Abstract
Background:
An emerging strategy to treat symptoms of gastrointestinal (GI) dysmotility utilizes the administration of isolated bacteria. However, the underlying mechanisms of action of these bacterial agents are not well established. Here, we elucidate a novel approach to promote intestinal motility by exploiting the biochemical capability of specific bacteria to produce the serotonin (5-HT) precursor, tryptophan (Trp).
Methods:
Mice were treated daily for one week by oral gavage of Bacillus (B.) subtilis (R0179), heat-inactivated R0179, or a tryptophan synthase-null strain of B. subtilis (1A2). Tissue levels of Trp, 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) were measured and changes in motility were evaluated.
Key Results:
Mice treated with B. subtilis R0179 exhibited greater colonic tissue levels of Trp and the 5-HT breakdown product, 5-HIAA compared to vehicle-treated mice. Furthermore, B. subtilis treatment accelerated colonic motility in both healthy mice as well as in a mouse model of constipation. These effects were not observed with heat-inactivated R0179 or the live 1A2 strain that does not express tryptophan synthase. Lastly, we found that the prokinetic effects of B. subtilis R0179 were blocked by co-administration of a 5-HT4 receptor (5-HT4R) antagonist and were absent in 5-HT4R knockout mice.
Conclusions and Inferences:
Taken together, these data demonstrate that intestinal motility can be augmented by treatment with bacteria that synthesize Trp, possibly through increased 5-HT signaling and/or actions of Trp metabolites, and involvement of the 5-HT4R. Our findings provide mechanistic insight into a transient and predictable bacterial strategy to promote GI motility.
Keywords: enteric nervous system, gut microbiota, intestinal motility, microbial metabolites, tryptophan synthase
Graphical Abstract
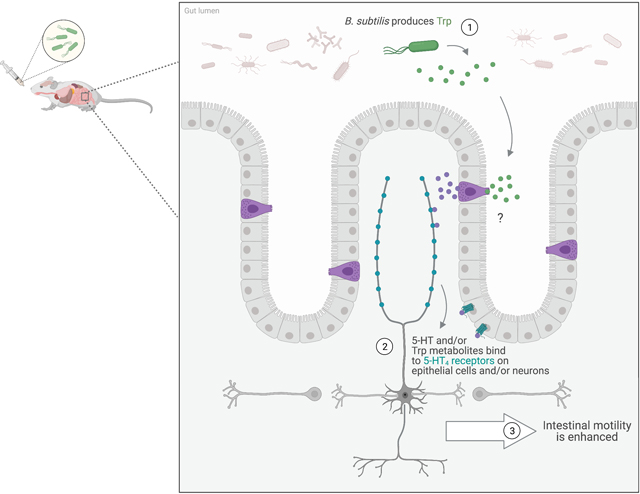
B. subtilis-produced tryptophan (Trp) increased colonic levels of Trp and the serotonin (5-HT) metabolite, 5-Hydroxyindoleacetic acid, and also enhanced colonic motility in normal and constipated mice. These actions were not seen with heat-inactivated B. subtilis or with a B. subtilis strain incapable of synthesizing Trp. Furthermore, the prokinetic effects were not seen when 5-HT4 receptors were blocked or knocked out, demonstrating that the 5-HT4 receptor is involved in the prokinetic actions of B. subtilis. The “?” indicates that we are still not sure whether the prokinetic effect involves increased 5-HT signaling and/or actions of Trp metabolites.
INTRODUCTION
It is widely accepted that the gut microbiome plays an active role in contributing to our health and well-being. Indeed, the tens of trillions of microorganisms lining the gastrointestinal (GI) tract interact dynamically to influence the physiological homeostasis of the host.1 As such, an emerging field of research investigates potential interventions to manipulate the existing gut microbiota or additions of exogenous bacteria to affect pathologic conditions and dysbiosis.
Healthy GI function necessitates a series of coordinated neural reflexes and signaling molecules. Serotonin (5-hydroxytryptamine; 5-HT) is known to play an integral role in regulating intestinal functions including motility, secretion, and blood flow, as well as delivering signals to the central nervous system (CNS).2 The majority of the body’s 5-HT is synthesized in the epithelium of the gut by enterochromaffin (EC) cells from the precursor tryptophan (Trp) using the rate-limiting enzyme tryptophan hydroxylase 1 (Tph1). Once synthesized, 5-HT is released in response to luminal chemical and mechanical stimuli to activate a host of GI reflexes, including peristalsis and secretion. It is known that 5-HT exerts its effects on GI function through the activation of a variety of receptors, including the 5-HT4 receptor.1,3
Mounting evidence points to a key role of gut microbes in influencing the host epithelial 5-HT signaling as well as 5-HT-mediated gut functions.4 Both germ-free mice and antibiotic-treated animals exhibit decreased levels of colonic 5-HT and decreased colonic Tph1 mRNA expression, which are accompanied by functional deficits in GI motility.5–9 Moreover, gut bacteria produce microbial metabolites that can stimulate 5-HT biosynthesis and release, and induce prokinetic effects on GI motility.4 One critical microbially-mediated process is the regulation of Trp metabolism.10 Trp, as an essential amino acid, cannot be synthesized de novo by the body and instead is acquired through dietary intake in sufficient quantities to meet metabolic demands. Notably, some bacteria are enzymatically capable of synthesizing Trp using tryptophan synthase or degrading Trp using tryptophanase.10
The administration of isolated bacterial strains, commonly referred to as “probiotics”, is an emerging field of research that seeks to beneficially alter host physiology for therapeutic ends. These live microorganisms, given commonly at doses of billions of colony forming units (CFUs) per day, are thought to exert pleiotropic effects on the host without inducing dysbiosis or major shifts in the microbiota composition.11 Orally delivered bacteria can influence mucosal serotonergic signaling by promoting the bioavailability of host 5-HT or through indirect means such as stimulating the secretion of microbial metabolites that act on the serotonergic system.5,12–14 While targeted bacterial treatments offer exciting potential to alter gut function in a transient, inducible, and reversible manner, there is a growing need for the elucidation of mechanisms underlying the effects of specific probiotic bacterial species and strains. This will facilitate optimization of desired outcomes.
In this study, we investigated a novel strategy to promote 5-HT-mediated intestinal function by attempting to increase Trp availability using the biochemical properties of specific bacteria. We utilized the bacterial strain Bacillus (B.) subtilis R0179 that is known to express tryptophan synthase, a key enzyme that synthesizes Trp from indole. We tested the hypothesis that oral administration of these Trp-synthesizing bacteria alters the availability of the amino acid for absorption in the intestinal lumen and ultimately induce prokinetic effects on GI function.
MATERIALS AND METHODS
Animals
The current study used male C57BL/6J mice 8–9 weeks old (Jackson Labs; Bar Harbor, ME, USA), and male and female 5-HT4 receptor (5-HT4R) KO mice and their wild type littermates aged 8–9 weeks and bred in-house on an SV129 background (Dr. Valerie Compan, Université Montpellier, via Dr. David Linden, Mayo Clinic). All animals were housed and cared for in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals as well as the Office of Animal Care Management of the University of Vermont. Mice were weighed and monitored daily. All experimental protocols were approved by the University of Vermont Institutional Animal Care and Use Committee (Protocols# PROTO202000040; PROTO202100028).
Bacteria
The experiments primarily utilized Bacillus (B.) subtilis strain R0179, provided by Lallemand Health Solutions, Inc. (Montreal, QC, CAN), which has been used in probiotic formulations and is known to be safe in humans.
Freeze-dried spores of B. subtilis R0179 were stored at 4°C. Each day of treatment, the bacteria were suspended at a concentration of 1010 CFU mL−1 in phosphate buffered saline (PBS) and administered by oral gavage with a 100 μL dose per mouse per day. Mice in the vehicle control group received a daily oral gavage of 100 μL PBS.
To validate our experimental approach, we tested for the presence of the R0179 strain of B. subtilis in fecal samples collected from experimental and control mice. A QIAamp PowerFecal DNA Kit (Qiagen; Germantown, MD, USA) was used to extract DNA from stool samples collected on the last day of treatment. PCR assays, using primers designed and tested by Lallemand to be specific to the R0179 strain of B. subtilis (5’ GTT GAA CAG CGT GTC AGT CG 3’; 5’ TCA AAC ATA GCA GTT GCG GC 3’), confirmed the presence of the experimental strain in bacteria-treated mice. No bacterial cross-contamination was present in stool samples from mice in the control group.
In addition to the vehicle group, the bacterial experiments for the study utilized two additional control treatments. Heat-inactivated B. subtilis R0179 was generated by autoclaving B. subtilis R0179 spores for 40 minutes. The heat-killed spores were then combined with PBS and incubated for 30 minutes in a 56ºC water bath, plated on LB agar plates, and incubated in a 37ºC warm room for 24 hours to confirm cell death. Heat-inactivated B. subtilis R0179 was administered by oral gavage with a 100 μL dose per mouse per day.
The B. subtilis strain 1A2, which has a non-revertible loss of function mutation in the tryptophan synthase gene, and the wildtype strain B. subtilis 1A1 were obtained as spore-containing discs from the Bacillus Genetic Stock Center (Ohio State University, Columbus, OH, USA). The spore-containing discs were plated on Luria-Bertani (LB) agar, covered with 1 mL LB liquid medium, and incubated at 37ºC overnight. Growth from this plate was streaked for isolation on another LB agar plate for 37ºC overnight incubation. One colony was then isolated and transferred to a 3 mL aliquot of LB liquid medium for repeat incubation at 37ºC overnight. 50% glycerol stock was then created using 500 μL glycerol and 500 μL water before being stored at −80ºC. For daily gavage, one LB agar plate was inoculated with the bacterial glycerol stock and incubated at 37ºC overnight. One single colony was then incubated at 37ºC overnight with 3 mL LB liquid medium. The gavage solution was created by combining 10 μL fresh bacterial culture with 3 mL PBS each day for a concentration of 1010 CFU mL-1. Mice received daily oral gavages of 100 μL per day.
Incubation times for the desired bacterial concentration of 1010 CFU mL−1 were determined using a 96-well plate serial dilution procedure. The optical density of the liquid cultures was measured daily at 600 nm to ensure the desired bacterial concentration was being reached.
High performance liquid chromatography with electrochemical detection
Measurements of Trp, 5-HT, and 5-hydroxyindoleacetic acid (5-HIAA) in intestinal tissue were obtained using high performance liquid chromatography (HPLC) with electrochemical detection. Upon euthanasia, 0.5 cm of distal colon tissue was collected from experimental mice, flash frozen in liquid nitrogen, and stored at −80ºC.
For processing, tissue samples were thawed, homogenized in 800 μL 0.1 M perchloric acid (Sigma Aldrich; St. Louis, MO, USA), and centrifuged for 15 minutes at 13,000 x g at 4ºC. The supernatant was collected and centrifuged once more for 15 minutes at 13,000 x g at 4ºC. The resultant supernatant was processed through a series of filtration steps using 17 mm Nylon 0.45 μm and 0.2 μm syringe filters (Thermo Scientific; Waltham, MA, USA). The final retention volume was 400–500 μL per sample, which was aliquoted and stored at −80ºC until analysis by HPLC.
For analysis, 20 μL of each sample was injected into the HPLC system. The system includes a Shimadzu LC-10ADvp pump (Shimadzu Corp.; Kyoto, Japan) and a Coulochem II electrochemical detector (ESA Inc.; Chelmsford, MA, USA). The mobile phase was comprised of 90 mM dihydrogen phosphate, 50 mM citric acid, 4% acetonitrile, and 50 μM EDTA at a pH of 3, and was pumped through a Microsorb 100–3 C18 100 × 4.6 mm column (Agilent Technologies, Inc.; Santa Clara, CA, USA) at a flow rate of 0.4 mL min-1.
Calibration curves were generated for each metabolite based on injections of known standard concentrations (Sigma Aldrich). Trp, 5-HT, and 5-HIAA were identified by their expected retention times in chromatographs generated using PC software (Chromperfect; Denville, NJ, USA). Concentrations of Trp, 5-HT, and 5-HIAA were quantified by calculating the area under the curve in the chromatographs for each injected sample, and then were normalized to tissue weight.
Whole Gut Transit Time
Whole gut transit time is a net measure of intestinal function that encompasses gastric emptying, small bowel transit, and colonic transit times. Non-fasted mice were orally gavaged with 300 μL non-nutrient solution made of 6% carmine red, 0.5% methylcellulose, and tap water. Carmine red is nonabsorbable in the gut lumen and therefore provides a visual indication of transit time through the entire GI tract. Following gavage, mice were placed individually in empty cages with access to food and Napa Nectar ad libitum. The whole gut transit time was calculated by the amount of time elapsed from time of gavage to time of expulsion of the first carmine red-positive fecal pellet.
Colonic Motility
Colonic motility was assessed using the bead expulsion assay. Following an acclimation period during which mice were placed in separate cages for one hour, non-fasted mice were then lightly anesthetized with 3% isoflurane and a size 10 bead was inserted 2 cm into the distal colon using a blunt gavage needle. Colonic motility time was calculated by the amount of time elapsed from insertion of bead to its expulsion.
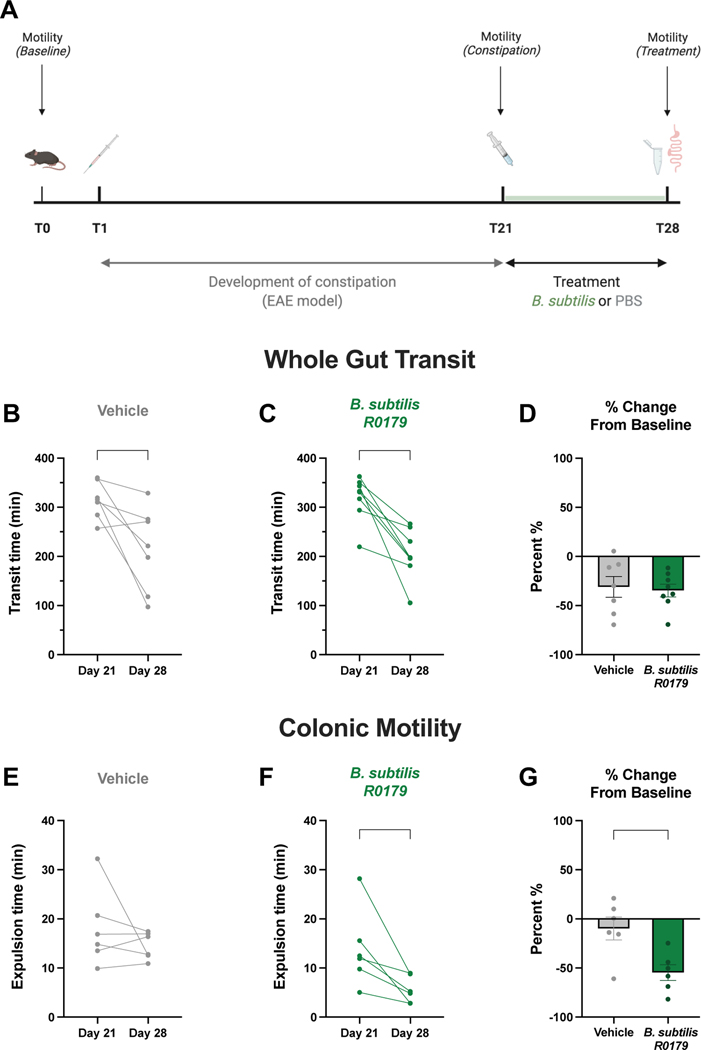
Experimental autoimmune encephalomyelitis (EAE) induction
Mice were immunized at 8 weeks old with mouse spinal cord homogenate (MSCH) using the complete Freund’s adjuvant (CFA) plus pertussis toxin (PTX) single-inoculation protocol.15 Mice received three posterior subcutaneous injections (50 μL each) of an emulsion of CFA containing 200 μg mL−1 Mycobacterium tuberculosis H37RA (Becton Dickinson & Co.; Franklin Lakes, NJ, USA) and 25 mg mL−1 MSCH. These injections were followed by intraperitoneal injection of 200 ng PTX on day 0 and again on day 2 of induction.
Mice were weighed and monitored daily beginning at 10 days post-induction. Cumulative clinical disease scores were recorded daily as previously described.16 Constipation, the hallmark symptom of GI dysmotility in this model, was assessed on day 21 post-induction. Following the assessment, mice displaying constipation were treated for one week with B. subtilis R0179 or vehicle control.
5-HT4R antagonist treatment
Mice received daily oral gavages of a 100 μL solution containing 1010 CFU mL−1 of B. subtilis R0179 in addition to a 5-HT4R selective antagonist GR113808 (1 mg kg−1; Tocris Bioscience; Bristol, UK).
Tryptophan diets
Mice were fed a control normal diet (Baker Amino Acid Diet 5CC7, TestDiet; St. Louis, MO, USA) with 0.2% Trp for 10 days prior to the start of the experiment. Baseline motility was assessed following this period. Mice were then placed on diets that had been modified based on the control diet to consist of no added Trp (Trp–; 0.0%, 575J, TestDiet) or twice the amount of Trp (Trp2x; 0.45%, LT570, TestDiet) for 10 days. All diets contained the same nutritional profiles except for these modifications in Trp content. Diets were fed ad libitum in irradiated pellet form in cage hoppers with full access to fresh water at all times. Motility was assessed following 10 days of modified diet access.
Statistics
All statistical analyses were performed using GraphPad Prism (v.9, GraphPad Software; San Diego, CA, USA). Data are presented as individual values or as mean ± standard error of the mean (SEM). Statistical differences were determined by analysis using unpaired Student’s t-test, paired Student’s t-test, or one-way ANOVA with Tukey’s post-hoc multiple comparisons test. Outliers were determined using a Q test, and values that were greater than 2 standard deviations from the mean were excluded. Statistical significance is considered as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
RESULTS
While Trp synthesis is a well-recognized feature of B. subtilis, we confirmed that the R0179 strain produces Trp by comparing Trp levels in tryptic soy broth growth medium at the beginning and end of a 48-hour incubation period. In this experiment, a 78% increase of Trp in the medium was detected following incubation with R0179, confirming that tryptophan synthase is active in this R0179 strain of B. subtilis (data not shown).
In our experiments, we administered B. subtilis daily for the entire treatment course. The presence of the R0179 strain of B. subtilis was detected in the feces of bacteria-treated mice collected upon euthanasia as validation of our experimental approach. In a separate study, we found that the R0179 strain is not detected in feces collected 24, 48, or 72 hours after a single gavage, suggesting that B. subtilis R0179 does not colonize the GI tract.
B. subtilis treatment increased colonic Trp and 5-HIAA levels
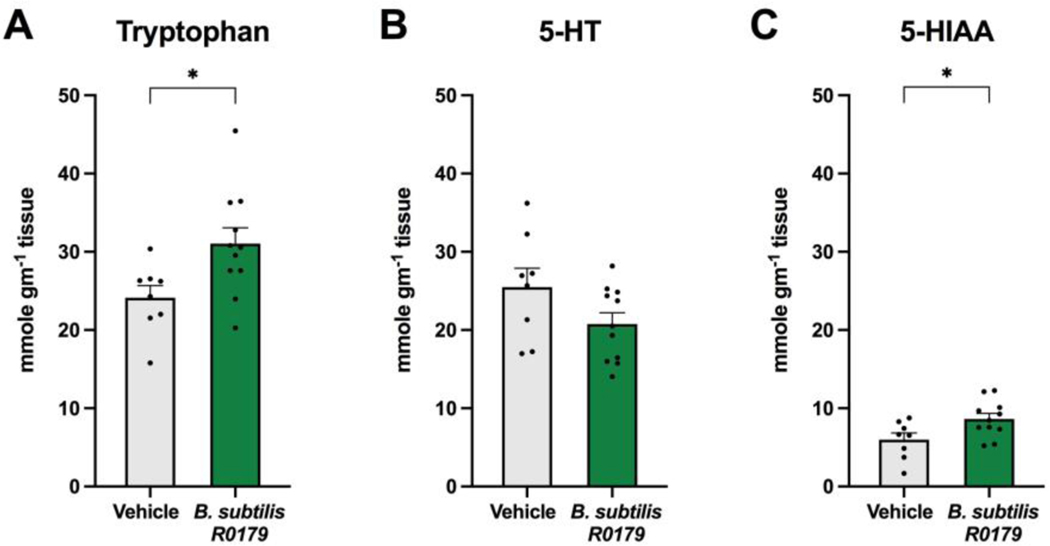
To determine whether B. subtilis R0179 treatment alters intestinal 5-HT signaling, concentrations of Trp, 5-HT, and 5-HIAA in distal colon tissue were measured by HPLC. Mice treated with B. subtilis R0179 exhibited greater levels of Trp in the distal colon compared to vehicle-treated mice (Fig. 1a). While there was no significant difference in colonic 5-HT levels (Fig. 1b), bacteria-treated mice displayed greater levels of the 5-HT breakdown product, 5-HIAA (Fig. 1c).
Figure 1. B. subtilis R0179 treatment increases colonic Trp and 5-HIAA levels.
To determine whether one week of treatment with the Trp-synthesizing strain B. subtilis R0179 alters colonic metabolite levels in mice, distal colon tissue was collected at day 7 upon euthanasia and analyzed by HPLC. Mice that received B. subtilis (n=11) displayed significantly higher levels of colonic Trp and 5-HIAA compared to vehicle control mice (n=8). Data are shown as mean ± SEM. Unpaired Student’s t-test. * p < 0.05.
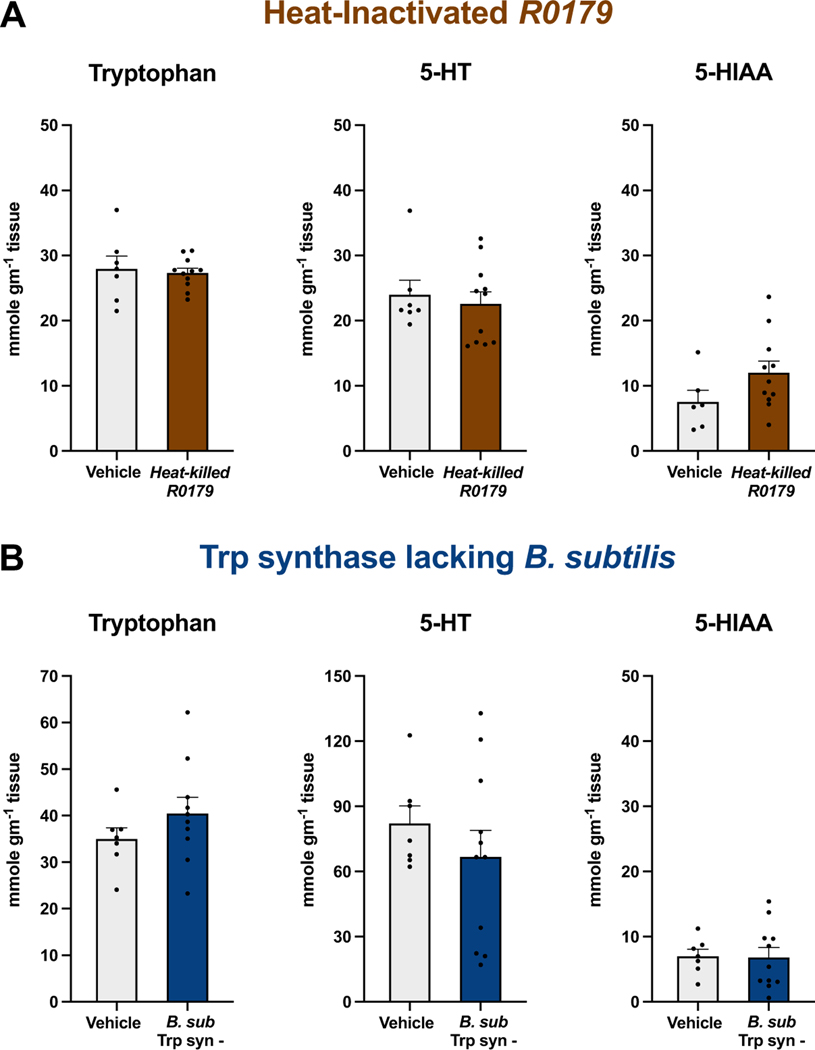
To examine whether B. subtilis R0179-induced changes were a consequence of the bacteria’s metabolic activity, we analyzed distal colon samples from mice treated with heat-inactivated R0179. Treatment with heat-inactivated B. subtilis R0179 did not alter levels of Trp, 5-HT, or 5-HIAA in distal colon tissue compared to vehicle treatment (Fig. 2a), demonstrating that administration of viable B. subtilis R0179 spores is required to induce alterations in metabolite levels.
Figure 2. Treatment with either heat-inactivated B. subtilis R0179 or with a non-Trp-synthesizing strain of B. subtilis does not alter colonic metabolite levels.
(A) To determine whether the B. subtilis R0179-induced increases in colonic Trp and 5-HIAA levels were due to the live nature of the administered bacteria, mice were treated for one week with heat-inactivated B. subtilis R0179. Analysis of distal colon tissue via HPLC revealed no significant differences in colonic Trp, 5-HT, or 5-HIAA between mice treated with heat-inactivated B. subtilis R0179 (n=11) and vehicle control (n=7). (B) To examine whether the altered metabolite levels from B. subtilis R0179 treatment were due to the Trp-synthesizing property of this bacterial strain, mice were treated for one week with B. subtilis 1A2 (B. sub Trp syn-), a strain that lacks tryptophan synthase, the key enzyme in the synthesis of Trp from indole. Mice that received this non-Trp-synthesizing strain (n=11) displayed no significant differences in colonic Trp, 5-HT, or 5-HIAA compared to vehicle-treated mice (n=7). Data are shown as mean ± SEM. Unpaired Student’s t-test.
The R0179 strain of B. subtilis expresses the enzyme tryptophan synthase, which synthesizes Trp from indole. To determine whether the altered colonic metabolite levels from B. subtilis R0179 treatment were due to this strain’s Trp-synthesizing property, we analyzed distal colon tissue of mice treated with a B. subtilis strain that is a tryptophan auxotroph due to mutation of the tryptophan synthase gene (B. subtilis 1A2). Mice treated with B. subtilis 1A2 exhibited no differences in colonic Trp, 5-HT, or 5-HIAA levels compared to vehicle-treated mice (Fig. 2b).
B. subtilis treatment induced prokinetic effects on colonic motility
The findings described above demonstrate that B. subtilis R0179 increases intestinal Trp and 5-HIAA content. We therefore sought to examine whether bacteria treatment consequently alters motility, which is a 5-HT-associated gut function. To assess changes in intestinal function in response to B. subtilis treatment, we performed validated motility assays at the beginning and end of a 7-day daily treatment period.
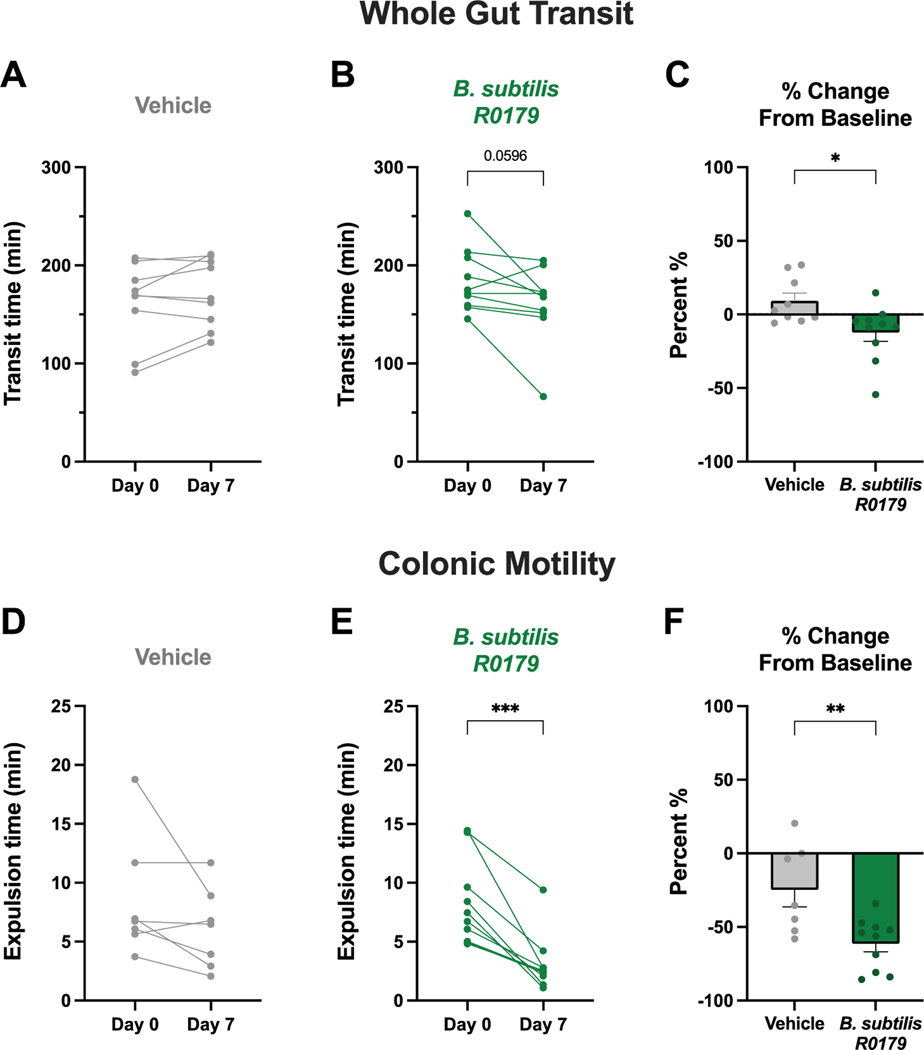
Whole gut transit time is a non-localized measure of intestinal function comprised of the net motility times of gastric emptying, small bowel transit, and colonic transit. Following a week of treatment with B. subtilis R0179, there was no change in whole gut transit time in either vehicle-treated mice (Fig. 3a) or bacteria-treated mice (Fig. 3b). However, when normalized to baseline, the effects of vehicle vs. B. subtilis R0179 were significantly different, with the B. subtilis R0179 group being faster and the vehicle group being slightly slower than baseline values (Fig. 3c).
Figure 3. B. subtilis treatment produces prokinetic actions on colonic motility.
To investigate the functional effects of B. subtilis R0179 treatment on intestinal motility, two different motility assays were performed at baseline and again after one week of treatment. Whole gut transit time is a non-localized, net motility measure encompassing gastric emptying, small bowel transit, and colonic transit. There was no change in whole gut transit time in vehicle-treated mice (A; n=9) or in mice that received B. subtilis (B; n=10) following a week of treatment. The bead expulsion assay was used to assess bacteria-induced changes in motility localized to the colon, the region of the GI tract that contains the highest numbers and biodiversity of bacteria. There was no change in colonic motility in vehicle-treated mice (D; n=7), but B. subtilis-treated mice (E; n=10) exhibited significantly faster colonic motility times following a week of treatment. Furthermore, there was a significantly different percent change from baseline between B. subtilis and vehicle control treatment groups (C, F). Data are shown as individual animals (A,B,D,E), paired Student’s t-test, or mean ± SEM (C,F), unpaired Student’s t-test. * p < 0.05, ** p < 0.01, *** p < 0.001.
To evaluate colonic motility, we employed the bead expulsion assay. Mice that received B. subtilis R0179 exhibited significantly faster bead expulsion on day 7 compared to baseline (Fig. 3e), whereas vehicle-treated mice displayed no change (Fig. 3d). Furthermore, the treatment groups were significantly different when the findings were evaluated as percent change from baseline (Fig. 3f). The data from these functional assays indicate that B. subtilis R0179 produces prokinetic effects on intestinal motility that are localized to the colon.
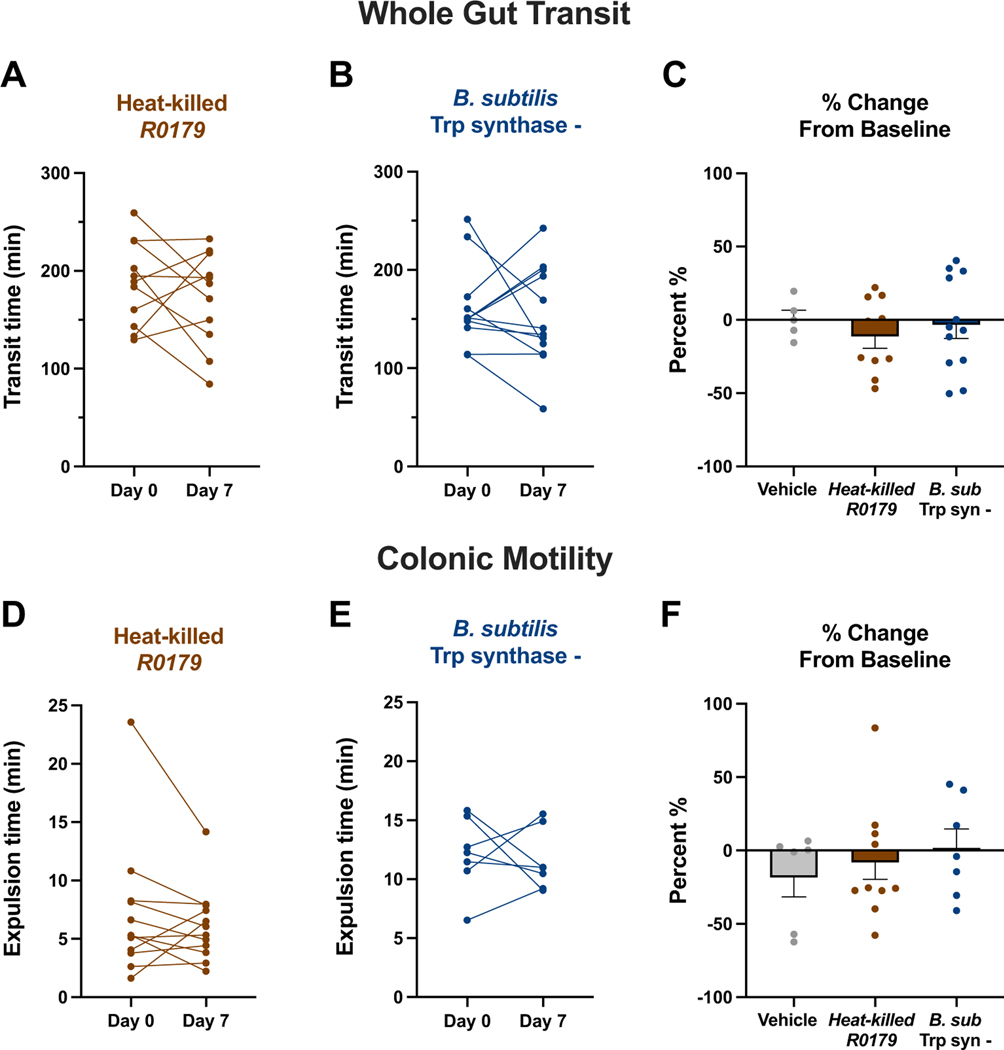
To further elucidate that the prokinetic effects were produced only by the viable, Trp-synthesizing strain B. subtilis R0179, we assessed changes in intestinal function in mice treated with heat-inactivated B. subtilis R0179 spores as well as mice treated with the tryptophan synthase-null strain B. subtilis 1A2. There were no significant changes in either whole gut transit times (Fig. 4a-c) or colonic motility times (Fig. 4d-f) in mice receiving either of these treatments. Notably, treatment with B. subtilis 1A1, a wildtype strain that closely resembles the 1A2 strain, but which can produce Trp, induced faster colonic motility (n=9, P<0.05; data not shown). Taken together, these results indicate that the prokinetic effects of B. subtilis are due to viable bacteria and the presence of a functional tryptophan synthase gene.
Figure 4. Heat-inactivated B. subtilis R0179 and the non-Trp-synthesizing strain B. subtilis 1A2 treatments do not induce prokinetic effects on GI motility.
To assess whether the prokinetic effects of B. subtilis were due to the bacteria’s live nature as well as its Trp-synthesizing capability, motility was assessed following a week of treatment with either heat-inactivated B. subtilis R0179 or with the tryptophan synthase lacking strain B. subtilis 1A2 (B. sub Trp syn-). (A-C) Mice that received heat-inactivated B. subtilis R0179 (n=11) or B. subtilis 1A2 (n=12) did not exhibit changes in whole gut transit time after a week of treatment. These treatment groups were not significantly different from each other or vehicle (n=5) in percent change from baseline. (D-F) In addition to whole gut transit, there was no significant change in colonic motility times in mice treated with either heat-inactivated B. subtilis R0179 (n=12) or B. subtilis 1A2 (n=7). Furthermore, there were no differences between both bacteria treatments or vehicle (n=6). Data are shown as individual animals (A,B,D,E), paired Student’s t-test, or mean ± SEM (C,F), one-way ANOVA with Tukey’s post-hoc multiple comparisons test.
B. subtilis restores colonic motility in a model of constipation
Since we detected prokinetic effects of B. subtilis R0179 treatment in healthy mice, we next sought to investigate whether bacteria treatment could promote motility in the context of GI dysfunction. Similar to patients with multiple sclerosis, mice induced with the murine model of multiple sclerosis, EAE, exhibit features of constipation including slower whole gut transit and slower colonic motility.16 We therefore used this model to test whether B. subtilis R0179 treatment could improve motility in mice with constipation.
We assessed intestinal motility in mice at day 0, and after the development of constipation at post-induction day 21. Mice displaying constipation were treated with either B. subtilis R0179 or vehicle for one week to evaluate changes in motility due to bacteria treatment (Fig. 5a). Constipated mice exhibited faster whole gut transit times following a week of treatment with B. subtilis R0179 (Fig. 5c). This recovery was seen in vehicle-treated mice as well (Fig. 5b), and there was no significant difference between groups in the percentage change from baseline (Fig. 5c), suggesting that there is a possibility for natural recovery from EAE-induced dysmotility in the net motility measure of whole gut transit.
Figure 5. B. subtilis treatment restores colonic motility in constipated mice.
To investigate whether treatment with B. subtilis could restore intestinal function in a model of constipation, mice were induced with experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis that exhibits features of constipation. (A) Schematic of experimental time course with development of constipation for three weeks beginning on day 1 post-induction, and treatment for one week beginning at the height of constipation on day 21. Only mice that were symptomatic for constipation received bacteria or vehicle treatment. (B-D) Following a week of treatment with B. subtilis, constipated mice displayed faster whole gut transit times on day 28 compared to the height of constipation on day 21 (n=8). This prokinetic effect on whole gut transit was seen in vehicle-treated mice as well (n=7). (E-G) B. subtilis-treated mice (n=6) exhibited significantly faster colonic motility times as assessed by the bead expulsion assay, whereas there was no significant change in colonic motility in vehicle-treated mice (n=6). Moreover, there was a significant difference between treatment groups in percent change from baseline following one week of treatment. Data are shown as individual animals (B,C,E,F), paired Student’s t-test, or mean ± SEM (D,G), unpaired Student’s t-test. * p < 0.05, ** p < 0.01.
To examine whether B. subtilis R0179 induces prokinetic effects localized to the colon in constipated animals, as was the case in healthy mice (Fig. 3), we performed the bead expulsion assay following a week of bacteria treatment. We found that B. subtilis R0179-treated constipated mice displayed significantly faster colonic motility times after the treatment course (Fig. 5f). Notably, there was no significant change in colonic motility time in vehicle-treated mice (Fig. 5e). Taken together, these findings demonstrate that B. subtilis R0179 treatment restores colonic motility function in a mouse model of constipation.
B. subtilis enhanced intestinal motility through 5-HT4Rs
5-HT signals through a variety of receptors to regulate intestinal motility.2 The 5-HT4R, which is located on enteric neurons as well as on intestinal epithelial cells, is known to play an important role in enhancing intestinal motility and secretion.3 Notably, 5-HT4R stimulation produces prokinetic effects, and agonists at the 5-HT4R promote colonic propulsive motility and are used to treat constipation.2,3,17 Thus, we sought to further elucidate the mechanisms by which B. subtilis R0179 exerts prokinetic effects and investigate whether the 5-HT4R plays a role.
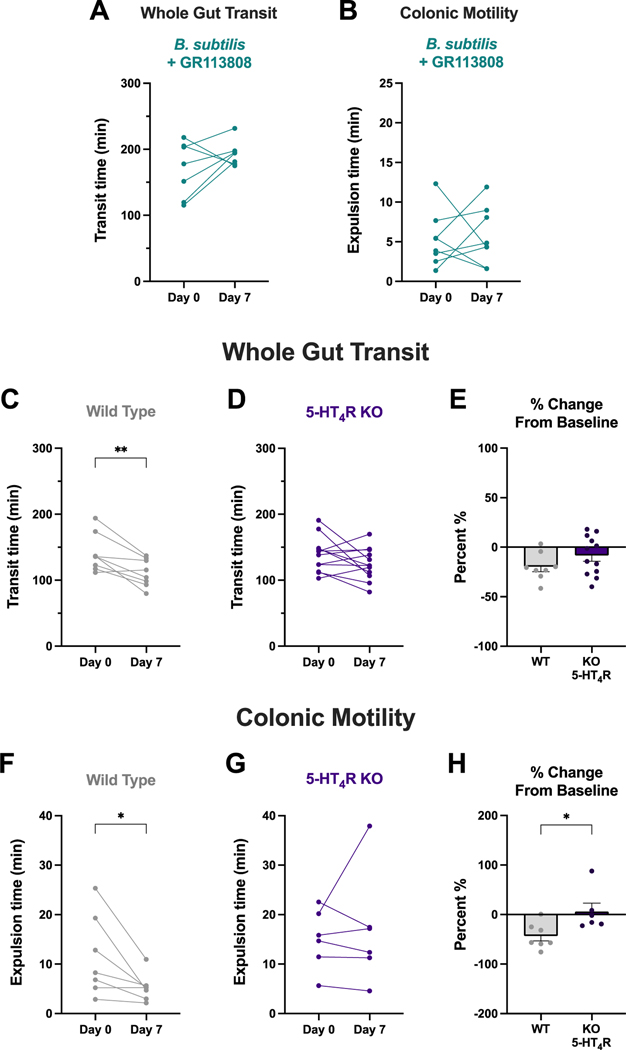
To test the involvement of 5-HT4Rs in the prokinetic actions of B. subtilis, we first employed a pharmacological approach to block the 5-HT4R with a selective antagonist. We treated mice daily with B. subtilis R0179 + GR113808 via oral gavage for one week. Treatment with the 5-HT4R antagonist GR113808 blocked the prokinetic effects of B. subtilis R0179 alone in whole gut transit (Fig. 6a) and colonic motility (Fig. 6b).
Figure 6. The prokinetic actions of B. subtilis involve the 5-HT4 receptor.
The 5-HT4 receptor (5-HT4R) was targeted both pharmacologically and genetically to determine whether this receptor plays a role in B. subtilis-induced prokinetic effects on colonic motility. (A-B) Mice that were co-treated with B. subtilis and GR113808, a 5-HT4R antagonist, exhibited no changes in whole gut transit time (n=7) or colonic motility (n=8) following a week of treatment. (C-E) Following a week of B. subtilis treatment, wild type (WT) littermate control mice displayed significantly faster whole gut transit times (n=8), whereas there was no change in whole gut transit in 5-HT4R KO mice (n=12). The two groups were not significantly different in percent change from baseline. (F-H) Wild type littermate controls exhibited significantly faster colonic motility times after a week of B. subtilis treatment (n=7), whereas there were no changes in colonic motility in 5-HT4R KO mice (n=6). Furthermore, there was a significant difference in percent change from baseline between wild type and KO mice. Data are shown as individual animals (A,B,C,D,F,G), paired Student’s t-test, or mean ± SEM (E,H), unpaired Student’s t-test. * p < 0.05, ** p < 0.01.
We also utilized a molecular approach to target the 5-HT4R by administering B. subtilis to 5-HT4R knockout mice and their wild type littermate controls. Following a week of B. subtilis R0179 treatment, wild type littermate mice exhibited significantly faster whole gut transit times (Fig. 6c) and colonic motility times (Fig. 6f), which is consistent with bacteria-induced prokinetic actions in other mouse strains. However, B. subtilis R0179 treatment did not elicit any changes to intestinal motility in 5-HT4R KO mice (Fig. 6d,g). Taken together, these findings demonstrate that the 5-HT4R plays a pivotal role in the prokinetic actions of B. subtilis R0179.
Dietary increasesd in Trp do not enhance intestinal motility
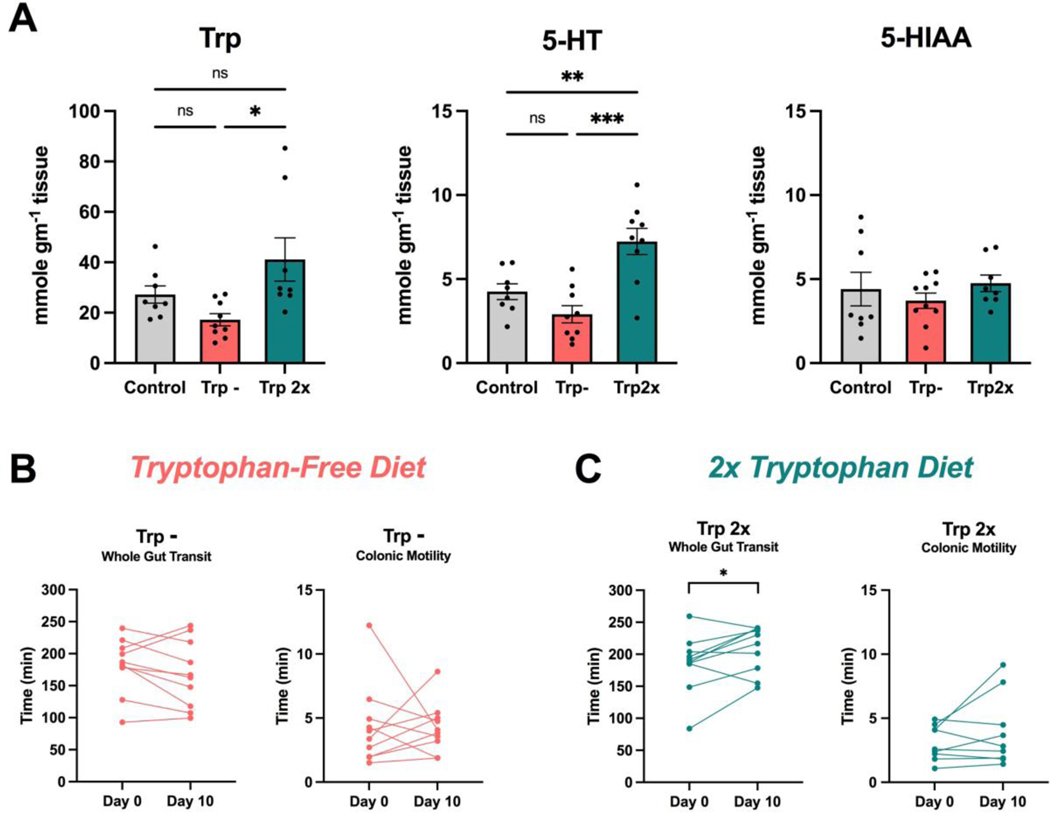
The traditional approach to modifying Trp availability is to alter the concentration of this essential amino acid in the diet. To determine whether dietary manipulations of Trp alter colonic 5-HT signaling and intestinal motility, we fed mice chow that contained no Trp (Trp–) or twice as much Trp (Trp2x) compared to a control chow. Mice on the Trp2x diet exhibited significantly elevated levels of colonic Trp compared to mice on the Trp– diet (Fig. 7a). Furthermore, mice on the Trp2x diet displayed significantly elevated colonic 5-HT levels compared to mice on both the control and Trp– diets. There was no difference in colonic 5-HT levels between the Trp– and control-diet fed mice.
Figure 7. Modifications of Trp through the diet do not induce prokinetic effects on gut motility.
To assess whether dietary-based alterations in Trp availability induce changes in colonic 5-HT signaling and intestinal motility, mice were fed for 10 days with either chow with no Trp (Trp -) or chow with twice as much Trp (Trp2x). (A) Mice that were fed the Trp2x diet (n=9) displayed significantly elevated levels of colonic Trp compared to mice that were fed the Trp - diet (n=9), and significantly elevated 5-HT compared to mice on the Trp - and control diets (n=8). (B) There was no significant change in whole gut transit or colonic motility following 10 days on a Trp– diet (n=10). (C) Mice exhibited slower whole gut transit time after 10 days on the Trp2x diet (n=10). There was no significantly change in colonic motility. Data are shown as mean ± SEM (A), one-way ANOVA with Tukey’s post-hoc multiple comparisons test, or as individual animals (B,C), paired Student’s t-test. * p < 0.05, ** p < 0.01, *** p < 0.001.
Assessments of intestinal function demonstrated that mice on the Trp2x diet displayed significantly slower whole gut transit times after consuming a Trp2x diet for 10 days (Fig. 7c), whereas there was no change in whole gut transit time in mice that consumed the Trp– diet (Fig. 7b). Furthermore, there was no significant effect of either the Trp2x or Trp– diets on colonic motility. Taken together, these findings suggest that dietary supplementation of Trp does not induce prokinetic actions in the gut.
DISCUSSION
The use of orally administered bacteria as therapeutic agents is an emerging strategy for the alleviation of GI symptoms, particularly constipation. As with standard pharmacology-based approaches, a deeper understanding of the mechanisms of actions of these agents is a necessary component of their development into predictable and individualized treatments for GI dysmotility. In this study, we explored a novel bacteria-mediated strategy to alter Trp-associated function in the gut. We tested the hypothesis that administration of the Trp-synthesizing strain B. subtilis R0179 alters Trp availability leading to prokinetic actions on GI motility. We found that B. subtilis R0179 administration to mice with an intact microbiome increased Trp and 5-HIAA levels in the colon, and accelerated colonic motility in both healthy and constipated mice. Notably, our findings indicate that these effects are dependent on spore viability as well as the ability to synthesize Trp, and that 5-HT4Rs are involved in mediating the prokinetic response. Collectively, these data establish that orally administered B. subtilis can enhance intestinal motility.
Gut bacteria influence the serotonergic signaling in the gut
Our findings contribute to a growing body of research into how gut microbes can markedly impact host 5-HT signaling.4 Previous studies have shown that both germ-free and antibiotic-treated animals display deficits in colonic 5-HT levels and decreased Tph1 expression, demonstrating that normal epithelial 5-HT signaling is microbiota-dependent.5,6,9 Furthermore, microbially-secreted metabolites, including short-chain fatty acids and secondary bile acids, stimulate host 5-HT biosynthesis and release, which promotes prokinetic actions within the gut.5,9,18
Administration of isolated bacterial strains that alter host epithelial serotonin signaling is gaining credibility as a potential therapeutic strategy to promote gut function. This work has involved studies of strain-specific effects of various bacteria acting through different mechanisms to ultimately promote 5-HT biosynthesis.5,12,14 While it is widely accepted that the gut microbiota serve an active role in Trp metabolism, our study is the first to our knowledge to exploit the biochemical capacity of certain bacteria that synthesize Trp to promote intestinal motility. While B. subtilis R0179 treatment did not produce detectable changes in colonic 5-HT levels, it is likely that tissue metabolite levels measured by HPLC do not capture the intracellular dynamics of 5-HT synthesis and release. Greater colonic 5-HIAA levels and an elevated 5-HIAA/5-HT ratio (p≤0.001) exhibited in B. subtilis-treated mice lends support to our hypothesis that B. subtilis R0179 promotes 5-HT synthesis in the gut; however, follow-up studies will be required to draw definitive conclusions about 5-HT synthesis, release, and site of action induced by bacterial treatment.
One caveat in understanding the data presented here is the question of how colonic tissue Trp concentrations are elevated. Under normal conditions, it appears that Trp is absorbed in the small intestine. Following oral gavage of an amino acid mixture, the highest luminal concentrations of Trp are detected in the jejunum and ileum, whereas the amino acid is present at very low levels in the cecum, and at indetectable levels in the colon19. Furthermore, it is not clear whether and how Trp can be absorbed by colonocytes. Intestinal absorption of Trp occurs primarily through the cotransporter B0AT1 (SLC6A19)20,21, and relative measurements of mRNA expression of the SLC6A19 gene in rats reveal that B0AT1 is predominantly located in the membranes of jejunal and ileal enterocytes, with little or no expression in the cecum or proximal colon22. On the other hand, in studies of amino acid absorption, Chen and colleagues reported that absorptive transfer of Trp in the cecum and proximal colon is comparable to that of the jejunum22. These findings could account for elevated tissue Trp levels in the colon due to the presence of Trp producing bacteria, but the question of how the Trp is absorbed in the colon remains unresolved. While dietary Trp appears to be completely absorbed in the small bowel, it is possible that Trp provided by commensal bacteria and bacteria provided in probiotic formulations could influence Trp levels in the colonic epithelium.
Because of the potential biological effects of heat-inactivated bacteria, our study utilized an additional bacterial control treatment to determine whether the prokinetic actions of B. subtilis R0179 were due to the presence of a functional tryptophan synthase gene. We treated mice with B. subtilis 1A2, which contains a non-revertible loss of function mutation in the tryptophan synthase gene. Due to this strain incongruence between 1A2 and R0179, we also assessed prokinetic actions of the wildtype strain, B. subtilis 1A1, and found that treatment with B. subtilis 1A1 produced prokinetic actions in the colon, which is consistent with the effects of R0179. In contrast, B. subtilis 1A2 treatment produced no effects on intestinal motility. Taken together, these data support the necessary role of a functional tryptophan synthase gene in the prokinetic actions induced by tryptophan-synthesizing bacteria.
B. subtilis as a prokinetic agent in the treatment of constipation
Clinical constipation is characterized by a variety of symptoms including infrequent bowel movements, hard stools, and distressful feelings of bloating and abdominal pain. A meta-analysis estimates that the global prevalence of constipation is ~14% in the adult population.23 Pharmacological interventions for patients who do not respond to first-line modifications to diet and lifestyle include osmotic and stimulant laxatives, prosecretory agents, and agents that target the serotonergic system. However, effective treatment options are limited.
Mounting evidence suggests that dysbiosis of the intestinal microbiota may contribute to chronic constipation.24–26 Our finding that B. subtilis treatment restores colonic dysmotility in a mouse model of constipation implicates its potential as a prokinetic agent for the treatment of constipation symptoms. Studies in humans treated with a combination of B. subtilis R0179 and Enterococcus faecium R0026 demonstrate effectiveness of these bacterial agents in treating constipation.27 The current study provides a potential mechanism for this effect.
Enteric 5-HT4Rs mediate the prokinetic actions of B. subtilis
5-HT signals through a variety of receptors that are expressed by many cell types within the gut, including intestinal epithelial cells and enteric neurons.2 The 5-HT4 receptor subtype has been targeted for its function as a prokinetic receptor in the GI tract. Previous work in our lab has demonstrated that 5-HT4R expression is differentially distributed in the intestinal mucosa along the GI tract, such that the expression levels are greatest in the colon in mice.3 Activation of colonic mucosal 5-HT4Rs evokes 5-HT release by EC cells and mucus release by goblet cells, both of which can lead to alleviation of constipation symptoms.3 Furthermore, luminally acting 5-HT4R agonists have been shown to elicit prokinetic effects on GI function and promote colonic propulsive motility, lending support for the notion that mucosal 5-HT4Rs act as important pharmacological targets in the treatment of constipation.3,17
Our findings that the prokinetic actions of B. subtilis were blocked by co-administration of a 5-HT4R antagonist and were absent in 5-HT4R KO mice demonstrate the involvement of the 5-HT4R in enhancing intestinal motility with B. subtilis treatment. It is worth noting that these experiments did not differentiate between epithelial and neuronal 5-HT4Rs, as the KO mice are characterized by a full-body KO of the 5-HT4R. Indeed, 5-HT4Rs are known to also be located on enteric nerve terminals, and previous studies demonstrate that activation of neuronal 5-HT4Rs facilitates synaptic transmission and increases neurotransmitter release from myenteric neurons.28–30 Since we have found that the prokinetic actions of B. subtilis are more profound in our colonic motility assay as compared to the whole gut transit assay, we would therefore hypothesize that mucosal 5-HT4Rs are ultimately mediating these bacterially induced effects.
In addition to 5-HT, other bioactive metabolites may also play a role in promoting GI function. One such molecule is tryptamine, a Trp-derived indoleamine. In the gut, tryptamine has been shown to act as a ligand for the epithelial 5-HT4R.31,32 Indeed, tryptamine-induced 5-HT4R activation increases colonic secretion which accelerates whole gut transit in mice, and stimulates mucus release from goblet cells.32,33 It is therefore possible that B. subtilis-induced increases in luminal Trp availability could lead to the conversion of Trp into tryptamine to play a role in enhancing intestinal motility through activation of the 5-HT4R.
Dietary-mediated increases in Trp slow intestinal motility
Since Trp is an essential amino acid, the body’s only source of Trp is through dietary consumption. It is known that Trp-varying diets can alter circulating Trp availability.34,35 However, this is a dynamic process that involves competition between Trp and other amino acids for absorption through transporters into enterocytes.36
The advantages of manipulating Trp through the diet are obvious. Consuming specific foods to benefit our health is common practice in our society and requires little extra work beyond acquiring the necessary dietary components. In contrast, altering Trp levels with bacteria is a more elaborate approach with greater hurdles to overcome, including preparation of the consistent dosage as well as the inherent unknowns regarding the consumption of live microorganisms that could interact with the host microbiome.
Our studies reveal that increasing Trp levels through dietary manipulations produces the opposite effects on colonic 5-HT motility compared to those seen following B. subtilis treatment. Since dietary Trp is absorbed primarily in the small intestine, it may be the case that too much Trp absorption leads to a detrimental effect on the 5-HT system, such that it induces a slowing of gut function in the upper GI tract. Our approach for altering Trp availability through a bacterially-mediated mechanism, rather than through the diet, is unique in that it localizes the effects of increased Trp to the colon, thereby acting primarily to stimulate colonic motility.
Concluding remarks
Taken together, our findings highlight the therapeutic potential of Trp-synthesizing B. subtilis to enhance motility in the gut. Bacteria that are capable of altering intestinal Trp availability represent a novel approach to altering intestinal motility via activation of 5-HT4 receptors. Future studies will be required to determine whether this involves increased 5-HT synthesis and release and/or actions of Trp metabolites such as tryptamine. Further elucidation of the mechanisms of action of specific bacteria such as this one will drive the field forward towards the targeted use of isolated bacteria to promote intestinal function in a transient and predictive manner.
Acknowledgments:
The authors wish to thank Dr. David Mokler for valuable advice and loan of equipment for the HPLC studies, Dr. Bhavik Patel for valuable advice, and Ms. Molly Hurd for genotyping and proofing the manuscript. The Graphical Abstract was created with Created with BioRender.com. Funding for this project was provided by NIH grants AT011203 and DK131044.
Footnotes
Disclosure: The authors have no conflicts to disclose.
REFERENCES
- 1.Cryan JF, O’Riordan KJ, Cowan CSM, et al. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99(4):1877–2013. [DOI] [PubMed] [Google Scholar]
- 2.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10(8):473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman JM, Tyler K, MacEachern SJ, et al. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142(4):844–854.e844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legan TB, Lavoie B, Mawe GM. Direct and indirect mechanisms by which the gut microbiota influence host serotonin systems. Neurogastroenterology & Motility. 2022;n/a(n/a):e14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ge X, Ding C, Zhao W, et al. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J Transl Med. 2017;15(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vicentini FA, Keenan CM, Wallace LE, et al. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome. 2021;9(1):210–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasa L, Abecia L, Forcén R, et al. Antibiotic-Induced Depletion of Murine Microbiota Induces Mild Inflammation and Changes in Toll-Like Receptor Patterns and Intestinal Motility. Microbial Ecology. 2015;70(3):835–848. [DOI] [PubMed] [Google Scholar]
- 9.Reigstad CS, Salmonson CE, Rainey JF, 3rd, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29(4):1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agus A, Planchais J, Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23(6):716–724. [DOI] [PubMed] [Google Scholar]
- 11.Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Medicine. 2016;8(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nzakizwanayo J, Dedi C, Standen G, Macfarlane WM, Patel BA, Jones BV. Escherichia coli Nissle 1917 enhances bioavailability of serotonin in gut tissues through modulation of synthesis and clearance. Scientific Reports. 2015;5(1):17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandić AD, Woting A, Jaenicke T, et al. Clostridium ramosum regulates enterochromaffin cell development and serotonin release. Scientific Reports. 2019;9(1):1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engevik MA, Luck B, Visuthranukul C, et al. Human-Derived Bifidobacterium dentium Modulates the Mammalian Serotonergic System and Gut-Brain Axis. Cell Mol Gastroenterol Hepatol. 2021;11(1):221–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teuscher C, Noubade R, Spach K, et al. Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(21):8024–8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spear ET, Holt EA, Joyce EJ, et al. Altered gastrointestinal motility involving autoantibodies in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2018;30(9):e13349-e13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konen JR, Haag MM, Guseva D, et al. Prokinetic actions of luminally acting 5-HT4 receptor agonists. Neurogastroenterology & Motility. 2021;33(4):e14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukumoto S, Tatewaki M Fau - Yamada T, Yamada T Fau - Fujimiya M, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. 2003(0363–6119 (Print)). [DOI] [PubMed] [Google Scholar]
- 19.Singer D, Camargo SMR, Ramadan T, et al. Defective intestinal amino acid absorption in Ace2 null mice. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2012;303(6):G686–G695. [DOI] [PubMed] [Google Scholar]
- 20.Böhmer C, Bröer A, Munzinger M, et al. Characterization of mouse amino acid transporter B0AT1 (slc6a19). Biochem J. 2005;389(Pt 3):745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keszthelyi D, Troost FJ, Masclee AA. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol Motil. 2009;21(12):1239–1249. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Dinges MM, Green A, Cramer SE, Larive CK, Lytle C. Absorptive transport of amino acids by the rat colon. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2019;318(1):G189–G202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suares NC, Ford AC. Prevalence of, and Risk Factors for, Chronic Idiopathic Constipation in the Community: Systematic Review and Meta-analysis. Official journal of the American College of Gastroenterology | ACG. 2011;106(9). [DOI] [PubMed] [Google Scholar]
- 24.Parthasarathy G, Chen J, Chen X, et al. Relationship Between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology. 2016;150(2):367–379.e361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mancabelli L, Milani C, Lugli GA, et al. Unveiling the gut microbiota composition and functionality associated with constipation through metagenomic analyses. Scientific Reports. 2017;7(1):9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Yu Y-B. Intestinal microbiota and chronic constipation. Springerplus. 2016;5(1):1130–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tompkins TA, Xu X, Ahmarani J. A comprehensive review of post-market clinical studies performed in adults with an Asian probiotic formulation. Benef Microbes. 2010;1(1):93–106. [DOI] [PubMed] [Google Scholar]
- 28.Pan H, Galligan JJ. 5-HT1A and 5-HT4 receptors mediate inhibition and facilitation of fast synaptic transmission in enteric neurons. Am J Physiol. 1994;266(2 Pt 1):G230–238. [DOI] [PubMed] [Google Scholar]
- 29.Liu M, Geddis MS, Wen Y, Setlik W, Gershon MD. Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2005;289(6):G1148–1163. [DOI] [PubMed] [Google Scholar]
- 30.Ren J, Zhou X, Galligan JJ. 5-HT4 receptor activation facilitates recovery from synaptic rundown and increases transmitter release from single varicosities of myenteric neurons. Am J Physiol Gastrointest Liver Physiol. 2008;294(6):G1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams BB, Van Benschoten AH, Cimermancic P, et al. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell host & microbe. 2014;16(4):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattarai Y, Williams BB, Battaglioli EJ, et al. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell host & microbe. 2018;23(6):775–785.e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhattarai Y, Jie S, Linden DR, et al. Bacterially Derived Tryptamine Increases Mucus Release by Activating a Host Receptor in a Mouse Model of Inflammatory Bowel Disease. iScience. 2020;23(12):101798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int J Tryptophan Res. 2009;2:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernstrom JD. Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids. 2013;45(3):419–430. [DOI] [PubMed] [Google Scholar]
- 36.Bröer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008;88(1):249–286. [DOI] [PubMed] [Google Scholar]