Abstract
Objective
This study applied a recently developed statistical method to compare the mean cost trajectories between non-Hispanic White (NHW) and non-Hispanic Black (NHB) patients with localized prostate cancer conditioning on patients’ survival.
Methods
In this observational study, we modeled cost trajectories of NHW and NHB patients with localized prostate cancer for three survival durations: 24, 48 and 72 months. We also compared the cost trajectories between NHW and NHB stratified by comorbidities scores.
Principal Findings
We find that the mean cost trajectories of NHB were significantly higher than the trajectories of NHW in the last 12 months before death, regardless of survival duration and patients’ baseline comorbidity scores. For patients with comorbidity score ≥ 2, mean cost trajectories within the first year of diagnosis for NHB were significantly higher than those for NHW, except for the subgroup of patients with comorbidity 2–3 and whose survival length was 72 months.
Conclusions
Our results suggested that a higher proportion of NHB patients with high comorbidity scores likely contribute to their higher end-of-life costs than those for NHW patients. To narrow the gap in healthcare-related financial burden between NHB and NHW patients with localized prostate cancer, policy makers need to explore strategies to better manage comorbidities.
Keywords: Health Care Costs, Health Care Disparities, Chronic Disease
Precis:
Higher levels of comorbidities of non-Hispanic Black patients with localized prostate cancer likely contribute to their higher end-of-life costs than those of non-Hispanic White patients.
INTRODUCTION
Medical costs in the United States have increased in the past decades.1 Cancer related medical costs nearly doubled between 1987 and 2005.2 Meanwhile, medical costs varied among different racial/ethnic groups.3 Understanding the medical cost trajectories of patients with cancer by race/ethnicity will inform policy makers of the cost accumulation process during different phases of cancer care; thus offering important insights on how racial/ethnicity disparities can potentially manifest into higher medical costs for racial/ethnic minorities. This study used a cohort of patients with localized prostate cancer (PC) to examine whether there is a racial/ethnic difference in medical cost trajectories and explore the underlying reasons.
Localized PC is the second most prevalent cancer among US men (next to skin cancer).4 The projected national expenditures for PC exceeded $20 billion in 2020.5 Patients diagnosed with PC may receive immediate definitive treatment (i.e., radiation or surgery), undergo active surveillance or opt for watchful waiting as the first course of treatment.6 Using data from 1995–2004, Wilson et al. found for patients with PC the average treatment cost per patient was $11,495 within the first 6 months of diagnosis, ranging from $2,586 for watchful waiting to $24,204 for radiation; the average 6-month cost reduced to $3,044 afterwards.7
Non-Hispanic white (NHW) and non-Hispanic black (NHB) patients with PC often have different disease progression because of biological factors 8,9 and initial treatment approaches.6 A total of 150 NHW and 234 NHB per 100,000 men in the US had PC between 2003 and 2007.10 In general, NHB patients present with more advanced disease at diagnosis and have shorter progression-free survival than NHW patients.11 However, NHB patients with PC are less likely to undergo active interventions, such as immediate active treatment or active surveillance (which does not include watchful waiting), than NHW patients.6 A study has found that for patients with a Gleason score 7 or higher, 24% of NHW and 16% of NHB patients underwent prostatectomy between 2004 and 2015.6
Patients with PC had long survival and many of these patients had other comorbidities throughout their lives while having PC. The 10-year relative survival rate in the absence of other causes of death for PC was close to 100%,12 with 75% of patients dying of other causes.13 About 60% of patients diagnosed with PC between 2003 and 2017 were 65 years or older,12 and their underlying comorbidities influenced choice of initial treatment and subsequent surveillance activities throughout their lifetimes. The interaction between patients’ baseline comorbidities and initial treatment has far-reaching impact on total medical costs beyond the first year of diagnosis, resulting from varying patterns of cost trajectories. Given the well-documented disparities in PC between NHB and NHW patients,6,11 comparing lifetime medical cost trajectories between NHB and NHW patients with PC will provide important policy insights on the long-term economic burdens borne by these patients, and reveal key areas to intervene to decrease cancer disparities.
This study estimates and compares the medical cost trajectories between NHB and NHW patients with PC using a recently developed statistical method.14,15 This approach of quantifying medical cost trajectories has two advantages. First, this method models survival and longitudinal medical cost data jointly. Accounting for survival when estimating medical cost trajectories is important given the documented racial/ethnic disparities in survival.11 Second, the method can depict and compare the monthly cost trajectories over time for different patient subgroups and test for the statistical significance of differences in cost trajectories between different groups at selected time intervals. This method fully utilizes information available in longitudinal cost data (subject to characteristics of medical costs data, such as high skewness, zero-inflation, and heteroskedasticity) to obtain in-depth understanding of the cost trajectory of patients with cancer throughout their lifetime. To the best of our knowledge, this is the first paper on estimating and comparing the mean medical cost trajectories conditional on survival for different subgroup of patients with localized PC. This information helps health policy makers better understand the financial burden of a disease throughout its life course conditional on length of survival, and such knowledge is critically important in future planning and allocation of healthcare resources.
METHODS
Data Resources and Patient Cohort
We used the Surveillance, Epidemiology, and End Results Program (SEER)-Medicare to construct our study cohort of NHB and NHW patients diagnosed with PC between 2003 and 2015. SEER-Medicare is a population-based database containing Medicare claims for cancer patients who resided in one of the 18 SEER registry regions, representing 34.6% of the US population.16 The algorithm employed to construct our study cohort is described below and illustrated in Figure 1. We identified 509,198 patients who were older than 65 when diagnosed with PC, and whose survival status recorded in Medicare was consistent with SEER-Medicare records. We excluded patients for whom PC was not the first and only cancer . To ensure completeness of Medicare claims data from the time of diagnosis, we excluded patients whose PC was not diagnosed between 2003 and 2015 . We further excluded patients whose PC was reported from autopsy or death certificate only . Next, we excluded patients who did not have Medicare fee-for-service Parts A and B enrollment during the month of PC diagnosis . The cohort was then restricted to patients with PC using SEER historic stage A, and we excluded those with PC other than localized stage . We further excluded patients whose race/ethnicity was not NHW or NHB . Lastly, we calculated patients’ modified Charlson comorbidity scores17 using Medicare claims from inpatient, outpatient, and physician professional services within the 12 months before the month of cancer diagnosis and excluded patients with missing comorbidity scores . Our final study cohort consisted of 141,604 patients with PC, of those 123,738 were NHW and 17,866 were NHB. Consistent with previous research,18 we quantified patients’ medical costs by aggregating Medicare paid amount from all Medicare Parts A and B claims, including inpatient, outpatient, carrier, durable medical equipment, home health agency, and hospice for each month starting from the month of diagnosis till December 31, 2016 or the end of follow-up (whichever came first). Motivated by the different distribution of comorbidities between NHW and NHB patients, we estimated the cost trajectories by stratifying patients into two groups: comorbidity 0–1 versus 2+. To explore the impact of comorbidities on the difference in the cost trajectories observed between NHB and NHW patients, we further stratified patients with comorbidities 2+ in two subgroup analyses: (a) comorbidity 2 vs. 3+, and (b) comorbidity 2–3 vs. 4+.
Figure 1.
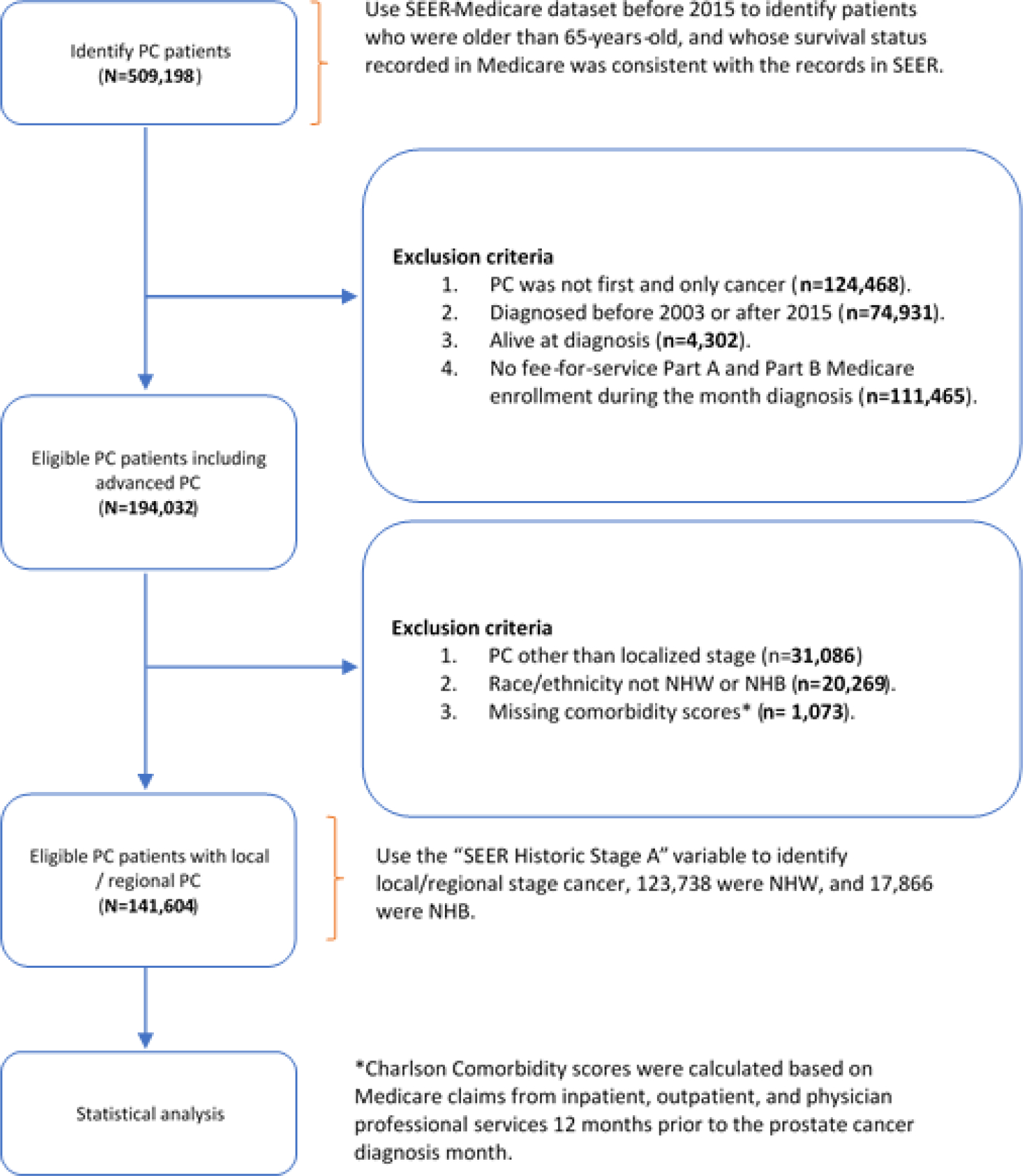
Study cohort ascertainment process.
Statistical Model
For patient , let the medical costs for month be . denotes the time to death measured from diagnosis, which could be right-censored due to loss of follow-up. The goal is to estimate the mean cost trajectory at population level given the time to death at , using the following bivariate cost trajectory surface model.
In this model, is the month after cancer diagnosis which is properly defined within ; the maximum follow-up time is 120 months (10 years) after cancer diagnosis. We used 120 months to ensure sufficient sample size for our analysis. The conditional expectation of the incident cost given survival duration can be represented as a bivariate function of and . A novel aspect of this model, as articulated in the recent literature,14,15 is that the survival time is taken into consideration when we calculate the population average of the incident cost at month . The overall goal of this study is to quantify the population mean cost trajectory over time until a terminal event such as death. The mean cost trajectory is of interest to policy makers because it reveals how medical costs vary across phases of care which is of direct relevance to healthcare decision-making. Most existing methods in medical cost analysis focus on the total or cumulative medical cost,5 which does not provide details on healthcare costs across different phases of the disease continuum, from initial diagnosis to death. Thus, they are unable to capture the heterogeneity of the cost trajectory among patients with various demographic characteristics. The bivariate cost trajectory surface model was estimated by a recently developed method.14,15 With the estimated trajectories, we applied a novel statistical test (described below) to compare two cost trajectory surfaces. Such comparison allowed us to test the hypothesis on the difference in costs between NHW and NHB patients at any selected time interval, properly accounting for survival differences.
To compare the cost differences for each of the two aforementioned time intervals, let two medical cost trajectory surfaces be and for a prespecified bivariate region . The null hypothesis is that there is no statistically significant difference in the cost trajectories of the two groups: . We use quadratic B-spline19 basis function with ten equally spaced knots to approximate the surfaces, i.e., for group , with spline parameters . A test statistic can be written as:
where are estimated by fitting the model, and the design matrix .
Intuitively, quantifies the volume between two cost trajectory surfaces. A large value of provides evidence against the null hypothesis, meaning that the difference between and in region is large. Existing methods considered testing nonparametric functions in the additive mixed models in a prespecified univariate interval.20,21 We extended the test statistic to the bivariate region, in which the distribution of under the null hypothesis can be approximated by a scaled chi-squared distribution. This extension will allow researchers to examine and test for cost differences in any region defined by the cost month and survival, and thus reveal details in these longitudinal cost data. For example, we can apply this method to test whether the difference between NHB and NHW patients in the cost surfaces for the first 12 months after diagnosis among patients with survival times between 5 and 10 years (i.e., the region of ) was statistically significant.
All analyses were conducted in SAS 9.4 and R 3.6.3. his study is exempt for human subject review from the Institutional Review Board at the authors’ institute.
RESULTS
Table 1 shows the summary statistics. A significantly higher proportion of NHB patients were at younger age at diagnosis (65–74 years) than NHW patients (71.5% vs. 64.2%). Notably, a significantly higher proportion of NHB patients had baseline comorbidity score 2 or higher at the time of diagnosis (40% vs. 29%). Compared to NHB patients, a significantly higher proportion of NHW patients received immediate definitive treatment. Specifically, among NHW patients 45.1% had radiation therapy and 30.7% underwent surgery, compared to 43.3% and 22.5%, respectively, for NHB patients. NHB patients also had shorter median survival than NHW patients (9.0 vs. 10.4 years). Overall, the median monthly medical cost was lower than the mean monthly cost, suggesting the distribution of medical costs for our patient cohort was highly skewed to the right. The mean monthly cost without considering survival time was higher for NHB than NHW patients ($1808.70 vs $1502.60) whereas the opposite was observed in the median monthly cost. This pattern suggests that the cost distribution of NHB patients is more skewed than that of NHW patients.
Table 1.
Summary statistics of the study sample.
| All (n=141,604) | White (n=123,738) | Black (n=17,866) | |||||
|---|---|---|---|---|---|---|---|
| Frequency | Percent* | Frequency | Percent | Frequency | Percent | P-value** | |
|
| |||||||
| Age group | <0.0001 | ||||||
| 65–74 | 91,926 | 64.9 | 79,215 | 64.2 | 12,711 | 71.5 | |
| 75–84 | 42,481 | 30.0 | 37,977 | 30.5 | 4,504 | 24.9 | |
| 85+ | 7,197 | 5.1 | 6,546 | 5.3 | 651 | 3.6 | |
| Comorbidity score # | <0.0001 | ||||||
| 0 | 63,732 | 45.2 | 57,092 | 46.1 | 6,640 | 37.2 | |
| 1 | 35,159 | 24.9 | 31,135 | 25.2 | 4,024 | 22.5 | |
| 2 | 19,866 | 14.1 | 17,029 | 13.8 | 2,837 | 15.9 | |
| 3 | 10,320 | 7.3 | 8,650 | 7.0 | 1,670 | 9.3 | |
| 4 | 5,952 | 4.2 | 4,828 | 3.9 | 1,124 | 6.3 | |
| 4+ | 6,575 | 4.7 | 5,004 | 4.0 | 1,571 | 8.8 | |
| Having radiation therapy as first-line treatment ^ | <0.0001 | ||||||
| Yes | 63,533 | 44.9 | 55,789 | 45.1 | 7,744 | 43.3 | |
| No | 78,071 | 55.1 | 67,949 | 54.9 | 10,122 | 56.7 | |
| Having surgery as first-line treatment & | <0.0001 | ||||||
| Yes | 41,987 | 29.7 | 37,975 | 30.7 | 4,012 | 22.5 | |
| No | 99,617 | 70.3 | 85,763 | 69.3 | 13,854 | 77.5 | |
| Cancer grade at diagnosis | <0.0001 | ||||||
| Grade I | 6,686 | 4.7 | 5,885 | 4.8 | 801 | 4.5 | |
| Grade II | 60,443 | 42.7 | 53,079 | 42.9 | 7,364 | 41.2 | |
| Grade III | 70,235 | 49.6 | 61,125 | 49.4 | 9,110 | 51.0 | |
| Grade IV | 339 | 0.2 | 288 | 0.2 | 51 | 0.3 | |
| Cell type not determined | 3,901 | 2.8 | 3,361 | 2.7 | 540 | 3.0 | |
|
| |||||||
| All (n=141,604) | White (n=123,738) | Black (n=17,866) | |||||
| Mean | Median | Mean | Median | Mean | Median | ||
|
| |||||||
| Monthly cost, $ | 1,536.9 | 131.5 | 1,502.6 | 134.4 | 1,808.7 | 110.1 | |
| Survival, years | 10.4 | 10.6 | 9.0 | ||||
Note:
Charlson Comorbidity score was estimated using Medicare claims.
Radiation therapy with/without chemotherapy, or radiation therapy and surgery with/without chemotherapy.
Surgery only, surgery with/without chemotherapy, or radiation therapy and surgery with/without chemotherapy.
Column percentage
P-value is calculated by univariate chi-squared test.
Figure 2A plots the average monthly medical cost for NHW versus NHB patients not conditioning on survival duration. The data show that NHB patients had higher monthly cost within the first 1–2 month of diagnosis, and higher cost for the NHB group was again observed starting around 10 months after cancer diagnosis. Figure 2A also exhibits high variation in patient-level monthly medical cost without considering the length of survival duration. Figure 2B shows that, overall, NHB patients had a lower probability of survival than NHW patients. These observations reaffirmed the importance of our model’s feature of estimating monthly medical cost given survival time.
Figure 2. Plots of motivation factors:
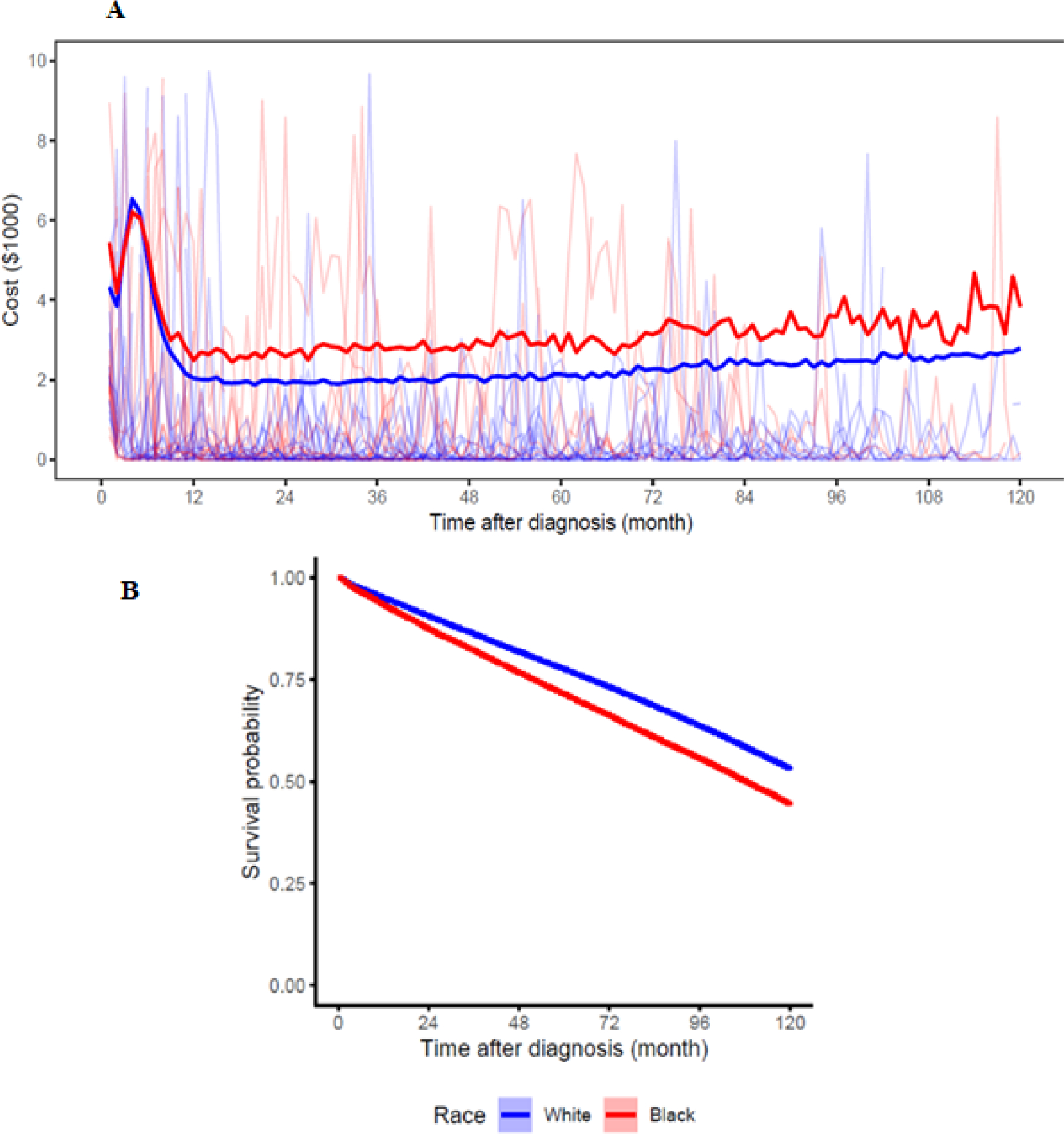
(A) pointwise average cost trajectories, with 100 random samples of the data are plotted in the background, and (B) Kaplan-Meier survival curve for NHW and NHB patients.
Figure 3A presents the estimated mean medical costs trajectories for NHW (left) and NHB (right) patients in a 3-dimensional surface that plots cost by “time after cancer diagnosis” and “survival duration from cancer diagnosis”. For example, the measure of “24 months” on the “time after cancer diagnosis” axis reflects patients’ monthly cost 24 months after initial cancer diagnosis. Note that the estimated monthly costs are properly defined at 24 months for patients whose survival time exceeds 24 months. Different colors represent different levels of medical cost, with blue representing the lowest range of cost and red representing the highest range. Given the survival duration from cancer diagnosis, the mean medical cost trajectory is U-shaped. Putting together all the trajectories forms a “smooth” 3-dimension surface visually. This surface is on a triangular region because the “time after diagnosis” cannot be larger than “survival duration from cancer diagnosis” (“time to death” axis).
Figure 3:
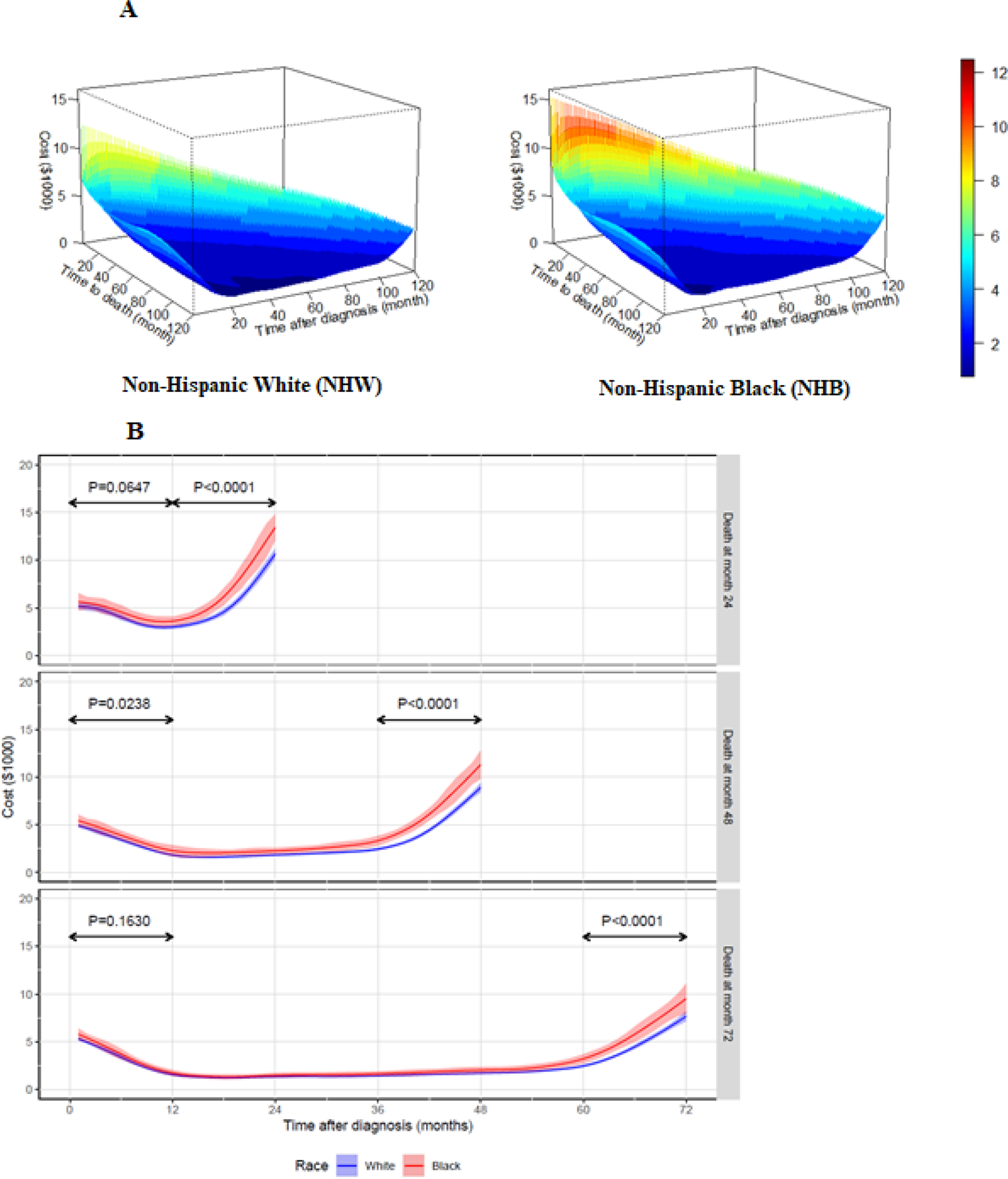
Cost trajectory by race/ethnicity group
The three-dimension medical cost surface is nonlinear and triangle-bathtub shaped, with the cost at the left and diagonal edges higher than the central surface (Figure 3A). The left-edge shows the average medical costs for the first month after cancer diagnosis (when ) for various survival durations. The diagonal edge (when ) represents the average medical cost for the last month of life for various survival durations. If we visually compare the diagonal-edges, costs at the last month of life were higher for NHB patients than NHW patients for any given survival time.
Figure 3B shows the estimated monthly cost trajectories when the survival time (month) , and 72; the three panels show the cost trajectories based on race/ethnicity for survival time after cancer diagnosis. Pointwise 95% confidence intervals are shown in the shaded color bands. The estimated cost trajectories in Figure 3B are all U-shaped. That is, during the first 12 months after the initial diagnosis of PC, a proportion of patients would undergo immediate definitive treatments, which then lead to higher mean total costs in this duration. High costs were also observed in the last year of life.
Figure 3B shows a steeper increase in the monthly costs for the year preceding death, i.e., end-of-life care for NHB patients compared to NHW patients. For example, for NHB patients who survived two years (Figure 3B, top panel), the end-of-life monthly medical costs increased from approximately $2,000 at 12-months before death to around $15,000 at the month of death for patients; the increase was from $2,000 to $10,000 for NHW patients. We also observed the end-of-life cost to be slightly lower as survival time lengthened. Between 12 months after cancer diagnosis and 12 months before death, the monthly medical costs were between $1000 and $3000, and NHB patients had slightly higher care costs than NHW patients. The confidence interval depicted in shaded area shows little to no difference in monthly costs between NHW and NHB from the diagnosis until the last year before death.
We tested whether the observed difference in medical cost trajectory between NHW and NHB patients was statistically different for two periods: within 12 months of cancer diagnosis ; and within 12 months before death . Although medical cost trajectory in the last year of life was significantly higher for NHB patients compared to NHW patients for the survival durations of 24, 48, and 72 months (all ), the difference in monthly costs within 12 months of diagnosis was only significant for the survival duration of 48 months .
We conducted subgroup analyses to investigate whether the disparities in cost trajectories between NHB and NHW patients could be explained by the difference in the distribution of comorbidity scores between the two groups. Figure 4A presents the estimated medical cost trajectories stratified by comorbidity score (0–1 and 2+) and race/ethnicity group (NHW and NHB). For patients with comorbidity 2+, NHB patients had significantly higher medical cost than NHW patients in the last 12 months of life for survival time 24, 48 and 72 months ( for all three survival lengths), as well as within 12 months of diagnosis (all ). For patients with comorbidity score 0–1, medical costs in the last 12 months of life remained higher for NHB patients (, and 0.0083 for survival time 24, 48, and 72 months, respectively). However, no statistically significant difference was observed in cost trajectories between NHW and NHB patients during the first 12 months after cancer diagnosis among patients who died within 24 months of diagnosis , and monthly costs in this duration were higher for NHW patients than NHB patients among those who died in 48 and 72 months ( and 0.0026, respectively).
Figure 4.
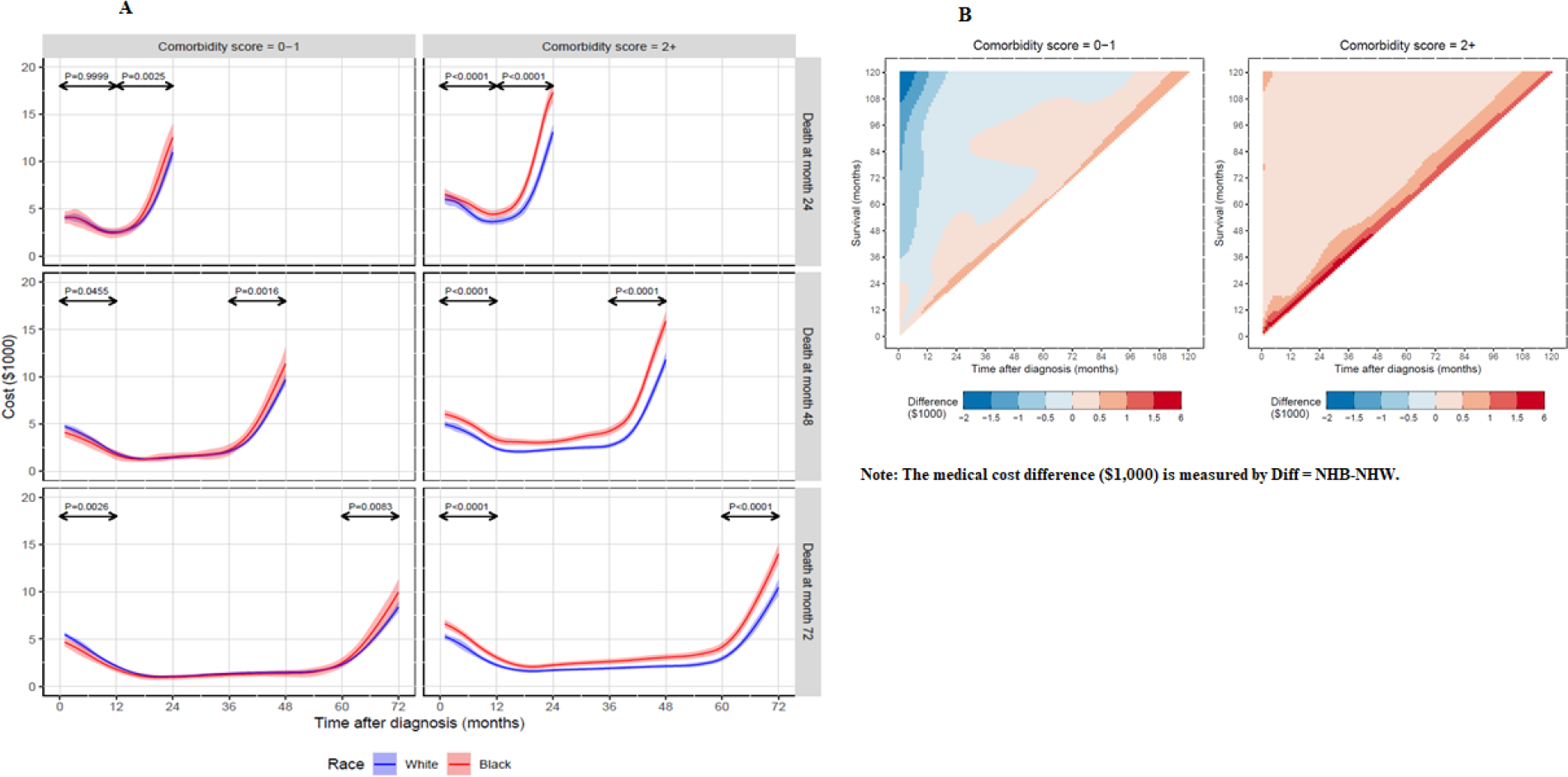
Estimated medical cost trajectories by race group, survival durations, and comorbidities.
Figure 4B shows the two-dimension heatmaps for the estimated medical cost difference between NHB and NHW patients (calculated using the mean monthly cost of NHB patients minus the mean monthly cost of NHW patients) by comorbidity group (0–1 and 2+). By visual inspection, the medical cost difference between NHB and NHW patients was larger for patients with comorbidity 2+ (Figure 4B, right panel) than those with comorbidity 0–1 (Figure 4B, left panel). For patients with comorbidity 2+, NHB patients had higher medical costs than NHW patients for any survival duration and time after cancer diagnosis, as shown by different shades of red on the right panel of Figure 4B. For those with comorbidity score 0–1, the consistent pattern is that the closer to the diagonal (which represents the month of death), the higher the medical cost difference between NHB and NHW patients, with higher costs in the NHB group.
We then focused on patients with a comorbidity score of 2+ by further stratifying patients into two subgroups by levels of comorbidities: (a) comorbidity score 2 vs. 3+ (Figure 5A) and (b) 2–3 vs. 4+ (Figure 5B). The pattern of higher end-of-life medical costs for NHB than NHW patients was consistently observed for all comorbidity subgroups at each survival duration. When restricting to patients with the same level of comorbidities (i.e., comorbidity of 2 in Figure 5A, left panel) or lower range (i.e., comorbidity 2–3 in Figure 5B, left panel), NHB patients still had higher costs in the first 12 months after cancer diagnosis in most cases. However, the pattern of higher medical costs for NHB patients was more pronounced for the higher comorbidity group in each of the two subgroups.
Figure 5.

Estimated medical cost trajectories by race group, survival durations, and comorbidities subgroups.
DISCUSSION
This study analyzed SEER-Medicare data for patients diagnosed with PC between 2003 and 2015 to estimate their monthly medical cost trajectories and compare the trajectories between NHB and NHW patients at varying lengths of survival. Motivated by the observation that a larger proportion of NHB patients had higher baseline comorbidity scores than NHW patients, we conducted a series of subgroup analyses by stratifying patients in to several comorbidity subgroups, including comorbidity score of 0–1 vs. 2+, and 2 vs. 3+ as well as 2–3 vs 4+ among those with comorbidity score ≥ 2 to gain a better understanding on the role of underlying comorbidities in cost trajectories between NHB and NHW patients.
The innovative statistical model allowed us to identify the time segments in which racial/ethnic differences in cost trajectory were statistically significant. The model also allowed us to produce the accompanying graphics, offering unique visualization of cost trajectories in multi-dimensions. The estimated cost trajectories were all U-shaped, which was consistent with the pattern of cancer care costs documented.22 The higher cost trajectories revealed among patients who died within a relatively short period since cancer diagnosis possibly reflected some overlap of intensive cancer treatment in the initial period and the intense end-of-life care. A consistent pattern across all analyses was that medical costs in the last year of life were significantly higher for NHB patients with PC than their NHW counterparts, regardless of survival duration and comorbidity scores. For costs within the first 12 months of diagnosis, the pattern of higher costs among NHB patients was only observed among those with comorbidity score 2 and higher.
Our results reaffirmed that total medical costs in the last year life were higher for NHB than NHW patients.23,24 Several reasons may contribute to this pattern. First, it is well-documented that NHB patients can have less access to high-quality healthcare.25 It is therefore possible that the cumulative effects of delayed care and suboptimal treatment result in higher end-of-life cost. Second, research has shown that NHB patients had lower utilization of hospice care than NHW patients.26,27 Factors associated with lower use of hospice among NHB patients included knowledge, culture, trust of the health system, among others.28 Third, the higher end-of-life costs observed among NHB patients may be driven by the fact that NHB patients overall had more comorbidities than NHW patients.29 We found that the difference in the end-of-life cost between NHW and NHB was more pronounced among patients with comorbidity score 2 and higher, particularly among patients with comorbidity 4 and higher.
The role of comorbidity, especially multiple comorbidities, in the cost trajectories of cancer patients is complex. It is not uncommon for cancer patients to have multiple comorbidities.30 Understanding the role of comorbidities is important in the examination of cost trajectories for patients with PC because of their long survival. On one hand, baseline comorbidities may influence patients’ choice of initial treatment as well as subsequent disease surveillance activities.31,32 On the other hand, the impact of baseline comorbidities on cost can manifest over time and the combined effect from multiple chronic conditions can be more than the sum of the cost of each individual condition.33,34 For patients with comorbidity 0–1, total costs would mostly be driven by cancer treatment, whereas for those with higher comorbidity scores, total cost would include a mix of treatment for cancer as well as other conditions. The stronger impact of cancer treatment on the medical cost for patients with low comorbidity (comorbidity 0–1) is evident from their cost trajectory within the first 12 months of diagnosis. For this group, a higher proportion of NHW patients received surgery or radiation within the first year of their diagnosis than NHB patients (32.2% vs. 22.7% for surgery and 45.2% vs. 42.8% for radiation). This likely explains the pattern of higher or similar costs found in NHW patients in this 12-month duration among the low-comorbidity group. An opposite pattern was found among patients with comorbidity score 2 or higher. This pattern was likely driven by a higher proportion of NHB patients with comorbidity scores greater than 2; these patients will incur costs from their cancer care as well as management of their large number of underlying comorbidities. Subgroup analyses that further stratifying this group into moderate to high comorbidity groups suggested that although the higher first 12-month costs observed in the NHB patients were likely driven by the distributional effort from having a higher proportion of these patients in high comorbidity group, there could be other factors unobserved from claims data, such as disease severity, because the same pattern (although a smaller magnitude) was also observed when limiting to the same comorbidity score. To effectively reduce financial burden of medical care to the healthcare system, patients and their families, policy makers should consider approaches in better managing patients’ comorbidities, especially among vulnerable populations.
This study had limitations. We stratified patients’ comorbidity group using baseline comorbidity scores, which may change over time. We did not evaluate cost trajectories based on patients’ comorbidity scores beyond their baseline comorbidity, because our current model is not able to handle time-dependent covariates; and future research needs to address the issue of incorporating time-dependent covariates. Secondly, our analysis was based on claims for Medicare fee-for-service plans. Patients who switched to Medicare Advantage were analyzed as censored data. In addition, we did not include prescription drug expenditures from Part D. Lastly, because of small number of observations for other race/ethnicity groups, we limited the comparison of medical costs trajectories between NHW and NHB patients.
Conclusion
This study used a novel statistical model to evaluate, visualize, and compare medical cost trajectories of patients with prostate cancer at different time periods between different racial/ethnicity subgroups. Our results highlighted the critical role of patients’ baseline comorbidities in the initial and end-of-life segment of medical cost trajectories. Our results emphasized the importance of managing co-existing comorbidities to reduce the racial/ethnic disparities in healthcare-related financial burden.
What is known on this topic?
Medical costs differ by phases of disease progress and survival durations.
A larger proportion of NHB had higher comorbidity scores than NHW.
Immediate active treatment for localized prostate cancer is different for NHB and NHW.
What this study adds?
Medical costs in the last 12 months before death were higher for NHB patients with localized prostate cancer than those for their NHW counterparts, regardless of survival duration and comorbidity score.
Higher medical costs for NHB patients in the first 12 months of diagnosis were only observed among patients with comorbidity score ≥ 2, particularly for patients with comorbidity score 3+ or 4+.
Underlying baseline comorbidities may have contributed to the overall higher healthcare spending observed among NHB than NHW.
Acknowledgments:
The authors thank Gary Deyter, technical writer from the Department of Health Services Research at The University of Texas MD Anderson Cancer Center, for proofreading the manuscript.
Funding/Support:
This work was supported in part by the National Cancer Institute (R01 CA207216, R01 CA225646, and CCSG P30 CA016672). The funder had no role in the design or conduct of the study; analysis or interpretation of the data; review or approval of the manuscript; or the decision to submit the manuscript for publication.
Role of the Funders/Sponsors:
The funder had no role in the design or conduct of the study; analysis or interpretation of the data; review or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr Shih reported receiving consulting fees, travel, and accommodations for serving on a grants review panel for Pfizer Inc, and an advisory board for AstraZeneca in 2019. Dr Shih is an editor for Value in Health and had no role in the peer-review process of this article. No other disclosures were reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Peterson-KFF. National Health Spending Explorer. https://www.healthsystemtracker.org/health-spending-explorer/. Published 2022. Accessed September 20, 2022.
- 2.Tangka FK, Trogdon JG, Richardson LC, Howard D, Sabatino SA, Finkelstein EA. Cancer treatment cost in the United States. Cancer. 2010;116(14):3477–3484. [DOI] [PubMed] [Google Scholar]
- 3.Dieleman JL, Chen C, Crosby SW, et al. US Health Care Spending by Race and Ethnicity, 2002–2016. JAMA. 2021;326(7):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henley SJ, Ward EM, Scott S, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Hall IJ, Filson C, Howard DH. Trends in the use of active surveillance and treatments in Medicare beneficiaries diagnosed with localized prostate cancer. Urologic Oncology: Seminars and Original Investigations. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson LS, Tesoro R, Elkin EP, et al. Cumulative cost pattern comparison of prostate cancer treatments. Cancer. 2007;109(3):518–527. [DOI] [PubMed] [Google Scholar]
- 8.Lillard JW Jr, Moses KA, Mahal BA, George DJ. Racial disparities in Black men with prostate cancer: A literature review. Cancer. 2022;128(21):3787–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin DN, Starks AM, Ambs S. Biological determinants of health disparities in prostate cancer. Curr Opin Oncol. 2013;25(3):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brawley OW. Trends in Prostate Cancer in the United States. JNCI Monographs. 2012;2012(45):152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chornokur G, Dalton K, Borysova ME, Kumar NB. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate. 2011;71(9):985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC. Prostate cancer incidence and survival by stage and race/ethnicity, United States, 2001–2017. 2020;69(41). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson JE, Andren O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291(22):2713–2719. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Wu CH, Ning J, Huang X, Tina Shih YC, Shen Y. Semiparametric Estimation of Longitudinal Medical Cost Trajectory. J Am Stat Assoc. 2018;113(522):582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, Shen Y, Shih YT, Xu Y, Li L. Statistical modeling of longitudinal medical cost trajectory: renal cell cancer care cost analyses. Biostatistics. 2020. [DOI] [PubMed] [Google Scholar]
- 16.NIH. SEER-Medicare: Brief Description of the SEER-Medicare Database. https://healthcaredelivery.cancer.gov/seermedicare/overview/. Published 2018. Accessed April 22, 2019.
- 17.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 18.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100(9):630–641. [DOI] [PubMed] [Google Scholar]
- 19.Ruppert D, Wand MP, Carroll RJ. Semiparametric regression during 2003–2007. Electron J Stat. 2009;3:1193–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L A Class of Permutation Tests for the Equality of Two Marginal Survival Functions Using Paired Censored Data. Communications in Statistics - Simulation and Computation. 2014;43(10):2498–2507. [Google Scholar]
- 21.Zhang D, Lin X. Hypothesis testing in semiparametric additive mixed models. Biostatistics. 2003;4(1):57–74. [DOI] [PubMed] [Google Scholar]
- 22.Brown ML, Riley GF, Schussler N, Etzioni R. Estimating Health Care Costs Related to Cancer Treatment from SEER-Medicare Data. Medical Care. 2002;40(8):IV104–IV117. [DOI] [PubMed] [Google Scholar]
- 23.Hanchate A, Kronman AC, Young-Xu Y, Ash AS, Emanuel E. Racial and Ethnic Differences in End-of-Life Costs: Why Do Minorities Cost More Than Whites? Archives of Internal Medicine. 2009;169(5):493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byhoff E, Harris JA, Langa KM, Iwashyna TJ. Racial and Ethnic Differences in End-of-Life Medicare Expenditures. Journal of the American Geriatrics Society. 2016;64(9):1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riley WJ. Health disparities: gaps in access, quality and affordability of medical care. Trans Am Clin Climatol Assoc. 2012;123:167–172; discussion 172–164. [PMC free article] [PubMed] [Google Scholar]
- 26.Ornstein KA, Roth DL, Huang J, et al. Evaluation of Racial Disparities in Hospice Use and End-of-Life Treatment Intensity in the REGARDS Cohort. JAMA Network Open. 2020;3(8):e2014639–e2014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith AK, Earle CC, McCarthy EP. Racial and Ethnic Differences in End-of-Life Care in Fee-for-Service Medicare Beneficiaries with Advanced Cancer. Journal of the American Geriatrics Society. 2009;57(1):153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazargan M, Bazargan-Hejazi S. Disparities in Palliative and Hospice Care and Completion of Advance Care Planning and Directives Among Non-Hispanic Blacks: A Scoping Review of Recent Literature. American Journal of Hospice and Palliative Medicine®. 2021;38(6):688–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogle KS, Swanson GM, Woods N, Azzouz F. Cancer and comorbidity: redefining chronic diseases. Cancer. 2000;88(3):653–663. [DOI] [PubMed] [Google Scholar]
- 30.Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA: A Cancer Journal for Clinicians. 2016;66(4):337–350. [DOI] [PubMed] [Google Scholar]
- 31.Bradley CJ, Dahman B, Anscher M. Prostate cancer treatment and survival: evidence for men with prevalent comorbid conditions. Med Care. 2014;52(6):482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taplin SH, Barlow W, Urban N, et al. Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst. 1995;87(6):417–426. [DOI] [PubMed] [Google Scholar]
- 33.McPhail SM. Multimorbidity in chronic disease: impact on health care resources and costs. Risk Manag Healthc Policy. 2016;9:143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cortaredona S, Ventelou B. The extra cost of comorbidity: multiple illnesses and the economic burden of non-communicable diseases. BMC Med. 2017;15(1):216–216. [DOI] [PMC free article] [PubMed] [Google Scholar]


