Abstract
Background:
Little is known whether electronic cigarettes (ECIG) increase vulnerability to future atherosclerotic cardiovascular disease (CVD). We determined, using an ex vivo mechanistic atherogenesis assay, whether proatherogenic changes including monocyte trans-endothelial migration (MTEM) and monocyte derived foam cell formation (MDFCF) are increased in people who use ECIGs.
Methods:
In a cross-sectional single-center study using plasma and peripheral blood mononuclear cells (PBMCs) from healthy participants who are non-smokers (NS) or with exclusive use of ECIGs or tobacco cigarettes (TCIGs), autologous PBMCs with patient plasma and pooled PBMCs from healthy NS with patient plasma were utilized to dissect patient-specific ex vivo proatherogenic circulating factors present in plasma and cellular factors present in monocytes. Our main outcomes were MTEM (% of blood monocyte cells that undergo TEM through a collagen gel) and MDFCF as determined by flow cytometry and the median fluorescence intensity (MFI) of the lipid-staining fluorochrome BODIPY in monocytes of participants in the setting of an ex vivo model of atherogenesis.
Results:
Study participants (n=60) had median age of 24.0 years [interquartile range (IQR) 22.0–25.0 years] and 31 were females. MTEM was increased in people who exclusively used TCIGs (n=18) [median, IQR: 2.30 (1.29–2.82, p<0.001) and in people who exclusively used ECIGs (n=21) [median, IQR: 1.42 (0.96–1.91, p<0.01)] compared to NS controls (n=21) [median, IQR: 1.05 (0.66–1.24)]. MDFCF was increased in people who exclusively used TCIGs [median, IQR: 2.01 (1.59–2.49, p<0.001)] and in people who exclusively used ECIGs [median, IQR: 1.54 (1.10–1.86, p<0.001)] compared to NS controls [median, IQR: 0.97 (0.86–1.22)]. Both MTEM and MDFCF were higher in TCIG smokers compared to ECIG users and in ECIG users who were former smokers vs ECIG users who were never smokers (p<0.05 for all comparisons).
Conclusions:
The finding of alterations in proatherogenic properties of blood monocytes and plasma in TCIG smokers compared to NS validates this assay as a strong ex vivo mechanistic tool with which to measure proatherogenic changes in people who use ECIGs. Similar yet significantly less severe alterations in proatherogenic properties of monocytes and plasma were detected in the blood from ECIGs users. Future studies are necessary to determine whether these findings are attributable to a residual effect of prior smoking or are a direct effect of current ECIG use.
Graphical Abstract
INTRODUCTION
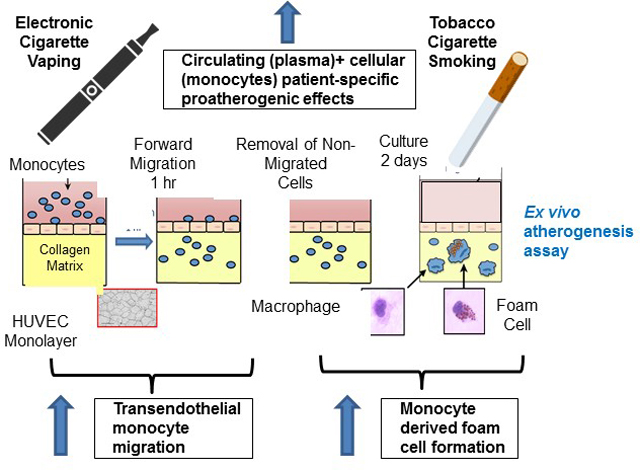
Electronic cigarettes (ECIGs), which remain very popular1, 2, have been available in the US for over 15 years, yet their contribution to cardiovascular risks has not been fully elucidated. In contrast, there is a large body of data informing our mechanistic understanding of atherosclerosis, a slowly progressive, inflammatory process in which circulating monocytes attach to endothelial cells, then migrate into the subintimal space, where they phagocytize oxidized lipids and become foam cells, generating the fatty streak. Foam cells then secrete pro-inflammatory cytokines, ultimately leading to the progressive process of inflammatory atherosclerosis that, unchecked, may present clinically as an acute coronary syndrome and even sudden death3. The role of tobacco cigarettes (TCIGs) in increasing oxidative stress and inflammation, thereby accelerating this process, is widely accepted4. Whether ECIGs, too, promote this process of monocyte trans-endothelial migration and foam cell formation is unknown.
We utilized our previously developed and validated in vitro model of early atherosclerotic events in which monocyte trans-endothelial migration and foam cell formation can be quantified5, 6. In the present study, we first determined if this novel assay was able to detect enhanced monocyte trans-endothelial migration (MTEM) and foam cell formation in young people who exclusively smoke TCIGs. Then we turned to otherwise healthy young people who exclusively use ECIGs to determine if using ECIGs also elicits similar proatherogenic changes, portending increased cardiovascular risk.
METHODS
Data are available upon reasonable request to the corresponding author.
Study Participants
Healthy participants meeting eligibility criteria7 were enrolled: 1) TCIG smokers (exclusive TCIG smoking >1 year, 2). ECIG users (exclusive ECIG use >1 year), and non-smokers (NS) (neither TCIGs or ECIGs >1 year). The experimental protocol was approved by the Institutional Review Board at the University of California, Los Angeles, and written informed consent was obtained from each participant.
Experimental session
Blood was drawn for cotinine levels and for peripheral blood mononuclear cells (PBMC) collection, and processed as previously described7, 8.
In vitro atherogenesis assay
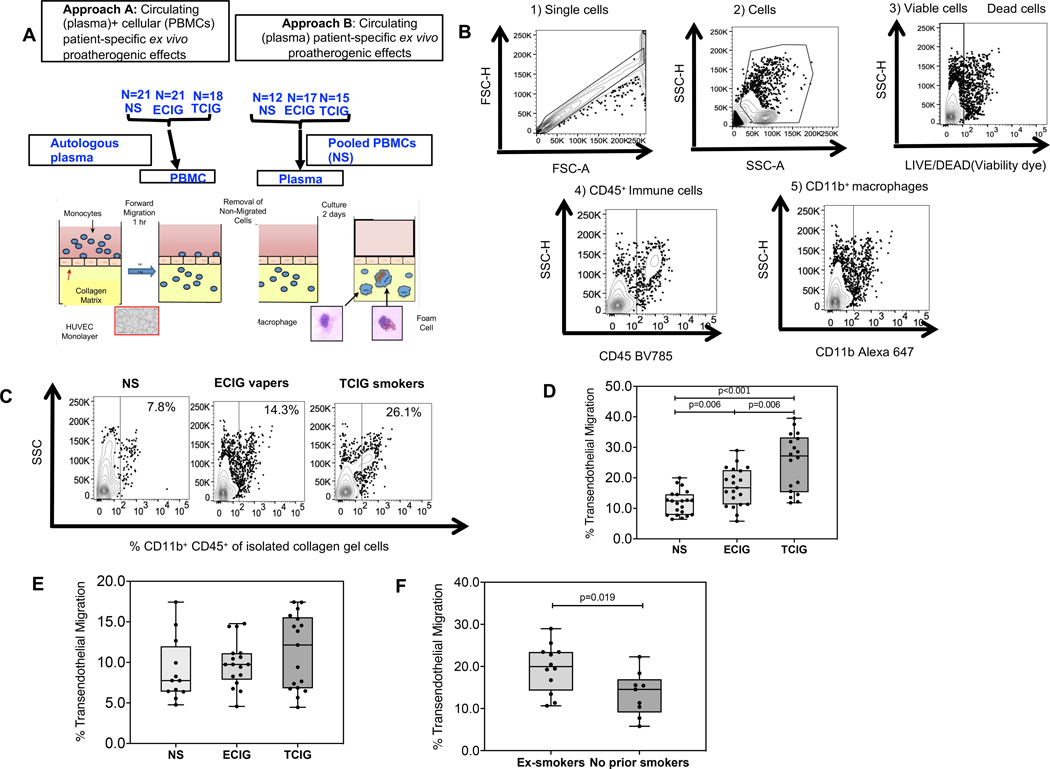
Briefly, PBMCs were added to tumor necrosis factor -activated human umbilical vein endothelial cells monolayers on type I collagen gels to transmigrate over 1 hour in the presence of 10% v/v plasma in serum-free media and form foam cells over 48 hours as previously described5, 6. To dissect circulating (present in plasma) and cellular (present in monocytes) patient-specific ex vivo proatherogenic effects we utilized autologous patient plasma and PBMCs. To dissect only circulating (present in plasma) patient-specific ex vivo proatherogenic effects we utilized autologous patient plasma and pooled PBMC from non-smokers (Figure 1A). Lipids present in the patient plasma drive ex vivo monocyte derived foam cell formation (MDFCF). Flow cytometry was utilized to determine the number of CD45+ CD11b+ macrophages inside the collagen gel (measure of MTEM) and the median fluorescence intensity (MFI) of BODIPY inside the transmigrated gel macrophages (measure of lipid content in macrophages and MDFCF). The gating strategy is shown in Figure 1B.
Figure 1: Transendothelial chemotaxis of monocytes among groups.
A. Optimal microscopy and flow cytometry was used to determine the percent of monocytes that underwent forward transendothelial migration (TEM) in collagen gel coated with HUVEC endothelial cells after 1 hour exposure to pooled plasma from healthy study participants as indicated in methods. The compared groups were nonsmokers (NS, light grey) (n=21), electronic-cigarette vapers (ECIG-vapers, grey)(n=21) and tobacco cigarette smokers (TCIG-smokers, dark grey)(n=18). Two different approaches were utilized to dissect the proatherogenic properties of circulating factors in plasma compared to cells. B. Gating flow cytometry strategy in macrophages isolated from collagen gels. C. Representative data of % transmigrated CD45+CD11b+ macrophages isolated from collagen gels among compared groups. D. % TEM migration among compared groups (Approach A) expressed relatively to the mean of the non-smoker group. Autologous plasma and cells were used for the atherogenesis assay. E. % TEM migration among compared groups (Approach B) expressed relatively to the mean of the non-smoker group. Patient plasma and pooled PBMCs from NS were used for the atherogenesis assay. F. % TEM migration among ECIG groups who were ex smokers versus never smokers. Summary data are shown as box and whisker plots with minimum, median, interquartile range (IQR) and maximum values. Mann Whitney test was used to compare 2 groups.
Statistical analysis
Kruskal—Wallis analysis of variance was used to compare the 3 groups and, if the results were statistically significant (P < 0.05), then the Mann—Whitney test was used to compare individual 2 groups. This study, largely exploratory, was not powered to detect effect sizes with adjustments for multiple comparisons. Our study is a small single-center study, and not powered to detect effect sizes with adjustment for multiple comparisons. Rather, consistency, direction, and magnitude of the effect in conjunction with the nominal p values were considered in order to help distinguish true and false-positive findings9.
RESULTS
Baseline characteristics
Participants in the 3 groups (TCIG smokers vs ECIG users vs NS, were not different by age, median [interquartile range (IQR), (24.0 [23.0–24.8] vs 23.0 [22.0–25.0] vs 23.0 [21.0–26.0] years; p=0.33), sex (female/male/, 9/9 vs 12/9 vs 10/11; p =0.85), body mass index (BMI), median [IQR], (21.9 [19.8–24.1] vs 22.5 [21.1–24.0] vs 22.5 [20.2–24.1] kg/m2; p = 0.49), and all participants had at least some college education. Tobacco product use burden, as estimated by plasma cotinine, was not different between TCIG smokers vs ECIG users (plasma cotinine median [IQR] 72.0 [28.0–143.5] vs 56.0 [22.0–165.0] ng/ml, p=0.58).
TCIG smokers have increased proatherogenic MTEM
Using autologous PBMCs and plasma, compared to NS, TCIG smokers had ~110% mean increase in MTEM (Figure 1C, D).
To dissect whether the patient-specific ex vivo proatherogenic effects of TCIG smoking in our model of atherogenesis was secondary to circulating factors present in plasma or due to cellular factors present in monocytes, we then utilized patient specific plasma and pooled PBMCs from the NS controls (Approach B)(Figure 1A). There was no difference in MTEM between TCIG smokers and NS controls (Figure 1E), consistent with the notion that the ex vivo proatherogenic increased MTEM observed with autologous monocytes and plasma from TCIG smokers compared to NS was secondary to effects of TCIG smoking on monocytes rather than circulating factors.
ECIG users have increased proatherogenic MTEM
Using autologous patient-specific monocytes and plasma (Approach A), compared to the NS controls, ECIG users had ~40% mean increase in MTEM (p<0.01) (Figure 1C, D). This increase was significantly less than in the TCIG smokers (p<0.01) (Figure 1C, D).
To determine if this ex vivo proatherogenic finding in ECIG users was a residual effect from prior TCIG smoking, or attributable to current ECIG use, the ECIG group was divided into ECIG-former smokersand ECIG-never smokers. Current ECIG use, as judged by plasma cotinine levels, was similar in people who were (n=12), and were not (n=9), former smokers (plasma cotinine median[IQR] 78.0 [25.8–179.3] vs 27.0 [4.5–141.5] ng/ml, p=0.34, respectively). MTEM was significantly greater in ECIG-former smokers compared to ECIG-never smokers (p<0.05) (Figure 1F).
Healthy PBMCs and patient plasma were again used to dissect whether the patient-specific ex vivo proatherogenic effects of ECIG use in our model of atherogenesis was secondary to circulating factors present in plasma or due to cellular factors present in monocytes. We found no difference in MTEM between ECIG users and NS (Figure 1E), suggesting that MTEM in ECIG users was secondary to effects of ECIG use on monocytes rather than circulating factors.
TCIG smokers have increased proatherogenic MDFCF
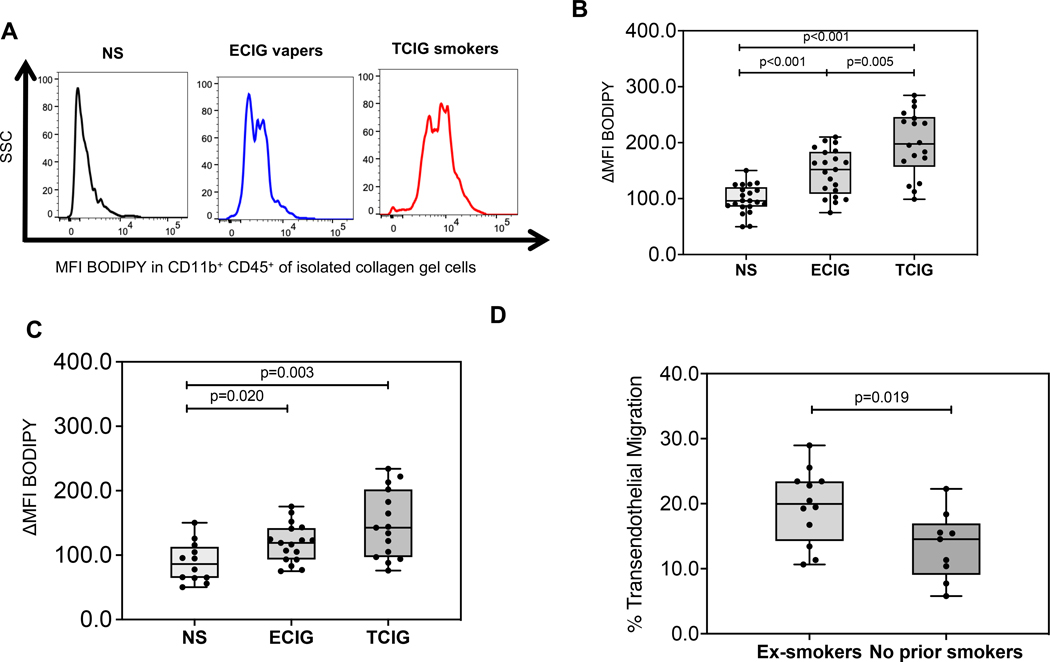
Using autologous patient-specific monocytes and plasma, compared to NS, TCIG smokers had ~100% relative mean increase in the BODIPY MFI in CD11b+ transmigrated macrophages (Figure 2A, B). Using healthy PBMCs and patient plasma, we found TCIG smokers had ~ 67% mean increase in BODIPY MFI compared to NS (Figure 2C), suggesting that the increased MDFCF in TCIG smokers compared to NS was secondary to effects of TCIG smoking on both monocytes and circulating factors.
Figure 2: Monocyte derived foam cell formation among groups.
A. Flow cytometry was used to determine the median fluorescence intensity (MFI) of the lipid-staining fluorochrome BODIPY in monocyte derived CD11b+ macrophages that underwent TEM and were isolated from the collagen gel after 48-hour exposure to autologous plasma as indicated in methods. Fluorescence intensity of a positive cell population was compared to a negative cell population (fluorescence minus one negative control for staining) (ΔMFI). The compared groups were nonsmokers (NS, light grey) (n=21), electronic-cigarette vapers (ECIG-vapers, grey) (n=21) and tobacco cigarette smokers (TCIG-smokers, dark grey) (n=18). B. MFI BODIPY among compared groups (Approach A) expressed relatively to the mean of the non-smoker group. C. MFI BODIPY among compared groups (Approach B) expressed relatively to the mean of the non-smoker group. Summary data are shown as box and whisker plots with minimum, median interquartile range (IQR) and maximum values. Mann Whitney test was used to compare 2 groups (*p < 0.05, **p < 0.01, ***p < 0.001).
Summary data (mean, SEM) are shown. Mann Whitney test was used to compare 2 groups.
ECIG users have increased proatherogenic MDFCF
Compared to NS, ECIG users had ~50% mean increase in BODIPY MFI in CD11b+ transmigrated macrophages (p<0.05) (Figure 2A, B). The mean BODIPY MFI in CD11b+ transmigrated macrophages was ~50% lower in ECIG users compared to TCIG smokers (Figure 2A, B). We then determined whether this intermediate level of MDFCF seen in ECIG users was a residual effect from prior TCIG smoking. MDFCF was significantly greater in ECIG-former smokers compared to ECIG-never smokers (p<0.05) (Figure 2D).
Using healthy PBMCs and patient plasma, we found that ECIG users had a mean 34% increase in BODIPY MFI in CD11b+ transmigrated macrophages compared to NS (Figure 2C)(p<0.05), suggesting that the ex vivo proatherogenic increased MDFCF observed in ECIG users compared to NS was secondary to effects of ECIG use on both monocytes and circulating factors.
DISCUSSION
To our knowledge, this is among the first studies in humans to confirm early alterations in proatherogenic properties of blood monocytes and plasma collected from otherwise healthy young people who smoke TCIGs compared to people who do not use tobacco products. Our findings in TCIG smokers compared to NS controls are consistent with the notion that this atherogenesis assay is a strong mechanistic tool to predict future cardiovascular risk in humans given established link of TCIGs with future cardiovascular disease9, and provides a rationale for its use in assessing proatherogenic changes in young people who use ECIGs. Similar yet significantly less severe alterations in proatherogenic properties were detected in blood monocytes and plasma collected in otherwise healthy young people who use ECIGs compared to people who do not use tobacco products. Both primary endpoints and key steps in early atherosclerosis, including monocyte trans-endothelial migration and foam cell formation, were significantly increased in people who use ECIGs compared to NS, but were significantly lower than in the TCIG smokers. Consistent with these data, we have previously reported an increase in established contributors to atherogenesis such as polyunsaturated fatty acids and oxidative biomarkers10, 11, proatherogenic proteins of the toll like receptor 4-inflammasome-IL-6 axis in immune cell subtypes7, 12, cellular oxidative stress8, 13 in otherwise healthy people who smoke TCIGs or use ECIGs compared to NS.
Since ECIG users and TCIG smokers have increased cellular oxidative stress compared to non-smokers 8, 11, and given that redox changes in biology of tissue macrophages may persist long term14, we then asked the question whether these intermediate levels MTEM and foam cell formation seen in people who use ECIGs was a residual effect from prior smoking or was attributable to current ECIG use. Although all ECIG users who were former smokers had quit smoking more than 1 year prior to study enrollment, both proatherogenic processes were significantly greater in current ECIG users who were former smokers compared to ECIG users who were never smokers, consistent with a residual effect of prior TCIG smoking. These data suggest that exclusive ECIG use does not contribute to atherosclerosis, but this needs to be confirmed in a larger study.
The strengths of our study are the careful covariate phenotyping of our study population and established physiologically relevant model of atherogenesis able to detect key proatherogenic properties of monocytes in people who smoke TCIGs that could then be applied to people who use ECIGs. Although our study is a small study, we were able to find a consistent increase in MTEM and MDFCF in otherwise healthy young people who exclusively use either ECIGs or TCIGs compared to non-smokers. Additional larger studies that also include former TCIG smokers who do not use ECIGs will help if there is an additive or even synergistic effect between TCIG and ECIG use.
In conclusion, the finding that key proatherogenic properties of monocytes are elevated in otherwise healthy young people who use either TCIGs or ECIGs compared to non-smokers has public health implications. Our data provide urgency to messaging that ECIG use is not harmless, and that smoking cessation efforts must be redoubled.
Supplementary Material
Highlights.
Little is known whether electronic cigarettes (ECIG) increase vulnerability to future atherosclerotic cardiovascular disease.
Using our in vitro model of early atherosclerotic events quantifying monocyte trans-endothelial migration (MTEM) and foam cell formation (MDFCF), we found that MTEM and MDFCF were increased in tobacco cigarette (TCIG) smokers and ECIG users compared to non-smoking (NS) controls.
Both MTEM and MDFCF were higher in TCIG smokers compared to ECIG users and in ECIG users who were former smokers vs ECIG users who were never smokers.
Future studies are necessary to determine whether these findings are attributable to a residual effect of prior smoking or are a direct effect of current ECIG use.
Acknowledgements
Sources of Funding
This work was supported by the Tobacco-Related Disease Research Program (TRDRP) under the contract number TRDRP 28IR-0065 (HRM). This work was also supported in part by NIH grants R01AG059501 (to TK). The flow cytometry machine used in the study was purchased through the UCLA Center for AIDS Research (P30AI28697) grant.
Non-standard abbreviations and acronyms
- ECIG
electronic cigarette
- TCIG
tobacco cigarette
- MTEM
monocyte trans-endothelial migration
- NS
non-smokers
- PBMC
peripheral blood mononuclear cells
- IQR
interquartile range
- MDFCF
monocyte derived foam cell formation
- MFI
median fluorescence intensity
Footnotes
Disclosures
None
REFERENCES
- 1.Cooper M, Park-Lee E, Ren C, Cornelius M, Jamal A and Cullen KA. Notes from the Field: E-cigarette Use Among Middle and High School Students - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1283–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornelius ME, Loretan CG, Wang TW, Jamal A and Homa DM. Tobacco Product Use Among Adults - United States, 2020. MMWR Morb Mortal Wkly Rep. 2022;71:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falk E. Pathogenesis of atherosclerosis. Journal of the American College of Cardiology. 2006;47:C7–12. [DOI] [PubMed] [Google Scholar]
- 4.Ambrose JA and Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. Journal of the American College of Cardiology. 2004;43:1731–7. [DOI] [PubMed] [Google Scholar]
- 5.Angelovich TA, Hearps AC, Maisa A, Kelesidis T and Jaworowski A. Quantification of Monocyte Transmigration and Foam Cell Formation from Individuals with Chronic Inflammatory Conditions. J Vis Exp. 2017. [DOI] [PMC free article] [PubMed]
- 6.Angelovich TA, Hearps AC, Oda MN, Borja MS, Huynh D, Homann S, Jaworowski A and Kelesidis T. Dysfunctional high-density lipoprotein from HIV+ individuals promotes monocyte-derived foam cell formation in vitro. AIDS. 2017;31:2331–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelesidis T, Zhang Y, Tran E, Sosa G and Middlekauff HR. Expression of Key Inflammatory Proteins Is Increased in Immune Cells From Tobacco Cigarette Smokers But Not Electronic Cigarette Vapers: Implications for Atherosclerosis. J Am Heart Assoc. 2021;10:e019324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelesidis T, Tran E, Arastoo S, Lakhani K, Heymans R, Gornbein J and Middlekauff HR. Elevated Cellular Oxidative Stress in Circulating Immune Cells in Otherwise Healthy Young People Who Use Electronic Cigarettes in a Cross-Sectional Single-Center Study: Implications for Future Cardiovascular Risk. J Am Heart Assoc. 2020;9:e016983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan MS, Greevy RA, Tindle HA, Vasan RS, Lipworth L, Aldrich MC, Lloyd-Jones DM and Freiberg MS. Inclusion of Smoking Data in Cardiovascular Disease Risk Estimation. JAMA Cardiol. 2022;7:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta R, Lin Y, Luna K, Logue A, Yoon AJ, Haptonstall KP, Moheimani R, Choroomi Y, Nguyen K, Tran E, Zhu Y, Faull KF, Kelesidis T, Gornbein J, Middlekauff HR and Araujo JA. Electronic and Tobacco Cigarettes Alter Polyunsaturated Fatty Acids and Oxidative Biomarkers. Circ Res. 2021;129:514–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moheimani RS, Bhetraratana M, Yin F, Peters KM, Gornbein J, Araujo JA and Middlekauff HR. Increased Cardiac Sympathetic Activity and Oxidative Stress in Habitual Electronic Cigarette Users: Implications for Cardiovascular Risk. JAMA Cardiol. 2017;2:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelesidis T, Zhang Y, Tran E, Sosa G and Middlekauff HR. Increased Expression of Proatherogenic Proteins in Immune Cell Subtypes in Tobacco Cigarette Smokers But Not in Electronic Cigarette Vapers. Can J Cardiol. 2021;37:1175–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelesidis T, Tran E, Nguyen R, Zhang Y, Sosa G and Middlekauff HR. Association of 1 Vaping Session With Cellular Oxidative Stress in Otherwise Healthy Young People With No History of Smoking or Vaping: A Randomized Clinical Crossover Trial. JAMA Pediatr. 2021;175:1174–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brune B, Dehne N, Grossmann N, Jung M, Namgaladze D, Schmid T, von Knethen A and Weigert A. Redox control of inflammation in macrophages. Antioxid Redox Signal. 2013;19:595–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.