Abstract
Treatment discontinuation during clinical trials in schizophrenia is a critical challenge, especially for longer-term interventions in the early course. This research explored predictors of treatment discontinuation in an outpatient early course schizophrenia sample (N = 102) during an 18-month multi-site trial of Cognitive Enhancement Therapy (n = 58) and Enriched Supportive Therapy (n = 44). Fifty-three (52%) participants discontinued, with no significant difference between the treatment groups in discontinuation rate. Univariate and multivariate binary logistic regression models explored differences in key demographic and cognitive and behavioral outcomes between participants who completed and discontinued treatment. Significant multivariate predictors of discontinuation included IQ (linear) and problem solving (curvilinear). The concave shape of the problem solving prediction demonstrated that initially as scores were increasing the probability of non-completion was increasing. However, after a score of 41 (below average problem solving), the probability of being a non-completer decreased as performance increased. Non-completers had significantly lower IQ scores compared to completers. Post-hoc analyses indicated that participants who discontinued prior to mid-treatment exhibited the greatest intellectual challenges, with comparisons moderate-to-large in strength. IQ and problem solving are likely important factors to assess at pre-treatment in early course schizophrenia trials to identify those most vulnerable to discontinuation.
Keywords: schizophrenia, early course, clinical trial, treatment discontinuation, psychosocial treatment, IQ, problem solving
1. Introduction
Treatment discontinuation during treatment trials in schizophrenia is a major challenge, and the consequences extend beyond diminished intervention research fidelity. Participants who discontinue treatment, especially in trials of longer-term evidence-based psychosocial interventions, have limited opportunities for improvement and recovery. For example, our recent 18-month confirmatory trial of two psychosocial interventions for early course schizophrenia (N = 102), Cognitive Enhancement Therapy (CET) compared to Enriched Supportive Therapy (EST), demonstrated that the largest functional benefits of CET were among participants who completed treatment (Wojtalik et al., 2022). The differential effect of CET vs. EST on social adjustment more than doubled from intent-to-treat (d = 0.23) to treatment completer analyses (d = 0.51). Wojtalik and colleagues (2022) concluded that CET is an effective intervention for early course schizophrenia, particularly for participants retained for the full treatment protocol.
The rate of treatment discontinuation in the CET trial mentioned above was higher relative to previous CET trials (Eack et al., 2009; Hogarty et al., 2004), with 53 (52%) participants discontinuing treatment (Wojtalik et al., 2022). Unfortunately, high treatment attrition rates are a frequent occurrence among clinical trials for schizophrenia, especially in long duration trials. For example, the NIMH-funded Recovery After an Initial Schizophrenia Episode (RAISE) Early Treatment Program trial reported an attrition rate of 43% over 24 months (Kane et al., 2015). The Lambeth Early Onset (LEO) 18-month randomized trial of specialized care for early psychosis in London observed an average 61% rate of treatment discontinuation from psychosocial treatments (Craig et al., 2004). In general, the average rate of discontinuation from psychosocial treatment trials in schizophrenia is estimated to be approximately 14% (Szymczynska et al., 2017). However, closer examination of these attrition rates reveals that discontinuation from psychosocial treatments is quite heterogeneous with rates ranging from 0% to as high as 63% (Szymczynska et al., 2017). Similarly, specific to cognitive remediation for schizophrenia, meta-analyses report average attrition rates from 13.7% to 16.58% with specific trial discontinuation rates ranging from 0% to 48% (Altman et al., 2022; Revell et al., 2015; Vita et al., 2022, 2021). Given the relative consistency of high treatment attrition rates among psychosocial clinical trials in schizophrenia, especially those longer in duration, factors that contribute to premature discontinuation warrant careful scrutiny.
In a meta-analysis of dropout across 74 psychosocial treatment trials for schizophrenia, age, sex, illness duration, treatment duration and setting, and study quality significantly moderated dropout rates (Villeneuve et al., 2010). However, in an updated meta-analysis of 43 psychosocial trials with sample sizes ≥ 100, these same factors were not replicated as significant predictors of treatment discontinuation (Szymczynska et al., 2017). The only significant predictor of treatment dropout was number of intervention sessions, such that more sessions increased the risk of treatment discontinuation within the experimental arm (Szymczynska et al., 2017). In observational community-based service studies of schizophrenia, other risk factors of treatment discontinuation include substance abuse (current and past), unemployment, social functioning, and insight (Nose et al., 2003), in addition to lack of family involvement, intellectual ability, symptom severity, and duration of untreated psychosis (Doyle et al., 2014; Johansen et al., 2011). The evidence above supports a greater risk of discontinuation in longer duration treatment trials, but other factors that are barriers to an individual with schizophrenia remaining engaged with longer-term interventions remains unclear, especially in the early course population.
Wojtalik et al. (2022) commented in their study that treatment completers and non-completers significantly differed in baseline age, IQ, illness duration, and antipsychotic medication dose, important factors that may contribute to likelihood of treatment discontinuation during a longer duration clinical trial. As such, the goal of this research was to further investigate baseline demographic, as well pre-treatment cognitive and behavioral impairments, as possible predictors of psychosocial treatment discontinuation in early course schizophrenia. The elucidation of risk and protective factors that predict discontinuation informs the development of strategies to address engagement and retention challenges in future longer-term clinical trials in the early course of schizophrenia.
2. Methods
2.1. Participants
This research included 102 outpatients with early course schizophrenia who participated in a large two-site (Pittsburgh, PA: n = 53; Boston, MA: n = 49) 18-month confirmatory trial of CET (n = 58) compared to EST (n = 44). Participants were included based on the following criteria: (1) a diagnosis of schizophrenia, schizoaffective or schizophreniform disorders established by the Structured Clinical Interview for the DSM-IV-TR (SCID) (First et al., 2002), (2) age 18–55, (3) IQ ≥ 80, (4) read and speak fluent English, (5) antipsychotic medication adherence, (6) stable positive symptoms, (7) significant social and cognitive disability, and (8) ≤ 10-year illness duration. Based on the current literature, wherein no consensus exists on the definition of early phase schizophrenia, the onset of first psychotic symptoms within 10 years is consistent with the range of illness durations used to characterize the early course of the condition (Newton et al., 2018). Further, because CET and EST are recovery-phase interventions and the duration of untreated psychosis often exceeds one year after first episode (Addington et al., 2015), the 10-year illness duration permitted inclusion of early course patients who were more likely to be in a recovery phase and thus more amenable to a long duration intervention. Notably, the average illness duration of the sample was under four years (Table 1). Overall, as seen in Table 1, this early course sample was young (i.e., mid-twenties), predominantly male (75%), and just over half identified their race as white (57%). Most participants reported some college education (73%), although less than a third were working at the time of enrollment (31%). The majority of the participants were diagnosed with schizophrenia (80%), and just under half reported a past substance use diagnosis (49%).
Table 1.
Comparison of Baseline Characteristics Between Treatment Completers and Non-Completers During an 18-Month Multi-Site Randomized Trial of Cognitive Enhancement Therapy for Early Course Schizophrenia
| Overall Sample | Completers | Non-Completers | Completers vs. | |
|---|---|---|---|---|
| (N = 102) | (n = 49) | (n = 53) | Non-Completers | |
|
| ||||
| Variable | M (SD) / N (%) | M (SD) / N (%) | M (SD) / N (%) | p j |
| Age | 24.76 (5.44) | 26.36 (6.58) | 23.28 (3.58) | 0.004 |
| Sex (% male) | 76 (75%) | 38 (78%) | 38 (72%) | 0.650 |
| Racial Minority (%) | 44 (43%) | 21 (43%) | 23 (43%) | 1.000 |
| African American | 24 (24%) | 11 (22%) | 13 (25%) | - |
| White | 58 (57%) | 28 (57%) | 30 (57%) | - |
| Hispanic | 1 (1%) | 0 (0%) | 1 (2%) | - |
| Asian | 5 (5%) | 4 (8%) | 1 (2%) | - |
| Hawaiian/Pacific Islander | 1 (1%) | 1 (2%) | 0 (0%) | - |
| More than one race | 7 (7%) | 2 (4%) | 5 (9%) | - |
| Other | 6 (6%) | 3 (6%) | 3 (6%) | - |
| IQa | 107.83 (10.40) | 110.43 (10.91) | 105.43 (9.38) | 0.015 |
| Education (% some college)b | 69 (73%) | 37 (77%) | 32 (70%) | 0.487 |
| Employed (% employed)c | 31 (31%) | 13 (27%) | 18 (35%) | 0.398 |
| Illness Length (years)c,d | 3.69 (2.28) | 4.32 (2.47) | 3.09 (1.94) | 0.006 |
| Schizophrenia Diagnosis (% with) | 82 (80%) | 37 (76%) | 45 (85%) | 0.319 |
| Substance Use Disorder History (% with)e | 50 (49%) | 21 (43%) | 29 (55%) | 0.243 |
| Antipsychotic Dose (CPZ equivalent mg/day)f | 429.60 (331.70) | 520.65 (377.00) | 345.68 (260.22) | 0.008 |
| Antipsychotic Adherence (% adherent)c | 75 (74%) | 39 (80%) | 36 (69%) | 0.262 |
| MCCB Overall Cognition Compositeg | 35.94 (11.63) | 36.80 (12.52) | 35.14 (10.81) | 0.476 |
| Processing Speed Subscalec,g | 38.32 (11.79) | 38.92 (13.05) | 37.75 (10.56) | 0.621 |
| Attention/Vigilance Subscalec,g | 38.62 (11.65) | 38.92 (12.02) | 38.94 (11.39) | 0.779 |
| Working Memory Subscalec,g | 42.81 (9.97) | 44.43 (8.96) | 41.29 (10.70) | 0.114 |
| Verbal Learning Subscalec,g | 41.88 (9.60) | 42.27 (9.98) | 41.52 (9.32) | 0.698 |
| Visual Learning Subscalec,g | 40.68 (11.37) | 39.88 (12.11) | 41.44 (10.68) | 0.492 |
| Problem Solving Subscalec,g | 42.66 (11.11) | 45.18 (12.54) | 40.29 (9.07) | 0.026 |
| Social Cognition Subscaleg,h | 44.88 (13.40) | 44.84 (14.35) | 44.92 (12.56) | 0.975 |
| Social Adjustment Compositei | 50.03 (9.95) | 48.53 (8.80) | 51.42 (10.81) | 0.144 |
| Symptom Compositei | 50.22 (10.19) | 49.73 (9.18) | 50.67 (11.11) | 0.641 |
Note. CPZ = Chlorpromazine, MCCB = Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery
Measured with Wechsler Abbreviated Scale of Intelligence 2nd Edition (WASI-II), Vocabulary and Matrix Reasoning subtests.
Based on a total sample of 94 with available data (Completer: n = 48, Non-Completer: n = 46).
Based on a total sample of 101 with available data (Completer: n = 49, Non-Completer: n = 52).
Since the onset of psychotic symptoms.
Confirmed by the Structured Clinical Interview for the DSM-IV-TR (SCID) (First et al., 2002) and defined as meeting substance abuse or dependence criteria 3 months or longer prior to enrollment in the trial.
Based on a total sample of 98 with available data (Completer: n = 47, Non-Completer: n = 51).
Age- and sex-corrected T-score with a mean of 50 (SD = 10), with higher scores indicating better cognition.
Based on a total sample of 100 with available data (Completer: n = 49, Non-Completer: n = 51).
T-score with mean of 50 (SD = 10), with higher scores indicating better outcome.
Results from independent samples t-tests or Fisher’s exact tests, two-tailed.
This trial used a randomized-controlled design with assessment raters blind to treatment allocation (clinicaltrials.gov identifier: NCT01561859). A computer-generated randomization program administered by an independent database manager randomly assigned eligible participants on a within-site basis to either CET (n = 58) or EST (n = 44). Due to the group format for social-cognitive training in CET, a higher ratio was allocated to CET in the randomization procedure to facilitate rapid formation of the groups. After randomization, participants completed study assessments at baseline (before starting treatment), 9-month (mid-treatment), and 18-month (end of treatment) time points. A more detailed description of the trial can be found in Wojtalik et al. (2022). This trial was approved annually by the Institutional Review Boards at the University of Pittsburgh and Beth Israel Deaconess Medical Center, and participants provided written informed consent prior to participation.
2.2. Measures
2.2.1. Demographic and Clinical Predictors.
The baseline demographic and clinical predictors used for this research included age, sex, race, IQ, education, employment status, illness length, schizophrenia diagnosis, history of a substance use diagnosis, and antipsychotic medication characteristics (dose and adherence). See Table 1 for additional details.
2.2.2. Cognitive and Behavioral Predictors.
Cognitive performance was assessed with the field standard Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) (Nuechterlein et al., 2008). The scaled T-scores (M = 50, SD = 10) generated from the MCCB, adjusted for age and sex (Kern et al., 2008), include an overall composite of cognitive performance, as well as domain scores for processing speed, attention/vigilance, working memory, verbal learning, visual learning, problem-solving, and social cognition.
The behavioral predictors for this research included the baseline social adjustment and symptom composite indexes (T-scores, M = 50, SD = 10) calculated for the main effects analyses (Wojtalik et al., 2022). The social adjustment composite consisted of scores from the Major Role Adjustment Inventory (Hogarty et al., 1974), Social Adjustment Scale-II (Schooler et al., 1979), Global Assessment of Functioning Scale (Endicott et al., 1976), and the social contacts (item 2) and usefully employed (item 3) items from the Strauss-Carpenter Outcome Scale (Strauss and Carpenter Jr., 1972). The symptom composite included scores from the Brief Psychiatric Rating Scale (Overall and Gorham, 1962), Raskin Depression Scale (Raskin et al., 1969), Covi Anxiety Scale (Lipman, 1982), Wing Negative Symptom Scale (Wing, 1961), Scale for the Assessment of Negative Symptoms (Andreasen, 1983), Scale of the Assessment of Positive Symptoms (Andreasen, 1984), and the Montgomery-Asberg Depression Rating Scale (Montgomery and Åsberg, 1979). All behavioral measures listed above are validated and commonly used measures in the schizophrenia literature. Higher T-scores on the cognitive, social adjustment, and symptom predictor variables are indicative of better performance or outcome (see Table 1).
2.2.3. Treatment Discontinuation (Outcome).
Figure 1 presents the CONSORT diagram of enrollment during the trial, with the overall intent-to-treat attrition rate at 49%. As noted in Figure 1, three CET participants who discontinued treatment returned to complete 18-month assessments and were included in the intent-to-treat sample for the main outcome analyses (Wojtalik et al., 2022). For the purposes of this research to examine predictors of treatment discontinuation, these participants were counted as treatment non-completers. As such, of the 102 eligible and randomized participants, 49 (48%) completed full treatment protocols and 53 (52%) discontinued treatment early. Of the 53 participants who discontinued treatment, 32 (60%) were in the CET group and 21 (40%) in the EST group. These rates of treatment discontinuation did not significantly differ between groups (p = 0.549), nor did the rate of treatment discontinuation between study locations (Pittsburgh: n = 26, 49%; Boston: n = 27, 51%; p =.559). As observed in Figure 1, the majority (n = 40, 75%) of the participants who discontinued treatment dropped during the first half of the trial (i.e., between baseline and 9-month time points).
Figure 1. CONSORT Diagram of Participant Flow Throughout an 18-Month Multi-Site Trial of Cognitive Enhancement Therapy (CET) Versus Enriched Supportive Therapy (EST) for Early Course Schizophrenia.
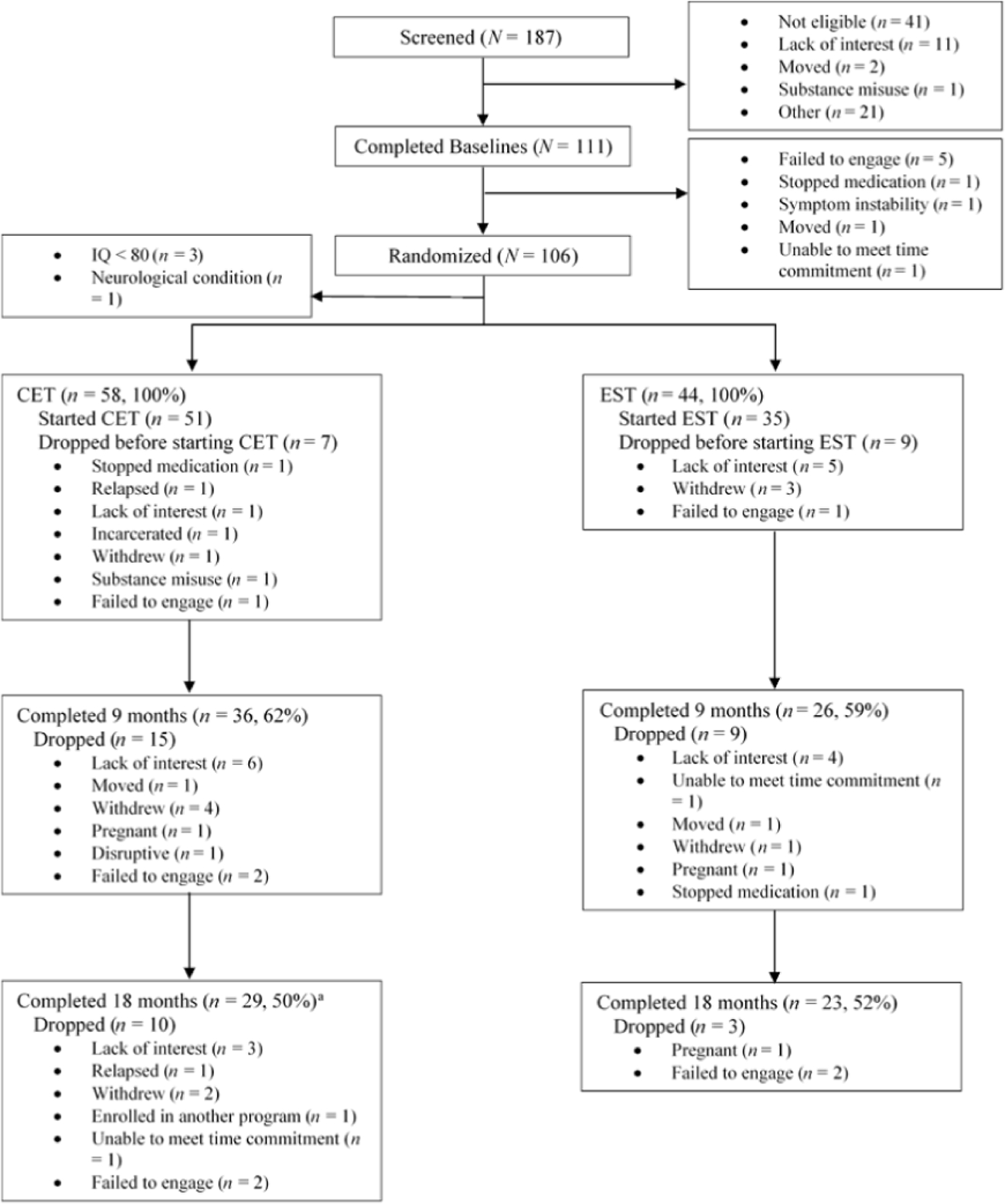
(Note. Originally published in Wojtalik et al. (2022) as supplemental material. aThree CET subjects who dropped prior to 18-months returned to complete 18-month assessments. These subjects were counted as treatment non-completers for the current research; treatment completers: n = 49, treatment non-completers: n = 53.)
2.3. Treatments
All participants were required to maintain adherence to their prescribed antipsychotic medication during the trial. CET and EST are both behavioral interventions with 18-month protocols that have been studied previously in early course schizophrenia (Eack et al., 2009). In the this trial, CET was the study intervention and EST was the active comparison, and both interventions are described briefly below. Full descriptions of CET and EST can be reviewed within their respective manuals (Hogarty, 2002; Hogarty and Greenwald, 2006)
2.3.1. Cognitive Enhancement Therapy (CET).
CET is a cognitive remediation intervention that uses a comprehensive approach, targeting both neurocognitive and social-cognitive impairments associated with the condition of schizophrenia. Neurocognition is targeted with 60 computer-based 1-hour training sessions that begin with the domain of attention (Ben-Yishay et al., 1987), followed by memory and problem-solving exercises over the 18 months of treatment (Bracy, 1994). Computer training is conducted in participant pairs to increase comfort and socialization. After approximately 3 months of attention training, 3 to 4 sets of neurocognitive training pairs are joined together to being the 1.5 hour weekly social-cognitive group sessions. There are 45 structured social-cognitive groups, which occur alongside the weekly neurocognitive computer-based training sessions for the duration of treatment. The social-cognitive curriculum is designed to facilitate experiential learning opportunities, such as participating in unrehearsed social exchanges with another participant and receiving subsequent feedback on the use of social-cognitive abilities. The social-cognitive exercises are designed to improve such abilities as perspective-taking, non-verbal communication, emotion management and recognition, and using foresight in social situations. The beneficial effects of CET on cognition and social adjustment for individuals living with schizophrenia are supported by nearly 20 years of research (Eack et al., 2015, 2010, 2009; Hogarty et al., 2004; Wojtalik et al., 2022).
2.3.2. Enriched Supportive Therapy (EST).
EST is aimed at reducing the risk of relapse by aiding participants in identifying signals of internal distress and improving coping skills. EST is provided in individual format in two phases based on the basic and intermediate stages of Personal Therapy (Hogarty, 2002). Each of the two phases are roughly 9 months in duration, although participants can progress through EST at their own pace. During the first phase, participants are provided psychoeducation about schizophrenia, learning about the relationship between stress vulnerability and risk of relapse, along with skills to reduce or avoid stressful experiences. The second phase involves helping participants gain awareness of their own internal signals of distress and practicing healthy coping techniques.
2.4. Data Analysis
All analyses were conducted in R (version: 4.2.0). To identify baseline predictors of psychosocial treatment discontinuation in early course schizophrenia, analyses began with univariate binary logistic regression models exploring potential differences between treatment completers (n = 49) and non-completers (n = 53) on key demographic and clinical characteristics, as well as the main pre-treatment cognitive and behavioral (social adjustment and symptom composites) outcomes from the primary analyses (Wojtalik et al., 2022). Significant (p < 0.05) univariate predictors were then entered into a multivariate binary logistic regression analysis. Logistic regression assumptions and model fit were explored at both the univariate and multivariate level, along with inspection for potential outliers. Model fit was examined using a likelihood ratio test and Nagelkerke’s R2 (Nagelkerke, 1991). Multicollinearity diagnostics were explored using variance inflation factor (VIF) and tolerance values for each estimated predictor (Field et al., 2012). Predictors with a VIF above 10 and tolerance below 0.1 indicate the presence of multicollinearity and require removal from the model (Myers, 1990). The assumption of linearity was examined using the Box-Tidwell Transformation, which involves calculating an interaction term between each predictor and the log of itself and including this term in the logit model (Hosmer and Lemeshow, 1989). A significant interaction term indicates a violation of the assumption of linearity of the relationship between a continuous predictor and the binary outcome (Field et al., 2012). For predictors with a significant Box-Tidwell Transformation (i.e., p < 0.05), violations of linearity were further confirmed visually with scatterplots of residual vs. predicted values. The quadratic term of such predictors were added to the model using the ‘poly’ function to create orthogonal polynomial terms. Change in fit was examined to ensure the addition of a quadratic term improved the model. Next, although the rate of treatment discontinuation was not significantly different between CET and EST, interactions between treatment assignment and significant predictors from the multivariate model were explored to identify potential treatment-specific predictors of attrition. All univariate and multivariate models adjusted for study location and designated the treatment completers as the reference group.
Lastly, significant linear predictors within the final multivariate model were further explored with post-hoc pairwise comparisons of participants who completed treatment (n = 49), discontinued treatment early before mid-treatment (n = 40), and discontinued treatment later after mid-treatment (n = 13) to explore possible differences related to the time of treatment discontinuation during the trial. Early discontinuation of treatment was defined as dropping anytime from baseline to mid-treatment (month 9), prior to the completion of 9-month assessments. Late discontinuation of treatment was defined as dropping from treatment any time after completion of 9-month assessments (mid-treatment) but prior to completing the full treatment protocol at 18 months (Figure 1). A false-discovery rate p-value adjustment was used to correct for multiple comparisons in the post-hoc analyses (Benjamini and Hochberg, 1995). Comparison effect sizes were calculated using the ‘effectsize’ package (Ben-Shachar et al., 2020) and presented as Hedges’ g.
3. Results
3.1. Univariate Analyses
During testing of model fit and assumptions of logistic regression, the problem solving subscale (Box-Tidwell Transformation: p = 0.008) and symptom composite (Box-Tidwell Transformation: p = 0.042) violated the assumption of linearity (residual vs. fitted value scatterplots in supplementary material). Quadratic terms for the problem solving subscale and symptom composite were generated and included in each respective univariate model, which significantly improved model fit (problem solving: χ2 = 8.93, df = 1, p = 0.003; symptom composite: χ2 = 4.09, df = 1, p = 0.043). Table 2 presents the results from the univariate logistic regression models, with age, IQ, illness duration, antipsychotic medication dose, and problem solving (linear and quadratic) emerging as significant predictors of treatment discontinuation. Overall, relative to participants who completed treatment, participants who discontinued treatment were younger, had lower IQ scores, shorter illness durations, were prescribed lower doses of antipsychotic medication, and had poorer problem-solving scores (see Table 1 as well).
Table 2.
Univariate Logistic Regression Models Predicting Treatment Discontinuation During an 18-Month Multi-Site Randomized Trial of Cognitive Enhancement Therapy for Early Course Schizophrenia (N = 102)
| Variable | b | SE | z-value | p-value | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
| Age | −0.12 | 0.05 | −2.62 | 0.009 | 0.88 (0.79, 0.96) |
| Sex (Male vs. Female) | −0.38 | 0.47 | −0.82 | 0.414 | 0.68 (0.26, 1.69) |
| Race (white vs. non-white) | −0.00 | 0.40 | −0.01 | 0.992 | 0.99 (0.45, 2.19) |
| IQ | −0.05 | 0.02 | −2.46 | 0.014 | 0.94 (0.90, 0.98) |
| Education (no college vs. some college) | −0.39 | 0.47 | −0.82 | 0.411 | 0.99 (0.26, 1.70) |
| Employed (not employed vs. employed) | 0.40 | 0.44 | 0.92 | 0.358 | 1.49 (0.63, 3.58) |
| Illness Length (years) | −0.25 | 0.10 | −2.59 | 0.010 | 0.77 (0.63, 0.93) |
| Schizophrenia Diagnosis (Schizophrenia vs. Schizoaffective) | 0.58 | 0.51 | 1.14 | 0.253 | 1.78 (0.66, 5.02) |
| Substance Use Disorder History (with vs. without) | 0.46 | 0.40 | 1.15 | 0.249 | 1.58 (0.72, 3.51) |
| Antipsychotic Dose (CPZ equivalent mg/day) | −0.00 | 0.00 | −2.56 | 0.011 | 0.99 (0.99, 0.99) |
| Antipsychotic Adherence (non-adherent vs. adherent) | 0.65 | 0.48 | 1.35 | 0.178 | 1.91 (0.75, 5.07) |
| Treatment Group (CET vs. EST) | 0.30 | 0.40 | 0.74 | 0.458 | 1.34 (0.61, 2.98) |
| MCCB Overall Cognition Composite | −0.01 | 0.02 | −0.75 | 0.456 | 0.98 (0.95, 1.02) |
| Processing Speed Subscale | −0.01 | 0.02 | −0.47 | 0.639 | 0.99 (0.95, 1.02) |
| Attention/Vigilance Subscale | 0.00 | 0.02 | 0.27 | 0.789 | 1.00 (0.97, 1.03) |
| Working Memory Subscale | −0.03 | 0.02 | −1.63 | 0.102 | 0.96 (0.92, 1.00) |
| Verbal Learning Subscale | −0.01 | 0.02 | −0.53 | 0.596 | 0.98 (0.94, 1.03) |
| Visual Learning Subscale | 0.01 | 0.02 | 0.64 | 0.520 | 1.01 (0.97, 1.04) |
| Problem Solving Subscalea | 0.38 | 0.15 | 2.49 | 0.013 | 1.46 (1.10, 2.01) |
| Problem Solving Subscale2 | −0.01 | 0.00 | −2.74 | 0.006 | 0.99 (0.99, 0.99) |
| Social Cognition Subscale | 0.00 | 0.02 | 0.03 | 0.976 | 1.00 (0.97, 1.03) |
| Social Adjustment Composite | 0.03 | 0.02 | 1.34 | 0.179 | 1.02 (0.98, 1.07) |
| Symptom Compositea | −0.36 | 0.19 | −1.87 | 0.061 | 0.70 (0.47, 0.99) |
| Symptom Composite2 | 0.00 | 0.00 | 1.91 | 0.057 | 1.00 (1.00, 1.01) |
Note. CPZ = Chlorpromazine, CET = Cognitive Enhancement Therapy, EST = Enriched Supportive Therapy, MCCB = Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery
Problem Solving Subscale2 and Symptom Composite2 are quadratic terms.
All models adjusted for study site and designated the treatment completers (n = 49) as the reference group.
Box-Tidell Transformation statistically significant (i.e., p < .05) indicating a violation of linearity assumption, quadratic term included in the model.
3.2. Multivariate Analysis
The aforementioned significant univariate predictors of treatment discontinuation were entered into the multivariate logistic regression model. Fit statistics indicated this model was significant (χ2 = 29.99, df = 7, p <0.001), explained 35.8% of the variance (Nagelkerke’s R2) in treatment discontinuation, and violated no assumptions. The results of the multivariate model are presented in Table 3. Significant multivariate predictors of treatment discontinuation included IQ and both the linear and quadratic effects for problem solving. With regard to IQ, the direction of the predicted effect was negative, such that treatment non-completers had significantly lower IQ scores relative to completers. The odds ratio for IQ indicated that for every 1-point increase in IQ score, the likelihood of discontinuing treatment decreased by 6% (Figure 2). Said more meaningfully, for every 0.5 standard deviation increase in IQ score (5.20 points), a participant was 4.88 times less likely to be a treatment non-completer. As seen in Figure 2, the curvilinear relationship between treatment discontinuation and problem solving was concave in shape. The point at which the curve bends (i.e., slope = 0) was calculated to be at a problem solving score of approximately 41 (Osborne, 2015, Eq. 7.8.). Taken together, the linear and quadratic coefficients indicate that the probability of being a treatment non-completer increased as problem solving performance scores increased (linear term) until scores around 41, after which the probability of being a treatment non-completer decreased as problem solving scores increased (quadratic term).
Table 3.
Final Multivariate Logistic Regression Model Predicting Treatment Discontinuation During an 18-Month Multi-Site Randomized Trial of Cognitive Enhancement Therapy for Early Course Schizophrenia (N = 102)
| Variable | b | SE | z-value | p-value | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
| (Intercept) | 2.24 | 4.45 | 0.50 | 0.615 | 9.40 (0.00, 65408.09) |
| Study Site (Pittsburgh vs. Boston) | −0.29 | 0.51 | −0.58 | 0.563 | 0.74 (0.27, 2.01) |
| Age | −0.09 | 0.05 | −1.80 | 0.073 | 0.91 (0.81, 1.00) |
| IQ | −0.06 | 0.03 | −2.28 | 0.023 | 0.94 (0.88, 0.98) |
| Illness Length (years) | −0.12 | 0.13 | −0.94 | 0.349 | 0.89 (0.69, 1.13) |
| Antipsychotic Dose (CPZ equivalent mg/day) | −0.00 | 0.00 | −1.72 | 0.085 | 0.99 (0.99, 1.00) |
| Problem Solving Subscale | 0.44 | 0.17 | 2.62 | 0.009 | 1.55 (1.14, 2.22) |
| Problem Solving Subscale2 | −0.01 | 0.00 | −2.65 | 0.008 | 0.99 (0.99, 0.99) |
Note. CPZ = Chlorpromazine,
Problem Solving Subscale2 is the quadratic term.
All models adjusted of study location and designated the treatment completers (n = 49) as the reference group. Multivariate model fit: χ2 = 29.99, df = 7, p <0.001, AIC = 119.05, Nagelkerke’s R2 = 0.358
Figure 2.
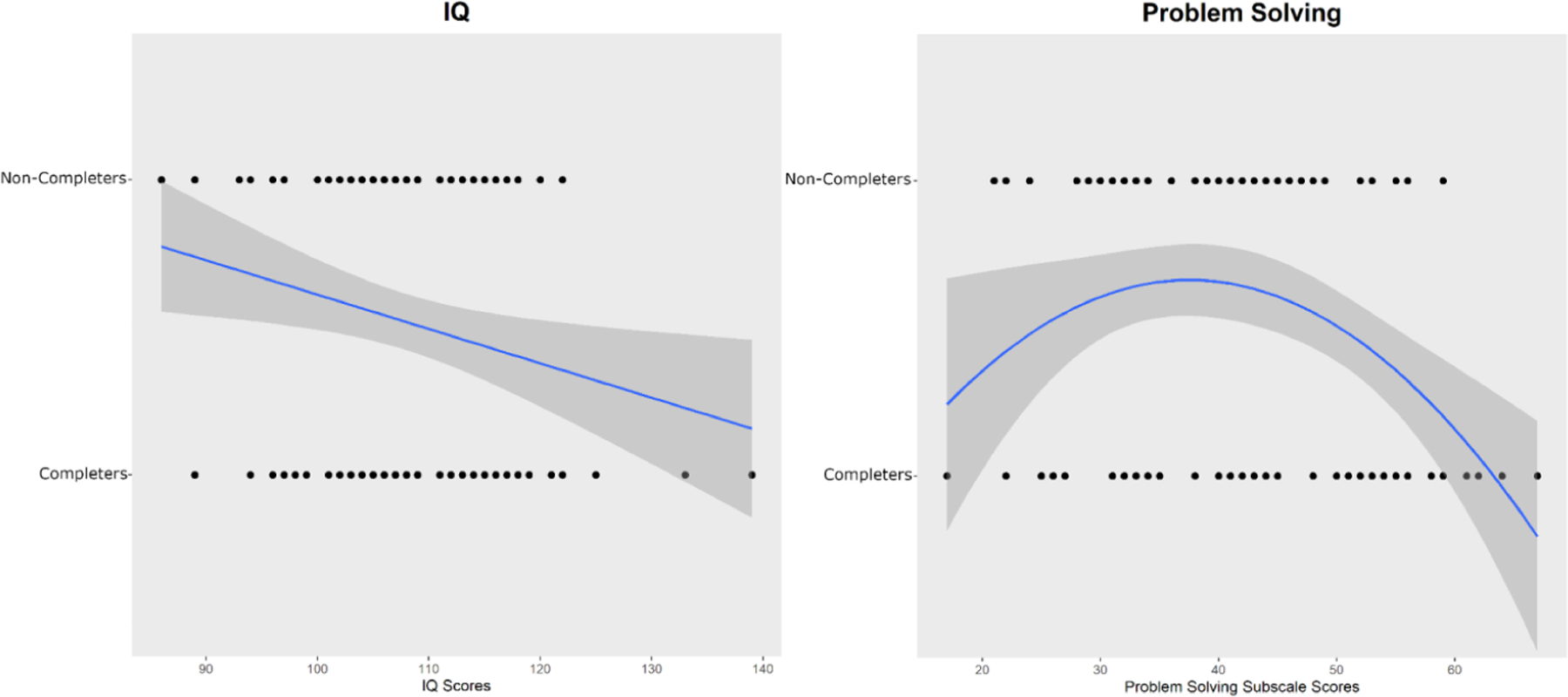
Significant Multivariate Predictors of Treatment Discontinuation (Completer: n = 49 vs. Non-Completer: n = 53).
Regarding the remaining predictors, age, antipsychotic dose, and illness duration did not emerge as significant multivariate predictors of treatment dropout (Table 3). Finally, treatment assignment (CET vs. EST) interactions with IQ (p = 0.377) and problem solving (linear: p = 0.961; quadratic: p = 0.988) were not significant, indicting no treatment-specific predictors.
3.3. Post-Hoc Analyses
Overall post-hoc analyses of possible differences by time of treatment discontinuation were significant for IQ (Figure 3, panel A), but not for problem solving (Figure 3, panel B). Pairwise comparisons revealed that the IQ scores of participants who dropped early from treatment (M = 103.88, SD = 9.51) were significantly lower when compared to both treatment completers (M = 110.43, SD = 10.91) and those who discontinued treatment late (M = 110.23, SD = 7.40), with the strength of these comparisons being moderate-to-large in size (Figure 3, panel A). The adjusted p-value maintained significance for early drop vs. treatment completers, but did not retain significance for early drop vs. late drop. Participants who completed treatment and discontinued treatment late exhibited similar IQ scores. Participants who discontinued treatment early also had the lowest average problem solving score (M = 40.21, SD = 9.10), which was significantly different from the average score of treatment completers (M = 45.18, SD = 12.54), with a moderate effect size (Figure 3, panel B). This comparison between early drop and completers did not survive p-value adjustment. Participants who discontinued treatment early and late (M = 40.54, SD = 9.62) did not significantly differ, nor did completers and late drop participants in average problem solving performance.
Figure 3. Pairwise Comparisons of IQ (A) and Problem Solving Scores (B) between Treatment Completers (n = 49) and Non-Completers who Discontinued Treatment Early (n = 40) and Late (n = 13).
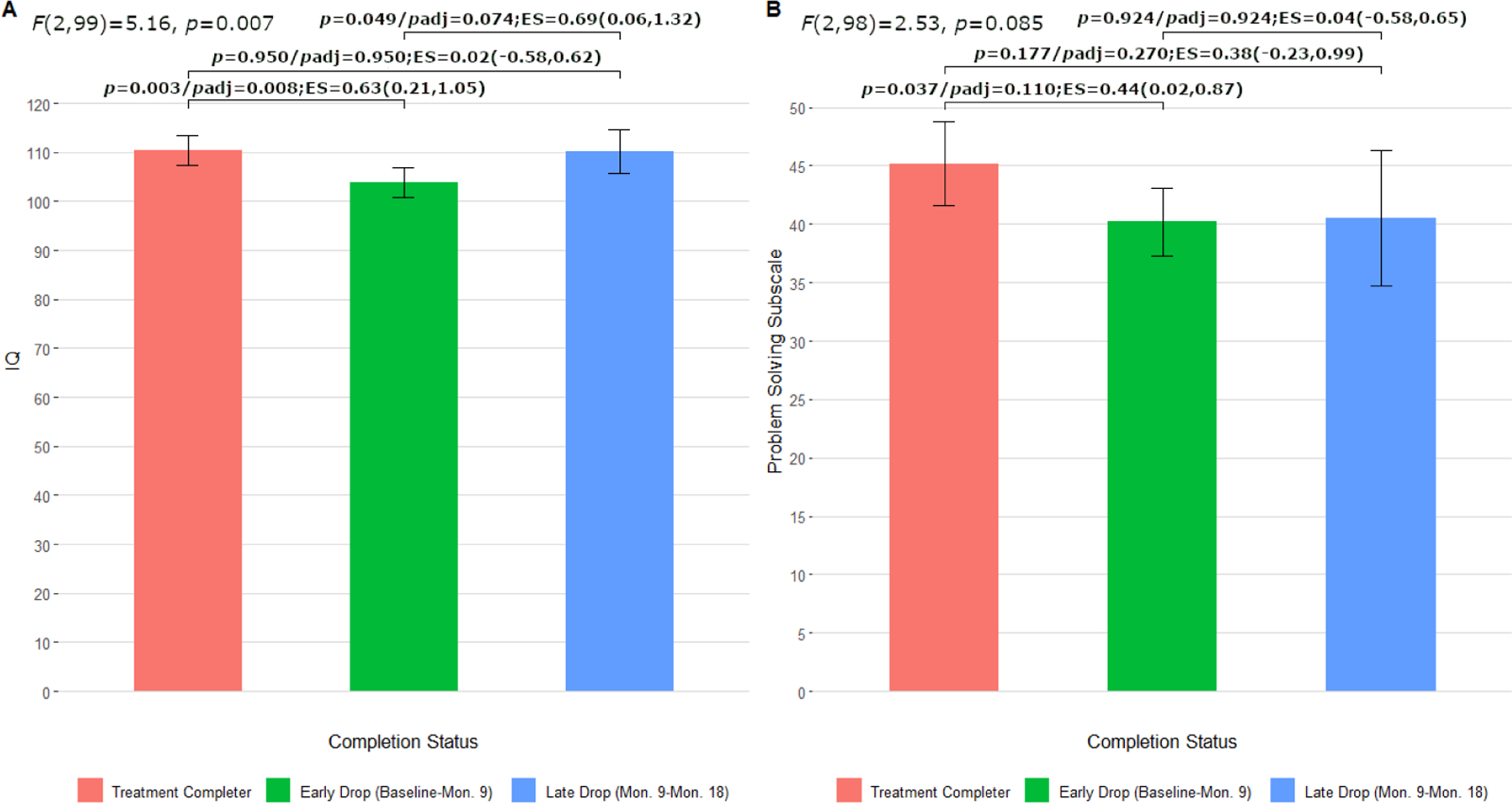
(Note. ES = effect size presented as Hedges’ g [95% CI])
4. Discussion
Factors that contribute to treatment discontinuation during longer-term clinical trials among individuals with schizophrenia are poorly understood, obscuring the systematic identification of patients at greatest risk for dropout. Consequently, high attrition rates during treatment trials, especially those with extensive durations, present a substantial challenge (Craig et al., 2004; Kane et al., 2015; Petersen et al., 2005). The early course of schizophrenia is a critical phase for reducing the severe and debilitating nature of this condition (Correll et al., 2018; Keshavan et al., 2006). An increased understanding of predictors for treatment discontinuation during long duration trials is needed for this population, with implications for enhanced research fidelity, as well as therapeutic engagement and opportunities for recovery.
Within a recent confirmatory trial of two 18-month psychosocial interventions for early course schizophrenia (Wojtalik et al., 2022), CET compared to EST, 52% of participants discontinued treatment early, with no significant difference in rate of discontinuation. Although these rates of treatment discontinuation were high for a CET trial (Eack et al., 2009; Hogarty et al., 2004), likely due to the early course nature of the population, they provided an opportunity for the current research to identify potentially important pre-treatment demographic, clinical, cognitive, and behavioral predictors of treatment discontinuation during a long duration trial. Findings indicated that IQ and MCCB problem solving performance were significant multivariate predictors of treatment discontinuation, which are discussed in detail below. While not statistically significant, it is noteworthy that age and antipsychotic dose demonstrated a trend-level (p < 0.085) relationship, such that younger age and lower antipsychotic dose were associated with a likely greater risk of treatment non-completion.
The findings indicated that lower IQ scores were significantly associated with an increased likelihood of treatment discontinuation, with the lowest IQ scores having a moderate-to-large prediction of earlier drop out, before mid-treatment, in the trial. IQ test performance in schizophrenia has previously been implicated in predicting level of treatment engagement (Kukla et al., 2014), albeit not during long duration clinical trials. In a study of first-episode service use, Johansen et al. (2011) observed that one of the strongest correlates of low service engagement was poor similarities subscale performance on the Wechsler Abbreviated Scale of Intelligence (WASI). In fact, it is not unexpected to identify a link between IQ and psychosocial treatment discontinuation in the early course of schizophrenia, as IQ is predictive of clinical status and outcome. Longitudinal studies in first episode psychosis demonstrate that low IQ is a significant predictor of more severe and disabling illness trajectories (Leeson et al., 2011, 2009; Wang et al., 2016), and moderates response to psychosocial treatment (Seccomandi et al., 2021, 2020). In contrast, recent meta-analytic evidence of cognitive remediation for schizophrenia indicates that lower premorbid IQ is significantly associated with greater acceptability and larger functional gains (Vita et al., 2022, 2021). However, the average duration of cognitive remediation in these meta-analyses was 15.2 (range: 3–104; Vita et al., 2021) and 15.82 (range: 2–104; Vita et al., 2022) weeks, which is around 3.5 months. Given the 18-month duration of the current trial and observation of a significant association between lower IQ and increased risk of dropout, it may be that duration of cognitive remediation is moderating this relationship, a critical research question to explore in future meta-analyses. It is possible that patients with greater intellectual challenges in long duration clinical trials feel early on that their participation is not personally beneficial or meeting their needs, increasing the likelihood to discontinue treatment (Kreyenbuhl et al., 2009). Nevertheless, these preliminary results point to a particularly vulnerable subpopulation with early course schizophrenia, characterized by lower IQ scores, at increased risk of treatment discontinuation in longer duration clinical trials.
For problem solving, a significant curvilinear relationship with treatment discontinuation was observed, with the peak of the curve occurring at a score of approximately 41 on this MCCB subscale. The inverted U-shape pattern was such that, for scores below 41, as performance scores increased the likelihood of treatment discontinuation increased (Figure 2). However, for scores above 41, as problem solving scores increased the likelihood of discontinuing treatment decreased. One possibly helpful conceptualization of this pattern is to use the descriptive categorization of MCCB performance in first-episode schizophrenia outlined by McCleery (2014). As such, it can be interpreted that patients with unimpaired (T-scores: ≥ 45) to below average (T-scores: 40–44) problem solving ability were the least likely to discontinue treatment. The curvilinear relationship also suggests that those with severe (T-scores < 20) to moderate (T-scores 20–34) impairment in problem solving were also at a lower risk of discontinuation. In contrast, the greatest risk for treatment dropout was among patients with mild (T-scores 35–39) impairment scores. Additional post-hoc analyses were conducted using the descriptive variables listed in Table 1 to identify unique characteristics among participants with moderate to severe problem solving impairments that may contribute to reduced risk of discontinuation. No significant differences emerged across groups based on degree of cognitive impairment (all p > .123, severe/moderate: n = 31; mild: n = 8; below average: n = 18; unimpaired; n = 44). The MCCB problem solving subscale may be a quick and convenient tool to identify participants at the greatest likelihood to discontinue long-term treatment, but the utility of using MCCB cognitive profiles in this manner requires examination in a larger sample. Future research with more power and differential problem solving measures is also needed to confirm the nonlinear relationship observed in this research.
Of the seven cognitive domains assessed on the MCCB, it is not surprising that problem solving would emerge as a unique predictor of treatment discontinuation during a long duration psychosocial treatment trial. Problem solving is an executive function skill that involves the use of foresight, planning, and flexibility to find solutions to an identified problem or goal. This domain of cognitive impairment is observable in the early phases of the condition (McCleery et al., 2014; Mesholam-Gately et al., 2009) and is significantly related to poor functional outcomes (Green, 2006; Jaeger et al., 2003; Lysaker et al., 1995). Previous evidence has demonstrated that first episode patients with stronger executive function are at a reduced likelihood to discontinue antipsychotic medication over the course of one year (Robinson et al., 2002). Although, Lepage et al. (2010) found no significant relationship between problem solving performance and medication adherence over 6 months in the first episode population. In the context of the current psychosocial treatment trial, participants with mild problem solving impairments likely struggled to foresee the beneficial effects of “sticking with” a psychosocial treatment that was 18 months in duration, in addition to the effortful planning required for continued attendance (Morris et al., 1995). Further, these participants may have perceived specific psychosocial intervention strategies that required greater cognitive resources as less salient or too difficult, which would discourage engagement. Future research may benefit from examining the relationship between treatment engagement and participants subjective sense of mastery and self-efficacy during long duration trials. One possible explanation for those with severe to moderate problem-solving difficulties at a lower risk of discontinuation is the need for more resources and social supports to function in the community. It is possible that such supports may have helped these participants stay motivated to continue treatment, such as helping participants organize transportation to their CET/EST and other research-related sessions, increasing their likelihood of completing. It will be important in the future to examine if IQ and problem solving, among other key variables, were moderators of treatment response during the trial, along with consideration of treatment adaptations for those with lower IQ scores and mild to moderate problem solving difficulties to optimize patient-intervention fit.
The findings of this research also have implications that preliminarily inform the development of strategies to address engagement and retention challenges in long duration clinical trials. While cognitive remediation programs focusing exclusively on problem solving in schizophrenia have demonstrated limited generalizability as standalone interventions, falling out of favor for comprehensive approaches (Bellack et al., 1996; McGurk et al., 2007; Revell et al., 2015; Wykes et al., 2011), they may have an innovative utility for increasing treatment engagement among patients with lower IQ and/or mild to moderate problem solving impairment. Early problem-solving remediation work by Medalia and colleagues (Medalia et al., 2002, 2001) indicated that training strategies that promote intrinsic motivation have significant therapeutic benefit on problem solving gains in the condition. Therefore, it is possible that such training strategies offered at pretreatment in longer duration trials could serve to increase retention, and is an important future research direction derived from this research. It is also likely that individuals with more severe illness/cognitive impairment might respond better to cognitive adaptation approaches, as opposed to cognitive remediation, such as the compensatory approach to improving cognitive impairment in schizophrenia (Allott et al., 2016).
This research has several limitations that require consideration. To start, treatment discontinuation in the early course of schizophrenia is a multifaceted and dynamic challenge. There is likely a myriad of factors that lead to treatment dropout over the course of a psychosocial clinical trial beyond the baseline individual-level factors examined here, as evidenced by the 35.8% of variance explained in the logit model. While the results of this research should be considered preliminary, they also provide impetus for continued investigation of predictors of treatment discontinuation, including environmental- (i.e., family support, access to transportation) and societal-level (i.e., stigma, discrimination) factors (Dixon et al., 2016; Fontanella et al., 2014), in addition to trial characteristics (Spineli et al., 2013). Such information is vital to the development of evidence-informed strategies to address higher rates of treatment discontinuation frequently observed in early course psychosocial clinical trials. Additionally, the use of the composite indexes, while statistically practical to reduce type I error, may conceal significant associations between treatment discontinuation and specific measures included in the social adjustment and symptom composites. There also may be concern of multicollinearity between IQ and problem solving as they were moderately correlated in the current study (r = 0.27, p = 0.005). Despite being significantly correlated, problem solving ability and other aspects of intellectual functioning (such as verbal memory) are separable variables. Previous research has demonstrated that problem solving performance on the MCCB only explains 31% of variance in IQ scores among patients with schizophrenia (Mohn et al., 2014). As a reminder, the assumption of multicollinearity was not violated and these variables were entered into the multivariate model using orthogonal terms. There are also limitations to generalizability and statistical power. This study included subjects with an IQ ≥ 80, which is common practice in cognitive remediation trials, but implications remain unknown for those below this IQ threshold. The odds ratio for IQ suggests that a 5-point difference (0.5 standard deviation) in scores places participants at nearly a five times greater likelihood of either completing or not completing an 18-month psychosocial intervention. While statistically meaningful in this research, a 5-point difference in community settings with patients with more heterogeneous IQ scores may be less clinically meaningful. Future research is needed to further investigate the relationship between IQ and likelihood of non-completion during long-term psychosocial treatment trials that do not include an IQ eligibility criterion. Generalizability to community-based clinics is also limited by the design of the current research, as the aim was to conduct an examination of predictors of treatment dropout in the context of a clinical trial. It will be important for effectiveness trials of psychosocial interventions for early course schizophrenia to evaluate factors associated with treatment discontinuation. Lastly, while the post-hoc analyses were informative, the loss and unequal distribution of statistical power as a result of examining three subgroups (early dropout, late dropout, completers) warrants caution in interpretation.
In conclusion, the findings preliminarily implicate IQ and MCCB problem solving performance as tangible baseline predictors of treatment discontinuation in long-term psychosocial clinical trials for early course schizophrenia. Retention and engagement in psychosocial interventions during the early course of schizophrenia is critical to address for reducing long-term disability. Continued research is needed on the role of IQ and problem solving, among the identification of other important factors, in treatment discontinuation during long duration psychosocial treatment trials to inform the development of evidence-informed engagement strategies specific to the early course of the condition.
Highlights.
Treatment discontinuation from clinical trials in schizophrenia is a common occurrence, especially in long duration trials
Predictors of discontinuation during an 18-month psychosocial treatment trial for early course schizophrenia were explored
IQ (linear) and problem solving (curvilinear) were significant predictors
Pre-treatment assessment of IQ and/or problem solving may help identify participants most vulnerable to discontinuation
Acknowledgements
This research was supported by NIMH grant R01 MH092440 (MSK and SME).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest Statement
Declarations of interest: none
References
- Addington J, Heinssen RK, Robinson DG, Schooler NR, Marcy P, Brunette MF, Correll CU, Estroff S, Mueser KT, Penn D, Robinson JA, Rosenheck RA, Azrin ST, Goldstein AB, Severe J, Kane JM, 2015. Duration of untreated psychosis in community treatment settings in the United States. Psychiatr. Serv 66, 753–756. 10.1176/appi.ps.201400124 [DOI] [PubMed] [Google Scholar]
- Allott KA, Killackey E, Sun P, Brewer WJ, Velligan DI, 2016. Feasibility and acceptability of cognitive adaptation training for first-episode psychosis. Early Interv. Psychiatry 10, 476–484. 10.1111/eip.12207 [DOI] [PubMed] [Google Scholar]
- Altman RAE, Tan EJ, Rossell SL, 2022. Factors impacting access and engagement of cognitive remediation therapy for people with schizophrenia: A systematic review. Can. J. Psychiatry 68, 139–151. 10.1177/07067437221129073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N, 1984. Scale for the Assessment of Positive Symptoms University of Iowa Press, Iowa City, IA. [Google Scholar]
- Andreasen N, 1983. Scale for the Assessment of Negative Symptoms University of Iowa Press, Iowa City, IA. [Google Scholar]
- Bellack AS, Blanchard JJ, Murphy P, Podell K, 1996. Generalization effects of training on the Wisconsin card sorting test for schizophrenia patients. Schizophr. Res 19, 189–194. 10.1016/0920-9964(95)00067-4 [DOI] [PubMed] [Google Scholar]
- Ben-Shachar MS, Lüdecke D, Makowski D, 2020. effectsize: Estimation of effect size indices and standardized parameters. J. Open Source Softw 5, 2815. [Google Scholar]
- Ben-Yishay Y, Piasetsky EB, Rattok J, 1987. A systematic method for ameliorating disorders in basic attention., in: Neuropsychological Rehabilitation Guilford Press, New York, NY, US, pp. 165–181. [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 57, 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Bracy OL, 1994. PSSCogRehab
- Correll CU, Galling B, Pawar A, Krivko A, Bonetto C, Ruggeri M, Craig TJ, Nordentoft M, Srihari VH, Guloksuz S, Hui CLM, Chen EYH, Valencia M, Juarez F, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, Marcy P, Addington J, Estroff SE, Robinson J, Penn D, Severe JB, Kane JM, 2018. Comparison of Early Intervention Services vs Treatment as Usual for Early-Phase Psychosis: A Systematic Review, Meta-analysis, and Meta-regression. JAMA Psychiatry 75, 555–565. 10.1001/jamapsychiatry.2018.0623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig TKJ, Garety P, Power P, Rahaman N, Colbert S, Fornells-Ambrojo M, Dunn G, 2004. The Lambeth Early Onset (LEO) Team: randomised controlled trial of the effectiveness of specialised care for early psychosis. BMJ 329, 1067. 10.1136/bmj.38246.594873.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon LB, Holoshitz Y, Nossel I, 2016. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry 15, 13–20. 10.1002/wps.20306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R, Turner N, Fanning F, Brennan D, Renwick L, Lawlor E, Clarke M, 2014. First-Episode Psychosis and Disengagement From Treatment: A Systematic Review. Psychiatr. Serv 65, 603–611. 10.1176/appi.ps.201200570 [DOI] [PubMed] [Google Scholar]
- Eack SM, Greenwald DP, Hogarty SS, Cooley SJ, DiBarry AL, Montrose DM, Keshavan MS, 2009. Cognitive Enhancement Therapy for early-course schizophrenia: Effects of a two-year randomized controlled trial. Psychiatr. Serv 60, 1468–1476. 10.1176/ps.2009.60.11.1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Cho RY, Prasad KMR, Greenwald DP, Hogarty SS, Keshavan MS, 2010. Neuroprotective Effects of Cognitive Enhancement Therapy Against Gray Matter Loss in Early Schizophrenia: Results From a 2-Year Randomized Controlled Trial. Arch. Gen. Psychiatry 67, 674–682. 10.1001/archgenpsychiatry.2010.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty SS, Greenwald DP, Litschge MY, McKnight SAF, Bangalore SS, Pogue-Geile MF, Keshavan MS, Cornelius JR, 2015. Cognitive Enhancement Therapy in substance misusing schizophrenia: Results of an 18-month feasibility trial. Schizophr. Res 161, 478–483. 10.1016/J.SCHRES.2014.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J, 1976. The Global Assessment Scale: A Procedure for Measuring Overall Severity of Psychiatric Disturbance. Arch. Gen. Psychiatry 33, 766–771. 10.1001/archpsyc.1976.01770060086012 [DOI] [PubMed] [Google Scholar]
- Field A, Miles J, Field Z, 2012. Discovering Statistics Using R Sage, London. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured Clinical Interview For DSM-IV-TR Axis I Disorders, Research Version, Patient Edition Biometrics Research, New York State Psychiatric Institute, New York, NY. [Google Scholar]
- Fontanella CA, Guada J, Phillips G, Ranbom L, Fortney JC, 2014. Individual and Contextual-Level Factors Associated with Continuity of Care for Adults with Schizophrenia. Adm. Policy Ment. Heal. Ment. Heal. Serv. Res 41, 572–587. 10.1007/s10488-013-0500-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, 2006. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry 153, 321–330. 10.1176/AJP.153.3.321 [DOI] [PubMed] [Google Scholar]
- Hogarty GE, 2002. Personal Therapy for Schizophrenia and Related Disorders: A Guide to Individualized Treatment Guilford Press, New York, NY. [Google Scholar]
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue-Geile M, Keshavan M, Cooley S, DiBarry AL, Garrett A, Parepally H, Zoretich R, 2004. Cognitive Enhancement Therapy for schizophrenia: Effects of a 2-year randomized trial on cognition and behavior. Arch. Gen. Psychiatry 61, 866–876. 10.1001/archpsyc.61.9.866 [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Goldberg SC, Schooler NR, 1974. Drug and Sociotherapy in the Aftercare of Schizophrenic Patients: III. Adjustment of Nonrelapsed Patients. Arch. Gen. Psychiatry 31, 609–618. 10.1001/archpsyc.1974.01760170011002 [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Greenwald DP, 2006. Cognitive Enhancement Therapy: The Training Manual University of Pittsburgh Medical Center, Pittsburgh, PA. [Google Scholar]
- Hosmer DW, Lemeshow S, 1989. Applied Logistic Regression Wiley, New York, NY. [Google Scholar]
- Jaeger J, Berns SM, Czobor P, 2003. The Multidimensional Scale of Independent Functioning: A new instrument for measuring functional disability in psychiatric populations. Schizophr. Bull 29, 153–167. 10.1093/oxfordjournals.schbul.a006987 [DOI] [PubMed] [Google Scholar]
- Johansen R, Hestad K, Iversen VC, Agartz I, Sundet K, Andreassen OA, Melle I, 2011. Cognitive and Clinical Factors Are Associated With Service Engagement in Early-Phase Schizophrenia Spectrum Disorders. J. Nerv. Ment. Dis 199. 10.1097/NMD.0b013e31820bc2f9 [DOI] [PubMed] [Google Scholar]
- Kane JM, Robinson DG, Schooler NR, Mueser KT, Penn DL, Rosenheck RA, Addington J, Brunette MF, Correll CU, Estroff SE, Marcy P, Robinson J, Meyer-Kalos PS, Gottlieb JD, Glynn SM, Lynde DW, Pipes R, Kurian BT, Miller AL, Azrin ST, Goldstein AB, Severe JB, Lin H, Sint KJ, John M, Heinssen RK, 2015. Comprehensive Versus Usual Community Care for First-Episode Psychosis: 2-Year Outcomes From the NIMH RAISE Early Treatment Program. Am. J. Psychiatry 173, 362–372. 10.1176/appi.ajp.2015.15050632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RSE, Mesholam-Gately R, Mintz J, Seidman LJ, Stover E, Marder SR, 2008. The MATRICS Consensus Cognitive Battery, Part 2: Co-Norming and Standardization. Am. J. Psychiatry 165, 214–220. 10.1176/appi.ajp.2007.07010043 [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Roberts M, Wittmann D, 2006. Guidelines for clinical treatment of early course schizophrenia. Curr. Psychiatry Rep 8, 329–334. 10.1007/s11920-006-0070-7 [DOI] [PubMed] [Google Scholar]
- Kreyenbuhl J, Nossel IR, Dixon LB, 2009. Disengagement From Mental Health Treatment Among Individuals With Schizophrenia and Strategies for Facilitating Connections to Care: A Review of the Literature. Schizophr. Bull 35, 696–703. 10.1093/schbul/sbp046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukla M, Davis LW, Lysaker PH, 2014. Cognitive Behavioral Therapy and Work Outcomes: Correlates of Treatment Engagement and Full and Partial Success in Schizophrenia. Behav. Cogn. Psychother 42, 577–592. 10.1017/S1352465813000428 [DOI] [PubMed] [Google Scholar]
- Leeson VC, Barnes TRE, Hutton SB, Ron MA, Joyce EM, 2009. IQ as a predictor of functional outcome in schizophrenia: A longitudinal, four-year study of first-episode psychosis. Schizophr. Res 107, 55–60. 10.1016/j.schres.2008.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson VC, Sharma P, Harrison M, Ron MA, Barnes TRE, Joyce EM, 2011. IQ Trajectory, Cognitive Reserve, and Clinical Outcome Following a First Episode of Psychosis: A 3-Year Longitudinal Study. Schizophr. Bull 37, 768–777. 10.1093/schbul/sbp143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Bodnar M, Joober R, Malla A, 2010. Is there an association between neurocognitive performance and medication adherence in first episode psychosis? Early Interv. Psychiatry 4, 189–195. 10.1111/j.1751-7893.2010.00174.x [DOI] [PubMed] [Google Scholar]
- Lipman RS, 1982. Differentiating anxiety and depression in anxiety disorders: use of rating scales. Psychopharmacol. Bull 18, 69–77. [PubMed] [Google Scholar]
- Lysaker P, Bell M, Beam-Goulet J, 1995. Wisconsin card sorting test and work performance in schizophrenia. Psychiatry Res 56, 45–51. 10.1016/0165-1781(94)02641-U [DOI] [PubMed] [Google Scholar]
- McCleery A, Ventura J, Kern RS, Subotnik KL, Gretchen-Doorly D, Green MF, Hellemann GS, Nuechterlein KH, 2014. Cognitive functioning in first-episode schizophrenia: MATRICS Consensus Cognitive Battery (MCCB) Profile of Impairment. Schizophr. Res 157, 33–39. 10.1016/J.SCHRES.2014.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT, 2007. A meta-analysis of cognitive remediation in schizophrenia. Am. J. Psychiatry 164, 1791–1802. 10.1176/appi.ajp.2007.07060906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia A, Revheim N, Casey M, 2002. Remediation of problem-solving skills in schizophrenia: Evidence of a persistent effect. Schizophr. Res 57, 165–171. 10.1016/S0920-9964(01)00293-6 [DOI] [PubMed] [Google Scholar]
- Medalia A, Revheim N, Casey M, 2001. The remediation of problem-solving skills in schizophrenia. Schizophr. Bull 27, 259–267. 10.1093/oxfordjournals.schbul.a006872 [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ, 2009. Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology 23, 315–336. 10.1037/A0014708 [DOI] [PubMed] [Google Scholar]
- Mohn C, Sundet K, Rund BR, 2014. The relationship between IQ and performance on the MATRICS consensus cognitive battery. Schizophr. Res. Cogn 1, 96–100. 10.1016/j.scog.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Åsberg M, 1979. A New Depression Scale Designed to be Sensitive to Change. Br. J. Psychiatry 134, 382–389. 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- Morris RG, Rushe T, Woodruffe PWR, Murray RM, 1995. Problem solving in schizophrenia: A specific deficit in planning ability. Schizophr. Res 14, 235–246. 10.1016/0920-9964(94)00044-9 [DOI] [PubMed] [Google Scholar]
- Myers RH, 1990. Classical and Modern Regression with Applications, 2nd ed. Duxbury Press, Boston. [Google Scholar]
- Nagelkerke NJD, 1991. A note on a general definition of the coefficient of determination. Biometrika 78, 691–692. [Google Scholar]
- Newton R, Rouleau A, Nylander A-G, Loze J-Y, Resemann HK, Steeves S, Crespo-Facorro B, 2018. Diverse definitions of the early course of schizophrenia—a targeted literature review. npj Schizophr 4, 21. 10.1038/s41537-018-0063-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose M, Baebui C, Tansella M, 2003. How often do patients with psychosis fail to adhere to treatment programmes? A systematic review. Psychol. Med 33, 1149–1160. 10.1017/S0033291703008328 [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, Gold JM, Goldberg T, Heaton RK, Keefe RSE, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR, 2008. The MATRICS Consensus Cognitive Battery, Part 1: Test Selection, Reliability, and Validity. Am. J. Psychiatry 165, 203–213. 10.1176/appi.ajp.2007.07010042 [DOI] [PubMed] [Google Scholar]
- Osborne JW, 2015. Best Practices in Logistic Regression SAGE Publications, Inc, Thousand Oaks, CA. [Google Scholar]
- Overall JE, Gorham DR, 1962. The brief psychiatric rating scale. Psychol. Rep 10, 799–812. [Google Scholar]
- Petersen L, Jeppesen P, Thorup A, Abel M-B, Øhlenschlæger J, Christensen TØ, Krarup G, Jørgensen P, Nordentoft M, 2005. A randomised multicentre trial of integrated versus standard treatment for patients with a first episode of psychotic illness. BMJ 331, 602. 10.1136/bmj.38565.415000.E01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin A, Schulterbrandt J, Reatig N, McKeon JJ, 1969. Replication of factors of psychopathology in interview, ward behavior and self-report ratings of hospitalized depressives. J. Nerv. Ment. Dis 148, 87–98. 10.1097/00005053-196901000-00010 [DOI] [PubMed] [Google Scholar]
- Revell ER, Neill JC, Harte M, Khan Z, Drake RJ, 2015. A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr. Res 168, 213–222. 10.1016/j.schres.2015.08.017 [DOI] [PubMed] [Google Scholar]
- Robinson DG, Woerner MG, Alvir JMJ, Bilder RM, Hinrichsen GA, Lieberman JA, 2002. Predictors of medication discontinuation by patients with first-episode schizophrenia and schizoaffective disorder. Schizophr. Res 57, 209–219. 10.1016/S0920-9964(01)00312-7 [DOI] [PubMed] [Google Scholar]
- Schooler N, Weissman M, Hogarty GE, 1979. Social Adjustment Scale for Schizophrenics, in: Hargreaves W, Attkisson C, Sorenson J (Eds.), Resource Material for Community Mental Health Program Evaluators. DHHS pub no (ADM) 79328 National Institute of Mental Health, Rockville, MD. [Google Scholar]
- Seccomandi B, Agbedjro D, Bell M, Keefe RSE, Keshavan M, Galderisi S, Fiszdon J, Mucci A, Cavallaro R, Bechi M, Ojeda N, Peña J, Wykes T, Cella M, 2021. Can IQ moderate the response to cognitive remediation in people with schizophrenia? J. Psychiatr. Res 133, 38–45. 10.1016/j.jpsychires.2020.12.013 [DOI] [PubMed] [Google Scholar]
- Seccomandi B, Tsapekos D, Newbery K, Wykes T, Cella M, 2020. A systematic review of moderators of cognitive remediation response for people with schizophrenia. Schizophr. Res. Cogn 10.1016/j.scog.2019.100160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spineli LM, Leucht S, Cipriani A, Higgins JPT, Salanti G, 2013. The impact of trial characteristics on premature discontinuation of antipsychotics in schizophrenia. Eur. Neuropsychopharmacol 23, 1010–1016. 10.1016/j.euroneuro.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Strauss JS, Carpenter WT Jr., 1972. The Prediction of Outcome in Schizophrenia: I. Characteristics of Outcome. Arch. Gen. Psychiatry 27, 739–746. 10.1001/archpsyc.1972.01750300011002 [DOI] [PubMed] [Google Scholar]
- Szymczynska P, Walsh S, Greenberg L, Priebe S, 2017. Attrition in trials evaluating complex interventions for schizophrenia: Systematic review and meta-analysis. J. Psychiatr. Res 90, 67–77. 10.1016/j.jpsychires.2017.02.009 [DOI] [PubMed] [Google Scholar]
- Villeneuve K, Potvin S, Lesage A, Nicole L, 2010. Meta-analysis of rates of drop-out from psychosocial treatment among persons with schizophrenia spectrum disorder. Schizophr. Res 121, 266–270. 10.1016/j.schres.2010.04.003 [DOI] [PubMed] [Google Scholar]
- Vita A, Barlati S, Ceraso A, Deste G, Nibbio G, Wykes T, 2022. Acceptability of cognitive remediation for schizophrenia: A systematic review and meta-analysis of randomized controlled trials. Psychol. Med 1–11. 10.1017/S0033291722000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita A, Barlati S, Ceraso A, Nibbio G, Ariu C, Deste G, Wykes T, 2021. Effectiveness, core elements, and moderators of response of cognitive remediation for schizophrenia: A systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 78, 848–858. 10.1001/jamapsychiatry.2021.0620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Ho NF, Sum MY, Collinson SL, Sim K, 2016. Impact of duration of untreated psychosis and premorbid intelligence on cognitive functioning in patients with first-episode schizophrenia. Schizophr. Res 175, 97–102. 10.1016/J.SCHRES.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Wing JK, 1961. A simple and reliable subclassification of chronic schizophrenia. J. Ment. Sci 107, 862–875. 10.1192/bjp.107.450.862 [DOI] [PubMed] [Google Scholar]
- Wojtalik JA, Mesholam-Gately RI, Hogarty SS, Greenwald DP, Litschge MY, Sandoval LR, Shashidhar G, Guimond S, Keshavan MS, Eack SM, 2022. Confirmatory efficacy of Cognitive Enhancement Therapy for early schizophrenia: Results from a multi-site randomized trial. Psychiatr. Serv 73, 501–509. 10.1176/appi.ps.202000552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P, 2011. A meta-analysis of cognitive remediation for schizophrenia: Methodology and effect sizes. Am. J. Psychiatry 168, 472–485. 10.1176/appi.ajp.2010.10060855 [DOI] [PubMed] [Google Scholar]


