Abstract
Localized provoked vulvodynia (LPV) affects ~14 million people in the US (9% of women), destroying lives and relationships. LPV is characterized by chronic pain (> 3 months) upon touch to the vulvar vestibule, which surrounds the vaginal opening. Many patients go months or years without a diagnosis. Once diagnosed, the treatments available only manage the symptoms of disease and do not correct the underlying problem. We have focused on elucidating the underlying mechanisms of chronic vulvar pain to speed diagnosis and improve intervention and management. We determined the inflammatory response to microorganisms, even members of the resident microflora, sets off a chain of events that culminates in chronic pain. This agrees with findings from several other groups, which show inflammation is altered in the painful vestibule. The vestibule of patients is acutely sensitive to inflammatory stimuli to the point of being deleterious. Rather than protect against vaginal infection, it causes heightened inflammation that does not resolve, which coincides with alterations in lipid metabolism that favor production of proinflammatory lipids and not pro-resolving lipids. Lipid dysbiosis in turn triggers pain signaling through the transient receptor potential vanilloid subtype 4 receptor (TRPV4). Treatment with specialized pro-resolving mediators (SPMs) that foster resolution reduces inflammation in fibroblasts and mice and vulvar sensitivity in mice. SPMs, specifically maresin 1, act on more than one part of the vulvodynia mechanism by limiting inflammation and acutely inhibiting TRPV4 signaling. Therefore, SPMs or other agents that target inflammation and/or TRPV4 signaling could prove effective as new vulvodynia therapies.
Vulvar Pain
Everyone with a vulva is likely to experience pain or discomfort in this area at some point in their life, even if only transiently, such as during pregnancy, after childbirth, or during menopause1, 2. Despite the ubiquity of vulvar pain, our fundamental understanding of the mechanisms involved are limited, and epidemiological studies indicate it is both underdiagnosed and seldom discussed3–26. Since the year 2000, fewer than 200 NIH grants have been funded on “vulvar pain,” which includes projects focused on endometriosis and pelvic pain27.
Patients with vulvar pain or sexual dysfunction often do not vocalize these concerns to their medical providers28–31. Epidemiological studies show patients perceive their provider is not equipped to discuss topics pertaining to sexual health and many fear judgement or embarrassment32. Patient perception may reflect a limited focus on sexual medicine training in many medical school curricula28–32. Most patients look for providers to initiate the conversation. When these conversations do not take place, patient needs go unmet.
Yet, vulvar pain remains a common and complex problem20, 21, 33–36. It can be acute and may resolve with time or treatment, or it can be chronic23, 37, 38. Vulvodynia is the most common cause of chronic dyspareunia (painful intercourse), affecting anywhere from 8–12% of women in the United States20, 33, 34. Lifetime risk has been estimated even higher, suggesting that up to a third of persons with vulvas could be affected16. If a patient has chronic vulvar pain that does not resolve for at least 3 months and cannot be explained by any other cause, they usually receive a vulvodynia diagnosis23, 37. Vulvodynia can develop at any age and affects a large portion of reproductive age individuals. Vulvodynia destroys the patient’s quality of life and has a negative impact on their relationships; it can make it difficult to sit, wear pants, ride a bicycle, use a tampon or menstrual cup, or engage in sexual activity18, 19, 23, 24, 36–42. Because vulvodynia is a diagnosis of exclusion, patients suffer months to years before a diagnosis. Once diagnosed, treatment is often “trial and error,” escalating from less to more invasive and often culminating in surgical procedures to remove the affected tissue, essentially amputating a portion of the vulva.
The most common type of vulvodynia is localized provoked vulvodynia4, 11–13, 43. This is pain that occurs upon touch (mechanical allodynia) and is localized to the vulvar vestibule, or ring of tissue surrounding the vaginal opening. Adjacent areas are non-painful to touch, while the vestibule is extremely sensitive, particularly between the 5 and 7-o’clock positions (Figure 1). Removal of this tissue during a surgical procedure termed a vestibulectomy is often curative and carries over a 90% satisfaction rate3, 22, 42, 44–47. However, due to its invasive nature and inherent risk, vestibulectomy is often delayed until all other treatments fail.
Figure 1. Anatomy of Localized Provoked Vulvodynia.
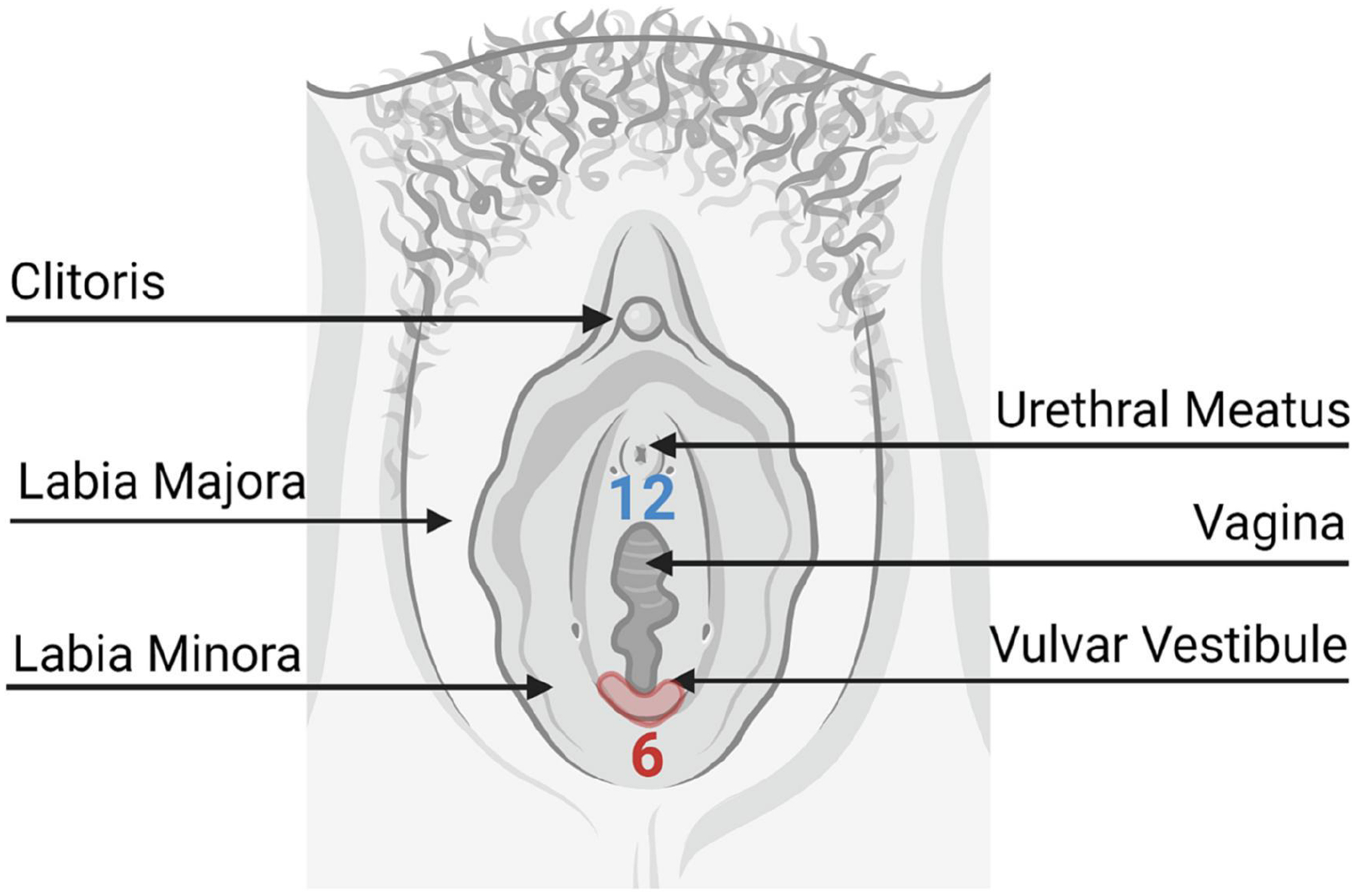
Pain is usually located at the posterior part of the vulvar vestibule between 5 and 7-o’clock (red shading). The red numbers orient the clock with the 12-o’clock position near the clitoris.
Treatment for vulvodynia is trial and error1, 9, 10, 22–24, 37, 38, 40–42, 44, 47–50, usually beginning with conservative management, including use of hypoallergenic soaps and lotions, wearing exclusively cotton undergarments, and avoiding wearing tight clothing or causing friction to the affected area. Most patients then move onto topical therapies, such as lidocaine, which is applied to the area before inserting anything into the vagina. This can be helpful, but it only temporarily reduces sensation in the area and can be transferred to the partner during intercourse. Gabapentin, selective serotonin reuptake inhibitors (SSRIs), and serotonin and norepinephrine reuptake inhibitor (SNRIs) are the next line, sometimes applied topically or injected, but typically dosed orally. Patients may try botulinum toxin injection into the area, which is thought to act on substance P to reduce pain. Patients also engage in cognitive behavioral therapy, bio-feedback, mediation and mindfulness, and relationship, psychiatric, or sexual counseling. When these fail, eligible patients usually elect vestibulectomy.
Vulvodynia can be further sub-divided into categories based on clinical presentation, but little to no mechanistic, scientific, or biological evidence is available to distinguish these categories5, 9, 10, 23, 37. Patients may have generalized vulvodynia, where the entire vulva is affected, making them ineligible for surgery. Patients can also have unprovoked or spontaneous pain. Patients can have primary or secondary vulvodynia, referring to whether they experienced a pain-free intercourse period before developing pain (secondary) or whether pain was experienced at the first attempt at tampon use or intercourse (primary). Because there is limited scientific information, it is difficult to discern if these presentations are manifestations of the same disease or unique disease entities. Clearly, there are knowledge gaps, leading to treatment delays and failures and an overall poor quality of life in vulvodynia patients. Although there is some evidence to support the use of any approved vulvodynia therapy, there is no level 1 evidence for their use, meaning most treatments have not been demonstrated more effective in blinded randomized control trials. More recently, our group and others have begun to unravel the biological mechanisms at play in vulvodynia with a focus on developing more effective mechanism-based therapies through the use of cellular and mouse models of disease10–12, 15, 26, 43, 51–59.
Connections Between Inflammation and Vulvar Pain
Yeast Infections and Vulvodynia
In patients with secondary vulvodynia, which appears more common than primary vulvodynia, there is a tipping point where patients go from pain-free to painful intercourse5, 10, 23, 37, 48. The most common warning sign is the occurrence of transient bouts of pain immediately following intercourse. It is difficult to discern a clear environmental cause for vulvodynia, as patients recall numerous factors that could have precipitated their pain, including sexual assault, vulvovaginal infections, childbirth, injury, abuse, trauma, and more20, 21, 36, 60. However, the most common precipitating factor cited by more than 70% of patients is a previous history of chronic or recurrent yeast infection8. Patients recall experiencing a yeast infection that never fully cleared or 4 or more yeast infections in the past year. It is important to note that the majority of yeast infections are self-diagnosed and treated with over-the-counter antifungals61–63. Few patients undergo laboratory testing. Therefore, it is possible that other agents, such as bacteria, viruses, or irritants could play a role.
There is strong evidence from patients, human cells, and animal studies to implicate yeast, especially Candida albicans, in the onset of vulvodynia. C. albicans is among the most common agents that cause vulvovaginal yeast infection14, 64, 65. Other non-albicans Candida, such as C. glabrata and C. tropicalis come in close second. Patients with vulvodynia can show cutaneous hypersensitivity to C. albicans, similar to an allergic reaction, suggestive of an overall sensitivity to this yeast species66. Human fibroblasts taken from painful areas of the vestibule of localized provoked vulvodynia (LPV) patients also show a hypersensitivity to yeast, where less than 100 yeast cells elicit an inflammatory response characterized by elevated levels of interleukin-6 (IL-6) and prostaglandin E2 (PGE2)11. Patients with vulvodynia do not have active yeast infections at the time of diagnosis23, 37; this data provides evidence that a small number of yeast, which are beneath the clinical limit of detection, can cause a significant inflammatory response in painful areas of the vestibule. This response is specific to the painful area of the vestibule and does not occur in cells taken from an adjacent non-painful site in the external vulva, which require numbers of yeast commiserate with an active infection to elicit a response11. Fibroblasts from the painful vestibule also show enhanced sensitivity to C. glabrata, C. tropicalis, Saccharomyces cerevisiae, and zymosan, a yeast cell wall extract from Saccharomyces, compared to non-painful sites in the patient and from the same anatomical sites in patients without vulvodynia15. Furthermore, repeated vulvovaginal infection with C. albicans or injection of zymosan into the vulva of several mouse strains (e.g. CD-1, BALB/c, and C57/BL-6) results in persistent sensitivity to touch (up to 16 weeks), while animals receiving saline remain insensitive13, 14.
Inflammation and Vulvar Fibroblasts
We developed a fibroblast model for studying vulvodynia where each patient serves as their own control by taking 6 mm biopsies from both the painful vestibule and external non-painful vulva (Figure 2). Biopsies are collected during a scheduled vestibulectomy for LPV patients and during another gynecologic surgery in patients without a history of vulvar disease (controls), which minimizes patient discomfort and infection risk. Each biopsy tissue can then be partitioned into up to 3 individual pieces for development of primary fibroblast stains and other approaches, such as histology, proteomics, transcriptomics, and lipidomics (Figure 2). This design facilitates paired analysis and allows for the comparison of painful to non-painful areas in both cases and controls to pinpoint what is happening at the painful vulvar vestibule. The high satisfaction rate with vestibulectomy44–46 supports the hypothesis that there is something unique about the vestibule that can lead to chronic pain.
Figure 2. LPV Model.
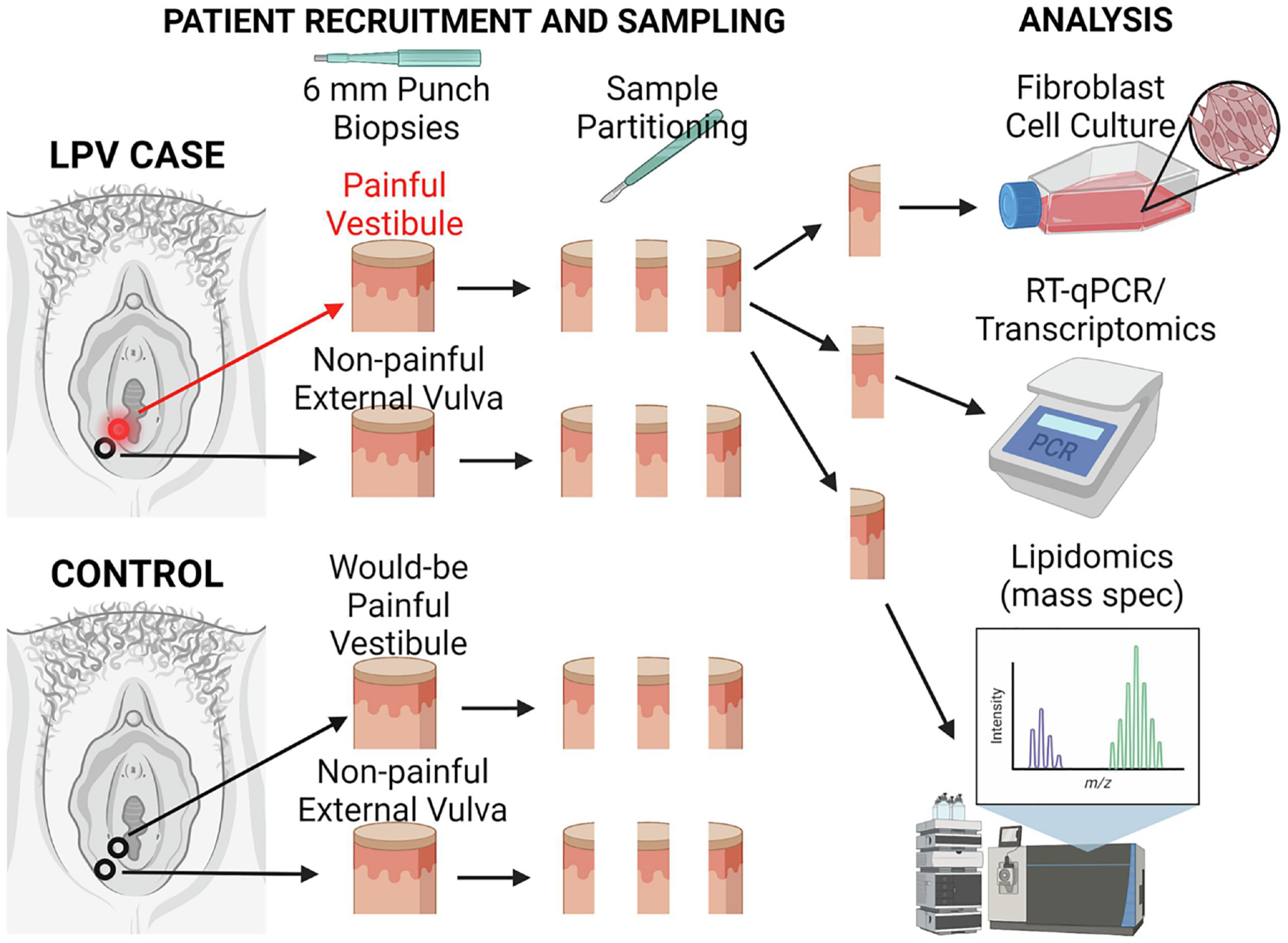
6 mm punch biopsies are collected during surgery from both LPV cases and from healthy controls (no vulvar disease). These are then sectioned into 3 pieces, each for a specific type of analysis, namely fibroblast isolation and culture, analysis of gene expression, and quantification of lipid profiles. An n of 1 represents 4 tissue samples, with only one painful sample from the vestibule of a case, while the remaining 3 samples, whether from case or control, are from non-painful areas of the vestibule and external vulva.
Pursuant to the possible connection between vulvovaginal yeast infection and vulvodynia, we introduced live yeast infection into our model and found that fibroblasts from the painful vestibule are indeed more sensitive to yeast than non-painful areas from cases and controls, measured by IL-6 and PGE2 production11, 15. Furthermore, we determined the response of fibroblasts to C. albicans could predict the patient threshold at the site from which the fibroblasts were obtained15. This finding 1) implicates inflammation in the vulvodynia mechanism, 2) demonstrates that the model correlates with key patient measures, and 3) establishes IL-6 and PGE2 as surrogate measures of pain, facilitating high through-put mechanistic studies that are impossible to conduct in the patient.
We considered the mechanisms by which fibroblasts might recognize yeast, focusing on recognition of conserved pathogen-associated molecular patterns (PAMPs) by host pattern recognition receptors (PRRs). Based on the chronicity of pain, we initially focused on PRRs involved in recognizing β-glucan, a key component of the C. albicans biofilm matrix associated with chronic infection67–71. We later expanded our scope to toll-like receptors12, which can recognize specific components of the yeast cell, as well as bacteria and viruses72–74. We also looked at bradykinin receptors58, which are not PRRs, but are involved in pain signaling. All the receptors studied were determined to be significantly more highly expressed in the painful vestibule compared to non-painful sites, likely accounting for enhanced sensitivity to C. albicans10–13, 58.
We found that sensitivity to inflammatory stimuli was also elevated in the vestibule of patients without vulvodynia albeit to much lesser extent10–13, 58. These findings suggest that pain in the vestibule may be an extreme of a normally protective response to inflammatory stimuli (Figure 3). Considering the physical location of the vestibular tissue and its unique embryonic origin, it makes sense that the vestibule serves as a protective barrier to vaginal infection, much like a moat around a castle75–77. This exaggerated response is deleterious and could explain the evolution of chronic pain, one of the cardinal symptoms of inflammation (dolor).
Figure 3. Inflammatory Mechanisms of Vulvodynia.
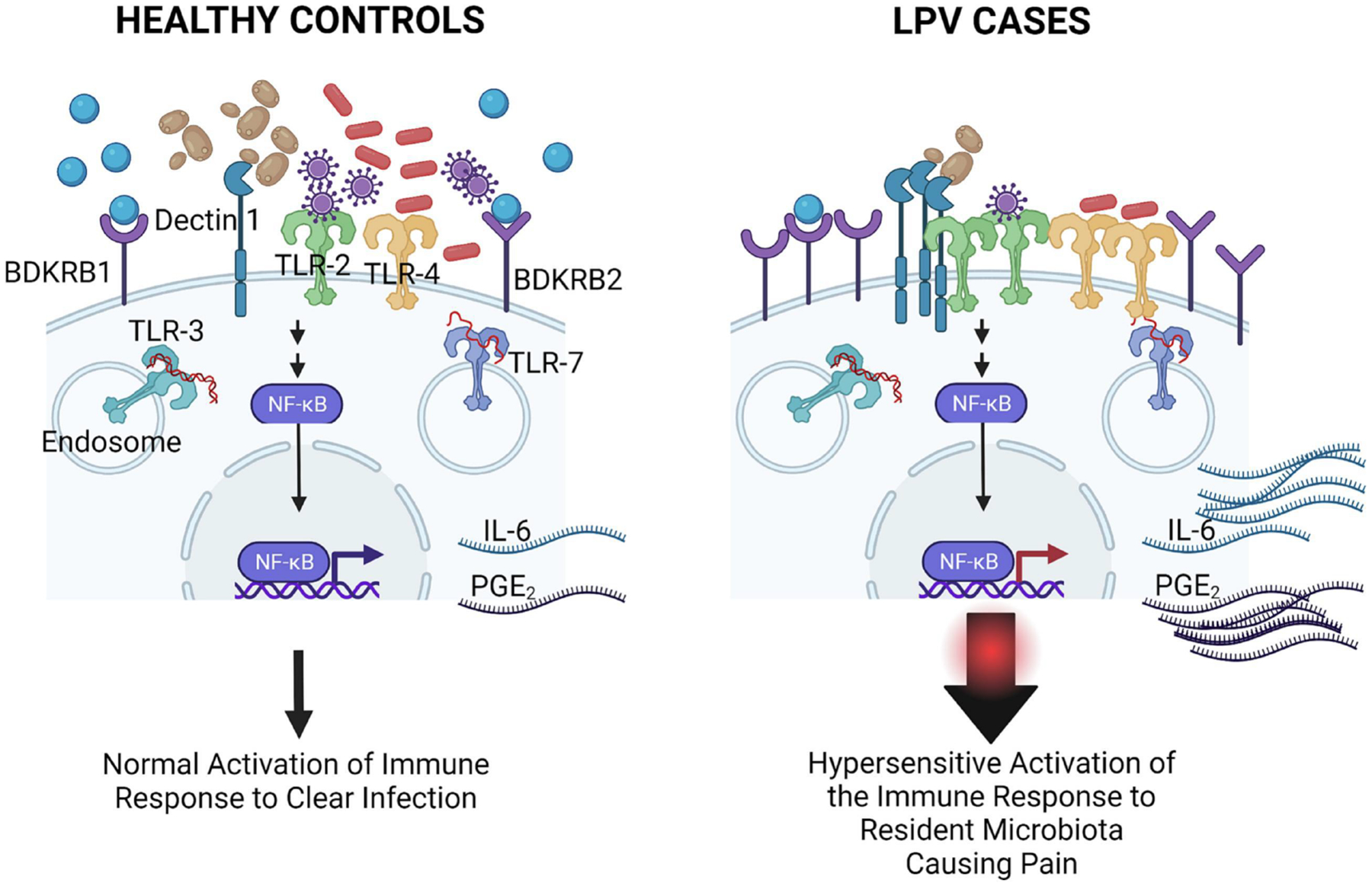
In patients with localized provoked vulvodynia, an abberation of a normally protective response against infection is exeracerbated by the elevated expression and function of nearly a dozen different receptors involved in innate immune responses (dectin-1, TLRs) and pain signaling (bradykinin receptors). This significantly increases levels of IL-6 and PGE2, which has been associated with pain in vulvodynia. On the left side, healthy controls are depicted, which express normal levels of receptors, responding only to infectious threats, which subsequently triggers immune clearance, after which inflammation resolves. On the right, LPV cases express abnormally high levels of receptors and respond to low numbers of organisms, even the resident flora. This triggers an abnormally strong response that does not resolve and leads to elevated inflammatory mediators associated with pain.
Knocking down or impairing the function of any one receptor reduces the response to inflammatory stimuli, but does not ablate this response10–12, 58. Consistent with the literature, we determined activation of these receptors in turn triggers nuclear factor kappa B (NFκB) signaling in vulvar fibroblasts73, 78–80. Blocking NFκB activity using Bay-11–7082 ablates inflammatory mediatory production in vestibular fibroblasts, pointing to NFκB as a master regulator of inflammation in vulvodynia10–12, 58. Based on the link between inflammatory mediator production in fibroblasts and pain in the patient, reducing IL-6 and PGE2 is likely to have analgesic properties. However, the connection between inflammation and pain in vulvodynia warrants further investigation. While IL-6 and PGE2 are surrogate measures of pain, an animal model of vulvodynia was necessary to quantify sensitivity indicative of pain.
Rodent Models of Vulvar Allodynia
One potential barrier to the development of novel therapeutics is the need for preclinical models of disease. There are a few rodent models of vulvar pain that use allergens or irritants (e.g. haptens, oxazolone, streptozotocin, and methylisothiazolinone) to provoke vulvar sensitivity, but it is unclear if these exposures model any natural causes of vulvodynia81–88. In a model developed by Farmer et al. mice were infected vulvovaginally with C. albicans or zymosan was injected into the midline vulvar skin immediately posterior to the vaginal opening14. Farmer determined vulvovaginal exposure to yeast or yeast products resulted in sustained allodynia, similar to what is observed in LPV patients. This is a key piece of evidence supporting the hypothesis that chronic yeast infection incites vulvar pain. However, the Farmer model was not designed to test therapeutic interventions. Therefore, we worked to expand the model to improve reproducibility, investigator comfort and reliability, and create a sustained window of stable allodynia (~16 weeks) for traditional pharmacokinetic testing13.
The disease pattern in our model is similar to human allodynia; there is a clear inflammatory response during the induction phase in mice receiving zymosan injections13. After the first few weeks, the response tapers off and there is no active inflammatory response at the time of treatment, while mice remain exquisitely sensitive to touch (< 1 g force elicits a response). Congruent with the role of inflammation in LPV, we found that interrupting this process via twice daily topical application of the specialized pro-resolving mediator (SPM) maresin 1 increased sensitivity thresholds to pre-induction baselines in less than 4 weeks, effectively alleviating pain. SPMs are small lipids involved in the resolution of inflammation. Unlike steroids or non-steroidal anti-inflammatory drugs (NSAIDs), SPMs do not risk compromising immune defense at a site that must respond to environmental threats89, 90. Rather, SPMs help to resolve inflammation faster and more efficiently, helping restore homeostasis, clear infection, and heal wounds89–102. There is a growing body of evidence to support their use as analgesic agents13, 103–106. SPMs are naturally produced, safe, and do not result in dependence.
Lipid Dysbiosis and Vulvar Pain
Why do the symptoms of vulvodynia persist?
For a long time, the resolution of inflammation was thought to be an inactive process mediated by a progressive tapering off of inflammatory signals as infection resolves and immune cells lyse and are no longer recruited to the site of infection89–91, 100, 101, 107, 108. However, with the discovery of SPMs, it became apparent that resolution is an active process governed by the secretion of lipid mediators that have specific and profound effects on immune cells that foster immune clearance and the return to homeostasis. The balance between resolution and inflammation has sometimes been referred to as a “Goldilocks mechanism” where everything needs to be “just right” to ensure resolution is sufficient but not overzealous. As discussed, in LPV, there is a heightened response to inflammatory stimuli in the vulvar vestibule, which represents an extreme of a naturally protective response (Figure 3). However, this does not explain why resolution fails to quell this deleterious response nor how inflammation leads to vulvar allodynia.
The chronicity of inflammation led us to hypothesize that a defect in the resolution machinery could account for unresolved inflammation. Therefore, we elected to use targeted lipidomic analysis to quantify the abundance of lipids involved in both resolution and inflammation in vulvar tissues collected from LPV patients and healthy controls. Punch biopsies from painful sites in the vestibule and non-painful sites in the external vulva of LPV patients were analyzed along with matched biopsies from controls. One-third of each 6 mm biopsy was used for lipidomic analysis from a total of 10 case and 10 control patients. Three lipids implicated in resolution (12- hydroxyeicosatetraenoic acid (HETE), 8(9)-eicosatrienoic acid (EET), and 14(15)-EET) were found to be highly and significantly reduced in the painful vestibule compared to non-painful areas from both cases and controls109–111. We also observed concomitant increases in lipids involved in inflammation, such as PGE2. These findings indicated that there may be a deficit in the resolution machinery that accounts for the chronicity of inflammation. We therefore hypothesized that adding exogenous SPMs could overcome this deficit by compensating for the reduction in pro-resolving lipids. We elected to focus on SPMs, because our pilot experiments indicated they were reduced in vulvodynia patients, are already under development for human use, and likely effective at lower doses than their precursor lipids (e.g. 8(9)-EET).
Pro-resolving mediators reduce inflammation and vulvar pain
We used our fibroblast model to screen commercially available SPMs across a variety of dosages and dosing strategies in cells from both painful and non-painful areas13. We first explored a pre-treatment model of vulvodynia, where we treated cells with the SPMs, then initiated inflammation using recombinant human interleukin-1β (IL-1β). IL-1β, an endogenous inflammatory stimulus, elicits a predictable and reproducible response, making it a useful tool for inducing inflammatory responses in human fibroblasts11–13, 15, 58. Although, there are no laboratory tests for vulvodynia at present, it is our goal to identify an LPV lipid signature using targeted lipidomics. This signature could be used to screen asymptomatic patients and intervene before the onset of pain and comorbid conditions linked to pain. It could also be used as an objective clinical trial measure or clinical metric by which to assess the progression and remission of symptoms. Early screening and prevention could be used in patients with warning signs, such as a family history of vulvodynia or someone who is experiencing transient bouts of pain following intercourse. Vulvodynia may be heritable112–116. This signature might also prove useful in establishing biological classifications of disease to help better define our current clinical classifications (e.g. provoked vs. unprovoked).
Using the prevention strategy, we were able to significantly attenuate the production of IL-6 and PGE2 in response to IL-1β for most, but not all SPMs13. Resolvin D1, aspirin triggered resolvin D1, and the precursor of resolvin D1 (17S-hydroxy docosahexaenoic acid; HDHA) were generally not successful in reducing mediator levels, while the others were highly effective in reducing IL-6 and many also reduced PGE2. Therefore, we elected to try a treatment strategy, which is currently the most likely scenario as patients present with symptoms for a duration greater than 3 months. Our results for the treatment strategy were comparable; most SPMs reduced IL-6 and several also reduced PGE2. This gave us a list of SPMs with greatest potential for reduction of inflammation in vulvodynia, which included maresin 1, lipoxin A4, and resolvin D2.
Before moving to testing analgesia in mice, we first determined that the mouse vulva both produces SPMs and expresses the majority of the G-coupled protein receptors (GPCRs) that recognize various SPMs. We also determined that explants of mouse vulvar tissue cultured as 3D tissue biopsies were highly responsive to maresin 1, lipoxin A4, and resolvin D2, demonstrated through their ability to reduce PGE213. Of the three, maresin 1 reduced PGE2 to the greatest magnitude, placing maresin 1 as a front runner for future drug development.
We went on to test the analgesic effects of maresin 1 and docosahexanoic acid (DHA) in our mouse model of vulvodynia13. DHA, a dietary polyunsaturated fatty acid, is a precursor for maresin synthesis. Maresin 1 was highly effective in returning sensitivity thresholds to their starting baselines. In models of tactile or mechanical allodynia, a von Frey probe is used to measure the tolerance to pressure, as is done clinically to determine thresholds in humans. The greater the force withstood without withdrawal, the lower the sensitivity. Mice with high sensitivity will react and withdraw or evade the probe with little to no force applied. Mice receiving maresin 1 recovered to and exceeded their starting baseline after 4 weeks of twice-daily treatment weekdays and once-daily treatment on weekends. Mice receiving vehicle also recovered during this period, but not to the magnitude of those receiving maresin 1 and did not exceed baseline. Mice that did not develop allodynia that received maresin 1 showed no significant difference in their thresholds compared to baseline. The vehicle contained dimethyl sulfoxide (DMSO), which has analgesic properties on its own117. Although this may not be ideal for detecting differences in a laboratory experiment, any benefit added by the vehicle would be welcome in a clinical application. This data demonstrated that SPMs have a high potential for therapeutic translation in humans; clinical trials will be necessary to move forward. Currently, it is unclear whether chronic SPM treatment would be needed to maintain its effects in humans. Mice recover completely after a short duration of treatment, but this remains to be tested in vulvodynia patients. Although SPMs represent a promising avenue for therapeutic development, this did not explain the cause for this deficit, nor how inflammation might elicit pain in the vulva.
Neuro-inflammatory Mechanisms of Vulvar Pain
Pools of lipids involved in pain signaling are sustained in the painful vestibule
In exploring the mechanisms responsible for deficiencies in pro-resolving lipids using a targeted lipidomic analysis panel comprised of ~150 pro-resolving and inflammatory mediators, we made an unexpected finding. There are 4 EETs that are implicated in resolution: 5(6)-EET, 8(9)-EET, 11(12)-EET, and 14(15)-EET109, 111. Since half of these were significantly less abundant in the painful vestibule, we became interested in their metabolism. EETs are produced by members of the cytochrome peroxidase (CYP) 450 family from the polyunsaturated dietary fatty acid arachidonic acid118, 119. EETs can be broken down to their less active, but not completely inactive dihydroxyeicosatrienoic acid (DHET) forms by an enzyme known as soluble epoxide hydrolase (sEH)109, 111, 118, 119. Previous studies have shown that inhibiting sEH can have pro-resolving and even analgesic properties by reducing EET degradation and thus maintaining EET pools109, 111, 119. Therefore, we wanted to determine if EET levels were reduced in the painful vestibule because of enhanced sEH degradation or reduced production by CYP450. Our lipidomic analysis also measured DHETs, which allowed us to calculate a ratio of active EET over inactive DHET for each of the 4 EETs. We anticipated a decrease in this ratio for at least 8(9)-EET and 14(15)-EET based on their reduced abundance in the painful vestibule. However, only one EET ratio showed a significant difference, which was 5(6)-EET. Surprisingly, 5(6)-EET active pools were enhanced in the painful vestibule, which appeared to be the result of a reduction in the 5(6)-DHET breakdown product. However, our lipidomic screen does not measure other metabolites of 5(6)-EET, and sustained DHETs levels do not preclude the involvement of sEH.
5(6)-EET is exceedingly short-lived and difficult to measure120. Therefore, its sustained presence in the painful vestibule was noteworthy. Although there is empiric evidence for the role of 8(9)-EET, 11(12)-EET, and 14(15)-EET in resolution109, 111, there are no publications that demonstrate such a role for 5(6)-EET. The most established role for 5(6)-EET is activation of the transient receptor potential vanilloid subtype 4 receptor implicated in pain signaling, specifically mechanical allodynia121. Although inflammation is related to pain, this was the first evidence for enhanced pain signaling in the vestibule of LPV patients suggestive of a neuro-inflammatory mechanism. However, the relationship between inflammation, lipids, and TRPV4 signaling would prove to be even more complex than we initially hypothesized.
TRPV4 signaling is elevated in painful areas and fed by inflammation
We next determined that 1) TRPV4 is more highly expressed in the painful vestibule, and 2) knocking it down reduces inflammatory mediator production in vulvar fibroblasts. Heightened receptor expression (TRPV4) combined with heightened levels of the receptor ligand (5(6)-EET) could lead to enhanced pain signaling. However, the relationship with inflammation was less clear. We hypothesized that activation of TRPV4 would foster increases in PGE2 and IL-6, which is consistent with the literature. TRPV4 activation often increases inflammatory mediator production through activation of downstream inflammatory pathways122–128. Therefore, we challenged vulvar fibroblasts with increasing concentrations of the TRPV4 synthetic activator 4alpha-Phorbol 12,13-didecanoate (4αPDD) expecting to see concomitant increases in PGE2 and IL-6. However, even the highest doses of 4αPDD, which showed some cellular toxicity, did not increase levels over the vehicle control. This was surprising, but it fit with other observations that 4αPDD was unable to activate calcium flux in fibroblasts, while they were responsive to the positive controls adenosine diphosphate (ADP) and histamine. TRPV4 is a non-specific cation channel that is permeable to calcium129, 130. Measuring intracellular calcium is a convenient and sensitive measurement of calcium flux through the channel.
In taking a step back, we realized that we were missing a key element necessary to best model vulvodynia, which was ongoing inflammation. In vulvodynia, patients appear to have chronic, low levels of inflammation that likely play a role in pain. Therefore, we pre-treated cells with IL-1β overnight before challenging with 4αPDD for our calcium flux experiments. We found that with IL-1β pre-treatment, cells responded to 4αPDD, even at the lowest dose. When we simultaneously treated cells with IL-1β and increasing doses of 4αPDD, we saw a dramatic increase in IL-6 and PGE2 levels, which were up to 5-fold higher than in cells treated with IL-1β alone. Altogether these results indicate concomitant inflammation is required for TRPV4 signaling to proceed in vulvar fibroblasts. Because levels of IL-6 and PGE2 could be reduced with an inhibitor of TRPV4 (HC064047) without any added 4αPDD, we wondered if inflammation alone was sufficient to initiate calcium signaling. We found that IL-1β (endogenous stimulus) and Poly(I:C), an exogenous stimulus that mimics viral RNA, could initiate calcium flux without an activator of TRPV4, indicating inflammation is both necessary and sufficient for TRPV4 signaling. In addition, we determined that TRPV4 activity is highest in the painful vestibule in response to 4αPDD, Poly(I:C), and the natural activator, 5(6)-EET, suggesting that changes in lipid profiles in the vulvar vestibule play a role in disease.
Inflammation induces changes in lipid profiles fostering inflammation and TRPV4 signaling
To investigate how inflammation influences the vulvar lipidome, we treated fibroblasts with IL-1β, Poly(I:C), or vehicle. We found that lipids involved in the resolution of inflammation (e.g. lipoxin B4, maresin 1, EETs) were significantly reduced in the painful vestibule of cells treated with endogenous or exogenous inflammatory stimuli. At the same time, lipids involved in inflammation (e.g. prostaglandins, leukotrienes) increased with inflammatory stimulation. The greatest degree of change occurred with Poly(I:C) treatment, suggesting that cells are more responsive to exogenous stimuli, consistent with the idea that chronic vulvovaginal infection precipitates vulvodynia symptoms. Poly(I:C) stimulates toll-like receptor 3 (TLR-3), which is more highly expressed in the painful vestibule and is involved in the recognition of nucleic acids from various microorganisms12, 131. As discussed earlier, yeast infections have been implicated in the onset of vulvodynia, although most yeast infections are not diagnosed clinically. The TLR receptors, which are involved in the inflammatory response in vulvodynia, recognize yeast cells and their products, but they also recognize bacteria and viruses12, 72–74. Therefore, it is unclear if yeast species are the causative agent or whether there are other triggers. This is an area of current investigation. Nonetheless, inflammatory stimuli clearly alter lipid profiles in the painful vestibule, which favors inflammation and TRPV4 pain signaling, while reducing the resolution capacity, as evidenced by reductions in pro-resolving lipids in vestibular tissue and fibroblasts. The combination of heightened inflammatory responses to even the resident microbiota and a defect in the ability to quell this response, which is linked pain signaling, represents the “perfect storm,” eliciting chronic low levels of inflammation and pain in LPV patients. Targeting TRPV4 with HC067047 reduces inflammatory signaling, suggesting targeting TRPV4 could be another option for therapeutic development.
Treatment Strategies Structured Around the Known Vulvodynia Mechanism
Reducing inflammation and enhancing resolution through the application of SPMs and their precursors appears to be a viable treatment strategy for vulvodynia, especially given the profound analgesic properties of maresin 1 and DHA13. We have also explored the use of fish oils that are naturally enhanced for DHA and intermediates of SPM synthesis, such as 14-HDHA. These too are highly effective in reducing signs of pain in mice with vulvar allodynia. Whether this will translate to significant effects in humans remains to be seen. However, there is good reason to believe translation is likely. The use of a natural product could lead to faster implementation; SPMs cannot be purified in sufficient quantities from dietary sources, warranting chemical synthesis and FDA-regulated drug development steps.
In exploring the TRPV4 pathway, we became curious as to whether maresin 1 would impact TRPV4 signaling; SPMs, especially resolvins, have been shown to reduce the activity of closely related TRPV1103, 106, 132–136. TRPV1 recognizes capsaicin, the spicy component in chili peppers, and has been implicated in peripheral neuropathy pain123–126. We found that maresin 1 was able to impede TRPV4 signaling on the order of seconds, demonstrating an acute effect. This helps to explain maresin’s impressive pain-reducing capacity in our mouse model; it can quell the inflammation that is necessary to elicit TRPV4 signaling and it can act on TRPV4 acutely. Maresin 1, and possibly other SPMs, act on multiple parts of the established LPV mechanism. We have found that inflammatory signaling helps to increase the expression of TRPV4, while TRPV4 activation results in the augmentation of inflammatory signaling. This represents a feed-forward loop causing amplification of the signals involved in inflammation and pain signaling, much like a snowball rolling down a hill. Maresin 1 is able to reduce the size of the snowball by both quelling inflammation and inhibiting TRPV4 signaling (Figure 4).
Figure 4. Neuro-inflammatory Mechanism of Vulvodynia and Proposed Action of Maresin 1.
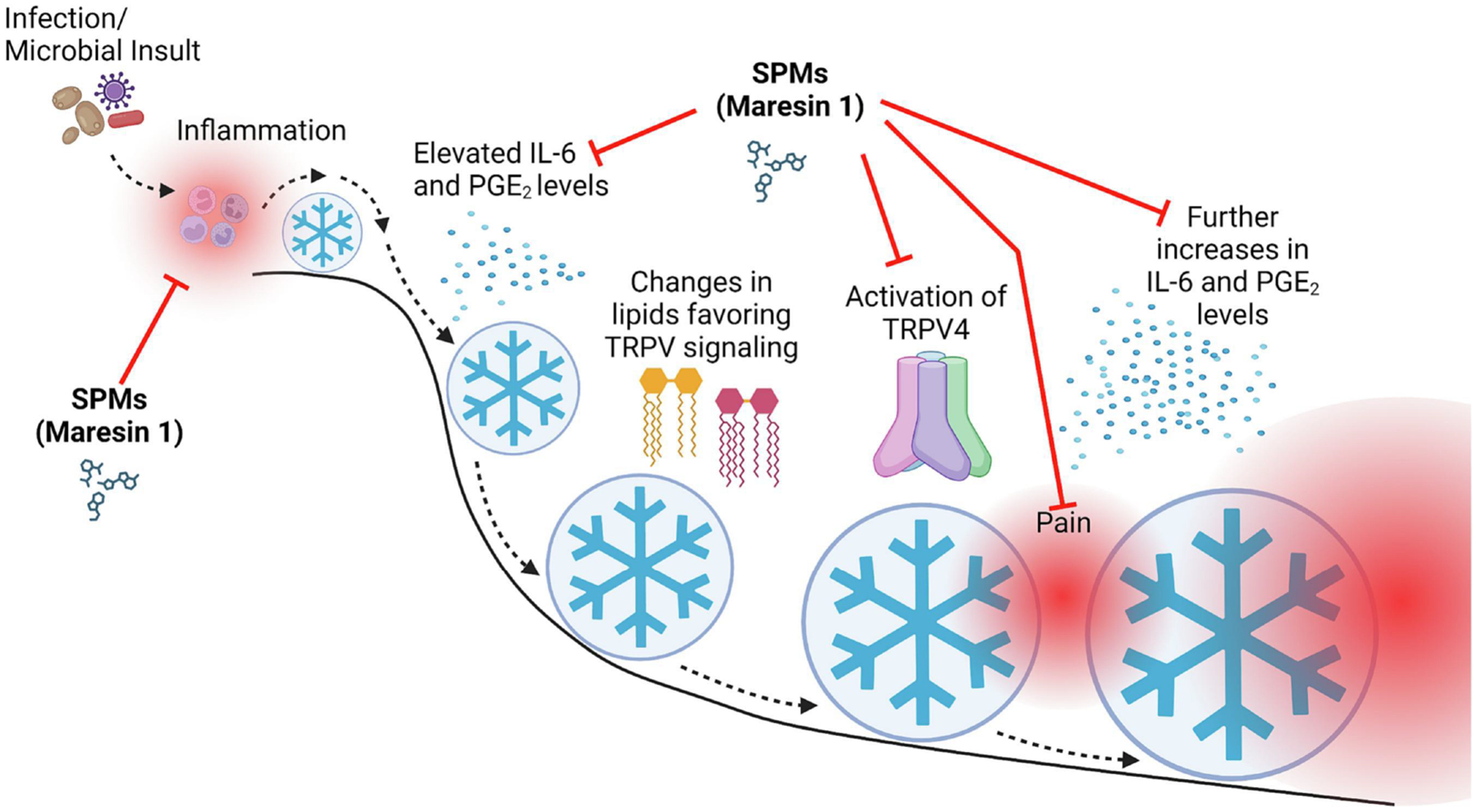
Abberant responses to inflammatory stimuli, as a result of heightened expression of receptors involved in innate immune recognition and a subsequent failure to resolve these responses due to reduced pro-resolving mediator levels, leads to chronic inflammation and changes in lipid profiles that favor the activation of the TRPV4 pain signaling pathway. When TRPV4 is activated, inflammatory mediators are further elevated, and TRPV4 expression is up-regulated, creating a feed-forward loop that amplifies inflammatory and pain signals. Much like a snowball rolling down a hill (depicted by the blue circle with snowflake symbol), this feed-forward loop gains momentum leading to a failure to resolve inflammation and thus sustained pain signaling. Application of SPMs, specifically maresin 1, inhibits several aspects of this mechanism, which is denoted by the red arrows. Maresin 1 application would thus significantly attenuate the feed-forward loop and reduce the size of or eliminate the “snowball.” We do not have any conflicts of interest to disclose.
Although, we have thus far only scratched the surface of the LPV mechanism, we have identified a class of molecules with high therapeutic capacity, which offer almost no toxicity as they are naturally produced or based on naturally produced molecules. As we continue to investigate the mechanism, we are likely to identify other analgesic targets. However, SPMs may show superior pain-relieving capacity as they act on more than one part of the pathway. We have identified other therapeutic options that could involve the use of off-label therapies such as antifungals, which also target CYP450137 and may reduce 5(6)-EET levels, or NSAIDs that target cyclooxygenase-2 (COX-2) and thus the production of PGE2138. Even diet modification to reduce sources of arachidonic acid synthesis could be another possibility, especially given that there is some evidence for the benefits of a low oxalate diet in vulvodynia, which is naturally low in sources of linoleic acid, from which arachidonic acid is produced139, 140. Improved understanding of the biological mechanisms that precipitate and sustain vulvodynia symptoms will only serve to enhance the odds of identifying promising new therapeutic avenues.
Conclusion
There is considerable work to be done, but SPMs show promise for the development of new therapeutics for LPV. They are safe and naturally produced, although drug development steps are necessary for chemically synthesized SPMs. Natural products, such as purified fish oil could represent another option, although this may not be as potent as SPMs, especially considering the apparent dysregulation of lipid metabolism in LPV patients. Natural products also pose other challenges for drug development, because they may not be patentable products. LPV appears to arise from an extreme of a natural process that provides immune defense to limit vaginal infection10–13, 15, 58. However, in patients, whether from environmental exposure, genetics, or a combination of these, a “perfect storm” occurs, where patients have enhanced inflammation without the ability to fully resolve this inflammation. This in turn leads to changes in lipid profiles and ultimately the activation of pain signaling pathways.
Fibroblasts are not sensory cells, but they may act as feeder cells to amplify inflammation and pain signals; PGE2 sensitizes neurons141, 142. Enhanced understanding of the LPV mechanism has uncovered new therapeutic targets and may help to diagnose and assess patients at earlier disease states to limit comorbid conditions and distress. Inflammation may be local in vulvodynia, but even local inflammation has systemic effects, altering brain chemistry and inducing sickness behaviors143–149. Inflammation is a key trigger that initially pushes the snowball down the hill. We have identified several possibilities to prevent or limit inflammation and thus the size of the snowball or prevent its formation entirely. The logical next step is to implement this knowledge clinically through FDA-approved trials, while continuing our investigations of the vulvodynia mechanism.
Current Knowledge Gaps and Future Directions
Although our mouse model of vulvodynia mimics the human disease in several key ways, it is impossible to recapitulate every aspect of human disease in an animal model, especially when the mechanisms of that disease are not yet fully elucidated. There is strong suspicion that vulvodynia is genetically inherited; a vulvodynia diagnosis is more likely if you have a first degree relative with this disease115. The data we have collected thus far points to a deficit in the metabolism of AA that favors inflammation while resolution is diminished. There are several well-defined small nucleotide polymorphisms (SNPs) associated with neuropathic pain that occur in the lipoxygenase genes, a key family of enzymes that metabolize AA150. One of our key future directions is to conduct genomic sequencing to identify potential SNPs or genetic changes that could explain the observed metabolic changes. This could lead to discovery of new screening tools for vulvodynia, help us to better understand the mechanism of disease, and give us additional parameters we can model in mice to more faithfully recapitulate human vulvodynia.
In addition to furthering our mechanistic understanding of disease, we plan to trial topical SPM therapy in vulvodynia patients. The University of Rochester was issued US patent 11400057 “Treatment of Vulvar Pain” for exclusive topical use of SPMs and similar preparations (e.g. fish oil) in vulvodynia patients. We are in the process of planning an industry sponsored FDA phase I/II trial using SPMs exclusively licensed to the sponsor. We anticipate that SPM therapy will improve patient reported outcomes, but there are several steps to complete, including formulation, stability programs, safety, and efficacy testing. Unfortunately, only 11% of successful animal trials translate to FDA approved applications in humans151. Therefore, while we move towards a clinical trial, we are continuing our mechanistic investigations to leave the door open for other therapeutic options and diagnostic strategies. There is still no objective test to diagnose vulvodynia, which is a significant reason many patients suffer for years before they are diagnosed and treated23, 37.
Acknowledgements
We have no conflicts to disclose. This work was funded by NIH-NICHD R01 HD092334 and R01 HD069313 and the National Vulvodynia Association. All figures were created with BioRender.com.
Abbreviations
- 4αPDD
4alpha-Phorbol 12,13-didecanoate
- ADP
adenosine diphosphate
- COX-2
cyclooxygenase-2
- CYP450
cytochrome peroxidase 450
- DHA
docosahexanoic acid
- DHET
dihydroxyeicosatrienoic acid
- DMSO
dimethyl sulfoxide
- EET
eicosatrienoic acid
- GPCR
G-coupled protein receptor
- HDHA
docosahexaenoic acid
- HETE
hydroxyeicosatetraenoic acid
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- LPV
localized provoked vulvodynia
- PAMP
pathogen associated molecular pattern
- PGE2
prostaglandin E2
- PRR
pathogen recognition receptor
- NIH
National Institutes of Health
- NSAIDs
non-steroidal anti-inflammatory drugs
- NFκB
nuclear factor kappa B
- sEH
soluble epoxide hydrolase
- SNPs
small nucleotide polymorphisms
- SNRI
serotonin and norepinephrine reuptake inhibitor
- SPM
specialized pro-resolving mediator
- SSRI
selective serotonin reuptake inhibitor
- TLR-3
toll-like receptor 3
- TRPV4
transient receptor potential vanilloid subtype 4 receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest
References
- 1.Danby CS, Margesson LJ. Approach to the diagnosis and treatment of vulvar pain. Dermatologic therapy. 2010;23(5):485–504. Epub 2010/09/28. doi: 10.1111/j.1529-8019.2010.01352.x. [DOI] [PubMed] [Google Scholar]
- 2.Simonelli C, Eleuteri S, Petruccelli F, Rossi R. Female sexual pain disorders: dyspareunia and vaginismus. Current opinion in psychiatry. 2014;27(6):406–12. Epub 2014/09/12. doi: 10.1097/YCO.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 3.Andrews JC. Vulvodynia interventions--systematic review and evidence grading. Obstetrical & gynecological survey. 2011;66(5):299–315. doi: 10.1097/OGX.0b013e3182277fb7. [DOI] [PubMed] [Google Scholar]
- 4.Bohm-Starke N. Medical and physical predictors of localized provoked vulvodynia. Acta obstetricia et gynecologica Scandinavica. 2010;89(12):1504–10. doi: 10.3109/00016349.2010.528368. [DOI] [PubMed] [Google Scholar]
- 5.Bornstein J, Goldstein AT, Stockdale CK, Bergeron S, Pukall C, Zolnoun D, Coady D, consensus vulvar pain terminology committee of the International Society for the Study of Vulvovaginal D, International Society for the Study of Women’s Sexual H, International Pelvic Pain S. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistent Vulvar Pain and Vulvodynia. The journal of sexual medicine. 2016;13(4):607–12. Epub 2016/04/06. doi: 10.1016/j.jsxm.2016.02.167. [DOI] [PubMed] [Google Scholar]
- 6.Damsted-Petersen C, Boyer SC, Pukall CF. Current perspectives in vulvodynia. Women’s health. 2009;5(4):423–36. doi: 10.2217/whe.09.30. [DOI] [PubMed] [Google Scholar]
- 7.Dargie E, Holden RR, Pukall CF. The Vulvar Pain Assessment Questionnaire: Factor Structure, Preliminary Norms, Internal Consistency, and Test-Retest Reliability. The journal of sexual medicine. 2017;14(12):1585–96. Epub 2017/12/05. doi: 10.1016/j.jsxm.2017.10.072. [DOI] [PubMed] [Google Scholar]
- 8.Donders G, Bellen G. Characteristics of the pain observed in the focal vulvodynia syndrome (VVS). Med Hypotheses. 2012;78(1):11–4. doi: 10.1016/j.mehy.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 9.Eppsteiner E, Boardman L, Stockdale CK. Vulvodynia. Best practice & research Clinical obstetrics & gynaecology. 2014;28(7):1000–12. doi: 10.1016/j.bpobgyn.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Falsetta ML, Foster DC, Bonham AD, Phipps RP. A review of the available clinical therapies for vulvodynia management and new data implicating proinflammatory mediators in pain elicitation. BJOG : an international journal of obstetrics and gynaecology. 2017;124(2):210–8. doi: 10.1111/1471-0528.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falsetta ML, Foster DC, Woeller CF, Pollock SJ, Bonham AD, Haidaris CG, Stodgell CJ, Phipps RP. Identification of novel mechanisms involved in generating localized vulvodynia pain. American journal of obstetrics and gynecology. 2015. doi: 10.1016/j.ajog.2015.02.002; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falsetta ML, Foster DC, Woeller CF, Pollock SJ, Bonham AD, Piekna-Przybylska D, Maggirwar SB, Haidaris CG, Phipps RP. Toll-Like Receptor Signaling Contributes to Proinflammatory Mediator Production in Localized Provoked Vulvodynia. Journal of lower genital tract disease. 2018;22(1):52–7. doi: 10.1097/LGT.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falsetta ML, Wood RW, Linder MA, Bonham AD, Honn KV, Maddipati KR, Phipps RP, Haidaris CG, Foster DC. Specialized Pro-resolving Mediators Reduce Pro-nociceptive Inflammatory Mediator Production in Models of Localized Provoked Vulvodynia. The journal of pain : official journal of the American Pain Society. 2021;22(10):1195–209. Epub 2021/04/05. doi: 10.1016/j.jpain.2021.03.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farmer MA, Taylor AM, Bailey AL, Tuttle AH, MacIntyre LC, Milagrosa ZE, Crissman HP, Bennett GJ, Ribeiro-da-Silva A, Binik YM, Mogil JS. Repeated vulvovaginal fungal infections cause persistent pain in a mouse model of vulvodynia. Sci Transl Med. 2011;3(101):101ra91. doi: 10.1126/scitranslmed.3002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster D, Falsetta M, Woeller C, Pollock S, Song K, Bonham A, Haidaris C, Stogell C, Messing S, Iadarola M, Phipps R. Site-specific mesenchymal control of inflammatory pain to yeast challenge in vulvodynia afflicted and pain-free women. Pain. 2015;156(3):386–96; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groysman V Vulvodynia: new concepts and review of the literature. Dermatol Clin. 2010;28(4):681–96. doi: 10.1016/j.det.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Harlow BL, Caron RE, Parker SE, Chatterjea D, Fox MP, Nguyen RHN. Recurrent Yeast Infections and Vulvodynia: Can We Believe Associations Based on Self-Reported Data? Journal of women’s health. 2017;26(10):1069–76. Epub 2017/07/08. doi: 10.1089/jwh.2016.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NIH. Multidisciplinary Research in Vulvodynia (R01) 2016. Available from: https://grants.nih.gov/grants/guide/pa-files/pa-16-102.html.
- 19.Pukall CF, Goldstein AT, Bergeron S, Foster D, Stein A, Kellogg-Spadt S, Bachmann G. Vulvodynia: Definition, Prevalence, Impact, and Pathophysiological Factors. The journal of sexual medicine. 2016;13(3):291–304. Epub 2016/03/06. doi: 10.1016/j.jsxm.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Reed BD, Harlow SD, Sen A, Legocki LJ, Edwards RM, Arato N, Haefner HK. Prevalence and demographic characteristics of vulvodynia in a population-based sample. American journal of obstetrics and gynecology. 2012;206(2):170 e1–9. doi: 10.1016/j.ajog.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed BD, Legocki LJ, Plegue MA, Sen A, Haefner HK, Harlow SD. Factors associated with vulvodynia incidence. Obstetrics and gynecology. 2014;123(2 Pt 1):225–31. doi: 10.1097/AOG.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen NO, Dawson SJ, Brooks M, Kellogg-Spadt S. Treatment of Vulvodynia: Pharmacological and Non-Pharmacological Approaches. Drugs. 2019;79(5):483–93. Epub 2019/03/09. doi: 10.1007/s40265-019-01085-1. [DOI] [PubMed] [Google Scholar]
- 23.Stockdale CK, Lawson HW. 2013 Vulvodynia Guideline update. Journal of lower genital tract disease. 2014;18(2):93–100. Epub 2014/03/19. doi: 10.1097/LGT.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 24.Wesselmann U, Bonham A, Foster D. Vulvodynia: Current state of the biological science. Pain. 2014. doi: 10.1016/j.pain.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wylie KR. Vulvodynia - a painful condition; an inflammatory process? BJOG : an international journal of obstetrics and gynaecology. 2017;124(2):219. Epub 2016/11/20. doi: 10.1111/1471-0528.14415. [DOI] [PubMed] [Google Scholar]
- 26.Zanotta N, Campisciano G, Scrimin F, Ura B, Marcuzzi A, Vincenti E, Crovella S, Comar M. Cytokine profiles of women with vulvodynia: Identification of a panel of pro-inflammatory molecular targets. European journal of obstetrics, gynecology, and reproductive biology. 2018;226:66–70. Epub 2018/06/01. doi: 10.1016/j.ejogrb.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 27.Vulvar Pain [Internet]2000–2023 [cited March 13, 2023]. Available from: https://reporter.nih.gov/.
- 28.Parish SJ, Clayton AH. Sexual medicine education: review and commentary. The journal of sexual medicine. 2007;4(2):259–67; quiz 68. Epub 2007/03/21. doi: 10.1111/j.1743-6109.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- 29.Rubin ES, Rullo J, Tsai P, Criniti S, Elders J, Thielen JM, Parish SJ. Best Practices in North American Pre-Clinical Medical Education in Sexual History Taking: Consensus From the Summits in Medical Education in Sexual Health. The journal of sexual medicine. 2018;15(10):1414–25. Epub 2018/10/10. doi: 10.1016/j.jsxm.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Shindel AW, Parish SJ. Sexuality education in North American medical schools: current status and future directions. The journal of sexual medicine. 2013;10(1):3–17; quiz 8. Epub 2013/01/25. doi: 10.1111/j.1743-6109.2012.02987.x. [DOI] [PubMed] [Google Scholar]
- 31.Solursh DS, Ernst JL, Lewis RW, Prisant LM, Mills TM, Solursh LP, Jarvis RG, Salazar WH. The human sexuality education of physicians in North American medical schools. Int J Impot Res. 2003;15 Suppl 5:S41–5. Epub 2003/10/11. doi: 10.1038/sj.ijir.3901071. [DOI] [PubMed] [Google Scholar]
- 32.Kingsberg SA, Schaffir J, Faught BM, Pinkerton JV, Parish SJ, Iglesia CB, Gudeman J, Krop J, Simon JA. Female Sexual Health: Barriers to Optimal Outcomes and a Roadmap for Improved Patient-Clinician Communications. Journal of women’s health. 2019;28(4):432–43. Epub 2019/02/05. doi: 10.1089/jwh.2018.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harlow BL, Kunitz CG, Nguyen RH, Rydell SA, Turner RM, MacLehose RF. Prevalence of symptoms consistent with a diagnosis of vulvodynia: population-based estimates from 2 geographic regions. American journal of obstetrics and gynecology. 2014;210(1):40 e1–8. doi: 10.1016/j.ajog.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? Journal of the American Medical Women’s Association. 2003;58(2):82–8. [PubMed] [Google Scholar]
- 35.Nguyen RH, Turner RM, Rydell SA, Maclehose RF, Harlow BL. Perceived stereotyping and seeking care for chronic vulvar pain. Pain medicine. 2013;14(10):1461–7. doi: 10.1111/pme.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed BD, Harlow SD, Sen A, Edwards RM, Chen D, Haefner HK. Relationship between vulvodynia and chronic comorbid pain conditions. Obstetrics and gynecology. 2012;120(1):145–51. doi: 10.1097/AOG.0b013e31825957cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haefner HK, Collins ME, Davis GD, Edwards L, Foster DC, Hartmann ED, Kaufman RH, Lynch PJ, Margesson LJ, Moyal-Barracco M, Piper CK, Reed BD, Stewart EG, Wilkinson EJ. The vulvodynia guideline. Journal of lower genital tract disease. 2005;9(1):40–51. [DOI] [PubMed] [Google Scholar]
- 38.Sadownik LA. Etiology, diagnosis, and clinical management of vulvodynia. Int J Womens Health. 2014;6:437–49. doi: 10.2147/IJWH.S37660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeon Y, Kim Y, Shim B, Yoon H, Park Y, Shim B, Jeong W, Lee D. A retrospective study of the management of vulvodynia. Korean journal of urology. 2013;54(1):48–52. doi: 10.4111/kju.2013.54.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kingdon J. Vulvodynia: a comprehensive review. Nurs Womens Health. 2009;13(1):48–57; quiz 8. doi: 10.1111/j.1751-486X.2009.01373.x. [DOI] [PubMed] [Google Scholar]
- 41.Nunns D, Mandal D, Byrne M, McLelland J, Rani R, Cullimore J, Bansal D, Brackenbury F, Kirtschig G, Wier M, British Society for the Study of Vulval Disease Guideline G. Guidelines for the management of vulvodynia. The British journal of dermatology. 2010;162(6):1180–5. doi: 10.1111/j.1365-2133.2010.09684.x. [DOI] [PubMed] [Google Scholar]
- 42.Stenson AL. Vulvodynia: Diagnosis and Management. Obstet Gynecol Clin North Am. 2017;44(3):493–508. Epub 2017/08/06. doi: 10.1016/j.ogc.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Akopians AL, Rapkin AJ. Vulvodynia: The Role of Inflammation in the Etiology of Localized Provoked Pain of the Vulvar Vestibule (Vestibulodynia). Semin Reprod Med. 2015;33(4):239–45. Epub 2015/07/02. doi: 10.1055/s-0035-1554919. [DOI] [PubMed] [Google Scholar]
- 44.Tommola P, Unkila-Kallio L, Paavonen J. Surgical treatment of vulvar vestibulitis: a review. Acta obstetricia et gynecologica Scandinavica. 2010;89(11):1385–95. doi: 10.3109/00016349.2010.512071. [DOI] [PubMed] [Google Scholar]
- 45.Tommola P, Unkila-Kallio L, Paavonen J. Long-term follow up of posterior vestibulectomy for treating vulvar vestibulitis. Acta obstetricia et gynecologica Scandinavica. 2011;90(11):1225–31. doi: 10.1111/j.1600-0412.2011.01248.x. [DOI] [PubMed] [Google Scholar]
- 46.Tommola P, Unkila-Kallio L, Paavonen J. Long-term well-being after surgical or conservative treatment of severe vulvar vestibulitis. Acta obstetricia et gynecologica Scandinavica. 2012;91(9):1086–93. doi: 10.1111/j.1600-0412.2012.01466.x. [DOI] [PubMed] [Google Scholar]
- 47.Wu C, Goldstein A, Klebanoff JS, Moawad GN. Surgical management of neuroproliferative-associated vestibulodynia: a tutorial on vestibulectomy with vaginal advancement flap. American journal of obstetrics and gynecology. 2019;221(5):525 e1–e2. Epub 2019/08/14. doi: 10.1016/j.ajog.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Fischer G Management of vulvar pain. Dermatologic therapy. 2004;17(1):134–49. [DOI] [PubMed] [Google Scholar]
- 49.Spoelstra SK, Dijkstra JR, van Driel MF, Weijmar Schultz WC. Long-term results of an individualized, multifaceted, and multidisciplinary therapeutic approach to provoked vestibulodynia. The journal of sexual medicine. 2011;8(2):489–96. doi: 10.1111/j.1743-6109.2010.01941.x. [DOI] [PubMed] [Google Scholar]
- 50.Tieu KD, MacGregor JL. Successful treatment of vulvodynia with botulinum toxin A. Archives of dermatology. 2011;147(2):251–2. doi: 10.1001/archdermatol.2010.443. [DOI] [PubMed] [Google Scholar]
- 51.Barry CM, Matusica D, Haberberger RV. Emerging Evidence of Macrophage Contribution to Hyperinnervation and Nociceptor Sensitization in Vulvodynia. Front Mol Neurosci. 2019;12:186. Epub 2019/08/27. doi: 10.3389/fnmol.2019.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leclair CM, Goetsch MF, Korcheva VB, Anderson R, Peters D, Morgan TK. Differences in primary compared with secondary vestibulodynia by immunohistochemistry. Obstetrics and gynecology. 2011;117(6):1307–13. doi: 10.1097/AOG.0b013e31821c33dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leclair CM, Leeborg NJ, Jacobson-Dunlop E, Goetsch MF, Morgan TK. CD4-positive T-cell recruitment in primary-provoked localized vulvodynia: potential insights into disease triggers. Journal of lower genital tract disease. 2014;18(2):195–201. doi: 10.1097/LGT.0b013e3182a55591. [DOI] [PubMed] [Google Scholar]
- 54.Reed BD, Plegue MA, Sen A, Haefner HK, Siddiqui J, Remick DG. Nerve Growth Factor and Selected Cytokines in Women With and Without Vulvodynia. Journal of lower genital tract disease. 2018;22(2):139–46. Epub 2018/03/24. doi: 10.1097/LGT.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 55.Tommola P, Butzow R, Unkila-Kallio L, Paavonen J, Meri S. Activation of vestibule-associated lymphoid tissue in localized provoked vulvodynia. American journal of obstetrics and gynecology. 2015;212(4):476 e1–8. doi: 10.1016/j.ajog.2014.10.1098. [DOI] [PubMed] [Google Scholar]
- 56.Tommola P, Unkila-Kallio L, Paetau A, Meri S, Kalso E, Paavonen J. Immune activation enhances epithelial nerve growth in provoked vestibulodynia. American journal of obstetrics and gynecology. 2016;215(6):768 e1–e8. Epub 2016/07/28. doi: 10.1016/j.ajog.2016.07.037. [DOI] [PubMed] [Google Scholar]
- 57.Baker D, Peresleni T, Kocis C. Inflammatory Markers in Vestibulodynia. Obstetrics and gynecology. 2016;127(Suppl 1):1S–2S. [Google Scholar]
- 58.Falsetta ML, Foster DC, Woeller CF, Pollock SJ, Bonham AD, Haidaris CG, Phipps RP. A role for bradykinin signaling in chronic vulvar pain. The journal of pain : official journal of the American Pain Society. 2016. doi: 10.1016/j.jpain.2016.07.007; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foster DC, Piekarz KH, Murant TI, LaPoint R, Haidaris CG, Phipps RP. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: implications for vulvar vestibulitis. American journal of obstetrics and gynecology. 2007;196(4):346 e1–8. doi: 10.1016/j.ajog.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 60.Arnold LD, Bachmann GA, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstetrics and gynecology. 2006;107(3):617–24. doi: 10.1097/01.AOG.0000199951.26822.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lipsky MS, Taylor C. The use of over-the-counter antifungal vaginitis preparations by college students. Fam Med. 1996;28(7):493–5. Epub 1996/07/01. [PubMed] [Google Scholar]
- 62.Mendling W, Brasch J, German Society for G, Obstetrics, Working Group for I, Infectimmunology in G, Obstetrics, German Society of Dermatology tBoGD, German Speaking Mycological S. Guideline vulvovaginal candidosis (2010) of the German Society for Gynecology and Obstetrics, the Working Group for Infections and Infectimmunology in Gynecology and Obstetrics, the German Society of Dermatology, the Board of German Dermatologists and the German Speaking Mycological Society. Mycoses. 2012;55 Suppl 3:1–13. Epub 2012/05/02. doi: 10.1111/j.1439-0507.2012.02185.x. [DOI] [PubMed] [Google Scholar]
- 63.Hani U, Shivakumar HG, Vaghela R, Osmani RA, Shrivastava A. Candidiasis: a fungal infection--current challenges and progress in prevention and treatment. Infect Disord Drug Targets. 2015;15(1):42–52. Epub 2015/03/27. doi: 10.2174/1871526515666150320162036. [DOI] [PubMed] [Google Scholar]
- 64.Giraldo P, von Nowaskonski A, Gomes FA, Linhares I, Neves NA, Witkin SS. Vaginal colonization by Candida in asymptomatic women with and without a history of recurrent vulvovaginal candidiasis. Obstetrics and gynecology. 2000;95(3):413–6. [DOI] [PubMed] [Google Scholar]
- 65.Peters BM, Palmer GE, Nash AK, Lilly EA, Fidel PL Jr., Noverr MC. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infection and immunity. 2014;82(2):532–43. doi: 10.1128/IAI.01417-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramirez De Knott HM, McCormick TS, Do SO, Goodman W, Ghannoum MA, Cooper KD, Nedorost ST. Cutaneous hypersensitivity to Candida albicans in idiopathic vulvodynia. Contact Dermatitis. 2005;53(4):214–8. doi: 10.1111/j.0105-1873.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- 67.Nett JE, Sanchez H, Cain MT, Andes DR. Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J Infect Dis. 2010;202(1):171–5. doi: 10.1086/651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taff HT, Nett JE, Zarnowski R, Ross KM, Sanchez H, Cain MT, Hamaker J, Mitchell AP, Andes DR. A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLoS pathogens. 2012;8(8):e1002848. doi: 10.1371/journal.ppat.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alves CT, Wei XQ, Silva S, Azeredo J, Henriques M, Williams DW. Candida albicans promotes invasion and colonisation of Candida glabrata in a reconstituted human vaginal epithelium. J Infect. 2014. doi: 10.1016/j.jinf.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 70.Chaffin WL. Candida albicans cell wall proteins. Microbiol Mol Biol Rev. 2008;72(3):495–544. doi: 10.1128/MMBR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harriott MM, Lilly EA, Rodriguez TE, Fidel PL Jr., Noverr MC. Candida albicans forms biofilms on the vaginal mucosa. Microbiology. 2010;156(Pt 12):3635–44. doi: 10.1099/mic.0.039354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bourgeois C, Kuchler K. Fungal pathogens-a sweet and sour treat for toll-like receptors. Frontiers in cellular and infection microbiology. 2012;2:142. doi: 10.3389/fcimb.2012.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doyle SL, O’Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochemical pharmacology. 2006;72(9):1102–13. doi: 10.1016/j.bcp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 74.Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2012;10(2):112–22. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chadha S, Gianotten WL, Drogendijk AC, Weijmar Schultz WC, Blindeman LA, van der Meijden WI. Histopathologic features of vulvar vestibulitis. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 1998;17(1):7–11. [DOI] [PubMed] [Google Scholar]
- 76.Friedrich EG Jr. The vulvar vestibule. The Journal of reproductive medicine. 1983;28(11):773–7. [PubMed] [Google Scholar]
- 77.Farage M, Maibach H. Lifetime changes in the vulva and vagina. Archives of gynecology and obstetrics. 2006;273(4):195–202. Epub 2005/10/07. doi: 10.1007/s00404-005-0079-x. [DOI] [PubMed] [Google Scholar]
- 78.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. The Journal of experimental medicine. 2003;197(9):1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luqman S, Pezzuto JM. NFkappaB: a promising target for natural products in cancer chemoprevention. Phytotherapy research : PTR. 2010;24(7):949–63. doi: 10.1002/ptr.3171. [DOI] [PubMed] [Google Scholar]
- 80.Lawrence T The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor perspectives in biology. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arriaga-Gomez E, Kline J, Emanuel E, Neamonitaki N, Yangdon T, Zacheis H, Pasha D, Lim J, Bush S, Boo B, Mengistu H, Kinnamon R, Shields-Cutler R, Wattenberg E, Chatterjea D. Repeated Vaginal Exposures to the Common Cosmetic and Household Preservative Methylisothiazolinone Induce Persistent, Mast Cell-Dependent Genital Pain in ND4 Mice. Int J Mol Sci. 2019;20(21). Epub 2019/10/31. doi: 10.3390/ijms20215361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boo B, Kamath R, Arriaga-Gomez E, Landry J, Emanuel E, Joo S, Saldias Montivero M, Martinov T, Fife BT, Chatterjea D. Tetrahydrocannabinol Reduces Hapten-Driven Mast Cell Accumulation and Persistent Tactile Sensitivity in Mouse Model of Allergen-Provoked Localized Vulvodynia. Int J Mol Sci. 2019;20(9). Epub 2019/05/06. doi: 10.3390/ijms20092163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pierce AN, Zhang Z, Fuentes IM, Wang R, Ryals JM, Christianson JA. Neonatal vaginal irritation results in long-term visceral and somatic hypersensitivity and increased hypothalamic-pituitary-adrenal axis output in female mice. Pain. 2015;156(10):2021–31. Epub 2015/06/23. doi: 10.1097/j.pain.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ali G, Subhan F, Abbas M, Zeb J, Shahid M, Sewell RD. A streptozotocin-induced diabetic neuropathic pain model for static or dynamic mechanical allodynia and vulvodynia: validation using topical and systemic gabapentin. Naunyn Schmiedebergs Arch Pharmacol. 2015;388(11):1129–40. Epub 2015/07/03. doi: 10.1007/s00210-015-1145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Awad-Igbaria Y, Dadon S, Shamir A, Livoff A, Shlapobersky M, Bornstein J, Palzur E. Characterization of Early Inflammatory Events Leading to Provoked Vulvodynia Development in Rats. J Inflamm Res. 2022;15:3901–23. Epub 2022/07/19. doi: 10.2147/JIR.S367193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rangappa S, Shankar VK, Jo S, Repka MA, Murthy SN. Chemotherapeutic Agent-Induced Vulvodynia, an Experimental Model. AAPS PharmSciTech. 2021;22(3):95. Epub 2021/03/10. doi: 10.1208/s12249-021-01969-0. [DOI] [PubMed] [Google Scholar]
- 87.Akbar S, Subhan F, Karim N, Shahid M, Ahmad N, Ali G, Mahmood W, Fawad K. 6-Methoxyflavanone attenuates mechanical allodynia and vulvodynia in the streptozotocin-induced diabetic neuropathic pain. Biomed Pharmacother. 2016;84:962–71. Epub 2016/10/21. doi: 10.1016/j.biopha.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 88.Aman U, Subhan F, Shahid M, Akbar S, Ahmad N, Ali G, Fawad K, Sewell RD. Passiflora incarnata attenuation of neuropathic allodynia and vulvodynia apropos GABA-ergic and opioidergic antinociceptive and behavioural mechanisms. BMC Complement Altern Med. 2016;16:77. Epub 2016/02/26. doi: 10.1186/s12906-016-1048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 2017;31(4):1273–88. Epub 2017/01/15. doi: 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Serhan CN, Chiang N, Dalli J, Levy BD. Lipid mediators in the resolution of inflammation. Cold Spring Harbor perspectives in biology. 2014;7(2):a016311. Epub 2014/11/02. doi: 10.1101/cshperspect.a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40(3):315–27. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buckley CD, Gilroy DW, Serhan CN, Stockinger B, Tak PP. The resolution of inflammation. Nature reviews Immunology. 2013;13(1):59–66. doi: 10.1038/nri3362. [DOI] [PubMed] [Google Scholar]
- 93.Chiurchiu V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, Serhan CN. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med. 2016;8(353):353ra111. Epub 2016/08/26. doi: 10.1126/scitranslmed.aaf7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cianci E, Recchiuti A, Trubiani O, Diomede F, Marchisio M, Miscia S, Colas RA, Dalli J, Serhan CN, Romano M. Human Periodontal Stem Cells Release Specialized Proresolving Mediators and Carry Immunomodulatory and Prohealing Properties Regulated by Lipoxins. Stem Cells Transl Med. 2016;5(1):20–32. Epub 2015/11/27. doi: 10.5966/sctm.2015-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Croasdell A, Thatcher TH, Kottmann RM, Colas RA, Dalli J, Serhan CN, Sime PJ, Phipps RP. Resolvins attenuate inflammation and promote resolution in cigarette smoke-exposed human macrophages. Am J Physiol Lung Cell Mol Physiol. 2015;309(8):L888–901. Epub 2015/08/25. doi: 10.1152/ajplung.00125.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dakin SG, Colas RA, Wheway K, Watkins B, Appleton L, Rees J, Gwilym S, Little C, Dalli J, Carr AJ. Proresolving Mediators LXB4 and RvE1 Regulate Inflammation in Stromal Cells from Patients with Shoulder Tendon Tears. Am J Pathol. 2019;189(11):2258–68. Epub 2019/08/23. doi: 10.1016/j.ajpath.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fonseca FC, Orlando RM, Turchetti-Maia RM, de Francischi JN. Comparative effects of the omega3 polyunsaturated fatty acid derivatives resolvins E1 and D1 and protectin DX in models of inflammation and pain. J Inflamm Res. 2017;10:119–33. Epub 2017/09/19. doi: 10.2147/JIR.S142424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annual review of physiology. 2014;76:467–92. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Serhan CN. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol Aspects Med. 2017;58:1–11. Epub 2017/03/07. doi: 10.1016/j.mam.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Seminars in immunology. 2015. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Serhan CN, Chiang N, Dalli J. New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol Aspects Med. 2018;64:1–17. Epub 2017/08/15. doi: 10.1016/j.mam.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luo X, Gu Y, Tao X, Serhan CN, Ji RR. Resolvin D5 Inhibits Neuropathic and Inflammatory Pain in Male But Not Female Mice: Distinct Actions of D-Series Resolvins in Chemotherapy-Induced Peripheral Neuropathy. Frontiers in pharmacology. 2019;10:745. Epub 2019/07/25. doi: 10.3389/fphar.2019.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tao X, Lee MS, Donnelly CR, Ji RR. Neuromodulation, Specialized Proresolving Mediators, and Resolution of Pain. Neurotherapeutics. 2020;17(3):886–99. Epub 2020/07/23. doi: 10.1007/s13311-020-00892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang L, Terrando N, Xu ZZ, Bang S, Jordt SE, Maixner W, Serhan CN, Ji RR. Distinct Analgesic Actions of DHA and DHA-Derived Specialized Pro-Resolving Mediators on Post-operative Pain After Bone Fracture in Mice. Frontiers in pharmacology. 2018;9:412. Epub 2018/05/17. doi: 10.3389/fphar.2018.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26(4):1755–65. Epub 2012/01/19. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiang N, Serhan CN. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med. 2017;58:114–29. Epub 2017/03/25. doi: 10.1016/j.mam.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chiang N, Serhan CN. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem. 2020;64(3):443–62. Epub 2020/09/05. doi: 10.1042/EBC20200018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87(11):992–8. Epub 2000/11/25. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 110.Maddipati KR. Non-inflammatory Physiology of “Inflammatory” Mediators - Unalamation, a New Paradigm. Front Immunol. 2020;11:580117. Epub 2020/10/30. doi: 10.3389/fimmu.2020.580117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thomson SJ, Askari A, Bishop-Bailey D. Anti-inflammatory effects of epoxyeicosatrienoic acids. Int J Vasc Med. 2012;2012:605101. Epub 2012/08/01. doi: 10.1155/2012/605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goldstein AT, Belkin ZR, Krapf JM, Song W, Khera M, Jutrzonka SL, Kim NN, Burrows LJ, Goldstein I. Polymorphisms of the androgen receptor gene and hormonal contraceptive induced provoked vestibulodynia. The journal of sexual medicine. 2014;11(11):2764–71. Epub 2014/09/05. doi: 10.1111/jsm.12668. [DOI] [PubMed] [Google Scholar]
- 113.Jeremias J, Ledger WJ, Witkin SS. Interleukin 1 receptor antagonist gene polymorphism in women with vulvar vestibulitis. American journal of obstetrics and gynecology. 2000;182(2):283–5. Epub 2000/02/29. doi: 10.1016/s0002-9378(00)70212-2. [DOI] [PubMed] [Google Scholar]
- 114.Kalfon L, Azran A, Farajun Y, Golan-Hamu O, Toben A, Abramov L, Yeshaya A, Yakir O, Zarfati D, Falik Zaccai TC, Bornstein J. Localized Provoked Vulvodynia: Association With Nerve Growth Factor and Transient Receptor Potential Vanilloid Type 1 Genes Polymorphisms. Journal of lower genital tract disease. 2019;23(1):58–64. Epub 2018/11/13. doi: 10.1097/LGT.0000000000000445. [DOI] [PubMed] [Google Scholar]
- 115.Morgan TK, Allen-Brady KL, Monson MA, Leclair CM, Sharp HT, Cannon-Albright LA. Familiality analysis of provoked vestibulodynia treated by vestibulectomy supports genetic predisposition. American journal of obstetrics and gynecology. 2016;214(5):609 e1–7. Epub 2015/12/03. doi: 10.1016/j.ajog.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 116.Reed BD, Harlow SD, Plegue MA, Sen A. Genetic differences may reflect differences in susceptibility to vulvodynia in general or in spontaneous remission propensity. The journal of sexual medicine. 2015;12(2):578. Epub 2014/11/27. doi: 10.1111/jsm.12775. [DOI] [PubMed] [Google Scholar]
- 117.Morris RW. Analgesic and local anesthetic activity of dimethyl sulfoxide. J Pharm Sci. 1966;55(4):438–40. Epub 1966/04/01. doi: 10.1002/jps.2600550421. [DOI] [PubMed] [Google Scholar]
- 118.Pearson T, Warren AY, Barrett DA, Khan RN. Detection of EETs and HETE-generating cytochrome P-450 enzymes and the effects of their metabolites on myometrial and vascular function. Am J Physiol Endocrinol Metab. 2009;297(3):E647–56. Epub 2009/06/25. doi: 10.1152/ajpendo.00227.2009. [DOI] [PubMed] [Google Scholar]
- 119.Hammock BD, McReynolds CB, Wagner K, Buckpitt A, Cortes-Puch I, Croston G, Lee KSS, Yang J, Schmidt WK, Hwang SH. Movement to the Clinic of Soluble Epoxide Hydrolase Inhibitor EC5026 as an Analgesic for Neuropathic Pain and for Use as a Nonaddictive Opioid Alternative. Journal of medicinal chemistry. 2021;64(4):1856–72. Epub 2021/02/09. doi: 10.1021/acs.jmedchem.0c01886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zosmer A, Elder MG, Sullivan MH. The production of progesterone and 5,6-epoxyeicosatrienoic acid by human granulosa cells. J Steroid Biochem Mol Biol. 2002;81(4–5):369–76. Epub 2002/10/04. doi: 10.1016/s0960-0760(02)00116-4. [DOI] [PubMed] [Google Scholar]
- 121.Berna-Erro A, Izquierdo-Serra M, Sepulveda RV, Rubio-Moscardo F, Donate-Macian P, Serra SA, Carrillo-Garcia J, Peralvarez-Marin A, Gonzalez-Nilo F, Fernandez-Fernandez JM, Valverde MA. Structural determinants of 5’,6’-epoxyeicosatrienoic acid binding to and activation of TRPV4 channel. Sci Rep. 2017;7(1):10522. Epub 2017/09/07. doi: 10.1038/s41598-017-11274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ito M, Ono K, Hitomi S, Nodai T, Sago T, Yamaguchi K, Harano N, Gunnjigake K, Hosokawa R, Kawamoto T, Inenaga K. Prostanoid-dependent spontaneous pain and PAR2-dependent mechanical allodynia following oral mucosal trauma: involvement of TRPV1, TRPA1 and TRPV4. Mol Pain. 2017;13:1744806917704138. Epub 2017/04/07. doi: 10.1177/1744806917704138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kaneko Y, Szallasi A. Transient receptor potential (TRP) channels: a clinical perspective. British journal of pharmacology. 2014;171(10):2474–507. Epub 2013/10/10. doi: 10.1111/bph.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mickle AD, Shepherd AJ, Mohapatra DP. Sensory TRP channels: the key transducers of nociception and pain. Prog Mol Biol Transl Sci. 2015;131:73–118. Epub 2015/03/07. doi: 10.1016/bs.pmbts.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB. Regulation of Pain and Itch by TRP Channels. Neuroscience bulletin. 2018;34(1):120–42. Epub 2017/12/29. doi: 10.1007/s12264-017-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moran MM, Szallasi A. Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. British journal of pharmacology. 2018;175(12):2185–203. Epub 2017/09/20. doi: 10.1111/bph.14044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Poole DP, Amadesi S, Veldhuis NA, Abogadie FC, Lieu T, Darby W, Liedtke W, Lew MJ, McIntyre P, Bunnett NW. Protease-activated receptor 2 (PAR2) protein and transient receptor potential vanilloid 4 (TRPV4) protein coupling is required for sustained inflammatory signaling. The Journal of biological chemistry. 2013;288(8):5790–802. Epub 2013/01/05. doi: 10.1074/jbc.M112.438184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang C, Ning LP, Wang YH, Zhang Y, Ding XL, Ge HY, Arendt-Nielsen L, Yue SW. Nuclear factor-kappa B mediates TRPV4-NO pathway involved in thermal hyperalgesia following chronic compression of the dorsal root ganglion in rats. Behav Brain Res. 2011;221(1):19–24. Epub 2011/03/02. doi: 10.1016/j.bbr.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 129.Darby WG, Grace MS, Baratchi S, McIntyre P. Modulation of TRPV4 by diverse mechanisms. Int J Biochem Cell Biol. 2016;78:217–28. Epub 2016/07/19. doi: 10.1016/j.biocel.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 130.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97(9):908–15. Epub 2005/09/24. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 131.Chiba Y, Mizoguchi I, Mitobe K, Higuchi K, Nagai H, Nishigori C, Mizuguchi J, Yoshimoto T. IL-27 enhances the expression of TRAIL and TLR3 in human melanomas and inhibits their tumor growth in cooperation with a TLR3 agonist poly(I:C) partly in a TRAIL-dependent manner. PloS one. 2013;8(10):e76159. doi: 10.1371/journal.pone.0076159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. 17(R)-resolvin D1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. British journal of pharmacology. 2012;165(3):683–92. Epub 2011/07/02. doi: 10.1111/j.1476-5381.2011.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. British journal of pharmacology. 2010;161(3):707–20. Epub 2010/10/01. doi: 10.1111/j.1476-5381.2010.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jo YY, Lee JY, Park CK. Resolvin E1 Inhibits Substance P-Induced Potentiation of TRPV1 in Primary Sensory Neurons. Mediators of inflammation. 2016;2016:5259321. Epub 2016/10/16. doi: 10.1155/2016/5259321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Park CK, Xu ZZ, Liu T, Lu N, Serhan CN, Ji RR. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci. 2011;31(50):18433–8. Epub 2011/12/16. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Payrits M, Horvath A, Biro-Suto T, Erostyak J, Makkai G, Saghy E, Pohoczky K, Kecskes A, Kecskes M, Szolcsanyi J, Helyes Z, Szoke E. Resolvin D1 and D2 Inhibit Transient Receptor Potential Vanilloid 1 and Ankyrin 1 Ion Channel Activation on Sensory Neurons via Lipid Raft Modification. Int J Mol Sci. 2020;21(14). Epub 2020/07/28. doi: 10.3390/ijms21145019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kjaerstad MB, Nielsen F, Nohr-Jensen L, Zwisler S, Brosen K, Andersen HR. Systemic uptake of miconazole during vaginal suppository use and effect on CYP1A2 and CYP3A4 associated enzyme activities in women. Eur J Clin Pharmacol. 2010;66(12):1189–97. Epub 2010/10/07. doi: 10.1007/s00228-010-0906-2. [DOI] [PubMed] [Google Scholar]
- 138.Kawabata A Prostaglandin E2 and pain--an update. Biological & pharmaceutical bulletin. 2011;34(8):1170–3. [DOI] [PubMed] [Google Scholar]
- 139.Greenstein A, Militscher I, Chen J, Matzkin H, Lessing JB, Abramov L. Hyperoxaluria in women with vulvar vestibulitis syndrome. The Journal of reproductive medicine. 2006;51(6):500–2. Epub 2006/07/19. [PubMed] [Google Scholar]
- 140.Harlow BL, Abenhaim HA, Vitonis AF, Harnack L. Influence of dietary oxalates on the risk of adult-onset vulvodynia. The Journal of reproductive medicine. 2008;53(3):171–8. Epub 2008/04/30. [PubMed] [Google Scholar]
- 141.Reinold H, Ahmadi S, Depner UB, Layh B, Heindl C, Hamza M, Pahl A, Brune K, Narumiya S, Muller U, Zeilhofer HU. Spinal inflammatory hyperalgesia is mediated by prostaglandin E receptors of the EP2 subtype. The Journal of clinical investigation. 2005;115(3):673–9. doi: 10.1172/JCI23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shapiro H, Singer P, Ariel A. Beyond the classic eicosanoids: Peripherally-acting oxygenated metabolites of polyunsaturated fatty acids mediate pain associated with tissue injury and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2016;111:45–61. Epub 2016/04/14. doi: 10.1016/j.plefa.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 143.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12(5):255–69. Epub 2005/09/17. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 144.Elmquist JK, Scammell TE, Saper CB. Mechanisms of CNS response to systemic immune challenge: the febrile response. Trends Neurosci. 1997;20(12):565–70. Epub 1998/01/07. doi: 10.1016/s0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- 145.Knorr C, Marks D, Gerstberger R, Muhlradt PF, Roth J, Rummel C. Peripheral and central cyclooxygenase (COX) products may contribute to the manifestation of brain-controlled sickness responses during localized inflammation induced by macrophage-activating lipopeptide-2 (MALP-2). Neuroscience letters. 2010;479(2):107–11. Epub 2010/06/29. doi: 10.1016/j.neulet.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 146.Pentreath VW, Baugh PJ, Lavin DR. Sleeping sickness and the central nervous system. Onderstepoort J Vet Res. 1994;61(4):369–77. Epub 1994/12/01. [PubMed] [Google Scholar]
- 147.Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain, behavior, and immunity. 2004;18(5):407–13. Epub 2004/07/22. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 148.Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120(3):277–86. Epub 2010/07/21. doi: 10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- 149.Zhang H, Ching S, Chen Q, Li Q, An Y, Quan N. Localized inflammation in peripheral tissue signals the CNS for sickness response in the absence of interleukin-1 and cyclooxygenase-2 in the blood and brain. Neuroscience. 2008;157(4):895–907. Epub 2008/10/28. doi: 10.1016/j.neuroscience.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zheng Z, Li Y, Jin G, Huang T, Zou M, Duan S. The biological role of arachidonic acid 12-lipoxygenase (ALOX12) in various human diseases. Biomed Pharmacother. 2020;129:110354. Epub 2020/06/17. doi: 10.1016/j.biopha.2020.110354. [DOI] [PubMed] [Google Scholar]
- 151.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10(4):283–94. Epub 2009/03/05. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]


