Abstract
Objectives:
The mGlu2/3 receptor agonist LY379268 was previously found to reduce sucrose-seeking, but not sucrose-taking, in male rats. The present study explored the generality of this effect across sexes. In addition, the effect of the drug on motivation to receive sucrose was assessed with a progressive ratio (PR) schedule of reinforcement.
Methods:
Adult male and female Long-Evans rats (N = 91) were challenged with LY379268 in three experiments: 1. fixed ratio (FR) schedule of reinforcement (taking), 2. extinction of responding previously reinforced on the FR (seeking), or 3. responding reinforced on a progressive ratio (PR) schedule of reinforcement (motivation). For each experiment, rats first responded for 10% liquid sucrose on a FR in 10 daily 2-h sessions. For the PR study, this was followed with training on a PR for 7 daily 3-h sessions. Rats were then challenged in a counterbalanced order with LY379268 (0, 1.5, 3, 6 mg/kg; IP; 30-minute pretreatment) on test days followed by either three reacquisition days of FR (Experiments 1 and 2) or PR (Experiment 3) responding.
Results:
Female rats responded more for sucrose on both the FR and PR. LY379268 reduced responding in all three experiments, but the dose-response differed by experiment. LY379268 challenge to sucrose taking on the FR produced an inverted U-shaped function while extinction responding and responding for sucrose on the PR were decreased dose-dependently with PR responding insensitive to the 1.5 mg/kg dose. There were no sex-dependent effects of the drug on sucrose-directed responding.
Conclusions:
The generality of the anti-taking, -seeking, and -motivation effects of LY379268 across male and female rats support further evaluation of this and other compounds that modulate synaptic glutamate as potential anti-addiction pharmacotherapies.
Keywords: craving, food addiction, glutamate, LY379268, mGlu2, mGlu3, motivation, rat, sex difference
Overconsumption of added sugars is endemic in the United States and a large proportion of the populace is overweight and obese (74% of adults; CDC 2022). Most sugar in the diet of individuals in the United States is found in sugar-sweetened beverages, desserts, and snacks (CDC 2021). Cheap commercially available sugar has been available for just over 100 years (Yudkin 1972). Sugar in this form is more like a drug than a food. That is, the excessive taking and seeking of palatable food, often high in sugar, resembles substance use disorder (O’Connor & Kenny 2022). Even more, sucrose-directed behavior may be especially persistent as the neurobiological substrates of motivation to acquire and consume sweet and typically high calorie foods are physiologically redundant (Ahmed et al. 2013).
Sucrose taking and seeking by rats provides a preclinical model of sucrose-directed behavior to gauge interest and motivation to consume sucrose. Results derived with this model may also enhance scientific understanding of reward-directed behaviors, such as substance use disorder, due to the shared neurocircuitry mediating the reinforcing properties of foods (e.g. sucrose) and most drugs of abuse (Volkow et al. 2017), and craving for those substances (Koban et al. 2022). Increased knowledge of the neurobiological substrates of sucrose taking and seeking could inform approaches to reduce reward-directed behaviors that are common across reward classes.
LY379268 agonizes mGlu2/3 autoreceptors resulting in decreased evoked glutamate release (Cartmell & Schoepp 2000). Bossert et al. (2006a) reported LY379268 reduced sucrose seeking, but not sucrose taking by rats. LY379268 had previously been found to decrease heroin seeking, but not heroin taking (Bossert et al. 2005) and subsequently, to reduce cocaine seeking (Lu et al. 2007) by rats. These results from a rat model of seeking and taking therefore reveal a role for increased glutamate in seeking, a behavior roughly translatable to craving in humans (Nicolas et al. 2022). In addition, there are sex differences in the prevalence and severity of substance use disorders (Becker et al. 2017; Nicolas et al. 2022) and in disordered eating and obesity (Reagan & Hersch 2005; Anversa et al. 2021). However, preclinical assessment of pharmacotherapies that could be used to treat the addiction behaviors that characterize drug and food addiction, such as craving, have been conducted primarily with male rodents. The studies cited above with LY379268 were all conducted with male rats.
Therefore, the aim of the present study was to explore the generality of the anti-seeking (“anti-craving”) effect of LY379268 across sexes. In addition, the effect of the drug on both taking (Fixed Ratio, FR responding) and motivation to receive sucrose (Progressive Ratio, PR responding) was assessed. Effects of the drug on PR responding has not yet been reported. It is important to consider the generality of effect of reducing glutamate signaling across schedules that tap into different aspects of reinforcement as results will inform how drugs that reduce glutamate availability might translate as pharmacotherapeutics.
Methods
Subjects
Separate groups of male and female adult Long-Evans rats bred at Western Washington University (WWU) were used for each experiment. Animals were housed in clear cages (width × depth × height: 20 × 30 × 20 cm) hung on Lab Products Inc. racks (Seaford, DE, USA). Subjects were individually housed under a reverse light cycle (lights off 0700–1900 h) at postnatal day 70 with commencement of experiments at approximately postnatal day 90. The outbred rat strain was sustained with random pairs of unrelated subjects and new outside breeders from Charles River (Wilmington, MA, USA) every 10 months. Experiments were conducted between 0800–1300 h 7 days a week. Mazuri Rodent Pellets (Purina Mills Inc., Saint Louis, MO, USA) and water were provided ad libitum in home cages and operant conditioning chambers except water was removed from both for the 17 h prior to, and during, the first day of training. Water for the study was from a common source of filtered water. Body weights were recorded before each experiment and every M,W,F thereafter. Welfare of subjects was provided for (PHS 2015) and experiments were approved by the WWU Institutional Animal Care and Use Committee.
Operant conditioning chambers
Med Associates operant conditioning chambers (30 × 20 × 24 cm; St. Albans, VT, USA) included a retractable lever, a stationary lever, four infrared photobeams, a 2 kHz tone generator (15 dB over ambient noise), a white stimulus light above the retractable lever, and a red house light on the opposite wall. Activation of an infusion pump resulted in sucrose delivery into a receptacle to the right of the active lever. Each chamber was enclosed in a light and sound-attenuated cabinet with a fan providing air flow and white noise.
Operant conditioning procedures
For each of the experiments rats first responded for 10% liquid sucrose on a FR schedule of reinforcement in 10 daily 2-h sessions. The session started with illumination of the house light and extension of the retractable lever. A response on that “Active” lever resulted in a 5 s illumination of the stimulus light and activation of the tone generator. Concurrent with this tone+light stimulus, the infusion pump delivered .2 mL sucrose. For the next 35 s subsequent lever responses were recorded but had no consequence. This response contingency can be summarized as a FR1 schedule of reinforcement with a 40 s post-reinforcer timeout. Extinction testing (Seeking; Experiment 2) consisted of rats making responses under these same conditions except sucrose syringes were absent. That is, an active lever response produced the tone+light cue and activated the empty syringe pump. After 40 s an active lever response would again produce these outcomes. PR training and testing sessions (Experiment 3) were up to 3 h long with rats allowed to respond on an escalating response/reinforcement requirement of 1, 2, 4, 6, 9, 12, 16, 20, 28, 36, 48, 63, 83, 110, 145, 191, 251, 331, 437, 575, 759, 999, (999 repeats) (Richardson & Roberts 1996). Different from the FR training, a response on the retractable lever (Active lever) resulted in three simultaneous consequences: retraction of the lever, presentation of the 5 s tone+light stimulus, and delivery of .4 mL sucrose. After this 5 s set of events, the lever was re-extended. The session ended if a subject did not respond for more than 30 min.
Figure 1 depicts the designs of the three experiments. Experiment 1 (Taking) examined the effects of LY379268 on responding for sucrose on a FR schedule with 3 self-administration sessions between each drug challenge session. Experiment 2 (Seeking) included drug challenges where rats responded in extinction conditions. Three self-administration sessions occurred between each drug challenge. For Experiment 3 (Motivation), rats responded on the PR schedule for 7 days prior to drug challenges; this followed the initial 10 days of FR training. Drug challenges on the PR were separated by 3 days of responding on the PR.
Figure 1.

Schematic of Experiments 1,2,3.
Drug
LY379268 was purchased from Tocris Bioscience (Minneapolis, MN, USA). An initial 18 mg/mL stock solution was prepared as follows: LY379268 was dissolved 100 mM NaOH with alternating vortexing and sonication and then clear solution was brought to a pH of 6.5 with 4 N HCl and to volume with sterile saline. Vehicle was prepared using a similar mixture of sterile saline, 100 mM NaOH, and 4 N HCl. Rats were accustomed to the injection procedure by administration of saline the two afternoons prior to the first drug challenge. Rats were then challenged in a counterbalanced order with LY379268 on each test day (0, 1.5, 3, 6 mg/kg; IP; 30-minute pretreatment). Vehicle was the 0 dose.
Statistical analyses
Data from each experiment were analyzed separately as were each dependent measure. Training and Testing results were also analyzed separately. Training data were analyzed with two-way repeated measure Analysis of Variance (ANOVA) (2-way RMANOVA) with the factors Time and Sex. Testing data were analyzed with 2-way RMANOVA with the factors Dose and Sex. Significant main effects and interactions were followed by Tukey’s tests as appropriate. Partial eta squared (η2p) is provided as an indication of effect size. Grouped data in the text are presented as mean ± standard error of the mean (SEM) while in the Figures grouped data are presented as estimated marginal mean ± SEM. Statistical analyses were performed with open source Jamovi version 2.2.5 (The Jamovi Project, Sydney, Australia) and for analysis with covariate, (Experiment 2, see below) IBM SPSS Statistics for Windows version 28 (IBM Corp., Armonk, NY, USA). Figures were constructed with Jamovi.
Results
Group sizes and average body weights at the start of the study are provided for each experiment below. Only significant main effects and interactions are indicated in the text. Active lever statistics are presented in the text and Figures as a measure of response rate (responses per session). Supplemental data (Grimm et alia Supplemental.xlsx) includes inferential statistics and figures for all dependent measures (active lever, reinforcers, inactive lever, locomotor activity) in addition to reinforcers adjusted by body weight for all experiments except for Experiment 2 Testing (extinction responding). Break points defined as the final ratio earned for Experiment 3 Training and Testing are also provided in the Supplemental file. Break point is sometimes reported as the number of reinforcers earned; break point measured as the final ratio is problematic (especially with large final ratios) as the scaling from one ratio to the next is non-linear. As noted by Richardson & Roberts (1996), a log transformation of these data to avoid violation of homogeneity of variance results in the number of reinforcers earned. That is, presenting PR data as reinforcers earned provides the same information as break points. In the present study, we presented total active lever responses in the Figures to allow comparison across the FR and PR experiments. All Figures include individual data points. Ranges for Y-axes were determined by the Jamovi software and are not editable.
Experiment 1 FR (Taking)
There were 12 female and 12 male subjects with initial body weights: females (196.6 ± 2.8 g), males (328.8 ±3.6 g). For Active lever Training there were significant effects of Day F(9,198) = 7.7, p < .001, η2p = .3, and Sex F(1,22) = 10.4, p < .01, η2p = .3. Post-hoc tests revealed that responses increased over Days 2–8 compared to Day 1 then settled at Day 1 levels for Days 9 and 10 (p’s < .05). As indicated in Figure 2, females responded at a higher rate than males. For Active lever Testing there were significant effects of Dose F(3,66) = 5.3, p < .01, η2p = .2, and Sex F(1,22) = 9.8, p < .01, η2p = .3. Post-hoc tests revealed a significant decrease in responses compared to the 0 dose when rats received either the 1.5 or 6 mg/kg dose (p’s < .05). Females responded at a higher rate than males.
Figure 2.
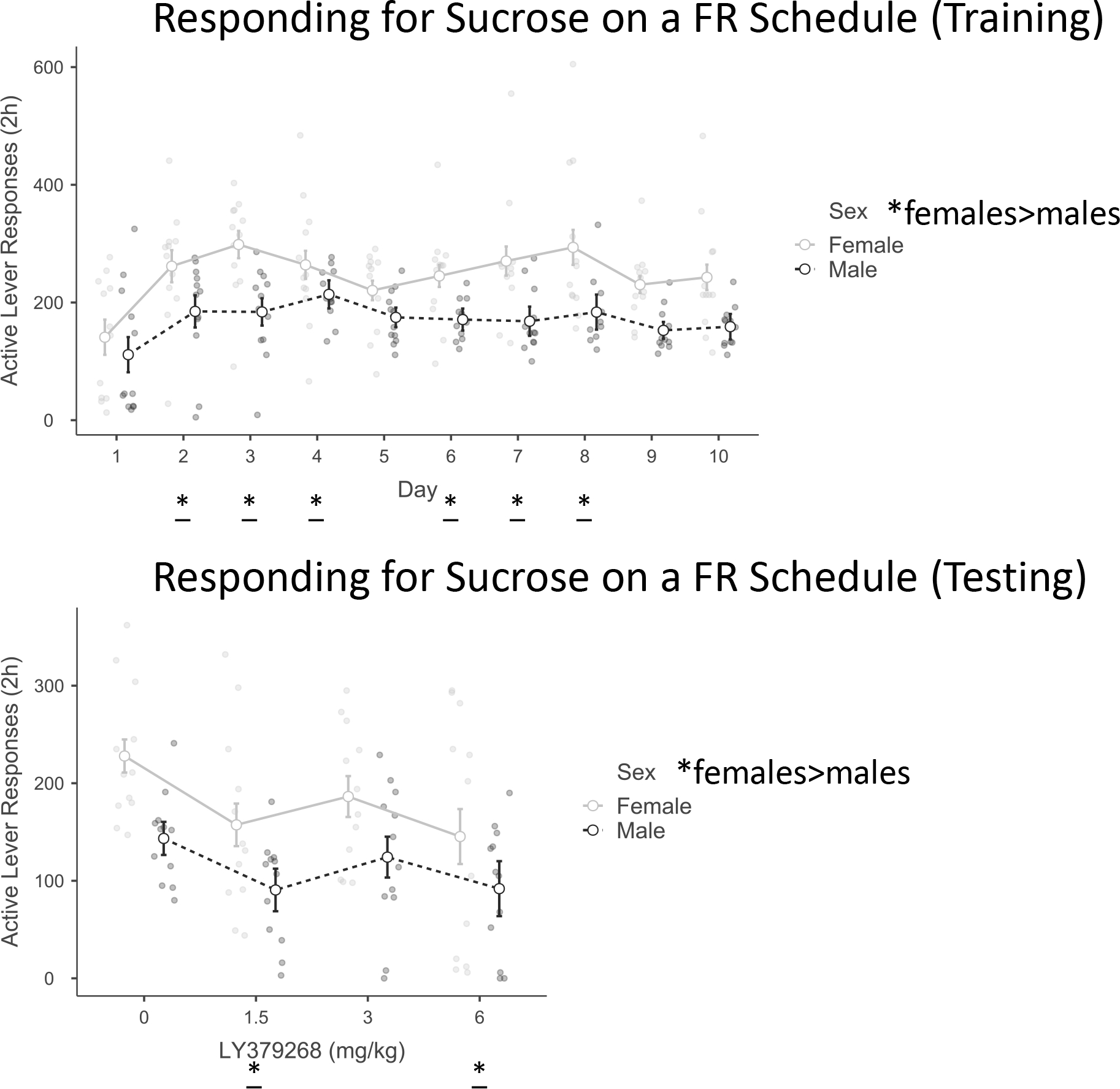
Responding for sucrose on a FR schedule (sucrose taking)
In Experiment 1, females responded at a higher rate than males in both training and testing. LY379268 (0, 1.5, 3, 6 mg/kg; IP; 30-minute pretreatment) decreased active lever responses after the 1.5 and 6, but not 3 mg/kg doses compared to the 0 dose. Grouped data are presented as estimated marginal mean ± SEM. * main effect of sex, p<0.05; * main effect vs. Day 1 of Training or vs. 0 dose of Testing, p<0.05.
Experiment 2 FR Extinction (Seeking)
There were 16 female and 14 male subjects with initial body weights: females (219.3 ± 4.4 g), males (375.1 ± 8.6 g). For Active lever Training there were significant effects of Day F(9,252) = 9.6, p < .001, η2p = .3 and Sex F(1,28) = 14.5, p < .001, η2p = .3. Post-hoc tests revealed that responses increased over Days 2–10 compared to Day 1 (p’s < .05). As indicated in Figure 3, females responded at a higher rate than males. For Active lever Testing there were significant effects of Dose F(3,84) = 9.5, p < .001, η2p = .3, and Sex F(1,28) = 8.6, p < .01, η2p = .2. Post-hoc tests revealed a significant decrease in responses compared to the 0 dose when rats received either the 1.5, 3, or 6 mg/kg dose (p’s < .05). Females responded at a higher rate than males.
Figure 3.
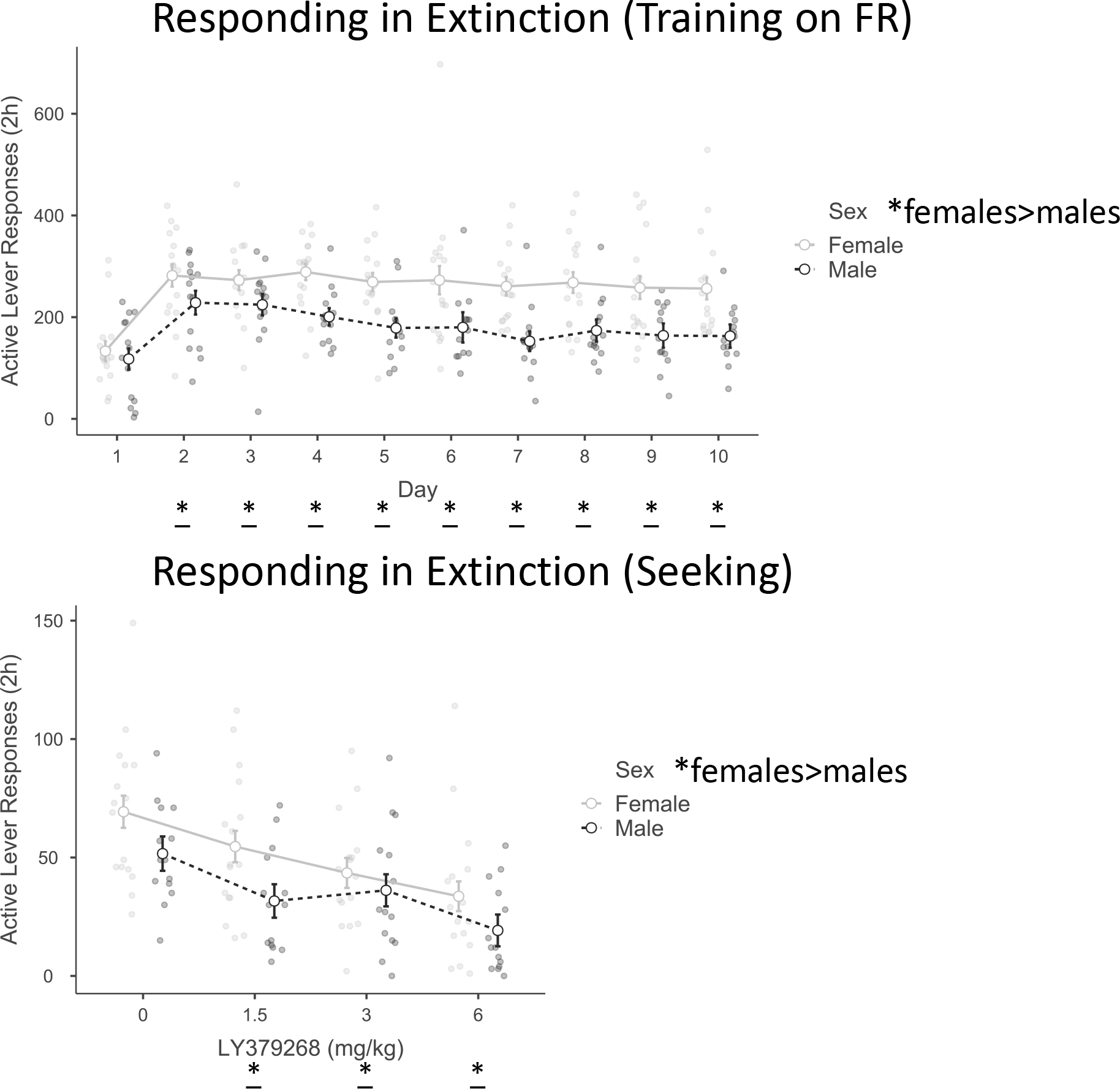
Responding in extinction (sucrose seeking)
In Experiment 2, females responded at a higher rate than males in both training and testing. LY379268 (0, 1.5, 3, 6 mg/kg; IP; 30-minute pretreatment) decreased active lever responses after all 3 doses compared to the 0 dose. Grouped data are presented as estimated marginal mean ± SEM. * main effect of sex, p<0.05; * main effect vs. Day 1 of Training or vs. 0 dose of Testing, p<0.05.
To determine whether the main effect of sex for sucrose seeking could be accounted for solely due to higher response rate for sucrose during training, the 2-way RMANOVA was run again with active lever responses on day 10 of training as a covariate. The sex difference in active lever seeking was still statistically significant F(1,27) = 4.3, p < .05 , η2p = .1 as was the effect of drug Dose F(3,81) = 4.9, p < .01, η2p = .2. This result replicates a similar calculation in Grimm et al. (2022) where sucrose taking (Active lever responding) did not explain the sex difference in sucrose seeking. This supports the conclusion that females seek sucrose at a higher rate than males.
Experiment 3 PR (Motivation)
There were 16 female and 16 male subjects with initial body weights: females (231.1 ± 5.6 g), males (373.3 ± 8.3 g). For FR Active lever Training there were significant effects of Day F(9,270) = 3.0, p < .01, η2p = .1, and Sex F(1,30) = 22.7, p < .001, η2p = .4. There was a significant Day X Sex interaction F(9,270) = 2.2, p < .05, η2p = .1. Post-hoc tests after the interaction revealed that responses did not increase over the 10 days of training. This may have been due to a high amount of variability in responses related to early acquisition. There were some specific days with significant post hocs for sex later in Training (Supplemental data). To verify the sex difference in Training, a subsequent 2-way RMANOVA was conducted for days 8–10 only. The main effect of Sex remained F(1,20) = 16.7, p < .001, η2p = .4. As indicated in Figure 4, females responded at a higher rate than males. There was no significant effect of Day nor a significant Day X Sex interaction from this ANOVA.
Figure 4.
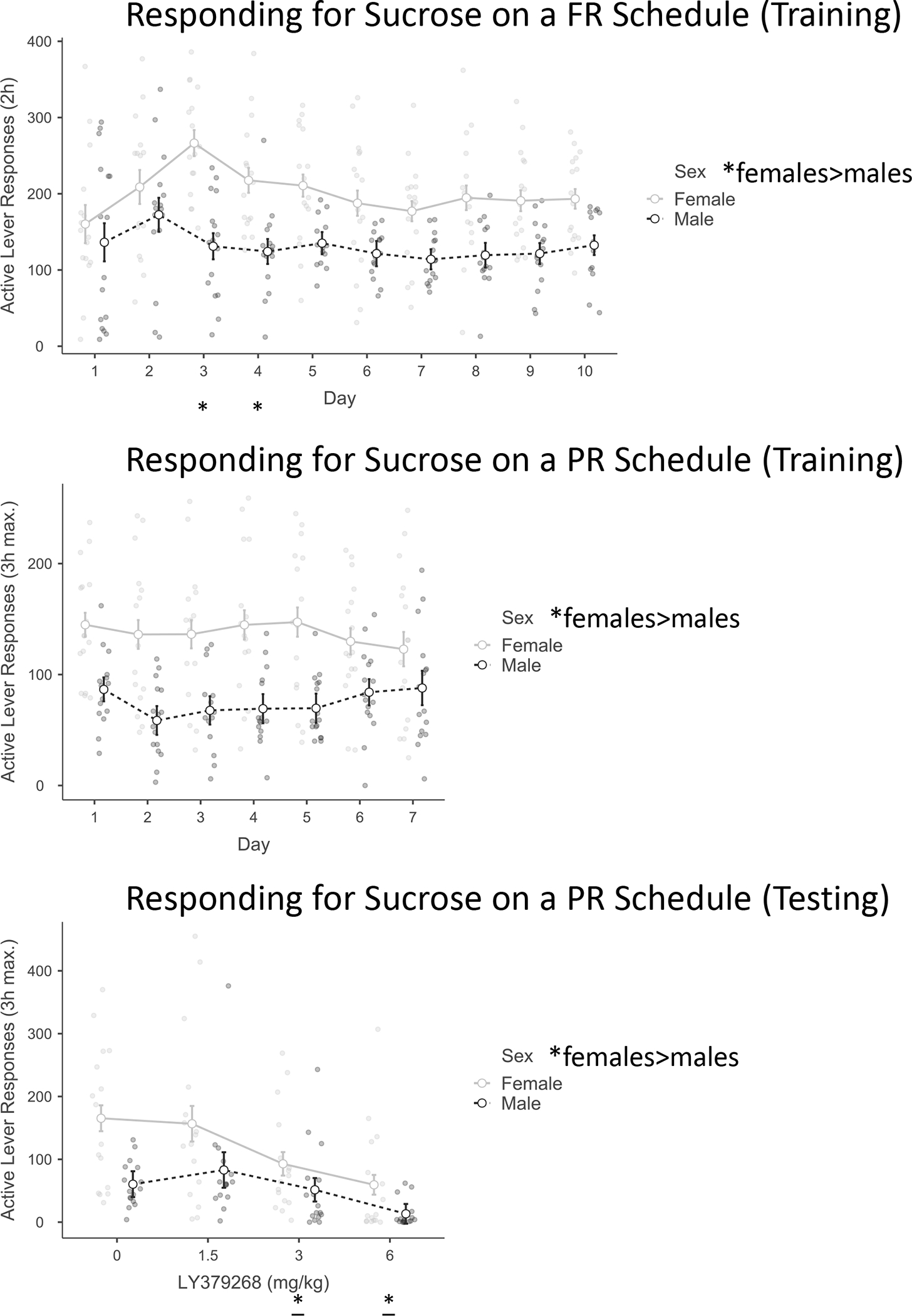
Responding for sucrose on a PR schedule (motivation for sucrose)
In Experiment 3, females responded at a higher rate than males in both training (FR and then PR) and testing (PR). LY379268 (0, 1.5, 3, 6 mg/kg; IP; 30-minute pretreatment) decreased active lever responses after the 3, 6, but not 1.5 mg/kg doses compared to the 0 dose. Grouped data are presented as estimated marginal mean ± SEM. * main effect of sex or significant sex difference at that Day of Training, p<0.05; * main effect vs. 0 dose of Testing, p<0.05.
For PR Active lever Training there was a significant effect of Sex F(1,30) = 21.5, p < .001, η2p = .4. Females responded at a higher rate than males. A lack of effect of Day indicates the response rate was steady over the 7 days of Training. For Active lever Testing there were significant effects of Dose F(3,90) = 11.8, p < .001, η2p = .3, and Sex F(1,30) = 8.4, p < .01, η2p = .2. Post-hoc tests revealed a significant decrease in responses compared to the 0 dose when rats received either the 3 or 6 mg/kg dose (p’s < .05). Females responded at a higher rate than males (Figure 4).
Discussion
As reported previously (Grimm et al. 2022), female Long-Evans rats responded at higher rates for sucrose on both FR and PR schedules of reinforcement. Intake of sucrose by females remained higher when adjusted for body weight (Supplementary data). Sucrose seeking was also at a higher rate for females, and this was not explained by their higher response rate across training. LY379268 reduced rate of responding in all experiments with no sex interaction. The low (1.5 mg/kg) and high (6 mg/kg) doses reduced responses in the FR experiment following an inverted-U dose-effect while PR and Extinction responses were decreased dose-dependently. For the PR experiment, only the medium (3 mg/kg) and high doses of LY379268 reduced the number of responses while all three doses reduced the number of responses in the Extinction experiment.
Sex differences
Table 1 provides a summary of sex differences in Experiments 1, 2, and 3. As noted above, females responded at higher rates for sucrose on both FR and PR schedules of reinforcement (Table 1), consistent with previous observations in Grimm et al. (2022). This sex difference was larger when body weight was accounted for. In fact, across the 3 experiments, for both FR and PR Training, body weight correction revealed that females consumed double the amount of sucrose compared to males (Supplemental data). Females also responded at a higher rate during extinction. The sex difference in extinction in Grimm et al. was larger than in the present study. For the vehicle condition, 2 h active lever responses in the previous study were 194.6 ± 18.6 (females) vs. 65.8 ± 12.5 (males) while in the present study active lever responses were 69.3 ± 7.9 (females) vs. 51.6 ± 5.6 (males). The higher response rates in the previous study were likely due to the fact that subjects had a history of PR training that produces high response rates. In addition, in the present study, rats were tested more times under extinction conditions (4 times in the present study vs. 3 in the previous).
Table 1.
Sex differences for all experiments for Training dependent measures and for the LY379268 0 dose Test for Experiment 2. Symbols indicate female (♀), male (♂), no difference (n.d.).
| Experiments 1, 2, and 3. FR responding for sucrose | |
| Active lever | ♀>♂ |
| Reinforcers | ♀>♂ |
| mL per g | ♀>♂ |
| Inactive lever | n.d.1 |
| Locomotion | ♀>♂ |
| Experiment 2. Responding in extinction (0 dose LY379268) | |
| Active lever | ♀>♂ |
| Reinforcers (cues) | n.d. |
| Inactive lever | n.d. |
| Locomotion | n.d. |
| Experiment 3. PR responding for sucrose | |
| Active lever | ♀>♂ |
| Reinforcers | ♀>♂ |
| mL per g | ♀>♂ |
| Inactive lever | ♀>♂ |
| Locomotion | ♀>♂ |
Notes:
(n.d. however ♀>♂, p=.055)
As reviewed in Grimm et al. (2022), the greater intake and motivation of females to consume, and to seek, sucrose corroborates several other previous reports with rats and mice. Furthermore, where sex differences were not reported on these measures, the negative effect may have been due to differences in strain of animals examined, type of reinforcer (pellet vs. liquid), or because intake was not corrected for body weight (Grimm et al.). Another limitation is the potential for estrous cycle-related effects on reinforcement to mask a sex difference. Very few studies include cyclicity as a measure, including the present study, as initial examination for sex differences often consist of examining first for an overall sex-related effect to be followed up with measures or manipulations (estrous cycle tracking, gonadectomies) should there be a sex difference (Beltz et al. 2019). Our lab is conducting estrous cycle tracking studies to correlate stage of cycle with ongoing sucrose taking, however the present study was not designed to be powered enough to connect stage of cycle with effect of LY379268 on sucrose taking and seeking.
Drug effects
Table 2 summarizes LY379268 effects for each dependent measure across the 3 experiments. As noted above, LY3279268 reduced sucrose taking on both FR and PR schedules of reinforcement and reduced sucrose seeking measured as responses under extinction conditions. The effect of the drug on some animals was to nearly completely suppress responding at a particular dose. However, for those animals, responding was not consistently suppressed upon testing with another dose for that same animal across counterbalanced drug challenges (data not shown). This indicates that the experience of LY379268 did not produce an aversive that carried over to subsequent testing. There were no sex differences in the LY3279268 effects. There is only one other published study that has reported on the effects of LY379268 in both males and females. Garceau et al. (2022) reported decreased expression of Pavlovian Instrumental Transfer (PIT), a measure of conditioned reinforcement (water as the reinforcer, CS was an auditory stimulus) after drug challenge; the decrease was similar extent in both male and female rats.
Table 2.
Effects of LY379268 on all dependent measures. There were no LY379268 X Sex interactions. Symbols indicate dose effects compared to 0 dose; increase (↑), decrease (↓), no change (n.c.).
| LY379268 (mg/kg) | 1.5 | 3 | 6 |
|---|---|---|---|
| Experiment 1. FR responding for sucrose | |||
| Active lever | ↓ | n.c. | ↓ |
| Reinforcers | ↓ | n.c. | ↓ |
| mL per g | ↓ | n.c. | ↓ |
| Inactive lever | n.c. | n.c. | n.c. |
| Locomotion | ↓ | n.c. | ↓ |
| Experiment 2. Responding in extinction | |||
| Active lever | ↓ | ↓ | ↓ |
| Reinforcers (cues) | n.c. | n.c. | ↓ |
| Inactive lever | n.c. | n.c. | n.c. |
| Locomotion | n.c. | n.c. | n.c. |
| Experiment 3. PR responding for sucrose | |||
| Active lever | n.c. | ↓ | ↓ |
| Reinforcers | n.c. | ↓ | ↓ |
| mL per g | n.c. | ↓ | ↓ |
| Inactive lever | n.c. | n.c. | n.c. |
| Locomotion | n.c. | n.c. | n.c. |
Anti-taking and seeking effects of LY379268 have been evaluated for both drug and non-drug (food, water) reinforcers in over a dozen published preclinical studies since in 2004. LY379268 decreases reward seeking and taking. For example, systemic LY379268 reduces responding for, or in the presence of, cues previously associated with intake of alcohol (Windisch & Czachowski 2018), heroin (Bossert et al. 2004, 2005), methamphetamine (Kufahl et al. 2013), cocaine (Baptista et al. 2004), sucrose (Bossert et al. 2006a; Kufahl et al. 2013, Windisch & Czachowski; present study), sweetened condensed milk (Baptista et al. 2004), and water (Garceau et al. 2022). Systemic LY379368 also reduces self-administration of cocaine (Baptista et al. 2004), methamphetamine (Crawford et al. 2013), and sucrose (Bossert et al. 2006a; Windisch & Czachowski; present study). The effects listed above are dose-dependent and in studies where LY379268 reduces both taking and seeking, there is a difference between a dose that reduces taking and a dose that reduces seeking. This was the case in the present study with sucrose where 3 mg/kg decreased active lever responses for a sucrose-paired cue but not for sucrose itself on the FR. Baptista et al. reported a similar finding with cocaine where 1 mg/kg reduced cocaine seeking but 3 mg/kg was required to reduce cocaine taking. As an interesting caveat to these findings, we observed an inverted-U with 1.5 and 6, but not 3 mg/kg reducing FR sucrose taking. Additionally, Baptista et al. reported no effect of their highest dose (3 mg/kg) on rats responding for sweetened condensed milk. It could be that a higher dose (e.g. 6 mg/kg) would have reduced sweetened condensed milk taking. Indeed, while Bossert et al. showed no significant effect of 3 mg/kg LY379268 on FR sucrose taking, it is noted in their Methods that unpublished pilot work with 6 mg/kg did reduce sucrose taking. The highest dose tested by Crawford et al. on sucrose (pellet) taking on the PR was 1 mg/kg on the PR and as with the present study, that did not reduce responding for sucrose on the PR schedule. Higher doses (3 and 6 mg/kg) were required. LY379268 also reduces the abstinence-dependent increase in reward seeking (incubation of craving) of cocaine (Lu et al. 2007) and sucrose (Uejima et al. 2007). Furthermore, 5 injections of LY379268 spaced every 12 h (1 or 3 mg/kg) decreased the alcohol deprivation effect but had no effect on body weight or water intake (Vengeliene & Spanagel 2022).
These preclinical results indicate an important role of glutamate in several brain regions in drug seeking. For example, glutamate antagonist delivered into the nucleus accumbens, ventral tegmental nucleus (VTA), or dorsal hippocampus reduces cocaine seeking (Cornish et al. 1999; You et al. 2007; Xie et al. 2010). Furthermore, introduction of a lever previously associated with cocaine delivery initiates cocaine seeking and predicts a spike in VTA glutamate; as noted above, infusion of glutamate antagonists into the VTA decreases that cocaine seeking (You et al.). Bossert and colleagues have examined effects of LY379268 delivered directly to the brain on both drug and sucrose seeking. Intra-VTA or intra-nucleus accumbens shell delivery of LY379268 (Bossert et al. 2004, 2006b) reduces heroin context-induced reinstatement of heroin seeking. Intra-central nucleus of the amygdala LY379268 delivery decreased incubation of sucrose (Uejima et al. 2007) and cocaine craving (Lu et al. 2007). Intra-nucleus accumbens core delivery of LY379268 has yielded different effects on sucrose seeking. Myal et al. (2015) reported an increase in sucrose seeking in adult but not adolescent rats while Windisch & Czachowski (2018) reported a decrease in both sucrose taking and seeking subsequent to delivery of the drug into the nucleus accumbens core of adult rats. The specific mGlu2 positive allosteric modulator BINA was without effect.
Without side-by-side comparisons of behavior (taking, seeking) changes due to intracranial LY379268 it is impossible to gauge whether one brain region/system is more sensitive to decreased glutamate release. The only study so far to do this is Windisch & Czachowski (2018) with LY379268 delivered into the nucleus accumbens shell prior to sucrose taking or seeking; both were reduced after drug microinjection. In contrast, the same manipulation decreased ethanol seeking but not taking (Windisch & Czachowski). Further studies are required to delineate the neural substrates of anti-sucrose taking and anti-sucrose seeking effects of LY379268. Regions beyond the nucleus accumbens, VTA, and hippocampus should be considered given the contribution of several cortical, subcortical, and brain stem regions to motivated behaviors (Cromwell et al. 2020).
In the present study, the dose-related effects of LY379268 varied across experiments. For FR sucrose taking, the dose-response effect of LY379268 was inverted-U shaped where 1.5 mg/kg and 6 mg/kg doses reduced active lever responses; but the 3mg/kg dose was without effect. In contrast, for active lever responding in extinction and on the PR, LY379268 had linear dose-related effects. For extinction, all 3 doses reduced active lever responses; for PR, the 3 and 6 mg/kg doses reduced active lever responses, but the 1.5 mg/kg dose did not. LY379268 also had effects on other dependent measures (reinforcers, mL per g) that varied by experiment. For example, the 3 mg/kg dose did not alter the number of reinforcers earned in Experiments 1 (sucrose FR) or 2 (sucrose-paired tone+light FR) but did reduce the number of reinforcers earned in Experiment 3 (sucrose PR). In addition, at the same doses that reduced sucrose taking in Experiment 1 (sucrose FR), LY379268 reduced locomotor activity. However, LY379268 had no effect on locomotion in the other 2 experiments. Perhaps most salient was that LY379268 reduced both absolute sucrose taking (reinforcers) and ml per g at the 3 mg/kg dose when rats responded on the PR (Experiment 3) but not on the FR (Experiment 1). In contrast, LY379268 at a low (1.5 mg/kg) dose had a preferential effect of producing decreased sucrose taking and seeking on the FR, but not taking on the PR (Table 2).
These differences in dose-effects are important to consider as each experiment aimed to examine the effect of LY379268 on a particular aspect of reinforcement. Self-administration on the FR (Experiment 1) is a measure of reinforcement value (taking), responding in extinction (Experiment 2) a measure of conditioned responding (seeking), and self-administration on the PR (Experiment 3) a measure of motivation to acquire reinforcement. It is important to consider that each of these constructs overlap as do the procedures to measure them. For example, sucrose self-administration on the FR and PR is determined to some extent by conditioned responding in the sucrose-predictive environment and motivation to acquire reinforcement is perhaps inextricably tied to any measure of taking or seeking. Nonetheless, dose-effects across each condition may provide insight into underlying mechanisms. For example, the fact that 1.5 mg/kg robustly reduced responding for sucrose on the FR (all dependent measures except inactive lever responding) but not responding in Extinction (only active lever responding decreased) or responding for sucrose on the PR (no dependent measures reduced) could mean that the drug has a dose-dependent impact on the neural systems that differentially mediate these behaviors.
Translational implications of the present results, in conjunction with previous reports, are that drugs that blunt glutamate release or stabilize glutamate extrasynaptic/synaptic homeostasis (Kalivas 2009) could reduce food and drug seeking, and possibly taking, in humans. These would be preferred outcomes for individuals who struggle with food-focused thoughts and behaviors as well as substance use disorders. Preliminary clinical studies in this area include administration of n-acetylcysteine. One theory is that this compound interacts with the glutamate transporter 1; this results in more extrasynaptic glutamate. This glutamate agonizes autoreceptors that signal intracellularly to decrease glutamate release (Woodcock et al. 2021). Presently, n-acetylcysteine has shown promise in clinical trials to reduce drug craving (e.g. Woodcock et al.). In addition, Azhari et al. (2021) administered ketamine (a glutamate NMDA antagonist) to individuals with cannabis use disorder which resulted in reduced cannabis intake. Further evaluation of compounds that affect glutamate signaling tone, LY379268 included, is warranted with the caveat that some compounds such as ketamine have abuse potential.
Conclusion
Preclinical and clinical results support further evaluation of compounds found to modulate glutamate signaling with the goal to reduce the reinforcing properties (unconditioned, conditioned) of food and drugs of abuse. The present results indicate that the glutamate autoreceptor agonist LY379268 reduces both sucrose taking and seeking in male and female adult rats, including a reduction in motivation to respond for sucrose assessed using the PR schedule of reinforcement. Further studies are needed to evaluate brain regions and systems responsible for these effects. Such studies could help differentiate how drugs such as LY379268 affect taking vs. seeking in rats (use vs. craving in humans). In addition, as we have done here, further studies need to incorporate sex as a variable; almost none of the studies conducted in this area included females or had the statistical power to determine sex differences.
Supplementary Material
Acknowledgements:
The authors thank Malina Rossow, Chelsea Batten, and Alexandra Gilsrud for their help with data collection. This project is dedicated in memory of our colleague Ismael Bhandal.
Source of funding:
NIH DA016285-05 and the State of Washington
Footnotes
Conflicts of interest: none declared
Supplemental data: sucrose deliveries, inactive lever, photobeam breaks, break points (Excel)
Supplemental Digital Content: Grimm et alia Supplemental.xlsx
References
- Ahmed SH, Guillem K, & Vandaele Y (2013). Sugar addiction: pushing the drug-sugar analogy to the limit. Curr Opin Clin Nutr Metab Care, 16(4), 434–439. [DOI] [PubMed] [Google Scholar]
- Anversa RG, Muthmainah M, Sketriene D, Gogos A, Sumithran P, & Brown RM (2021). A review of sex differences in the mechanisms and drivers of overeating. Front Neuroendocrinol, 63, 100941. [DOI] [PubMed] [Google Scholar]
- Azhari N, Hu H, O’Malley KY, Blocker ME, Levin FR, & Dakwar E (2021). Ketamine-facilitated behavioral treatment for cannabis use disorder: A proof of concept study. Am J Drug Alcohol Abuse, 47(1), 92–97. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, & Weiss F (2004). Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci, 24(20), 4723–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, McClellan ML, & Reed BG (2017). Sex differences, gender and addiction. J Neurosci Res, 95(1–2), 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz AM, Beery AK, & Becker JB (2019). Analysis of sex differences in pre-clinical and clinical data sets. Neuropsychopharmacology, 44(13), 2155–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Busch RF, & Gray SM (2005). The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport, 16(9), 1013–1016. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, & Shaham Y (2006). Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology, 31(10), 2197–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, & Shaham Y (2004). A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci, 24(47), 10726–10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Sheffler-Collins SI, & Ghitza UE (2006). The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav Brain Res, 173(1), 148–152. [DOI] [PubMed] [Google Scholar]
- Cartmell J, & Schoepp DD (2000). Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem, 75(3), 889–907. [DOI] [PubMed] [Google Scholar]
- CDC (2021). Get the Facts: Added Sugars. Retrieved September 1, 2022 from https://www.cdc.gov/nutrition/data-statistics/added-sugars.html
- CDC (2022). Overweight & Obesity. Retrieved September 1, 2022 from https://www.cdc.gov/obesity/index.html
- Cornish JL, Duffy P, & Kalivas PW (1999). A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience, 93(4), 1359–1367. [DOI] [PubMed] [Google Scholar]
- Crawford JT, Roberts DC, & Beveridge TJ (2013). The group II metabotropic glutamate receptor agonist, LY379268, decreases methamphetamine self-administration in rats. Drug Alcohol Depend, 132(3), 414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Abe N, Barrett KC, Caldwell-Harris C, Gendolla GHE, Koncz R, & Sachdev PS (2020). Mapping the interconnected neural systems underlying motivation and emotion: A key step toward understanding the human affectome. Neurosci Biobehav Rev, 113, 204–226. [DOI] [PubMed] [Google Scholar]
- Garceau C, Marsault J, Robinson MJF, & Samaha AN (2022). Metabotropic group II glutamate receptors mediate cue-triggered increases in reward-seeking behaviour. Psychopharmacology (Berl). [DOI] [PubMed] [Google Scholar]
- Grimm JW, North K, Hopkins M, Jiganti K, McCoy A, Sulc J, MacDougall D, & Sauter F (2022). Sex differences in sucrose reinforcement in Long-Evans rats. Biol Sex Differ, 13(1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW (2009). The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci, 10(8), 561–572. [DOI] [PubMed] [Google Scholar]
- Koban L, Wager TD, & Kober H (2022). A neuromarker for drug and food craving distinguishes drug users from non-users. Nat Neurosci. Dec 19. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Watterson LR, Nemirovsky NE, Hood LE, Villa A, Halstengard C, Zautra N, & Olive MF (2013). Attenuation of methamphetamine seeking by the mGluR2/3 agonist LY379268 in rats with histories of restricted and escalated self-administration. Neuropharmacology, 66, 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, & Shaham Y (2007). Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry, 61(5), 591–598. [DOI] [PubMed] [Google Scholar]
- Myal S, O’Donnell P, & Counotte DS (2015). Nucleus accumbens injections of the mGluR2/3 agonist LY379268 increase cue-induced sucrose seeking following adult, but not adolescent sucrose self-administration. Neuroscience, 305, 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas C, Zlebnik NE, Farokhnia M, Leggio L, Ikemoto S, & Shaham Y (2022). Sex Differences in Opioid and Psychostimulant Craving and Relapse: A Critical Review. Pharmacol Rev, 74(1), 119–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor R, & Kenny P (2022). Utility of ‘substance use disorder’ as a heuristic for understanding overeating and obesity. Prog Neuro-Psychopharmacology Biol Psychiatry, 118, 110580. [DOI] [PubMed] [Google Scholar]
- PHS (2015). PHS Policy on Humane Care and Use of Laboratory Animals. Retrieved September 1, 2022 from https://olaw.nih.gov/policies-laws/phs-policy.htm
- Reagan P, & Hersch J (2005). Influence of race, gender, and socioeconomic status on binge eating frequency in a population-based sample. Int J Eat Disord, 38(3), 252–256. [DOI] [PubMed] [Google Scholar]
- Richardson NR, & Roberts DC (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods, 66(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Uejima JL, Bossert JM, Poles GC, & Lu L (2007). Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav Brain Res, 181(2), 292–296. [DOI] [PubMed] [Google Scholar]
- Vengeliene V & Spanagel R (2022). mGlu2 mechanism-based interventions to treat alcohol relapse. Frontiers in Pharmacology, 13, 10.3389/fphar.2022.985954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA, & Baler R (2017). The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci, 18(12), 741–752. [DOI] [PubMed] [Google Scholar]
- Windisch KA, & Czachowski CL (2018). Effects of group II metabotropic glutamate receptor modulation on ethanol- and sucrose-seeking and consumption in the rat. Alcohol, 66, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock EA, Lundahl LH, Khatib D, Stanley JA, & Greenwald MK (2021). N-acetylcysteine reduces cocaine-seeking behavior and anterior cingulate glutamate/glutamine levels among cocaine-dependent individuals. Addict Biol, 26(2), e12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, & Fuchs RA (2010). Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl), 208(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Azari S, & Wise RA (2007). A role for conditioned ventral tegmental glutamate release in cocaine seeking. J Neurosci, 27(39), 10546–10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin J (1972). Sugar and disease. Nature, 239(5369), 197–199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


