Abstract
Background:
Aviptadil (vasoactive intestinal peptide) is a lung-protective neuropeptide and remdesivir is a nucleotide prodrug of an adenosine analog, both of which may improve outcomes for COVID-19 patients with acute hypoxemic respiratory failure.
Methods:
Daily 12-hour infusions of aviptadil or placebo for three successive days and daily infusions of remdesivir or placebo for up to 10 successive days were studied using a master protocol for adults hospitalized for COVID-19 with acute hypoxemic respiratory failure. Participants could be randomized to both study treatments in a 2×2 factorial design or to just one of the agents. For both treatment comparisons, the primary outcome, assessed at Day 90, was a 6-category ordinal outcome ranging from return to home with liberation from supplemental oxygen (recovery) for ≥77 days to death. Mortality through Day 90 was a key secondary outcome. The independent data and safety monitoring board recommended stopping the aviptadil trial on May 25, 2022 for futility. On June 9, 2022, the sponsor stopped the trial of remdesivir due to slow enrollment.
Findings:
Four hundred seventy-one of the 640 planned participants were randomized to aviptadil/placebo, among whom 461 participants (231 allocated to aviptadil and 230 to placebo) received any infusion of blinded aviptadil. For the aviptadil/placebo comparison, at entry, 271 (59%) were on high flow nasal oxygen or non-invasive ventilation and 190 (41%) on mechanical ventilation or ECMO. The odds ratio (aviptadil/placebo) of being in a better outcome category at Day 90 was 1.11 (95% CI: 0.80–1.55; p=0.54). Cumulative mortality through Day 90 was 38% (n=86) for aviptadil and 36% (n=83) for placebo (HR 1.04; 95% CI: 0.77–1.41). A composite safety outcome including death, organ failure, serious infection, serious adverse events, and grade 3 or 4 adverse events through Day 5 occurred in 63% (n=146) of aviptadil and 56% (n=129) of placebo participants (OR 1.40; 95% CI: 0.94–2.08). Eighty-seven participants were randomized to remdesivir/placebo.
Interpretation:
Among patients with COVID-19-associated acute hypoxemic respiratory failure, aviptadil, compared to placebo, did not significantly improve clinical outcomes through Day 90. The smaller than planned sample size for the remdesivir trial does not permit definitive conclusions regarding safety or efficacy.
INTRODUCTION
SARS-CoV-2 infection may lead to acute hypoxemic respiratory failure, including acute respiratory distress syndrome (ARDS), which is often lethal. Even with adherence to current treatment guidelines, 60-day mortality remains 30–50% among COVID-19 patients with acute hypoxemic respiratory failure.1,2 3
Aviptadil, the synthetic form of the 28-amino acid neuropeptide vasoactive intestinal peptide (VIP), has been proposed as a treatment for acute hypoxemic respiratory failure based on pleoiotropic lung-protective effects including increases in surfactant production, decreased cytopathy, and modulated inflammatory response in monocytes.4,5 A Phase 2/3 randomized trial suggested possible efficacy in COVID-19 respiratory failure.6 Remdesivir, a small molecule antiviral, improves outcomes among hospitalized patients in general, but data among critically ill patients are less robust.7
ACTIV (Accelerating COVID-19 Therapeutic Interventions and Vaccines / Therapeutics for Inpatients with COVID-19) is an NIH-led public/private partnership established in April 2020 to advance COVID-19 therapeutics and vaccines. ACTIV-3b, Therapeutics for Severely Ill Inpatients with COVID-19 (TESICO), which focuses on patients with COVID-19 critical illness with hypoxemic respiratory failure, is one of several ACTIV therapeutic master protocols enabling simultaneous evaluation of multiple agents.8 The ACTIV agent selection committee selected aviptadil for study within TESICO.9 We report here the results of the trials comparing intravenous aviptadil and intravenous remdesivir each with placebo among patients with COVID-19 acute hypoxemic respiratory failure.
METHODS
Trial Design and Oversight
For the TESICO trial of aviptadil and remdesivir, depending on eligibility, consenting participants could be randomized to both study treatments or matching placebo in a 2×2 factorial design or to just one of the agents (details in the Supplementary Appendix, Section 2 and Figure S1). A factorial design was used for sample size efficiency because an interaction was considered unlikely. Randomization to only one of the two factors was permitted in order to include participants who were not eligible for one of the investigational agents.
The TESICO protocol was approved by a governing institutional review board for each enrolling site. Written informed consent for trial participation was obtained from each enrolled participant or a legally authorized representative. The trial was overseen by an independent data and safety monitoring board (DSMB) and conducted under an investigational new drug protocol with the U.S. Food and Drug Administration.
Target Population
Hospitalized adult patients were eligible if they had acute hypoxemic respiratory failure due to confirmed SARS-CoV-2 infection and were within four days of onset of respiratory failure. Acute hypoxemic respiratory failure was defined pragmatically as hypoxemia (defined clinically, with no specific threshold required) caused by COVID-19 pneumonia plus receipt of high-flow nasal oxygen (HFNO), non-invasive ventilation (NIV), invasive mechanical ventilation (IMV), or extracorporeal membrane oxygenation (ECMO).10,11
Agent-specific exclusion criteria for aviptadil included refractory hypotension, severe diarrhea, or end-stage liver disease. Participants were excluded from the factorial randomization if they did not meet the eligibility criteria for both aviptadil and remdesivir. If eligible for one of the treatments, participants could be randomized separately to that active agent/placebo. A full list of eligibility criteria is provided in the Supplementary Appendix (Section 2).
Randomization and Blinding
Participants were randomized with a web-based application. For each site, randomization was stratified by disease severity (HFNO/NIV or IMV/ECMO), and four strata defined by remdesivir and aviptadil eligibility: 1) eligible for randomization to aviptadil and remdesivir in the 2×2 factorial; participants were equally randomized to either intravenous aviptadil, aviptadil matching placebo, intravenous remdesivir, or remdesivir matching placebo (1:1:1:1), with participants in the factorial receiving blinded infusions of both agents; 2) eligible for randomization to aviptadil only because remdesivir was started prior to randomization; 3) eligible for randomization to aviptadil only because remdesivir was contraindicated; and 4) eligible for randomization to remdesivir only because aviptadil was contraindicated (Figure S1). For participants in strata 2 through 4, randomization was (1:1) to the active agent or matching placebo. Permuted block randomization was used to generate the randomization schedules for each site (see Supplementary Appendix Section 2). Research staff, clinical personnel, and participants were blinded to trial group assignment; an unblinded pharmacist prepared blinded study product for infusion on site.
Interventions/Treatments
Aviptadil acetate was administered as a daily 12-hour infusion for three days, targeting 600 pmol/kg on infusion day 1, 1200 pmol/kg on infusion day 2, and 1800 pmol/kg on infusion day 3. Remdesivir was administered as a 200 mg loading dose, followed by 100 mg daily maintenance doses for up to a 10-day total course. For participants assigned placebo for either agent, matched saline placebo was administered in identical volumes.
All infusions of aviptadil/placebo were administered in an intensive care or step-down unit; participants discharged from intensive care or step-down unit did not continue dosing of aviptadil/placebo. If respiratory failure had resolved on or before Day 5, clinical teams were allowed to stop blinded remdesivir/placebo after 5 doses. Neither agent was continued after hospital discharge if the treatment course had not been completed.
Expected side effects of aviptadil included hypotension and diarrhea. Aviptadil/matching placebo was contraindicated on a given infusion day for participants receiving ≥ 0.1 mcg/kg/min of norepinephrine or equivalent vasopressor or with > 3 liquid stools in the preceding 24 hours. Blood pressure was monitored per local clinical guidelines but at least every 2 hours for the duration of the aviptadil infusion and for 2 hours thereafter. Study management guidelines stipulated responses to worsening hypotension with pauses, decreases, or discontinuation of the aviptadil/placebo infusion and to substantial diarrhea with oral loperamide and/or changes in the infusion. The grading system used for hypotension and other adverse events is given in the Supplemental Appendix (Section 2).
Sites were strongly encouraged to administer glucocorticoids per NIH treatment guidelines.12 By design, participants were either receiving remdesivir prior to randomization or were randomized to remdesivir versus remdesivir placebo, unless contraindicated. Use of antiplatelet/anticoagulant treatment and immunomodulators were at the discretion of treating clinicians.
Primary and Secondary Outcomes for Each Treatment Comparison
The primary outcome was a 6-category ordinal outcome defining the participant’s status at Day 90: (1) at home (defined as the type of residence before hospitalization) and off oxygen (recovered) for at least 77 days, (2) at home and off oxygen for 49–76 days, (3) at home and off oxygen for 1–48 days, (4) not hospitalized but either on supplemental oxygen or not at home, (5) hospitalized or in hospice care, or (6) dead (see Supplementary Appendix Section 2). Mortality through Day 90 was a secondary endpoint; all participants were also followed for mortality through Day 180. Adverse events of any grade severity were collected during each infusion and 2 hours after infusion completion. Composite safety outcomes were assessed through Day 5, Day 28, and Day 90. These outcomes are defined in the Supplementary Appendix (Section 2).
Baseline Serologic and Virologic Assays
Serostatus (Genscript cPass and Quanterix HD-X) and antigen (Quanterix Simoa) testing were performed centrally on cryopreserved plasma obtained at baseline, while viral strain was determined using strain-specific PCR for Delta or Omicron variants on mid-turbinate samples obtained at baseline. Further details of laboratory methods are presented in the Supplementary Appendix (Section 2).
Statistical Analysis
The enrollment target was 640 participants for both the aviptadil versus matched placebo comparison and for the remdesivir versus matched placebo comparison. With the planned factorial design, it was assumed that the total sample size for the two trials would be substantially less than 1,280. This sample size target for each trial provided 80% power to detect a common odds ratio (OR) of 1.5 for the primary ordinal outcome at the (two-sided) 0.05 level of significance. More detailed sample size assumptions are provided in the Supplementary Appendix (Section 2).
The independent DSMB regularly reviewed interim analyses; on May 25, 2022 the DSMB reviewed a pre-specified futility analysis using conditional power estimates which were based on 70% of the planned number of participants in the aviptadil/placebo group with the Day 90 primary outcome. Following this review, the DSMB recommended stopping randomization of aviptadil versus placebo because it was highly unlikely that statistical significance would be achieved with full enrollment (conditional power was 12.4%, assuming an OR of 1.5 for the as-yet unobserved data). Randomization to the aviptadil trial of the master protocol was stopped at the time of this recommendation. At this review, the DSMB also noted that while there were no safety concerns for the remdesivir/placebo comparison, given the slow enrollment there may be operational reasons to close that trial as well. The investigator and sponsor agreed to end the remdesivir/placebo trial on June 9, 2022. Guidelines given to the DSMB for the futility assessment by the blinded investigator team are provided in the Supplementary Appendix (Section 2).
The analysis cohort for treatment comparisons of aviptadil versus matching placebo was randomized participants who received any amount of aviptadil or placebo (modified intention-to-treat). The primary analysis used a proportional odds model to estimate the OR of a better outcome on aviptadil compared to placebo for the 6-category ordinal outcome at Day 90.13 The model was stratified by disease severity (HFNO/NIV or IMV/ECMO) at study entry. Treatment comparisons for the primary outcome were based on participants for whom the Day 90 outcome was ascertained. An analysis that considered missing Day 90 outcome data was also carried out and is described in the Supplementary Appendix (Section 2). Death through Day 90 and through Day 180 and composite safety outcomes through Days 28 and 90 were compared between treatment groups using Cox proportional hazards models stratified by disease severity; hazard ratios (HRs) and 95% confidence intervals (CIs) are cited. Follow-up was censored using the date of withdrawal/loss-to-follow-up, or Day 90 (or 180), whichever was earlier. Treatment groups were also compared using Fine-Gray models stratified by disease severity at study entry to estimate sub-hazard ratios (sHR) for secondary outcomes that have been used in other trials, including time to discharge, time to discharge home, and time to discharge home for 14 days; cumulative incidence was estimated using the Aalen and Johansen method and compared using Gray’s method.14–17 Twenty-three exploratory subgroup analyses (15 of which were prespecified) were performed to assess treatment effect heterogeneity by baseline characteristics. The heterogeneity of ORs and HRs for subgroups were assessed by including an interaction term in the corresponding regression models. One of the subgroups considered for the aviptadil comparison was defined according to whether remdesivir was randomized as part of the 2×2 factorial, whether remdesivir was initiated prior to randomization, or whether remdesivir was contraindicated. For participants enrolled in the 2×2 factorial, the effect of aviptadil versus matched placebo was also compared for those randomly assigned remdesivir with those assigned matching placebo for remdesivir. Results of the subgroup analyses should be interpreted with caution because there was no adjustment for type 1 error.
Analyses for remdesivir versus matching placebo were carried out with similar methods.
For both the aviptadil and remdesivir efficacy comparisons, an OR >1 for the primary endpoint and a sHR >1 for Fine-Gray models indicate superiority of the active treatment compared to placebo. Treatment comparisons for safety outcomes that include death are presented such that HRs <1 or ORs <1 indicate a more favorable outcome for the active treatment. Statistical analyses were performed using SAS (version 9.4) or R (version 4.1).
Role of the funding source
Investigators from NIH were directly involved in all aspects of this study, including study design, data collection, data analysis, data interpretation, and writing of the report. All analyses of biological material were done in a blinded manner at laboratories affiliated with the funding source; data were sent to the statistical and data management center at the University of Minnesota for linkage to the trial database. Several representatives from NIH are part of the writing group for the manuscript. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing official policies, either expressed or implied, of the National Institutes of Health or Department of Veterans Affairs.
RESULTS
Participants
From April 21, 2021 to May 24, 2022, participants were enrolled at 28 sites in the United States (Table S1). For the aviptadil comparison, 471 participants were randomized to aviptadil or matched placebo. The mITT population comprised 461 participants (Figures S1 and S2) who received at least a partial infusion of aviptadil (n=231) or aviptadil matched placebo (n=230).
For the remdesivir comparison, 87 participants (Figures S1 and S3) were randomized to remdesivir or matched placebo and all received some infusion of remdesivir (n=44) or remdesivir matched placebo (n=43). Eighty-five participants, i.e., those enrolled in the 2×2 factorial, were included in the mITT analyses for both agents. Reasons for not receiving any infusion of blinded aviptadil are presented in Table S2.
Baseline characteristics for the aviptadil/placebo comparison by treatment group are summarized in Table 1 and Tables S3–S11 for the mITT population. Overall, for the 461 participants in the mITT population, median age was 57 (IQR 46, 66) years; 39% (n=178) were female; 53% (n=246) reported non-White race or Hispanic ethnicity. Ninety-four percent (n=431) were in an intensive care unit at baseline, with 59% (n=271) receiving HFNO or NIV, 40% (n=185) receiving IMV, and 1% (n=5) ECMO. Median times from hospital admission and from onset of respiratory failure to randomization were 2 (IQR: 2,4) days and 2 (IQR: 2, 3) days, respectively, 39% (n=179) met Berlin criteria for ARDS, while 97% (n=445) of participants met modified Berlin criteria (bilateral infiltrates and SF ratio <315).11 Ninety-five percent (n=440) of participants were prescribed glucocorticoids, and 95% (n=436) were prescribed antiplatelet/anticoagulant treatment at baseline. Most participants (74%, n=305 of 414 with positive nucleocapsid RT-PCR results) were infected with the Delta variant.
Table 1.
Baseline Characteristics: Modified Intention to Treat Cohort for Aviptadil versus Placebo Comparison.
| Characteristic | Aviptadil n=231 | Aviptadil Placebo n=230 | Total N=461 |
|---|---|---|---|
|
Agent randomization/eligibility stratum, n(%)
Factorial (both agents) |
39 (16.9) |
46 (20.0) |
85 (18.4) |
| Aviptadil only, remdesivir contraindicated | 12 (5.2) | 10 (4.3) | 22 (4.8) |
| Aviptadil only, current/prior remdesivir use | 180 (77.9) | 174 (75.7) | 354 (76.8) |
| Age in years, median (IQR) | 58 (46, 67) | 57 (46, 66) | 57 (46, 66) |
| Female sex, n (%) | 94 (40.7) | 84 (36.5) | 178 (38.6) |
|
Race/ethnicity, n (%)
White |
102 (44.2) |
113 (49.1) |
215 (46.6) |
| Black | 40 (17.3) | 33 (14.3) | 73 (15.8) |
| Hispanic | 62 (26.8) | 57 (24.8) | 119 (25.8) |
| Asian | 7 (3.0) | 10 (4.3) | 17 (3.7) |
| Other | 20 (8.7) | 17 (7.4) | 37 (8.0) |
|
Body mass index in kg/m2, n (%)
30–39.9 |
92 (40.4) |
89 (39.0) |
181 (39.7) |
| ≥40.0 | 55 (24.1) | 61 (26.8) | 116 (25.4) |
| Days since onset of respiratory failure, median (IQR) | 2 (2, 3) | 2 (2, 3) | 2 (2, 3) |
|
Baseline respiratory support, n (%) HFNO device |
127 (55.0) |
118 (51.3) |
245 (53.1) |
| NIV | 9 (3.9) | 17 (7.4) | 26 (5.6) |
| IMV | 93 (40.3) | 92 (40.0) | 185 (40.1) |
| ECMO | 2 (0.9) | 3 (1.3) | 5 (1.1) |
| SF ratio (SPO2/FiO2) at randomization, median (IQR) | 134 (100, 176) | 131 (98, 186) | 133 (99, 182) |
| Estimated PF ratio (PaO2/FiO2) at randomizationa, median (IQR) | 83 (43, 133) | 79 (40, 145) | 74 (64, 86) |
| Bilateral lung infiltrates and SF ratio < 315, n (%) | 224 (97.4) | 221 (96.1) | 445 (96.7) |
| ARDS by Berlin criteriab, n (%) | 89 (38.7) | 90 (39.1) | 179 (38.9) |
| Vasopressor use at randomization, n (%) | 35 (15.2) | 29 (12.6) | 64 (13.9) |
|
Co-existing chronic illnessc n (%) Hypertension |
99 (42.9) |
92 (40.0) |
191 (41.4) |
| Diabetes mellitus | 82 (35.5) | 71 (30.9) | 153 (33.2) |
| Renal impairment | 45 (19.5) | 38 (16.5) | 83 (18.0) |
| Heart failure | 24 (10.4) | 15 (6.5) | 39 (8.5) |
| Immunocompromisedd n (%) | 36 (15.6) | 32 (13.9) | 68 (14.8) |
|
SARS-CoV-2 Vaccination Statuse, n (%)
No vaccine dose received Medication use prior to randomization, n% |
149 (64.5) |
151 (65.7) |
300 (65.1) |
| Remdesivir | 177 (76.6) | 172 (74.8) | 349 (75.7) |
| Corticosteroid | 219 (94.8) | 221 (96.1) | 440 (95.4) |
| Immunomodulator | 78 (33.8) | 78 (33.9) | 157 (33.9) |
|
Infecting variant (among the N=414 with positive nucleocapsid RT-PCRf), n (%)
Delta |
155 (74.9) |
150 (72.5) |
305 (73.7) |
| Omicron | 25 (12.1) | 25 (12.1) | 50 (12.1) |
| Other | 27 (13.0) | 32 (15.5) | 59 (14.3) |
| Genscript neutralising anti-spike antibody positiveg, n (%) | 157 (70.7) | 156 (70.0) | 313 (70.3) |
| BioRad anti-nucleocapsid antibody positiveh, n (%) | 171 (77.0) | 182 (81.6) | 355 (79.3) |
| Nucleocapsid antigen concentration (pg/mL)I, median (IQR) | 1246 (95, 6114) | 1502 (224, 6406) | 1294 (150, 6200) |
| Positive (concentration ≥3 pg/mL), n (%) | 211 (95.0) | 211 (94.6) | 422 (94.8) |
Abbreviations:HFNO, high flow nasal oxygen; NIV, non-invasive ventilation; IMV, invasive mechanical ventilation; ECMO; extracorporeal membrane oxygenation; IQR, interquartile range; n, number; kg, kilogram; m, meter; Covid-19, coronavirus disease 2019; RT-PCR, reverse transcription-polymerase chain reaction
Estimated PF ratio computed as PF ratio = (SF ratio – 64)/0.84. Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007 Aug;132(2):410–7. doi: 10.1378/chest.07-0617. Epub 2007 Jun 15. PMID: 17573487.
Bilateral lung infiltrates and PF ratio < 300 and (IMV or ECMO) at baseline.
Full list of co-existing chronic illness in the Supplementary Appendix (Table S6)
Immunocompromised defined as receiving antirejection medications, biologic medications to treat autoimmune disease or cancer, human immunodeficiency virus, or other immunosupmpressive condition
Not vaccinated = No known vaccine doses received before randomization (includes 18 with unknown vaccination status; 6 aviptadil, 12 placebo)
SARS-CoV-2 Delta variant was determined from a mid-turbinate swab at baseline based on RT-PCR detection of the N-terminal domain of the Delta spike. Omicron variant was determined by similar method. Data available for 414; 207 aviptadil and 207 placebo.
GenScript cPass surrogate SARS-CoV-2 neutralization assay (anti-spike); positive: ≥30% binding inhibition. Data available for 445; 222 aviptadil, 223 placebo.
BioRad Platelia anti-nucleocapsid assay (total antibody); positive: ≥1.0 sample/cutoff ratio. Data available for 445; 222 aviptadil, 223 placebo.
Quanterix Simoa nucleocapsid antigen; positive: ≥3 pg/mL. Data available for 445; 222 aviptadil, 223 placebo.
Seventy-six percent (n=349) of participants assigned aviptadil or placebo were receiving remdesivir prior to randomization.
Baseline characteristics for the remdesivir/placebo comparison by treatment group are summarized in Section 5 of the Supplemental Appendix (Tables S12–S20).
Aviptadil
Adherence to Infusions and Concomitant Treatments
Infusions of aviptadil or matching placebo were closely monitored (Table S21). On the first infusion day (600 pmol/kg), 91% (n=211 aviptadil and n=210 placebo) participants received a full dose (defined as at least 90% of the prescribed volume), with no difference by treatment group. On subsequent infusion days (1200–1800 pmol/kg), fewer participants received a full dose (83% (n=190) for aviptadil vs. 91% (n=208) for placebo on the second day, 75% (n=170) vs. 88% (n=197) on the third day), with participants in the aviptadil group consistently receiving less study infusion than the placebo group.
The great majority of participants in both treatment groups continued glucocorticoids through Day 5 and antiplatelet/anticoagulant treatment through Day 7 (Table S22).
Efficacy Summary
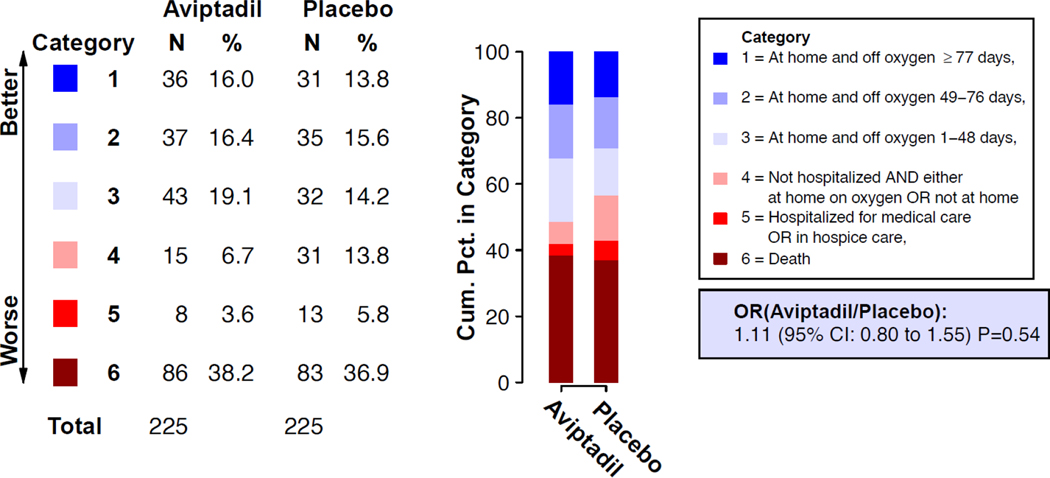
The OR for being in a better category of the primary efficacy endpoint for aviptadil vs. placebo at Day 90, from a model stratified by baseline disease severity, was 1.11 (95% CI: 0.80–1.55; p=0.54) (Figure 1). The p-value corresponding to the test of proportional odds was 0.078; ORs for dichotomized outcomes for the 6 categories of the primary ordinal outcome are summarized in Table 2. The cumulative distribution of supplemental oxygen-free days at home through Day 90 (“days recovered”) is shown in Figure S4. The unadjusted OR for the 6-category Day 90 outcome, and the ORs after adjustment for other randomization stratification variables were consistent with the primary analysis (Table S23).
Figure 1.
Primary 6-category ordinal outcome at Day 90 for the aviptadil versus placebo comparison. Category percentages at Day 90 for the aviptadil group and the placebo group are shown. The summary odds ratio (OR) was estimated with the use of a proportional odds model that was stratified by disease severity at entry. An OR > 1.0 favors aviptadil. Estimated for the 450 participants with known status on Day 90. See Supplementary Appendix Figure S4 for related figure that includes imputation for those with unknown Day 90 status.
Table 2:
Summary of Major Efficacy Outcomes for the Aviptadil versus Placebo Comparison: Modified Intention to Treat Cohort
| Aviptadil | Placebo | |||||
|---|---|---|---|---|---|---|
| Primary Outcome at Day 90 | No. in Group | No. with Event (%in Group) | No. in Group | No. with Event (% in Group) | Odds Ratio for Aviptadil/Placebo (95% CI) | p value |
| 6-category primary ordinal outcome at Day 90a,b | 225 | 225 | 1.11 (0.80, 1.55) | 0.54 | ||
| Dichotomized outcomes for the 6-category primary ordinal outcome at Day 90a,c Category 1 Category 2–6 (reference) Category 1–2 Category 3–6 (reference) Category 1–3 Category 4–6 (reference) Category 1–4 Category 5–6 (reference) Category 1–5 Category 6 (reference) |
225 |
36 (16.0) 189 (84.0) 73 (32.4) 152 (67.6) 116 (51.6) 109 (48.4) 131 (58.2) 94 (41.8) 139 (61.8) 86 (38.2) |
225 |
31 (13.8) 194 (86.2) 66 (29.3) 159 (70.7) 98 (43.6) 127 (56.4) 129 (57.3) 96 (42.7) 142 (63.1) 83 (36.9) |
1.19 (0.71, 2.01) 1.16 (0.77, 1.73) 1.38 (0.95, 2.00) 1.04 (0.71, 1.51) 0.94 (0.64, 1.38) |
0.51 0.48 0.09 0.85 0.77 |
| Dichotomized outcomes for the 6-category primary ordinal outcome at Day 90a,c Category 1 Category 2–6 (reference) Category 1–2 Category 3–6 (reference) Category 1–3 Category 4–6 (reference) Category 1–4 Category 5–6 (reference) Category 1–5 Category 6 (reference) |
225 |
36 (16.0) 189 (84.0) 73 (32.4) 152 (67.6) 116 (51.6) 109 (48.4) 131 (58.2) 94 (41.8) 139 (61.8) 86 (38.2) |
225 |
31 (13.8) 194 (86.2) 66 (29.3) 159 (70.7) 98 (43.6) 127 (56.4) 129 (57.3) 96 (42.7) 142 (63.1) 83 (36.9) |
1.19 (0.71, 2.01) 1.16 (0.77, 1.73) 1.38 (0.95, 2.00) 1.04 (0.71, 1.51) 0.94 (0.64, 1.38) |
0.51 0.48 0.09 0.85 0.77 |
| Other Efficacy Outcomes Through Day 90 | No. in Group | No. with Event (Estimated Cumulative % in Group) | No. in Group | No. with Event (Estimated Cumulative % in Group) | Hazard Ratio or Sub-Hazard Ratio for Aviptadil/Placebo (95% CI) | p value |
| Deathd | 231 | 86 (37.5) | 230 | 83 (36.2) | 1.04 (0.77, 1.41) | 0.78 |
| Dischargede | 231 | 139 (60.4) | 230 | 138 (60.3) | 0.99 (0.78, 1.24) | 0.90 |
| Discharged homee | 231 | 132 (57.4) | 230 | 133 (58.1) | 0.97 (0.77, 1.23) | 0.81 |
| Discharged home for 14 consecutive days (sustained recovery) e | 231 | 126 (55.1) | 230 | 128 (56.2) | 0.97 (0.76, 1.23) | 0.78 |
| Death, end-organ failure or serious infectiond | 231 | 165 (71.6) | 230 | 158 (69.0) | 1.13 (0.91, 1.40) | 0.29 |
| Worsening respiratory failure or deathd | 231 | 106 (46.2) | 230 | 106 (46.3) | 1.00 (0.77, 1.31) | 0.98 |
| Hospital readmission or death, after initial discharged,f | 139 | 10 (7.8) | 138 | 15 (11.2) | 0.66 (0.30, 1.47) | 0.31 |
| Through Day 180 | ||||||
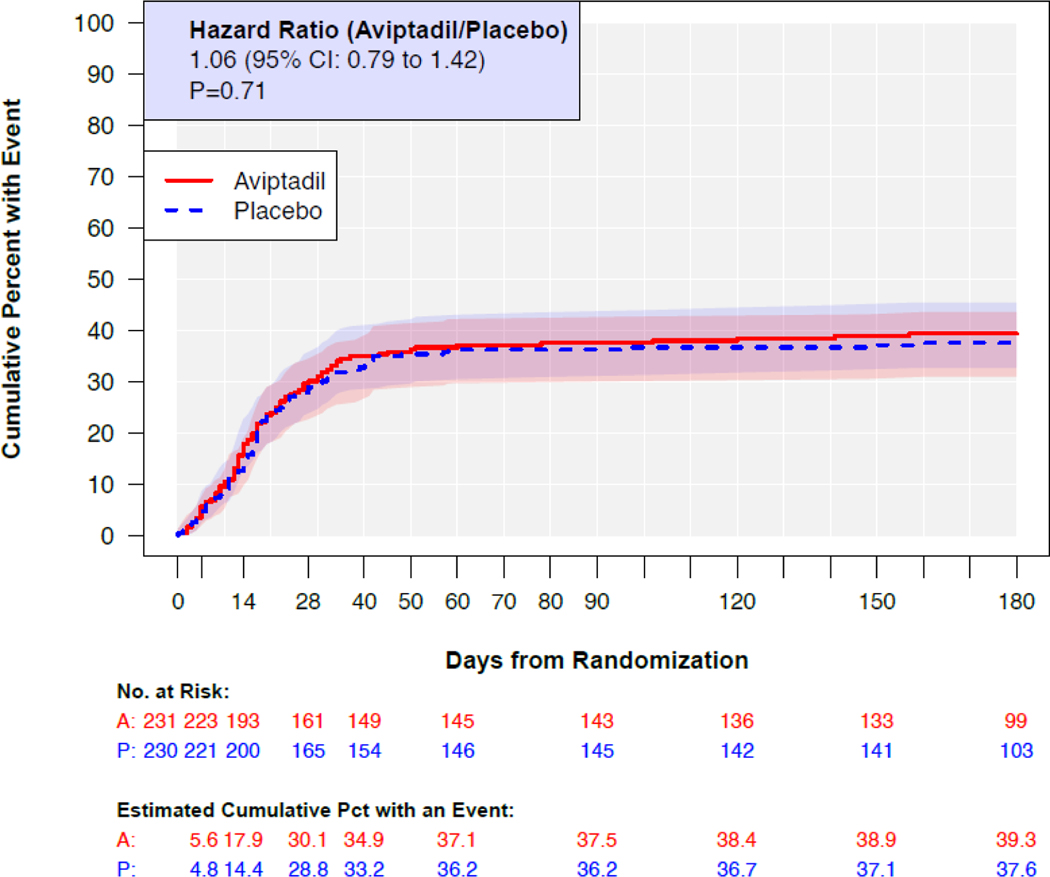
| Deathd | 231 | 90 (39.3) | 230 | 86 (37.6) | 1.06 (0.79, 1.42) | 0.71 |
Category 1: At home and off oxygen ≥ 77 days (best) Category 2: At home and off oxygen 49–76 days Category 3: At home and off oxygen 1–48 days Category 4: Discharged, but not at home, or at home requiring supplemental oxygen Category 5: Hospitalized or receiving hospice care Category 6: Died (worst)
Summary odds ratio for being in a better category, aviptadil vs. placebo. Proportional odds regression model with 1 indicator for treatment group stratified by disease severity. Computed for the 450 participants with known status at day 90; refer to the Supplementary Appendix (Figure S4) for the odds ratio after imputation for those with missing data.
Odds ratio for being in a better category compared to designated reference group, aviptadil vs. placebo. Logistic regression model with 1 indicator for treatment group stratified by disease severity.
Hazard ratio for time to first event, aviptadil vs. placebo. Cox proportional hazards regression model with 1 indicator for treatment group stratified by disease severity.
Sub-hazard ratio for time to first event, aviptadil vs. placebo. Fine-Gray model considering death a competing risk with 1 indicator for treatment group stratified by disease severity.
Among participants who were discharged from the index hospital. Time=0 set to the date of discharge.
Eleven participants (2% of the mITT population) with follow-up through Day 90 (6 assigned aviptadil and 5 placebo) had an unknown status for the Day 90 ordinal primary outcome. Sensitivity analyses that imputed the outcome category for participants with unknown Day 90 status were consistent with the primary analysis (OR=1.10; 95% CI: 0.79–1.52; Figure S5).
Through Day 90, 86 participants in the aviptadil group and 83 in the placebo group died. The cumulative percent who died through Day 90 was 38% and 36% in the aviptadil and placebo groups respectively (Figure 2) with a HR for aviptadil compared to placebo of 1.04 (95% CI: 0.77–1.41). By Day 180, the percentages who died were 39% (90 deaths) and 38% (86 deaths), respectively with a corresponding HR of 1.06 (95% CI: 0.79, 1.42).
Figure 2.
Kaplan-Meier estimates of cumulative mortality through Day 180 for the aviptadil group and the placebo group. Hazards ratio (HR) estimated with a proportional hazards regression model stratified by disease severity at entry. A HR > 1.0 favors placebo. Estimated for all 461 in the mITT cohort.
Secondary efficacy outcomes are further summarized in Table 2, Figures S6–S13 and Table S24. For each of these measures, the 95% CI for the relative treatment difference includes 1.0.
Safety Summary
Infusion reactions were significantly more common for participants treated with aviptadil compared to placebo (Table 3 and Tables S25–S32). Twenty-four (10%) aviptadil and 13 (6%) placebo participants had an infusion discontinued due to an adverse event; 71 (31%) and 35 (15%) experienced an infusion pause due to an adverse event (Table 3). Most adverse events during or within 2 hours of completing the infusion were grade 1 or 2. Diarrhea, facial flushing, tachycardia and hypotension occurred more frequently on aviptadil than placebo; these events were more common for aviptadil than placebo on the second and third infusion days, when higher aviptadil doses were targeted (Table 3).
Table 3.
Safety Outcomes for the Aviptadil versus Placebo Comparison: Modified Intention to Treat Cohort
| Outcomes | Aviptadil (n=231), n (%) | Placebo (n=230), n (%) | Odds or Hazard Ratio for Aviptadil/Placebo (95% CI) | p value |
|---|---|---|---|---|
| Infusion reactions of any grade, Days 0–2 a | ||||
| Any reaction | 200 (86.6) | 132 (57.4) | -- | <0.001 |
| Facial flushing | 34 (14.7) | 14 (6.1) | -- | 0.002 |
| Diarrhea | 92 (39.8) | 26 (11.3) | -- | <0.001 |
| Tachycardia | 22 (9.5) | 11 (4.8) | -- | 0.049 |
| Hypotension | 135 (58.4) | 95 (41.3) | -- | <0.001 |
| Infusion adjustments due to AEb, Days 0–2 | ||||
| Pause | 71 (30.7) | 35 (15.2) | -- | |
| Reduction in rate | 53 (22.9) | 20 (8.7) | -- | |
| Discontinuation | 24 (10.4) | 13 (5.7) | ||
| Safety through Day 5 c | ||||
| Composite safety outcome of SAE, Grade 3/4 AE, Organ Failure/Serious Infection, or Death | 146 (63.2) | 129 (56.1) | 1. | 0.10 |
| Select components | ||||
| Deathc | 13 (5.6) | 11 (4.8) | 1.19 (0.52, 2.71) | 0.68 |
| Hypotension or shockc,d | 69 (29.9) | 64 (27.8) | 0.62 | |
| Diarrheac,e | 4 (1.7) | 2 (0.9) | 0.42 | |
| Cardiacc,f | 24 (10.4) | 22 (9.6) | -- | 0.76 |
| Renalc,g | 20 (9.4) | 12 (5.4) | -- | 0.10 |
| Barotraumac,h | 5 (2.2) | 1 (0.4) | 0.14 | |
| Safety through Day 28 i | ||||
| Composite safety outcome of SAE, Grade 3/4 AE, Organ Failure/Serious Infection, or Death | 181 (78.4) | 172 (74.8) | 1.17 (0.95,1.44) | 0.15 |
| Select components | ||||
| Deathi | 69 (29.9) | 66 (28.7) | 1.05 (0.75, 1.47) | 0.77 |
| Hypotension or shockd,i | 112 (48.5) | 114 (49.6) | -- | 0.82 |
| Diarrheae,i | 7 (3.0) | 4 (1.7) | -- | 0.37 |
| Cardiac f,i | 56 (24.2) | 49 (21.3) | -- | 0.49 |
| Renalg,i | 41 (19.2) | 40 (17.9) | 0.56 | |
| Barotrauma h,i | 11 (4.8) | 14 (6.1) | 0.55 |
SAE=serious adverse event; AE=adverse event; MedDRA=Medical Dictionary for Regulatory Activities; SOC=System Organ Class.
P-value for comparing infusion reactions between treatment groups from Cochran-Mantel-Haenszel tests, stratified by disease severity at baseline.
Infusion adjustments are not mutually exclusive, see Table S17 for mutually exclusive representation.
Odds ratio for experiencing the event, aviptadil vs. placebo. Logistic regression model with 1 indicator for treatment group, stratified by disease severity at baseline. P-values for treatment group comparisons for select components from separate similar logistic regression models.
Any grade 3/4 hypotension during infusion, any organ failure report of hypotension with vasopressor use, any grade 3/4 AE, SAE, or death with MedDRA PT for hypotension, shock, shock haemorrhagic, distributive shock
Any grade 3/4 AE report if diarrhea. Per protocol, infusion-related diarrhea events excluded unless event led to discontinuation of blinded aviptadil.
Any grade 3/4 AE, SAE, or death with MedDRA Cardiac SOC, and any organ failure report of myocardial infarction, congestive heart failure III/IV, or atrial/ventricular tachyarrhythmia
Any grade 3/4 AE, SAE, or death with MedDRA PT for acute kidney injury, and organ failure report of new renal replacement therapy. Limited to patients without dialysis at baseline, Aviptadil= 213 and Placebo= 223
Any grade 3/4 AE, SAE, or death with MedDRA PT for pneumomediastinum or pneumothorax.
Hazard ratio for time to first event, aviptadil vs. placebo. Cox proportional hazards regression model with 1 indicator for treatment group stratified by disease severity. P-values for treatment group comparisons for select components from separate similar logistic regression models.
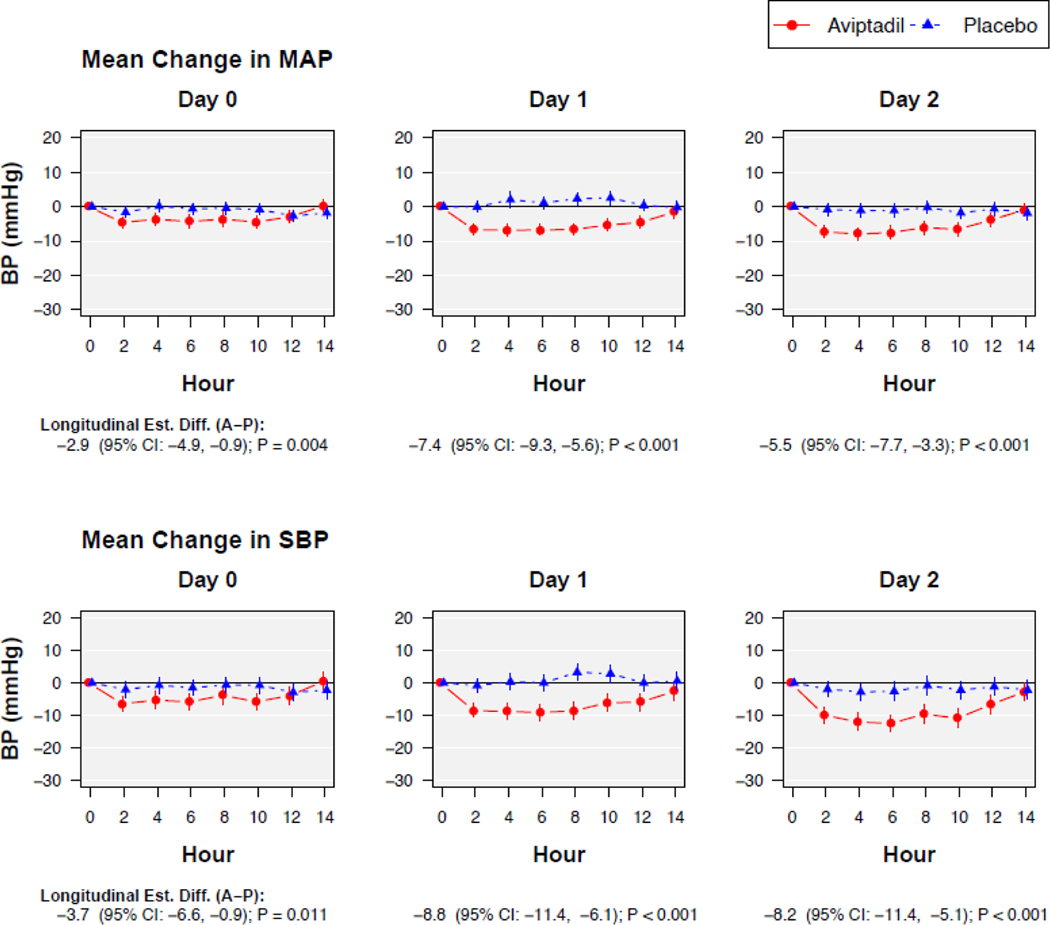
On average, on the second and third infusion days, mean arterial pressure (MAP) was 5–7mmHg lower among aviptadil vs. placebo participants during each infusion (p<0.001), but returned to parity by 2 hours after completing the infusion (Figure 3). Further details on hypotension adverse events associated with the infusion are provided in Table S32.
Figure 3.
Average change in mean arterial pressure (MAP) and systolic blood pressure (SBP) during the blinded aviptadil infusions on days 0, 1, and 2. MAP and SBP were reported pre-infusion (hour=0), every 2 hours peri-infusion, and 2 hours after the infusion ended.
The primary safety outcome of death, serious adverse events, organ failure, serious infection, or grade 3 or 4 adverse events through Day 5 (Tables 3 and S33–S46) was experienced by 63% (n=146) of participants in the aviptadil compared to 56% (n=129) in the placebo groups, OR 1.40 (95% CI 0.94–2.08), p=0.10. Using data through Day 28, the percentages were 78% (n=181) versus 75% (n=172) in the aviptadil and placebo groups, respectively, with HR 1.17 (95% CI 0.95–1.44), p=0.15 (Tables 3 and S47–S61 and Figure S14). While hypotension was more common in the aviptadil than placebo groups during the infusion, the percentage of participants with potentially serious renal complications of hypotension through Day 28, among those without dialysis at entry, were similar for the aviptadil and placebo groups, 19% (n=41/213) and 18% (n=40/223), respectively (Table 3).
Summaries of serious adverse events, including organ failure and serious infections, and cardiovascular events through Day 90, and laboratory abnormalities through Day 5 are in the Supplemental Appendix (Tables S62–S66).
Subgroup Analyses
The estimated OR (aviptadil/placebo) for the primary ordinal outcome did not vary according to disease severity (Figure S15). ORs for those receiving HFNO or NIV at entry and IMV or ECMO at entry were 1.04 (95% CI: 0.67–1.60) and 1.21 (95% CI: 0.73–2.04), respectively (p=0.71 for interaction). ORs for the primary endpoint varied across four subgroups defined by use (or nonuse) of remdesivir (p=0.038). Among the 84 participants enrolled in the factorial, ORs (aviptadil/placebo) were 2.11 (95% CI: 0.69–6.46) for those randomized to remdesivir and 3.78 (95% CI: 1.17–12.24) for those randomized to the remdesivir placebo. For those for whom remdesivir was contraindicated (n=22), the OR was 0.64 (95% CI: 0.10–4.08); and for those from whom remdesivir had been started prior to randomization (n=344), the majority of participants, the OR was 0.93 (95% CI: 0.64–1.36). For the 84 participants in the factorial study, ORs (aviptadil/placebo) for those also assigned remdesivir as compared to placebo for remdesivir did not vary (p=0.33) (Figure S15).
There was also possible evidence of heterogeneity for subgroups defined by race/ethnicity. This apparent heterogeneity arose because of an OR (2.71, 95% CI: 1.37–5.33) indicating a possible favorable effect of aviptadil for those of non-Black Hispanic ethnicity and ORs indicating possible unfavorable effects (OR 0.78, 95% CI: 0.34–1.83 for Black participants, and OR 0.76, 95% CI: 0.46–1.23 for non-Hispanic White participants) for other race/ethnicity groups (Figure S15). Subgroup analyses for Day 90 mortality (Figure S16 and Table S67) and the Day 5 and 28 composite safety outcomes are summarized in Figures S17–S18.
Follow-up results for the 84 participants randomized to remdesivir vs. placebo are presented in Section 7 of the Supplementary Appendix.
DISCUSSION
In this multicenter phase III trial of participants hospitalized for COVID-19-associated respiratory failure, treatment with aviptadil did not improve the primary ordinal outcome or survival at Day 90 compared to placebo. Other secondary endpoints also did not demonstrate efficacy for aviptadil. Safety analyses were largely consistent with the known safety profile of aviptadil—hypotension, diarrhea, and facial flushing were the most common and prominent events associated with infusion.
This trial specifically addresses patients with critical COVID-19 acute hypoxemic respiratory failure, who suffer the highest COVID-19-related mortality.18–20 In vitro, aviptadil has four potential mechanisms of action relevant to COVID-19 acute hypoxemic respiratory failure, including suppression of SARS-CoV-2 replication, decrease in viral cytopathy, modulation of monocyte-derived inflammation, and increases in surfactant production.5 Based on multiple complementary mechanisms of action, experience with pre-pandemic ARDS, and an exploratory efficacy signal from a previous Phase 2/3 randomized trial,6 aviptadil was a logical lung-specific agent for investigation in COVID-19 acute hypoxemic respiratory failure. While in the present study aviptadil infusion appeared safe in a closely monitored, largely ICU, setting in which on-treatment hypotension can be promptly addressed, we found no evidence to suggest that aviptadil has a therapeutic role for patients with COVID-19 acute hypoxemic respiratory failure.
Although more than half of enrolled participants reported Hispanic ethnicity or non-White race, the trial had limited power to explore subgroups based on race/ethnicity. A possible favorable effect of aviptadil observed among Hispanic participants (and a possibly similar effect among patients in the remdesivir factorial, who were more often Hispanic) must be interpreted with caution, due to the absence of an overall treatment effect as well as the large number of subgroups assessed.
Strengths and limitations
This trial demonstrates the feasibility of robust trials specific to critical COVID-19 acute hypoxemic respiratory failure, with enhanced safety monitoring adapted to the specific patient population. Study drug was infused with intensive monitoring, structured management guidance for hypotension, and specific grading tables for hypotension.
The safety protocols used within this trial aimed to balance potential risks and benefits, emphasizing infusion pauses and dose reduction rather than substantial increases in vasopressor therapy and/or fluid loading to counter the hypotensive effects of aviptadil, an investigational agent with unproven clinical benefit. This approach resulted in 24% of assigned participants not receiving the full prescribed dose of aviptadil across the three infusion days, although 97% of participants received 50% or more of the target dose. While it is therefore possible that the dose or duration of aviptadil used (3 days of escalating doses, 12 hours per day) was not ideal, we employed dosing conventions used in prior studies at the time of study launch that pharmacokinetic modeling suggests achieve adequate lung concentrations.5,21,22 Furthermore, the average decrease in MAP of 5–7 mmHg on the second and third days of infusion suggests that a higher dose of aviptadil would not be well tolerated. Given the lack of efficacy and increasing intolerance on later dosing days, we believe it unlikely that longer courses of treatment would have a benefit.
Methodological innovations include use of a novel, patient-centered endpoint and a pragmatic definition of respiratory failure that included patients receiving HFNO.10,11 The primary endpoint combined mortality and the speed with which survivors recovered; the definition of recovery was patient-centered given the substantial burden of new supplemental oxygen and/or failure to return home. The inclusion of patients receiving HFNO is justified based on the homogeneity of this population (all had COVID-19 pneumonia as the cause of their hypoxemic respiratory failure), the fact that 40% of patients receiving HFNO at baseline subsequently underwent invasive mechanical ventilation, and the concordance of results between patients receiving HFNO or invasive mechanical ventilation at baseline.
Additional strengths of this trial include rigorous safety protocols for the study of a vasodilating agent in patients with or at risk for hypotension, the racial/ethnic diversity of participants, use of evidence-based standard of care therapy, and standardized ascertainment of endpoints. Specifically, ascertainment of the primary, patient-centered endpoint through Day 90 was >95%, suggesting that (with appropriate resources and training) outcomes beyond hospital mortality can be reliably measured in this complex and critically ill population.
Our trial has other limitations. First, the trial has limited power for subgroup analyses. Second, while the trial was performed after SARS-CoV-2 vaccines were generally available, most participants had not been vaccinated, limiting generalization to a more vaccinated population. Third, while the vast majority of trial participants received corticosteroids, NIH COVID-19 treatment guidelines changed during the conduct of the TESICO trial, adding second-line immune-suppression for hospitalized patients with rapidly worsening hypoxemia, and only 34% (n=156) of trial participants received second-line immune-suppression at enrollment. Fourth, enrollment in the remdesivir factor of the 2×2 factorial was limited by extensive pretreatment in the patient population, and as a consequence, planned comparisons of remdesivir with placebo are substantially underpowered. Fifth, we did not collect formal screening logs from enrolling sites, although we enrolled with broad eligibility criteria at diverse sites, and the enrolled trial population was ethnically diverse, suggesting good generalizability.
In summary, we found no evidence that intravenous aviptadil improved clinical outcomes among patients with COVID-19-associated acute hypoxemic respiratory failure; inferences regarding remdesivir are limited by sample size.
Supplementary Material
Research in Context.
Evidence before this study
Mortality remains high among hospitalized patients with COVID-19 who experience critical hypoxemic respiratory failure. While certain immunomodulators (especially glucocorticoids and perhaps baricitinib and tocilizumab) have suggested efficacy in this critically ill population, mortality remains high. Lung-specific therapies are an important priority for patients with this condition, given the strong association between the severity of lung failure and ultimate patient outcomes. Aviptadil, the synthetic form of Vasoactive Intestinal Peptide, is a neuropeptide hormone with positive pleiotropic effects in preclinical models. A phase 2/3 trial of intravenous aviptadil among hospitalized patients with COVID-19 and respiratory failure suggested the possibility of efficacy for intravenous aviptadil in this population. In addition, prior trials suggested the possibility that the antiviral remdesivir may have efficacy in this population, although this is not certain. We serially searched MEDLINE in English from 1970 to 2021 for (“aviptadil” or “remdesivir”) and (“COVID” or “novel coronavirus” or SARS-CoV-2).
Added value of this study
In this multicenter Phase 3 trial, we evaluated three daily infusions of intravenous aviptadil vs. placebo and/or 10 days of intravenous remdesivir in the setting of standard care (including glucocorticoid therapy). Aviptadil did not improve the primary endpoint of recovery status at Day 90; nor did aviptadil improve any other endpoint. The anticipated safety profile was confirmed—flushing, hypotension, and diarrhea were all more common in the aviptadil-assigned group. Few patients were randomized to the remdesivir vs. placebo comparison.
Implications of all the available evidence
Aviptadil does not appear to have a role in the treatment of critical respiratory failure among patients hospitalized with COVID-19. Other lung-related therapies should be sought in this severely underserved population.
ACKNOWLEDGMENTS
We thank the participants and families whose collaborative spirit and dedication to science at a time of great personal stress made this trial possible. We also thank the members of the ACTIV-3b / TESICO data and safety monitoring board — Graeme A. Meintjes, M.B., Ch.B., Ph.D. (chair), Wendy Armstrong, M.D., David Glidden, Ph.D., Jesse Hall, M.D., Yvonne Maldonado, Ph.D., Barbara E. Murray, M.D., Stuart Campbell Ray, M.D., Valeria Cavalcanti Rolla, M.D., Ph.D., Haroon Saloojee, M.B., B.Ch., Anastasios A. Tsiatis, Ph.D., Paul A. Volberding, M.D., Rieke van der Graaf, Ph.D., and Sally Hunsberger, Ph.D. (executive secretary) — for their review of the protocol and their guidance based on interim reviews of the data.
Funding:
Supported by the U.S. Operation Warp Speed program, the National Institute of Allergy and Infectious Diseases (NIAID), Division of Clinical Research (DCR) and Leidos Biomedical Research for the INSIGHT Network, and the National Heart, Lung, and Blood Institute and the Research Triangle Institute for the Prevention and Early Treatment of Acute Lung Injury (PETAL) Network and the Cardiothoracic Surgical Trials Network (CTSN). The NIAID, DCR funded this project through a subcontract,18X107C, from Leidos Biomedical Prime Contract HHSN261200800001E, NIH. The research was also, in part, funded by NIAID grant U01-AI136780 and the National Institutes of Health (NIH) Agreement 1OT2HL156812–01. Trial medication was donated by NRx Pharmaceuticals (aviptadil) and Gilead Sciences, Inc. (remdesivir). The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the NIH.
(Funded by the National Institutes of Health (NIH) and others. TESICO ClinicalTrials.gov number, NCT04843761)
Declaration of Interests
CEB reports funding from the National Institutes of Health (NIH) for the Aviptadil study, during the conduct of the study. BG reports grants from the NIH, during the conduct of the study. SS reports a grant from the NIH, during the conduct of the study. ANP reports grants from the William T. Grant Foundation (WT), the National Institute for Health and Care Research (NIHR), UK Research and Innovation (UKRI), and the Bill and Melinda Gates Foundation (BMGF) and consulting fees from BMGF, outside of the submitted work. IDP reports funding from NIH and the National Institute of General Medical Sciences (NIGMS), during the conduct of the study, a grant from Janssen for the study of influenza patient reported outcomes and a contract with Regeneron for a COVID-19 therapy trial, outside of the submitted work. JRB reports a grant from the NIH, during the conduct of the study, grants from the NIH, Quantum Leap Healthcare Collaborative, and Sedana Medical, consulting fees from Sedana Medical, and compensation from Hamilton Medical for participation as a Medical Monitor, outside of the submitted work. ESH reports study materials from NeuroRx, Inc. and Gilead through the National Heart, Lung, and Blood Institute (NHLBI) subcontract, during the conduct of the study, subcontracts with Bristol Meyers Squibb (BMS), Allergan, Gilead, and Janssen for Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-1) clinical trial, subcontracts with Astra Zeneca, Brii Biosciences, Vir Biotechnology, Inc., and Eli Lilly for Therapeutics for Inpatients with COVID-19 (TICO) clinical trial, and additional subcontracts with Rigel, APEIRON Biologics AG and Trevena for Novel Experimental COVID-19 Therapies Affecting Host Response (NECTAR) clinical trial, outside of the submitted work. EM reports study materials from NeuroRx, Inc. and Gilead through NHLBI subcontract, during the conduct of the study, subcontracts with BMS, Allergan, Gilead, and Janssen for ACTIV-1 clinical trial, subcontracts with Astra Zeneca, Brii Biosciences, Vir Biotechnology, Inc., and Eli Lilly for TICO clinical trial, and additional subcontracts with Rigel, APEIRON Biologics AG and Trevena for NECTAR clinical trial, outside of the submitted work. MAGB reports study materials from NeuroRx, Inc. and Gilead through NHLBI subcontract, during the conduct of the study, subcontracts with BMS, Allergan, Gilead, and Janssen for ACTIV-1 clinical trial, subcontracts with Astra Zeneca, Brii Biosciences, Vir Biotechnology, Inc., and Eli Lilly for TICO clinical trial, and subcontracts with Rigel, APEIRON Biologics AG and Trevena for NECTAR clinical trial, outside of the submitted work. KSM reports grants and contracts from NIH, NHLBI, and the Society for Critical Care Medicine, participation as a steering committee member for Roivant/Kinevant Sciences, and employment as a Clinical Research Physician at Chiesi USA, Inc., outside of the submitted work. JM reports receiving study materials and funding from the Albert Einstein College of Medicine for the study protocol, during the conduct of the study. CH reports funding from the National Institute of Allergy and Infectious Diseases (NIAID) in the form of per patient payments for “A Multicenter, Adaptive, Randomized, Blinded Controlled Trial of the Safety and Efficacy of Investigational Therapeutics for Hospitalized Patients with Acute Respiratory Distress Syndrome Associated with COVID-19”, during the conduct of the study. AK reports grants from Eli Lilly, AstraZeneca, 4D Medical, United Therapeutics, Regeneron Pharmaceuticals, and Dompe Pharmaceuticals and consulting fees from Dompe Pharmaceuticals for clinical trial design, outside of the submitted work. AD reports grants from NHLBI Prevention and Early Treatment of Acute Lung Injury (PETAL) network and the Centers for Disease Control and Prevention (CDC) and Data Safety Monitoring Board (DSMB) or Advisory Board participation for Alung Technologies, outside of the submitted work. SD reports a grant from Chest Sonosite Ultrasound to study of incidence of Deep Vein Thrombosis (DVT) in COVID-19 patients, during the conduct of the study. AJG reports payment from Sound Pharmaceuticals for participation as a Medical Monitor for a COVID therapeutic trial, outside of the submitted work. USS reports consulting fees from Shionogi, Paratek, and ViiV Healthcare for participation on Advisory Boards and speaking fees from Shionogi and Paratek, outside of the submitted work. NJJ reports grants from CDC, Department of Defense (DOD), and NIH and participation on a DSMB, outside of the submitted work. MAM reports grants from NIH and NIAID, during the conduct of the study. NA reports funding from NIH, during the conduct of the study. JDC reports grants from NIH and DOD, outside of the submitted work. DDM reports funding from the Danish National Research Foundation (DNRF126), during the conduct of study. AAG reports funding from NIH, during the conduct of the study, grants or contracts from NIH, DOD, CDC, Faron Pharmaceuticals, and Abbvie and participation on a DSMB or Advisory Board for NIH, outside of the submitted work. WHS reports funding from NIH and NIAID, during the conduct of the study. CFO reports contracts with NIH and NHLBI, outside of the submitted work. BTT reports a grant from NHLBI, consulting fees from Bayer, Genetec, and Novartis, and participation on a DSMB or Advisory Board for Aperion, outside of the submitted work. VK reports subcontracts with University of Minnesota, NIAID, and NIH for the TICO and Therapeutics for Severely Ill Inpatients with COVID-19 (TESICO) platform trials, outside of the submitted work. AGB reports a grant from University of Minnesota, during the conduct of the study, grants from the Medical Reserve Corps (MRC) and UKRI, payment from NIAID for participation on a COVID-19 Vaccines DSMB, and participation on the World Health Organization (WHO) Trial Data and Safety Monitoring Committee (DSMC), outside of the submitted work. JDN reports grants from NIAID, NIH, and Leidos Biomedical, outside of the submitted work. HCL reports employment from NIAID, during the conduct of study.
Footnotes
For the ACTIV-3b / Therapeutics for Severely Ill Inpatients with COVID-19 (TESICO) Study Group
A full list of members of the ACTIV-3b/TESICO Study Group is provided in the Supplementary Appendix.
Contributions of Writing Committee Members
Responsible for decision to submit the manuscript: Brown, Neaton, Lane, Barkauskas.
Directly accessed and verified the underlying data: Sharma, Grund.
Composed the initial manuscript: Brown, Barkauskas (no outside medical writer was used).
Conceptualization: Lundgren, Lane, Neaton, Gelijns, Thompson, Kan, Davey, Babiker, Polizzotto, Brown, Ginde, Barkauskas.
Investigation: Brown, Barkauskas, Grund, Sharma, Phillips, Leither, Peltan, Lanspa, Gilstrap, Mourad, Lane, Beitler, Serra, Garcia, Almasri, Fayed, Hubel, Harris, Middleton, Barrios, Mathews, Goel, Acquah, Mosier, Hypes, Campbell, Khan, Hough, Wilson, Levitt, Duggal, Dugar, Goodwin, Terry, Chen, Torbati, Iyer, Sandkovsky, Johnson, Robinson, Matthay, Aggarwal, Douglas, Casey, Hache-Marliere, Youssef, Knemdirim, Leshnower, Awan, Pannu, O’Mahony, Manian, Hayanga, Wortmann, Tomazini, Miller, Jensen, Murray, Bickell, Zitakia, Burris, Higgs, Natarajan, Dewar, Schechner, Kang, Arenas-Pinto, Hudson, Ginde, Self, Rogers, Oldmixon, Morin, Sanchez, Weintrob, Cavalcanti, Davis-Karim, Engen, Denning, Thompson, Gelijns, Kan, Davey, Lundgren, Babiker, Neaton, Lane.
Data curation: Sharma, Grund, Neaton.
Formal analysis: Phillips, Sharma, Grund, Babiker, Neaton.
Funding acquisition: Lundgren, Lane, Neaton, Gelijns, Thompson, Kan, Davey, Babiker.
Supervision: Lane, Lundgren, Neaton, Gelijns, Thompson, Kan, Davey, Babiker, Higgs.
Review & editing manuscript: Brown, Barkauskas, Grund, Sharma, Phillips, Leither, Peltan, Lanspa, Gilstrap, Mourad, Lane, Beitler, Serra, Garcia, Almasri, Fayed, Hubel, Harris, Middleton, Barrios, Mathews, Goel, Acquah, Mosier, Hypes, Campbell, Khan, Hough, Wilson, Levitt, Duggal, Dugar, Goodwin, Terry, Chen, Torbati, Iyer, Sandkovsky, Johnson, Robinson, Matthay, Aggarwal, Douglas, Casey, Hache-Marliere, Youssef, Knemdirim, Leshnower, Awan, Pannu, O’Mahony, Manian, Hayanga, Wortmann, Tomazini, Miller, Jensen, Murray, Bickell, Zitakia, Burris, Higgs, Natarajan, Dewar, Schechner, Kang, Arenas-Pinto, Hudson, Ginde, Self, Rogers, Oldmixon, Morin, Sanchez, Weintrob, Cavalcanti, Davis-Karim, Engen, Denning, Thompson, Gelijns, Kan, Davey, Lundgren, Babiker, Neaton, Lane.
Contributor Information
Samuel M. Brown, Department of Pulmonary/Critical Care Medicine, Intermountain Medical Center, Murray, Utah, USA. Division of Pulmonary/Critical Care Medicine, University of Utah, Salt Lake City, UT, USA.
Christina Barkauskas, Division of Pulmonary/Critical Care Medicine, Duke Health, Durham, NC, USA.
Birgit Grund, School of Statistics, University of Minnesota; Minneapolis, MN, USA.
Shweta Sharma, Division of Biostatistics, School of Public Health, University of Minnesota; Minneapolis, MN, USA.
Andrew N. Phillips, Institute for Global Health, University College London, United Kingdom Lindsay Leither DO; Department of Pulmonary/Critical Care Medicine, Intermountain Medical Center, Murray, Utah, USA. Division of Pulmonary/Critical Care Medicine, University of Utah, Salt Lake City, UT, USA.
Ithan D. Peltan, Department of Pulmonary/Critical Care Medicine, Intermountain Medical Center, Murray, Utah, USA. Division of Pulmonary/Critical Care Medicine, University of Utah, Salt Lake City, UT, USA.
Michael Lanspa, Department of Pulmonary/Critical Care Medicine, Intermountain Medical Center, Murray, Utah, USA. Division of Pulmonary/Critical Care Medicine, University of Utah, Salt Lake City, UT, USA.
Daniel Gilstrap, Division of Pulmonary, Allergy and Critical Care Medicine, Duke Health, Durham, NC, USA.
Ahmad Mourad, Division of Infectious Diseases, Duke University School of Medicine, Durham, NC, USA.
Kathleen Lane, Surgical Office of Clinical Research, Cardiothoracic Surgical Division, Duke University School of Medicine, Durham, NC, USA.
Jeremy R. Beitler, Columbia Respiratory Critical Care Trials Group and Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University. Center for Acute Respiratory Failure, New York-Presbyterian Hospital, New York, NY, USA.
Alexis L. Serra, Columbia Respiratory Critical Care Trials Group and Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University. Center for Acute Respiratory Failure, New York-Presbyterian Hospital, New York, NY, USA.
Ivan Garcia, Columbia Respiratory Critical Care Trials Group and Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University. Center for Acute Respiratory Failure, New York-Presbyterian Hospital, New York, NY, USA.
Eyad Almasri, Department of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, UCSF Fresno, Fresno, CA, USA.
Mohamed Fayed, Department of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, UCSF Fresno, Fresno, CA, USA.
Kinsley Hubel, Department of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, UCSF Fresno, Fresno, CA, USA.
Estelle S. Harris, Department of Medicine, Division of Pulmonary and Critical Care Medicine, University of Utah, Salt Lake City, UT, USA.
Elizabeth A. Middleton, Department of Medicine, Division of Pulmonary and Critical Care Medicine, University of Utah, Salt Lake City, UT, USA.
Macy AG Barrios, Department of Medicine, Division of Pulmonary and Critical Care Medicine, University of Utah, Salt Lake City, UT, USA.
Kusum S. Mathews, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine; Department of Emergency Medicine; Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Neha N. Goel, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine; Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Samuel Acquah, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine; Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Jarrod Mosier, Department of Emergency Medicine and Division of Pulmonary, Allergy, Critical Care and Sleep, Department of Medicine, University of Arizona College of Medicine and Banner University Medical Center-Tucson, Tucson, AZ, USA.
Cameron Hypes, Department of Emergency Medicine and Division of Pulmonary, Allergy, Critical Care and Sleep, Department of Medicine, University of Arizona College of Medicine, Tucson, AZ, USA.
Elizabeth Campbell, Department of Emergency Medicine, University of Arizona College of Medicine, Tucson, AZ, USA.
Akram Khan, Department of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, Oregon Health & Science University, Portland, OR, USA.
Catherine L. Hough, Department of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, Oregon Health & Science University, Portland, OR, USA.
Jennifer G. Wilson, Department of Emergency Medicine, Stanford University School of Medicine, Palo Alto, CA, USA.
Joseph E. Levitt, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, Stanford University School of Medicine, Palo Alto CA, USA.
Abhijit Duggal, Department of Critical Care, Respiratory Institute, Cleveland Clinic, Cleveland OH, USA.
Siddharth Dugar, Department of Critical Care, Respiratory Institute, Cleveland Clinic, Cleveland OH, USA.
Andrew J. Goodwin, Division of Pulmonary, Critical Care, Allergy and Sleep Medicine, Medical University of South Carolina, Charleston, SC, USA.
Charles Terry, Division of Pulmonary, Critical Care, Allergy and Sleep Medicine, Medical University of South Carolina, Charleston, SC, USA.
Peter Chen, Women’s Guild Lung Institute, Department of Medicine and Biomedical Sciences, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Sam Torbati, Department of Emergency Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Nithya Iyer, Division of Pulmonary/Critical Care, Baylor University Medical Center and Texas A&M School of Medicine. Dallas, TX, USA.
Uriel Sandkovsky, Division of Infectious Diseases, Baylor University Medical Center. Dallas, TX, USA.
Nicholas J. Johnson, Department of Emergency Medicine and Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, University of Washington/Harborview Medical Center, Seattle, WA, USA.
Bryce RH Robinson, Department of Surgery, University of Washington/Harborview Medical Center, Seattle, WA, USA.
Michael Matthay, Cardiovascular Research Institute and Departments of Medicine and Anesthesia, University of California-San Francisco, San Francisco, CA, USA.
Neil R Aggarwal, Department of Medicine, Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Ivor S. Douglas, Department of Medicine, Denver Health Medical Center and Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Jonathan D. Casey, Division of Allergy, Pulmonary and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Manuel Hache-Marliere, Jacobi Medical Center/Montefiore Medical Center - Albert Einstein College of Medicine, New York, NY, USA.
J. Georges Youssef, Department of Pulmonary/Critical Care Medicine, Weill Cornell Medical College.
J. C. Walter, Jr., Transplant Center Advanced Lung Diseases Program, Houston Methodist Hospital, Houston, TX, USA
William Nkemdirim, Montefiore Medical Center - Albert Einstein College of Medicine, New York, NY, USA.
Brad Leshnower, Division of Cardiothoracic Surgery, Emory University School of Medicine, Atlanta, GA, USA.
Omar Awan, Department of Medicine, Division of Pulmonary, Critical Care, and Sleep Disorders Medicine, VA Medical Center and George Washington University, Washington, DC, USA.
Sonal Pannu, Department of Medicine, Division of Pulmonary Critical Care and Sleep, Ohio State University, Columbus, OH, USA.
Darragh Shane O’Mahony, Department of Acute Care Medicine, Swedish Medical Center, Seattle, WA, USA.
Prasad Manian, Division of Pulmonary and Critical Medicine, Baylor College of Medicine & Texas Heart Institute, Houston, TX, USA.
J. W. Awori Hayanga, Department of Cardiovascular and Thoracic Surgery. Heart and Vascular Institute, West Virginia University, Morgantown, WV, USA.
Glenn W. Wortmann, Infectious Diseases Section, MedStar Washington Hospital Center and Georgetown University, Washington, DC, USA.
Bruno M. Tomazini, Brazilian Research in Intensive Care Network (BRICNet), Brazil. HCor Research Institute, São Paulo, Brazil.
Robert F Miller, Institute for Global Health, University College London, UK.
Jens-Ulrik Jensen, Section of Respiratory Medicine, Department of Medicine, Herlev-Gentofte Hospital, Hellerup, Denmark. CHIP Center of Excellence for Health, Immunity, and Infections and Department of Infectious Diseases, Rigshospitalet; Copenhagen, Denmark. Department of Clinical Medicine, Faculty of Health Sciences, University of Copenhagen, Denmark.
Daniel D. Murray, CHIP Center of Excellence for Health, Immunity, and Infections, Rigshospitalet; Copenhagen University Hospital; Copenhagen, Denmark.
Nina A. Bickell, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Jigna Zatakia, Icahn School of Medicine at Mount Sinai, NY, USA.
Sarah Burris, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Elizabeth S. Higgs, National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA.
Ven Natarajan, Frederick National Laboratory for Cancer Research, Frederick MD, USA.
Robin L. Dewar, Frederick National Laboratory for Cancer Research, Frederick MD, USA.
Adam Schechner, Frederick National Laboratory for Cancer Research, Frederick MD, USA.
Nayon Kang, Contractor | Medical Science and Computing, Bethesda, MD, USA.
Alejandro Arenas-Pinto, The Medical Research Council Clinical Trials Unit at UCL, University College London; London, UK. Institute for Global Health, University College London; London, UK.
Fleur Hudson, The Medical Research Council Clinical Trials Unit at UCL, University College London; London, United Kingdom.
Adit A Ginde, Department of Emergency Medicine, University of Colorado School of Medicine, Aurora, CO, USA.
Wesley H. Self, Department of Emergency Medicine, Vanderbilt University Medical Center; Nashville, TN, USA.
Angela J. Rogers, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, Stanford University School of Medicine, Palo Alto CA, USA, Palo Alto, CA, USA.
Cathryn Oldmixon, Massachusetts General Hospital, Boston, MA, USA.
Haley Morin, Stanford University, Palo Alto, CA, USA.
Adriana Sanchez, Veteran Affairs Medical Center, Washington, DC, USA.
Amy C. Weintrob, Infectious Diseases Section, Washington, DC VA Medical Center, Washington, DC, USA.
Alexandre Biasi Cavalcanti, HCor Research Institute, São Paulo, Brazil.
Anne Davis-Karim, Cooperative Studies Program, Clinical Research Pharmacy Coordinating Center, Office of Research & Development, Department of Veterans Affairs, Albuquerque, NM, USA.
Nicole Engen, Division of Biostatistics, School of Public Health, University of Minnesota; Minneapolis, MN, USA.
Eileen Denning, Division of Biostatistics, School of Public Health, University of Minnesota; Minneapolis, MN, USA.
B. Taylor Thompson, Division of Pulmonary and Critical Care, Department of Medicine, Massachusetts General Hospital and Harvard Medical School; Boston, MA, USA.
Annetine C. Gelijns, Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai; New York, NY, USA.
Virginia Kan, Infectious Diseases Section, VA Medical Center, Washington, DC, USA.
Victoria J. Davey, United States Department of Veterans Affairs; Washington, DC, USA.
Jens D. Lundgren, CHIP Center of Excellence for Health, Immunity, and Infections, Rigshospitalet; Copenhagen University Hospital; Copenhagen, Denmark.
Abdel G. Babiker, The Medical Research Council Clinical Trials Unit at UCL, University College London; London, United Kingdom.
James D. Neaton, Division of Biostatistics, School of Public Health, University of Minnesota; Minneapolis, MN, USA.
H. Clifford Lane, National Institute of Allergy and Infectious Diseases; Bethesda, MD, USA.
DATA SHARING STATEMENT
Deidentified data and supporting documentation, including the protocol, statistical analysis plan, informed consent document, and data dictionary will be made available to researchers after approval of a proposal for use of the data. Proposals for data use should be submitted by means of the research proposal form on the INSIGHT website (www.insight-trials.org).
REFERENCES
- 1.Peltan ID, Caldwell E, Admon AJ, et al. Characteristics and Outcomes of US Patients Hospitalized With COVID-19. American journal of critical care : an official publication, American Association of Critical-Care Nurses 2022; 31(2): 146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasan SS, Capstick T, Ahmed R, et al. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert review of respiratory medicine 2020; 14(11): 1149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merchant HA, Kow CS, Hasan SS. COVID-19 first anniversary review of cases, hospitalization, and mortality in the UK. Expert review of respiratory medicine 2021; 15(8): 973–8. [DOI] [PubMed] [Google Scholar]
- 4.Said SI. Vasoactive intestinal peptide. Journal of endocrinological investigation 1986; 9(2): 191–200. [DOI] [PubMed] [Google Scholar]
- 5.Temerozo JR, Sacramento CQ, Fintelman-Rodrigues N, et al. VIP plasma levels associate with survival in severe COVID-19 patients, correlating with protective effects in SARS-CoV-2-infected cells. J Leukoc Biol 2022; 111(5): 1107–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youssef JG, Lavin P, Schoenfeld DA, et al. The Use of IV Vasoactive Intestinal Peptide (Aviptadil) in Patients With Critical COVID-19 Respiratory Failure: Results of a 60-Day Randomized Controlled Trial. Critical care medicine 2022; 50(11): 1545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amstutz A, Speich B, Mentré F, et al. Effects of remdesivir in patients hospitalised with COVID-19: a systematic review and individual patient data metaanalysis of randomised controlled trials. Lancet Respir Med 2023, 10.1016/S2213-2600(22)00528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaVange L, Adam SJ, Currier JS, et al. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV): Designing Master Protocols for Evaluation of Candidate COVID-19 Therapeutics. Ann Intern Med 2021; 174(9): 1293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchman TG, Draghia-Akli R, Adam SJ, et al. Accelerating Coronavirus Disease 2019 Therapeutic Interventions and Vaccines-Selecting Compounds for Clinical Evaluation in Coronavirus Disease 2019 Clinical Trials. Critical care medicine 2021; 49(11): 1963–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthay MA, Thompson BT, Ware LB. The Berlin definition of acute respiratory distress syndrome: should patients receiving high-flow nasal oxygen be included? Lancet Respir Med 2021; 9(8): 933–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown SM, Peltan ID, Barkauskas C, et al. What Does Acute Respiratory Distress Syndrome Mean during the COVID-19 Pandemic? Ann Am Thorac Soc 2021; 18(12): 1948–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. 2022. https://www.covid19treatmentguidelines.nih.gov/ (accessed 5/25/2022. [PubMed] [Google Scholar]
- 13.McCullagh P.Regression models for ordinal data. Journal of the Royal Statistical Society: Series B (Methodological) 1980; 42(2): 109–27. [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94(446): 496–509. [Google Scholar]
- 15.Zhou B, Latouche A, Rocha V, Fine J. Competing risks regression for stratified data. Biometrics 2011; 67(2): 661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aalen OO, Johansen S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scandinavian Journal of Statistics 1978: 141–50. [Google Scholar]
- 17.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. The Annals of statistics 1988: 1141–54. [Google Scholar]
- 18.Group RC, Horby PW, Mafham M, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, openlabel, platform trial. medRxiv 2021: 2021.06.15.21258542. [Google Scholar]
- 19.Investigators RECOVERY. Dexamethasone in Hospitalized Patients with Covid-19. New England Journal of Medicine 2020; 384(8): 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ACTIV-3–Therapeutics for Inpatients with COVID-19 (TICO) Study Group. Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial. Lancet Respir Med 2022; 10(10): 972–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kane MG, O’Dorisio TM, Krejs GJ. Production of secretory diarrhea by intravenous infusion of vasoactive intestinal polypeptide. N Engl J Med 1983; 309(24): 1482–5. [DOI] [PubMed] [Google Scholar]
- 22.Virgolini I, Raderer M, Kurtaran A, et al. Vasoactive intestinal peptide-receptor imaging for the localization of intestinal adenocarcinomas and endocrine tumors. N Engl J Med 1994; 331(17): 1116–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data and supporting documentation, including the protocol, statistical analysis plan, informed consent document, and data dictionary will be made available to researchers after approval of a proposal for use of the data. Proposals for data use should be submitted by means of the research proposal form on the INSIGHT website (www.insight-trials.org).