Abstract
Background:
Immune-checkpoint inhibitors (ICI) are approved for multiple cancers but can result in ICI-associated myocarditis, an infrequent but life-threatening condition. Elevations in cardiac biomarkers, specifically troponin-I (cTnI), troponin-T (cTnT) and creatine-kinase (CK) are used for diagnosis. However, the association between temporal elevations of these biomarkers with disease trajectory and outcomes has not been established.
Methods:
We analyzed the diagnostic accuracy and prognostic performances of cTnI, cTnT and CK in ICI-myocarditis (n=60) followed-up for a year in two cardio-oncology units (APHP.Sorbonne, Paris, France & Heidelberg, Germany). A total of 1751 (one cTnT assay-type), 920 (4 cTnI assay-types), and 1191 CK sampling time points were available. Major adverse cardio-myotoxic events (MACE) were defined as heart failure, ventricular arrhythmia, atrioventricular/sinus block requiring pacemaker, respiratory muscle failure requiring mechanical ventilation, and sudden cardiac death. Diagnostic performance of cTnI and cTnT were also assessed in an international ICI-myocarditis registry.
Results:
Within 72h of admission, cTnT, cTnI or CK were increased compared to upper reference limit (URL) in 56/57(98%), 37/42(88%, p=0.03 vs. cTnT), 43/57(75%, p<0.001 vs. cTnT), respectively. This higher rate of positivity for cTnT (93%) vs. cTnI (64%,p<0.001) on admission was confirmed in 87 independent cases from an international registry (13 countries). In the Franco-German cohort, 24/60 (40%) patients developed at least one MACE (52 MACE in total, median time to first MACE=5[2-16]days). The highest value of cTnT/URL within the first 72h of admission performed best in terms of association with MACE within 90days (AUC=0.84) than CK/URL (AUC=0.70). A cTnT/URL≥32 within ≤72h of admission was the best cut-off associated with MACE within 90days (Hazard-ratio=11.1(95%CI=3.2, 38.0), p<0.001), after adjustment for age and sex. cTnT was increased in all patients within 72hours of the first MACE (23/23, 100%) while cTnI and CK values were <URL in 2/19 (11%) and 6/22 (27%) of patients (p<0.001).
Conclusions.
cTnT is associated with MACE, and is sensitive for diagnosis and surveillance in ICI-myocarditis. A ratio of cTnT/URL<32 within ≤72h of diagnosis is associated with a subgroup at low-risk of MACE. Potential differences in diagnostic and prognostic performances between cTnT and cTnI as a function of the assays used deserve further evaluation in ICI-myocarditis.
Keywords: Myocarditis, Cardio-oncology, Pharmacology, Immune-checkpoint inhibitors, Adverse drug reactions, Biomarkers, Troponins
Introduction.
Immune-Checkpoint inhibitors (ICI) are a potent class of oncology therapies used to treat up to 50% of cancer types.1, 2 Currently approved ICI are monoclonal antibodies targeting four inhibitory immune checkpoints: CTLA4 (Cytotoxic T-Lymphocyte Associated protein 4), PD1 (Programmed cell Death protein 1) and its ligand (PDL1), and LAG3 (Lymphocyte Activation Gene-3).3 By virtue of activating the adaptive immune system in fighting cancer, ICI can result in immune-related toxicities that can affect any organ.1 ICI-induced myocarditis is one such toxicity which, although infrequent, can result in mortality in up to ~50% of affected patients.4, 5 ICI-myocarditis often presents concurrently with other myotoxicities including symptomatic myositis (~30-35% of the time) and may lead to fatal respiratory muscle failure.4-9 Mechanistically, ICI-myocarditis is associated with macrophage and T-cell infiltration into muscles and associated myocyte death.10-13 The diagnosis of ICI-myocarditis is challenging and a combination of biomarkers, cardiac imaging, and endomyocardial biopsy is needed to confirm the diagnosis.14, 15 Cardiac biomarkers, including high-sensitive cardiac troponin-T (cTnT), cardiac troponin-I (cTnI) and creatine kinase (CK), are sensitive (though not specific) for the detection of myocarditis.16 However, there are no comparative data on performance of different biomarker and commercial assays for diagnosis of ICI-myocarditis, particularly when considering the analytical differences between contemporary and high-sensitive troponin assays.17-20 Available data regarding use of cardiac biomarkers for risk prediction of major adverse cardiac and respiratory muscle failure events (MACE) in patients with ICI-myocarditis are limited and most risk prediction tools use appearance of pathological electrocardiographic features or signs and symptoms of clinical heart failure.5, 21 Most ICI-myocarditis reports used blood analysis for cardiac troponins to detect cardiac injury, which is currently part of most diagnostic criteria.14, 17 Increased levels of CK in blood have also been used for ICI-myocarditis diagnosis and potentially prognostication.13, 14, 16, 17 However, the diagnostic and predictive performance of these different cardiac biomarker for the diagnosis and prediction of MACE in ICI-myocarditis is unknown. Herein, we investigated their value for diagnosis, risk assessment and surveillance in ICI-myocarditis.
Methods.
Patient cohort
We included consecutive patients (n=60) admitted for ICI-myocarditis (having at least an histologic examination of cardiac biopsy specimens and/or cardiac magnetic resonance imaging consistent with myocarditis and presentation not explained by other conditions, (see Supplementary-Table-1 for detailed diagnostic criteria)9 into a Franco-German study at the university hospitals of Heidelberg (Heidelberg, Germany) or Pitié-Salpêtrière (AP-HP; Sorbonne, Paris, France) between 2018 and 2020. Data from the initial hospital stay and subsequent one-year follow-up visits were prospectively gathered and analyzed. Collected and adjudicated MACE events included : heart failure (requiring hospitalization); ventricular arrhythmias (including non-sustained events); high-degree atrioventricular or sinus blocks requiring pacemaker implantation; respiratory muscle failure requiring mechanical ventilation support; and sudden cardiac death. Death related to MACE was termed as ‘cardiomyotoxicity-related’ death. The study protocol was approved by the Ethics Committee / institutional review board of both institutions (Heidelberg University: S-286/2017, 390/2011; APHP-Sorbonne: APHP-CSE-20-37_JOCARDITE; NCT04637672). The investigation conforms with the principles outlined in the Declaration of Helsinki. Written informed consent was gathered from the participating patients.
Measurement of cardiac and muscular circulating biomarkers
In the index Franco-German cohort, cTnT was measured in 1751 samples (n=60 patients with at least one measurement), cTnI in 920 samples (n=55) and CK in 1191 samples (n=60) over a median follow-up of 354 days, interquartile range [85-360]. Blood samples were collected as clinically indicated up to one year after diagnosis of ICI-myocarditis and were subsequently analyzed at different time intervals in days (d) after first hospital admission for ICI-myocarditis: 0-3d (i.e diagnosis phase), 4-7d, 8-14d, 15-30d, 31-90d, 91-180d and 181-360d. Across the whole surveillance period, the median available number of CK/cTnI/cTnT samples per patient was 15[10-23], 14[7-20], and 21[14-39]; respectively. For a detailed outline of the different assays used and their individual characteristics including limit of detection, 10% coefficient of variation, and 99th and 95th percentile (for troponins, and CK, respectively) upper reference limit of normal population values (URL); refer to Supplementary-Table-2. The magnitude of correlations between cTnT and cTnI levels as a function of the 3 main different types of cTnI assays used was moderate (rho≈0.6), and not dependent on the type of cTnI assay (Supplementary-Figure-1). Blood sampling of cTnT and cTnI was considered concomitant if performed within 6 hours.
We externally validated our results concerning cTnI and cTnT diagnostic properties using an international registry collecting ICI-myocarditis worldwide (n=659 as of July 2022, 13 countries, see Appendix for full list of contributing centers).21, 22 A total of 87 independent cases (different from the Heidelberg/APHP.Sorbonne discovery cohort) in which both cTnI or cTnT were available were used for this validation. In this international registry, cTnI and cTnT and their URL were entered by contributors but data regarding the assays used were not collected. International ICI-myocarditis registry ethical approval has already been described elsewhere (NCT04294771).21, 22
Determination of cTnI circulating auto-antibody titers
We assessed (as previously described in other cardiac prevalent diseases)23-29 whether ICI-myocarditis was associated with the presence of anti-cTnI antibodies, potentially interfering with cTnI assays. Sera samples (n=242 in total, n=7/patient[4-11]) from all patients prospectively included at APHP.Sorbonne (n=29 patients, Paris, France)15 were used to detect circulating anti-cTnI IgM or IgG during the course of their care. Ninety-six well plates were coated with anti-cTnI diluted in coating buffer (0.1M NaHCO3/ 34mM Na2CO3, pH=9.5) and then incubated overnight at 4°C. All washing steps were performed with 1x PBS/0.05% Tween20 three times each. One percent Gelatine (Cold Water Fish, Sigma)/1x PBS was used for blocking. After 2h incubation at 37°C, half of the plate was coated with human cTnI for another 2h at room temperature (RT) while the other half served as control, thus, only coated with 1x PBS/1%BSA/0.1% Tween20. The dilution series of the serum samples were as follows: 1/40, 1/80, 1/160, and 1/320. For 2h, the plates were incubated at RT. Horseradish peroxidase (HRP) anti-human IgG or anti-human IgM (diluted 1/7500 with 1x PBS/1% BSA/0.1% Tween20, 1h incubation at RT) was used as detection-antibody. Blue Star HRP-Substrate from Diarect was applied for 10min (IgG) or 25 min (IgM) at RT. The reaction was stopped with 0.3M H2SO4. Finally, the absorbance was measured at 450nm. We used a hybrid antibody construct (Fc-fragment = humanIgG + Fab-fragment=mouse anti human cTnI; provided by Roche Diagnostics®, Mannheim, Germany) as a positive control. To calculate the cTnI titres, the optical densities on both halves of the plate of each sample and dilution were subtracted. Total IgG and IgM antibody titers were measured in all tested samples. Total antibody endpoint titers for each sample were calculated as the highest positive dilution of antibody.30, 31
Pathology findings and troponins expression in skeletal muscles
In all ICI-myocarditis patients included in the APHP.Sorbonne cohort (n=29), we systematically performed a peripheral muscle biopsy and searched for concurrent ICI-myositis on pathology, as ICI-myocarditis and ICI-myositis frequently co-occur and have similar pathophysiology.5, 13, 32 We sought to determine whether skeletal muscle injury attributed to ICI-myositis is a source of cTnT or cTnI release, as non ICI-mediated myositis is associated with increases in cTnT but not cTnI.33 We performed bulk transcriptomics using muscle biopsies from 6 ICI-myositis patients compared to 6 controls with normal muscle (both groups collected at APHP.Sorbonne, France)31 seeking for differential expression between TNNT2 (encoding cTnT) versus TNNI3 (encoding cTnI). RNA was extracted from 20 slices of 20 μm using QIAzol Lysis reagent and RNeasy Plus Universal Mini Kit (Qiagen, Germany) following the manufacturer’s protocol. Only samples with RIN (RNA Integrity Number)>7 determined on the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) were then processed. Non-strand-oriented libraries were prepared following the NEBNEXT single cell/Low input RNA library prep kit protocol from NEB, starting from 20 ng of high-quality total RNA. Paired-end (2×75bp) sequencing was performed on an Illumina Nextseq 500 platform. RNA-sequencing analyses were performed using STAR program version 2.7.1a and GRCh 38 release 96 reference genomes for sequences alignment. Data were analyzed using R-program version 4.0.2 and are expressed as log2 fold change (Log2FC), adjusted p-value using DESeq2 method. Volcano plot of differentially expressed genes was created using package ‘EnhancedVolcano’.
To investigate whether cTnT may be expressed in ICI-myositis muscle samples, we performed an immunohistochemistry staining on 3μm sections of formalin-fixed/parrafin-embedded (FFPE) muscle tissue of a representative patient. Staining was performed using anti-cTnT antibody (#ab91605, Abcam, Amsterdam, Netherlands; clone:EPR3695). After the addition of a biotinylated secondary antibody, the reaction product was visualized on a Benchmark XT immunostainer (Roche Ventana, Darmstadt, Germany). Images were acquired on a Nikon Eclipse Ti2 at 20X magnification. After image acquisition, image tiles were stitched together with the microscope’s native software (NIS-Elements).
Statistical analysis
Data were analyzed using R-software. Quantitative data were presented as median and interquartile range [IQR] and were compared using Mann-Whitney test. Biomarker measurements were compared to their respective 99th and 95th percentiles URL for troponins and CK, respectively. Calculated ratios (cTnT/URL, cTnI/URL, CK/URL) were subsequently normalized by logarithmic transformation. Kaplan Meier analyses and multivariate logistic Cox regression models (adjusted on age and sex) were computed using the survival package (Version 3.1-8). ROC (Receiver-Operator curves) and AUC (Area under the curve) analysis were calculated by the plotROC package (version 2.2.1). AUC and its [95th confidence intervals] were calculated by using the pROC package with 2000 stratified bootstrap replicates each. The correlation between concomitant cTnI and cTnT plasma levels was calculated using spearman’s test (rho). Comparison of these different correlations as a function of cTnI assay used was calculated with the Fisher z test for independent correlations as implemented in the R cocor package.34 Non-linear mixed effect (nlme) models were used to study if the ratio of cTnT/URL over cTnI/URL (as a proxy of their divergence) were influenced by the following fixed effects variables (time-period after ICI-myocarditis diagnosis, inclusion center, presence or not of IgG or IgM anti-cTnI antibodies, age and sex), integrating the following random effects (patients’ identity, and cTnI assay types). Sensitivity analyses were performed to study the time-dependent evolution of the ratio of cTnT/URL over cTnI/URL using nlme models (with similar explaining covariates) in subgroups of patients with the same cTnI assay available. A serum sample identifying detectable IgG or IgM anti-cTnI (at least 1/40 for each) was considered positive for a 10days time period, except if plasmapheresis was performed within this time-period. Data will be made available to other researchers upon reasonable request to corresponding authors.
Results.
Population studied
Our cohort included 60 consecutive patients admitted with ICI-myocarditis. The median age was 71 [61-80] years, 35% were female and the median follow-up was 354 [85-360] days. All patients were promptly hospitalized upon suspicion of ICI-myocarditis with diagnosis confirmed via endomyocardial biopsy or cardiac MRI. Each patient was serially assessed for circulating biomarker (CK/cTnI/cTnT) of myotoxicity; these biomarkers were analyzed within the first 3 days (median number[IQR] of tests/patient for CK=3[2-4], cTnI=3[1-3], cTnT=4[3-4]). The clinical and demographic characteristics of this cohort are displayed in Table-1. Most patients had definite ICI-myocarditis (48/60, 80%) as determined by a diagnostic level of certainty; the rest had probable or possible myocarditis (Supplementary-Table-1).9 A total of 63% of patients received anti-PD1 monotherapy, 22% anti-PDL1 monotherapy and 15% received a combination of anti-PD1 and anti-CTLA4. Most patients had non-small cell lung cancer (40%) or malignant melanoma (20%). Most patients were symptomatic at initial presentation (44/60; 73%) while a small subset was identified asymptomatically as part of a systematic screening strategy (16/60; 27%). A total of 24/60 (40%) patients developed at least one MACE (52 total MACE events detailed in Table-1). Overall mortality and ICI cardio-myotoxicity related death occurred in 30/60 (50%) and 9/60 (15%) patients, respectively. Cardio-myotoxicity related deaths occurred earlier after ICI-myocarditis diagnosis versus non-myocarditis related deaths (17[8-38] vs. 107[59-271] days, p<0.001). Causes of death are detailed in Table-1.
Table 1.
Characteristics of the Franco-German cohort
| Overall cohort (n=60) | MACE (n=24) | No MACE (n=36) | p-value | ||
|---|---|---|---|---|---|
| Age (years; median [IQR]) | 71 [61-80], n=60 | 70 [60-80], n=24 | 73 [62-77], n=36 | 0.84 | |
| Sex (female) | 21/60 (35%) | 8/24 (33%) | 13/36 (36%) | 0.99 | |
| Symptomatic at admission (Yes) | 44/60 (73%) | 20/24 (83%) | 24/36 (67%) | 0.23 | |
| Follow-up after diagnosis (days, median [IQR]) | 354[85-360], n=60 | 105[23-360], n=24 | 360[135-360], n=36 | 0.04 | |
| Patients with MACE (24 patients, 52 events) | 24/60 (40%) | NA | |||
| - Respiratory failure | 12/24* (50%) 12/52 (23%) |
||||
| - Ventricular arrythmias | 9/24* (38%) 19/52 (37%) |
||||
| - Pacemaker implantation | 8/24* (33%) 8/52 (15%) |
||||
| - Heart failure | 12/24* (50%) 12/52 (23%) |
||||
| - Sudden cardiac death | 1/24* (4%) 1/52 (2%) |
||||
| Overall mortality (1-year follow-up) | 30/60 (50%) | 14/24 (58%) | 16/36 (44%) | 0.43 | |
| Cause of death: | <0.001 | ||||
| - Cancer progression | 13/30 (43%) | 1/14 (7%) | 12/16 (75%) | ||
| - Myotoxicity | 9/30 (29%) | 9/14# (64%) | 0/16 (0%) | ||
| - Infection | 6/30 (20%) | 5/14# (36%) | 2/16 (13%) | ||
| - Digestive hemorrhage | 1/30 (3%) | 0/14 (0%) | 1/16 (6%) | ||
| - Unknown | 1/30 (3%) | 0/14 (0%) | 1/16 (6%) | ||
| Time to first MACE (days, median [IQR]) | NA | 5 [2-16] | NA | NA | |
| cTnT/URL ratio at diagnosis (median [IQR])** | 29 [10-69], n=57 | 59 [43-182], n=22 | 16 [4-34], n=35 | <0.001 | |
| cTnI/URL ratio at diagnosis (median [IQR])** | 14 [6-61], n=42 | 38 [11-522], n=15 | 10 [2-57], n=27 | 0.04 | |
| CK/URL ratio at diagnosis (median [IQR])** | 6 [1-24], n=57 | 12 [4-42], n=23 | 2 [1-11], n=34 | 0.002 | |
| cTnT/URL ratio during MACE (median [IQR])† | NA | 90 [45-314], n=45 | NA | NA | |
| cTnI/URL ratio during MACE (median [IQR])† | NA | 50 [8-409], n=42 | NA | NA | |
| CK/URL ratio during MACE (median [IQR])† | NA | 5 [1-10], n=46 | NA | NA | |
| Drugs | Anti-PD1 | 38/60 (63%) | 17/24 (71%) | 21/36 (58%) | 0.46 |
| Anti-PD1 + Anti-CTLA4 | 9/60 (15%) | 2/24 (8%) | 7/36 (19%) | ||
| Anti-PDL1 | 13/60 (22%) | 5/24 (21%) | 8/36 (22%) | ||
| Tumor | Non-small cell lung cancer | 24/60 (40%) | 10/24 (42%) | 14/36 (39%) | 0.62 |
| Melanoma | 12/60 (20%) | 3/24 (13%) | 9/36 (25%) | ||
| Renal cell carcinoma | 6/60 (10%) | 2/24 (8%) | 4/36 (12%) | ||
| Hepato-carcinoma | 4/60 (7%) | 1/24 (4%) | 3/36 (11%) | ||
| Squamous cell carcinoma | 3/60 (5%) | 2/24 (8%) | 1/36 (3%) | ||
| Other‡ | 11/60 (18%) | 6/24 (25%) | 5/36 (14%) | ||
Abbreviations: CTLA4 (Cytotoxic T-Lymphocyte Associated protein 4); IQR: interquartile range; MACE, major adverse cardiomyotoxic event; NA, not applicable; PD1 (Programmed cell Death protein 1) and its ligand (PDL1); SD, standard deviation; URL: upper reference limit being upper 99th percentile of normal values for troponins and 95th for CK.
Statistics: Proportions were compared using Fisher's exact test or Chi-Square test, as appropriate. Quantitative values between MACE and no MACE groups were compared using a Mann-Whitney test.
One patient may develop more than one MACE (n=52 total number of events in 24 patients).
Maximal value available within 3 days of presentation.
The closest measured value within 3 days of the occurrence of a MACE. When two different types of MACE occurred concurrently, only one time-point with a biomarker value available was used for calculation (48 timepoints in total)
Other cancers involved thymoma (3), colorectal carcinoma (2), sarcoma (1), malignant histiocytosis (1), urothelial carcinoma (1), pleural mesothelioma (1), endometrial carcinoma (1), cancer of unknown primary (1)
One patient died of the combination of a septic shock and a severe cardio-myotoxicity
Time course of cTnT, TnI and CK in patients with ICI-myocarditis
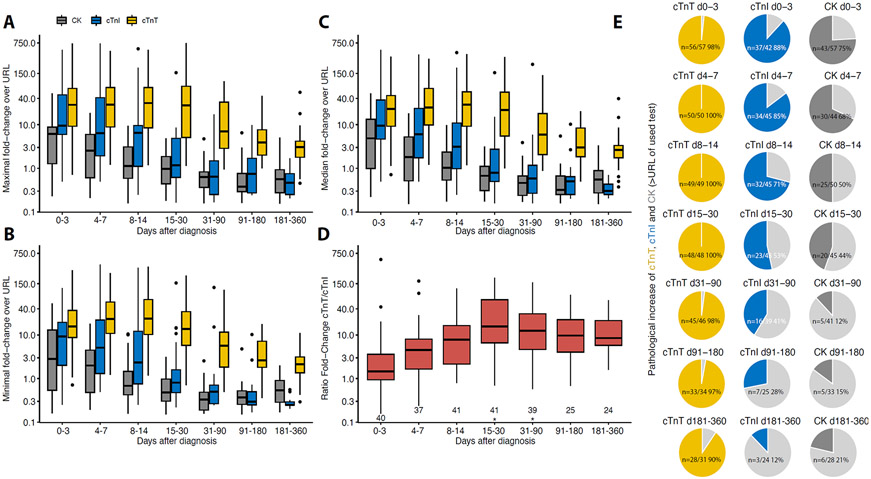
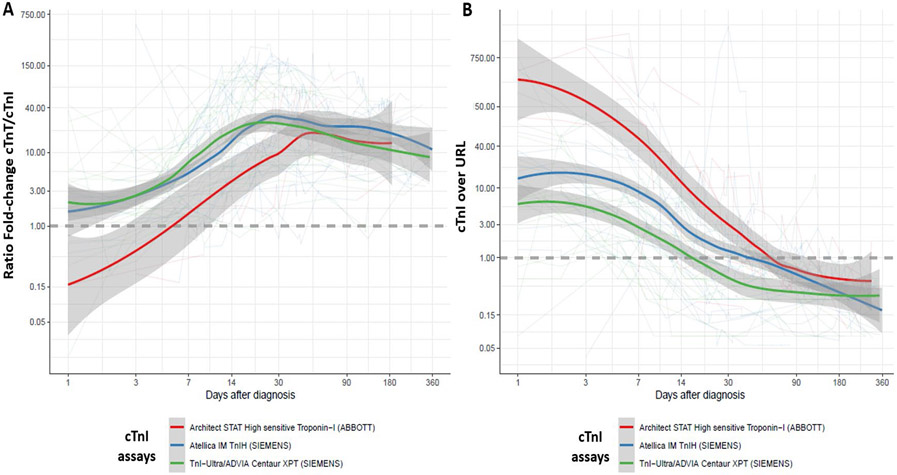
Within 72 hours of admission, cTnT, cTnI or CK were increased compared to upper reference limit (URL) with varying degrees. cTnT was elevated in 56/57(98%), compared to cTnI (37/42, 88%, p=0.03 vs. cTnT) and CK (43/57, 75%, p<0.001 vs. cTnT), respectively. Within 72h after admission, maximum blood concentrations expressed as multiples of URL were higher for cTnT (median=40[10-70]) compared to cTnI (median=12[6-64]; p=0.03 vs. cTnT) and CK (median=7[2-9]; p<0.001 vs. cTnT). These biomarkers were serially measured during hospitalization and their time-dependent concentration changes following presentation are displayed in Figure-1. Peak values were observed for cTnT on day 7[3-16], for cTnI on day 3[2-10] (p=0.03 vs. cTnT), and for CK on day 1[1-5] (p<0.001 vs. cTnT) after initial ICI- myocarditis diagnosis. Figure-1 shows a faster decline in maximal (Figure-1A), minimal (Figure-1B) and median (Figure-1C) CK and cTnI levels during the early phase of the ICI-myocarditis (weeks) in contrast to a more prolonged elevation of TnT lasting several months. Minimal circulating levels of cTnT, cTnI and CK were below URL between day 15-30 after admission for ICI-myocarditis in 2%, 65% and 78% of cases; and in 11%, 87%, 95% between day 31-90 (p<0.001 at all times, more extended follow-up data are shown in Figure-1E), respectively. In patients in whom measured biomarkers normalized during the follow-up, the median time to first value below URL was longer for cTnT (133[50-247]days), compared to cTnI (17[10-14]days), and CK (12[6-23]days). Maximum discrepancy between ratio of cTnT/URL over cTnI/URL (maximal ratio=14.6[4.8-64.3]) during follow-up was identified between day 15-30 after diagnosis (Figure-1D). Using non-linear mixed models in the patients having concomitant cTnT and cTnI levels available (n=55 patients; n=761 timepoints), we confirmed that the ratio of cTnT/URL over cTnI/URL was significantly associated with time to admission for ICI myocarditis (p<0.001 for days 15 to 90 after diagnosis vs. other time periods; Figure-1D), after adjusting on fixed effect variables (age, p=0.02; inclusion center, p=0.72 and sex, p=0.04; Supplementary-Table-3A for detailed results and Supplementary-Table-2 for age and sex-specific display) and integrating random effects (subject and types of cTnI assays used). This latter analysis performed in subgroups of patients having only the same type of cTnI assay available showed similar time-dependent increase of the ratio of cTnT/URL over cTnI/URL through time (Supplementary-Table-4A-C for detailed nlme models results and Figure-2 for evolution of cTnI/URL or ratio of cTnT/URL over cTnI/URL through time as a function of cTnI assays).
Figure 1: Time course of troponins and creatine kinase (CK) after admission for ICI-myocarditis.
URL stands for 99th percentile upper reference limit for troponins and 95th percentile for CK. Evolution of maximal (A), minimal (B), and median (C) values (median, IQR in the boxplots) of cardiac troponin-T (cTnT)/URL, cardiac troponin-I (cTnI)/URL and CK/URL ratios over time after initial diagnosis of ICI-myocarditis within specific timeframes (x-axis) in follow-up. (D) Ratio of maximum cTnT/URL over cTnI /URL over time after initial diagnosis of ICI-myocarditis within specific timeframes (x-axis) in follow-up. Nonlinear mixed models p-values are shown (*<0.001, See Supplementary Table-2A). For D, n of patients available for each biomarker at each time frame is just above the x-axis. (E) Proportion of patients with biomarkers above URL over time after diagnosis are displayed, numbers indicate patients with abnormal values. Light grey area represents the proportion of patients with biomarker levels below URL. Minimal values within the indicated time period were used for figure E (d for days).
Figure 2. LOESS (Locally Estimated Scatterplot Smoothing) of the mean (and standard-error) of the ratio of cTnT/URL over cTnI /URL (A) and cTnI/URL (B) over time after initial admission for ICI-myocarditis in a one-year follow-up as a function of cTnI assays.
Predictors of MACE in ICI-myocarditis
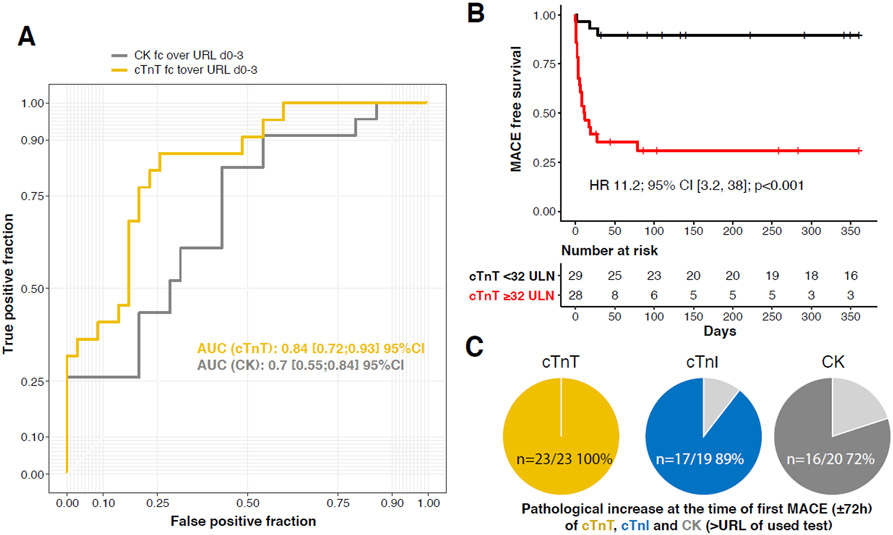
We next investigated the association with MACE of the levels of troponins and CK at index admission and during the course of their surveillance. Characteristics of ICI-myocarditis patients with MACE compared to patients without MACE during follow-up are shown in Table-1. The maximal cTnT/URL value measured within 72h of admission performed best in predicting MACE (AUC=0.84(0.72-0.93)) during follow-up compared to CK/URL (AUC=0.70(0.55-0.84)) (Figure-3A). Based on ROC analysis, we found a maximal cTnT/URL value within ≤72 hours of admission for ICI-myocarditis above ≥32 to be the most associated with MACE during follow-up (Cox regression hazard-ratio=11.1, 95% confidence interval=3.2, 38.0, p<0.001, adjusted for age and sex, Figure-3B). The sensitivity, specificity, positive and negative predictive values of this cTnT/URL threshold to predict MACE was 86% (66-96%) , 74% (58-86%), 68% (51-85%) and 90% (79-100%), respectively. This latter cTnT/URL threshold was also associated with all-cause mortality (HR=2.4 (1.1, 5.1), p=0.03; Supplementary-Figure-3) but not with non-MACE related mortality (HR=1.2 (0.5, 3.1), p=0.71; Supplementary-Figure-3) during the one-year follow-up. MACE in the 3/29 patients with cTnT/URL<32, were non-fatal and occurred after hospitalization discharge except for one ventricular tachycardia diagnosed at day 2 after admission for ICI-myocarditis. Notably, cTnT/URL values (58[33-130]) were abnormal in all patients within 3days of the first MACE (23/23 patients) while cTnI/URL (14[2-150]) and CK/URL (7[0.5-20]) values were normal in 2/19 (11%) and 6/22 (27%) of patients, respectively (p<0.001) (Figure-3C). Kinetic changes of mostly declining or normalizing CK and cTnI despite persistently high or even increasing cTnT levels at the time of MACE in the 24 ICI-myocarditis patients developing a MACE are shown in Supplementary-Figure-4.
Figure 3. Cardiac biomarkers as predictors of MACE.
(A) Maximal cardiac biomarkers values within 72h of ICI myocarditis diagnosis as a predictor of MACE within 90 days with receiver operating curve of cTnT/URL, and CK/URL. (B) MACE over a one-year time-course after diagnosis as a function of cTnT/URL value above and below 32 (n=57) using maximal cTnT values within 72h of ICI myocarditis diagnosis. (C) Proportion of patients with biomarkers above URL before first MACE are displayed in yellow (cTnT), blue (cTnI) and dark grey (CK). Light grey area represents the proportion of patients with biomarker levels below URL.
Abbreviations: AUC, area under the curve; MACE, major adverse cardio-myotoxic event; fc, fold-change; URL, upper reference limit being upper 99th percentile of normal values for troponins and 95th for CK; 95%CI, 95% confidence interval; HR, hazard ratio.
External validation of cTnT, cTnI diagnostic value in patients with ICI-myocarditis
The external validation cohort included 87 patients from an international registry (cases described in the discovery Franco-German cohort were not included) who had both initial cTnI and cTnT measurements within 72 hours of admission. While 64% patients (56/87) with ICI-myocarditis had an elevated cTnI/URL on admission, the respective percentage was 93% (81/87) for cTnT/URL (Mac Nemar Test, p<0.001). This discrepancy also persisted for peak troponin values (cTnI/URL>1 in 58/79 (73%) vs. TnT/URL>1 in 76/79 (96%), Mac Nemar Test, p<0.001).
Pathobiology of cardio-muscular biomarkers in ICI-myocarditis
We first combined the external international validation cohort with the Franco-German discovery cohort to analyze differences in the clinico-demographical features of ICI-myocarditis patients having both cTnI and cTnT increased over URL (n=109/134, 81%; cTnT+/cTnI+) vs. those having cTnI below URL despite having cTnT increased over URL (25/134, 19%; cTnT+/cTnI−). Age, sex, past cardiovascular medical history, and diagnostic certainty criteria for ICI-myocarditis were similar (Table-2). However, ICI-myocarditis patients with cTnT+/cTnI+ had more severe phenotypes than those with cTnT+/cTnI− with increased admission and peak cTnT and CK levels, more abnormal admission echocardiogram and electrocardiogram, and shorter time to onset after ICI start (Table-2). Concurrent association with ICI-myositis was similar between cTnT+/cTnI+ (74/109, 68%) and cTnT+/cTnI− (16/25, 64%, p=0.71) groups, but myasthenia-like features (potentially leading to respiratory muscle failure) were more prevalent in cTnT+/cTnI+ (43/109, 39%) vs. cTnT+/cTnI− patients (4/25, 16%, p=0.03).
Table 2.
Demographics and diagnostic characteristics of ICI-myocarditis cases reported in the international redcap (including the Franco-German discovery cohort) based on cardiac troponins (T, cTnT & I, cTnI) assays results. Only cases with both cTnI and cTnT available are displayed.
| cTnI+ & cTnT+ (n=109) |
cTnI− & cTnT+ (n=25) |
p-value | |
|---|---|---|---|
| Age at hospital admission (years) | 71 [62-78] (n=108) | 74 [62-79] (n=25) | 0.47 |
| Female | 39/108 (36%) | 7/25 (28%) | 0.49 |
| Medical History | |||
| Coronary Artery Disease | 21/109 (19%) | 5/25 (20%) | 0.99 |
| Heart Failure | 5/109 (5%) | 3/24 (13%) | 0.16 |
| Cardiovascular Risk Factors | |||
| Body Mass Index | 26 [22-28] (n=107) | 28 [27-30] (n=25) | 0.004 |
| Dyslipidemia | 46/108 (43%) | 16/24 (67%) | 0.04 |
| Diabetes | 27/108 (25%) | 11/25 (44%) | 0.08 |
| Hypertension | 69/109 (63%) | 17/25 (68%) | 0.82 |
| History of smoking | 55/109 (50%) | 14/25 (56%) | 0.66 |
| Days since first ICI dose to presentation | 28 [20-51] (n=106) | 56 [30-229] (n=25) | 0.002 |
| Admission Symptoms | |||
| Fatigue | 27/109 (25%) | 9/25 (36%) | 0.32 |
| Chest pain | 20/109 (18%) | 6/25 (24%) | 0.58 |
| Dyspnea | 41/109 (38%) | 9/25 (36%) | 0.99 |
| Syncope | 2/109 (2%) | 1/25 (4%) | 0.47 |
| Abnormal admission electrocardiogram | 87/109 (80%) | 14/25 (56%) | 0.02 |
| Admission Echocardiography | |||
| Regional Wall Motion Abnormality | 33/104 (32%) | 2/23 (9%) | 0.04 |
| Left Ventricular Ejection Fraction (%) | 58 [55-64] (n=94) | 60 [58-64] (n=24) | 0.17 |
| Initial cTnI (multiple of institution URL) | 8 [2-34] (n=108) | NA | |
| Initial cTnI (>URL) | 96/108 (98%) | NA | |
| Peak cTnI (multiple of institution URL) | 13 [4-48] (n=104) | NA | |
| Peak cTnI (>URL) | 104/104 (100%) | NA | |
| Initial cTnT (multiple of institution ULN) | 33 [11-78] (n=108) | 6 [2-24] (n=25) | 0.002 |
| Initial cTnT (>URL) | 106/108 (98%) | 23/25 (92%) | 0.16 |
| Peak cTnT (multiple of institution URL) | 64 [20-130] (n=107) | 11 [4-47] (n=25) | <0.001 |
| Peak cTnT (>URL) | 107/107 (100%) | 25/25 (100%) | |
| Initial CK (multiple of institution URL) | 7 [1-21] (n=105) | 2 [0-4] (n=22) | 0.001 |
| Peak CK (multiple of institution URL) | 10 [2-21] (n=105) | 2 [1-4] (n=22) | <0.001 |
| Diagnostic Criteria* | 0.15 | ||
| Definite | 69/109 (63%) | 12/25 (48%) | |
| Probable | 23/109 (21%) | 5/25 (20%) | |
| Possible | 17/109 (16%) | 8/25 (32%) | |
| Confirmed Myocarditis (Histology or cMRI) | 80/101 (79%) | 16/23 (70%) | 0.41 |
| Cardiac pathology supporting diagnosis | 52/79 (66%) | 7/17 (41%) | 0.10 |
| cMRI supporting diagnosis | 52/86 (60%) | 15/22 (68%) | 0.63 |
| Other irAE | |||
| Myositis | 74/109 (68%) | 16/25 (64%) | 0.81 |
| Myasthenia-gravis like syndrome | 43/109 (39%) | 4/25 (16%) | 0.04 |
As defined in the following publication (14)
Abbreviations: ICI: Immune checkpoint inhibitors; URL: upper reference limit of institution’s lab; CK: creatinine kinase; cMRI: cardiac magnetic resonance imaging; irAE: immune related adverse event; NA: not available
Statistics: Results are provided as median with interquartile range [25%-75%] and number (%). Proportions were compared using Fisher's exact test or Chi-Square test, as appropriate. Quantitative values were compared using a Mann-Whitney test.
Patients with ICI-myocarditis enrolled at APHP.Sorbonne in the discovery cohort were systematically and prospectively evaluated for concomitant ICI-myositis. Almost all of these patients (26/29, 90%) had ICI-myositis with T-cells and macrophages inflammatory cells mostly associated with myocytes death on peripheral muscle biopsy; a figure similar to what is found in ICI-myocarditis on endomyocardial pathology.7, 13 This finding further supported that ICI-myocarditis was overwhelmingly part of a systemic ICI-myotoxicity sharing similar pathophysiology with ICI-myositis.
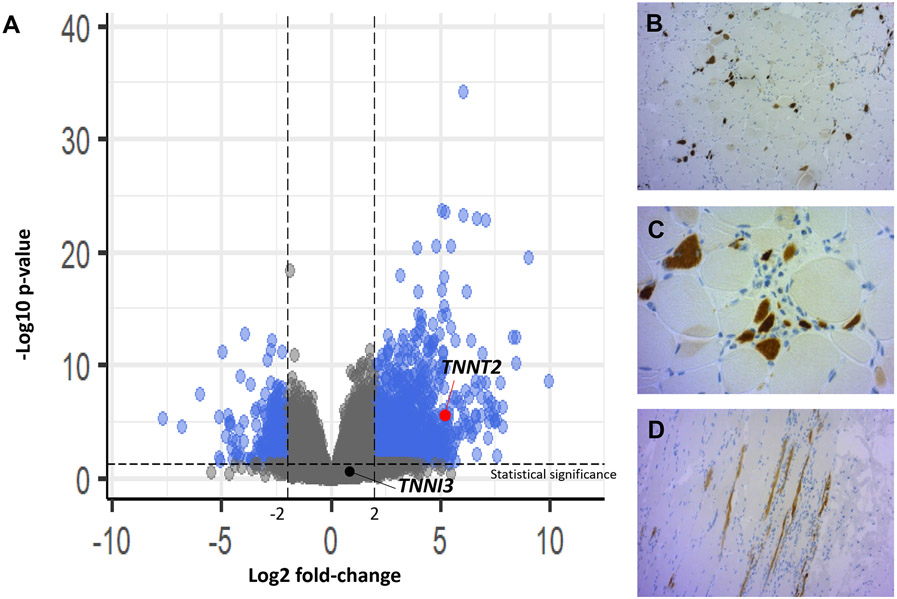
We hypothesized that cTnT more thoroughly captured the overall cardio-muscular burden induced by ICI-myotoxicity compared to cTnI, as this discrepancy in troponins prognostic value have been previously described in non-ICI cardio-muscular diseases.33, 35-38 Therefore, we searched for differences in the gene expression coding for cTnT (TNNT2) vs cTnI (TNNI3) in ICI-myositis peripheral muscle samples versus normal controls (n=6 each, Figure-4). We confirmed that TNNT2 had higher RNA expression in ICI-myositis vs. controls (Log2 fold-change=5.2, adjusted-p=0.3x106). No such increase was observed with TNNI3 (Log2 fold-change=0.96, adjusted-p=0.2). Immunohistochemistry showed protein expression of cTnT in the muscle sample of a patient with ICI-myositis (Figure-4B-D).
Figure 4: Pathobiology of cardio-muscular biomarkers in ICI-myotoxicity patients.
Volcano plot showing the distribution of all differentially expressed genes (n=17070) in muscle samples from patients with ICI-myotoxicities (n=6, concomitant ICI-myocarditis and ICI-myositis in n=5 and one ICI myositis not screened for concomitant myocarditis) vs. normal skeletal muscle samples (n=6). Blue dots represent significantly upregulated and downregulated genes (at least >log2(∣2∣)), after adjustment for multiple testing. TNNT2 gene (coding for cTnT) is significantly overexpressed in ICI-myotoxicity patients (Log2 fold-change:5.2, adjusted-p:0.3x10−6; red dot) whereas TNNI3 (coding for cTnI) is not (Log2 fold-change:0.96, adjusted-p=0.2; black dot) (panel A). Immunostaining (immunochemistry identifying protein expression– hematoxylin counterstaining) showing transversal sections of skeletal muscle fibers positive for cTnT (brown) (100X, panel B; 400X, panel C) adjacent to inflammatory cells (cluster of nuclei with blue staining) from a representative patient with ICI-myotoxicity. Longitudinal section of a skeletal muscle sample showing a linear pattern of positive immunostaining for cTnT tracking the morphology of muscle fibers (200X, panel D).
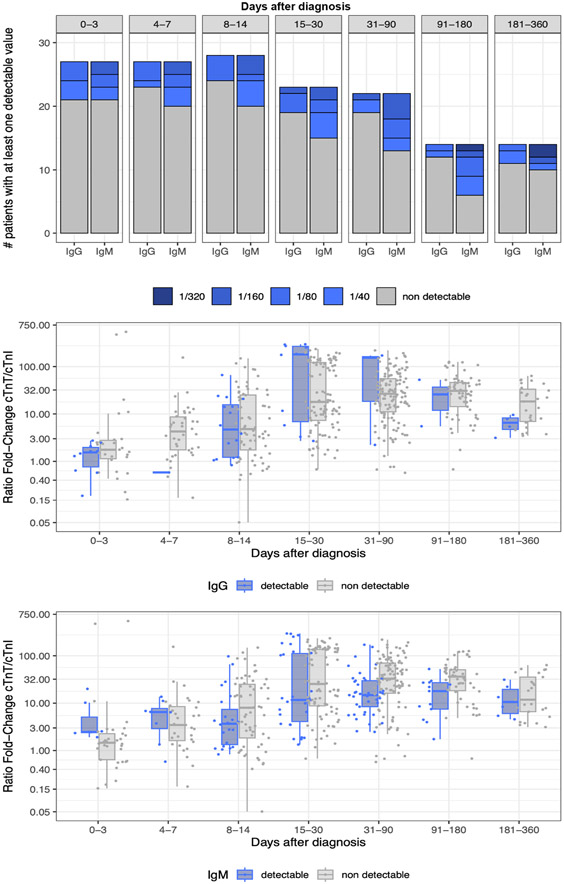
We additionally assessed whether presence of anti-cTnI antibodies could be interfering with cTnI assays and thus eventually contributing to the differences observed in cTnI vs cTnT circulating levels in ICI-myocarditis. In the French cases, IgM and IgG anti-cTnI antibodies were searched serially upon evolution of the disease (Figure-5A). Forty-one percent of the patients (12/29) had at least one detectable anti-cTnI IgM levels above 1/40; 31% above 1/80; 17% above 1/160 and 7% above 1/320. Similarly, 9/29, 31% had anti-cTnI IgG above 1/40; 17% above 1/80 and 3% above 1/160. No patient was detected with anti-cTnI IgG levels over 1/320. Using non-linear mixed models in these latter 29 patients on 493 available time-points with cTnT/URL over cTnI/URL ratio and IgM/IgG status available; we did not find any association between expression of detectable anti-cTnI IgM or IgG and cTnT/URL over cTnI/URL ratio (Figure-5 & Supplementary-Table-3B), after adjusting on cTnI assay types, patient’s identity, age, sex and time from ICI-myocarditis diagnosis.
Figure 5: Anti-cTnI IgG/IgM antibodies and influence on cTnT/cTnI ratio.
Evolution of anti-cTnI IgG and IgM antibodies circulating levels (at least on detectable value >1/40 in each time frame) over time in ICI-myocarditis in the French discovery cohort (A). Influence of detectable levels of anti-cTnI IgG (B) or IgM (C) on cTnT/cTnI ratio over their respective URL (99th percentile upper reference limit). No significant interaction between anti-cTnI IgG or IgM levels and cTnT/cTnI ratio over their respective URL was identified (Supplementary-Table-3B for detailed statistics).
Discussion.
Herein, we investigated a prospective cohort of 60 ICI-myocarditis patients from two cardio-oncology programs where cTnT, cTnI and CK were collected as clinically indicated during the first year of follow-up after diagnosis. This cohort is unique given the frequency of measurements of cardio-muscular biomarkers, particularly within 72 hours after admission. At the time of initial diagnosis, cTnT was more often elevated compared to cTnI and CK. This higher sensitivity for ICI-myocarditis of cTnT compared to cTnI or CK was also observed in an independent international cohort. These data are in contrast with current ICI-myocarditis diagnostic guidelines which specifically recommend cTnI.14 Our data also highlight an early peak of cTnI and CK within hours of initial presentation, followed by a normalization over several days. In contrast, cTnT peaked within days after the initial presentation, but remained persistently elevated for months. In our cohort, we identified a significant difference in the association of each biomarker elevation and kinetics with MACE with peak cTnT being a stronger prognosticator than peak CK. Repeated measurement of cTnT within the first few days of presentation may help to capture the peak value of cTnT; allowing for identification of a subgroup of patients associated with a low-risk of event; when cTnT/URL is <32. Further studies are required to assess eventual differences in the diagnostic and prognostic performances of the multiple cTnI assays available commercially. Given the technical limitation in comparing the quantitative magnitude of cTnI/URL increase (Figure 2B) at admission with the 4 different cTnI assays used in our study, we did not investigate cTnI association with MACE.
Troponins as a diagnostic tool for ICI-myocarditis
To date, most ICI-myocarditis cases reported in the literature were identified using cTnI, because cTnI assays are widely available due to multiple vendors and are often preferred over cTnT given the recent recommendations for diagnosis of ICI-myocarditis.14 cTnI is considered by some to be more cardiac specific than cTnT and therefore more suitable for diagnosis of ICI-myocarditis.36-38 However, in the few reported cases where ICI-myocarditis was diagnosed despite negative troponins, the troponin assay used was for cTnI.39 Those findings are in line with our results showing that ~10-20% of our cases lack an increase of cTnI on admission, despite cTnT being positive. The reason of the discrepancy between cTnT and cTnI is unclear. It has been reported that of patients who develop ICI-myocarditis, 2/4 patients developed anti troponin-I antibodies vs 0/4 in ICI treated control patients, possibly interfering with cTnI assays.40 In preclinical models of myocarditis including genetic animal models with global PD-1 deletion, production of antibodies against cardio-muscular antigens, including troponin-I were felt to cause myocardial damage.26 Interestingly, interference between cTnI and anti-cTnI antibodies have previously and consistently been reported in humans.23, 24 25-29 In our cohort, up to half of the patients had detectable anti-cTnI IgM levels during ICI-myocarditis course, with highest proportion observed in survivors after a month of initial presentation. This proportion is much higher (but of indeterminate clinical significance) than what have been observed in dilated or post-ischemic cardiomyopathies patients.31 However, the presence of these latter anti-cTnI autoantibodies were not associated with differences in the ratio of cTnT/URL over cTnI/URL; therefore not supporting a major analytical interference between anti-cTnI antibodies and cTnI circulating levels in ICI-myocarditis.
Troponins as a prognosticator of MACE in ICI-myocarditis
Differences between cTnT and cTnI blood kinetics and prognostic implications has been assessed in various research settings including cardiac ischemia,41-47 cardiac hypertrophy,48 diabetes,49 general population,50 and patients affected by neuromuscular disorders.33, 35-38 In these studies, cTnT was shown to be associated with overall mortality while cTnI was more often associated with cardiovascular specific mortality.48 In addition, cTnT may be elevated due to coexisting non-cardiac pathologies including muscular disorders with regenerating muscle potentially expressing cTnTwhile cTnI is not.50-52 In our study, cTnI normalized within days while cTnT remained elevated for over three months in ≥90% of patients. These differences between cTnI and cTnT blood concentration cannot be explained by their plasma half-life (previously determined in the setting of isolated myocardial injury or ischemia), which is only slightly longer for cTnT compared to cTnI, but still within a range of few hours for both.41, 42, 47 The fact that ICI myositis was almost universally present in our cohort of ICI-myocarditis (90% of patients with available muscular biopsy) highlights that ICI-myocarditis almost always occurred in the context of a systemic ICI-myotoxicity. Interestingly, this damaged peripheral muscle expressed specifically more cTnT versus cTnI RNA. This latter finding may have contributed to the better diagnostic and prognostic accuracy of cTnT vs. cTnI in ICI-myocarditis, because cTnT levels may have reflected more appropriately the overall cardio-muscular burden of ICI induced myotoxicity.
Study limitations
While careful attention was paid to prospectively collect cTnT, cTnI and CK biomarkers in the standard of care of our Franco-German index cohort, some timepoints were missing given the prolonged follow-up. Extended follow-up may have occurred and biomarkers collected in clinics closer to patient’s main residence, which explains an additional confounder of different cTnI assay measurements (various providers, variable sensitivity detection with differences in high-sensitive and contemporary assays favoring heterogeneity bias, Supplementary-Table-2);18-20 this concern was less of an issue with cTnT, given a single vendor (All using the Elecsys high-sensitive assay by Roche®). Though, adjustment on the types of cTnI assays (in the multivariate model we used) did not blunt the discrepancy observed between cTnT and cTnI circulating levels evolution after admission for ICI-myocarditis. While these latter points may be seen as limitations of our study, they reflect use of these biomarkers in the real-life setting. Other subgroup analysis by co-prescribed cancer or ICI drugs may be worth pursuing but our cohort was too small and heterogenous to allow for such analysis. Another important limitation is that these findings reflect the biomarker use and MACE incidence of the symptomatic ICI-myocarditis cases, which is an emerging and very recently described disease.5, 7 With the better awareness concerning ICI-myocarditis, we expect an identification of patients at a much earlier stage or even while asymptomatic during systematic troponin/CK screening strategies in ICI treated patients. Therefore, our findings and conclusion might need to be reevaluated in this latter situation specifically. Our troponin prognostic cut-off threshold needs to be validated in independent cohorts, completed prospectively to evaluate if the low-risk population can be managed in an outpatient setting. Lastly, our data suggesting that cTnI may less sensitively detect ICI-myocarditis, need to be interpreted knowing that some of the signal for cTnT may come from the concomitant ICI-myositis. Therefore, acknowledging for these competing issues, cTnT may not be as ideal to evaluate strictly the cardiovascular component of the ICI-myotoxicity disease state.
Conclusion
cTnT is a sensitive biomarker for the diagnosis of ICI-myocarditis and is associated with MACE. A ratio of cTnT/URL<32 within ≤72h of diagnosis is associated with a subgroup at low-risk of MACE. Differences in diagnostic and prognostic performances between cTnT and cTnI in ICI-myocarditis deserve further evaluation.
Supplementary Material
Clinical Perspective.
What is new?
Circulating levels of cTnI and CK normalized earlier in the course of ICI-myocarditis while cTnT levels continued to stay elevated. cTnT was increased in all patients at the time of the first major adverse cardiac and respiratory muscle failure events (MACE) while cTnI and CK were within normal ranges in up to one quarter of patients with MACE.
A cTnT level less than 32x the upper reference limit within 3 days of an ICI-myocarditis diagnosis was associated with a minimal risk of MACE
When diagnosing or surveilling ICI-myocarditis, a normal cTnI value may justify a confirmatory cTnT evaluation to avoid missing active cardio-muscular pathologic involvement.
What are the clinical implications?
Circulating levels of cTnT are associated with MACE and are more often elevated at the time of MACE in ICI-myocarditis patients compared to CK and cTnI.
Kinetic changes of circulating levels of cTnT within the first 72 hours of admission in ICI-myocarditis are associated with risk of MACE in ICI-myocarditis.
While suspecting or following-up an ICI-myocarditis, a normal cTnI value may justify a complementary cTnT evaluation to avoid missing an active ICI-myotoxic active process.
Acknowledgement
We thank Monika Arnold, and Ines Ludwig for support with clinical data collection and patient care. We thank Mr Franck Bournot for his help in getting the technical details of all the assays used herein.
Sources of funding
LHL reported receiving grants LE3570/2-1; 3570/3-1 from the Deutsche Forschungsgemeinschaft (DFG) and grant 01KC2006B from the Bundesministerium für Forschung (BMBF) outside the submitted work. JJM is supported by National Institutes of Health grants (R01HL141466, R01HL155990, R01HL156021, and R01HL160688).
Conflict of interest Disclosures
L.H.L. has served on the advisory board for Daiichi Sankyio, Senaca, and Servier as an external expert for Astra Zeneca and received speakers’ honoraria from Novartis and MSD. JES has served as consultant for BMS, AstraZeneca, BeiGene, IPSEN, EISAI, Novartis and had received grants from BMS, and Novartis. NLP is a Cancer Prevention Research Institute of Texas (CPRIT) Scholar and Andrew Sabin Family Foundation Fellow. NLP is supported by CPRIT RP200670 and by NIH/NCI 1P01CA261669-01. JJM has served on advisory boards for Bristol-Myers Squibb, Takeda, AstraZeneca, Myovant, Kurome Therapeutics, Kiniksa Pharmaceuticals, Daiichi Sankyo, CRC Oncology, BeiGene, Prelude Therapeutics, TransThera Sciences, Antev Ltd, IQVIA, AskBio, Lapcorp, Paladin, Quell Therapeutics, and Cytokinetics.
Non-standard Abbreviations and Acronyms.
- CK
creatine kinase
- CTLA4
Cytotoxic T-Lymphocyte Associated protein 4
- cTnI
cardiac troponin-I
- cTnT
cardiac troponin-T
- ICI
immune checkpoint inhibitors
- LAG3
Lymphocyte Activation Gene-3
- MACE
major adverse cardiac and respiratory muscle failure events
- PD1
Programmed cell Death protein 1
- PDL1
Programmed cell Death protein 1 ligand
- URL
upper reference limit
Appendix
Collaborator names for indexing
Franck Thuny
Shanthini Crusz
Aarti Asnani
Anja Karlstaedt
Fanny Rocher
Elise Paven
Michel Obeid
Wei Ting Chan
Danette L Flint
Dimitri Arangalage
Courand Pierre Yves
Martin Nicol
Cariou Eve
Stephane Ederhy
Yuichi Tamura
Roberta Florido
Sanjeev Francis
Darryl Leong
Nicolas Piriou
Nausheen Akhter
Sandhu Shahneen
Osnat Itzhaki
Manhal Habib
Pankit Vachhani
Giovanni Peretto
Han Zhu
Michal Laufer Perl
Mandar Aras
Joachim Alexandre
Carrie Lenneman
Salim Hayek
Joshua Levenson
Anita Deswal
Vlad Zaha
Elizabeth M Gaughan
Steven Ewer
Douglas Johnson
Lauren A Baldassarre
References
- 1.Johnson DB, Reynolds KL, Sullivan RJ, Balko JM, Patrinely JR, Cappelli LC, Naidoo J and Moslehi JJ. Immune checkpoint inhibitor toxicities: systems-based approaches to improve patient care and research. Lancet Oncol. 2020;21:e398–e404. [DOI] [PubMed] [Google Scholar]
- 2.Ribas A and Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geraud A, Gougis P, Vozy A, Anquetil C, Allenbach Y, Romano E, Funck-Brentano E, Moslehi JJ, Johnson DB and Salem JE. Clinical Pharmacology and Interplay of Immune Checkpoint Agents: A Yin-Yang Balance. Annu Rev Pharmacol Toxicol. 2021;61:85–112. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen LS, Cooper LT, Kerneis M, Funck-Brentano C, Silvain J, Brechot N, Hekimian G, Ammirati E, Ben M'Barek B, Redheuil A, Gandjbakhch E, Bihan K, Lebrun-Vignes B, Ederhy S, Dolladille C, Moslehi JJ and Salem JE. Systematic analysis of drug-associated myocarditis reported in the World Health Organization pharmacovigilance database. Nat Commun. 2022;13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano JP, Balko JM, Bonaca MP, Roden DM, Johnson DB and Moslehi JJ. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allenbach Y, Anquetil C, Manouchehri A, Benveniste O, Lambotte O, Lebrun-Vignes B, Spano JP, Ederhy S, Klatzmann D, Rosenzwajg M, Fautrel B, Cadranel J, Johnson DB, Moslehi JJ and Salem JE. Immune checkpoint inhibitor-induced myositis, the earliest and most lethal complication among rheumatic and musculoskeletal toxicities. Autoimmun Rev. 2020;19:102586. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA Jr., Anders RA, Sosman JA and Moslehi JJ. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med. 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Power JR, Alexandre J, Choudhary A, Ozbay B, Hayek SS, Asnani A, Tamura Y, Aras M, Cautela J, Thuny F, Gilstrap L, Arangalage D, Ewer S, Huang S, Deswal A, Palaskas NL, Finke D, Lehmann LH, Ederhy S, Moslehi J, Salem JE and International ICIMR. Association of early electrical changes with cardiovascular outcomes in immune checkpoint inhibitor myocarditis. Arch Cardiovasc Dis. 2022;115:315–330. [DOI] [PubMed] [Google Scholar]
- 9.Salem JE, Bretagne M, Abbar B, Leonard-Louis S, Ederhy S, Redheuil A, Boussouar S, Nguyen LS, Procureur A, Stein F, Fenioux C, Devos P, Gougis P, Dres M, Demoule A, Psimaras D, Lenglet T, Maisonobe T, De Chambrun MP, Hekimian G, Straus C, Gonzalez-Bermejo J, Klatzmann D, Rigolet A, Guillaume-Jugnot P, Champtiaux N, Benveniste O, Weiss N, Saheb S, Rouvier P, Plu I, Gandjbakhch E, Kerneis M, Hammoudi N, Zahr N, Llontop C, Morelot-Panzini C, Lehmann L, Qin J, Moslehi JJ, Rosenzwajg M, Similowski T and Allenbach Y. Abatacept/Ruxolitinib and Screening for Concomitant Respiratory Muscle Failure to Mitigate Fatality of Immune-Checkpoint Inhibitor Myocarditis. Cancer Discov. 2023;13:1100–1115. [DOI] [PubMed] [Google Scholar]
- 10.Moslehi JJ, Johnson DB and Sosman JA. Myocarditis with Immune Checkpoint Blockade. N Engl J Med. 2017;376:292. [DOI] [PubMed] [Google Scholar]
- 11.Palaskas NL, Segura A, Lelenwa L, Siddiqui BA, Subudhi SK, Lopez-Mattei J, Durand JB, Deswal A, Zhao B, Maximilian Buja L and Iliescu C. Immune checkpoint inhibitor myocarditis: elucidating the spectrum of disease through endomyocardial biopsy. Eur J Heart Fail. 2021;23:1725–1735. [DOI] [PubMed] [Google Scholar]
- 12.Finke D, Heckmann MB, Salatzki J, Riffel J, Herpel E, Heinzerling LM, Meder B, Völkers M, Müller OJ, Frey N, Katus HA, Leuschner F, Kaya Z and Lehmann LH. Comparative Transcriptomics of Immune Checkpoint Inhibitor Myocarditis Identifies Guanylate Binding Protein 5 and 6 Dysregulation. Cancers. 2021;13:2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ and Kerneis M. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. N Engl J Med. 2019;380:2377–2379. [DOI] [PubMed] [Google Scholar]
- 14.Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, Stewart GC, Choueiri TK, Di Carli M, Allenbach Y, Kumbhani DJ, Heinzerling L, Amiri-Kordestani L, Lyon AR, Thavendiranathan P, Padera R, Lichtman A, Liu PP, Johnson DB and Moslehi J. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation. 2019;140:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ederhy S, Devos P, Pinna B, Funck-Brentano E, Abbar B, Fenioux C, Cohen AA, Moslehi J, Bretagne M, Allenbach Y, Kharroubi D and Salem JE. (18)F-fluorodeoxyglucose positron emission tomography/computed tomography imaging for the diagnosis of immune checkpoint inhibitor-associated myocarditis. Arch Cardiovasc Dis. 2022;115:114–116. [DOI] [PubMed] [Google Scholar]
- 16.Vasbinder A, Chen Y, Procureur A, Gradone A, Azam TU, Perry D, Shadid H, Anderson E, Catalan T, Blakely P, Nelapudi N, Fardous M, Bretagne MC, Adie SK, Pogue KT, Leja M, Yentz S, Schneider B, Fecher LA, Lao CD, Salem J-E and Hayek SS. Biomarker Trends, Incidence, and Outcomes of Immune Checkpoint Inhibitor–Induced Myocarditis. JACC: CardioOncology. 2022;4:689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehmann LH, Cautela J, Palaskas N, Baik AH, Meijers WC, Allenbach Y, Alexandre J, Rassaf T, Muller OJ, Aras M, Asnani AH, Deswal A, Laufer-Perl M, Thuny F, Kerneis M, Hayek SS, Ederhy S, Salem JE and Moslehi JJ. Clinical Strategy for the Diagnosis and Treatment of Immune Checkpoint Inhibitor-Associated Myocarditis: A Narrative Review. JAMA Cardiol. 2021;6:1329–1337. [DOI] [PubMed] [Google Scholar]
- 18.Januzzi JL Jr., Mahler SA, Christenson RH, Rymer J, Newby LK, Body R, Morrow DA and Jaffe AS. Recommendations for Institutions Transitioning to High-Sensitivity Troponin Testing: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;73:1059–1077. [DOI] [PubMed] [Google Scholar]
- 19.Clerico A, Padoan A, Zaninotto M, Passino C and Plebani M. Clinical relevance of biological variation of cardiac troponins. Clin Chem Lab Med. 2021;59:641–652. [DOI] [PubMed] [Google Scholar]
- 20.Wu AHB, Christenson RH, Greene DN, Jaffe AS, Kavsak PA, Ordonez-Llanos J and Apple FS. Clinical Laboratory Practice Recommendations for the Use of Cardiac Troponin in Acute Coronary Syndrome: Expert Opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem. 2018;64:645–655. [DOI] [PubMed] [Google Scholar]
- 21.Power JR, Alexandre J, Choudhary A, Ozbay B, Hayek S, Asnani A, Tamura Y, Aras M, Cautela J, Thuny F, Gilstrap L, Arangalage D, Ewer S, Huang S, Deswal A, Palaskas NL, Finke D, Lehman L, Ederhy S, Moslehi J, Salem JE and International ICIMR. Electrocardiographic Manifestations of Immune Checkpoint Inhibitor Myocarditis. Circulation. 2021;144:1521–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nowatzke J, Guedeney P, Palaskas N, Lehmann L, Ederhy S, Zhu H, Cautela J, Francis S, Courand PY, Deswal A, Ewer SM, Aras M, Arangalage D, Ghafourian K, Fenioux C, Finke D, Peretto G, Zaha V, Itzhaki Ben Zadok O, Tajiri K, Akhter N, Levenson J, Baldassarre L, Power J, Huang S, Collet JP, Moslehi J, Salem JE and International ICImrc. Coronary artery disease and revascularization associated with immune checkpoint blocker myocarditis: Report from an international registry. Eur J Cancer. 2022;177:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippi G, Aloe R, Meschi T, Borghi L and Cervellin G. Interference from heterophilic antibodies in troponin testing. Case report and systematic review of the literature. Clin Chim Acta. 2013;426:79–84. [DOI] [PubMed] [Google Scholar]
- 24.Mair J, Lindahl B, Muller C, Giannitsis E, Huber K, Mockel M, Plebani M, Thygesen K and Jaffe AS. What to do when you question cardiac troponin values. Eur Heart J Acute Cardiovasc Care. 2018;7:577–586. [DOI] [PubMed] [Google Scholar]
- 25.Waliany S, Neal JW, Reddy S, Wakelee H, Shah SA, Srinivas S, Padda SK, Fan AC, Colevas AD, Wu SM, Witteles RM and Zhu H. Myocarditis Surveillance with High-Sensitivity Troponin I During Cancer Treatment with Immune Checkpoint Inhibitors. JACC CardioOncol. 2021;3:137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N and Honjo T. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477–83. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N and Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–22. [DOI] [PubMed] [Google Scholar]
- 28.Bockstahler M, Fischer A, Goetzke CC, Neumaier HL, Sauter M, Kespohl M, Muller AM, Meckes C, Salbach C, Schenk M, Heuser A, Landmesser U, Weiner J, Meder B, Lehmann L, Kratzer A, Klingel K, Katus HA, Kaya Z and Beling A. Heart-Specific Immune Responses in an Animal Model of Autoimmune-Related Myocarditis Mitigated by an Immunoproteasome Inhibitor and Genetic Ablation. Circulation. 2020;141:1885–1902. [DOI] [PubMed] [Google Scholar]
- 29.Waliany S, Lee D, Witteles RM, Neal JW, Nguyen P, Davis MM, Salem JE, Wu SM, Moslehi JJ and Zhu H. Immune Checkpoint Inhibitor Cardiotoxicity: Understanding Basic Mechanisms and Clinical Characteristics and Finding a Cure. Annu Rev Pharmacol Toxicol. 2021;61:113–134. [DOI] [PubMed] [Google Scholar]
- 30.Goser S, Andrassy M, Buss SJ, Leuschner F, Volz CH, Ottl R, Zittrich S, Blaudeck N, Hardt SE, Pfitzer G, Rose NR, Katus HA and Kaya Z. Cardiac troponin I but not cardiac troponin T induces severe autoimmune inflammation in the myocardium. Circulation. 2006;114:1693–702. [DOI] [PubMed] [Google Scholar]
- 31.Leuschner F, Li J, Goser S, Reinhardt L, Ottl R, Bride P, Zehelein J, Pfitzer G, Remppis A, Giannitsis E, Katus HA and Kaya Z. Absence of auto-antibodies against cardiac troponin I predicts improvement of left ventricular function after acute myocardial infarction. Eur Heart J. 2008;29:1949–55. [DOI] [PubMed] [Google Scholar]
- 32.Touat M, Maisonobe T, Knauss S, Ben Hadj Salem O, Hervier B, Aure K, Szwebel TA, Kramkimel N, Lethrosne C, Bruch JF, Laly P, Cadranel J, Weiss N, Behin A, Allenbach Y, Benveniste O, Lenglet T, Psimaras D, Stenzel W and Leonard-Louis S. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. 2018;91:e985–e994. [DOI] [PubMed] [Google Scholar]
- 33.du Fay de Lavallaz J, Prepoudis A, Wendebourg MJ, Kesenheimer E, Kyburz D, Daikeler T, Haaf P, Wanschitz J, Loscher WN, Schreiner B, Katan M, Jung HH, Maurer B, Hammerer-Lercher A, Mayr A, Gualandro DM, Acket A, Puelacher C, Boeddinghaus J, Nestelberger T, Lopez-Ayala P, Glarner N, Shrestha S, Manka R, Gawinecka J, Piscuoglio S, Gallon J, Wiedemann S, Sinnreich M, Mueller C and Investigators BX. Skeletal Muscle Disorders: A Noncardiac Source of Cardiac Troponin T. Circulation. 2022;145:1764–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher R. Statistical tests for research workers. 1925. [Google Scholar]
- 35.Chen TC, Liu HW, Russell A, Barthel BL, Tseng KW, Huang MJ, Chou TY and Nosaka K. Large increases in plasma fast skeletal muscle troponin I after whole-body eccentric exercises. J Sci Med Sport. 2020;23:776–781. [DOI] [PubMed] [Google Scholar]
- 36.Jaffe AS, Vasile VC, Milone M, Saenger AK, Olson KN and Apple FS. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol. 2011;58:1819–24. [DOI] [PubMed] [Google Scholar]
- 37.Rittoo D, Jones A, Lecky B and Neithercut D. Elevation of cardiac troponin T, but not cardiac troponin I, in patients with neuromuscular diseases: implications for the diagnosis of myocardial infarction. J Am Coll Cardiol. 2014;63:2411–20. [DOI] [PubMed] [Google Scholar]
- 38.Schmid J, Liesinger L, Birner-Gruenberger R, Stojakovic T, Scharnagl H, Dieplinger B, Asslaber M, Radl R, Beer M, Polacin M, Mair J, Szolar D, Berghold A, Quasthoff S, Binder JS and Rainer PP. Elevated Cardiac Troponin T in Patients With Skeletal Myopathies. J Am Coll Cardiol. 2018;71:1540–1549. [DOI] [PubMed] [Google Scholar]
- 39.Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, Monestier S, Grob JJ, Scemama U, Jacquier A, Lalevee N, Barraud J, Peyrol M, Laine M, Bonello L, Paganelli F, Cohen A, Barlesi F, Ederhy S and Thuny F. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity. Circulation. 2017;136:2085–2087. [DOI] [PubMed] [Google Scholar]
- 40.Dolladille C, Ederhy S, Allouche S, Dupas Q, Gervais R, Madelaine J, Sassier M, Plane AF, Comoz F, Cohen AA, Thuny FR, Cautela J and Alexandre J. Late cardiac adverse events in patients with cancer treated with immune checkpoint inhibitors. J Immunother Cancer. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnadottir A, Pedersen S, Bo Hasselbalch R, Goetze JP, Friis-Hansen LJ, Bloch-Munster AM, Skov Jensen J, Bundgaard H and Iversen K. Temporal Release of High-Sensitivity Cardiac Troponin T and I and Copeptin After Brief Induced Coronary Artery Balloon Occlusion in Humans. Circulation. 2021;143:1095–1104. [DOI] [PubMed] [Google Scholar]
- 42.Pickering JW, Young JM, George PM, Pemberton CJ, Watson A, Aldous SJ, Verryt T, Troughton RW, Richards AM, Apple FS and Than MP. Early kinetic profiles of troponin I and T measured by high-sensitivity assays in patients with myocardial infarction. Clin Chim Acta. 2020;505:15–25. [DOI] [PubMed] [Google Scholar]
- 43.Rubini Gimenez M, Twerenbold R, Reichlin T, Wildi K, Haaf P, Schaefer M, Zellweger C, Moehring B, Stallone F, Sou SM, Mueller M, Denhaerynck K, Mosimann T, Reiter M, Meller B, Freese M, Stelzig C, Klimmeck I, Voegele J, Hartmann B, Rentsch K, Osswald S and Mueller C. Direct comparison of high-sensitivity-cardiac troponin I vs. T for the early diagnosis of acute myocardial infarction. Eur Heart J. 2014;35:2303–11. [DOI] [PubMed] [Google Scholar]
- 44.Starnberg K, Friden V, Muslimovic A, Ricksten SE, Nystrom S, Forsgard N, Lindahl B, Vukusic K, Sandstedt J, Dellgren G and Hammarsten O. A Possible Mechanism behind Faster Clearance and Higher Peak Concentrations of Cardiac Troponin I Compared with Troponin T in Acute Myocardial Infarction. Clin Chem. 2020;66:333–341. [DOI] [PubMed] [Google Scholar]
- 45.Tveit SH, Cwikiel J, Myhre PL, Omland T, Berge E, Seljeflot I and Flaa A. Differential associations of cardiac troponin T and cardiac troponin I with coronary artery pathology and dynamics in response to short-duration exercise. Clin Biochem. 2021;88:23–29. [DOI] [PubMed] [Google Scholar]
- 46.van der Linden N, Wildi K, Twerenbold R, Pickering JW, Than M, Cullen L, Greenslade J, Parsonage W, Nestelberger T, Boeddinghaus J, Badertscher P, Rubini Gimenez M, Klinkenberg LJJ, Bekers O, Schoni A, Keller DI, Sabti Z, Puelacher C, Cupa J, Schumacher L, Kozhuharov N, Grimm K, Shrestha S, Flores D, Freese M, Stelzig C, Strebel I, Miro O, Rentsch K, Morawiec B, Kawecki D, Kloos W, Lohrmann J, Richards AM, Troughton R, Pemberton C, Osswald S, van Dieijen-Visser MP, Mingels AM, Reichlin T, Meex SJR and Mueller C. Combining High-Sensitivity Cardiac Troponin I and Cardiac Troponin T in the Early Diagnosis of Acute Myocardial Infarction. Circulation. 2018;138:989–999. [DOI] [PubMed] [Google Scholar]
- 47.Wereski R, Kimenai DM, Taggart C, Doudesis D, Lee KK, Lowry MTH, Bularga A, Lowe DJ, Fujisawa T, Apple FS, Collinson PO, Anand A, Chapman AR and Mills NL. Cardiac Troponin Thresholds and Kinetics to Differentiate Myocardial Injury and Myocardial Infarction. Circulation. 2021;144:528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyngbakken MN, Aagaard EN, Kvisvik B, Berge T, Pervez MO, Brynildsen J, Tveit A, Steine K, Rosjo H and Omland T. Cardiac Troponin I and T Are Associated with Left Ventricular Function and Structure: Data from the Akershus Cardiac Examination 1950 Study. Clin Chem. 2020;66:567–578. [DOI] [PubMed] [Google Scholar]
- 49.Tang O, Matsushita K, Coresh J, Ndumele C, McEvoy JW, Sharrett AR, Hoogeveen R, Ballantyne CM and Selvin E. High-Sensitivity Cardiac Troponin I and T for Cardiovascular Risk Stratification in Adults With Diabetes. Diabetes Care. 2020;43:e144–e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Welsh P, Preiss D, Hayward C, Shah ASV, McAllister D, Briggs A, Boachie C, McConnachie A, Padmanabhan S, Welsh C, Woodward M, Campbell A, Porteous D, Mills NL and Sattar N. Cardiac Troponin T and Troponin I in the General Population. Circulation. 2019;139:2754–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katrukha IA and Katrukha AG. Myocardial Injury and the Release of Troponins I and T in the Blood of Patients. Clin Chem. 2021;67:124–130. [DOI] [PubMed] [Google Scholar]
- 52.Lippi G and Cervellin G. Cardiac troponin T versus cardiac troponin I for mortality risk prediction: Is one biomarker better than the other? Clin Biochem. 2020;78:40–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.