Abstract
Aging is characterized by functional decline occurring alongside changes to several hallmarks of aging. One of the hallmarks includes attrition of repeated DNA sequences found at the ends of chromosomes called telomeres. While telomere attrition is linked to morbidity and mortality, whether and how it causally contributes to lifelong rates of functional decline is unclear. In this review, we propose the shelterin-telomere hypothesis of life history, in which telomere-binding shelterin proteins translate telomere attrition into a range of physiological outcomes, the extent of which may be modulated by currently understudied variation in shelterin protein levels. Shelterin proteins may expand the breadth and timing of consequences of telomere attrition, e.g., by translating early life adversity into acceleration of the aging process. We consider how the pleiotropic roles of shelterin proteins provide novel insights into natural variation in physiology, life history, and lifespan. We highlight key open questions that encourage the integrative, organismal study of shelterin proteins that enhances our understanding of the contribution of the telomere system to aging.
Keywords: telomere, shelterin proteins, aging, early life adversity, life history, physiology, metabolism, immune function, hallmarks of aging, lifespan, cancer
1. Introduction
Aging is characterized by the lifelong buildup of molecular damage and functional decline that increases vulnerability to morbidity and mortality (Gladyshev et al., 2021). Molecular damage induced by internal and external factors alters the cellular ‘hallmarks of aging,’ a set of age-associated markers that upon accentuation or reversal in the body can accelerate or decelerate the pace of aging, respectively (Kennedy et al., 2014; López-Otín et al., 2013, 2023). Several hallmarks of aging are central biomolecules that when damaged, result in genomic instability, epigenetic changes to gene packaging, and errors in gene transcription and translation into proteins. With advancing age, such damage cannot be effectively repaired or removed, thereby triggering secondary responses (i.e., cellular senescence, cell arrest) that fuel further damage and functional decline. The rate at which molecular and cellular damage accumulates over a lifetime varies by individual and can be a strong predictor of chronic disease and mortality (Niccoli & Partridge, 2012).
Rapid advancements in omics and computational techniques have leveraged these markers of cellular damage to predict aging in a variety of scientific disciplines. Longitudinal studies of human and non-human primates explore cellular damage factors that influence lifelong patterns of disease and longevity. On the other hand, shorter-lived animal models allow for more nuanced attention to the molecular mechanisms underlying aging and provide an opportunity to pinpoint the accumulation of lifelong damage starting at a younger age (Kinzina et al., 2019). Various biological aging ‘clocks’ use global assessments of these biomolecules to predict an individual’s ‘biological age’, with iterations trained to predict chronological age, physiological age, expected lifespan, and the rate of functional decline (Belsky et al., 2022; Horvath & Raj, 2018; Johnson et al., 2020; Meyer & Schumacher, 2021). The extent to which the cellular hallmarks of aging – and their respective biological clocks – causally drive the aging process is unclear and requires further exploration of the physiological and functional outcomes of such measures.
Telomere attrition is one such hallmark of aging that predicts disease risk and early mortality (Armanios & Blackburn, 2012; Wang et al., 2018). Telomeres are repeated sequences of DNA (i.e., TTAGGG) and associated proteins (i.e., shelterin complex) that cap the ends of eukaryotic chromosomes. Telomeres shorten as a consequence of imperfect copying of DNA during cellular replication, termed the end replication problem (Olovnikov, 1973). Because telomeres track the age (i.e., # divisions) of cells, telomere attrition is largely presented as a passive tracker of damage. Only upon reaching a critically short length does it act as a failsafe that removes old, dysfunctional cells. As described, telomeres should play no causal role in aging until sufficient damage has already occurred, but the reality is likely much more complex. In this review, we present telomere regulatory shelterin proteins as a potential nexus of telomere biology that contributes to aging and life history via telomere-dependent and independent functions, termed the shelterin-telomere hypothesis of life history. These proteins may expand the breadth and timing of consequences of variation in telomere length, and may act as a bridge translating environmental challenges into prolonged effects on the aging process prior to traditional senescence. Our goal is not to provide an exhaustive literature review on shelterin proteins, but rather to highlight and integrate key empirical and review papers in novel ways. We hope to encourage investigation of shelterin proteins across scientific disciplines so that we can expand our understanding of telomere attrition as a causal hallmark of aging.
2. Telomere Attrition as a Hallmark of Aging
Telomere length and attrition is a proposed cellular hallmark of aging that tracks chronological age to varying degrees in human and non-human vertebrates (Codd et al., 2022; Remot et al., 2021). While telomere length can be elongated by the enzyme telomerase, which synthesizes repetitive telomeric sequences (Blackburn et al., 1989), the action of telomerase is not typically enough to counteract telomere attrition during cell division. Telomere lengthening has been observed in some longitudinal datasets (Hatakeyama et al., 2016; Hoelzl et al., 2016; L. Liu et al., 2007; Spurgin et al., 2018; van Lieshout et al., 2019) but is typically attributed to measurement error or redistribution and replenishment of long-telomere cells by stem cells (Bateson & Nettle, 2017; Epel, 2012). In addition to natural shortening due to cell division, telomere attrition can be accelerated via stressors like infection, high aerobic activity, and competition in wild animals (Chatelain et al., 2020), as well as psychosocial stress, adverse childhood events, and low socioeconomic status in humans (Needham et al., 2012; Oliveira et al., 2016; Pepper et al., 2018; Shalev et al., 2013). While lifelong telomere length is strongly predicted by telomere length at birth (Benetos et al., 2013; Martens et al., 2021), exposure to adversity early in life, when telomere attrition is more pronounced, can produce variation among same-age individuals later in life (Boonekamp et al., 2014; Nettle et al., 2015; Spurgin et al., 2018), although strong longitudinal evidence in humans is scarce (Shalev et al., 2013).
Variation in telomere dynamics in response to internal and external stimuli is regulated by a complex set of factors. Metabolic regulators like glucocorticoids (GCs), thyroid, and growth hormones are thought to accelerate telomere attrition (Athanasoulia-Kaspar et al., 2018; Lee et al., 2021; Matsumoto et al., 2015; Pauliny et al., 2015; Stier et al., 2020). Such hormones can alter mitochondrial activity (Du et al., 2009; Short et al., 2008; Wrutniak-Cabello et al., 2001) and increase the production of reactive oxygen species (Costantini et al., 2011; Holzenberger et al., 2003; Venditti & Meo, 2006), which damage macromolecules like DNA. Oxidative damage to telomeric DNA seems particularly robust (Oikawa & Kawanishi, 1999; von Zglinicki, 2002) and might accelerate telomere attrition, although antioxidants can neutralize these reactive oxygen species to prevent telomere loss (Pineda-Pampliega et al., 2020; Serra et al., 2003; Tan et al., 2018). However, evidence for the role of reactive oxygen species is mixed (Boonekamp et al., 2017; Armstrong & Boonekamp, 2023; Reichert & Stier, 2017). Instead, telomere attrition may be a consequence of metabolic shifts that deprioritize telomere maintenance during energy-demanding processes like immune function and reproduction (Casagrande & Hau, 2019; Casagrande et al., 2020).
In addition, telomere attrition is considered an aging hallmark because it can mirror the pace of aging. Telomere attrition often predicts mortality in human and non-human vertebrates (Wang et al., 2018; Arbeev et al., 2020; Wilbourn et al., 2018), with early life changes to telomere length having a particularly robust and prolonged effect on aging outcomes. For example, same-aged juvenile zebra finches with the longest telomeres survive longer than their shorter-telomere counterparts (Haussmann et al., 2005; Heidinger et al., 2012). In humans, the buildup of shortened telomeres is also linked to higher risk of diseases like cardiovascular disease, Alzheimer’s disease, and Type 2 diabetes (Forero et al., 2016; Georgin-Lavialle et al., 2010; Yu & Koh, 2022; Zglinicki & Martin-Ruiz, 2005; Zhang et al., 2016). In addition, the genetic determinants of telomere length (based on single nucleotide polymorphisms, SNPs) also predict aging-related outcomes and cancer risk (Haycock et al., 2017; Kuo et al., 2019; Zhang et al., 2015), thereby suggesting that telomeres may play a causal role in disease state. Furthermore, experimental reversal of telomere attrition improves healthspan in rodents (de Jesus et al., 2011; de Jesus et al., 2012). While telomeres are at least correlates of aging, further assessment of the causal effects of telomere attrition need to be considered.
3. Shelterin proteins are key to the consequences of telomere attrition
The telomere’s structure largely consists of double-stranded DNA with a single-stranded tail, all of which is bound by six distinct shelterin proteins (de Lange, 2018; Figure 1A): telomeric repeat-binding factor 1 and 2 (TRF1 and TRF2), repressor activator protein 1 (RAP1; alias TRF2IP), TRF1-interacting nuclear factor 2 (TIN2; alias TINF2), TIN2-interacting protein 1 (TPP1), and protection of telomeres 1 (POT1). TRF1 and TRF2 bind double-stranded telomeric repeats with aid from TIN2, which also recruits TPP1 and POT1, while RAP1 associates with TRF2. Protein binding of telomeric DNA is highly conserved across major phyla, with some differences in shelterin protein composition across metazoans (Hockemeyer et al., 2006; Myler et al., 2021).
Figure 1. Telomere attrition increases the likelihood of extratelomeric shelterin actions.
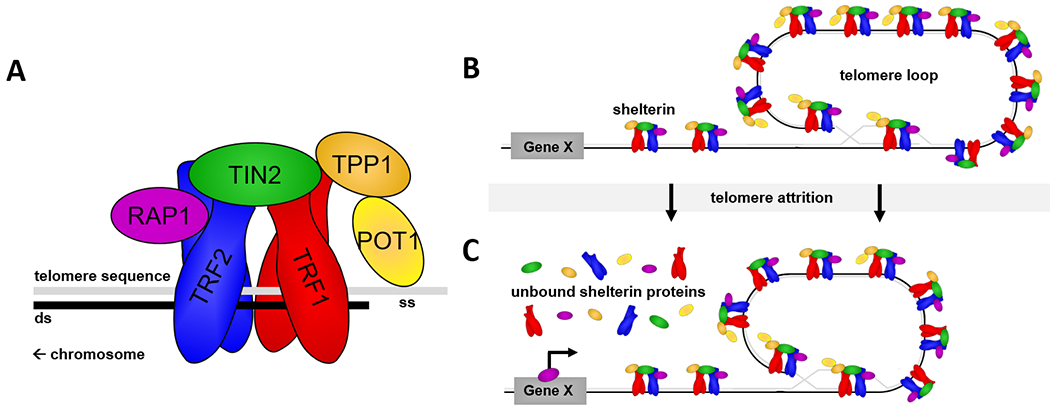
(A) The six-subunit shelterin complex binds to telomeric DNA (i.e., typically TTAGGG). TRF1 and TRF2 bind to the double-stranded (ds) telomere while POT1 binds the telomere’s single-stranded (ss) tail. (B) Shelterin complexes bind repeatedly along the telomere’s length and facilitate the formation of a telomere loop that sequesters the telomere end. (C) Telomere attrition may decrease the number of shelterin binding sites and increase unbound shelterin proteins (e.g., Mukherjee et al., 2018). This unbound fraction of shelterin proteins may then perform genomic and non-genomic (e.g., in the cytosol, mitochondria) extratelomeric actions, like RAP1 transcriptional regulation of genes (Gene “X” in figure).
Shelterin proteins act alongside telomeric DNA to stabilize the genome. Blunt telomere ends can be misidentified as DNA breaks, thereby triggering DNA repair mechanisms (Ciccia & Elledge, 2010) that may fuse chromosome ends and initiate cell-cycle arrest, genome instability, and cell death (Cleal & Baird, 2020). Shelterin proteins conceal these blunt ends within a protective cap (reviewed in de Lange, 2018; Doksani, 2019; Figure 1B). In particular, shelterin proteins facilitate telomere looping formations that hide the single-stranded telomere end within the double-stranded telomere to prevent DNA damage responses (Karlseder et al., 1999; Van Steensel et al., 1998). Similarly, POT1 binding of the single-stranded telomere can block master signaling pathways that initiate DNA damage responses (Denchi & de Lange, 2007; Hockemeyer et al., 2005). Overall, shelterin proteins stabilize telomeres, but critically short telomeres limit shelterin binding and trigger cellular consequences. As described below, shelterin proteins likely underlie many known and unknown consequences of telomere attrition.
First, shelterin proteins at critically short telomeres cannot effectively cap chromosome ends (Griffith et al., 1999), prompting shifts in many hallmarks of aging later in a cell’s life (Chakravarti et al., 2021; Figure 2). Cells with short, uncapped telomeres reach their ‘Hayflick Limit’ (i.e., maximum # cell divisions; Hayflick & Moorhead, 1961) and exhibit cellular senescence (Harley et al., 1990), which is characterized by changes in morphology, gene expression, and epigenetics that promote clearance of older damaged cells (Van Deursen, 2014). Critically short telomeres also repress regulatory sirtuins and induce epigenetic alterations (Amano et al., 2019; Amano & Sahin, 2019; Houtkooper et al., 2012) that compromise other hallmarks like mitochondrial function and protein homeostasis (Sahin et al., 2011; Westerheide et al., 2009). In addition, cellular senescence in short-telomere cells initiates distinct secretory phenotypes that alter intercellular communication (Coppé et al., 2008; Van Deursen, 2014). This increases pro-inflammatory cytokines and thus, ‘inflammaging,’ which influences healthy nearby cells, promotes tissue dysfunction, and further exacerbates telomere attrition (Franceschi et al., 2017).
Figure 2. Mechanistic links between shelterin proteins and other hallmarks of aging.
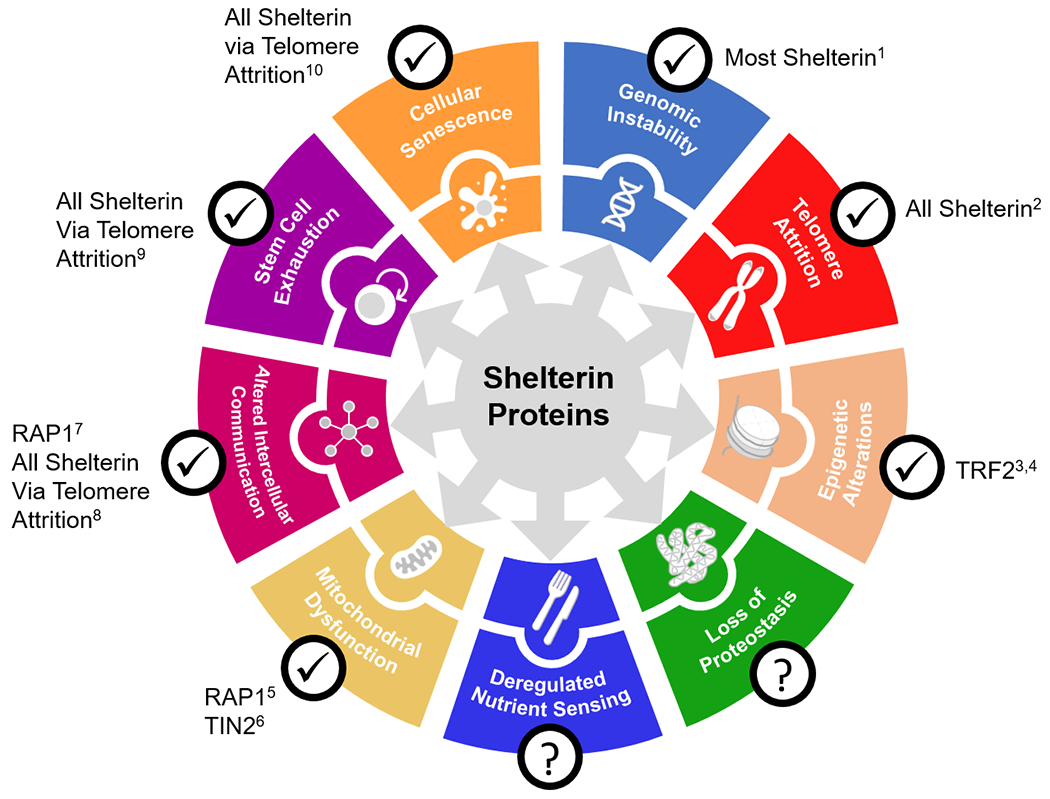
Dysfunctional shelterin protein regulation can induce genomic instability and telomere attrition, thereby contributing indirectly to cellular senescence and its byproducts: altered intercellular communication (e.g., inflammation) and exhaustion of stem cell populations. Shelterin proteins may also directly influence hallmarks of aging such as mitochondrial dysfunction, epigenetic alterations, and intercellular communication, likely alongside other unknown effects. Publications supporting such links are provided, but do not represent an exhaustive list. This figure was adapted from López-Otín et al., 2013, but note that an expanded list of hallmarks is included in López-Otín et al., 2023. References: 1de Lange, 2018; 2Lim et al., 2021; 3Sharma et al., 2021; 4Wang et al., 2007; 5Mukherjee et al., 2018; 6Mukherjee et al., 2019; 7Stock et al., 2022; 8Chen et al., 2012; 9Zhu et al., 2019; 10Martínez & Blasco, 2010.
In addition, the age at which senescence is triggered may be modulated by shelterin proteins via the enzyme telomerase, which can somewhat repair telomere damage. Many shelterin proteins negatively regulate telomerase activity by blocking it from binding the single-stranded telomere end (Ancelin et al., 2002; Loayza & de Lange, 2003; Smogorzewska et al., 2000; Van Steensel & De Lange, 1997) or more directly influencing telomerase expression (e.g., TRF2; Sharma et al., 2021). The TPP1-POT1 protein dimer is thought to play the most direct role (Wang et al., 2007; Xu et al., 2019). While POT1 inhibits telomerase activity via competitive binding at the telomere end (Laprade et al., 2020), POT1 and TPP1 together may act to enhance telomerase activity, especially at shorter telomeres (Wang et al., 2007; Hockemeyer & Collins, 2015). Age-related or environmentally-induced variation in shelterin abundance and availability, which to date is largely unexplored, may result in subtle but life-long variation in telomerase activity that builds into robust variation in telomere dynamics. Such shelterin mediation of telomerase may therefore influence at what age telomeres reach a critically short length to trigger cellular senescence and initiate a cascade of changes in other hallmarks of aging.
Moreover, while telomere attrition is largely tied to cellular senescence (Chakravarti et al., 2021), shelterin proteins may also translate telomere loss into cellular consequences prior to that trigger point. Beyond the telomere end, shelterin proteins also bind at other chromosomal sites, including interstitial telomeric sequences at intrachromosomal regions, subtelomeres immediately adjacent to the telomere, and to some extent, other coding regions of the genome (Martinez et al., 2010; Simonet et al., 2011; Yang et al., 2011). In addition, some shelterin proteins interact with proteins outside of the nucleus (e.g., Chen et al., 2012; Lian et al., 2013). When bound to DNA, shelterin proteins can modulate transcription, as shown with TRF2 (Biroccio et al., 2013; Mukherjee et al., 2018; Zhang et al., 2008), or via epigenetic modifications at those sites (Mukherjee et al., 2019; Mukherjee et al., 2018). Binding affinity of shelterin proteins is higher at telomeric vs extratelomeric sites (Biroccio et al., 2013; Diala et al., 2013; Yang et al., 2011), and so early shifts in shelterin binding should largely alter transcription without compromising telomere function, although additional studies are needed. Critically, shelterin transcriptional regulation (and likely other extratelomeric roles) can also be telomere-length dependent (Mukherjee et al., 2018), as telomere loss limits shelterin binding at telomeres and shunts them away from the telomere end (Gotta et al., 1996; Maillet et al., 1996; Marcand et al., 1996; Figure 1C). Complex interactions between shelterin proteins and telomere length may therefore influence aging rates prior to cellular senescence.
4. The shelterin-telomere hypothesis of life history
Although telomere length has largely been considered a passive tracker of damage, more recent evidence suggests that telomeres can actively regulate physiology and pace of life (e.g., telomere position effect; Baur et al., 2001; Robin et al., 2014). Based on previous biomolecular work, we propose the shelterin-telomere hypothesis of life history, in which shelterin proteins translate changes in telomere length into a range of physiological outcomes, the extent of which may be modulated by stress-induced or other sources of variation in shelterin protein binding, abundance, and availability. Telomere-independent and dependent variation in shelterin protein actions may play a causal role in generating variation in physiology, life histories, rates of aging, and lifespan, similar to previous hypotheses suggesting that telomeres are an active signal that regulates life history strategies (Bateson & Nettle, 2018; Casagrande & Hau, 2019; Giraudeau et al., 2019; Young, 2018). Support for this hypothesis requires an integrative and in-depth look at the environmental and molecular drivers of shelterin protein biology, which remains sparse outside of molecular research. In the following sections, we expand applications of shelterin protein biology to natural variation in physiology and life history and then highlight key open areas of research.
5. Applications of shelterin proteins to aging research
5.1. Shelterin proteins may generate variation in physiology and life history traits
Aging and lifespan are an integral part of life history theory. While the use and definition of life history theory greatly varies across disciplines (Daniel Nettle & Frankenhuis, 2019), it is generally defined as variation occurring as a consequence of natural selection and trade-offs among competing processes like self-maintenance (e.g., lifespan), growth, and reproduction (Roff, 2002; Stearns, 1992). Life history traits are often placed along a “slow-fast” continuum – also referred to in some fields as the pace-of-life syndrome (POLS), in which “slow” phenotypes include late maturation, slow reproduction, and longer lifespan, even applied at the individual level (Réale et al., 2010; see critiques of POLS: Montiglio et al., 2018; Royauté et al., 2018; Zietsch & Sidari, 2020). Initial descriptions of the POLS suggest that suites of physiological traits coevolve to influence life history (Ricklefs & Wikelski, 2002). Here, shelterin proteins may alter the expression of life history traits within and among species.
Metabolism is one trait proposed to coevolve with life history, in which some traits or strategies (e.g., reproduction, rapid growth) promote higher metabolic rates (Auer et al., 2018; Chung et al., 2018) that can trade-off with longevity (Glazier, 2015; Wikelski et al., 2003). That shelterin proteins are linked to metabolic dysfunction hints at their potential mechanistic role in individual and taxonomic-level variation in metabolism and other life-history traits. For example, RAP1 is associated with fatty liver, glucose intolerance, and obesity in mice (Martínez et al., 2013; Martinez et al., 2010; Yeung et al., 2013) and humans (Åberg et al., 2009; Meyre et al., 2004). Enhanced binding of mouse RAP1 at extratelomeric sites, which may be expected with advancing age or at a faster pace of life, reduces metabolic transcripts in the liver and promotes metabolic dysfunction (Stock et al., 2022). In addition, TIN2 alters major metabolic pathways and mitochondrial efficiency (Chen et al., 2012; Kim et al., 2017), which may affect the production of ATP vs metabolic heat. Shelterin proteins may not only promote age and telomere-dependent metabolic dysfunction, but may also play a role in intra- and interspecific metabolic rates via their telomere-independent availability. Shelterin protein binding may therefore alter metabolic function and influence energy allocation towards life history traits, but whether this varies across the life history spectrum is unclear.
Immune function is another trait linked to life history: fast vs slow strategies may differentially invest in costly immune traits (Demas et al., 1997; e.g., Jacques-Hamilton et al., 2017; Previtali et al., 2012; Saino et al., 1998; Tieleman, 2018) that in some cases trade off with competing processes (Nystrand & Dowling, 2020). Here, shelterin proteins may help generate immunological variation across life histories. For example, RAP1 may be a potent modulator of immune function, in which cytosolic RAP1 activates the NF-κB pathway (Lian et al., 2013; Teo et al., 2010), a master regulator of immune and inflammatory responses (Salminen et al., 2008). In the nucleus, higher RAP1 binding at extratelomeric sites also promotes transcription of pro-inflammatory cytokines and immune response pathways in mice (Stock et al., 2022). Thus, subtly higher RAP1 actions following either telomere attrition or high RAP1 availability (baseline or stress-induced) may alter immune function and contribute to intra- and interspecific variation in life histories and rates of aging. Clearly, future studies should further expand upon the physiological causes and consequences of shelterin protein biology.
5.2. Can shelterin proteins translate early life adversity into aging outcomes?
Early life adversity is a potent exposure linked to aging. In humans, early-life challenges like neglect, abuse, and low socioeconomic status predict later-life morbidity and early mortality (Barker et al., 2002; O’Rand & Hamil-Luker, 2005; Pesonen & Räikkönen, 2012), which is mirrored in studies of resource availability and environmental conditions in wild vertebrates (Lindström, 1999; Tung et al., 2016). Interestingly, adverse conditions in early life can accelerate telomere attrition (Ridout et al., 2018; Shalev, 2012), and such early telomere dynamics often predict adult survival (Benetos et al., 2013; Heidinger et al., 2012). However, whether telomere dynamics directly translate early life adversity into later health outcomes is unclear. Telomere length is often thought to simply track somatic damage (Kawanishi & Oikawa, 2004; von Zglinicki, 2002), at least until short telomeres induce senescent phenotypes (Van Deursen, 2014). However, telomere attrition at any age may also directly signal immediate shifts in physiology (Entringer et al., 2018). Shelterin proteins may be one such mediator linking early adversity to later life outcomes. According to the shelterin-telomere hypothesis of life history, early life adversity may accelerate telomere attrition and increase shelterin actions away from the telomere, and/or independently alter shelterin protein availability (e.g., via gene expression, protein synthesis or degradation, Figure 3). Such variation in shelterin protein abundance may then impact physiology and life history, as described above.
Figure 3. Proposed model for shelterin proteins linking early life adversity to aging and life history.
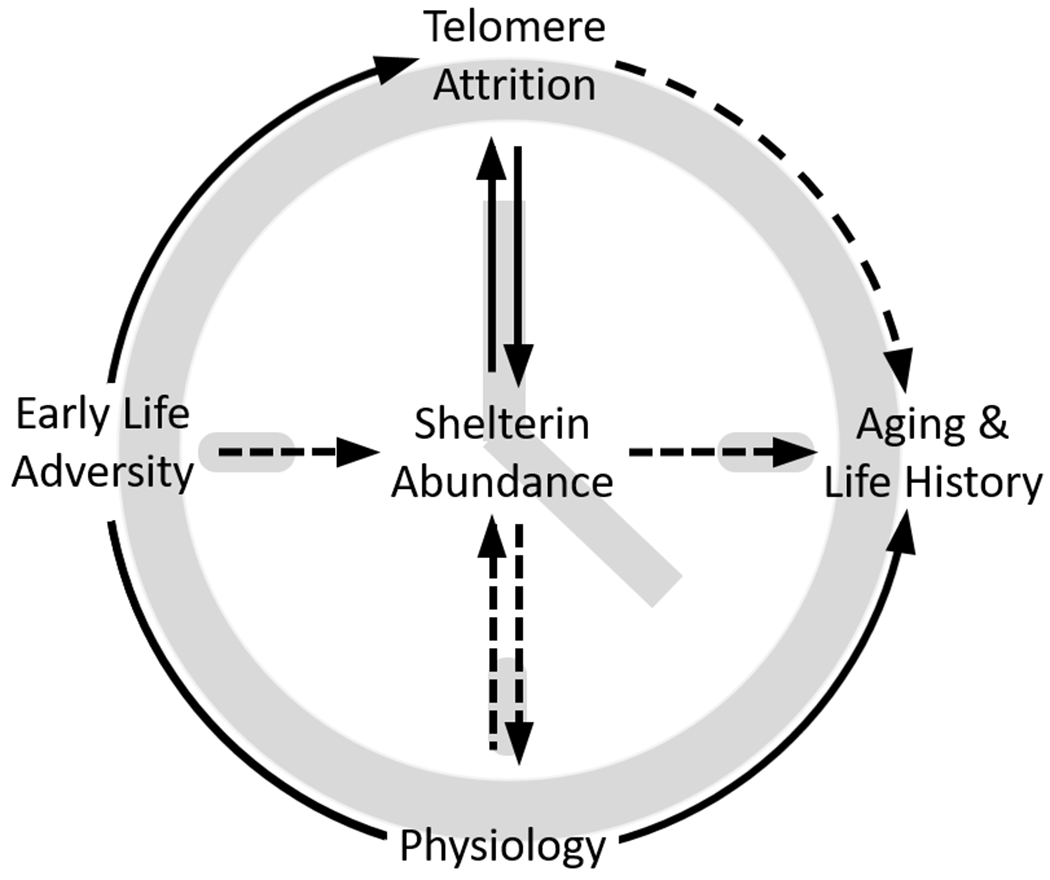
Following early life adversity, availability of extratelomeric shelterin proteins may be altered by stress-induced telomere attrition or changes to shelterin protein expression levels. Shelterin protein abundance may then directly influence physiological mechanisms related to aging and life history, e.g., metabolism and immune function. Solid lines represent supported causal relationships and broken lines represent theoretical or untested relationships. This simplified model does not include potential mediators like biological sex, age, or tissue type.
How early life adversity alters shelterin proteins and their downstream consequences depends on whether and how these proteins respond to stress. That stress may alter shelterin availability is gaining traction in kinesiology and evolutionary ecology. Exercise is considered a physiological stressor that increases gene expression of TRF1 and TRF2 in mice (Ludlow et al., 2017; Ludlow et al., 2012). Similar results are found in humans (but see Chilton et al., 2014; reviewed in Ludlow et al., 2013), in which chronic exercise increases several shelterin proteins in lymphocytes and monocytes, despite no changes to telomere length (Laye et al., 2012; Werner et al., 2009). Chronic stress may also prime higher basal levels of shelterin proteins, as seen in master athletes (Aguiar et al., 2021). In this context, high shelterin levels are considered a telo-protective benefit of exercise, but in the wild, there is evidence of decreasing shelterin levels following environmental stress. For example, in young birds, food limitation leads to lower POT1 gene expression (Wolf et al., 2022). In addition, higher nest temperatures marginally decrease TRF2 in blood (Stier et al., 2021), and limited food availability decreases POT1 gene expression in coral (Rouan et al., 2021). The timing, magnitude, and type of stressors may influence these shelterin profiles but requires further testing.
These theoretical shifts in shelterin availability with early life adversity may lead to long-term effects on life history and aging via their extratelomeric effects. However, whether up and downregulation of shelterin proteins have opposing effects is unclear and likely varies by shelterin protein, although we may generally expect a continuum of dose-dependent effects. Subtle increases in shelterin protein levels may safeguard DNA from damage (e.g., during exercise; Ludlow et al., 2017; Ludlow et al., 2012), but extreme upregulation could accelerate potential pro-aging effects of shelterin proteins. On the other hand, subtle decreases in shelterin proteins may physically promote repair by telomerase or grant a reprieve from shelterin’s pro-aging effects. Alternatively, lower shelterin abundance may reflect a temporary sacrifice of telomere maintenance in favor of survival or reproduction during stress (Casagrande & Hau, 2019). However, extreme downregulation may increase cancer risk and telomere fragility (Akincilar et al., 2021). The study of shelterin dynamics may thus reveal two sides of a coin that promote adaptive responses to and negative consequences of stress. Such outcomes may vary by stressor and depend on shelterin plasticity, heritability, and age-related changes over the lifetime, but these complex interactions are currently unclear. Overall, stress-related regulation of shelterin proteins is unstudied and presents a novel opportunity to understand mechanistic links between early life adversity and long-term aging outcomes.
5.3. Shelterin proteins and natural variation in longevity
Telomere attrition is a ‘protective’ mechanism that limits cellular proliferation and thus the development of cancers. However, abnormal upregulation of telomerase activity nullifies such protection and is observed in 90% of cancers (Akincilar et al., 2016). Telomerase activity not only repairs telomere length but also promotes cancer progression via changes to cell adhesion and migration, cellular replication, and apoptosis (Liu et al., 2016; Rahman et al., 2005; Zhou et al., 2009). Cancer biologists have found shelterin dysfunction to be a common trait among cancers (reviewed in Akincilar et al., 2021). Loss of any shelterin protein can disrupt the formation of shelterin complexes and lead to fragile telomeres that are susceptible to cancer. In addition, changes to shelterin proteins often enhance telomerase activity or can promote cancer directly, for example, via TRF2’s suppression of innate immune responses that protects cancer cells (Biroccio et al., 2013). Further elaboration on shelterin’s roles in cancer progression falls outside the bounds of this review (see Akincilar et al., 2021; Martínez & Blasco, 2010); however, this body of literature makes a case for shelterin proteins as mediators of cellular longevity that may also play a prominent role in natural variation in longevity in normal, healthy individuals.
Several studies have identified positive selection or increased abundance of shelterin proteins in long-lived species, including TRF1 in naked mole rats (NMRs) and TIN2 in long-lived bats and rodents (Kim et al., 2011; Ma et al., 2016; MacRae et al., 2015). Increased shelterin abundance may be selected for in long-lived species as a failsafe for cancer avoidance. In addition, TRF1 evolution in the NMR may facilitate life in subterranean, hypoxic environments: when the NMR TRF1 variant is transfected into mouse cells, those cells enhance energy production and telomere binding under hypoxia (Augereau et al., 2021). Therefore, selection on shelterin SNPs (and binding affinity) may be particularly salient in stressful environments. In addition, young birds with relatively low POT1 mRNA abundance are more likely to recruit into the breeding population (i.e., a proxy for survival; Wolf et al., 2022). That early shelterin levels predict survival in animals with high extrinsic mortality hints that shelterin proteins may push beyond survival to influence reproduction and resilience to environmental stress prior to extrinsic death. In fact, low-POT1 is linked to fitness in female birds; namely, they bred earlier and were better able to feed offspring during sickness (Wolf et al., 2021). The extratelomeric roles of shelterin proteins may in part dictate survival outcomes of everyday stressors, but as shown here, may also be commandeered to promote the evolution of long-lived species. Clearly, additional studies are needed across levels of biological complexity to explore selection on shelterin proteins across these levels.
6. Open Questions in Shelterin Protein Biology
Under the shelterin-telomere hypothesis of life history, the effects of shelterin proteins on physiology, life history, and aging depend on availability of telomere binding sites and fluctuations in shelterin protein availability. While the number of shelterin proteins ready for extratelomeric actions may be determined in part by the pace of telomere attrition and subsequent shunting of shelterin to other regions of the genome (Mukherjee et al., 2019), shelterin protein abundance may also independently change in response to a wide variety of factors, such as early-life adversity. Detrimental costs of extremely low and high shelterin levels (e.g., cancer; Akincilar et al., 2021) suggests there is an optimal shelterin abundance, yet the extent to which an individual can viably deviate from that optimum without negative consequence is unclear. Manipulations of both telomere-dependent and independent fractions of shelterin proteins are critical to understanding their relative variability and contributions to downstream effects. This may require the continued use of molecular techniques that analyze protein interactions with DNA (i.e., ChIP-seq) as well as measurement of telomere length alongside shelterin mRNA and protein abundance. Critically, effects of increased extratelomeric shelterin following telomere attrition may be exacerbated or nullified by respective increases or decreases in shelterin protein abundance. Thus, exploring the determinants and degree of natural variation in shelterin proteins is key (see Figure 4).
Figure 4. Open questions for the study of shelterin proteins in life history and aging.
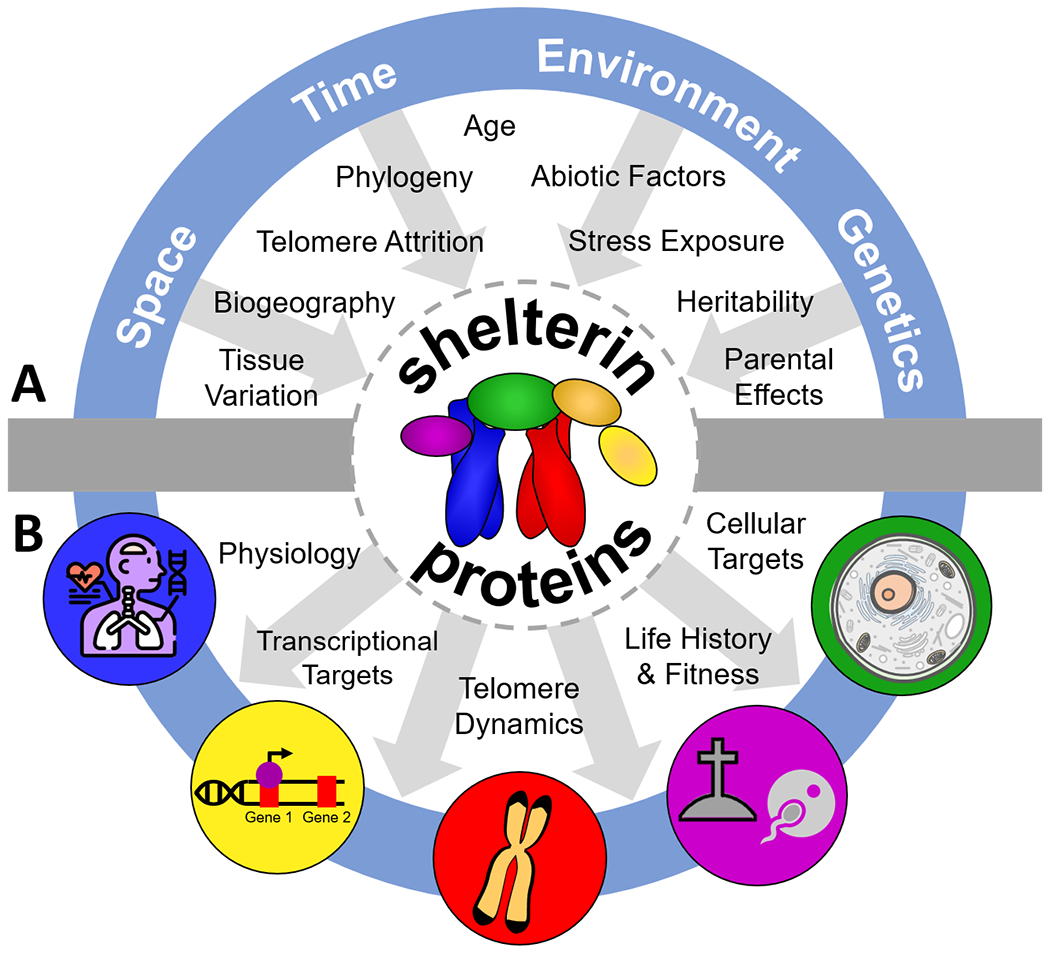
Integration of research across scientific fields is needed to understand the functionality and evolution of shelterin proteins in the context of life history and aging. (A) top half, impact on shelterin proteins: this includes research exploring natural and experimental variation in shelterin abundance across space (i.e., tissues, biogeography), time (i.e., with age, telomere attrition, and phylogeny), environmental conditions (i.e., stress exposure, abiotic factors), and with genetic factors (i.e., heritability, other parental effects). (B) bottom half, impact of shelterin proteins: there is a need to establish an expanded proximate understanding of each shelterin protein’s functional roles in transcription, telomere dynamics, and physiology across the cell and document the relationships between shelterin protein abundance and various aging, life history, and fitness outcomes.
How does shelterin protein abundance vary across time and tissues?
The strength of shelterin protein responses to stress may change over space and time. For example, not much is known about the ontogeny of shelterin proteins with age, but we may expect shelterin levels to loosely follow the age-related dynamics of telomere length and telomerase activity. As an example, telomere attrition is especially robust and variable early in life and then slows into adulthood (Frenck et al., 1998; Ridout et al., 2018; Spurgin et al., 2018), although this varies across tissues (Demanelis et al., 2020; Haussmann et al., 2004; Haussmann et al., 2007; Ulaner et al., 2001). Similarly, telomerase activity is downregulated in tissues with somatic cells early in development, and to a lesser extent in proliferating stem cells, a pattern that appears strongest in larger long-lived animals (Procházková Schrumpfová et al., 2019; Seluanov et al., 2007). We may therefore expect similar tissue-specific and age-related decreases in shelterin proteins, and in fact, several studies do show decreases in shelterin protein expression with age in mice and zebrafish (Ludlow et al., 2012; Wagner et al., 2017), which occur in a tissue and shelterin protein-specific manner. All in all, the strength of shelterin protein effects on physiology may be strongest earlier in life when telomere dynamics are most in flux. Future studies of shelterin levels across a species’ lifetime are vital to understanding such developmental variation.
How does shelterin protein abundance respond to stress?
While telomere attrition is typically accelerated by a variety of stressors in human and non-human vertebrates (Chatelain et al., 2020; Pepper et al., 2018), the extent to which shelterin proteins respond to stress is largely unknown. Current evidence shows both increasing and decreasing gene expression and/or protein levels in response to stressors like poor resource availability and intense exercise (Ludlow et al., 2017; Rouan et al., 2021; Wolf et al., 2022), but clearly, additional research on shelterin responses to essentially any stressor would offer valuable information. Whether the duration of stress exposure or certain types of stressors, e.g., metabolic stress vs perceived stress, more strongly influence shelterin protein abundance is unclear but may be revealed upon further investigation of the upstream regulators of shelterin protein transcription, translation, and degradation. For example, TRF2 appears regulated by pathways involved in cellular development and homeostasis, such as the Wnt/β-catenin signaling pathways (El Maï et al., 2014). Knowing which signaling pathways alter shelterin abundance may aid in predicting the environmental factors that most influence shelterin proteins dynamics.
What are the downstream physiological and functional consequences of shelterin proteins?
Robust manipulations of shelterin proteins have been key to identifying several mechanistic pathways that may respond to variation in shelterin expression, but whether this web of shelterin-regulated traits is expanded at a broader organismal level is unclear. Outside of lab manipulations of shelterin binding at telomeric vs extratelomeric sites, correlations of shelterin abundance (gene expression and protein levels) with ecologically-relevant traits are severely lacking. Beyond links to metabolism and immune function, shelterin proteins are also linked to neural development in humans and fish (e.g., TRF2; Jung et al., 2004; Ovando-Roche et al., 2014; Ovando Roche, 2013; Ying et al., 2022), and antioxidant gene expression in birds (e.g., POT1; Wolf et al., 2022). Notably, extratelomeric actions of shelterin proteins do not seem limited to the nucleus, e.g., TRF2 and RAP1 interactions with proteins in the cytoplasm. Altogether, shelterin proteins may contribute to many life history and fitness-related traits, and future studies examining natural variation in shelterin abundance may reveal additional key physiological connections.
How might selection act on shelterin protein abundance?
That shelterin protein abundance varies and likely alters physiology suggests that selection on shelterin proteins could contribute to broadscale variation in life history and aging. Variation in shelterin traits that may be the outcome of environmental selection pressures have already been reported. Several long-lived species like naked mole rats and certain bats exhibit positive selection on or increased abundance of shelterin proteins (Kim et al., 2011; Ma et al., 2016; MacRae et al., 2015). Selection for a longer lifespan may be stronger in certain environments, including hypoxic environments like that of the naked mole rat, and in species with unique metabolic requirements like flight in bats and birds (Omotoso et al., 2021). Sparse evidence shows that shelterin proteins can respond to environmental challenges (Ludlow et al., 2017; Rouan et al., 2021; Wolf et al., 2022) and that variation in POT1 gene expression is correlated with performance in birds (Wolf et al., 2022). Therefore, differential fitness by shelterin proteins in certain environments may select for certain shelterin traits. For example, the naked mole rat’s TRF1 protein has evolved in a hypoxic environment and facilitates telomere binding and novel glycolytic functions (Augereau et al., 2021), suggesting that selection on shelterin protein SNPs, and consequential binding affinity, may be evolutionarily relevant. Similar evolution of shelterin proteins may be the product of responses to other environments and life histories. Phylogenetic comparisons of variation in shelterin SNPs and shelterin abundance would shed light on this question.
7. Conclusion
Telomere attrition has long been established as a ‘hallmark of aging’ that predicts survival, health, and longevity; however, the mechanisms underlying such patterns are unclear. In this review, we highlighted pleiotropic roles of the telomere-binding shelterin proteins that may provide novel insights into natural variation in physiology, life history, and lifespan. Under the shelterin-telomere hypothesis of life history, shelterin proteins act as a nexus of telomere biology and other hallmarks of aging that translate telomere attrition into more immediate shifts in physiology before the onset of senescence. This fundamentally shifts our interpretation and use of telomere attrition from a passive tracker of damage to an active player that contributes to the lifelong pace of aging. To date, little work on shelterin proteins has been done outside of molecular biology, making integrative research on shelterin proteins a low hanging fruit that has potential to generate new and exciting insights into variation in life history and aging. We call attention to key questions for future research and encourage the integrative and organismal study of shelterin proteins to enhance our understanding of the telomere system and its contributions to aging.
Highlights:
Telomere attrition is a hallmark of aging linked to cellular senescence.
Regulatory shelterin proteins may translate telomere loss into shifts in physiology.
Shelterin proteins alter gene expression related to life history traits and aging.
Early adversity may alter lifetime aging via telomere loss and shelterin proteins.
We encourage several lines of integrative research on shelterin protein abundance.
Acknowledgements:
Many thanks to K.A. Rosvall for her intellectual feedback and encouragement of this review, and to the guest editors A. Bartolomucci, J. Tung, and K.M. Harris for inviting this contribution.
Funding:
S.E.W. supported by the National Institutes of Health T32 AG049676 to The Pennsylvania State University. I.S. supported by the National Institute of Environmental Health Sciences (U01 ES030949). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: none
References
- Åberg K, Dai F, Sun G, Keighley ED, Indugula SR, Roberts ST, Zhang Q, Smelser D, Viali S, & Tuitele J (2009). Susceptibility loci for adiposity phenotypes on 8p, 9p, and 16q in American Samoa and Samoa. Obesity, 17(3), 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar SS, Sousa CV, Santos PA, Barbosa LP, Maciel LA, Coelho-Júnior HJ, Motta-Santos D, Rosa TS, Degens H, & Simões HG (2021). Master athletes have longer telomeres than age-matched non-athletes. A systematic review, meta-analysis and discussion of possible mechanisms. Experimental Gerontology, 146, 111212. [DOI] [PubMed] [Google Scholar]
- Akincilar SC, Chan CHT, Ng QF, Fidan K, & Tergaonkar V (2021). Non-canonical roles of canonical telomere binding proteins in cancers. Cellular and molecular life sciences, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akincilar SC, Unal B, & Tergaonkar V (2016). Reactivation of telomerase in cancer. Cellular and molecular life sciences, 73(8), 1659–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano H, Chaudhury A, Rodriguez-Aguayo C, Lu L, Akhanov V, Catic A, Popov YV, Verdin E, Johnson H, & Stossi F (2019). Telomere dysfunction induces sirtuin repression that drives telomere-dependent disease. Cell metabolism, 29(6), 1274–1290. e1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano H, & Sahin E (2019). Telomeres and sirtuins: at the end we meet again. Molecular & Cellular Oncology, 6(5), e1632613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancelin K, Brunori M, Bauwens S, Koering C-E, Brun C, Ricoul M, Pommier J-P, Sabatier L, & Gilson E (2002). Targeting assay to study the cis functions of human telomeric proteins: evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Molecular and cellular biology, 22(10), 3474–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeev KG, Verhulst S, Steenstrup T, Kark JD, Bagley O, Kooperberg C, Reiner AP, Hwang S-J, Levy D, & Fitzpatrick AL (2020). Association of leukocyte telomere length with mortality among adult participants in 3 longitudinal studies. JAMA network open, 3(2), e200023–e200023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M, & Blackburn EH (2012). The telomere syndromes. Nature Reviews Genetics, 13(10), 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong E, & Boonekamp J (2023). Does oxidative stress shorten telomeres in vivo? A meta-analysis. Ageing Res Rev, 101854. doi: 10.1016/j.arr.2023.101854 [DOI] [PubMed] [Google Scholar]
- Athanasoulia-Kaspar AP, Auer MK, Stalla GK, & Jakovcevski M (2018). Shorter telomeres associated with high doses of glucocorticoids: the link to increased mortality? Endocrine Connections, 7(11), 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer SK, Dick CA, Metcalfe NB, & Reznick DN (2018). Metabolic rate evolves rapidly and in parallel with the pace of life history. Nature communications, 9(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augereau A, Mariotti M, Pousse M, Filipponi D, Libert F, Beck B, Gorbunova V, Gilson E, & Gladyshev VN (2021). Naked mole rat TRF1 safeguards glycolytic capacity and telomere replication under low oxygen. Science advances, 7(8), eabe0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsén T, & Osmond C (2002). Fetal origins of adult disease:strength of effects and biological basis. International journal of epidemiology, 31(6), 1235–1239. [DOI] [PubMed] [Google Scholar]
- Bateson M, & Nettle D (2017). The telomere lengthening conundrum–it could be biology. Aging Cell, 16(2), 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson M, & Nettle D (2018). Why are there associations between telomere length and behaviour? Philosophical Transactions of the Royal Society, B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Zou Y, Shay JW, & Wright WE (2001). Telomere position effect in human cells. Science, 292(5524), 2075–2077. [DOI] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Corcoran DL, Sugden K, Poulton R, Arseneault L, Baccarelli A, Chamarti K, Gao X, & Hannon E (2022). DunedinPACE, a DNA methylation biomarker of the pace of aging. Elife, 11, e73420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetos A, Kark JD, Susser E, Kimura M, Sinnreich R, Chen W, Steenstrup T, Christensen K, Herbig U, & von Bornemann Hjelmborg J (2013). Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell, 12(4), 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biroccio A, Cherfils-Vicini J, Augereau A, Pinte S, Bauwens S, Ye J, Simonet T, Horard B, Jamet K, & Cervera L (2013). TRF2 inhibits a cell-extrinsic pathway through which natural killer cells eliminate cancer cells. Nature cell biology, 15(7), 818–828. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Greider CW, Henderson E, Lee MS, Shampay J, & Shippen-Lentz D (1989). Recognition and elongation of telomeres by telomerase. Genome, 31(2), 553–560. [DOI] [PubMed] [Google Scholar]
- Boonekamp JJ, Bauch C, Mulder E, & Verhulst S (2017). Does oxidative stress shorten telomeres? Biology Letters, 13(5), 20170164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonekamp JJ, Mulder GA, Salomons HM, Dijkstra C, & Verhulst S (2014). Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc Biol Sci, 281(1785), 20133287. doi: 10.1098/rspb.2013.3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande S, & Hau M (2019). Telomere attrition: metabolic regulation and signalling function? Biology Letters, 15(3), 20180885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande S, Stier A, Monaghan P, Loveland JL, Boner W, Lupi S, Trevisi R, & Hau M (2020). Increased glucocorticoid concentrations in early life cause mitochondrial inefficiency and short telomeres. Journal of Experimental Biology, 223(15), jeb222513. [DOI] [PubMed] [Google Scholar]
- Chakravarti D, LaBella KA, & DePinho RA (2021). Telomeres: history, health, and hallmarks of aging. Cell, 184(2), 306–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelain M, Drobniak SM, & Szulkin M (2020). The association between stressors and telomeres in non-human vertebrates: a meta-analysis. Ecology letters, 23(2), 381–398. [DOI] [PubMed] [Google Scholar]
- Chen L-Y, Zhang Y, Zhang Q, Li H, Luo Z, Fang H, Kim SH, Qin L, Yotnda P, & Xu J (2012). Mitochondrial localization of telomeric protein TIN2 links telomere regulation to metabolic control. Molecular Cell, 47(6), 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton WL, Marques FZ, West J, Kannourakis G, Berzins SP, O’Brien BJ, & Charchar FJ (2014). Acute exercise leads to regulation of telomere-associated genes and microRNA expression in immune cells. PLoS One, 9(4), e92088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DJ, Healy TM, McKenzie JL, Chicco AJ, Sparagna GC, & Schulte PM (2018). Mitochondria, temperature, and the pace of life. Integrative and Comparative Biology, 58(3), 578–590. [DOI] [PubMed] [Google Scholar]
- Ciccia A, & Elledge SJ (2010). The DNA damage response: making it safe to play with knives. Molecular Cell, 40(2), 179–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleal K, & Baird DM (2020). Catastrophic endgames: emerging mechanisms of telomere-driven genomic instability. Trends in Genetics, 36(5), 347–359. [DOI] [PubMed] [Google Scholar]
- Codd V, Denniff M, Swinfield C, Warner S, Papakonstantinou M, Sheth S, Nanus D, Budgeon C, Musicha C, & Bountziouka V (2022). Measurement and initial characterization of leukocyte telomere length in 474,074 participants in UK Biobank. Nature Aging, 2(2), 170–179. [DOI] [PubMed] [Google Scholar]
- Coppé J-P, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez P-Y, & Campisi J (2008). Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS biology, 6(12), e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini D, Marasco V, & Moller AP (2011). A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B, 181, 447–456. doi: 10.1007/s00360-011-0566-2) [DOI] [PubMed] [Google Scholar]
- de Jesus BB, Schneeberger K, Vera E, Tejera A, Harley CB, & Blasco MA (2011). The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell, 10(4), 604–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus BB, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, & Blasco MA (2012). Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO molecular medicine, 4(8), 691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T (2018). Shelterin-mediated telomere protection. Annual review of genetics, 52, 223–247. [DOI] [PubMed] [Google Scholar]
- Demanelis K, Jasmine F, Chen LS, Chernoff M, Tong L, Delgado D, Zhang C, Shinkle J, Sabarinathan M, & Lin H (2020). Determinants of telomere length across human tissues. Science, 369(6509), eaaz6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Chefer V, Talan MI, & Nelson RJ (1997). Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 273(5), R1631–R1637. [DOI] [PubMed] [Google Scholar]
- Denchi EL, & de Lange T (2007). Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature, 448(7157), 1068–1071. [DOI] [PubMed] [Google Scholar]
- Diala I, Wagner N, Magdinier F, Shkreli M, Sirakov M, Bauwens S, Schluth-Bolard C, Simonet T, Renault VM, & Ye J (2013). Telomere protection and TRF2 expression are enhanced by the canonical Wnt signalling pathway. EMBO reports, 14(4), 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doksani Y (2019). The response to DNA damage at telomeric repeats and its consequences for telomere function. Genes, 10(4), 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, Khairova R, Zhou R, Yuan P, & Machado-Vieira R (2009). Dynamic regulation of mitochondrial function by glucocorticoids. Proceedings of the National Academy of Sciences, 106(9), 3543–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Maï M, Wagner K-D, Michiels J-F, Ambrosetti D, Borderie A, Destree S, Renault V, Djerbi N, Giraud-Panis M-J, & Gilson E (2014). The telomeric protein TRF2 regulates angiogenesis by binding and activating the PDGFRβ promoter. Cell reports, 9(3), 1047–1060. [DOI] [PubMed] [Google Scholar]
- Entringer S, de Punder K, Buss C, & Wadhwa PD (2018). The fetal programming of telomere biology hypothesis: an update. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1741), 20170151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E (2012). How “reversible” is telomeric aging? Cancer Prevention Research, 5(10), 1163–1168. [DOI] [PubMed] [Google Scholar]
- Forero DA, Gonzalez-Giraldo Y, Lopez-Quintern C, Castro-Vega LJ, Barreto GE, & Perry G (2016). Meta-analysis of telomere length in Alzheimer’s disease. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 71(8), 1069–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Vitale G, Capri M, & Salvioli S (2017). Inflammaging and ‘Garb-aging’. Trends in Endocrinology & Metabolism, 28(3), 199–212. [DOI] [PubMed] [Google Scholar]
- Frenck RW, Blackburn EH, & Shannon KM (1998). The rate of telomere sequence loss in human leukocytes varies with age. Proceedings of the National Academy of Sciences, 95(10), 5607–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgin-Lavialle S, Aouba A, Mouthon L, Londono-Vallejo JA, Lepelletier Y, Gabet AS, & Hermine O (2010). The telomere/telomerase system in autoimmune and systemic immune-mediated diseases. Autoimmunity Reviews, 9(10), 646–651. doi: 10.1016/j.autrev.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Giraudeau M, Angelier F, & Sepp T (2019). Do Telomeres Influence Pace-of-Life-Strategies in Response to Environmental Conditions Over a Lifetime and Between Generations? Bioessays, 41(3), 1800162. [DOI] [PubMed] [Google Scholar]
- Gladyshev VN, Kritchevsky SB, Clarke SG, Cuervo AM, Fiehn O, de Magalhães JP, Mau T, Maes M, Moritz RL, & Niedernhofer LJ (2021). Molecular damage in aging. Nature Aging, 1(12), 1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier DS (2015). Is metabolic rate a universal ‘pacemaker’for biological processes? Biological Reviews, 90(2), 377–407. [DOI] [PubMed] [Google Scholar]
- Gotta M, Laroche T, Formenton A, Maillet L, Scherthan H, & Gasser SM (1996). The clustering of telomeres and colocalization with Rap1, Sir3, and Sir4 proteins in wild-type Saccharomyces cerevisiae. The Journal of cell biology, 134(6), 1349–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JK, Bryant JE, Fordyce CA, Gilliland FD, Joste NE, & Moyzis RK (1999). Reduced telomere DNA content is correlated with genomic instability and metastasis in invasive human breast carcinoma. Breast cancer research and treatment, 54(1), 59–64. [DOI] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, & Greider CW (1990). Telomeres shorten during ageing of human fibroblasts. Nature, 345(6274), 458–460. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H, Yamazaki H, Nakamura K-I, Izumiyama-Shimomura N, Aida J, Suzuki H, Tsuchida S, Matsuura M, Takubo K, & Ishikawa N (2016). Telomere attrition and restoration in the normal teleost Oryzias latipes are linked to growth rate and telomerase activity at each life stage. Aging (Albany NY), 8(1), 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann MF, Winkler DW, Huntington CE, Nisbet IC, & Vleck CM (2004). Telomerase expression is differentially regulated in birds of differing life span. Annals of the New York Academy of Sciences, 1019(1), 186–190. [DOI] [PubMed] [Google Scholar]
- Haussmann MF, Winkler DW, Huntington CE, Nisbet IC, & Vleck CM (2007). Telomerase activity is maintained throughout the lifespan of long-lived birds. Experimental Gerontology, 42(7), 610–618. doi: 10.1016/j.exger.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Haussmann MF, Winkler DW, & Vleck CM (2005). Longer telomeres associated with higher survival in birds. Biol Lett, 1(2), 212–214. doi: 10.1098/rsbl.2005.0301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock PC, Burgess S, Nounu A, Zheng J, Okoli GN, Bowden J, Wade KH, Timpson NJ, Evans DM, & Willeit P (2017). Association between telomere length and risk of cancer and non-neoplastic diseases: a Mendelian randomization study. JAMA oncology, 3(5), 636–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L, & Moorhead PS (1961). The serial cultivation of human diploid cell strains. Experimental cell research, 25(3), 585–621. [DOI] [PubMed] [Google Scholar]
- Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, & Monaghan P (2012). Telomere length in early life predicts lifespan. PNAS, 109(5), 1743–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, & Collins K (2015). Control of telomerase action at human telomeres. Nature structural & molecular biology, 22(11), 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Daniels J-P, Takai H, & de Lange T (2006). Recent expansion of the telomeric complex in rodents: Two distinct POT1 proteins protect mouse telomeres. Cell, 126(1), 63–77. [DOI] [PubMed] [Google Scholar]
- Hockemeyer D, Sfeir AJ, Shay JW, Wright WE, & De Lange T (2005). POT1 protects telomeres from a transient DNA damage response and determines how human chromosomes end. The EMBO journal, 24(14), 2667–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzl F, Cornils JS, Smith S, Moodley Y, & Ruf T (2016). Telomere dynamics in free-living edible dormice (Glis glis): the impact of hibernation and food supply. Journal of Experimental Biology, 219(16), 2469–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, & Le Bouc Y (2003). IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature, 421(6919), 182–187. [DOI] [PubMed] [Google Scholar]
- Horvath S, & Raj K (2018). DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics, 19(6), 371–384. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Pirinen E, & Auwerx J (2012). Sirtuins as regulators of metabolism and healthspan. Nature reviews Molecular cell biology, 13(4), 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques-Hamilton R, Hall ML, Buttemer WA, Matson KD, da Silva AG, Mulder RA, & Peters A (2017). Personality and innate immune defenses in a wild bird: Evidence for the pace-of-life hypothesis. Hormones and behavior, 88, 31–40. [DOI] [PubMed] [Google Scholar]
- Johnson AA, Shokhirev MN, Wyss-Coray T, & Lehallier B (2020). Systematic review and analysis of human proteomics aging studies unveils a novel proteomic aging clock and identifies key processes that change with age. Ageing Research Reviews, 60, 101070. [DOI] [PubMed] [Google Scholar]
- Jung Y, Lee S, Bang S, Kim S, Choi K, Lee C, Lee S-G, Kim CJ, Song K, & Lee I (2004). TRF2 is in neuroglial cytoplasm and induces neurite-like processes. Febs Letters, 557(1-3), 129–132. [DOI] [PubMed] [Google Scholar]
- Karlseder J, Broccoli D, Dai Y, Hardy S, & de Lange T (1999). p53-and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science, 283(5406), 1321–1325. [DOI] [PubMed] [Google Scholar]
- Kawanishi S, & Oikawa S (2004). Mechanism of telomere shortening by oxidative stress. Annals of the New York Academy of Sciences, 1019(1), 278–284. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, & Pessin JE (2014). Geroscience: linking aging to chronic disease. Cell, 159(4), 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EB, Fang X, Fushan AA, Huang Z, Lobanov AV, Han L, Marino SM, Sun X, Turanov AA, Yang P, Yim SH, Zhao X, Kasaikina MV, Stoletzki N, Peng C, Polak P, Xiong Z, Kiezun A, Zhu Y, Chen Y, Kryukov GV, Zhang Q, Peshkin L, Yang L, Bronson RT, Buffenstein R, Wang B, Han C, Li Q, Chen L, Zhao W, Sunyaev SR, Park TJ, Zhang G, Wang J, & Gladyshev VN (2011). Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature, 479(7372), 223–227. doi: 10.1038/nature10533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Li F, He Q, Deng T, Xu J, Jin F, Coarfa C, Putluri N, Liu D, & Songyang Z (2017). Systematic analysis of human telomeric dysfunction using inducible telosome/shelterin CRISPR/Cas9 knockout cells. Cell discovery, 3, 17034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzina ED, Podolskiy DI, Dmitriev SE, & Gladyshev VN (2019). Patterns of aging biomarkers, mortality, and damaging mutations illuminate the beginning of aging and causes of early-life mortality. Cell reports, 29(13), 4276–4284. e4273. [DOI] [PubMed] [Google Scholar]
- Kuo CL, Pilling LC, Kuchel GA, Ferrucci L, & Melzer D (2019). Telomere length and aging-related outcomes in humans: A Mendelian randomization study in 261,000 older participants. Aging Cell, 18(6), e13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprade H, Querido E, Smith MJ, Guérit D, Crimmins H, Conomos D, Pourret E, Chartrand P, & Sfeir A (2020). Single-molecule imaging of telomerase RNA reveals a Recruitment-Retention model for telomere elongation. Molecular Cell. [DOI] [PubMed] [Google Scholar]
- Laye MJ, Solomon TP, Karstoft K, Pedersen KK, Nielsen SD, & Pedersen BK (2012). Increased shelterin mRNA expression in peripheral blood mononuclear cells and skeletal muscle following an ultra-long-distance running event. Journal of Applied Physiology, 112(5), 773–781. [DOI] [PubMed] [Google Scholar]
- Lee RS, Zandi PP, Santos A, Aulinas A, Carey JL, Webb SM, McCaul ME, Resmini E, & Wand GS (2021). Cross-species Association Between Telomere Length and Glucocorticoid Exposure. The Journal of Clinical Endocrinology & Metabolism, 106(12), e5124–e5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian S, Meng L, Liu C, Xing X, Song Q, Dong B, Han Y, Yang Y, Peng L, Qu L, & Shou C (2013). PRL-3 activates NF-κB signaling pathway by interacting with RAP1. Biochem Biophys Res Commun, 430(1), 196–201. doi: 10.1016/j.bbrc.2012.11.036 [DOI] [PubMed] [Google Scholar]
- Lim CJ, & Cech TR (2021). Shaping human telomeres: from shelterin and CST complexes to telomeric chromatin organization. Nature reviews Molecular cell biology, 22(4), 283–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström J (1999). Early development and fitness in birds and mammals. Trends in Ecology & Evolution, 14(9), 343–348. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu Q, Ge Y, Zhao Q, Zheng X, & Zhao Y (2016). hTERT promotes cell adhesion and migration independent of telomerase activity. Scientific reports, 6(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Bailey SM, Okuka M, Muñoz P, Li C, Zhou L, Wu C, Czerwiec E, Sandler L, & Seyfang A (2007). Telomere lengthening early in development. Nature cell biology, 9(12), 1436–1441. [DOI] [PubMed] [Google Scholar]
- Loayza D, & de Lange T (2003). POT1 as a terminal transducer of TRF1 telomere length control. Nature, 423(6943), 1013. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, & Kroemer G (2013). The hallmarks of aging. Cell, 153(6), 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, & Kroemer G (2023). Hallmarks of aging: An expanding universe. Cell. [DOI] [PubMed] [Google Scholar]
- Ludlow AT, Gratidao L, Ludlow LW, Spangenburg EE, & Roth SM (2017). Acute exercise activates p38 MAPK and increases the expression of telomere-protective genes in cardiac muscle. Experimental physiology, 102(4), 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow AT, Ludlow LW, & Roth SM (2013). Do telomeres adapt to physiological stress? Exploring the effect of exercise on telomere length and telomere-related proteins. BioMed research international, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow AT, Witkowski S, Marshall MR, Wang J, Lima LC, Guth LM, Spangenburg EE, & Roth SM (2012). Chronic exercise modifies age-related telomere dynamics in a tissue-specific fashion. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 67(9), 911–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Upneja A, Galecki A, Tsai Y-M, Burant CF, Raskind S, Zhang Q, Zhang ZD, Seluanov A, & Gorbunova V (2016). Cell culture-based profiling across mammals reveals DNA repair and metabolism as determinants of species longevity. Elife, 5, e19130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae SL, Zhang Q, Lemetre C, Seim I, Calder RB, Hoeijmakers J, Suh Y, Gladyshev VN, Seluanov A, & Gorbunova V (2015). Comparative analysis of genome maintenance genes in naked mole rat, mouse, and human. Aging Cell, 14(2), 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet L, Boscheron C, Gotta M, Marcand S, Gilson E, & Gasser SM (1996). Evidence for silencing compartments within the yeast nucleus: a role for telomere proximity and Sir protein concentration in silencer-mediated repression. Genes & development, 10(14), 1796–1811. [DOI] [PubMed] [Google Scholar]
- Marcand S, Buck SW, Moretti P, Gilson E, & Shore D (1996). Silencing of genes at nontelomeric sites in yeast is controlled by sequestration of silencing factors at telomeres by Rap 1 protein. Genes & development, 10(11), 1297–1309. [DOI] [PubMed] [Google Scholar]
- Martens DS, Van Der Stukken C, Derom C, Thiery E, Bijnens EM, & Nawrot TS (2021). Newborn telomere length predicts later life telomere length: Tracking telomere length from birth to child-and adulthood. EBioMedicine, 63, 103164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez P, & Blasco MA (2010). Role of shelterin in cancer and aging. Aging Cell, 9(5), 653–666. [DOI] [PubMed] [Google Scholar]
- Martínez P, Gómez-López G, García F, Mercken E, Mitchell S, Flores JM, de Cabo R, & Blasco MA (2013). RAP1 protects from obesity through its extratelomeric role regulating gene expression. Cell reports, 3(6), 2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez P, Thanasoula M, Carlos AR, Gómez-López G, Tejera AM, Schoeftner S, Dominguez O, Pisano DG, Tarsounas M, & Blasco MA (2010). Mammalian Rap1 controls telomere function and gene expression through binding to telomeric and extratelomeric sites. Nature cell biology, 12(8), 768–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto R, Fukuoka H, Iguchi G, Odake Y, Yoshida K, Bando H, Suda K, Nishizawa H, Takahashi M, & Yamada S (2015). Accelerated telomere shortening in acromegaly; IGF-I induces telomere shortening and cellular senescence. PLoS One, 10(10), e0140189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DH, & Schumacher B (2021). BiT age: A transcriptome-based aging clock near the theoretical limit of accuracy. Aging Cell, 20(3), e13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyre D, Lecoeur C, Delplanque J, Francke S, Vatin V, Durand E, Weill J, Dina C, & Froguel P (2004). A genome-wide scan for childhood obesity–associated traits in French families shows significant linkage on chromosome 6q22. 31-q23. 2. Diabetes, 53(3), 803–811. [DOI] [PubMed] [Google Scholar]
- Montiglio P-O, Dammhahn M, Dubuc Messier G, & Réale D (2018). The pace-of-life syndrome revisited: the role of ecological conditions and natural history on the slow-fast continuum. Behavioral Ecology and Sociobiology, 72(7), 1–9. [Google Scholar]
- Mukherjee AK, Sharma S, Bagri S, Kutum R, Kumar P, Hussain A, Singh P, Saha D, Kar A, & Dash D (2019). Telomere repeat-binding factor 2 binds extensively to extra-telomeric G-quadruplexes and regulates the epigenetic status of several gene promoters. Journal of biological chemistry, 294(47), 17709–17722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee AK, Sharma S, Sengupta S, Saha D, Kumar P, Hussain T, Srivastava V, Roy SD, Shay JW, & Chowdhury S (2018). Telomere length-dependent transcription and epigenetic modifications in promoters remote from telomere ends. PLoS genetics, 14(11), e1007782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myler LR, Kinzig CG, Sasi NK, Zakusilo G, Cai SW, & de Lange T (2021). The evolution of metazoan shelterin. Genes & development, 35(23-24), 1625–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham BL, Fernandez JR, Lin J, Epel ES, & Blackburn EH (2012). Socioeconomic status and cell aging in children. Social science & medicine, 74(12), 1948–1951. [DOI] [PubMed] [Google Scholar]
- Nettle D, & Frankenhuis WE (2019). The evolution of life-history theory: a bibliometric analysis of an interdisciplinary research area. Proceedings of the Royal Society B: Biological Sciences, 286(1899), 20190040. doi:doi: 10.1098/rspb.2019.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettle D, Monaghan P, Gillespie R, Brilot B, Bedford T, & Bateson M (2015). An experimental demonstration that early-life competitive disadvantage accelerates telomere loss. Proc Biol Sci, 282(1798), 20141610. doi: 10.1098/rspb.2014.1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccoli T, & Partridge L (2012). Ageing as a risk factor for disease. Current biology, 22(17), R741–R752. [DOI] [PubMed] [Google Scholar]
- Nystrand M, & Dowling D (2020). Effects of immune challenge on expression of life-history and immune trait expression in sexually reproducing metazoans—a meta-analysis. BMC biology, 18(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rand AM, & Hamil-Luker J (2005). Processes of cumulative adversity: Childhood disadvantage and increased risk of heart attack across the life course. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 60(Special_Issue_2), S117–S124. [DOI] [PubMed] [Google Scholar]
- Oikawa S, & Kawanishi S (1999). Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. Febs Letters, 453(3), 365–368. [DOI] [PubMed] [Google Scholar]
- Oliveira BS, Zunzunegui MV, Quinlan J, Fahmi H, Tu MT, & Guerra RO (2016). Systematic review of the association between chronic social stress and telomere length: A life course perspective. Ageing Research Reviews, 26, 37–52. [DOI] [PubMed] [Google Scholar]
- Olovnikov AM (1973). A theory of marginotomy: the incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. Journal of theoretical Biology, 41(1), 181–190. [DOI] [PubMed] [Google Scholar]
- Omotoso O, Gladyshev VN, & Zhou X (2021). Lifespan Extension in Long-Lived Vertebrates Rooted in Ecological Adaptation. Front Cell Dev Biol, 9, 704966. doi: 10.3389/fcell.2021.704966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovando-Roche P, Yu JS, Testori S, Ho C, & Cui W (2014). TRF2-mediated stabilization of hREST4 is critical for the differentiation and maintenance of neural progenitors. Stem Cells, 32(8), 2111–2122. [DOI] [PubMed] [Google Scholar]
- Ovando Roche P (2013). Role of telomere binding protein TRF2 in neural differentiation of human embryonic stem cells. [Google Scholar]
- Pauliny A, Devlin RH, Johnsson JI, & Blomqvist D (2015). Rapid growth accelerates telomere attrition in a transgenic fish. BMC evolutionary biology, 15(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper GV, Bateson M, & Nettle D (2018). Telomeres as integrative markers of exposure to stress and adversity: a systematic review and meta-analysis. Royal Society open science, 5(8), 180744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen A-K, & Räikkönen K (2012). The lifespan consequences of early life stress. Physiology & Behavior, 106(5), 722–727. [DOI] [PubMed] [Google Scholar]
- Pineda-Pampliega J, Herrera-Dueñas A, Mulder E, Aguirre JI, Höfle U, & Verhulst S (2020). Antioxidant supplementation slows telomere shortening in free-living white stork chicks. Proceedings of the Royal Society B, 287(1918), 20191917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previtali MA, Ostfeld RS, Keesing F, Jolles AE, Hanselmann R, & Martin LB (2012). Relationship between pace of life and immune responses in wild rodents. Oikos, 121(9), 1483–1492. [Google Scholar]
- Procházková Schrumpfová P, Fojtová M, & Fajkus J (2019). Telomeres in plants and humans: not so different, not so similar. Cells, 8(1), 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman R, Latonen L, & Wiman KG (2005). hTERT antagonizes p53-induced apoptosis independently of telomerase activity. Oncogene, 24(8), 1320–1327. [DOI] [PubMed] [Google Scholar]
- Réale D, Garant D, Humphries MM, Bergeron P, Careau V, & Montiglio P-O (2010). Personality and the emergence of the pace-of-life syndrome concept at the population level. Philosophical Transactions of the Royal Society B: Biological Sciences, 365(1560), 4051–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert S, & Stier A (2017). Does oxidative stress shorten telomeres in vivo? A review. Biology Letters, 13(12), 20170463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remot F, Ronget V, Froy H, Rey B, Gail lard JM, Nussey DH, & Lemaitre JF (2021). Decline in telomere length with increasing age across nonhuman vertebrates: A meta-analysis. Molecular Ecology. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, & Wikelski M (2002). The physiology/life-history nexus. Trends in Ecology & Evolution, 17(10), 462–468. [Google Scholar]
- Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, Price LH, & Tyrka AR (2018). Early life adversity and telomere length: a meta-analysis. Molecular psychiatry, 23(4), 858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin JD, Ludlow AT, Batten K, Magdinier F, Stadler G, Wagner KR, Shay JW, & Wright WE (2014). Telomere position effect: regulation of gene expression with progressive telomere shortening over long distances. Genes & development, 28(22), 2464–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff DADA (2002). Life history evolution. [Google Scholar]
- Rouan A, Pousse M, Tambutté E, Djerbi N, Zozaya W, Capasso L, Zoccola D, Tambutté S, & Gilson E (2021). Telomere dysfunction is associated with dark-induced bleaching in the reef coral Stylophora pistillata. Molecular Ecology. [DOI] [PubMed] [Google Scholar]
- Royauté R, Berdal MA, Garrison CR, & Dochtermann NA (2018). Paceless life? A meta-analysis of the pace-of-life syndrome hypothesis. Behavioral Ecology and Sociobiology, 72(3), 64. [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Müller FL, Guo M, Cooper M, Kotton D, Fabian AJ, & Walkey C (2011). Telomere dysfunction induces metabolic and mitochondrial compromise. Nature, 470(7334), 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino N, Calza S, & Møller AP (1998). Effects of a dipteran ectoparasite on immune response and growth trade-offs in barn swallow, Hirundo rustica, nestlings. Oikos, 217–228. [Google Scholar]
- Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, & Suuronen T (2008). Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Research Reviews, 7(2), 83–105. [DOI] [PubMed] [Google Scholar]
- Seluanov A, Chen Z, Hine C, Sasahara TH, Ribeiro AA, Catania KC, Presgraves DC, & Gorbunova V (2007). Telomerase activity coevolves with body mass not lifespan. Aging Cell, 6(1), 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra V, Von Zglinicki T, Lorenz M, & Saretzki G (2003). Extracellular superoxide dismutase is a major antioxidant in human fibroblasts and slows telomere shortening. Journal of biological chemistry, 278(9), 6824–6830. [DOI] [PubMed] [Google Scholar]
- Shalev I (2012). Early life stress and telomere length: investigating the connection and possible mechanisms: a critical survey of the evidence base, research methodology and basic biology. Bioessays, 34(11), 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Mill J, Arseneault L , & Caspi A (2013). Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Molecular psychiatry, 18(5), 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Mukherjee AK, Roy SS, Bagri S, Lier S, Verma M, Sengupta A, Kumar M , Nesse G, & Pandey DP (2021). Human telomerase is directly regulated by non-telomeric TRF2-G-quadruplex interaction. Cell reports, 35(7), 109154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Moller N, Bigelow ML, Coenen-Schimke J, & Nair KS (2008). Enhancement of muscle mitochondrial function by growth hormone. The Journal of Clinical Endocrinology & Metabolism, 93(2), 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet T, Zaragosi L-E, Philippe C, Lebrigand K, Schouteden C, Augereau A, Bauwens S, Ye J, Santagostino M, & Giulotto E (2011). The human TTAGGG repeat factors 1 and 2 bind to a subset of interstitial telomeric sequences and satellite repeats. Cell research, 21(7), 1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, & de Lange T (2000). Control of human telomere length by TRF1 and TRF2. Molecular and cellular biology, 20(5), 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurgin LG, Bebbington K, Fairfield EA, Hammers M, Komdeur J, Burke T, Dugdale HL, & Richardson DS (2018). Spatio-temporal variation in lifelong telomere dynamics in a long-term ecological study. Journal of Animal Ecology, 87(1), 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC (1992). The evolution of life histories (Vol. 249): Oxford University Press Oxford. [Google Scholar]
- Stier A, Hsu B-Y, Cossin-Sevrin N, Garcin N, & Ruuskanen S (2021). From climate warming to accelerated cellular ageing: an experimental study in wild birds. bioRxiv, 2021.2012.2021.473625. doi: 10.1101/2021.12.21.473625 [DOI] [Google Scholar]
- Stier A, Hsu B-Y, Marciau C, Doligez B, Gustafsson L, Bize P, & Ruuskanen S (2020). Born to be young? Prenatal thyroid hormones increase early-life telomere length in wild collared flycatchers. Biology Letters, 16(11), 20200364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock AJ, McDevitt RA, Puligilla C, Wang Y, Zhang Y, Wang K, Sun C, Becker KG, Lehrmann E, Wood WH 3rd, Gong Y, Aqdas M, Sung MH, Hoffmann V, Liu C, Gorospe M, Harrington L, Ferrucci L, & Liu Y (2022). Aberrant expression and localization of the RAP1 shelterin protein contribute to age-related phenotypes. PLoS Genet, 18(11), e1010506. doi: 10.1371/journal.pgen.1010506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BL, Norhaizan ME, Liew W-P-P, & Sulaiman Rahman H (2018). Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Frontiers in pharmacology, 9, 1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo H, Ghosh S, Luesch H, Ghosh A, Wong ET, Malik N, Orth A, De Jesus P, Perry AS, & Oliver JD (2010). Telomere-independent Rap1 is an IKK adaptor and regulates NF-κB-dependent gene expression. Nature cell biology, 12(8), 758–767. [DOI] [PubMed] [Google Scholar]
- Tieleman BI (2018). Understanding immune function as a pace of life trait requires environmental context. Behavioral Ecology and Sociobiology, 72(3), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J, Archie EA, Altmann J, & Alberts SC (2016). Cumulative early life adversity predicts longevity in wild baboons. Nature communications, 7(1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulaner GA, Hu JF, Vu TH, Giudice LC, & Hoffman AR (2001). Tissue-specific alternate splicing of human telomerase reverse transcriptase (hTERT) influences telomere lengths during human development. International journal of cancer, 91(5), 644–649. [PubMed] [Google Scholar]
- Van Deursen JM (2014). The role of senescent cells in ageing. Nature, 509(7501), 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lieshout SH, Bretman A, Newman C, Buesching CD, Macdonald DW, & Dugdale HL (2019). Individual variation in early-life telomere length and survival in a wild mammal. Molecular Ecology. [DOI] [PubMed] [Google Scholar]
- Van Steensel B, & De Lange T (1997). Control of telomere length by the human telomeric protein TRF1. Nature, 385(6618), 740–743. [DOI] [PubMed] [Google Scholar]
- Van Steensel B, Smogorzewska A, & De Lange T (1998). TRF2 protects human telomeres from end-to-end fusions. Cell, 92(3), 401–413. [DOI] [PubMed] [Google Scholar]
- Venditti P, & Meo SD (2006). Thyroid hormone-induced oxidative stress. Cellular and Molecular Life Sciences CMLS, 63(4), 414–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T (2002). Oxidative stress shortens telomeres. Trends In Biochemical Sciences, 27(7), 339–344. [DOI] [PubMed] [Google Scholar]
- Wagner K-D, Ying Y, Leong W, Jiang J, Hu X, Chen Y, Michiels J-F, Lu Y, Gilson E, & Wagner N (2017). The differential spatiotemporal expression pattern of shelterin genes throughout lifespan. Aging (Albany NY), 9(4), 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Podell ER, Zaug AJ, Yang Y, Baciu P, Cech TR, & Lei M (2007). The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature, 445(7127), 506. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhan Y, Pedersen NL, Fang F, & Hägg S (2018). Telomere length and all-cause mortality: a meta-analysis. Ageing Research Reviews, 48, 11–20. [DOI] [PubMed] [Google Scholar]
- Werner C, Fürster T, Widmann T, Pöss J, Roggia C, Hanhoun M, Scharhag J. r., Büchner N, Meyer T, & Kindermann W (2009). Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation, 120(24), 2438–2447. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Anckar J, Stevens SM, Sistonen L, & Morimoto RI (2009). Stress-Inducible Regulation of Heat Shock Factor 1 by the Deacetylase SIRT1. Science, 323(5917), 1063–1066. doi:doi: 10.1126/science.H65946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikelski M, Spinney L, Schelsky W, Scheuerlein A, & Gwinner E (2003). Slow pace of life in tropical sedentary birds: a common-garden experiment on four stonechat populations from different latitudes. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270(1531), 2383–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbourn RV, Moatt JP, Froy H, Walling CA, Nussey DH, & Boonekamp JJ (2018). The relationship between telomere length and mortality risk in non-model vertebrate systems: a meta-analysis. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1741), 20160447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SE, Sanders TL, Beltran SE, & Rosvall KA (2022). The telomere regulatory gene POT1 responds to stress and predicts performance in nature: Implications for telomeres and life history evolution. Molecular Ecology, 31(23), 6155–6171. doi: 10.1111/mec.16237 [DOI] [PubMed] [Google Scholar]
- Wrutniak-Cabello C, Casas F, & Cabello G (2001). Thyroid hormone action in mitochondria. J Mol Endocrinol, 26(1), 67–77. doi: 10.1677/jme.0.0260067 [DOI] [PubMed] [Google Scholar]
- Xu M, Kiselar J, Whited TL, Hernandez-Sanchez W, & Taylor DJ (2019). POT1-TPP1 differentially regulates telomerase via POT1 His266 and as a function of single-stranded telomere DNA length. Proceedings of the National Academy of Sciences, 116(41), 23527–23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Xiong Y, Kim H, He Q, Li Y, Chen R, & Songyang Z (2011). Human telomeric proteins occupy selective interstitial sites. Cell research, 21(7), 1013–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung F, Ramírez CM, Mateos-Gomez PA, Pinzaru A, Ceccarini G, Kabir S, Fernández-Hernando C, & Sfeir A (2013). Nontelomeric role for Rap1 in regulating metabolism and protecting against obesity. Cell reports, 3(6), 1847–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Y, Hu X, Han P, Mendez-Bermudez A, Bauwens S, Eid R, Tan L, Pousse M, Giraud-Panis M-J, & Lu Y (2022). The non-telomeric evolutionary trajectory of TRF2 in zebrafish reveals its specific roles in neurodevelopment and aging. Nucleic acids research, 50(4), 2081–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AJ (2018). The role of telomeres in the mechanisms and evolution of life-history trade-offs and ageing. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1741). doi: 10.1098/rstb.2016.0452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HJ, & Koh SH (2022). Is Telomere Length Shortening a Risk Factor for Neurodegenerative Disorders? Dement Neurocogn Disord, 21(3), 83–92. doi: 10.12779/dnd.2022.21.3.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zglinicki T. v., & Martin-Ruiz C (2005). Telomeres as biomarkers for ageing and age-related diseases. Current molecular medicine, 5(2), 197–203. [DOI] [PubMed] [Google Scholar]
- Zhang C, Doherty JA, Burgess S, Hung RJ, Lindström S, Kraft P, Gong J, Amos CI, Sellers TA, & Monteiro AN (2015). Genetic determinants of telomere length and risk of common cancers: a Mendelian randomization study. Human molecular genetics, 24(18), 5356–5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JW, Rane G, Dai XY, Shanmugam MK, Arfuso F, Sarny RP, Lai MKP, Kappei D, Kumar AP, & Sethi G (2016). Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Research Reviews, 25, 55–69. doi: 10.1016/j.arr.2015.11.006 [DOI] [PubMed] [Google Scholar]
- Zhang P, Pazin MJ, Schwartz CM, Becker KG, Wersto RP, Dilley CM, & Mattson MP (2008). Nontelomeric TRF2-REST interaction modulates neuronal gene silencing and fate of tumor and stem cells. Current biology, 18(19), 1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zheng D, Wang M, & Cong Y-S (2009). Telomerase reverse transcriptase activates the expression of vascular endothelial growth factor independent of telomerase activity. Biochemical and biophysical research communications, 386(4), 739–743. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Liu X, Ding X, Wang F, & Geng X (2019). Telomere and its role in the aging pathways: telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology, 20(1), 1–16. [DOI] [PubMed] [Google Scholar]
- Zietsch BP, & Sidari MJ (2020). A critique of life history approaches to human trait covariation. Evolution and Human Behavior, 41(6), 527–535. [Google Scholar]


