Abstract
Somatostatin (SST) is a neuropeptide widely expressed in the central nervous system with dense expression in limbic regions such as the extended amygdala. It has recently gained attention for playing a role in modulating alcohol use disorders and co-morbid neuropsychiatric disorders. However, the role of SST in the central nucleus of the amygdala (CeA), a key region for neuropeptide regulation of alcohol and anxiety related behaviors, in alcohol consumption has not been assessed. In this work we perform an initial examination of the interaction between the CeA SST system and binge ethanol intake. Binge intake is a dangerous pattern of excessive ethanol consumption associated with health complications and the transition into alcohol dependence. We use the Drinking in the Dark (DID) model of binge intake in C57BL/6J male and female mice to examine: 1) the impact of 3 DID cycles on CeA SST expression; 2) the effect of intra-CeA SST injection on binge-like ethanol consumption; and 3) if the SST receptor 2 or 4 (SST2R or SST4R) mediate any effect on consumption. Our results show binge-like ethanol intake decreases SST expression in the CeA, but not neighboring basolateral amygdala. We further found intra-SST CeA administration reduces binge ethanol intake. This decrease was replicated by the administration of an SST4R agonist. These effects were not sex-dependent. Overall, this work lends further support for SST playing a role in alcohol related behaviors and as a potential therapeutic target.
Keywords: Binge drinking, drinking in the dark, somatostatin, central nucleus of the amygdala, alcohol use disorder, neuropeptide
1.0. INTRODUCTION
Nearly 15 million individuals currently qualify as exhibiting an alcohol use disorder within the United States (National Institute on Alcohol Abuse and Alcoholism, 2022). Frequent engagement in binge alcohol (ethanol) consumption is thought to act as an early step in the transition to full alcohol dependence and is thus a critical intervention point. With approximately 25% of Americans over the age of 12 engaging in binge ethanol intake, understanding the neural mechanisms driving this behavior is important in both developing treatments for early intervention and identifying circuits of interest following the transition to full alcohol dependence and withdrawal [NIAAA]. Extensive research has identified the central nucleus of the amygdala (CeA) as a key mediator of alcohol-related behaviors [for review see:(N W Gilpin et al., 2015)]. The CeA is the primary output nucleus of the amygdala, serving as an integrative site which assigns emotional salience to incoming stimuli through extensive projections to other limbic and subcortical regions (Lalumiere, 2014; Tye et al., 2011). The CeA is predominantly composed of GABAergic cells which can be subdivided based, in part, on their neuropeptide expression pattern (Babaev et al., 2018). Overall, repeated binge-ethanol exposure significantly alters CeA GABAergic, inducing, in brief, a ‘two-punch’ increase in signaling of GABAergic cells expressing ‘pro-stress’ excitatory and decrease in GABAergic cells expressing ‘anti-stress’ inhibitory neuropeptides which significantly contributes to driving dangerous patterns of alcohol intake (N W Gilpin et al., 2015; Roberto et al., 2012, 2004; Silberman et al., 2009).
A significant CeA neuropeptide which has gained recent attention for playing a role in drug-related behavior is somatostatin (SST) (Robinson and Thiele, 2020). SST is a cyclic polypeptide widely expressed throughout the CNS and acts as an important neuro-transmitter/modulator and trophic factor (Babaev et al., 2018; Kubota et al., 2011; Schwartz et al., 1996). Five SST receptors (SSTRs) have thus far been identified, SSTR1–5, and are all Gi/o-coupled G-protein coupled receptors (GPCR). These receptors are expressed throughout the brain on astrocytes and numerous neural populations, including: glutamatergic principal; GABAergic inter- and projection-; and monoaminergic cells (Bell et al., 1995; Yamada et al., 1992). Unsurprisingly given this wide distribution, SST has been shown to play a role in numerous important behaviors (for review see (Engin and Treit, 2009; Robinson and Thiele, 2020)).
Importantly, given the well-known co-morbidity between alcohol use and anxiety/depressive disorders, amygdalar SST signaling potently modulates anxiety- and depression-like behaviors, with SSTR2 and SSTR4 thought to play particularly important roles (Butler et al., 2012; Engin and Treit, 2009; Fuchs et al., 2017; Lin and Sibille, 2015; Robinson and Thiele, 2020; Scheich et al., 2017, 2016; Stengel et al., 2013; Stengel and Taché, 2017; Yeung and Treit, 2012; Yu et al., 2016). Further, SST-expressing (SST+) cells are particularly vulnerable to stress-induced insult. For example, following chronic stress SST+ interneurons in the prefrontal cortex and hippocampus are specifically downregulated relative to other GABAergic subtypes and display significant transcriptome deregulations compared with pyramidal neurons (Banasr et al., 2017; Lin and Sibille, 2015). This vulnerability likewise exists during ethanol exposure. Acute ethanol increases SSTR expression while decreasing expression of SST itself, while in contrast chronic ethanol reduces both receptor and peptide expression in a brain region dependent manner (Barrios et al., 1990). Importantly, activation of the CNS SST system is sufficient to inhibit the stress response mediated by corticotropin releasing factor (Stengel and Taché, 2017), another neuropeptide critical in mediating binge-ethanol intake (Zorrilla et al., 2014).
The CeA has one of the highest densities of SST+ cells in the rodent brain (McCullough et al., 2018; Zhang et al., 2017). This system is therefore an ideal target for pharmacological intervention of ethanol-induced CeA pathology; however, to date, there have been no studies evaluating interaction between binge-ethanol consumption and the CeA SST system. Within this work we seek to assess, in male and female mice, 1) the impact of repeated cycles of binge-ethanol intake on CeA SST expression, and 2) the impact of microinjection of SST or a SSTR agonist into the CeA on binge-like drinking and anxiety-like behavior.
2.0. METHODS
2.1. Animals
Male and female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were ≥10 weeks old at experiment start. Mice were housed individually in an AAALAC accredited vivarium (22°C; reversed 12:12h light:dark cycle, lights on at 20:00) with ad libitum access to Prolab® RMH 3000 (Purina LabDiet®; St. Louis, MO) and water, unless otherwise stated. Prior to experiments, animals acclimated to the experimental housing environment for ≥1 week. All procedures were approved by the University of North Carolina Institutional Animal Care and Use Committee and followed the Guidelines for the Care and Use of Laboratory Animals.
2.2. “Drinking in the Dark” Procedures
Binge drinking was modeled using a 4-day DID paradigm as previously described(Robinson et al., 2019; Thiele et al., 2014). Three hours into the dark cycle (11:00), water bottles were removed and replaced with sipper tubes containing 20% (v/v) ethanol or 3% (w/v) sucrose in tap water for 2h. On the fourth day of each cycle (test day), animals were treated with drug or appropriate vehicle ~30 minutes prior to bottles on (except in the immunohistochemistry study, where no treatment was given). Each 4-day DID cycle was separated by a 3-day abstinence. In pharmacological studies tail blood samples (≈60μL) were taken immediately following DID procedure to determine BECs with a AM1 Alcohol Analyzer (Analox, London, UK).
2.3. Elevated Plus Maze Testing Procedures
Animals from both cohorts of experiment 2 that retained unobstructed cannula were used for EPM testing. Behavioral testing occurred at least one week following DID sucrose testing (at least 3 weeks after final binge-ethanol exposure). Animals were randomly assigned into treatment group and administered drug or appropriate vehicle treatment identically as described for DID testing, save animals only underwent one behavioral test (no Latin-square design was used). Testing was performed 4–6h after the initiation of their dark period. Animals were allowed to acclimate to the dimly lit testing room for ≥1 hour prior to test and animals remained in room alone during the test period. Videos were taken of the 5-minute EPM test then scored by hand by an experimenter blinded to treatment group.
2.4. Immunohistochemistry (IHC)
Mice underwent 3 cycles of ethanol DID or 3 cycles with water alone [Fig. 1a]. Three cycles were selected based on previous work in our lab (Robinson et al., 2019). Immediately following the final DID session (at approximately 13:00h) each mouse was administered 0.1 mL intraperitoneal (i.p.) injection ketamine/xylazine (6.67mg/0.1mL; 0.67 mg/0.1mL; in 0.9% saline) and perfused transcardially using 0.1M phosphate buffer saline (PBS; pH=7.4) and 4% paraformaldehyde in PBS (pH=7.4). After extraction, brains were post-fixed in 4% paraformaldehyde for 24–48h then sectioned at 40μm thickness (Leica VT1000S vibratome; Wetzlar, Germany). Following antigen retrieval (1h incubation in citrate buffer (10mM citric acid; .05% Tween20; pH=6.0) at 66°C), sections were used for SST immunoreactivity detection. At this time random identification numbers were assigned to blind experimenter to treatment. Sections were blocked in 3% horse serum (0.005% Tween20 in phosphate buffered saline pH=7.4) for 1h then incubated in primary anti-Somatostatin (1:500) (rat monoclonal, Millipore, Burlington, MA) for 72h at 5°C. Sections were then incubated 2h at room temperature in secondary solution (1:5000) (Alexa Fluor 647 donkey anti-rat, abcam, Boston, MA) then mounted onto glass slides and coverslipped in Shur Mount (General Data). One tissue set was run without primary antibody to confirm lack of non-specific florescence. Color images of the CeA and BLA were captured through a digital camera (Roper Scientific), mounted on an optical microscope (Leica DM6000), and positive fluorescence was quantified using FIJI (https://www.nature.com/articles/nmeth.2019). Individual CeA and BLA regions were sectioned as regions of interest (ROI) in FIJI for each image (guided by Paxinos The Mouse Brain in Stereotaxic Coordinates) and SST expression was determined by evaluating the percentage of each ROI area which expressed fluorescence. This method was selected as CeA and BLA sizes/shapes change along the rostral-caudal axis in the regions analyzed (bregma −0.82 to −1.82), incurring difficulty in interpreting absolute value measurements. Experimenter remained blinded to treatment group until following quantification. Unilateral percent area of florescence for 2–4 sections per animal were averaged together and used for analysis. The same slices were used to analyze the BLA and CeA, with 4 mice (3 water and 1 ethanol) excluded from BLA analysis due to excessive damage to the region during the slicing/straining processing. Representative images in figures were digitally adjusted (brightness/contrast/saturation) for best appearance in publication. All images were enhanced as a group (identical settings).
FIGURE 1. Binge-Like Ethanol Intake Reduces CeA SST Expression.
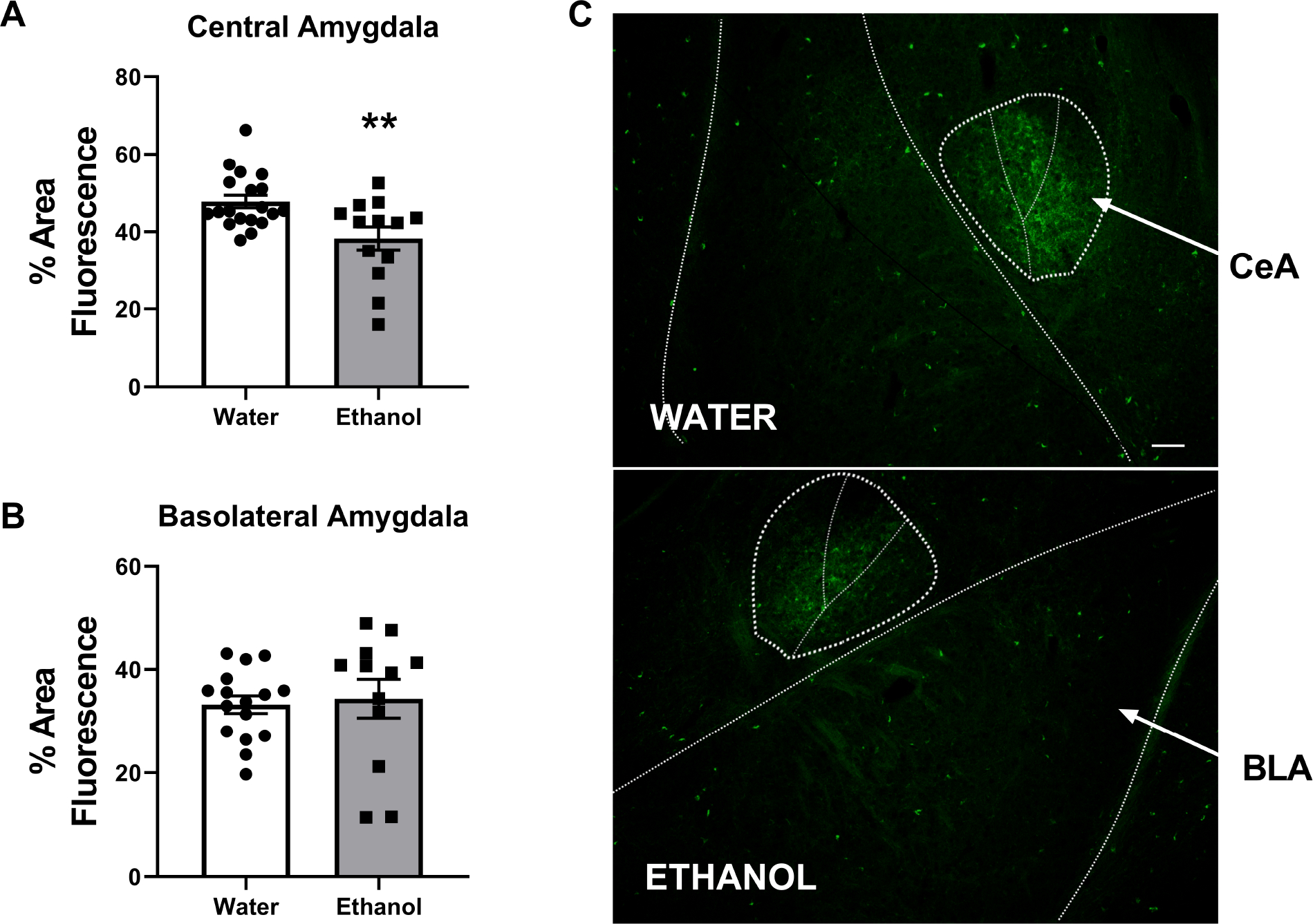
Three weeks of DID ethanol intake reduced SST expression in the A) central nucleus of the amygdala (CeA), but not B) basolateral nucleus of the amygdala (BLA) relative to water exposed animals. C) exemplar images of SST expression in the amygdala; scale bar = 200μm. ** = p < 0.01 in student’s t-test.
2.5. Surgeries
For all surgeries, animals were given intraperitoneal (i.p.) injections (1.5 mL/kg) of an anesthetizing cocktail of xylazine (10 mg/kg) and ketamine (100 mg/kg). In cannulation studies, using an Angle II™ Stereotax (Leica Instruments, Buffalo Grove, IL), bilateral guide cannula (26GA single cannula cut 5MM below pedestal; dummy cannula: 5MM with 0.5MM projection; injector: 5MM with 0.5MM projection) (Plastics One; Roanoke, VA) were lowered into the CeA (AP: −1.02, ML: ±2.25, DV: −4.4). Mice recovered for ≥1 week before experiment start.
2.6. Drug Administration
On DID test days animals underwent micro-injection of the experiment-specific drug or appropriate vehicle ~30 minutes prior to test start. Drugs were as follows (vehicle in each experiment was the solution respective drugs were dissolved in): Somatostatin, Tocris, Minneapolis, MN (3μg/0.5μl/side 5% DMSO in saline); L-054,264, Tocris, Minneapolis, MN (2.7μg/0.5μl/side made up 1:3 DMSO:Saline) (SST2R agonist); L-803,087 trifluoroacetate, Tocris, Minneapolis, MN (3μg/0.5μl/side made up 1:3 DMSO:Saline) (SST4R agonist)(Prévôt et al., 2017). A Latin-square design was used in each experiment. Animals were randomly assigned to drug or vehicle treatment on test day 1, then received the alternative treatment on test day 2 of a second DID cycle [See Fig. 2a]. In experiment 4 a third week was added, in which animals were randomly assigned to groups to receive one of three treatments on each of the three test days [See Fig. 4a]. Infusions for microinjected drugs occurred as previously published at a rate of 0.5 μL/min using a Hamilton syringe (Reno, NV) attached to a Harvard Apparatus PHD 2000 infusion pump (Holliston, MS)(Robinson et al., 2019). After infusion, injectors remained in guide cannula for an additional 0.5–1 min for diffusion. As animals underwent a 3-day abstinence from ethanol or sucrose following each test day, potential carry-over effects 24h following injection were not specifically evaluated. Following brain extraction and section mounting (see IHC methods) cannula placements were verified through use of a digital camera (Roper Scientific), mounted on an optical microscope (Leica DM6000).
FIGURE 2. Intra-CeA SST Reduces Binge-Like Ethanol Intake.
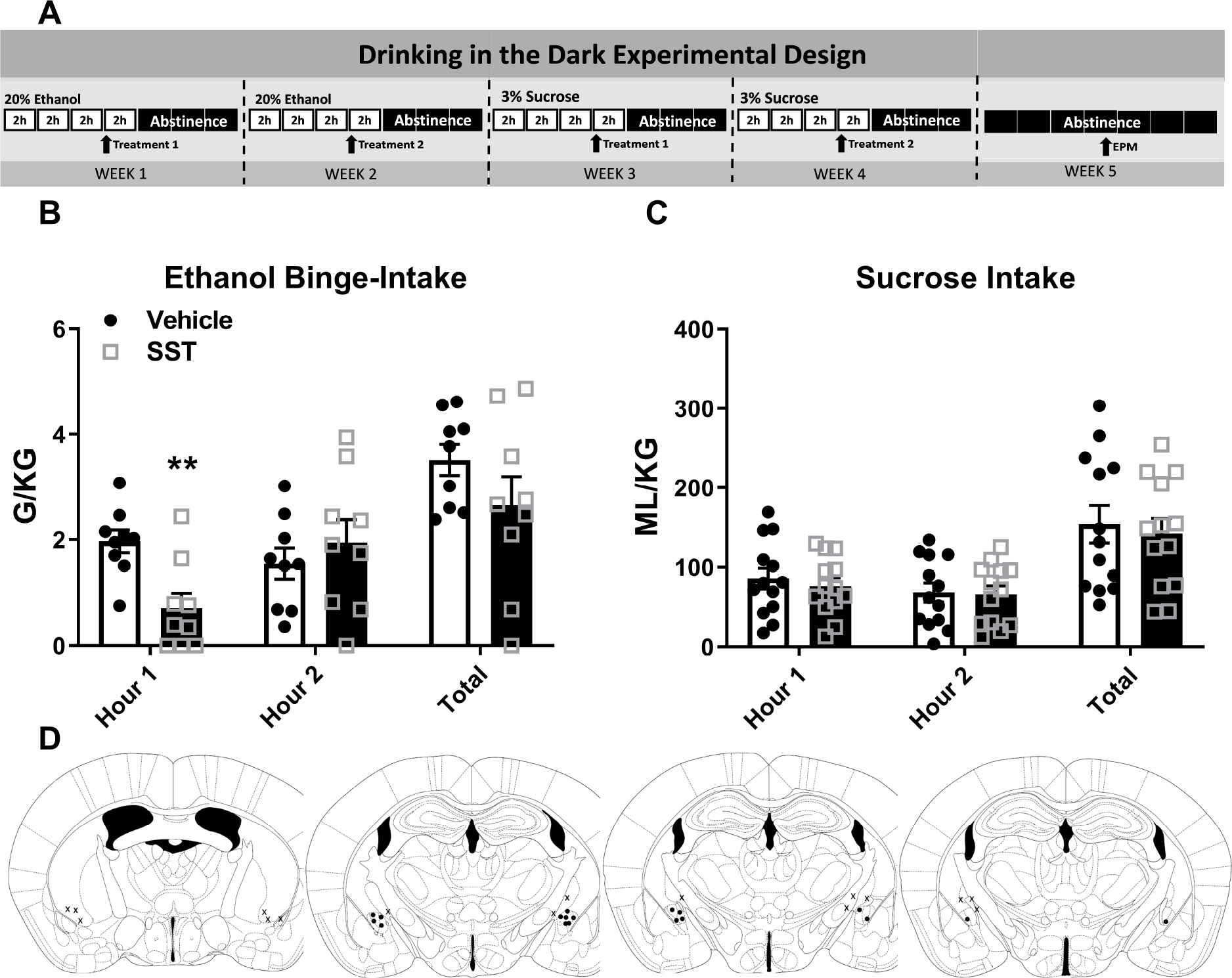
A) Experimental timeline. B) Intra-CeA microinjection of SST reduced binge-like ethanol intake in the first hour of the two hour DID test. C) This first hour reduction occurred in both male and female animals. D) Intra-CeA SST microinjection did not alter sucrose intake. E) Cannula placement. ** = p < 0.01 in Bonferroni’s multiple comparisons test. ## = p < 0.01 in Two-Way ANOVA.
FIGURE 4. Intra-CeA SST4R Agonism Reduces Binge-Like Ethanol Intake.
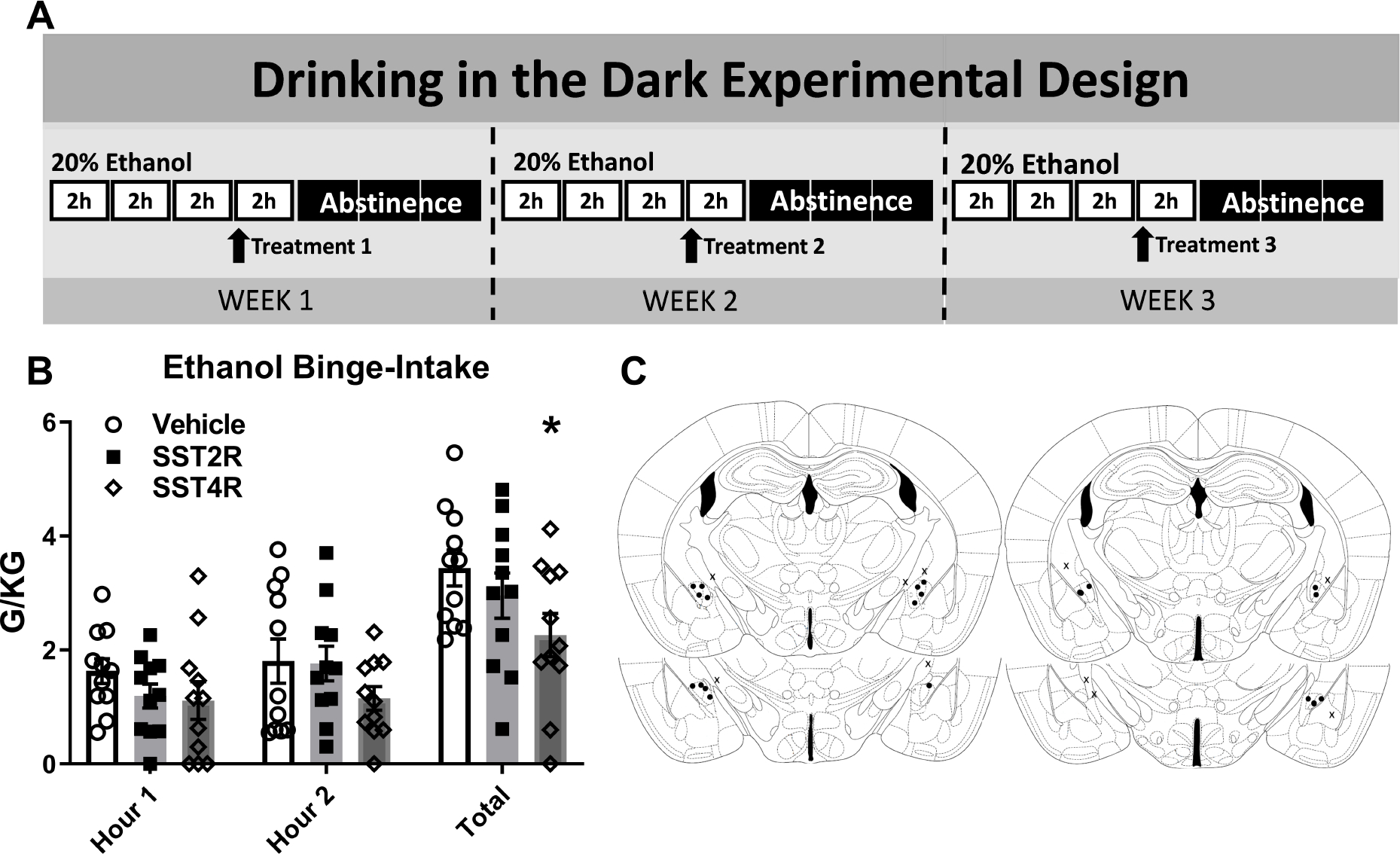
A) Experimental timeline. B) Intra-CeA microinjection of the SST4R agonist (L-803,087; 3μg/0.5μl/side), but not the SST2R agonist (L-054,264; 2.7μg/0.5μl/side) significantly reduced total binge-like ethanol intake in the two hour DID test. C) Cannula placement. * = p < 0.05 in Bonferroni’s multiple comparisons test.
2.7. Statistical Analysis
GraphPad Prism (GraphPad Software, Inc. La Jolla, Ca) was used to analyze and graph all data. T-tests, One- or Two-way ANOVAs and Dunnett or Bonferroni post-hoc tests were used as described in individual experiments. All data are reported as the mean ± standard error of the mean and considered significant if p<0.05. A trend towards significance was defined as p<0.06. Animals were removed from analysis if: 1) they were found by a Grubbs test (Alpha=0.05) to be a significant outlier; 2) due to cannula misplacement (uni or bilateral); or 3) due to cannula obstruction on day of microinjection. Cannula placement was determined by locating the end of the guide cannula and adding injector projection length (0.5MM).
3.0. Results
3.1. Three DID Cycles Reduces CeA SST Expression
SST immunoreactivity in the CeA and BLA following water (female (F) N=9; male (M) N=10) or 3 cycles of binge-like ethanol (female N=3; male N=10) was assessed. Two-way ANOVA revealed a significant impact of treatment in which in the CeA ethanol decreased SST expression [F(1,28)=5.73, p=0.02], with no effect of sex [F(1,28)=0.1, p=0.76; interaction: F(1,28)=0.1, p=0.76] (data not shown). Following combination of male and female mice, student’s t-test detected ethanol exposure resulted in a significant reduction in % area positive for SST fluorescence expression relative to water treated animals [ t=3.025, p=0.005] [Fig. 1a]. No impact was observed in the neighboring BLA [Treatment: F(1,24)=0.77, p=0.21; sex: [F(1,24)=1.2, p=0.28; interaction: F(1,24)=1.6, p=0.21] (data not shown) (water N=16; ethanol N=12) [t=0.3069, df=26; p=0.76] [Fig. 1b].
3.2. Intra-CeA SST Decreases Binge-Like Ethanol Intake
Two cohorts (total N=17, 8 excluded due to cannula obstruction or misplacement) underwent ethanol and sucrose intake. Treatment timeline detailed in Figure 2a. Two-way (treatment × time) ANOVAs were used to analyze the Latin Square data and t-test to analyze total intake. Bonferroni’s multiple comparisons test detected CeA microinjection of SST reduced total ethanol intake during hour 1 of the DID test alone (N=9) [Treatment: F(1,8)=4.27, p=0.073; time: F(1,8)=0.896, p=0.37; interaction: F(1,8)=27.19, p=.001; Bonferroni’s: hour 1: p=0.001; hour 2: 0.23]. T-test did not detect a reduction in total ethanol intake [t=2.005, df=9, p=0.76] [Fig. 2b]. This first hour reduction in ethanol intake occurred in both male and female animals (F N=5, M=4) [treatment: F(1,7)=14.98, p=0.01; sex: F(1,7)=0.233, p=0.64; interaction: F(1,7)=0.296, p=0.6] (data not shown). (BEC: vehicle: 94.17 ± 21.83 mg/dl; SST: 88.34 ± 27.23 mg/dl; t-test: t=0.2687, df=8, p=0.8; data not shown). Identical administration did not impact sucrose intake (N=13) [treatment: F(1,12)=0.384, p=0.55; time: F(1,12)=5.685, p=0.04; interaction: F(1,12)=0.349, p=0.57] [Fig. 2c]. Placement checks for experiment [Fig. 2d].
3.3. Intra-CeA SST Reduces Anxiety-Like Behavior and Not General Locomotion on the Elevated Plus Maze
Unpaired student’s t-test were used to analyze intra-CeA SST administration impact on behavior in the EPM behavioral assay. As no sex effect was observed in IHC or DID studies, sexes were analyzed together. SST administration increased % time spent in the open arms of the EPM (open arm time/(open+closed arm time)) (Vehicle N=7, SST N=4) [t-test: t=2.352, df=9, p=0.04] [Fig. 3a] without any such impact in number of total arm entries [t=1.054, df=9, p=0.34] [Fig. 3b] or open arm entries [t=0.47, df=9, p=0.93] [Fig. 3C].
FIGURE 3. Intra-CeA SST Reduces Anxiety-Like Behavior in the EPM.
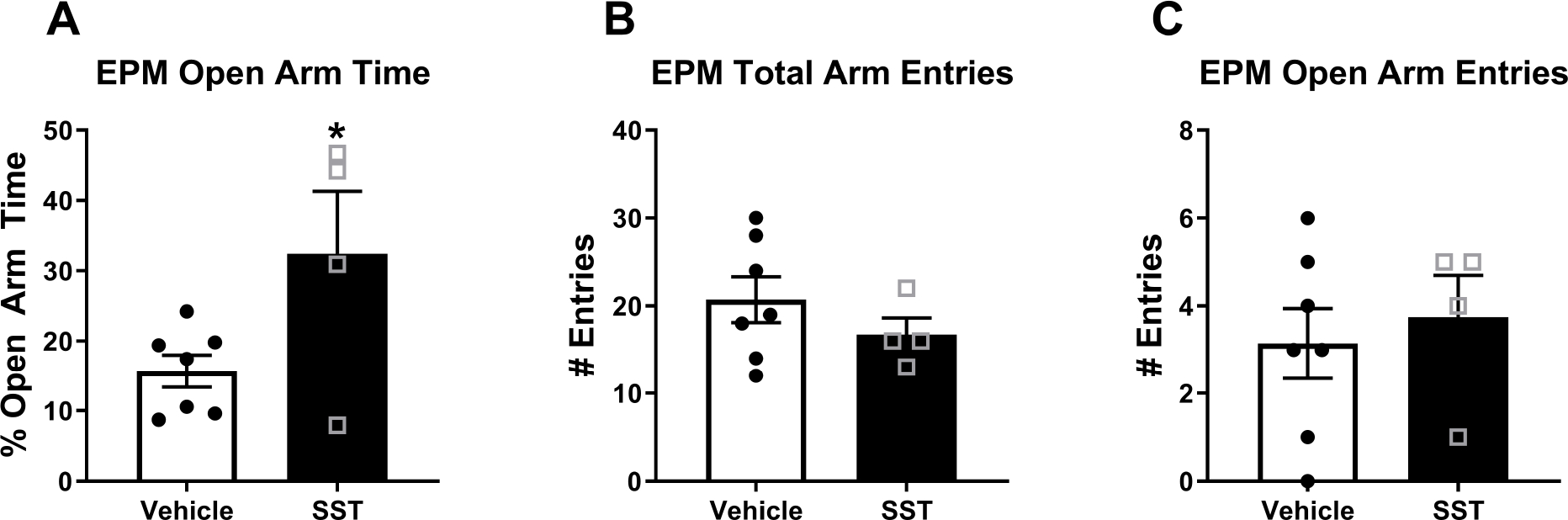
Intra-CeA SST microinjection significantly A) increased total time spent in the open arms of the EPM, B) with no impact on total arm or C) open arm entries. * = p = 0.04 in student’s t-test.
3.4. Intra-CeA SST4R Agonism Reduces Binge-Like Ethanol Consumption
One cohort (total N=18, 8 excluded due to cannula obstruction or misplacement) underwent three weeks of ethanol DID testing. As no impact of SST administration was observed in sucrose intake above, sucrose consumption was not evaluated in this test. As no sex effect was observed in IHC or DID studies, sexes were analyzed together. Treatment timeline detailed in Figure 4a. Two-way (treatment × time) ANOVAs were used to analyze the hourly Latin Square data and One-way ANOVAs to analyze total intake. Two-Way ANOVA detected no significant impact of treatment on hourly intake (N=10 (F=6, M=4)) [Treatment: F(2,20)=3.37, p=0.051; time: F(1,10)=2.28, p=0.16; interaction: F(2,20)=0.32, p=.73]. Total ethanol intake of the two-hour test period was evaluated using One-way ANOVA with Dunnett’s multiple comparisons test. Analysis revealed use of the SST4R agonist L-803,087, but not the SST2R agonist L-054,264, reduced total ethanol intake relative to vehicle treatment [F(1.532,13.79)=4.774, p=0.03; Bonferroni’s: SST2R p=0.11, SST4R=0.03] [Fig. 4b] (BEC: vehicle: 123.8 ± 12.6 ml/dl; SST2R: 88.1 ± 12.75 mg/dl; STT4R: 62.54 ± 15.14 mg/dl; One-way ANOVA: F(1.771, 15.94)=4.866, p=0.02; Bonferroni’s: SST2R p=0.1, SST4R p=0.04; data not shown). Placement checks for experiment [Fig. 4c].
4.0. DISCUSSION
In this work we demonstrate a novel role of the CeA SST system in regulating binge-like ethanol intake. Intra-CeA administration of synthetic SST reduced binge-like ethanol intake in both male and female animals. That the impact of intra-CeA SST occurred only during the first hour of testing is likely due to the short half-life of SST within the brain (Blake et al., 2004). This reduction is likely mediated by activation of the SST4R, as microinjection of a SST4R but not SST2R reduced total ethanol intake. However, as we did not conduct a full dose-response assessment with the SST2R agonist, and in light of the fact that there was a trend for reduced binge-like ethanol intake with this compound (Fig. 4), we cannot definitely rule out a potential role of the SS2R. Higher doses of the SST2R agonist may be required to significantly impact binge-like ethanol intake. We further found that when brains were collected immediately after the last drinking session following three weeks of binge-like ethanol consumption there was a significant reduction of SST expression in the CeA, but not the neighboring BLA, indicating a specific impact of binge-like ethanol intake on this nucleus. Finally, consistent with previous research here we found the CeA administration of SST was anxiolytic evidenced by a significant increase in open-arm time on the EPM test. This work, taken in context with previous literature, lends further support for the SST system as a potential therapeutic target for AUDs, even in their earliest stages.
It has been over 30 years since brain SST was first observed to be altered by ethanol exposure in rodents (Barrios et al., 1990) and to be reduced in post-mortem brain tissue of clinic AUD patients (Koponen et al., 1990). Despite this, intense attention has only recently been paid to the role of this densely expressed neuropeptide in AUDs. Our present findings are in keeping with the growing current literature in which binge-like ethanol intake is associated with reduced SST activity and enhanced SST activity induces decreased ethanol intake. Within the prelimbic subdivision of the medial prefrontal cortex (mPFC) binge-like or high dose ethanol exposure induces hypoactivity in SST+ cells (Dao et al., 2021; Li et al., 2021). Interestingly, the Dao study found both inhibition and activation of this SST+ population resulted in decreased binge-like ethanol intake. This finding was suggested to be due to the opposing impact of SST+ activity on glutamatergic and GABAergic signaling within the tightly regulated microcircuitry of the mPFC (Dao et al., 2021). Of interest, acute inhibition of cortical SST+ cells increase anxiety-like behavior, suggesting a potential mechanism through which decreased SST+ signaling in this region may impact the drive to consume ethanol (Soumier and Sibille, 2014).
SST+ cells comprise a significant portion of CeA cells, indeed representing the majority of cells within the lateral nucleus of the CeA (McCullough et al., 2018). In addition to sending outputs of its own, the lateral nucleus serves as an important afferent point in the CeA, acting to synthesize incoming information and potently modulate outgoing signals from the neighboring medial nucleus (itself containing a significant SST+ population) (McCullough et al., 2018). SST is thus poised to significantly impact behavior via both efferent projections to downstream regions and by regulation of CeA microcircuitry. SST+ CeA cells are notably involved in numerous behaviors known to influence initial ethanol intake and relapse following continued use and withdrawal. CeA SST+ cells are strongly activated by food and water intake in deprived animals, and activation of CeA SST+ cells both drive appetitive behavior (Kim et al., 2017). This population also suppresses active defensive behaviors via inhibition of non-SST+ CeA cells, resulting in gating of the fear response (Kim et al., 2017; Li et al., 2013). In keeping with this role of modulating the fear response, CeA SST+ cells are critically involved in fear learning and expression (Penzo et al., 2015). Given the high co-morbidity of AUDs and impaired fear-response behaviors, such as post-traumatic stress disorder, this may suggest impaired CeA SST+ activity as a contributing factor to both disorders. CeA SST+ has also recently been shown to play an important role in modulation of chronic pain, another condition frequently co-morbidly expressed with AUDs (Maleki et al., 2019; Wilson et al., 2019). In a neighboring region of the extended amygdala, the bed nucleus of the stria terminalis (BNST), chemogenetic activation of SST+ cells reduced binge-like ethanol intake in female mice, with no impact of inhibition (Suresh Nair et al., 2022). This may suggest increased BNST SST+ signaling serves a protective role against binge-like ethanol intake, particularly in female animals, but tonic SST+ signaling is not required to drive the initial behavior. Within the present work, SST system manipulation was found to reduce binge-like intake in both male and female mice, potentially indicating a more generalized role of CeA SST signaling or suggesting co-expressed peptides or neurotransmitters released by manipulation of the whole cell population may sex-specifically influence behavior. The extended amygdala is known to be highly sexually dimorphic both at baseline and in their signaling response to ethanol exposure, highlighting the need for further investigation into sex differences in AUDs and potential treatments (Hitzemann et al., 2022). Of note, activation of CeA SST+ cells indirectly increase BNST SST+ signaling, indicating perturbation of SST signaling within the CeA may have a significant impact on this BNST population(Ahrens et al., 2018). In the above work focused on manipulating SST+ populations, it is important to consider that the specific role of SST relative to co-expressed neuropeptides or the neurotransmitter GABA in specific behaviors remains to be fully evaluated. It is also important to note that while we demonstrated that binge-like ethanol drinking reduced SST levels within the CeA and that infusion of SST and SST4R agonist into the CeA blunted binge-like ethanol intake, we cannot definitively conclude that local CeA SST contributes to the modulating of binge-like ethanol intake as SST innervation from sources outside of the CeA (e.g., the BNST) may act on CeA SST receptors to modulate binge-like ethanol intake.
Here we found that CeA infusion of SST was anxiolytic in mice with a prior history of ethanol drinking as evidenced by increased open-arm time during an EPM test, which is consistent with previous work using ethanol-naïve mice (Yeung and Treit, 2012). There is a converging body of research highlighting an important role for SST in integrating emotional behaviors. For example, knock-out SST rodent models display enhanced emotionality and anxiety-like behaviors which are partially ameliorated through intra-amygdala SST injection (Kahl and Fendt, 2014; Lin and Sibille, 2015). SST knockout is likewise associated with increased stress hormone expression, including elevated cortisol levels (Lin and Sibille, 2015). Of note, intracerebroventricular (ICV) injection of SST is sufficient to alleviate several components of the stress response engaged by enhanced corticotropin releasing factor (CRF) signaling (Stengel and Taché, 2017). In keeping with this, SST signaling increases in the amygdala following acute stress, potentially counteracting the enhanced CRF signaling engaged during stress exposure(Brodin et al., 1994). Given the critical role CeA CRF is known to play in AUDs (Agoglia and Herman, 2018; Nicholas W. Gilpin et al., 2015), this suggests CeA SST signaling may influence alcohol-directed behaviors in part through regulating extended amygdala CRF signaling. Of note in this interpretation, however, is the finding that a majority of CeA SST+ cells co-express CRF (Kim et al., 2017; McCullough et al., 2018). This presents a more complicated image than two cell populations acting in opposition to regulate stress behavior. Rather, it suggests the intriguing possibility the same SST-CRF+ cells are poised to act in potentially opposing manners depending on the relative balance of co-expressed neuropeptide expression and release. Given this balance is likely disturbed during excessive ethanol intake, it is important to consider that the same CeA cell population could either inhibit or aggravate stress behaviors depending on the history of ethanol exposure. Of interest, CeA-nucleus accumbens regulation of binge-like ethanol intake was found to not involved activation of the SST system (Borrego et al., 2022). Together, these data may suggest CeA SST system regulation of binge-like ethanol intake is mediated by its impact on anxiety/depressive behaviors and regulation of stress-associated signaling mechanisms rather than influence on reward/appetitive signaling. That decreased SST expression is found in the prefrontal cortex and amygdala of patients with major depressive disorder, a disorder frequently co-occurring with AUDs, lends some clinical support to this idea (Guilloux et al., 2012; Lin and Sibille, 2015; Sibille et al., 2011; Tripp et al., 2011).
SST2R and SST4R were selected for closer examination in this work due to their well-known association with modulating amygdalar regulation of anxiety and depressive behaviors (for review see:(Robinson and Thiele, 2020)). Overall, while both receptors have been implicated in anxiety and depressive behaviors, evidence suggests a potential preferential role for SST2R in anxiety-related behaviors. SST2R knockout results in an overall increase in anxiety-like behavior, depressive-behavior, stress sensitivity, and cortisol levels(Prévôt et al., 2018, 2017; Viollet et al., 2000). ICV administration of a SST2R antagonist increases anxiety-like behavior on the EPM, a finding further supported by the finding exogenous SST-induced anxiolysis is blocked by intra-amygdalar administration of the same SST2R antagonist (Engin and Treit, 2009; Yeung and Treit, 2012). In contrast, SST4R impact on anxiety-like behavior has been mixed, with some studies finding SST4R likewise inhibits anxiety and stress behavior (Scheich et al., 2017, 2016) and others observing no impact on behavior (Nanda et al., 2008; Prévôt et al., 2017). SST4R has been consistently shown to modulate depressive-like behavior, with knock-out increasing and agonists decreasing measures of depressive behavior at baseline and following stress exposure(Prévôt et al., 2017; Scheich et al., 2017, 2016). That SST4R agonism decreased binge-like ethanol intake in this work is in keeping with the limited previous literature assessing the relationship between ethanol exposure and SST receptors. Low dose chronic ethanol exposure decreases nucleus accumbens SST4R expression in rats(Jonsson et al., 2014) and the SST4R promoter region is methylated in 22% of clinical AUD patients relative to 2% of the general population(Berent et al., 2017). SST4R has also recently been highlighted as a potential target for neuropathic pain treatment, against suggesting a potential role for the SST system in the co-morbidity of alcohol abuse and chronic pain(Kántás et al., 2019).
There are several caveats that need discussion. First, as noted above we did not perform dose-response analyses in the study that assessed the effect of SST2R and SST4R agonist on binge-like ethanol intake. Thus, while the dose of SST2R agonist that we chose did not significantly blunted binge-like ethanol intake, in the absence of assessment of higher doses we cannot rule out a potential role for the SST2R in the modulation of binge-like ethanol intake. Another caveat is that mice tested on the EPM and sucrose consumption tests had a prior history of binge-like ethanol intake. As noted above, while previous work demonstrates similar anxiolytic effects when SST was infused into the CeA of ethanol-naïve mice, it is possible that ethanol-induced plasticity prevented us from observing subsequent effects of CeA-infused SST on sucrose drinking. Related, it is also possible that SST blunted binge-like ethanol intake but not sucrose drinking because the 3% sucrose solution used in the present study was a more salient reinforcer for the mice relative to the 20% ethanol solution. Finally, while we assessed SST levels in mouse brains that were collected immediately after the last binge-like ethanol drinking session, it will be important in future work to assess SST levels after periods of abstinence following binge-like ethanol drinking.
In conclusion, these results support an important role of the CeA SST system in regulating binge-like drinking. This system is well-situated to exert significant influence over numerous behaviors associated with the motivation to consume ethanol, including anxiety, depressive, fear, and pain-related behavior, via modulating activity of CeA microcircuits and downstream efferent targets. We identify the SST4R as a notable mediator of this behavior, lending support to future investigation into this receptor and for its potential as a therapeutic target.
Highlights.
Binge-like ethanol intake reduces SST expression in the CeA
Intra-CeA SST administration reduces binge-like ethanol consumption and anxiety-like behavior on the elevated plus maze
Intra-CeA SST administration does not impact sucrose intake or general locomotion
Intra-CeA agonism of the SST 4, but not SST2, receptor decreases binge-like ethanol consumption
These effects appear to occur in both male and female mice
Acknowledgments:
Special thanks to Rhiannon Thomas, MS, for technical assistance and feedback.
Funding:
This work was supported by the National Institutes of Health [grant numbers: AA022048, AA013573, F32AA025811, and K99AA028268]
Footnotes
CRediT author statement: Stacey Robinson: Conceptualization, Methodology, Investigation, Writing- Original draft preparation. Todd Thiele: Supervision, Writing- Reviewing and Editing.
Competing Interests: The authors declare no competing financial interests. Dr. Thiele owns shares of Glauser Life Sciences, a company focusing on the development of therapeutics for mental health disorders. The work that is presented in this paper is not directly related to the scientific aims of Glauser Life Sciences.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agoglia AE, Herman MA, 2018. The center of the emotional universe: Alcohol, stress, and CRF1 amygdala circuitry. Alcohol 72, 61–73. 10.1016/j.alcohol.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens S, Wu MV, Furlan A, Hwang GR, Paik R, Li H, Penzo MA, Tollkuhn J, Li B, 2018. A central extended amygdala circuit that modulates anxiety. J. Neurosci. 38, 5567–5583. 10.1523/JNEUROSCI.0705-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaev O, Piletti Chatain C, Krueger-Burg D, 2018. Inhibition in the amygdala anxiety circuitry. Exp Mol Med 50, 18. 10.1038/s12276-018-0063-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Lepack A, Fee C, Duric V, Maldonado-Aviles J, DiLeone R, Sibille E, Duman RS, Sanacora G, 2017. Characterization of GABAergic marker expression in the chronic unpredictable stress model of depression. Chronic Stress (Thousand Oaks) 1. 10.1177/2470547017720459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios V, Rodríguez-Sánchez MN, Arilla E, 1990. Effects of acute and chronic ethanol administration and its withdrawal on the level and binding of somatostatin in rat brain. Clin Sci 79, 451–456. [DOI] [PubMed] [Google Scholar]
- Bell GI, Yasuda K, Kong H, Law SF, Raynor K, Reisine T, 1995. Molecular biology of somatostatin receptors. Ciba Found Symp 190, 65–68. [DOI] [PubMed] [Google Scholar]
- Berent D, Emilien G, Podgórski M, Kusideł E, Kulczycka-Wojdala D, Szymańska B, Macander M, Pawłowska Z, 2017. SSTR4, Childhood Adversity, Self-efficacy and Suicide Risk in Alcoholics. Transl. Neurosci. 8, 76–86. 10.1515/tnsci-2017-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake AD, Badway AC, Strowski MZ, Strowsk M, 2004. Delineating Somatostatins Neuronal Actions. Curr. Drug Target -CNS Neurol. Disord. 3, 153–160. 10.2174/1568007043482534 [DOI] [PubMed] [Google Scholar]
- Borrego MB, Grigsby KB, Townsley KG, Chan A, Firsick EJ, Tran A, Savarese A, Ozburn AR, 2022. Central nucleus of the amygdala projections onto the nucleus accumbens core regulate binge-like alcohol drinking in a CRF-dependent manner. Neuropharmacology 203, 108874. 10.1016/J.NEUROPHARM.2021.108874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin E, Rosén A, Schött E, Brodin K, 1994. Effects of sequential removal of rats from a group cage, and of individual housing of rats, on substance P, cholecystokinin and somatostatin levels in the periaqueductal grey and limbic regions. Neuropeptides 26, 253–260. 10.1016/0143-4179(94)90079-5 [DOI] [PubMed] [Google Scholar]
- Butler RK, White LC, Frederick-Duus D, Kaigler KF, Fadel JR, Wilson MA, 2012. Comparison of the activation of somatostatin- and neuropeptide Y-containing neuronal populations of the rat amygdala following two different anxiogenic stressors. Exp Neurol 238, 52–63. 10.1016/j.expneurol.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao NC, Brockway DF, Suresh Nair M, Sicher AR, Crowley NA, 2021. Somatostatin neurons control an alcohol binge drinking prelimbic microcircuit in mice. Neuropsychopharmacology 46, 1906–1917. 10.1038/S41386-021-01050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Treit D, 2009. Anxiolytic and antidepressant actions of somatostatin: the role of sst2 and sst3 receptors. Psychopharmacol. 206, 281–289. 10.1007/s00213-009-1605-5 [DOI] [PubMed] [Google Scholar]
- Fuchs T, Jefferson SJ, Hooper A, Yee P-HH, Maguire J, Luscher B, 2017. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol Psychiatry 22, 920–930. 10.1038/mp.2016.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA., Roberto M., 2015. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry 77, 859–869. 10.1016/j.biopsych.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, Tseng GC, Lewis DA, Sibille E, 2012. Molecular evidence for BDNF-and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol. Psychiatry 17, 1130–1142. 10.1038/mp.2011.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzemann R, Bergeson SE, Berman AE, Bubier JA, Chesler EJ, Finn DA, Hein M, Hoffman P, Holmes A, Kisby BR, Lockwood D, Lodowski KH, McManus M, Owen JA, Ozburn AR, Panthagani P, Ponomarev I, Saba L, Tabakoff B, Walchale A, Williams RW, Phillips TJ, 2022. Sex Differences in the Brain Transcriptome Related to Alcohol Effects and Alcohol Use Disorder. Biol. Psychiatry 91, 43–52. 10.1016/J.BIOPSYCH.2021.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S, Ericson M, Söderpalm B, 2014. Modest Long-Term Ethanol Consumption Affects Expression of Neurotransmitter Receptor Genes in the Rat Nucleus Accumbens. Alcohol. Clin. Exp. Res. 38, 722–729. 10.1111/acer.12307 [DOI] [PubMed] [Google Scholar]
- Kahl E, Fendt M, 2014. Injections of the somatostatin receptor type 2 agonist L-054,264 into the amygdala block expression but not acquisition of conditioned fear in rats. Behav. Brain Res. 10.1016/j.bbr.2014.02.011 [DOI] [PubMed] [Google Scholar]
- Kántás B, Börzsei R, Szőke É, Bánhegyi P, Horváth Á, Hunyady Á, Borbély É, Hetényi C, Pintér E, Helyes Z, 2019. Novel Drug-Like Somatostatin Receptor 4 Agonists are Potential Analgesics for Neuropathic Pain. Int. J. Mol. Sci. 20. 10.3390/IJMS20246245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S, 2017. Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron 93, 1464–1479.e5. 10.1016/j.neuron.2017.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koponen H, Reinikainen KJ, Jolkkonen JT, Riekkinen PJ, 1990. Somatostatin-like immunoreactivity in the CSF of patients with dementia associated with alcoholism. Acta Neurol. Scand. 82, 289–291. 10.1111/J.1600-0404.1990.TB03305.X [DOI] [PubMed] [Google Scholar]
- Kubota Y, Shigematsu N, Karube F, Sekigawa A, Kato S, Yamaguchi N, Hirai Y, Morishima M, Kawaguchi Y, 2011. Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex 21, 1803–1817. 10.1093/cercor/bhq252 [DOI] [PubMed] [Google Scholar]
- Lalumiere RT, 2014. Optogenetic dissection of amygdala functioning. Front Behav Neurosci 8, 107. 10.3389/fnbeh.2014.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B, 2013. Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci. 16, 332–9. 10.1038/nn.3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Cabrera-Garcia D, Salling MC, Au E, Yang G, Harrison NL, 2021. Alcohol reduces the activity of somatostatin interneurons in the mouse prefrontal cortex: A neural basis for its disinhibitory effect? Neuropharmacology 188, 108501. 10.1016/J.NEUROPHARM.2021.108501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LC, Sibille E, 2015. Somatostatin, neuronal vulnerability and behavioral emotionality. Mol Psychiatry 20, 377–387. 10.1038/mp.2014.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N, Tahaney K, Thompson BL, Oscar-Berman M, 2019. At the intersection of alcohol use disorder and chronic pain. Neuropsychology 33, 795–807. 10.1037/NEU0000558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough KM, Morrison FG, Hartmann J, Carlezon WA, Ressler KJ, 2018. Quantified Coexpression Analysis of Central Amygdala Subpopulations. eNeuro 5. 10.1523/ENEURO.0010-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda SA, Qi C, Roseboom PH, Kalin NH, 2008. Predator stress induces behavioral inhibition and amygdala somatostatin receptor 2 gene expression. Genes. Brain. Behav. 7, 639–48. 10.1111/j.1601-183X.2008.00401.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism, 2022. No Title [WWW Document]. Alcohol Facts Stat. URL https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics
- Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, Darvas M, Parada LF, Palmiter RD, He M, Huang ZJ, Li B, 2015. The paraventricular thalamus controls a central amygdala fear circuit. Nature 519, 455–459. 10.1038/NATURE13978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévôt TD, Gastambide F, Viollet C, Henkous N, Martel G, Epelbaum J, Béracochéa D, Guillou J-L, 2017. Roles of Hippocampal Somatostatin Receptor Subtypes in Stress Response and Emotionality. Neuropsychopharmacology 42, 1647–1656. 10.1038/npp.2016.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévôt TD, Viollet C, Epelbaum J, Dominguez G, Béracochéa D, Guillou JL, 2018. sst 2 -receptor gene deletion exacerbates chronic stress-induced deficits: Consequences for emotional and cognitive ageing. Prog. Neuro-Psychopharmacology Biol. Psychiatry 86, 390–400. 10.1016/j.pnpbp.2018.01.022 [DOI] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, Siggins GR, 2012. The central amygdala and alcohol: role of γ-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb Perspect Med 2, a012195. 10.1101/cshperspect.a012195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR, 2004. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci 24, 10159–10166. 10.1523/JNEUROSCI.3004-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SL, Thiele TE, 2020. A role for the neuropeptide somatostatin in the neurobiology of behaviors associated with substances abuse and affective disorders. Neuropharmacology 167. 10.1016/j.neuropharm.2020.107983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SL, Marrero IM, Perez-Heydrich CA, Sepulveda-Orengo MTT, Reissner KJ, Thiele TE, 2019. Medial prefrontal cortex neuropeptide Y modulates binge-like ethanol consumption in C57BL/6J mice. Neuropsychopharmacology 44, 1132–1140. 10.1038/s41386-018-0310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheich B, Csekő K, Borbély É, Ábrahám I, Csernus V, Gaszner B, Helyes Z, 2017. Higher susceptibility of somatostatin 4 receptor gene-deleted mice to chronic stress-induced behavioral and neuroendocrine alterations. Neuroscience 346, 320–336. 10.1016/j.neuroscience.2017.01.039 [DOI] [PubMed] [Google Scholar]
- Scheich B, Gaszner B, Kormos V, László K, Ádori C, Borbély É, Hajna Z, Tékus V, Bölcskei K, Ábrahám I, Pintér E, Szolcsányi J, Helyes Z, 2016. Somatostatin receptor subtype 4 activation is involved in anxiety and depression-like behavior in mouse models. Neuropharmacology 101, 204–215. 10.1016/j.neuropharm.2015.09.021 [DOI] [PubMed] [Google Scholar]
- Schwartz JP, Taniwaki T, Messing A, Brenner M, 1996. Somatostatin as a trophic factor. Analysis of transgenic mice overexpressing somatostatin in astrocytes. Ann N Y Acad Sci 780, 29–35. [DOI] [PubMed] [Google Scholar]
- Sibille E, Morris HM, Kota RS, Lewis DA, 2011. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int. J. Neuropsychopharmacol. 14, 721–34. 10.1017/S1461145710001616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Bajo M, Chappell AM, Christian DT, Cruz M, Diaz MR, Kash T, Lack AK, Messing RO, Siggins GR, Winder D, Roberto M, McCool BA, Weiner JL, 2009. Neurobiological mechanisms contributing to alcohol-stress-anxiety interactions. Alcohol 43, 509–519. 10.1016/j.alcohol.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumier A, Sibille E, 2014. Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology 39, 2252–62. 10.1038/npp.2014.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Rivier J, Taché Y, 2013. Modulation of the adaptive response to stress by brain activation of selective somatostatin receptor subtypes. Peptides 42, 70–77. 10.1016/j.peptides.2012.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Taché YF, 2017. Activation of Brain Somatostatin Signaling Suppresses CRF Receptor-Mediated Stress Response. Front Neurosci 11, 231. 10.3389/fnins.2017.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh Nair M, Dao NC, Lopez Melean D, Griffith KR, Starnes WD, Moyer JB, Sicher AR, Brockway DF, Meeks KD, Crowley NA, 2022. Somatostatin neurons in the bed nucleus of the stria terminalis play a sex-dependent role in binge Drinking. Brain Res. Bull. 186, 38–46. 10.1016/J.BRAINRESBULL.2022.05.010 [DOI] [PubMed] [Google Scholar]
- Thiele TE, Crabbe JC, Boehm SL, 2014. “Drinking in the Dark” (DID): a simple mouse model of binge-like alcohol intake. Curr Protoc Neurosci 68, 9.49.1–12. 10.1002/0471142301.ns0949s68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp A, Kota RS, Lewis DA, Sibille E, 2011. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol. Dis. 42, 116–24. 10.1016/j.nbd.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K, 2011. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362. 10.1038/nature09820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet C, Vaillend C, Videau C, Bluet-Pajot MT, Ungerer A, L’Héritier A, Kopp C, Potier B, Billard J, Schaeffer J, Smith RG, Rohrer SP, Wilkinson H, Zheng H, Epelbaum J, 2000. Involvement of sst2 somatostatin receptor in locomotor, exploratory activity and emotional reactivity in mice. Eur. J. Neurosci. 12, 3761–70. 10.1046/j.1460-9568.2000.00249.x [DOI] [PubMed] [Google Scholar]
- Wilson TD, Valdivia S, Khan A, Ahn HS, Adke AP, Gonzalez SM, Sugimura YK, Carrasquillo Y, 2019. Dual and Opposing Functions of the Central Amygdala in the Modulation of Pain. Cell Rep. 29, 332–346.e5. 10.1016/J.CELREP.2019.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Post SR, Wang K, Tager HS, Bell GI, Seino S, 1992. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci U S A 89, 251–255. 10.1073/pnas.89.1.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung M, Treit D, 2012. The anxiolytic effects of somatostatin following intra-septal and intra-amygdalar microinfusions are reversed by the selective sst2 antagonist PRL2903. Pharmacol Biochem Behav 101, 88–92. 10.1016/j.pbb.2011.12.012 [DOI] [PubMed] [Google Scholar]
- Yu K, Garcia da Silva P, Albeanu DF, Li B, 2016. Central Amygdala Somatostatin Neurons Gate Passive and Active Defensive Behaviors. J Neurosci 36, 6488–6496. 10.1523/JNEUROSCI.4419-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Yan C, Ren M, Li A, Quan T, Gong H, Yuan J, 2017. A platform for stereological quantitative analysis of the brain-wide distribution of type-specific neurons. Sci Rep 7, 14334. 10.1038/s41598-017-14699-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, Koob GF, 2014. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocr. 35, 234–244. 10.1016/j.yfrne.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]


