Abstract
Background:
The appropriate use of pulmonary artery catheters (PACs) in critically ill cardiac patients remains debated.
Objective:
We aimed to characterize the current use of PACs in cardiac intensive care units (CICUs) with attention to patient-level and institutional factors influencing their application, and explore the association with in-hospital mortality.
Methods:
The Critical Care Cardiology Trials Network is a multicenter network of CICUs in North America. Between 2017–2021, participating centers contributed annual 2-month snapshots of consecutive CICU admissions. Admission diagnoses, clinical and demographic data, use of PACs, and in-hospital mortality were captured.
Results:
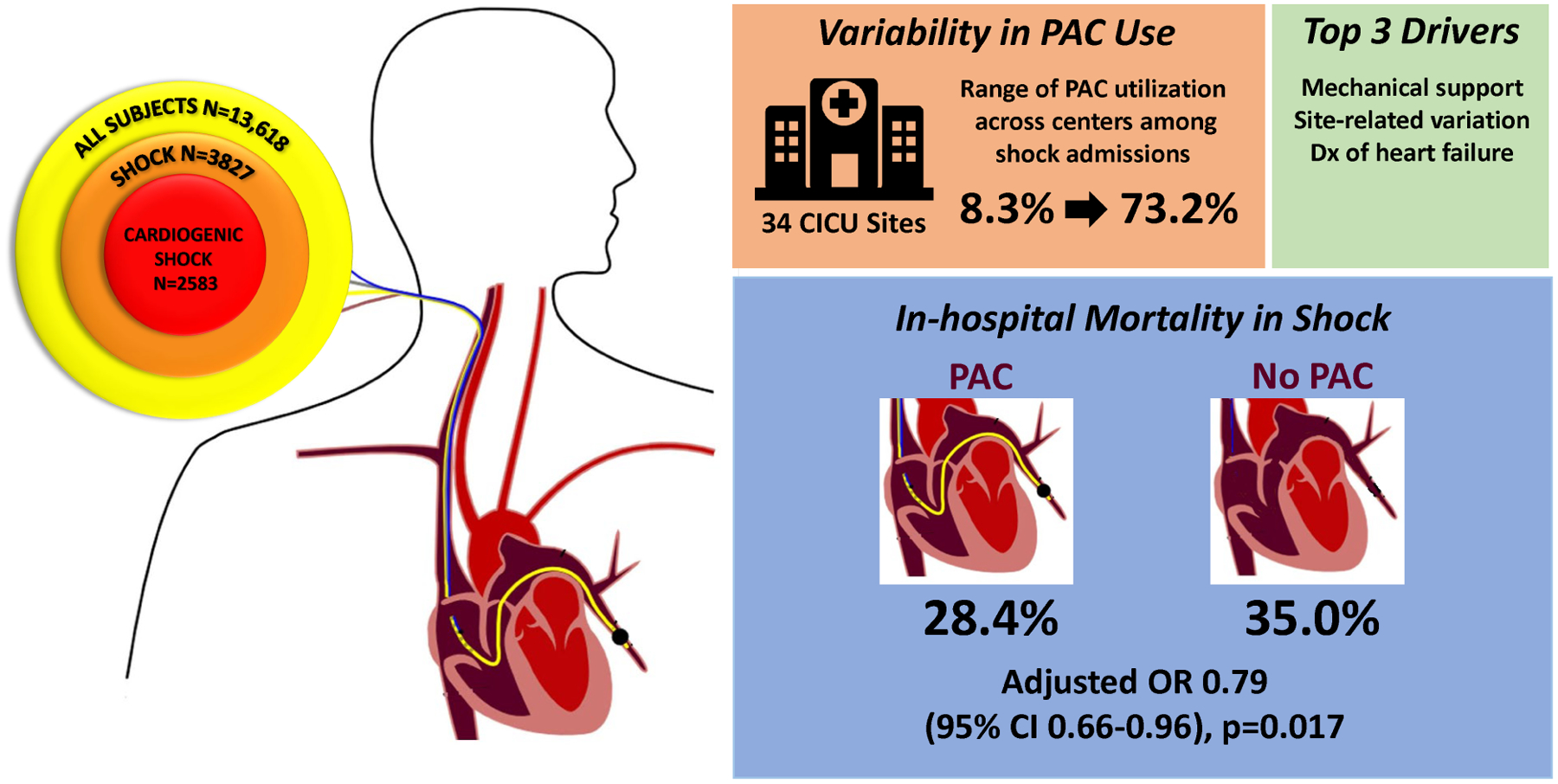
Among 13,618 admissions at 34 sites, 3827 were diagnosed with shock with 2583 of cardiogenic etiology. Use of mechanical circulatory support and heart failure were the patientlevel factors most strongly associated with a greater likelihood of use of a PAC (odds ratio 5.99, 95% confidence interval 5.15–6.98; p<0.001 and 3.33, 95% CI 2.91–3.81; p<0.001, respectively). The proportion of shock admissions with a PAC varied significantly by study center ranging from 8% to 73%. In analyses adjusted for factors associated with their placement, PAC use was associated with lower mortality in all shock patients admitted to a CICU (OR 0.79, 95% CI 0.66–0.96; p=0.017).
Conclusions:
There is wide variation in the use of PACs that is not fully explained by patient level-factors and appears driven in part by institutional tendency. PAC use was associated with higher survival in cardiac patients with shock presenting to CICUs. Randomized trials are needed to guide the appropriate use of PACs in cardiac critical care.
Keywords: Shock, Cardiac intensive care, Pulmonary artery catheter
Introduction
Invasive hemodynamic monitoring with a pulmonary artery catheter (PAC) has been used both as a diagnostic tool and to guide therapies in critically ill patients.1 However, the use of PACs precipitously declined following multiple trials that failed to demonstrate a benefit while revealing possible harm with routine use.2–8 For example, in the National Inpatient Sample, between 1993 and 2004, the use of PACs decreased by 65% in medical intensive care units.9 Focusing on patients with acute decompensated heart failure without shock in the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, PAC-guided therapy was not beneficial with respect to days alive out of the hospital.10 In light of the results of the ESCAPE trial, current American College of Cardiology/American Heart Association guidelines for management of heart failure (HF) recommend against routine invasive hemodynamic monitoring in acute HF (Class III: No benefit). However, in the absence of well-controlled comparative data, use of a PAC is recommended by expert opinion (Class I: Level of Evidence C) for HF patients with respiratory distress or signs of impaired perfusion in whom the adequacy or excess of ventricular filling pressures is difficult to clinically determine.11
Overall PAC use among patients with HF in the US continued to decline from 2004 to 2014, including in patients with cardiogenic shock.12,13 More recently, some experts have strongly advocated for invasive hemodynamic assessment to better guide therapeutic decision-making in patients with suspected shock.14 In addition, an important recent observational analysis suggested possible benefit from a complete invasive hemodynamic assessment.15 The latest European Society of Cardiology Guidelines for Acute and Chronic Heart Failure have included that a standardized team-based approach to the diagnosis and management of shock coupled with invasive hemodynamic monitoring may translate to improved survival.16 An expert consensus statement from the Society of Cardiovascular Angiography and Interventions (SCAI) stressed that defining shock phenotypes using invasive hemodynamics may help guide therapy, particularly with respect to identification of patients who may require isolated right ventricular or biventricular support.17 Given the continued debate regarding the use of PACs, we aimed to characterize their current use in contemporary dedicated cardiac intensive care units (CICUs), assess institutional and patient-level factors driving their application across all patients and among those with shock, and explore their association with in-hospital mortality.
Methods
Study Population
The Critical Care Cardiology Trials Network (CCCTN) is an investigator-initiated collaborative research network of American Heart Association Level 1 CICUs18 in the United States and Canada. Details regarding the data collection and structure of the CCCTN Registry have been reported.19 Scientific and operational oversight of the CCCTN is conducted by the TIMI Study Group (Boston, MA). Between September 2017 and September 2021, a total of 34 participating centers, 68% of which were transplant centers, contributed clinical data on all consecutive medical admissions to the CICU during annual 2-month collection periods. Patients admitted only for post-surgical recovery or medical ICU overflow were not included in this analysis cohort. The study protocol and waiver of informed consent were approved by the institutional review board at each of the participating institutions.
Placement of a PAC for ongoing bedside hemodynamic monitoring or as a discrete diagnostic assessment were recorded in the registry database. Shock was defined as systolic blood pressure <90 mm Hg for ≥30 minutes or the need for inotropic or vasopressor support, and evidence of end-organ hypoperfusion.20 Shock etiology was classified by the site investigator as cardiogenic, distributive, hypovolemic, or mixed according to prespecified definitions based either on invasive hemodynamic data or supportive clinical features.20 The decision to use a PAC was made at the discretion of the clinical team. When invasive hemodynamics were performed, a cardiac index <2.2 mL/min/m2 and pulmonary capillary wedge pressure or central venous pressure >15 mm Hg were considered indicative of cardiogenic shock (CS). When PACs were not used, assessment of CS was based on other supportive clinical features including low central venous oxygen saturation, echocardiographic evidence of impaired cardiac output, or peripheral hypoperfusion on physical exam. The mixed shock category included patients for whom there was >1 type of shock (e.g., CS and distributive) determined to be contributing to the clinical syndrome. Cases were reviewed by the central coordinating center via automated checks and manual reviews.
Admission diagnosis, cardiovascular procedures, and in-hospital mortality were recorded for all patients along with clinical and demographic data.
Statistical Analysis
Categorical variables are reported as counts and percentages. For continuous variables, data are reported as medians with 25th and 75th percentiles. Differences in baseline characteristics were evaluated with the chi-square test for categorical variables and Wilcoxon rank-sum test for continuous variables. Spearman’s correlation analysis was used to assess the relationship between continuous variables, including CS volume at each site and use of PAC. The SCAI staging system for cardiogenic shock21,22 was used to perform analyses of the relationship between shock severity and PAC use. For categorical analyses based on the institutional tendency for PAC use, sites were categorized from “low” to “high” users based on quartiles of PAC utilization.
Predictors of the use of a PAC were assessed using generalized linear mixed-effects models with variability in use of PACs between sites modeled as a random effect. Variables associated with the use of a PAC were determined using a backward selection procedure with retaining alpha level of 0.05. Among the selected variables, relative variability explained by individual covariates were estimated using the R2-based Pratt Index.23 The proportion (%) of total variability can explained by 3 components (Fixed, random, and error). Each of the variables in the model were modeled as fixed effects, except for clinical center. The association of hospital mortality with use of a PAC within each subpopulation of shock type was then assessed using generalized linear mixed-effect models accounting for propensity by adjusting for the variables associated with PAC use in each sub-population as well as for variability between sites modeled as a random effect. Results were considered statistically significant at a 2-sided p-value <0.05. Analyses were performed using SAS System V9.4 (SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics
Of the 13,618 admissions eligible for this analysis in the overall CICU population, 2719 (20.0%) received a PAC. Among the subgroup of admissions with shock (n=3827) and the further subset with CS (n=2583), a PAC was used in 45.8% and 55.6%, respectively.
The overall CICU population had a median age of 66 years, 37% were female, and 31% were non-White (Table 1). There was a high prevalence of medical comorbidities including chronic kidney disease (24.0%), coronary artery disease (37.4%) and heart failure (36.8%). The most common primary diagnoses on admission were acute coronary syndrome (29.4%), heart failure (15.7%), and valvular heart disease (7.8%). Mechanical ventilation was required in 21.0% of admissions. Mechanical circulatory support (MCS) was used in 28.4% of shock cases and 36.9% of CS cases. In-hospital mortality for all admissions to CICU was 12.1% and for admissions with shock was 32.0%.
Table 1.
Baseline Characteristics
| Characteristic | N | Overall | Use of PAC | p-value | ||
|---|---|---|---|---|---|---|
| No (N=10899) | Yes (N=2719) | |||||
| Demographics | Age (yrs) | 13618 | 66.0 (56.0–76.0) | 67.0 (57.0–77.0) | 64.0 (54.0–72.0) | <0.0001 |
| BMI (kg/m2) | 13477 | 27.8 (24.1–32.6) | 27.8 (24.1–32.6) | 27.6 (24.0–32.6) | 0.61 | |
| Female | 13618 | 5032 (37.0%) | 4156 (38.1%) | 876 (32.2%) | <0.0001 | |
| White | 11229 | 7750 (69.0%) | 6165 (69.7%) | 1585 (66.5%) | 0.0029 | |
| History | Diabetes mellitus | 13618 | 4747 (34.9%) | 3717 (34.1%) | 1030 (37.9%) | 0.0002 |
| Chronic kidney disease | 13618 | 3264 (24.0%) | 2426 (22.3%) | 838 (30.8%) | <0.0001 | |
| Significant pulmonary disease | 13618 | 2067 (15.2%) | 1586 (14.6%) | 481 (17.7%) | <0.0001 | |
| Significant dementia | 13618 | 253 (1.9%) | 238 (2.2%) | 15 (0.6%) | <0.0001 | |
| Active cancer | 13618 | 891 (6.5%) | 757 (6.9%) | 134 (4.9%) | 0.0001 | |
| Cardiovascular | Coronary artery disease | 13618 | 5093 (37.4%) | 4033 (37.0%) | 1060 (39.0%) | 0.056 |
| Cerebrovascular disease | 13618 | 1351 (9.9%) | 1065 (9.8%) | 286 (10.5%) | 0.24 | |
| Peripheral artery disease | 13618 | 1410 (10.4%) | 1153 (10.6%) | 257 (9.5%) | 0.084 | |
| Heart failure* | 13618 | 5006 (36.8%) | 3440 (31.6%) | 1566 (57.6%) | <0.0001 | |
| LVEF< 20% | 4856 | 1069 (22.0%) | 544 (16.3%) | 525 (34.4%) | <0.0001 | |
| 20 – 29% | 4856 | 1122 (23.1%) | 728 (21.9%) | 394 (25.8%) | ||
| 30 – 39% | 4856 | 764 (15.7%) | 540 (16.2%) | 224 (14.7%) | ||
| 40 – 49% | 4856 | 578 (11.9%) | 462 (13.9%) | 116 (7.6%) | ||
| ≥ 50% | 4856 | 1323 (27.2%) | 1054 (31.7%) | 269 (17.6%) | ||
| Heart transplant recipient | 13618 | 198 (1.5%) | 117 (1.1%) | 81 (3.0%) | <0.0001 | |
| Atrial fibrillation | 13618 | 3505 (25.7%) | 2638 (24.2%) | 867 (31.9%) | <0.0001 | |
| Ventricular arrhythmia | 13618 | 884 (6.5%) | 582 (5.3%) | 302 (11.1%) | <0.0001 | |
| Severe valvular disease | 13618 | 2121 (15.6%) | 1589 (14.6%) | 532 (19.6%) | <0.0001 | |
| Pulmonary hypertension | 13618 | 770 (5.7%) | 494 (4.5%) | 276 (10.2%) | <0.0001 | |
| Congenital heart disease | 13618 | 257 (1.9%) | 180 (1.7%) | 77 (2.8%) | <0.0001 | |
| Baseline | ALT (U/L) < 200 | 11072 | 10104 (91.3%) | 8048 (93.5%) | 2056 (83.5%) | <0.0001 |
| 200 – 799 | 11072 | 628 (5.7%) | 388 (4.5%) | 240 (9.7%) | ||
| 800 – 1999 | 11072 | 210 (1.9%) | 110 (1.3%) | 100 (4.1%) | ||
| ≥ 2000 | 11072 | 130 (1.2%) | 64 (0.7%) | 66 (2.7%) | ||
| Creatinine (mg/dL) | 13566 | 1.2 (0.9–1.8) | 1.1 (0.9–1.6) | 1.4 (1.0–2.2) | <0.0001 | |
| Lactate (mmol/L) | 7785 | 1.8 (1.2–3.1) | 1.7 (1.2–3.0) | 1.9 (1.2–3.3) | <0.0001 | |
| SOFA Score | 13618 | 3.0 (1.0–6.0) | 3.0 (1.0–6.0) | 6.0 (3.0–9.0) | <0.0001 | |
Statistics presented are median (25th, 75th percentiles) for continuous variables and count (%) for categorical variables.
LVEF rates were obtained within subjects with Heart Failure (HF). PAC: pulmonary artery catheter, BMI: Body Mass Index, LVEF: left ventricular ejection fraction, ALT: alanine aminotransferase, SOFA: Sequential Organ Failure Assessment
Patient-Level Factors Associated with Use of a PAC
First considering all admissions to the CICU, compared with admissions without use of a PAC, patients with a PAC were slightly younger (64 vs 67 years) and were less likely to be female (32.2% vs 38.1%; Table 1). The PAC group had higher rates of prior HF, left ventricular ejection fraction (LVEF) <20%, severe valvular disease, pulmonary hypertension, and congenital heart disease. In addition, the PAC group had higher presenting levels of creatinine, lactate, and alanine transaminase (Table 1). The primary admitting diagnoses with the highest proportions of PAC use were heart failure with or without shock (48.5%), pulmonary hypertension (45.0%), and complex congenital disease (36.4%; Figure 1). Among heart failure patients without shock admitted to the CICU, PACs were used in 30.2% of cases. Invasive hemodynamics were also obtained in a substantial proportion of admissions presenting with cardiac arrest (19.2%), ventricular arrhythmias (17.5%), valvular heart disease (14.8%), and acute coronary syndrome (14.6%). Among admissions with respiratory insufficiency as an indication for CICU care, 29.2% had a PAC; although rates were lower (14.1%) when patients with shock were excluded.
Figure 1.
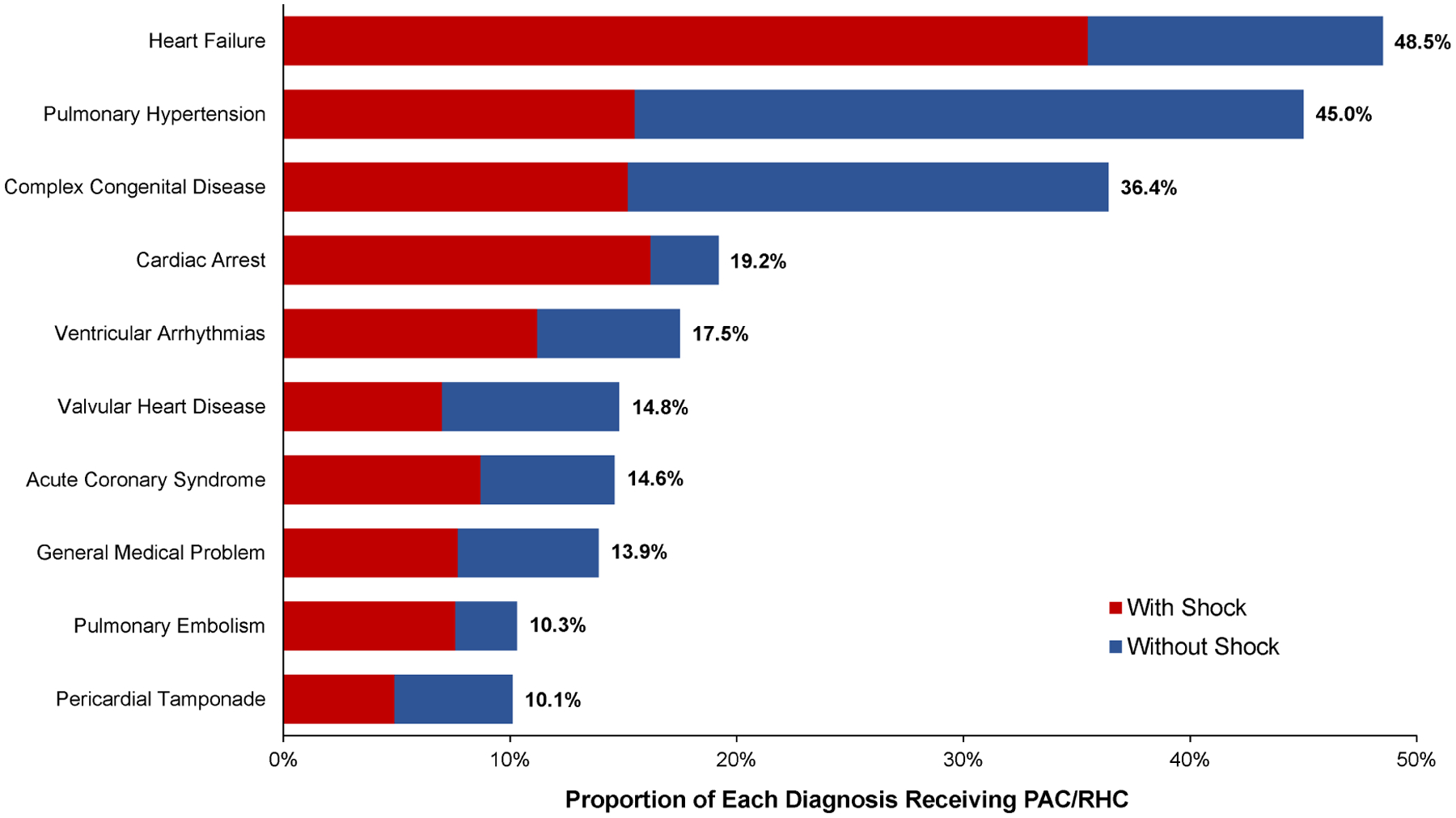
Proportion of admissions with PAC use by primary admission diagnosis. The total length of the bar represents the total proportion of admissions with each diagnosis, with or without shock, with a PAC. Red bars indicate the component for each admitting diagnosis contributed by admissions with shock. PAC: pulmonary artery catheter
In multivariable analysis, patient-level factors that were independently associated with a greater likelihood of PAC use in the overall CICU population included a history or primary diagnosis of HF, pulmonary hypertension, the presence of shock or hypotension, higher inotrope or vasopressor requirement, and use of MCS (Table 2). Older patients and those with elevated lactates were less likely to have invasive hemodynamic assessments.
Table 2.
Patient-Level Factors Associated with the Use of a Pulmonary Artery Catheter in the Overall CICU Cohort
| Adjusted OR (95% CI) | P-value | |
|---|---|---|
| Mechanical Circulatory Support | 5.99 (5.15, 6.98) | <0.0001 |
| Primary Diagnosis of Heart Failure | 3.33 (2.91, 3.81) | <0.0001 |
| Shock/Hypotension | 2.97 (2.59, 3.42) | <0.0001 |
| Maximum Number of Inotropes/Vasopressors (per pressor) | 1.39 (1.30, 1.48) | <0.0001 |
| History Heart Failure | 1.67 (1.48, 1.88) | <0.0001 |
| Pulmonary Hypertension 1 | 1.95 (1.60, 2.38) | <0.0001 |
| Severe Valvular Heart Disease 2 | 1.38 (1.20, 1.59) | <0.0001 |
| Alanine Transaminase 3 | 1.13 (1.06, 1.20) | 0.0003 |
| Male Sex | 1.15 (1.03, 1.30) | 0.014 |
| Age (per 5 years) | 0.94 (0.93, 0.96) | <0.0001 |
| Lactate (per log mmol/L) | 0.83 (0.75, 0.91) | <0.0001 |
N=13618.
History or primary diagnosis of pulmonary hypertension;
History or primary diagnosis of severe valvular heart disease;
Modeled per a priori category of increasing alanine transaminase
Turning to focus on admissions with shock, the baseline characteristics for the subgroup of admissions with shock are summarized in Supplemental Table S1. Shock admissions with a PAC were younger (63 vs 68 years), and less likely to be female (30.3% vs 38.4%). Compared with admissions managed without a PAC, the PAC group more commonly had a past history of heart failure (60.3% vs 46.1%), ventricular arrhythmias (11.4% vs 7.0%), and pulmonary hypertension (9.1% vs 7.3%). Admissions managed with MCS were significantly more likely to receive a PAC than those treated without MCS (73.2% vs. 34.9%, p<0.0001). The PAC was placed prior to initiation of MCS in 49% of cases. Multivariable modeling of the probability of having a PAC among patients with shock revealed a consistent set of independent clinical factors associated with higher probability of use, including management with MCS, a primary diagnosis or history of HF, higher use of vasoactive therapies, lower lactate, and higher SOFA scores (Supplemental Table S2). In a sensitivity analysis excluding patients who presented with cardiac arrest prior to CICU admission, elevated lactate remained a negative predictor of PAC use within the shock cohort.
In the overall population, the total proportion of variability in the use of a PAC explained by patient-level factors was 58%. Among shock admissions, use of MCS accounted for the highest proportion of the total variability (32%), followed by a primary diagnosis of heart failure (Table 3).
Table 3.
Proportion of Variability in PAC Use Accounted for by CICU Center and Patient-Level Factors
| All Admissions % (n=13618) | Shock % (n=3827) | Cardiogenic Shock % (n=2583) | |
|---|---|---|---|
| Participating Center | 14.3 | 14.5 | 18.3 |
| Mechanical Circulatory Support | 25.8 | 32.0 | 27.0 |
| Primary Diagnosis of Heart Failure | 12.8 | 7.1 | 4.0 |
| Shock/Hypotension | 9.7 | ||
| Pulmonary Hypertension | 2.7 | 0.6 | |
| History of Heart Failure | 2.6 | 2.3 | 2.9 |
| Max Number of Inotropes/Vasopressors | 2.0 | 3.1 | 0.6 |
| Severe Valvular Heart Disease | 0.9 | 1.1 | |
| Age (years) | 0.7 | 1.2 | 2.8 |
| Lactate (log mmol/L) | 0.3 | 0.9 | 0.9 |
| Male Sex | 0.2 | 0.9 | 0.8 |
| Alanine Transaminase | 0.2 | 0.5 | |
| SOFA Score | 1.0 | ||
| Cardiac Arrest | 0.6 |
Variable Definitions are the same as for Table 2
SOFA: sequential organ failure assessment
Findings were qualitatively similar in the key subgroup with CS. In multivariable analysis of the CS cohort, the clinical factors independently associated with higher use of a PAC were a history or primary diagnosis of heart failure, increasing numbers of vasopressors or inotropes, and MCS. The proportion of variability explained by each variable is shown in Table 3. Invasive hemodynamic assessment was performed in half of cases (50.1%) prior to initiation of MCS. In a sensitivity analysis testing MCS initiated prior to PAC placement as the covariate, the same relationship with higher PAC use was observed.
Variability of PAC Use by Institution
The proportion of all admissions with a PAC varied significantly by study center, ranging from 1.1% to 34.9%. This variability persisted when the analysis was restricted to admissions with shock (8.3% to 73.2%, Figure 2) and CS (8.3% to 77.4%, Figure 2), even among patients with CS receiving MCS (14.3% to 95.6%). There was a positive correlation between PAC utilization and volume of shock admissions both for all shock (r=0.58; p<0.001) and for CS (r=0.49; p=0.003; Figure 3A). Framed by quartiles of low to high PAC utilizers by site, the distribution of SCAI shock stage is shown in Figure 3B. Regardless of site utilization rate, the majority of PACs were used for patients with SCAI Stage D (“Deteriorating”) and Stage E (“Extremis”) with no significant heterogeneity across low to high utilizers (p=0.10). Moreover, there was no correlation between site volumes of SCAI Stage D and E CS with PAC utilization (Figure S1). In the mixed effects modeling, an estimated 14% of the variability of PAC use was explained by the participating center in the overall CICU cohort and in admissions with shock (Table 3).
Figure 2.
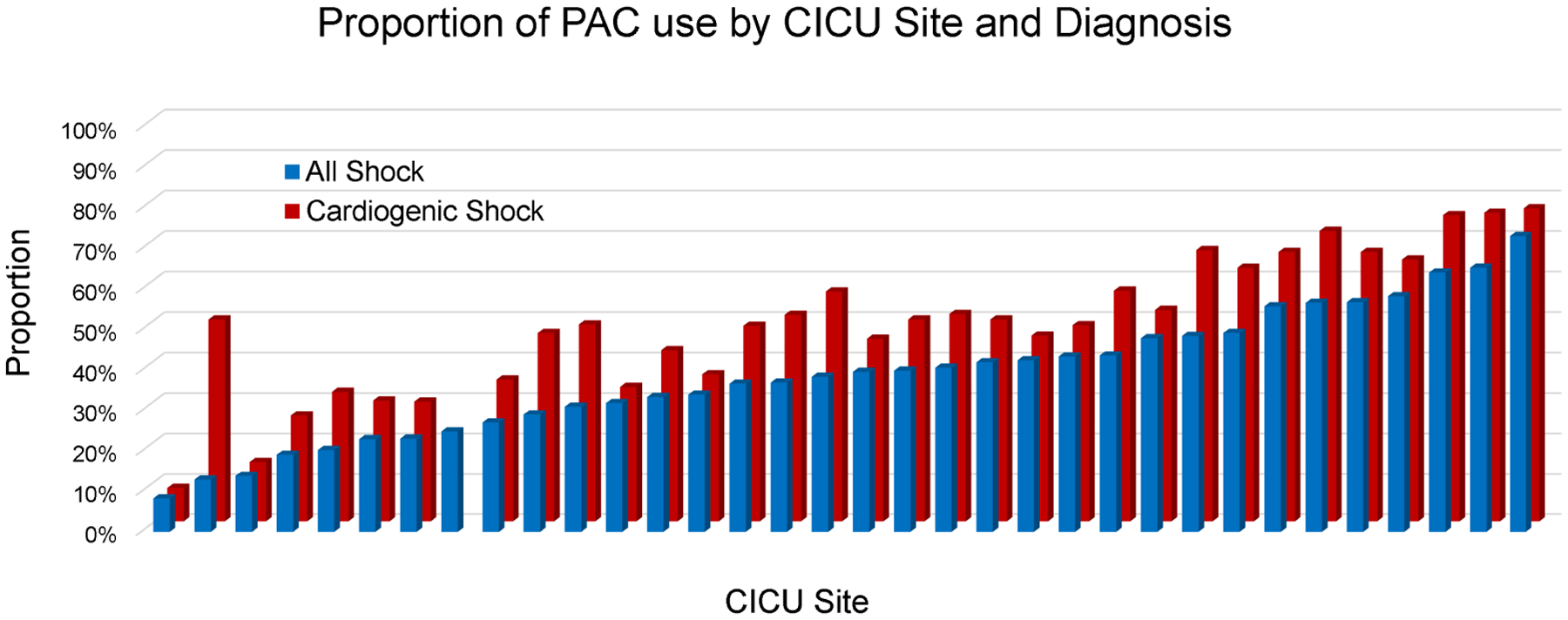
Proportion of shock admissions that received a PAC at each participating center. PAC: pulmonary artery catheter
Figure 3.
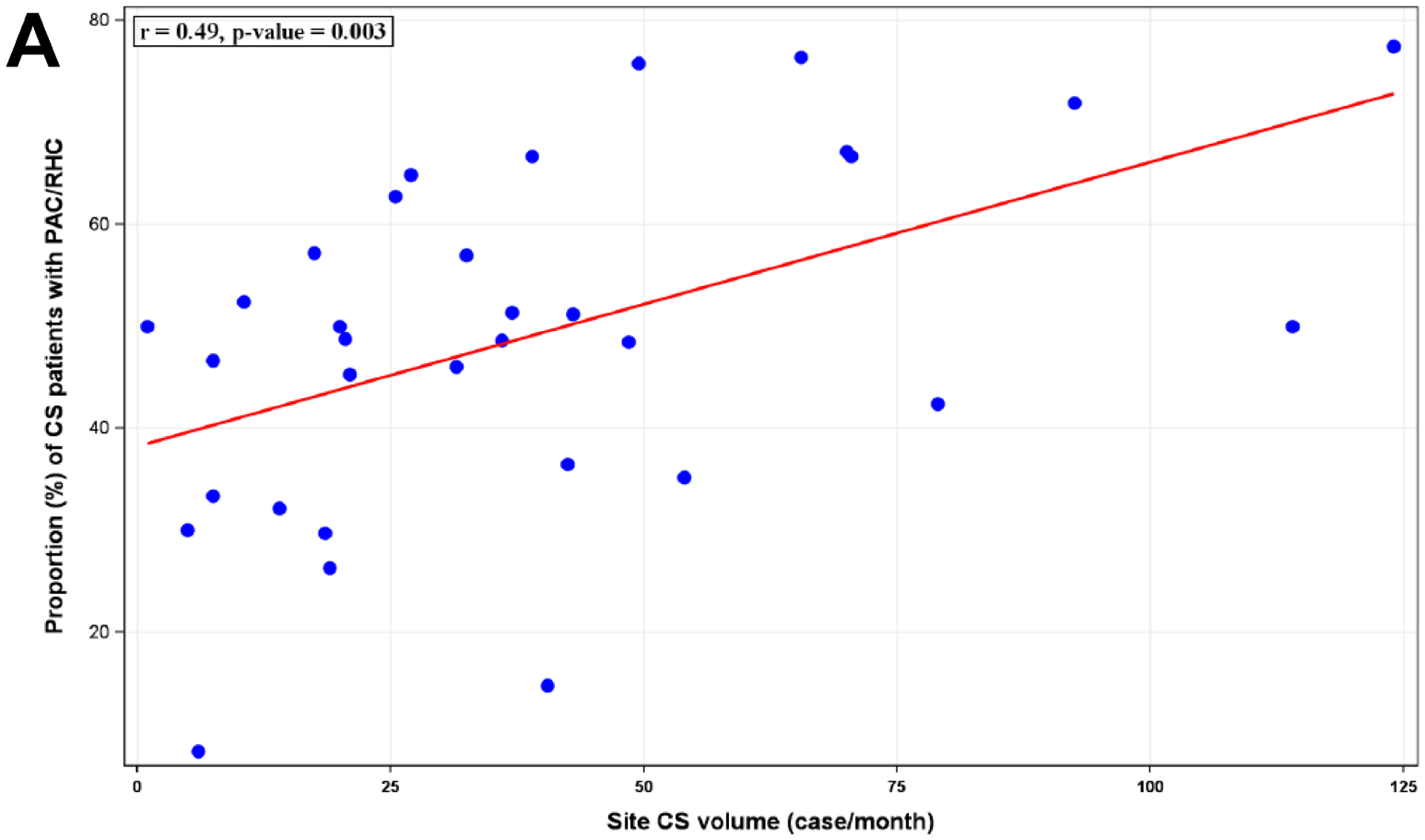
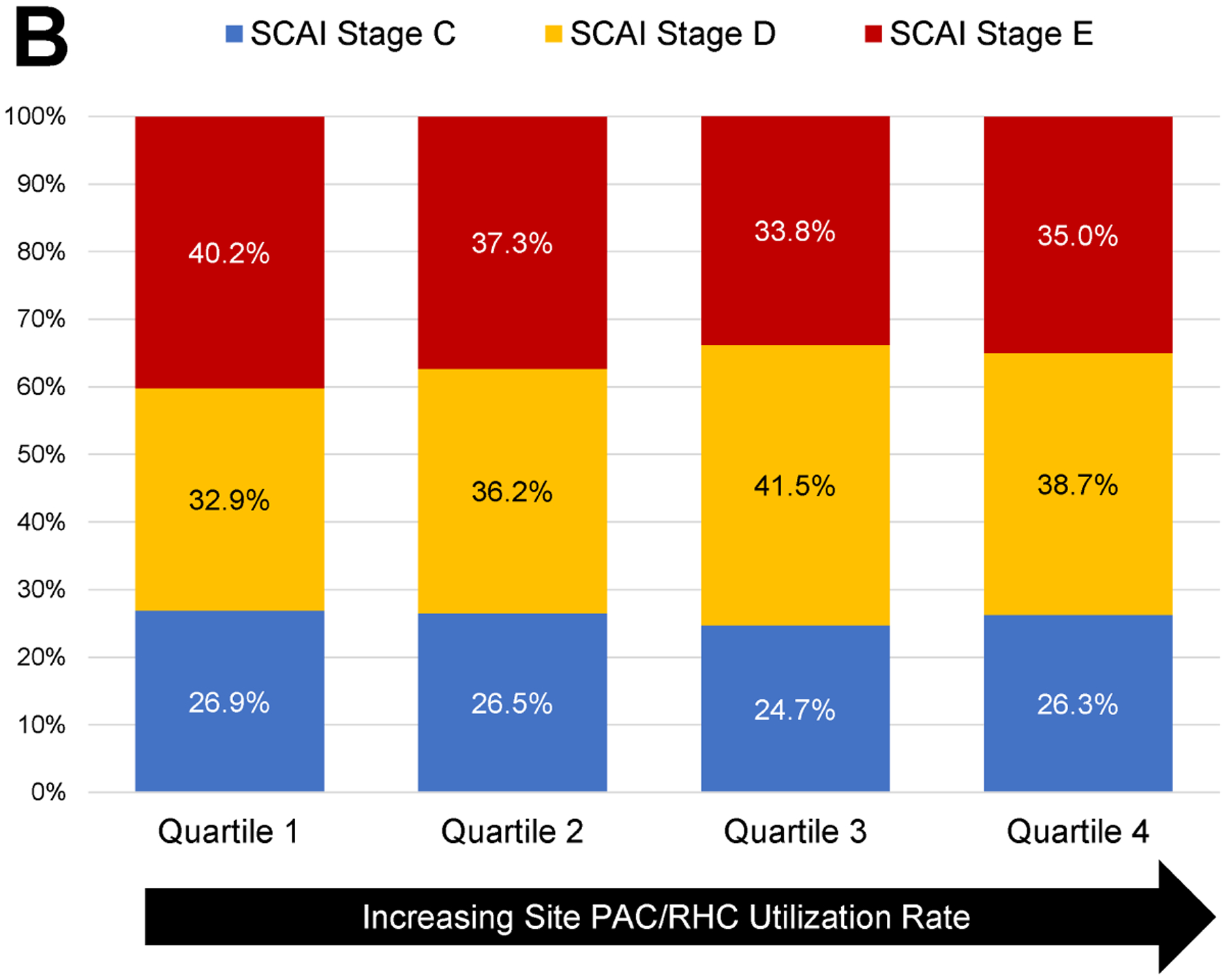
Relationship of PAC use to CS volume and shock severity. A: Correlation between the volume of CS cases per site and the proportion of CS patients receiving PAC. B: Shock severity distribution based on SCAI Shock Stage among CS admissions stratified by quartile of PAC utilization rates at each site. PAC: pulmonary artery catheter, CS: cardiogenic shock, SCAI: Society for Cardiovascular Angiography and Interventions.
Other Management Among Shock Patients With or Without a PAC
In an exploratory analysis limited to a subset of admissions with a snapshot of vasoactive therapies at 4 hours after CICU arrival and PAC placement, the use of a PAC was associated with more frequent use of inotropic agents and a trend toward less frequent use of inopressors and vasopressors (Supplemental Table S3). Moreover, in an additional exploratory analysis among a subset of admissions in the last 2 years of the analysis period (n=1341) when details of surgical MCS during the index hospitalization were captured, we found that management with a PAC among those with CS was associated with a higher rate of durable VAD implantation (4.7% vs. 0.7%, p<0.001).
Mortality among Shock Patients Managed With or Without a PAC
Among the 3827 admissions with shock, the in-hospital mortality rate was 28.4% in admissions managed with a PAC compared with 35.0% among admissions managed without a PAC (OR 0.73, 95% CI 0.63, 0.85; p<0.0001). Adjusted for variables associated with the propensity for placement of a PAC, PAC use among admissions with shock was associated with lower in-hospital mortality (adjusted-OR 0.79, 95% CI 0.66, 0.96; p=0.017). Among the subgroups of CS, mixed shock, or other shock, this pattern was directionally consistent with overlapping confidence intervals (Table 4). Moreover, there was no heterogeneity between those with acute myocardial infarction and heart failure related CS (adjusted ORs 0.78 [95% CI 0.55, 1.10] and 0.86 [95% CI 0.63, 1.16], respectively).
Table 4.
Association between PAC Use and In-Hospital Mortality2
| PAC Mortality (%) | No PAC Mortality (%) | Unadjusted | P-value | Adjusted* | P-value | |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||||
| Shock | 497/1751 (28.4) | 726/2076 (35.0) | 0.73 (0.63, 0.85) | <0.0001 | 0.79 (0.66, 0.96) | 0.017 |
| Cardiogenic shock | 371/1436 (25.8) | 366/1147 (31.9) | 0.74 (0.62, 0.88) | 0.001 | 0.88 (0.70, 1.10) | 0.25 |
| Mixed shock | 96/226 (42.5) | 169/379 (44.6) | 0.90 (0.64, 1.27) | 0.55 | 0.77 (0.50, 1.17) | 0.21 |
| Other shock | 30/89 (33.7%) | 191/550 (34.7%) | 0.96 (0.60, 1.55) | 0.87 | 0.73 (0.43, 1.23) | 0.23 |
Association was assessed with a generalized linear mixed-effect model accounting for variability of ICU centers as a random variable
Covariates used for adjustment were the covariates retained from model selections for each analysis cohorts as listed in Supplemental Table S6
PAC: pulmonary artery catheter OR: Odds Ratio, CS: cardiogenic shock
A sensitivity analysis restricted to sites with greater experience based on the top 50% of PAC volume showed consistent association between PAC use and lower mortality (adjusted-OR 0.75, 95% CI 0.59, 0.95; p=0.016). Moreover, adjustment for the presence of a shock team and for transplant center did not alter this finding (adjusted OR 0.80, 95% CI 0.66, 0.97; p=0.024) (Supplemental Table S4). Additionally, we found no definite heterogeneity in the relationship between PAC use and in-hospital mortality based on whether MCS was used or the timing relative to PAC placement (Supplemental Table S5).
In October 2018 the United Network for Organ Sharing (UNOS) allocation system for heart transplantation underwent a change emphasizing hemodynamic assessment to prioritize patients on the waitlist. This change occurred after the first data collection campaign. In our dataset, there was no heterogeneity in the association of PAC use with lower mortality among shock patients admitted to the CICU in the eras before and after the 2018 revision in the UNOS heart allocation system (p-interaction = 0.55).
Discussion
This analysis of use of PACs in contemporary CICUs among more than 13,000 total admissions including 3827 admissions with shock demonstrated four key findings. First, management of one in every five patients in contemporary Level 1 CICUs includes placement of a PAC, with use in nearly half of CICU admissions with shock. Second, there is important variability in the use of PACs across institutions that is not fully explained by patient-level factors. Third, heart failure and pulmonary hypertension are the primary diagnoses associated with use of a PAC in the overall CICU population. Features indicating shock as a likely diagnosis, along with indicators of worsening hemodynamics (such as increasing numbers of vasoactive medications or need for MCS) were associated with more use of PACs, whereas advanced age was associated with a less invasive approach. Fourth, an exploratory analysis of in-hospital mortality accounting for patient- and institution-related factors associated with the propensity for using invasive hemodynamics demonstrated an association between PAC use and higher survival in patients with shock triaged to the CICU (Central Figure).
Central Figure.
Pulmonary Artery Catheter Use in CICUs and Association With Mortality
Variability in Use of Invasive Hemodynamics in Shock
Despite the absence of high-quality randomized evidence, the use of PACs in contemporary CICUs is substantial. The overall rate of PAC among shock admissions in CCCTN was 45.8% with a high degree of variability in PAC use across institutions ranging from 8.3% to 73.2%. Overall, this rate is higher compared to that from a large administrative database showing PAC rates as low as 8.7% in CS.13 However, this rate is commensurate with other studies demonstrating PAC rates of 82% and 64% among patients with CS at tertiary centers in the US and Spain, respectively.15,24 Because CCCTN is comprised by specialized cardiac ICUs, the prevalence of PAC use is likely to be higher than among all CICUs nationally. However, our findings are of particular relevance and importance to contemporary practice in tertiary medical centers that provide care for complex patients with cardiogenic shock. Moreover, the variation in care observed in our report is consistent with a study of academic intensive care units that showed physician specialty and institutional norms strongly influenced PAC use.25 In our analysis, we observed a tendency towards more PAC use in centers with higher CS case volume; however, the distribution of shock severity was quite homogeneous across low to high utilizers of invasive hemodynamics. In addition, 14% of the overall variability in use among shock admissions was explained by institutional tendency rather than patient-related factors. The overall variability in use of PACs underscores a lack of evidence in the shock population that may inform clinicians and guidelines on the appropriate use of this tool.
Notably, PAC use was lower for older patients and, curiously, those with higher lactates after accounting for the diagnosis of shock and use of MCS. The reason for this latter finding is unclear; the observation may be related to complex clinical behaviors in which the lack of need for further diagnostics, and clinical assessment of prognosis vs. the perceived impact of invasive hemodynamic information dictate the clinician’s decision to use a PAC in shock cases.
Mortality with and without Use of PACs
Observational studies have suggested that sustained improvements in hemodynamics, exercise performance, and symptoms can be achieved with used of invasive hemodynamic monitoring to tailor therapy in select populations with advanced heart failure.26–30 Other studies that focused primarily on CS showed a favorable association of use of a PAC with lower mortality. For example, in a single-center observational study of 129 patients with CS, PAC use was associated with lower mortality at 30 days (adjusted HR 0.55, 95% CI 0.35, 0.86, p=0.008), particularly in patients without AMI.24 In a recent observational study among 1414 patients with CS, the Cardiogenic Shock Working Group identified an association of a complete hemodynamic assessment compared with no PAC assessment with higher in-hospital survival (adjusted OR 1.57; 95% CI 1.06, 2.33).15 In addition, in an analysis of administrative data from the Nationwide Readmissions Database including 236156 patient hospitalizations with cardiogenic shock between 2016 and 2017, use of a PAC was associated with a significantly lower in-hospital mortality (adjusted odds ratio [OR] 0.69; 95% CI, 0.66–0.72).31
The analytic cohort in our CCCTN study is a large population of consecutive admissions to the CICU which allows for a unique perspective on the epidemiology compared with other studies. The clinical review of individual complete medical records using centralized definitions with detailed classification of shock severity and presenting end-organ dysfunction are strengths of this analysis and this granularity of detail complements the methodology and findings from large administrative datasets.31 The design enabled the characterization of PAC use across the full spectrum of CICU admissions with and without shock. Moreover, our study population includes the full continuum of patients with shock triaged to CICUs, inclusive of patients with mixed shock etiologies where the dominant hemodynamics may also be less clear. In this broader population, the potential value of a PAC may also relate to providing diagnostic clarity in addition to guiding therapeutic decision-making. We found a favorable association between PAC use and mortality in this population of cardiac patients with shock admitted to a CICU with directional consistency across subtypes of shock. These findings support that similar observations using administrative data from centers with relatively low of use of PACSs are relevant in contemporary tertiary centers with specialized CICUs. The qualitative consistency of these findings across different datasets and methodological approaches lends support to a potential favorable impact of invasive hemodynamic assessment in this population.
Our findings add to observational data that, taken in aggregate, intriguingly point to a possible clinical benefit of an invasive hemodynamic strategy among patients with undifferentiated shock in CICUs and that are reassuring with respect to a lack of harm. It is possible that heterogeneity in the outcomes associated with PACs across past studies is related in part to differences in the interpretation and use of invasive hemodynamic data across specialtyspecific ICUs. Nevertheless, remaining uncertainty points to the value of future research, ideally randomized trials with larger cohorts, to rigorously assess the impact of PAC use in patients with shock.
Limitations
This analysis has several limitations. From a perspective of the descriptive epidemiology, the registry is limited to medical CICUs and thus cannot be applied to surgical patients. In addition, VA-ECMO exclusively occurs in the surgical ICU at some centers and would not be captured in this dataset unless care was transferred from the medical CICU. As well, our results may not be generalizable to the overall management of shock in non-cardiac ICUs. The CCCTN Registry collects 2-month snapshots at each center. While this strategy allows for the collection of consecutive admissions, it is not known whether seasonal variations in patient presentation would affect the results. Specific changes to the administration of inotropes, vasopressors, or diuretics timed after PAC placement were not captured in the database. Therefore, we are not able to rigorously assess how hemodynamic information influenced management decisions. Finally, and foremost with respect to the exploratory observational analysis of the association of PAC use with in-hospital mortality, this analysis is subject to inherent confounding both by indication and by other selection pressures. We have worked to mitigate such confounding by adjusting for covariates associated with the propensity for a PAC; however, unknown confounders may still exist. Given these uncertainties and the rapidly changing landscape of cardiogenic shock management, it is paramount that a strategy of invasive hemodynamic guided care be assessed in randomized clinical trials of critically ill cardiac patients who present with shock.
Conclusion
In this multicenter cohort, PAC use varied widely between institutions, with such variability explained in meaningful part by institutional tendency after accounting for patientspecific factors; pointing to uncertainty regarding the optimal use of PACs. We observed an association between PAC use and higher survival among cardiac patients admitted to CICUs with shock. These findings underscore the need for robust outcomes-based research to provide insight into the appropriate use of PACs in cardiac critical care.
Supplementary Material
Perspectives.
COMPETENCY IN SYSTEMS-BASED PRACTICE:
With the advent of cardiac critical care units, pulmonary artery catheters may help guide treatment decisions and improve survival for this unique subset of patients presenting with shock. Institutional preference is still a large driver of their use.
TRANSLATIONAL OUTLOOK:
Further research is needed to identify a cohort of patients that may benefit from invasive hemodynamic assessments in the current era of specialized cardiac intensive care units and protocol-based care for shock.
Funding:
Dr. Michael A. Solomon receives research support from the National Institutes of Health Clinical Center intramural research funds.
Disclosures:
Dr. David A. Morrow has received research grant support to Brigham and Women’s Hospital from Abbott and Abiomed and consulting fees from Abbott Laboratories.
Abbreviations
- CICU
cardiac intensive care unit
- CS
cardiogenic shock
- HF
heart failure
- ICU
intensive care unit
- MCS
mechanical circulatory support
- PAC
pulmonary artery catheter
- SCAI
Society for Cardiovascular Angiography and Interventions
- VA-ECMO
veno-arterial extracorporeal membrane oxygenation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chatterjee K The Swan-Ganz catheters: past, present, and future. A viewpoint. Circulation. Jan 6 2009;119(1):147–52. doi: 10.1161/CIRCULATIONAHA.108.811141 [DOI] [PubMed] [Google Scholar]
- 2.Robin ED. The cult of the Swan-Ganz catheter. Overuse and abuse of pulmonary flow catheters. Ann Intern Med. Sep 1985;103(3):445–9. doi: 10.7326/0003-4819-103-3-445 [DOI] [PubMed] [Google Scholar]
- 3.Guyatt G A randomized control trial of right-heart catheterization in critically ill patients. Ontario Intensive Care Study Group. J Intensive Care Med. Mar-Apr 1991;6(2):91–5. doi: 10.1177/088506669100600204 [DOI] [PubMed] [Google Scholar]
- 4.Connors AF Jr., Speroff T, Dawson NV, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA. Sep 18 1996;276(11):889–97. doi: 10.1001/jama.276.11.889 [DOI] [PubMed] [Google Scholar]
- 5.Afessa B, Spencer S, Khan W, LaGatta M, Bridges L, Freire AX. Association of pulmonary artery catheter use with in-hospital mortality. Crit Care Med. Jun 2001;29(6):1145–8. doi: 10.1097/00003246-200106000-00010 [DOI] [PubMed] [Google Scholar]
- 6.Chittock DR, Dhingra VK, Ronco JJ, et al. Severity of illness and risk of death associated with pulmonary artery catheter use. Crit Care Med. Apr 2004;32(4):911–5. doi: 10.1097/01.ccm.0000119423.38610.65 [DOI] [PubMed] [Google Scholar]
- 7.Harvey S, Harrison DA, Singer M, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet. Aug 6-12 2005;366(9484):472–7. doi: 10.1016/S0140-6736(05)67061-4 [DOI] [PubMed] [Google Scholar]
- 8.Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA. Oct 5 2005;294(13):1664–70. doi: 10.1001/jama.294.13.1664 [DOI] [PubMed] [Google Scholar]
- 9.Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993–2004. JAMA. Jul 25 2007;298(4):423–9. doi: 10.1001/jama.298.4.423 [DOI] [PubMed] [Google Scholar]
- 10.Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. Oct 5 2005;294(13):1625–33. doi: 10.1001/jama.294.13.1625 [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, et al. 2013. ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. Oct 15 2013;128(16):1810–52. doi: 10.1161/CIR.0b013e31829e8807 [DOI] [PubMed] [Google Scholar]
- 12.Khera R, Pandey A, Kumar N, et al. Variation in Hospital Use and Outcomes Associated With Pulmonary Artery Catheterization in Heart Failure in the United States. Circ Heart Fail. Nov 2016;9(11)doi: 10.1161/CIRCHEARTFAILURE.116.003226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez GA, Lemor A, Blumer V, et al. Trends in Utilization and Outcomes of Pulmonary Artery Catheterization in Heart Failure With and Without Cardiogenic Shock. J Card Fail. May 2019;25(5):364–371. doi: 10.1016/j.cardfail.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 14.Tehrani BN, Truesdell AG, Psotka MA, et al. A Standardized and Comprehensive Approach to the Management of Cardiogenic Shock. JACC Heart Fail. Nov 2020;8(11):879–891. doi: 10.1016/j.jchf.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garan AR, Kanwar M, Thayer KL, et al. Complete Hemodynamic Profiling With Pulmonary Artery Catheters in Cardiogenic Shock Is Associated With Lower In-Hospital Mortality. JACC Heart Fail. Nov 2020;8(11):903–913. doi: 10.1016/j.jchf.2020.08.012 [DOI] [PubMed] [Google Scholar]
- 16.McDonagh TA, Metra M, Adamo M, et al. 2021. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. Sep 21 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 17.Naidu SS, Baran DA, Jentzer JC, et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J Am Coll Cardiol. Mar 8 2022;79(9):933–946. doi: 10.1016/j.jacc.2022.01.018 [DOI] [PubMed] [Google Scholar]
- 18.Morrow DA, Fang JC, Fintel DJ, et al. Evolution of critical care cardiology: transformation of the cardiovascular intensive care unit and the emerging need for new medical staffing and training models: a scientific statement from the American Heart Association. Circulation. Sep 11 2012;126(11):1408–28. doi: 10.1161/CIR.0b013e31826890b0 [DOI] [PubMed] [Google Scholar]
- 19.Bohula EA, Katz JN, van Diepen S, et al. Demographics, Care Patterns, and Outcomes of Patients Admitted to Cardiac Intensive Care Units: The Critical Care Cardiology Trials Network Prospective North American Multicenter Registry of Cardiac Critical Illness. JAMA Cardiol. Sep 1 2019;4(9):928–935. doi: 10.1001/jamacardio.2019.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg DD, Bohula EA, van Diepen S, et al. Epidemiology of Shock in Contemporary Cardiac Intensive Care Units. Circ Cardiovasc Qual Outcomes. Mar 2019;12(3):e005618. doi: 10.1161/CIRCOUTCOMES.119.005618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. Jul 1 2019;94(1):29–37. doi: 10.1002/ccd.28329 [DOI] [PubMed] [Google Scholar]
- 22.Lawler PR, Berg DD, Park JG, et al. The Range of Cardiogenic Shock Survival by Clinical Stage: Data From the Critical Care Cardiology Trials Network Registry. Crit Care Med. Aug 1 2021;49(8):1293–1302. doi: 10.1097/CCM.0000000000004948 [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Zumbo BD, Wu AD. Relative Importance of Predictors in Multilevel Modeling. J Mod Appl Stat Meth. May 2014;13(1):2–22. [Google Scholar]
- 24.Rossello X, Vila M, Rivas-Lasarte M, et al. Impact of Pulmonary Artery Catheter Use on Short- and Long-Term Mortality in Patients with Cardiogenic Shock. Cardiology. 2017;136(1):61–69. doi: 10.1159/000448110 [DOI] [PubMed] [Google Scholar]
- 25.Koo KK, Sun JC, Zhou Q, et al. Pulmonary artery catheters: evolving rates and reasons for use. Crit Care Med. Jul 2011;39(7):1613–8. doi: 10.1097/CCM.0b013e318218a045 [DOI] [PubMed] [Google Scholar]
- 26.Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol. Sep 1997;30(3):725–32. doi: 10.1016/s0735-1097(97)00208-8 [DOI] [PubMed] [Google Scholar]
- 27.Steimle AE, Stevenson LW, Chelimsky-Fallick C, et al. Sustained hemodynamic efficacy of therapy tailored to reduce filling pressures in survivors with advanced heart failure. Circulation. Aug 19 1997;96(4):1165–72. doi: 10.1161/01.cir.96.4.1165 [DOI] [PubMed] [Google Scholar]
- 28.Stevenson LW, Dracup KA, Tillisch JH. Efficacy of medical therapy tailored for severe congestive heart failure in patients transferred for urgent cardiac transplantation. Am J Cardiol. Feb 15 1989;63(7):461–4. doi: 10.1016/0002-9149(89)90320-2 [DOI] [PubMed] [Google Scholar]
- 29.Lucas C, Johnson W, Hamilton MA, et al. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J. Dec 2000;140(6):840–7. doi: 10.1067/mhj.2000.110933 [DOI] [PubMed] [Google Scholar]
- 30.Chomsky DB, Lang CC, Rayos G, Wilson JR. Treatment of subclinical fluid retention in patients with symptomatic heart failure: effect on exercise performance. J Heart Lung Transplant. Aug 1997;16(8):846–53. [PubMed] [Google Scholar]
- 31.Ranka S, Mastoris I, Kapur NK, et al. Right Heart Catheterization in Cardiogenic Shock Is Associated With Improved Outcomes: Insights From the Nationwide Readmissions Database. J Am Heart Assoc. Sep 7 2021;10(17):e019843. doi: 10.1161/JAHA.120.019843 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


