Abstract
The first case of disseminated aspergillosis caused by Aspergillus ustus in an allogeneic peripheral stem cell transplant patient is described. The patient, a 46-year-old female with a history of myelodysplastic syndrome, underwent high-dose chemotherapy and total body irradiation prior to transplantation. She was released from the hospital 49 days posttransplant (p.t.) in a stable condition with an absolute neutrophil count (ANC) of 2,700 cells per μl. Multiple antimicrobial agents, including itraconazole (ITR), were prescribed during hospitalization and at the time of discharge. Three days after discharge, the patient was readmitted with hemorrhagic cystitis, persistent thrombocytopenia, and bilateral pulmonary consolidation, although no fever was present. The ANC at the time of readmission was 3,500. Upon detection of a pulmonary nodule (day 67 p.t.), a bronchoalveolar lavage was performed; the lavage fluid was positive for both cytomegalovirus and parainfluenza virus and negative for fungus. The patient was placed on ganciclovir. A biopsy specimen from a leg lesion also noted on day 67 p.t. revealed septate hyphae consistent with Aspergillus species, and a culture subsequently yielded Aspergillus ustus. Confirmation detection of A. ustus was made by demonstration of characteristic reproductive structures with the presence of Hülle cells. On day 67 p.t., ITR was discontinued and liposomal amphotericin B (AMB) was initiated. The patient’s condition worsened, and she died 79 days p.t. At the time of autopsy, septate hyphae were present in heart, thyroid, and lung tissues, with lung tissue culture positive for A. ustus. In vitro susceptibility testing indicated probable resistance to AMB but not to ITR. This case supports the need for the development of rapid methods to determine antifungal susceptibility.
Invasive aspergillosis, whether a focal disease or a disseminated infection involving multiple organ systems, has become the most common invasive mold infection. It is generally associated with severe immunosuppression, such as that occurring in patients undergoing high-dose chemotherapy followed by bone marrow transplantation, in patients undergoing solid organ transplantation, or, rarely, in patients with acquired immunodeficiency syndrome (1, 7, 14, 16, 20, 25, 26). Aspergillus fumigatus and Aspergillus flavus are the most common species associated with infection; however, reports of infection caused by other species such as Aspergillus terreus, Aspergillus niger, Aspergillus nidulans, and Aspergillus ustus have also been documented (3, 10, 13, 15, 27). Three cases of invasive infection caused by A. ustus have been reported in the literature (2, 27, 30). Two of these cases were endocarditis, one of which remained localized to the heart, while the other became disseminated, involving also the thyroid gland, lungs, kidneys, and peritoneum. The third case was primary cutaneous invasive aspergillosis in a liver transplant recipient (27). We report the first case of disseminated aspergillosis caused by A. ustus in a patient with myelodysplastic syndrome who underwent an allogeneic peripheral stem cell transplant from a related donor following myeloablative therapy.
Case report.
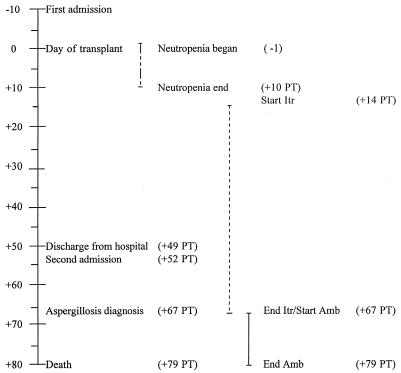
The patient was a 46-year-old female with a 2-year history of myelodysplastic syndrome complicated by persistent thrombocytopenia. She was admitted in March 1996 to the University of Nebraska Medical Center for evaluation and treatment. During a platelet transfusion, the patient developed an anaphylactic reaction requiring oxygen, steroids, and diphenhydramine hydrochloride (Benadryl). Subsequently, she was placed on high-dose steroids to decrease the antibody response to the donor platelets. In October 1996, the patient was readmitted with severe leg pain and continuing thrombocytopenia, at which time she was considered a candidate for myeloablative therapy with stem cell transplantation. Ten days after admission and following treatment with cyclophosphamide (Cytoxan) and total body irradiation, she received an allogeneic peripheral stem cell transplant (day 0) from her matched brother (Fig. 1). She experienced a 12-day period of neutropenia, and her hospitalization course was further complicated by a subdural hematoma, which necessitated two bur holes to evacuate pressure, and marked thrombocytopenia, which required daily platelet transfusions. In addition to routine immunosuppressive therapy, she continued to receive high-dose steroids to prevent anaphylaxis from the platelet transfusions. On day 14 posttransplant (p.t.), progressive pleural effusions were noted and a pleurocentesis was performed. Stains and cultures of the pleural fluid failed to reveal an infectious etiology. At this time, the patient was placed on empirical itraconazole (ITR) therapy (200 mg twice daily). She was discharged 49 days p.t. in stable condition with an absolute neutrophil count (ANC) of 2,700 cells per μl. Antimicrobial agents, including ITR, were prescribed during the hospitalization period and at the time of discharge. On day 52 p.t., the patient was readmitted with severe hematuria, persistent thrombocytopenia, and bilateral pulmonary consolidation visible on her chest radiograph. Her ANC at the time of admission was 3,500 cells per μl, and she did not have a fever. On day 55 p.t., she underwent a cystoscopy of her bladder with clot evacuation and fulguration of the bleeding points. Urine culture detected BK virus. The patient remained respirator dependent following the cystoscopy. At 67 days p.t., a chest radiograph revealed increased pulmonary consolidation with the formation of a nodule in the lower left lobe of the lung. A bronchoalveolar lavage was performed; the lavage fluid was positive for cytomegalovirus pp65 antigen and was tissue culture positive for cytomegalovirus as well as parainfluenza virus. No fungi were cultured from this specimen. Also on day 67 p.t., a necrotic skin lesion was noted on the lower left leg. Histopathology of the biopsy tissue revealed septate hyphae consistent with an Aspergillus sp. Culture of the specimen grew an unusual Aspergillus species (recognized 7 days after culture), which was subsequently identified by a reference laboratory as A. ustus. Based on the histologic findings of the skin biopsy, ITR therapy was stopped and intravenous liposomal amphotericin B (AMB) at a dose of 5 mg per kg of body weight was begun. The condition of the patient progressively deteriorated, and she died 79 days p.t. An autopsy was performed.
FIG. 1.
Time course illustrating the relationship of antifungal therapy to times of admission, transplant, aspergillosis diagnosis, and death. Itr, itraconazole; Amb, amphotericin B. Days before (−) and after (+) transplant are indicated by numbers.
Autopsy findings.
At the time of autopsy, the left lung contained a 2.5-cm nodule in the lower lobe which upon histopathological examination showed numerous septate branching hyphae. Part of this nodule was submitted for fungal culture, which grew A. ustus. Viral inclusions consistent with cytomegalovirus infection were also seen in lung tissue sections. Sections of the left atrium and right ventricle of the heart revealed areas of necrotic myocardium infiltrated by broad sheets of fungal hyphae morphologically consistent with an Aspergillus species. The necrotic areas were surrounded by lymphocytes, histocytes, and giant cells. The thyroid showed extensive replacement by large areas of necrosis which were filled with branching septate hyphae. The bladder was found to contain a 150-g blood clot with grossly hemorrhagic mucosa. Microscopic examination of the bladder mucosa showed mucosal ulceration and submucosal hemorrhage. No organisms were identified microscopically in the bladder tissue. No other organs contained evidence of Aspergillus hyphae. The cause of death was recorded as “fungal and cytomegaloviral sepsis arising in a setting of myelodysplastic syndrome, status postrelated to allogeneic peripheral stem cell transplantation.”
Mycology.
Tissue from the leg lesion biopsy on day 67 p.t. and from lung tissue obtained at the time of postmortem examination were plated on Sabouraud dextrose agar with chloramphenicol (SAB-C; Remel, Lenexa, Kans.) and incubated at 30°C. Both cultures revealed a rapidly growing mold which produced white colonies that subsequently became gray and radially folded. A 10-day-old slide culture prepared on Sabouraud dextrose agar (SAB; Remel) revealed fruiting structures consistent with an unusual Aspergillus species. A subculture of this leg lesion isolate was forwarded to the Fungus Testing Laboratory, Department of Pathology, University of Texas Health Science Center (UTHSC) at San Antonio, for further characterization and susceptibility testing and entered into the UTHSC stock collection under accession no. UTHSC 97-113.
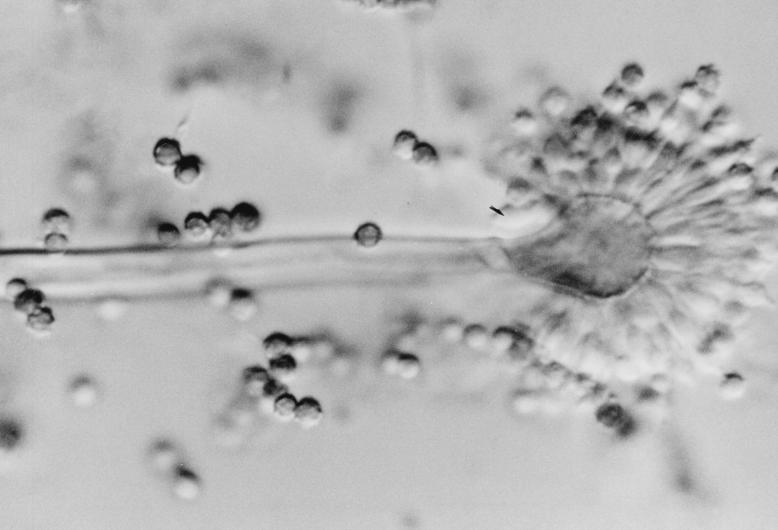
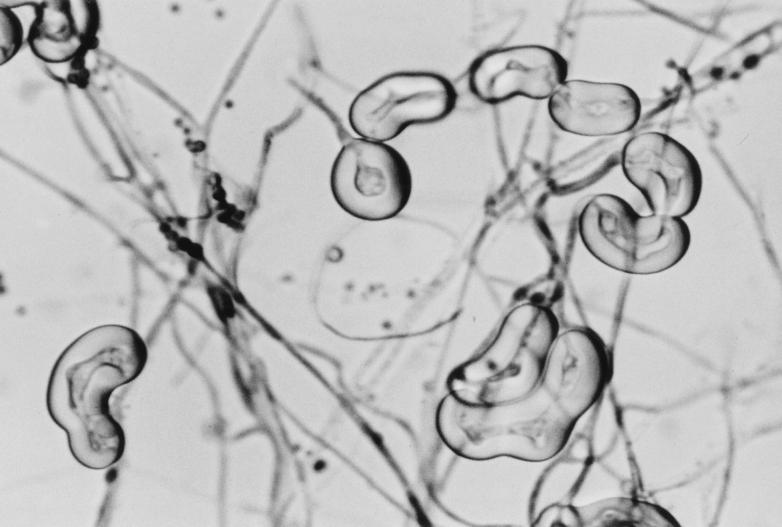
The isolate, forwarded on an SAB slant, was woolly and tan-brown to gray and produced a noticeable yellow diffusing pigment. Tease mounts of the isolate revealed a biseriate Aspergillus species with rough conidia. The isolate was subcultured onto a potato flakes agar (PFA) plate, a PFA slide culture, and PFA slants, prepared in-house, at 25°C (22). Colonies at 7 days were drab olivaceous-gray and woolly, with a yellow diffusing pigment, with the PFA plate producing a slight brownish exudate. Seven-day-old slide cultures revealed a radiate to loosely columnar biseriate Aspergillus species with smooth, brown, thick-walled conidiophores (mostly 200 to 280 μm long by 4 μm wide); small, subglobose, brown vesicles (8 to 14 μm in diameter); metulae (3 μm wide by 4 μm long); phialides (2.5 μm wide by 5 μm long); and very rough-walled, brown, globose conidia (2.5 to 3.9 μm in diameter) (Fig. 2). Elongate, irregular to curved Hülle cells were also present around the edge of the coverslip and measured approximately 20 by 50 to 20 by 60 μm (Fig. 3). Twelve-day-old subcultures on Czapek medium (Remel) at 25°C produced spreading colonies that were 45 mm in diameter. Colonies were cream to buff with the heaviest conidiation occurring in the drab, brown-gray central and submarginal area. Reverse colors were similar, with a slight yellow diffusing pigment. Twelve-day-old, 25°C subcultures on Emmons Sabouraud dextrose agar (E-SDA) plates (Remel) displayed flat, spreading, olivaceous-brown colonies with a slight yellow diffusing pigment. On the basis of these macroscopic and microscopic characteristics, the isolate was identified as A. ustus (Bainer) Thom et Church in the subgenus Nidulantes section Usti (Aspergillus ustus group) (11) and was deposited in the American Type Culture Collection under accession no. ATCC 201953.
FIG. 2.
Fruiting structures of A. ustus from 7-day-old PFA slide culture at 25°C demonstrating a small vesicle with metulae, phialides, and rough conidia. Under light microscopic illumination, these structures were brown in color. Magnification, ×460.
FIG. 3.
Hülle cells of A. ustus from 7-day-old PFA slide culture at 25°C. Magnification, ×460.
Although four other species of aspergilli are included in the A. ustus group, as defined by Raper and Fennell (21), A. ustus is by far the most common, being widely distributed in both temperate and tropical soils. Aspergillus puniceus Kwon et Fennell, the species most closely resembling A. ustus, is characterized by yellow masses of Hülle cells, yellow mycelium, and a conspicuous pink to red exudate and pink diffusing pigment. The three other species in the group, A. panamensis Raper et Thom, A. conjunctus Kown et Fennell, and A. deflectus Fennell et Raper, each have features quite distinct from A. ustus which excluded them from consideration. These included persistently radiate conidial heads and large vesicles in A. panamensis, conidia with distinct connectives and long, narrow Hülle cells in A. conjunctus, and nodding conidial heads in A. deflectus.
The use of the term group in the designation of Aspergillus species refers to species sharing several key features. These features include growth rates, temperature responses, macroscopic descriptions, microscopic characteristics (foot cells, conidiophores, vesicles, metulae, phialides, conidia, Hülle cells, and sclerotia, etc.), and teleomorphic connections. The reporting of Aspergillus groups gained acceptance with the seminal work of Raper and Fennel because major separating characteristics were usually clearly evident. Identification to the species level, however, was often more problematic due to difficulty in defining individual species in precise, unequivocal terms. Although Raper and Fennell and subsequent authors have clearly delineated species based upon macroscopic and microscopic characteristics (21) and scanning electron microscopy studies (12), the characterization of species at the molecular level is only in its infancy. Given our current technologies, however, the further separation of species and their connections to heretofore-unknown teleomorphs seems highly probable. Clearly, identification of aspergilli to the currently recognized species level provides a more precise identification of the etiologic agent than does a group designation. The continued use of the term group, however, does separate species that display different, easily recognizable characteristics.
Antifungal susceptibility studies.
The case isolate was evaluated by using the National Committee for Clinical Laboratory Standards macrobroth dilution method M27-A, which was modified for mold testing (18). Briefly, the case isolate and the Paecilomyces control strain (UTHSC 90-459) were grown on PFA for 14 days at 25°C to induce conidial formation. Mature PFA cultures were overlaid with sterile, distilled water, and suspensions were made by gently scraping the colonies with the tip of a Pasteur pipette. Heavy hyphal fragments were allowed to settle, and the upper, homogeneous conidial suspensions were removed. Conidia were counted with a hemocytometer, and the inoculum was standardized to 1.0 × 105 CFU per ml. Conidial suspensions were further diluted 1:10 in medium for a final inoculum concentration of 1.0 × 104 CFU per ml. Final drug concentration ranges were 0.03 to 16 μg per ml for AMB (E. R. Squibb & Sons, Princeton, N.J.) and 0.125 to 64 μg per ml ITR (Janssen Pharmaceutica, Titusville, N.J.). Amphotericin B was tested in antibiotic medium 3 (Difco, Detroit, Mich.), while ITR was tested in RPMI 1640 with l-glutamine and morpholinepropanesulfonic acid (MOPS) buffer at a concentration of 165 mM and without sodium bicarbonate (American Biorganics, Inc., Niagara Falls, N.Y.). Previously prepared frozen drug tubes containing 0.1 ml of drug were allowed to thaw and were inoculated with 0.9 ml of the conidial suspension. A drug-free growth tube containing the case isolate and control organism was included. The tubes were incubated at 35°C, and MICs were determined at 24 and 48 h. The concentrations of the first tubes that gave a score of 0 (optically clear) for AMB and a score of 2 (reduction in turbidity of >80% in contrast to that the drug-free control tube) for ITR were defined as the MICs. Minimum lethal concentrations (MLCs) were determined for AMB by plating 100 μl of the contents of the drug-free control tube, the MIC tube, and each tube with a concentration above the MIC onto an SDA plate incubated at 35°C. The MLC was defined as the lowest concentration of antifungal compound resulting in 5 or fewer colonies on the SDA plate (23). Results indicated probable in vitro resistance to AMB with MICs and MLCs at 24 h of 1 and 2 μg per ml, respectively, and at 48 h of 2 and 2 μg per ml, respectively, and susceptibility to ITR at 24 and 48 h at MICs of 0.125 and 0.5 μg per ml, respectively.
Discussion.
A recent 12-year retrospective analysis of 133 cases of confirmed invasive aspergillosis identified one case each of A. ustus and A. niger infection, while A. flavus and A. fumigatus were responsible for 88.7% of the cases and A. terreus was responsible for 9.8% (10). The case of disseminated aspergillosis caused by A. ustus is described here in detail and is the first case reported for a stem cell transplant recipient.
Our patient had several factors which placed her at high risk for the development of disseminated aspergillosis (1, 6). These included a long history of myelodysplastic syndrome complicated by prolonged pancytopenia, long-term steroid therapy, myeloablative therapy with neutropenia for a duration of 12 days, immunosuppressive therapy to prevent graft-versus-host disease, and multiple antimicrobial agent usage. Additionally, upon readmission, she was diagnosed with pneumonia caused by cytomegalovirus and parainfluenza virus. Even though immunosuppression is considered a major risk factor for invasive aspergillosis, it was not noted as a risk factor in the previous reports of infection caused by A. ustus, although other risk factors were mentioned in each case (2, 27, 30).
The primary site of infection in our patient was presumed to be respiratory, even though initial diagnosis of invasive aspergillosis was obtained following biopsy of a necrotic skin lesion. A lung nodule was discovered at 67 days p.t. and confirmed at the time of autopsy to contain septate branching hyphae consistent with an Aspergillus sp. Cultures of the lung nodule were positive for A. ustus. The lung is the most common primary site of invasive aspergillosis, with dissemination believed to occur from this focus of infection (16). Primary infection of other sites may also occur, with the sinus and skin being the most commonly reported (9, 16, 29). Stiller et al. described a case of primary cutaneous invasive aspergillosis caused by A. ustus in a liver transplant patient (27). This patient eventually died of hepatic failure with no evidence of disseminated disease.
The brain is the most frequent site of extrapulmonary involvement in invasive infection caused by A. fumigatus and A. flavus (9, 10). However, dissemination to the heart is common in infections caused by A. terreus (10). Involvement of the heart occurred in two of the three reported cases of invasive aspergillosis caused by A. ustus (2, 30). Carrizosa et al. described a case of primary endocarditis without dissemination in a patient with complications of rheumatic heart disease (2). Weiss and Thiemke described disseminated A. ustus infection in a patient with severe coronary artery disease with mitral regurgitation (30). Their patient underwent multiple surgeries, required steroids for bronchospasm, had adult-onset diabetes, and received multiple antimicrobial agents. A. ustus was found at the time of autopsy in the heart, as well as the thyroid, lungs, kidney, and peritoneum. Similarly, in our patient, septate branching hyphae characteristic of Aspergillus were noted in tissue from the heart and thyroid, as well as the left lung, upon postmortem examination. Only the left lung tissue was cultured, which became positive for A. ustus. Although not confirmed by culture, it is highly probable that the heart tissue was invaded by A. ustus. Multiple organ involvement by Aspergillus detected at the time of postmortem examination is common (28).
Even though AMB remains the treatment of choice for invasive aspergillosis, ITR has shown some effectiveness (1, 4, 26). Our patient received an empirical dose of ITR (200 mg twice daily) for 52 days prior to aspergillosis diagnosis. At the time of diagnosis, the patient was switched from ITR to liposomal AMB. Liposomal AMB has shown some effectiveness in the treatment of fungal infections in neutropenic patients (17, 19). Susceptibility testing of the A. ustus isolated in the present study showed it to be resistant to AMB and susceptible to ITR. Resistance to ITR has been demonstrated for other Aspergillus species (5). In vitro studies comparing the susceptibilities of clinical isolates of A. ustus to ITR and AMB have not been reported to our knowledge. Additionally, it is not generally known which therapeutic regimen is most useful in the treatment of invasive aspergillosis (4). Neutropenic patients with a solitary pulmonary lesion have benefited from aggressive surgical resection to improve long-term survival (24). Intravenous AMB appeared to be successful in treating invasive A. ustus infection in two previously reported cases (2, 27).
Nosocomial aspergillosis is common, especially when construction activity is present, as occurred at the time of diagnosis of aspergillosis in our patient (8). It is difficult to determine whether the infection was acquired in the hospital. Upon discharge, the patient was in stable condition with a normal chest radiograph. At readmission, pulmonary consolidation was noted, with a pulmonary nodule detected 12 days later. A bronchoalveolar lavage specimen was culture positive for cytomegalovirus and parainfluenza virus but negative for fungus; however, at the time of autopsy, the lung nodule contained septate hyphae and was culture positive for A. ustus. Since only 3 days had elapsed between discharge and readmission, and there was an extended time between readmission and the development of the pulmonary nodule, it is probable that the A. ustus causing invasive aspergillosis was hospital acquired.
This case extends the spectrum of invasive clinical infections caused by A. ustus and illustrates the importance of early recognition of patients infected with Aspergillus species. The identification and susceptibility results were not available prior to death in this case; however, with limited treatment options, the outcome would probably have remained unchanged. The use of molecular methods for a more timely diagnosis of invasive aspergillosis, along with advances in susceptibility testing and the development of new therapeutic options, is needed to improve the outcome in patients diagnosed with this disease (1, 4).
Acknowledgments
We thank Mary Parsons and Delinda Sundsboe of the Clinical Microbiology Laboratory, Mycology Section, at the University of Nebraska Medical Center for their assistance in maintaining the Invasive Mold Infections database.
ADDENDUM IN PROOF
A fourth case of invasive infection caused by A. ustus and the second recorded case of primary cutaneous invasive aspergillosis were recently reported in the literature (R. M. Ricci et al., J. Am. Acad. Dermatol. 38:397–398, 1998).
REFERENCES
- 1.Andriole V T. Aspergillus infections: problems in diagnosis and treatment. Infect Agents Dis. 1996;5:47–54. [PubMed] [Google Scholar]
- 2.Carrizosa J, Levison M E, Lawrence T, Kaye D. Cure of Aspergillus ustus endocarditis on a prosthetic valve. Arch Intern Med. 1974;133:486–490. [PubMed] [Google Scholar]
- 3.Casson D H, Riordan F A, Ladusens E J. Aspergillus endocarditis in chronic granulomatous disease. Acta Paediatr. 1996;85:758–759. doi: 10.1111/j.1651-2227.1996.tb14143.x. [DOI] [PubMed] [Google Scholar]
- 4.Denning D W. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608–615. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 5.Denning D W, Venkateswarlu K, Oakley K L, Anderson M J, Manning N J, Stevens D A, Warnock D W, Kelly S L. Itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1364–1368. doi: 10.1128/aac.41.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fridkin S K, Jarvis W R. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev. 1996;9:499–511. doi: 10.1128/cmr.9.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwen P C, Reed E C, Armitage J O, Bierman P J, Kessinger A, Vose J M, Arneson M A, Winfield B A, Woods G L. Nosocomial invasive aspergillosis in lymphoma patients treated with bone marrow or peripheral stem cell transplants. Infect Control Hosp Epidemiol. 1993;14:131–139. doi: 10.1086/646698. [DOI] [PubMed] [Google Scholar]
- 8.Iwen P C, Davis J C, Reed E C, Winfield B A, Hinrichs S H. Airborne fungal spore monitoring in a protective environment during hospital construction, and correlation with an outbreak of invasive aspergillosis. Infect Control Hosp Epidemiol. 1994;15:303–306. doi: 10.1086/646916. [DOI] [PubMed] [Google Scholar]
- 9.Iwen P C, Rupp M E, Hinrichs S H. Invasive mold sinusitis: 17 cases in immunocompromised patients and review of the literature. Clin Infect Dis. 1997;24:1178–1184. doi: 10.1086/513662. [DOI] [PubMed] [Google Scholar]
- 10.Iwen P C, Rupp M E, Langnas A N, Reed E C, Hinrichs S H. Invasive pulmonary aspergillosis due to Aspergillus terreus: 12-year experience and review of the literature. Clin Infect Dis. 1998;26:1092–1097. doi: 10.1086/520297. [DOI] [PubMed] [Google Scholar]
- 11.Klich M A, Pitt J I. A laboratory guide to common Aspergillus species and their teleomorphs. North Ryde, New South Wales, Australia: Commonwealth Scientific and Industrial Research Organization; 1988. [Google Scholar]
- 12.Kozakiewicz Z. Aspergillus species on stored products. Wallingford, Oxon, United Kingdom: International Mycological Institute; 1989. [Google Scholar]
- 13.Lawrence T, Schockman A T, McVaugh H I. Aspergillus infection of aortic prosthetic valves. Chest. 1971;60:406–414. doi: 10.1378/chest.60.4.406. [DOI] [PubMed] [Google Scholar]
- 14.Lortholary O, Dupont B. Antifungal prophylaxis during neutropenia and immunodeficiency. Clin Microbiol Rev. 1997;10:477–504. doi: 10.1128/cmr.10.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miles A M, Barth R H. Aspergillus peritonitis: therapy, survival, and return to peritoneal dialysis. Am J Kidney Dis. 1995;26:80–83. doi: 10.1016/0272-6386(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 16.Miller W T. Aspergillosis: a disease with many faces. Semin Roentgenol. 1996;31:52–66. doi: 10.1016/s0037-198x(96)80040-x. [DOI] [PubMed] [Google Scholar]
- 17.Mills W, Chopra R, Linch D C, Goldstone A H. Liposomal amphotericin B in the treatment of fungal infections in neutropenic patients: a single-center experience of 133 episodes in 116 patients. Br J Haematol. 1994;86:754–760. doi: 10.1111/j.1365-2141.1994.tb04825.x. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 19.Ng T T, Denning D W. Liposomal amphotericin B (AmBisome) therapy in invasive fungal infections. Evaluation of United Kingdom compassionate use data. Arch Intern Med. 1995;155:1093–1098. [PubMed] [Google Scholar]
- 20.Perfect, J. R., and W. A. Schell. 1996. The new fungal opportunists are coming. Clin. Infect. Dis. 22(Suppl. 2):S112–S118. [DOI] [PubMed]
- 21.Raper K B, Fennell D I. The genus Aspergillus. Baltimore, Md: The Williams & Wilkins Co.; 1965. [Google Scholar]
- 22.Rinaldi M G. Use of potato flakes agar in clinical mycology. J Clin Microbiol. 1982;15:1159–1160. doi: 10.1128/jcm.15.6.1159-1160.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinaldi M G, Howell A W. Antifungal antimicrobics: laboratory evaluation. In: Wentworth B, editor. Diagnostic procedures for mycotic and parasitic infections. 7th ed. Washington, D.C: American Public Health Association; 1988. pp. 325–356. [Google Scholar]
- 24.Robinson L A, Reed E C, Galbraith T A, Alonso A, Moulton A L, Fleming W H. Pulmonary resection for invasive Aspergillus infections in immunocompromised patients. J Thorac Cardiovasc Surg. 1995;109:1182–1197. doi: 10.1016/S0022-5223(95)70202-4. [DOI] [PubMed] [Google Scholar]
- 25.Singh N, Arnow P M, Bonham A, Dominguez E, Paterson D L, Pankey G A, Wagener M M, Yu V L. Invasive aspergillosis in liver transplant recipients in the 1990s. Transplantation. 1997;64:716–720. doi: 10.1097/00007890-199709150-00009. [DOI] [PubMed] [Google Scholar]
- 26.Stevens D A, Lee J Y. Analysis of compassionate use itraconazole therapy for invasive aspergillosis by the NIAID Mycoses Study Group criteria. Arch Intern Med. 1997;157:1857–1862. [PubMed] [Google Scholar]
- 27.Stiller M J, Teperman L, Rosenthal S A, Riordan A, Potter J, Shupack J L, Gordon M A. Primary cutaneous infection by Aspergillus ustus in a 62-year-old liver transplant recipient. J Am Acad Dermatol. 1994;31:344–347. doi: 10.1016/s0190-9622(94)70169-5. [DOI] [PubMed] [Google Scholar]
- 28.Vogeser M, Haas A, Aust D, Ruckdeschel G. Postmortem analysis of invasive aspergillosis in a tertiary care hospital. Eur J Clin Microbiol Infect Dis. 1997;16:1–6. doi: 10.1007/BF01575110. [DOI] [PubMed] [Google Scholar]
- 29.Wald A, Leisenring W, van Burik J A, Bowden R A. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–1466. doi: 10.1086/516480. [DOI] [PubMed] [Google Scholar]
- 30.Weiss L M, Thiemke W A. Disseminated Aspergillus ustus infection following cardiac surgery. Am J Clin Pathol. 1983;80:408–411. doi: 10.1093/ajcp/80.3.408. [DOI] [PubMed] [Google Scholar]