, Berumen ML, Tan KS, Huang D. 2021. Carlgren’s hesitation allayed: redescription and systematics of Heteranthus verruculatus Klunzinger, 1877 (Cnidaria, Actiniaria), with a redefinition of Heteranthidae Carlgren, 1900. Contrib Zool 90:155–182. doi:10.1163/18759866-BJA10015.
- Yap NWL, Tan R, Yong CLX, Tan KS, Huang D. 2019. Sea anemones (Cnidaria, Actiniaria) of Singapore: redescription and taxonomy of Phymanthus pinnulatus Martens in Klunzinger, 1877. Zookeys 840:1–20. doi:10.3897/zookeys.840.31390. [DOI] [PMC free article] [PubMed]
- Yong CLX, Yap NWL, Tan KS, Huang D. 2021. Reproduction in the tropical frilly sea anemone Phymanthus pinnulatus (Cnidaria, Actiniaria). Invertebr Biol 140:312313. doi:10.1111/ivb.12313.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sea anemones species included in our phylogenetic analyses. Information on voucher specimens and associated museum catalogue numbers are provided here, if available. Dashes denote that such information is absent. Accession numbers in bold denotes new sequences to this study. Abbreviations: AMNH, American Museum of Natural History, New York; CAS, California Academy of Sciences, San Francisco; Cat. nos., Catalogue numbers; KUNHM, University of Kansas Natural History Museum; ZRC, Zoological Records Collection, Lee Kong Chian Natural History Museum, Singapore.
Map of Singapore where specimens of both Macrodactyla aspera (Haddon & Shackleton, 1893) and Macrodactyla fautinae sp. nov. were collected for this study: 1, Pulau Ubin OBS Camp (1°25'07.7"N, 103°55'44.3"E); 2, Pasir Ris (1°22'56.45"N, 103°57'3.88"E); 3, Changi Region beaches [this area encompass the following localities that are in very close proximity to each other: Changi beach, Changi beach near Carpark 4; Changi Point beaches; Changi Point SAF Chalet beach; Changi Point Ferry Terminal beach] (1°23'33.34"N, 103°59'20.94"E); 4, Pulau Sekudu (1°24'19"N, 103°59'17"E); 5, Chek Jawa (1°24'25"N, 103°59'23"E); 6, Beting Bronok (1°26'13.00"N, 104°02'58.00"E); 7, Changi beach near Carpark 7 (1°22'26.03"N, 104°0'24.79"E); 8, Changi East next to Changi water Reclamation Plant (1°18'45"N, 104°00'31"E); 9, Cyrene Reef (Terumbu Pandan) (1°15'28"N, 103°45'19"E). White circles represent M. aspera, while stars (both black and white) denote the occurrences of M. fautinae sp. nov., with the single black star indicating the type locality of this new species. Numbers not accompanied by any symbols indicate the presence of both species occurring at the same site. See main-text for full details.
Distribution of Macrodactyla aspera (Haddon & Shackleton, 1893) and Macrodactyla fautinae sp. nov. 1. Murray Islands, Torres Straits. 2. Noumea, New Caledonia. 3. Perth, Western Australia. 4. Singapore. 5. Eilat, Israel. Symbols beside each number represent the occurrence of the sea anemones: circles being M. aspera, stars represents M. fautinae. Symbols that are coloured black denote the type locality of the species they represent. See main-text for full details.
Maximum likelihood phylogram (RAxML) of concatenated dataset (i.e., 12S, 16S, 28S and cox3). Bootstrap resampling values under ML, and posterior probability values of Bayesian inference (BI), are indicated at the branches as ML/BI. Only bootstrap values > 50 and posterior probability > 0.8 are shown, values less than these limits are denoted by a dash (-).
Maximum likelihood phylogram (RAxML) of 12S sequences. Bootstrap values (≥ 50) are indicated on branches.
Maximum likelihood phylogram (RAxML) of 16S sequences. Bootstrap values (≥ 50) are indicated on branches.
Maximum likelihood phylogram (RAxML) of 28S sequences. Bootstrap values (≥ 50) are indicated on branches.
Maximum likelihood phylogram (RAxML) of cox3 sequences. Bootstrap values (≥ 50) are indicated on branches.