Abstract
Background:
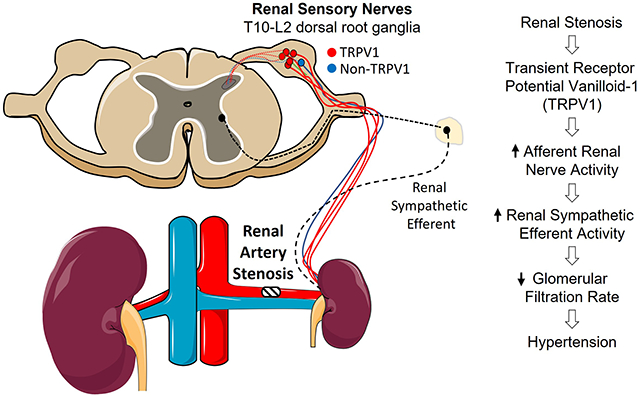
Renal denervation lowers arterial blood pressure (ABP) in both clinical populations and multiple experimental models of hypertension. This therapeutic effect is partly attributed to the removal of overactive renal sensory nerves. The transient receptor potential vanilloid 1 (TRPV1) channel is highly expressed in renal sensory nerves and detects changes in noxious and mechanosensitive stimuli, pH, and chemokines. However, the extent to which TRPV1 channels contribute to 2-kidney-1-clip (2K1C) renovascular hypertension has not been tested.
Methods:
We generated a novel Trpv1−/− rat using CRISPR/Cas9 and 26-bp deletion in exon 3 and induced 2K1C hypertension.
Results:
The majority (85%) of rat renal sensory neurons retrogradely labelled from the kidney were TRPV1-positive. Trpv1−/− rats lacked TRPV1 immunofluorescence in the dorsal root ganglia, had a delayed tail-flick response to hot but not cold water, and lacked an afferent renal nerve activity response to intrarenal infusion of the TRPV1 agonist capsaicin. Interestingly, 2K1C hypertension was significantly attenuated in male Trpv1−/− versus wild-type rats. 2K1C hypertension significantly increased the depressor response to ganglionic blockade, total renal nerve activity (efferent and afferent) and afferent renal nerve activity in wild-type rats, but these responses were attenuated in male Trpv1−/− rats. 2K1C hypertension was attenuated in female rats with no differences between female strains. Finally, glomerular filtration rate was reduced by 2K1C in wild-type rats but improved in Trpv1−/− rats.
Conclusion:
These findings suggest that renovascular hypertension requires activation of the TRPV1 channel to elevate renal afferent and sympathetic nerve activity, reduce glomerular filtration rate, and increase ABP.
Keywords: autonomic nervous system, blood pressure, hypertension, glomerular filtration rate, dorsal root ganglion, kidney
Graphical Abstract
INTRODUCTION
Total renal denervation lowers arterial blood pressure (ABP) in multiple clinical trials and several experimental models of hypertension 1–3. These anti-hypertensive effects are partly attributed to removal of renal sensory nerves. Renal sensory nerves innervate the pelvic wall, tubules, glomeruli and vasculature 4,5 and respond to changes in renal ischemia, increased pelvic pressure, and various chemokines 4–7. In turn, these fibers project centrally to cell bodies located in the lower thoracic and upper lumber dorsal root ganglia (DRG) to regulate sympathetic nerve activity (SNA) and ABP 8–10. Electrical stimulation of renal afferent nerves increases sympathetic nerve activity (SNA) and ABP 8,11–13. Renal afferent discharge or activity is elevated in deoxycorticosterone-salt rats 14 and renovascular 2-kidney-1-clip (2K1C) mice 8. Dorsal rhizotomy 15 or chemical ablation of renal sensory fibers 8,14,16,17 reduces ABP in these hypertension models. Importantly, selective ablation of renal sensory nerves reduces ABP to the same extent as total renal denervation in deoxycorticosterone-salt rats 14 and 2K1C mice 8.
TRPV1 is a nonselective cation channel that responds to noxious and mechanical stimuli, pH, and chemokines 18,19. Intrarenal artery or renal pelvic infusion of the TRPV1 agonist capsaicin increases afferent renal nerve activity (ARNA), SNA, and ABP 14,20,21. Much higher concentrations of TRPV1 agonists applied periaxonally are used to chemically ablate renal sensory nerves 8,14,22. Although this technique destroys TRPV1-expressing neurons, the approach does not assess the contribution of the TRPV1 channel. Therefore, the purpose of this study was to define the proportion of renal sensory neurons that express TRPV1 in the rat and the extent to which the TRPV1 channel is required for the elevated ARNA, total renal nerve activity (efferent and afferent), and ABP in a renal nerve dependent model of hypertension. To test this hypothesis, we generated a novel Trpv1−/− rat using CRISPR/Cas9 to produce 26-bp deletion in exon 3 and subsequently induced 2K1C renovascular hypertension.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. A detailed methods section is available in the online supplement.
Animals.
All of the experimental procedures conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh School of Medicine. Rats were housed in a temperature-controlled room (22±1ºC) with a 12-hour light-dark cycle (lights off 10AM-10PM). Experiments utilized both male and female Sprague-Dawley rats (8-10 weeks of age, Charles River Laboratories) or novel Trpv1−/− rats (SD-Trpv1em4Mcwi) and wild-type littermates (see Experiment 2). 2K1C hypertension: The right renal artery was isolated from the renal vein and nerve. A 0.5mm length of polytetrafluoroethylene catheter (0.016 x 0.022 in, Braintree Scientific SUBL-220) was cut longitudinally. The renal artery was placed inside the tubing through the longitudinal cut. Three 10-0 circumferential sutures were wrapped around the tubing to restore the original diameter (Figure S1). At the end of all experiments, animals were euthanized, and the left and right kidney mass was measured. In isoflurane-anesthetized Sprague-Dawley rats, 2K1C surgery decreased renal blood flow within 5 min in male and female rats by 39±4% (3.9±0.2 to 2.4±0.2 mL/min, P<0.01) and 32±3% (3.1±0.2 to 2.1±0.2 mL/min, P<0.01), respectively. The reduction in renal blood flow was not statistically different between male versus female rats (Figure S2).
Experiment 1: Quantification of TRPV1 Neurons in the Rat Renal Sensory Neurons
Male and female Sprague-Dawley rats with sham or 2K1C surgery received an injection of wheat germ agglutinin tagged with AlexaFluor 647 (WGA-647, 5%; ThermoFisher) or Cholera Toxin subunit B tagged with AlexaFluor647 (CTB-647, 2mg/mL; ThermoFisher) into the right kidney using a glass micropipette and coordinates relative to the renal hilum: 1.0-1.5 mm lateral and 1.5 mm ventral to the surface. Three injections (1uL each) were performed separated by 1.0 mm toward each pole using the same depth. At 5-7 days later, animals were perfused transcardially. The T7-L2 dorsal root ganglia ipsilateral to the injection were harvested, sectioned, and processed for TRPV1 and IB4 immunofluorescence using an anti-rabbit TRPV1 antibody 23 or Isolectin GS-IB4 binding 24.
Experiment 2: Construction and Validation of Trpv1−/− Rats
Trpv1−/− Sprague Dawley rats were generated at the Medical College of Wisconsin (Dr. Aron Geurts) by pronuclear injection of a CRISPR-Cas9 ribonucleoprotein targeting the sequence CTGCGATCATAGAGCCTCGG into one cell Crl:SD (Charles River Laboratories) rat embryos as described previously 25–27. A mutant strain (SD-Trpv1em4Mcwi) harboring a 26-bp frame-shift deletion in exon 3 (chr10:57,856,337-57,856,362 (rn7)) was established. The line is maintained by heterozygous Trpv1+/− breeding to produce wild-type and Trpv1−/− littermates for experiments. To validate Trpv1−/− rats, a series of both behavioral and functional tests were performed using both wild-type and Trpv1−/− littermates. Eye-Wipe Test: The number of eye wipes were quantified after a drop of the TRPV1 agonist capsaicin (100uM) or saline was topically applied to the left or right eye, respectively. Tail-Flick Test: the latency to a tail flick was quantified in response to 3 inches of the tail immersed in 50°C or 0°C water. Deletion of Trpv1 in mice disrupts responses to heat but not cold 28. TRPV1 immunofluorescence of DRG Neurons: The T8-L2 DRGs of these animals (n=4 per genotype) were harvested and processed for TRPV1 and IB4 immunofluorescence as described in Experiment 1. ARNA Responses to Intrarenal Capsaicin and Bradykinin. A separate set of wild-type and Trpv1−/− littermates (n=6 per genotype, 3M:3F) were anesthetized with Inactin and prepared for ARNA recordings from the right kidney. ARNA responses were tested to intrarenal artery infusion of the TRPV1 agonist capsaicin (0, 0.1, 1, and 10uM, 50uL) or bradykinin (0.1, 1, and 10uM, 50uL) at a rate of 0.01 mL/s via a heat-stretched catheter inserted into the adrenal artery and advanced to the junction of the renal artery as described previously 20,21,29.
Experiment 3: Renovascular Hypertension in Wild-Type and Trpv1−/− Rats
Male and female WT and Trpv1−/− littermates with PA-C10 telemetry units (DSI) received 2K1C surgery as described above. Telemetry data were collected for 3 days before and 28 days after 2K1C surgery and analyzed for beat to beat for systolic and diastolic ABP. Ganglionic blockade with hexamethonum (30mg/kg, ip) was performed between 1-3 pm at Day 0 (before the 2K1C surgery) and Day 28.
Experiment 4: Effect of TRPV1 Deletion on Total Renal SNA, ARNA, and Glomerular Filtration Rate in 2K1C Hypertension.
Male and female wild-type and Trpv1−/− littermates received sham or 2K1C surgery. At 21-28 days later, rats were anesthetized with Inactin as described in Experiment 2. Both kidneys were catheterized to collect urine and measure glomerular filtration rate using a continuous infusion of FITC-sinistrin (2mg/mL at 2mL/h, MediBeacon). Then, the renal nerve was isolated to record total renal SNA (efferent and afferent activity) and ARNA (see online supplement for detailed methods).
Statistical Analysis.
Data are presented as mean±SEM plus individual data points when possible. Data were analyzed using two or three-way ANOVAs (time, sham vs 2K1C surgery, male vs female) with repeated measures (Systat 10.2). When significant F values were obtained, a layered Bonferroni paired or independent t tests were performed to identify differences. P<0.05 was statistically significant for all comparisons. Group sizes are noted in the text and figure legends.
RESULTS
Experiment 1: Quantification of TRPV1 Neurons in Rat Renal Sensory Neurons
An initial set of experiments defined the number of renal sensory neurons that expressed TRPV1 immunofluorescence in both control and 2K1C rats. Figure 1A illustrates the greatest number of renal sensory neurons labelled from the right kidney were located in the T12 through L1 DRG. These numbers did not differ between male versus female rats nor between sham and 2K1C rats. The majority of renal sensory neurons were TRPV1-positive as revealed by immunofluorescence (Figure 1B, control: 84±4% vs 2K1C: 85±7%). On the other hand, a very small proportion were IB4+ (control: 5±1% vs 2K1C: 10±7%) or TRPV1 plus IB4+ (control: 4±1% vs 2K1C: 7±4%). Figure 1C-G illustrates an example of a rat DRG with retrogradely labelled cells from the kidney, TRPV1 immunofluorescence and IB4 labeling. In this example, the majority of retrogradely-labelled neurons from the kidney were TRPV1-positive but not IB4-positive. A representative injection site is illustrated in Figure S3. As expected, the renal mass of the clipped or right kidney was significantly lower than sham controls (see Figure S4).
Figure 1. Quantification of Rat Renal Sensory Neurons Expressing Transient Receptor Potential Vanilliod 1 (TRPV1).
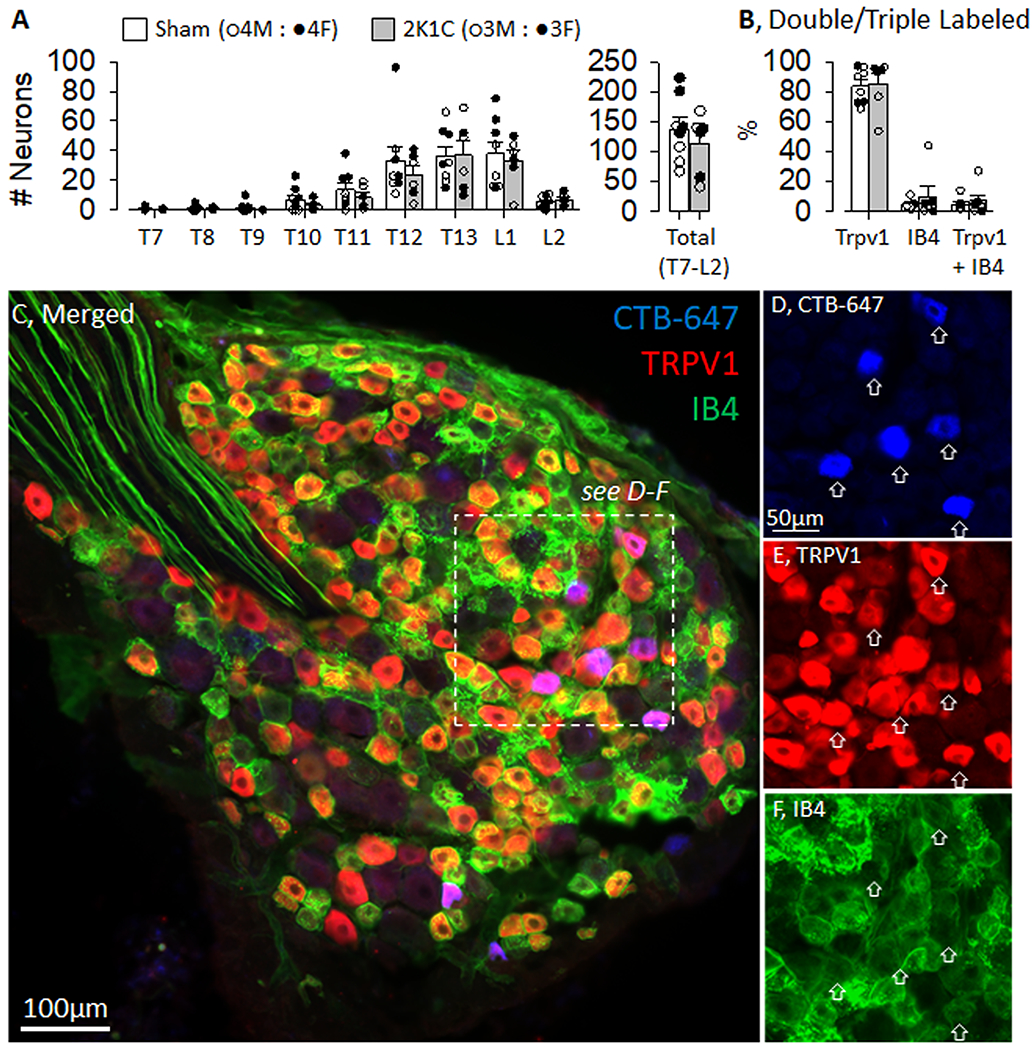
A, Number of retrogradely labelled neurons in the dorsal root ganglion at each thoracic and lumbar spinal segment in both control and 2-kidney-1-clip (2K1C) rats. B, The percentage of retrogradely labelled neurons that were Trpv1, IB4, or Trpv1 plus IB4 positive. C, Low power image of rat dorsal root ganglia processed for Cholera Toxin Subunit B (CTB-647, blue), TRPV1 (red), and IB4 (green). D-F, Inset images of CTB-647 (blue), Trpv1 (red), and IB4 (green). There were no differences in the number of neurons and proportion expressing Trpv1 versus IB4 between male vs female or sham versus 2K1C.
Experiment 2: Validation of Trpv1−/− Rat
Since the above findings indicate a majority of renal sensory neurons are TRPV1-positive, we developed a novel Trpv1−/− rat by a CRISPR-Cas9-mediated 26-bp frame-shift deletion in exon 3. The 26-bp deletion can be resolved using PCR and a 4% gel (see Figure S5). TRPV1 immunofluorescence was abundant in the T8-L2 DRG of wild-type rats but absent in Trpv1−/− rats (Figure 2A). Topical application of capsaicin to the eye evoked immediate and repetitive eye-wipes in wild-type rats, but this response was absent in Trpv1−/− rats (Figure 2B). Tail immersion in 50°C water evoked a tail-flick response in both genotypes, but the onset latency was significantly delayed in Trpv1−/− versus wild-type rats (Figure 2C). The tail-flick latency to 0°C water was not different between groups (Figure 2C). Tail immersion in room temperature water (22°C) did not evoke a response in the 120s testing period in either group (wild-type: 120±0 vs Trpv1−/−: 117±3 s). In a final set of experiments, ARNA responses to intrarenal artery infusion of the TRPV1 agonist capsaicin or bradykinin were tested. As expected, intrarenal artery infusion of capsaicin produced concentration-dependent increases in ARNA discharge and rectified/integrated ARNA of wild-type rats (Figure 2D). However, these responses were abolished in Trpv1−/− rats (Figure 2D). In fact, ARNA responses to capsaicin in Trpv1−/− rats were not different than those responses to infusion of saline. On the other hand, intrarenal infusion of bradykinin produced concentration-dependent increases in ARNA discharge and rectified/integrated ARNA in both wild-type and Trpv1−/− rats (Figure 2D). Responses to bradykinin did not differ between wild-type and Trpv1−/− rats. Area under the curve analysis of ARNA produced similar results (Figure S6). There were no differences between wild-type and Trpv1−/− rats in baseline mean ABP (115±4 vs 110±4 mmHg), heart rate (366±8 vs 348±18 bpm), ARNA discharge (24±8 vs 19±8 Hz) or ARNA voltage (0.18±0.04 vs 0.21±0.07 µV).
Figure 2. Validation of Transient Receptor Potential Vanilloid 1 Knockout (Trpv1−/−) Rats.
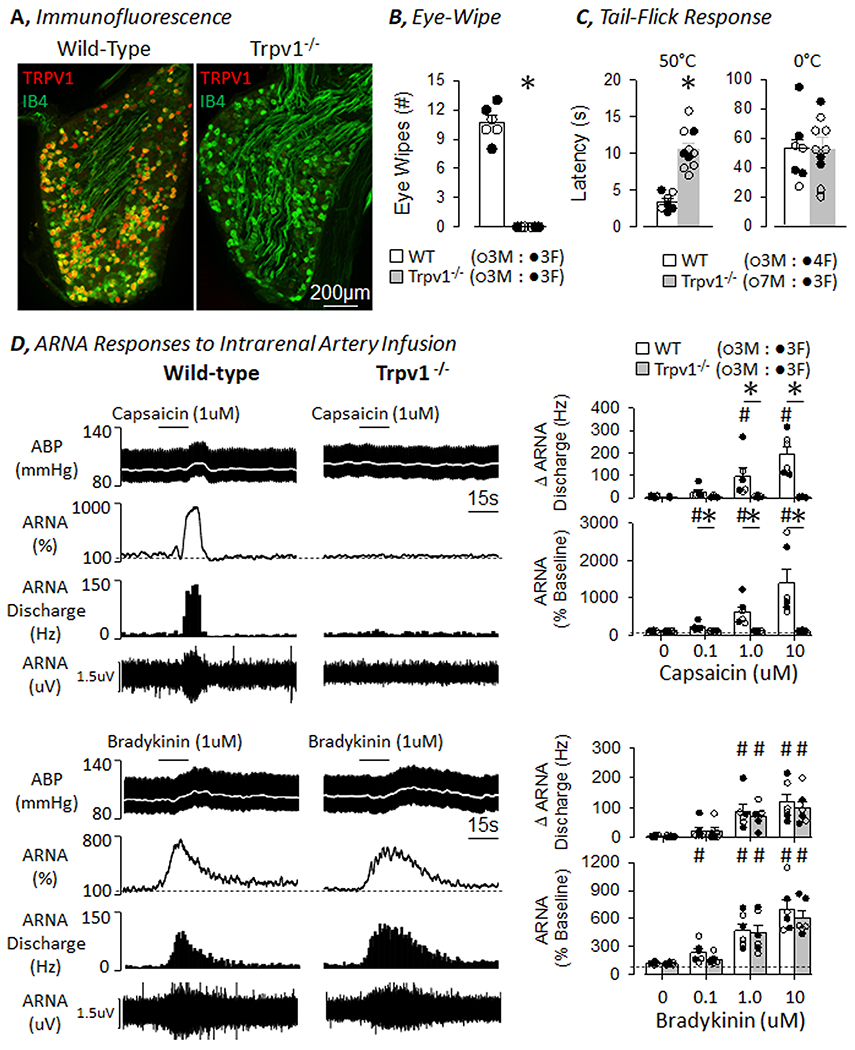
A, TRPV1 (RED) and IB4 (green) immunofluorescence of the DRG in wild-type and Trpv1−/− rat. Note, the absence of TRPV1 signal in the DRG of the Trpv1−/− rat. B, Number of eye-wipes to one drop of capsaicin (100uM) was absent in Trpv1−/− versus wild-type rats. C, Tail-flick latency was significantly delayed to hot (50°C) but not cold (0°C) water in Trpv1−/− versus wild-type rats. D, (TOP) Intrarenal artery infusion of capsaicin increased fferent renal nerve activity (ARNA) in wild-type but not Trpv1−/− rats. (BOTTOM) Intrarenal artery infusion of bradykinin increased ARNA in both wild-type and Trpv1−/− rats. Summary data presented as mean±SEM and individual data points are presented on the right. *P<0.05 Wild-type versus Trpv1−/− ; #P<0.05 vs saline or 0µM
Experiment 3: Hemodynamic Responses to 2K1C Hypertension in Wild-Type and Trpv1−/− Rats
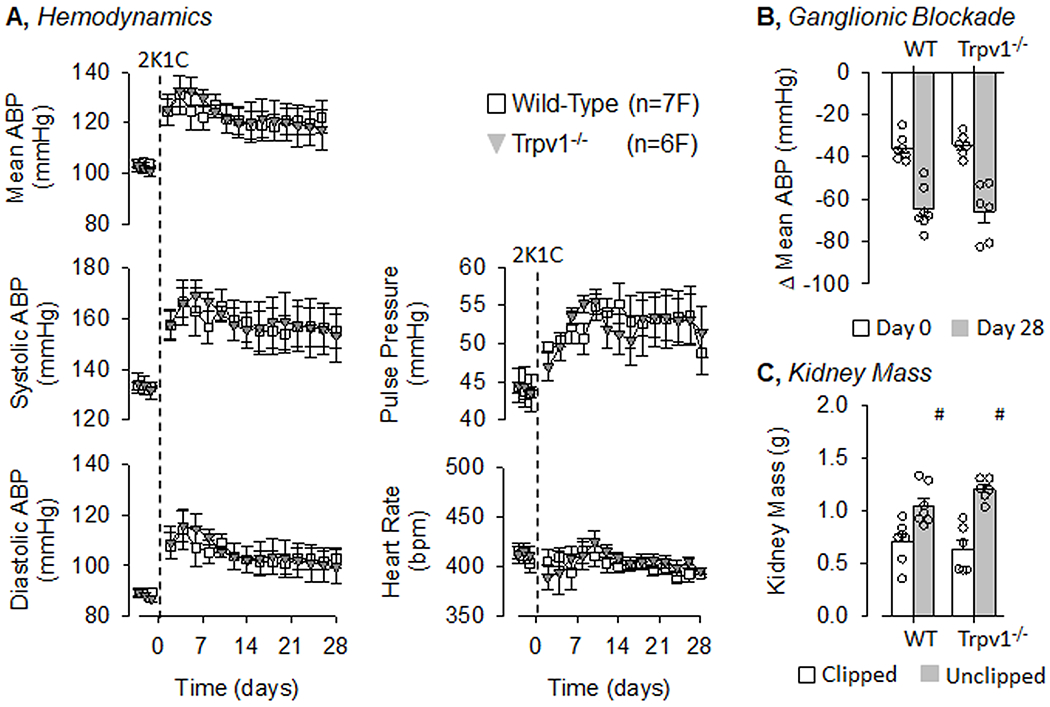
To test the extent by which TRPV1 channels contribute to renovascular hypertension, 2K1C was produced in both male and female wild-type and Trpv1−/− littermate rats. Since there was a significant sex difference in the hemodynamic profile of wild-type rats, male and female data are presented separately. Figure 3 illustrates summary data for male wild-type and Trpv1−/− rats that underwent 2K1C surgery on Day 0. There were no differences in baseline mean, systolic, or diastolic ABP as well as pulse pressure or heart rate between male wild-type and Trpv1−/− rats. 2K1C surgery significantly increased ABP in both groups at Day 2, and ABP remained significantly elevated through the remainder of the 28 days. However, Trpv1−/− rats had a significantly lower mean, systolic, and diastolic ABP than wild-type rats at Day 16 thru Day 28. 2K1C surgery significantly increased pulse pressure in both groups from Day 2 thru Day 28. Heart rate also increased in both groups from Day 8 thru Day 16. There were no differences in pulse pressure or heart rate between wild-type and Trpv1−/− rats. Depressor responses to ganglionic blockade on Day 0 were not statistically different between wild-type and Trpv1−/− rats (Figure 3B). The depressor response was larger in each genotype at Day 28 versus Day 0. However, the response was significantly attenuated in male Trpv1−/− versus wild-type rats. As expected, the clipped kidneys were significantly smaller than unclipped kidneys in both wild-type and Trpv1−/− rats (Figure 3C), but these values did not differ across genotypes. There were no differences in body weights between male wild-type and Trpv1−/− rats on Day 28 (377±20 vs 384±18g, respectively).
Figure 3. Hemodynamic responses to 2-kidney-1-Clip (2K1C) Hypertension in Wild-Type and Transient Receptor Potential Vanilloid 1 Knockout (Trpv1−/−) Male Rats.
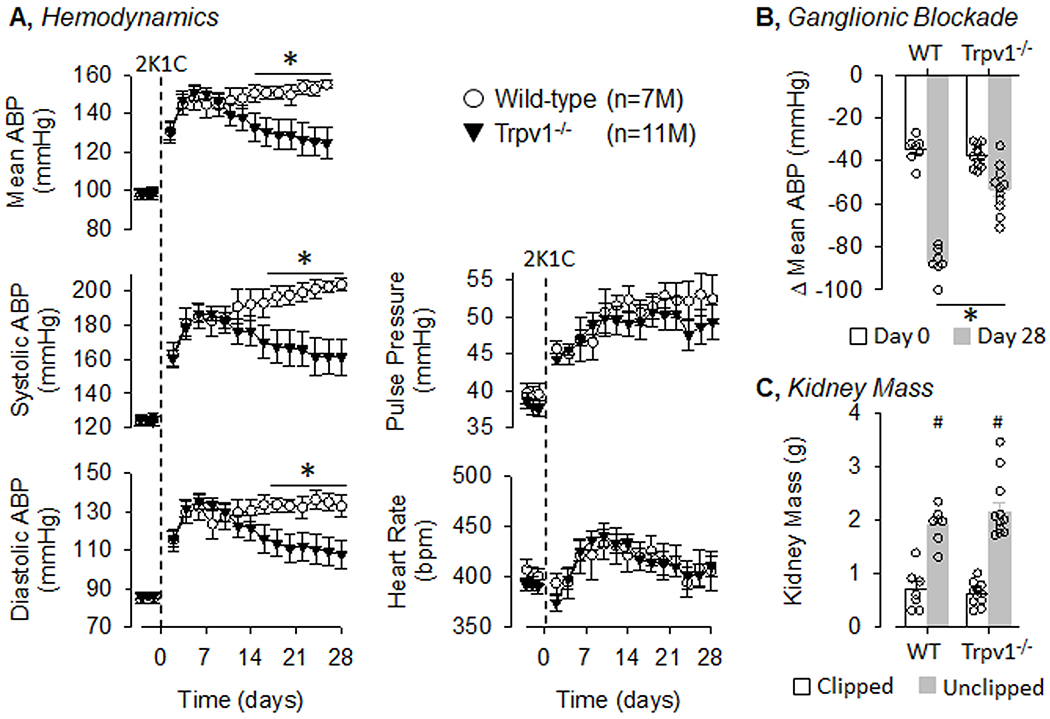
A, Mean±SEM of mean, systolic, and diastolic ABP as well as pulse pressure and heart rate in male wild-type and Trpv1−/− rats. Values were averaged between day and night periods. B, Mean±SEM and individual data points of the depressor response to ganglionic blockade with hexamethonium (30mg/kg, ip) on Day 0 and 28. C, Mean±SEM and individual data points of kidney mass for both clipped and unclipped kidneys in wild-type and Trpv1−/− rats. *P<0.05 wild-type vs Trpv1−/−, #P<0.05 unclipped vs clipped.
Figure 4 summarizes 24-h hemodynamic data for 2K1C hypertension in female rats. Similar to male rats, there were no significant differences in baseline ABP, pulse pressure, or heart rate between female genotypes. 2K1C surgery significantly increased mean, systolic, and diastolic ABP in both female wild-type and Trpv1−/− rats from Day 2 to Day 28. However, there were no statistical differences between female wild-type and Trpv1−/− rats at any time. 2K1C surgery increase pulse pressure but did not produce a statistically significant change in heart rate. Depressor responses of female rates to ganglionic blockade were not different between genotypes at Day 0 (Figure 4B). 2K1C surgery increased the magnitude of the depressor response on Day 28 but there were no differences between genotypes. As expected, the clipped kidneys were significantly smaller than unclipped kidneys in both female wild-type and Trpv1−/− rats (Figure 4C), but these values did not differ across genotypes. There were no differences in body weights between female wild-type and Trpv1−/− rats on Day 28 (280±10 vs 289±10g, respectively).
Figure 4. Hemodynamic Responses to 2-Kidney-1-Clip (2K1C) Hypertension in Wild-Type and Transient Receptor Potential Vanilloid 1 Knockout (Trpv1−/−) Female Rats.
A, Mean±SEM of mean, systolic, and diastolic ABP as well as pulse pressure and heart rate in female wild-type and Trpv1−/− rats. Values were averaged between day and night periods. B, Mean±SEM and individual data points of the depressor response to ganglionic blockade with hexamethonium (30mg/kg, ip) on Day 0 and 28. C, Mean±SEM and individual data points of kidney mass for both clipped and unclipped kidneys in wild-type and Trpv1−/− rats. Note, there were no statistical differences between genotypes of female rats for any reported variable.
It is noteworthy that the mean, systolic, and diastolic ABP was higher in male versus female wild-type rats from day 2 thru day 28 (P<0.05, Figures 3A and 4A). This higher level of ABP were associated with a greater depressor response to ganglionic blockade between male versus female wild-type rats (P<0.05, Figures 3B and 4B). There was not a statistical difference between male versus female Trpv1−/− rats.
Experiment 4: Total Renal SNA, ARNA, and GFR in 2K1C Hypertension of Wild-Type and Trpv1−/− rats
A separate set of male and female wild-type and Trpv1−/− rats underwent sham or 2K1C surgery to directly assess total renal SNA (efferent and afferent activity), ARNA and GFR between days 21-28. The differences in ABP of male wild-type and Trpv1−/− rats with 2K1C surgery measured via telemetry (Figure 3) were maintained using Inactin anesthesia (Figure 5). There were no differences in mean ABP between sham wild-type and Trpv1−/− rats. As expected, mean ABP was significantly elevated after 2K1C surgery, but mean ABP was significantly lower in male 2K1C Trpv1−/− versus wild-type rats. Total renal SNA and ARNA were recorded to the right or stenotic kidney. In male rats, nerve recordings were obtained in every sham animal and the majority of 2K1C rats (wild-type: 12/16 and Trpv1−/−: 9/10). Figure 5 illustrates no differences in total renal SNA between male wild-type and Trpv1−/− rats in the sham group. However, total renal SNA was significantly elevated in the male 2K1C wild-type but not Trpv1−/− rats. After severing the renal nerve between the electrode and spinal cord, ARNA was isolated and quantified. There were no differences in ARNA voltage or ARNA discharge between male wild-type and Trpv1−/− rats in the sham group. However, both ARNA voltage and ARNA discharge was significantly higher in male 2K1C wild-type but not Trpv1−/− rats. In fact, renal SNA or ARNA of 2K1C Trpv1−/− rats was not different from sham Trpv1−/− rats.
Figure 5. Mean Arterial Blood Pressure (ABP), Renal Sympathetic Nerve Activity (SNA) and Afferent Renal Nerve Activity (ARNA) in Male Wild-Type and Transient Receptor Potential Vanilloid 1 Knockout (Trpv1−/−) Rats.
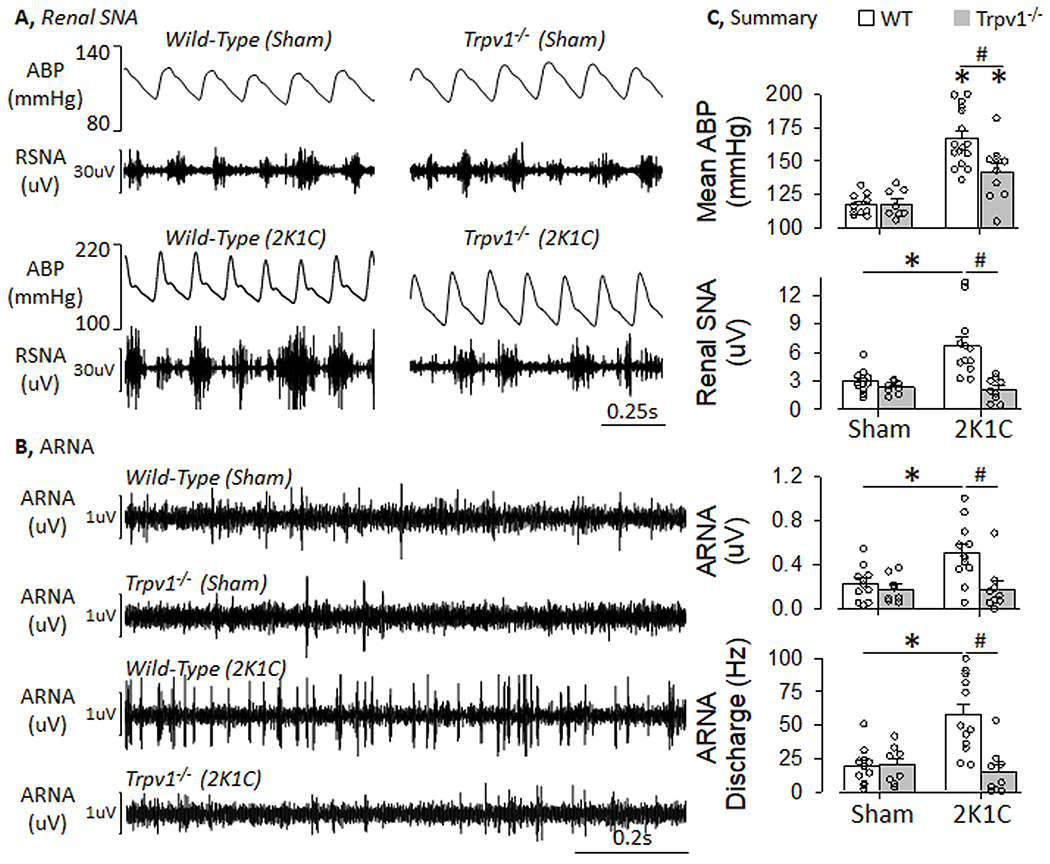
A, Examples (1 s) of arterial blood pressure (ABP) and RSNA for male wild-type and Trpv1−/− rats between Day 21-28 after sham or 2-kidney-1-clip (2K1C) surgery. B, Example (1s) of ARNA for male wild-type and Trpv1−/− rats between Day 21–28 after sham or 2K1C surgery. RSNA and ARNA were recorded from the right or clipped kidney. C, Mean±SEM and individual data points of mean ABP, renal SNA, rectified/integrated ARNA, and ARNA discharge. Summary values were derived from 15-min averages for each variable. Summary data for female rats is illustrated in Figure S3. *P<0.05 sham vs 2K1C wild-type rats, #P<0.05 wild-type vs Trpv1−/− rats with 2K1C
In female rats, viable nerve recordings were obtained in the majority of animals (sham wild-type: 9/10; sham Trpv1−/−: 9/9; 2K1C wild-type: 5/6; 2K1C Trpv1−/−: 5/5). Similar to male rats, there were no differences in total renal SNA of female wild-type and Trpv1−/− rats in the sham group (Figure S7). Total renal SNA was significantly higher in female 2K1C wild-type but not Trpv1−/− rats. It is noteworthy that the increase in total renal SNA of wild-type rats after 2K1C surgery was significantly greater in males versus females (P<0.05). Although there were no differences in ARNA voltage in female wild-type and Trpv1−/− rats in the sham group, 2K1C surgery significantly increased ARNA voltage and tended to increase ARNA discharge (P=0.07) in wild-type rats but not Trpv1−/− rats.
GFR was measured in the same animals presented in Figure 5 but before SNA recordings. Total GFR (both left and right kidney) was not different between male wild-type and Trpv1−/− rats of the sham group. Total GFR was significantly lower after 2K1C surgery in male wild-type rats. Interestingly, total GFR of 2K1C Trpv1−/− rats was improved and significantly higher than wild-type rats. In fact, there was not a statistically significant difference between male sham and 2K1C Trpv1−/− rats. Figure 6 also illustrates both GFR and kidney mass of the left and right kidney of sham and 2K1C rats. Although 2K1C surgery significantly decreased GFR in the right kidney of both wild-type and Trpv1−/− rats, GFR increased only in the left kidney of 2K1C Trpv1−/− rats. Only 12% (2/17) of the right kidneys in 2K1C wild-type rats produced urine whereas 30% (3/10) of the right kidneys in 2K1C Trpv1−/− rats did. As expected, 2K1C surgery increased left kidney mass but decreased right kidney mass. There were no differences in kidney mass between strains in the same surgery group.
Figure 6. Glomerular Filtration Rate (GFR) of Male Wild-Type and Transient Receptor Potential Vanilloid 1 Knockout (Trpv1−/−) Rats.
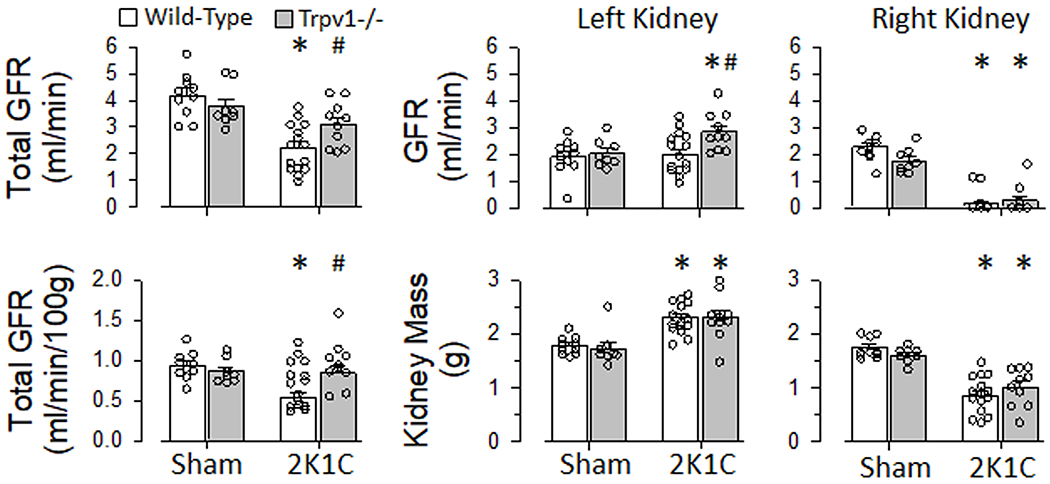
Total GFR, total GFR normalized to 100 g body weight, left and right kidney GFR, and left and right kidney mass of male wild-type and Trpv1−/− rats with sham or 2-kidney-1-clip (2K1C) surgery. *P<0.05 sham vs 2K1C; #P<0.05 wild-type vs Trpv1−/−
In females rats, the differences in ABP measured via telemetry between sham and 2K1C groups was maintained using Inactin anesthesia (Figure S7). There were no differences between strains in the same surgery group. Total GFR was not different between female wild-type and Trpv1−/− rats in the sham group but total GFR was significantly lower in females versus males (Figure 6 vs S6). 2K1C surgery significantly lowered total GFR in female wild-type rats but not female Trpv1−/− rats.
DISCUSSION
The study provides several key observations regarding the role of TRPV1 channels in renovascular hypertension. First, the majority of renal sensory neurons (~85%) in control and 2K1C rats were TRPV1-positive. Second, 2K1C hypertension was significantly attenuated in male Trpv1−/− versus wild-type rats. Third, ARNA, total renal SNA (efferent and afferent), and the depressor responses to ganglionic blockade were significantly attenuated in male 2K1C Trpv1−/− versus wild-type rats. Fourth, 2K1C hypertension in female rats, which was reduced compared to male rats, was not impacted by TRPV1 deletion. Finally, GFR was significantly reduced in 2K1C wild-type rats but improved in 2K1C Trpv1−/− rats. These findings suggest that renovascular hypertension requires activation of the TRPV1 channel to elevate ARNA and renal SNA, reduce GFR, and increase ABP.
High concentrations of TRPV1 agonists are used to chemically ablate sensory fibers 8,14,22. The validity of the approach assumes the majority of sensory neurons express the TRPV1 channel. We found that approximately 85% of rat DRG neurons labelled from the kidney were TRPV1-positive but IB4-negative as assessed by immunofluorescence. Importantly, the total number of retrogradely labelled neurons and proportion of TRPV1-positive neurons did not differ between sham and 2K1C rats. We readily acknowledge this approach is qualitative and cannot account for differences in mRNA expression or protein levels between sham and 2K1C groups. The kidney injections were targeted to the renal pelvic and adjacent to the renal hilus as preliminary experiments with injections in the renal cortex produced sparse labeling in the dorsal root ganglion. Therefore, our injections may have missed renal sensory fibers innervating the renal cortex. However, our findings are consistent with in vitro electrophysiological recordings show that capsaicin evoked an inward current response in approximately 70% of rat renal DRG neurons 30. It is noteworthy that unpublished observations in our laboratory suggest that a lower proportion of renal sensory neurons in the mouse are TRPV1-positive (~50-60%). The proportion of TRPV1 neurons in control or disease animals and across species may have important implications to interpret studies using chemical ablation techniques.
To test whether TRPV1 channel contributes to 2K1C hypertension, we generated a novel Trpv1−/− rat with a 26-bp deletion in exon 3. These animals lack TRPV1 immunofluorescence in the DRG and functional responses to capsaicin (eye wipes and ARNA) but displayed normal ARNA responses to intrarenal infusion of bradykinin. Moreover, Trpv1−/− rats had a delayed tail-flick latency to hot (50°C) but not cold (0°C) water – deletion of TRPV1 in mice disrupts responses to hot but not cold 28. Relative to wild-type littermates, Trpv1−/− rats had a similar baseline ABP, heart rate, depressor response to ganglionic blockade, ARNA, and total renal SNA (efferent and afferent), and GFR. Although 2K1C hypertension was not different between male wild-type and Trpv1−/− rats over the initial 14 days, ABP of Trpv1−/− rats was significantly lower than those of wild-type rats at day 16-28. The early phase (week 1) of 2K1C hypertension is mediated by a robust activation of the renin-angiotensin system 31,32, whereas the chronic phase (week 3-6) is mediated in part by a sympathetically-mediated increase in total peripheral resistance 8,33,34. Sympathoexcitation in the 2K1C model is partly driven by activation of renal afferent or sensory nerves. Chemical ablation of renal sensory fibers or dorsal rhizotomy lowers SNA and ABP in male 2K1C rodents 8,15–17. ARNA voltage and ARNA discharge is elevated in male 2K1C mice 8 and confirmed in male 2K1C wild-type rats of the present study. Interestingly, ARNA was significantly lower in male 2K1C Trpv1−/− versus wild-type rats. Moreover, the depressor response to ganglionic blockade and total renal SNA was significantly attenuated in male 2K1C Trpv1−/− versus wild-type rats. Although total renal SNA reported herein represents the combined renal efferent and afferent signal, subtraction of ARNA from renal SNA to isolate renal efferent sympathetic nerve activity did not change the statistical outcomes. Herein, we directly compared ARNA and total renal SNA between experimental groups but did not normalize values to a stimulus. Normalization to a stimulus assumes the circuitry mediating such a response has not changed between healthy and disease animals. On the other hand, the ability to detect differences in nerve voltage will depend on the variability of the recordings (due to number of axons, electrode configurations, and nerve dissection), magnitude of the effect, and sample size. In the present study, renal SNA of 2K1C rats was 2-3 fold greater than sham controls. Therefore, these data are consistent with the hypothesis that 2K1C hypertension requires TRPV1 to elevate ARNA, renal SNA, and ABP.
The current study revealed a clear sex difference in the hemodynamic responses to 2K1C surgery. In addition, there were no differences between female 2K1C wild-type and Trpv1−/− rats. Renal SNA and the depressor response to ganglionic blockade were greater in male versus female 2K1C wild-type rats. We have previously reported ARNA responses to intrarenal infusion of capsaicin, increased pelvic pressure, or renal artery occlusion did not differ between male versus female rats 20. These observations suggest that the attenuated 2K1C hypertension in female rats may be attributed to sex differences in central sympathetic networks. Indeed, many experimental models of neurogenic hypertension exhibit a sex-dependency and attenuated hypertension in female rodents 35. Finally, the majority of studies testing the contribution of renal sensory nerves to hypertension have exclusively used male rodents. Therefore, 2K1C hypertension in female rats may not require TRPV1 activation because the hypertension does not depend on renal sensory nerves.
An interesting observation in the current study was the deletion of TRPV1 improved total GFR in both male and female 2K1C rats. 2K1C surgery reduced total GFR in male and female wild-type rats, and this was largely attributed to the reduction in GFR of the stenotic kidney. Interestingly, GFR to the contralateral or left kidney increased in male 2K1C Trpv1−/− rats – the same tendency was also present in females but did not reach statistical significance. Multiple mechanisms may explain the improved GFR of 2K1C Trpv1−/− rats including differences in the renal renin-angiotensin system, tuberoglomerular feedback, and renal SNA. In regard to the latter, total renal SNA was significantly attenuated in male 2K1C Trpv1−/− versus wild-type rats. Although these recordings were performed to the stenotic and not contralateral kidney, renal SNA to the contralateral or unclipped kidney is elevated in 2K1C hypertension 16,17,36. Stimulation of renal sympathetic nerves produces frequency-dependent changes in vascular resistance and glomerular filtration rate 37. Therefore, we speculate that the improved GFR of the contralateral kidney in 2K1C Trpv1−/− rats is attributed to a lower renal SNA to the contralateral kidney.
A limitation of the present study is the use of a total body knockout of the TRPV1 channel. Therefore, these findings cannot be directly attributed to the expression of TRPV1 on renal sensory nerves versus other sensory nerves or cells. Future studies need to directly delete TRPV1 channels in renal sensory nerves or whether the renal sensory nerves contribute to 2K1C hypertension after deletion of the TRPV1 channel. Neonatal capsaicin treatment 38 or dorsal rhizotomy of the T9-L1 segments 39 produces salt-sensitive hypertension in rats. However, neonatal capsaicin treatment targets sensory neurons throughout the body and ablates neurons that do not express TRPV1 in the adult animal due to the developmental expression of TRPV1 40. Dorsal rhizotomy eliminates sensory input from the kidney but also many other tissues. On the other hand, periaxonal application of capsaicin to the renal nerve does not produce salt-sensitive hypertension 22. The discrepancies across these studies may be attributed to the technique and cell population affected by the treatment. However, the current study did not manipulate dietary salt intake in the 2K1C model nor test the extent by which the novel Trpv1−/− rats are salt-sensitive.
PERSPECTIVES
Total renal denervation lowers arterial ABP in multiple clinical trials and several experimental models of hypertension 1–3. These anti-hypertensive effects are partly attributed to removal of renal sensory nerves as chemical ablation of these fibers lowers ABP to the same extent in in deoxycorticosterone-salt rats 32 and 2K1C mice 8. The current findings suggest the renovascular hypertension requires the TRPV1 channel to elevate ARNA, renal SNA, reduce GFR and raise ABP. The TRPV1 channel responds to noxious and mechanical stimuli, pH, and chemokines 18,19. Renal afferent fibers are sensitive ischemia, increased pelvic pressure and various chemokines 4–7. In addition, TRPV1 channels are required for tissue inflammation in neuropathic pain models 18,19. Future studies are necessary to distinguish the mechanisms of TRPV1 channel activation (local metabolites, renal inflammation) and to extent to which these channels are required for other models of renal-nerve dependent hypertension.
Supplementary Material
NOVELTY AND RELEVANCE.
What is New?
The majority (85%) of rat renal sensory neurons in sham and 2-kidney-1-clip (2K1C) rats expressed the transient receptor potential vanilloid 1 (TRPV1) channel.
2K1C hypertension was significantly attenuated in male Trpv1−/− versus wild-type rats. The magnitude of the hypertension and effect of TRPV1 deletion was sex-dependent.
2K1C hypertension significantly increased afferent renal nerve activity, sympathetic efferent nerve activity, and reduced glomerular filtration rates. These effects were attenuated in the 2K1C Trpv1−/− rat.
What is Relevant?
Deletion of the TRPV1 channel attenuates sympathoexcitation and hypertension in the 2K1C renovascular model of hypertension.
Clinical/Pathophysiological Implications?
These findings suggest that renovascular hypertension requires the TRPV1 channel to elevate renal afferent and sympathetic nerve activity, reduce glomerular filtration rate, and increase arterial blood pressure.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Dr. Aron Geurts at Medical College of Wisconsin for generation of the Trpv1−/− rats and Dr. Alan Sved for comments on the manuscript.
SOURCE of FUNDING
The research was supported by NIH Grants HL145875 and HL152680.
Abbreviations:
- ABP
arterial blood pressure
- ARNA
afferent renal nerve activity
- DRG
dorsal root ganglion
- SNA
sympathetic nerve activity
- TRPV1
transient receptor potential vanilloid 1
- Trpv1−/−
transient receptor potential vanilloid 1 knockout
- 2K1C
2-kidney-1-clip
Footnotes
CONFLICT OF INTEREST/DISCLOSURES
The authors have no disclosures.
REFERENCES
- 1.Kiuchi MG, Esler MD, Fink GD, Osborn JW, Banek CT, Bohm M, Denton KM, DiBona GF, Everett THt, Grassi G, et al. Renal Denervation Update From the International Sympathetic Nervous System Summit: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:3006–3017. doi: 10.1016/j.jacc.2019.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osborn JW, Banek CT. Catheter-Based Renal Nerve Ablation as a Novel Hypertension Therapy: Lost, and Then Found, in Translation. Hypertension. 2018;71:383–388. doi: 10.1161/HYPERTENSIONAHA.117.08928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeLalio LJ, Sved AF, Stocker SD. Sympathetic Nervous System Contributions to Hypertension: Updates and Therapeutic Relevance. Can J Cardiol. 2020;36:712–720. doi: 10.1016/j.cjca.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol. 2015;308:R79–95. doi: 10.1152/ajpregu.00351.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osborn JW, Foss JD. Renal Nerves and Long-Term Control of Arterial Pressure. Compr Physiol. 2017;7:263–320. doi: 10.1002/cphy.c150047 [DOI] [PubMed] [Google Scholar]
- 6.Recordati GM, Moss NG, Genovesi S, Rogenes PR. Renal receptors in the rat sensitive to chemical alterations of their environment. Circ Res. 1980;46:395–405. [DOI] [PubMed] [Google Scholar]
- 7.Recordati GM, Moss NG, Waselkov L. Renal chemoreceptors in the rat. Circ Res. 1978;43:534–543. [DOI] [PubMed] [Google Scholar]
- 8.Ong J, Kinsman BJ, Sved AF, Rush BM, Tan RJ, Carattino MD, Stocker SD. Renal sensory nerves increase sympathetic nerve activity and blood pressure in 2-kidney 1-clip hypertensive mice. J Neurophysiol. 2019;122:358–367. doi: 10.1152/jn.00173.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciriello J, Calaresu FR. Central projections of afferent renal fibers in the rat: an anterograde transport study of horseradish peroxidase. J Auton Nerv Syst. 1983;8:273–285. [DOI] [PubMed] [Google Scholar]
- 10.Kuo DC, Nadelhaft I, Hisamitsu T, de Groat WC. Segmental distribution and central projections of renal afferent fibers in the cat studied by transganglionic transport of horseradish peroxidase. J Comp Neurol. 1983;216:162–174. doi: 10.1002/cne.902160205 [DOI] [PubMed] [Google Scholar]
- 11.Stella A, Golin R, Busnardo I, Zanchetti A. Effects of afferent renal nerve stimulation on renal hemodynamic and excretory functions. Am J Physiol. 1984;247:H576–583. doi: 10.1152/ajpheart.1984.247.4.H576 [DOI] [PubMed] [Google Scholar]
- 12.Stella A, Weaver L, Golin R, Genovesi S, Zanchetti A. Cardiovascular effects of afferent renal nerve stimulation. Clin Exp Hypertens A. 1987;9 Suppl 1:97–111. [DOI] [PubMed] [Google Scholar]
- 13.Caverson MM, Ciriello J. Contribution of paraventricular nucleus to afferent renal nerve pressor response. Am J Physiol. 1988;254:R531–543. doi: 10.1152/ajpregu.1988.254.3.R531 [DOI] [PubMed] [Google Scholar]
- 14.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting Afferent Renal Nerve Discharge and Renal Inflammation: Elucidating the Role of Afferent and Efferent Renal Nerves in Deoxycorticosterone Acetate Salt Hypertension. Hypertension. 2016;68:1415–1423. doi: 10.1161/HYPERTENSIONAHA.116.07850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyss JM, Aboukarsh N, Oparil S. Sensory denervation of the kidney attenuates renovascular hypertension in the rat. Am J Physiol. 1986;250:H82–86. doi: 10.1152/ajpheart.1986.250.1.H82 [DOI] [PubMed] [Google Scholar]
- 16.Lopes NR, Milanez MIO, Martins BS, Veiga AC, Ferreira GR, Gomes GN, Girardi AC, Carvalho PM, Nogueira FN, Campos RR, et al. Afferent innervation of the ischemic kidney contributes to renal dysfunction in renovascular hypertensive rats. Pflugers Arch. 2020;472:325–334. doi: 10.1007/s00424-019-02346-4 [DOI] [PubMed] [Google Scholar]
- 17.Nishi EE, Lopes NR, Gomes GN, Perry JC, Sato AYS, Naffah-Mazzacoratti MG, Bergamaschi CT, Campos RR. Renal denervation reduces sympathetic overactivation, brain oxidative stress, and renal injury in rats with renovascular hypertension independent of its effects on reducing blood pressure. Hypertens Res. 2019;42:628–640. doi: 10.1038/s41440-018-0171-9 [DOI] [PubMed] [Google Scholar]
- 18.Julius D TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833 [DOI] [PubMed] [Google Scholar]
- 19.Nilius B TRP channels in disease. Biochim Biophys Acta. 2007;1772:805–812. doi: S0925-4439(07)00040-3 [pii] 10.1016/j.bbadis.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 20.DeLalio LJ, Stocker SD. Impact of anesthesia, sex, and circadian cycle on renal afferent nerve sensitivity. Am J Physiol Heart Circ Physiol. 2021;320:H117–H132. doi: 10.1152/ajpheart.00675.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLalio LJ, Stocker SD. Impact of anesthesia and sex on sympathetic efferent and hemodynamic responses to renal chemo- and mechanosensitive stimuli. J Neurophysiol. 2021;126:668–679. doi: 10.1152/jn.00277.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foss JD, Wainford RD, Engeland WC, Fink GD, Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol. 2015;308:R112–122. doi: 10.1152/ajpregu.00427.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Li L, Li Y, Liang X, Sun Q, Yu H, Zhong J, Ni Y, Chen J, Zhao Z, et al. Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ influx. Cardiovasc Diabetol. 2015;14:22. doi: 10.1186/s12933-015-0183-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytol. 1990;19:789–801. doi: 10.1007/BF01188046 [DOI] [PubMed] [Google Scholar]
- 25.Fehrenbach DJ, Abais-Battad JM, Dasinger JH, Lund H, Keppel T, Zemaj J, Cherian-Shaw M, Gundry RL, Geurts AM, Dwinell MR, et al. Sexual Dimorphic Role of CD14 (Cluster of Differentiation 14) in Salt-Sensitive Hypertension and Renal Injury. Hypertension. 2021;77:228–240. doi: 10.1161/HYPERTENSIONAHA.120.14928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spires D, Ilatovskaya DV, Levchenko V, North PE, Geurts AM, Palygin O, Staruschenko A. Protective role of Trpc6 knockout in the progression of diabetic kidney disease. Am J Physiol Renal Physiol. 2018;315:F1091–F1097. doi: 10.1152/ajprenal.00155.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehrotra P, Ullah MM, Collett JA, Myers SL, Dwinell MR, Geurts AM, Basile DP. Mutation of RORgammaT reveals a role for Th17 cells in both injury and recovery from renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2020;319:F796–F808. doi: 10.1152/ajprenal.00187.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 8443 [pii] [DOI] [PubMed] [Google Scholar]
- 29.DeLalio LJ, Stocker SD. Sympathoexcitatory responses to renal chemosensitive stimuli are exaggerated at nighttime in rats. Am J Physiol Heart Circ Physiol. 2022;323:H437–H448. doi: 10.1152/ajpheart.00665.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Wang DH, Galligan JJ. P2Y2 receptors mediate ATP-induced resensitization of TRPV1 expressed by kidney projecting sensory neurons. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1634–1641. doi: 10.1152/ajpregu.00235.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cervenka L, Horacek V, Vaneckova I, Hubacek JA, Oliverio MI, Coffman TM, Navar LG. Essential role of AT1A receptor in the development of 2K1C hypertension. Hypertension. 2002;40:735–741. doi: 10.1161/01.hyp.0000036452.28493.74 [DOI] [PubMed] [Google Scholar]
- 32.Morton JJ, Wallace EC. The importance of the renin-angiotensin system in the development and maintenance of hypertension in the two-kidney one-clip hypertensive rat. Clin Sci (Lond). 1983;64:359–370. doi: 10.1042/cs0640359 [DOI] [PubMed] [Google Scholar]
- 33.Katholi RE, Whitlow PL, Winternitz SR, Oparil S. Importance of the renal nerves in established two-kidney, one clip Goldblatt hypertension. Hypertension. 1982;4:166–174. [PubMed] [Google Scholar]
- 34.Katholi RE, Winternitz SR, Oparil S. Decrease in peripheral sympathetic nervous system activity following renal denervation or unclipping in the one-kidney one-clip Goldblatt hypertensive rat. J Clin Invest. 1982;69:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hay M, Xue B, Johnson AK. Yes! Sex matters: sex, the brain and blood pressure. Curr Hypertens Rep. 2014;16:458. doi: 10.1007/s11906-014-0458-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi NF, Pajewski R, Chen H, Littrup PJ, Maliszewska-Scislo M. Hemodynamic and neural responses to renal denervation of the nerve to the clipped kidney by cryoablation in two-kidney, one-clip hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2016;310:R197–208. doi: 10.1152/ajpregu.00331.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75 [DOI] [PubMed] [Google Scholar]
- 38.Wang DH, Li J, Qiu J. Salt-sensitive hypertension induced by sensory denervation: introduction of a new model. Hypertension. 1998;32:649–653. doi: 10.1161/01.hyp.32.4.649 [DOI] [PubMed] [Google Scholar]
- 39.Kopp UC, Cicha MZ, Smith LA. Dietary sodium loading increases arterial pressure in afferent renal-denervated rats. Hypertension. 2003;42:968–973. doi: 10.1161/01.HYP.0000097549.70134.D8 [DOI] [PubMed] [Google Scholar]
- 40.Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D, et al. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31:5067–5077. doi: 31/13/5067 [pii] 10.1523/JNEUROSCI.6451-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


