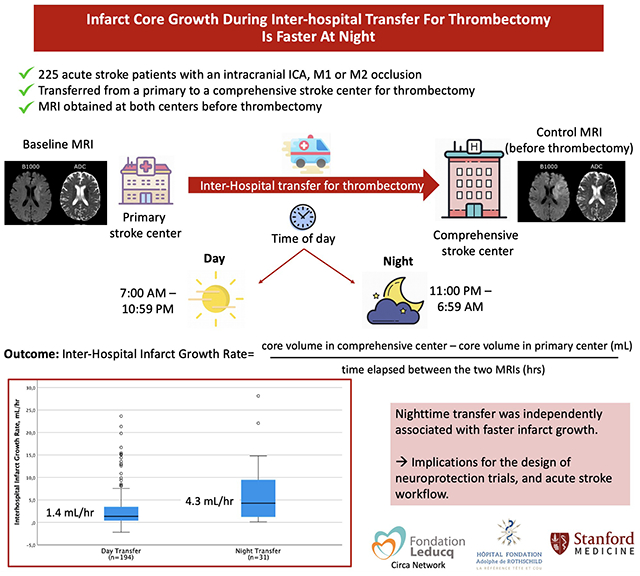
Abstract
Background:
Preclinical stroke models have recently reported faster infarct growth (IG) when ischemia was induced during daytime. Considering the inverse rest-activity cycles of rodents and humans, faster IG during the nighttime has been hypothesized in humans.
Methods:
We retrospectively evaluated acute ischemic stroke patients with a large vessel occlusion transferred from a primary to one of three French comprehensive stroke center, with an MRI obtained at both centers before thrombectomy. Inter-hospital IG rate was calculated as the difference in infarct volumes on the two diffusion-weighted imaging, divided by the time elapsed between the two MRIs. IG rate was compared between patients transferred during daytime (7:00-22:59) and nighttime (23:00-06:59) in multivariable analysis adjusting for occlusion site, NIHSS score, infarct topography and collateral status.
Results:
Out of the 329 patients screened, 225 patients were included. Inter-hospital transfer occurred during nighttime in 31 (14%) patients and daytime in 194 (86%). Median inter-hospital IG was faster when occurring at night (4.3 mL/hr, IQR 1.2-9.5) as compared to the day (1.4 mL/hr, IQR 0.4-3.5, P<0.001). In multivariable analysis, nighttime transfer remained independently associated with IG rate (P<0.05).
Conclusions:
Inter-hospital IG appeared faster in patients transferred at night. This has potential implications for the design of neuroprotection trials, and acute stroke workflow.
Graphical Abstract
INTRODUCTION
In acute ischemic stroke with a large vessel occlusion (LVO), the rate of the irreversibly injured ischemic brain tissue (‘infarct’) growth over time is highly variable across individuals.1,2 Clarifying the determinants of infarct growth (IG) rate is important to optimally select patients for trials of neuroprotective therapies, those with fast IG being more likely to benefit from these promising therapies, as well as for optimizing patient workflow. Recent studies in rodents found that IG is faster when focal ischemia is induced during the day, i.e. during their inactive phase.3 Moreover, the neuroprotective treatment benefits were only seen during their inactive phase.3 Considering the inverse rest-activity cycles of rodents and humans, faster IG during the nighttime has been hypothesized in humans. However, whether the time-of-day influences IG in human acute ischemic stroke with LVO is poorly known.2 Here, we aimed to study whether IG is faster at night.
METHODS
Our analysis was conducted according to the STROBE criteria for observational studies. The research was approved by the Rothschild Foundation Hospital review board. Each patient was informed of her/his participation in the study and was offered the possibility to withdraw. The data supporting the study findings are available upon reasonable request.
Data Sources
The data were extracted from the Infarct-Growth cohort, for which detailed methods and main results have been published recently.1 Data from acute stroke patients admitted to three French comprehensive stroke centers that fulfilled the following criteria were retrospectively collected for the present sub-study: (1) initial admission at a primary stroke center where a standard-of-care MRI was performed showing an infarct volume <70mL and an anterior circulation LVO, and (2) subsequent transfer to a comprehensive center where a standard-of-care MRI was repeated by protocol upon arrival (before thrombectomy), and demonstrated a stable vascular occlusion (see Supplemental Methods for details). MRI was the routine first-line imaging technique for acute stroke in all participating primary and comprehensive stroke centers. Center 1 collected data on all patients, regardless of whether thrombectomy was attempted following the control MRI, whereas centers 2 and 3 collected thrombectomy-treated patients only.1
Clinical and Radiological Data
Clinical variables routinely recorded in the acute stroke setting were collected. Time of transfer was operationally defined as the time of MRI in the primary stroke center, and was dichotomized into day (7:00-22:59) vs night (23:00-6:59), with time-epochs decided a priori based on the CIRCA network consortium recommendations.4 Infarct volumes on diffusion-weighted imaging (DWI) were manually outlined based on DWI signal intensity and encompassed the entire area of bright DWI signal intensity. Areas of decreased apparent diffusion coefficient with subtle DWI signal changes were also segmented. To ensure unbiased segmentations, infarct on the primary and comprehensive stroke center DWIs were outlined several days apart, blinded to all clinical and radiological data (including time-of-day). Inter-hospital IG rate was calculated as the difference in infarct volumes on the two DWIs studies, divided by the time elapsed between the two MRIs.1 Collateral status was evaluated using the hypoperfusion intensity ratio (HIR) on perfusion imaging whenever available, lower HIR indicating better collaterals.1 Details regarding imaging data are provided in Supplementals Methods.
Statistical Analysis
The association between inter-hospital IG rate and nighttime was assessed through β coefficient and its 95% Confidence Interval (CI), calculated in multivariable linear regression analysis, with log-transformed IG rate as the dependent variable to keep the linear assumption valid. Two different multivariable models were constructed, with and without HIR (see Supplemental Methods).1 Two sensitivity analyses were performed: (1) limited to patients transferred to center 1 (the only center that collected both thrombectomy-treated and untreated patients), and (2) with further adjustment on initial infarct volume in the first multivariable model.5
RESULTS
During the study period, 225 patients met inclusion criteria (Figure 1). Patient characteristics are detailed in Table. Inter-hospital transfer occurred during the day in 194 (86%) patients, and at night in 31 (14%). Patients transferred at night had more proximal occlusion site, larger infarcts in the primary center and higher HIR (i.e. poorer collaterals) but had otherwise similar baseline characteristics with patients transferred during daytime (Table).
Figure 1. Flow chart.
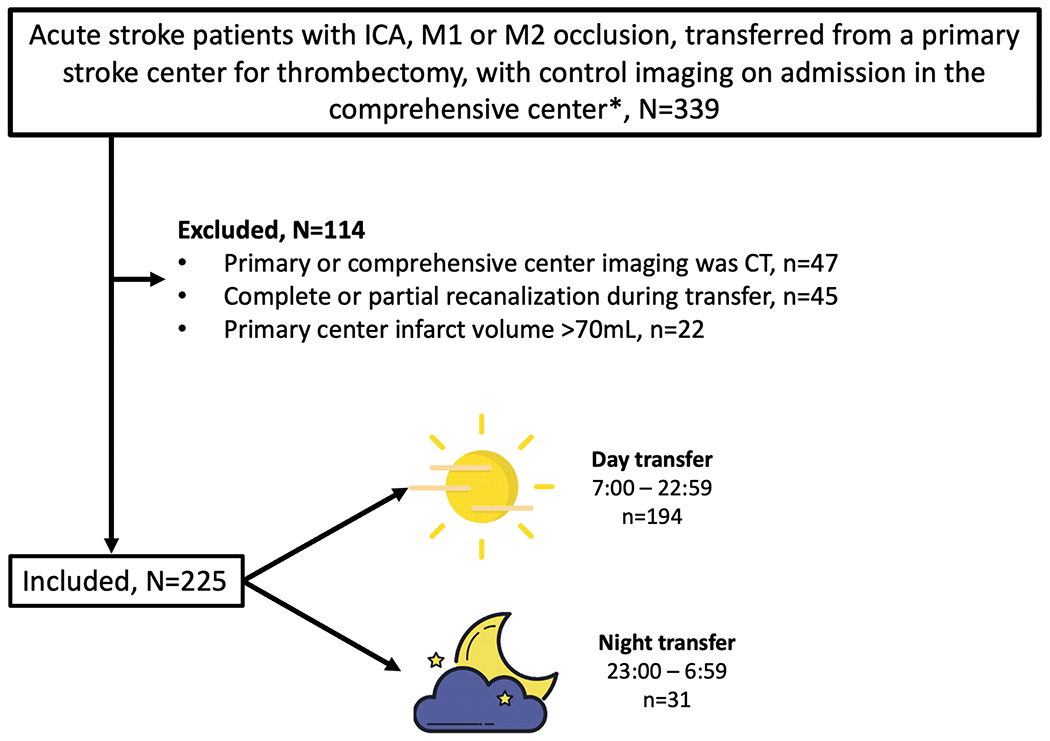
*for centers 2 and 3, data from patients who did not undergo groin puncture was not available.
Table.
Baseline comparison of patients transferred during daytime vs nighttime.
| Overall N=225 | Day transfer n=194 | Night transfer n=31 | P value | |
|---|---|---|---|---|
| Age, years | 72 (63-81) | 73 (63-82) | 70 (65-74) | 0.24 |
| Male | 109 (48%) | 90 (46%) | 19 (61%) | 0.12 |
| Hypertension | 147 (65%) | 130 (67%) | 17 (57%) | 0.19 |
| Diabetes | 48 (21%) | 39 (20%) | 9 (29%) | 0.26 |
| Clinical data (primary center) | ||||
| NIHSS score | 14 (9-19) | 14 (7-19) | 15 (10-20) | 0.25 |
| Glycemia, g/L | 1.20 (1.07-1.43) | 1.20 (1.07-1.42) | 1.19 (1.08-1.58) | 0.71 |
| Intravenous thrombolysis use | 122 (54%) | 107 (55%) | 15 (48%) | 0.48 |
| MRI characteristics (primary center) | ||||
| Last seen well to MRI time, hrs | 2.5 (1.6-6.1) | 2.4 (1.6-6.1) | 3.4 (2.2-5.1) | 0.29 |
| Occlusion site | 0.04 | |||
| ICA | 39 (17%) | 29 (15%) | 10 (32%) | |
| M1 | 147 (65%) | 132 (68%) | 15 (48%) | |
| M2 | 39 (17%) | 33 (17%) | 6 (19%) | |
| Core volume, mL | 9 (3-19) | 8 (2-17) | 17 (8-27) | <0.01 |
| Hypoperfusion Intensity Ratio* | 0.33 (0.19-0.47) | 0.30 (0.17-0.43) | 0.47 (0.36-0.53) | <0.01 |
Hypoperfusion Intensity Ratio was available in 158 patients (day transfer, n=136; night transfer, n=22), primarily during the MRI performed at the comprehensive stroke center (141/165, 89%)
Median primary-to-comprehensive center MRI delay was 3.0 hrs (IQR, 2.6-3.5), and was similar during daytime (3.0 hrs, IQR 2-7-3.5) and nighttime (2.8 hrs, 2.4-3.2; P=0.08). Median inter-hospital IG rate was 1.6 mL/hr (IQR, 0.6-4.6), and was significantly higher when occurring at night (4.3 mL/hr, IQR 1.2-9.5) as compared to the day (1.4 mL/hr, IQR 0.4-3.5, P<0.001; Figure 2). Faster IG during nighttime was observed in both the internal carotid artery (2.2 mL/hr [IQR, 0.7-6.8] during daytime vs 9.5 mL/hr [IQR, 6.3-12.0] during nighttime, P=0.009) and the M1/M2 (1.3 mL/hr [IQR, 0.4-3.1] during daytime and 2.0 mL/hr [IQR, 0.7-7.5] during nighttime, P=0.044) occlusion site subgroups. Nighttime transfer was independently associated with log-transformed inter-hospital IG rate following adjustment for baseline occlusion site, NIHSS score and infarct topography (β coefficient=0.17, 95%CI 0.07-0.27, P=0.001), as well as in the alternative model adjusted for HIR, occlusion site and infarct topography (n=158 patients with perfusion imaging; β coefficient=0.11; 95%CI 0.01-0.20, P=0.033). Similar results were found in the sensitivity analyses (Supplemental Results).
Figure 2. Relationship between Inter-hospital Infarct Growth Rate and Time of Transfer.
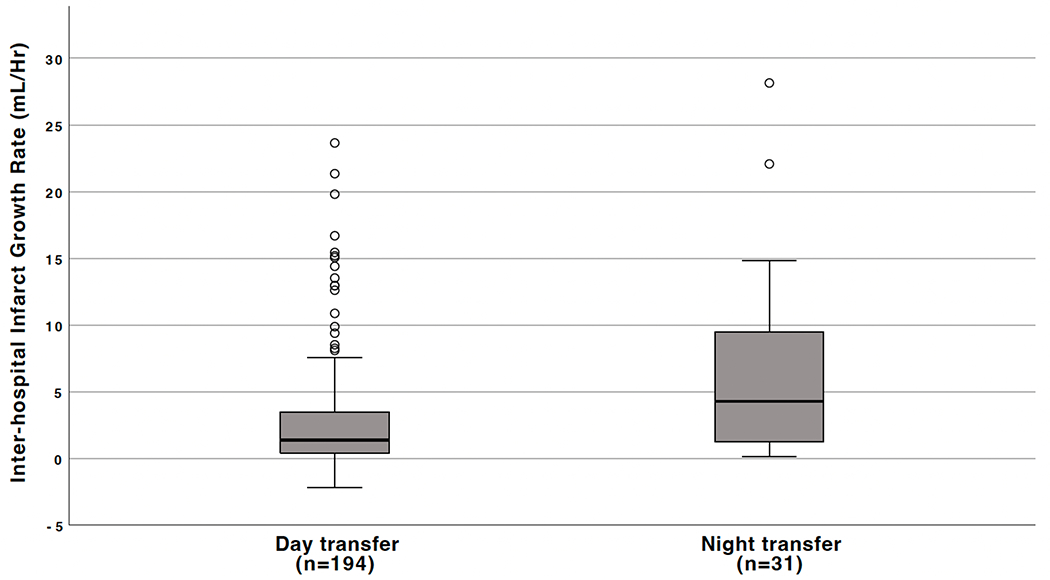
The box represents the upper and lower quartiles, the short black line within the box represents the median, the whiskers represent the 5th and 95th percentiles, and the circles extreme values.
DISCUSSION
Inter-hospital IG was faster at night in LVO-related stroke patients transferred from a primary to a comprehensive center for thrombectomy. These data expand the recent observations made in preclinical stroke models to humans,3 and are in line with one previous study reporting faster early IG at night in LVO-related stroke patients.2 This previous report studied IG rate based on a single CT-perfusion, defined as baseline infarct volume divided by onset-to-imaging time. However, this measure was typically imprecise for nighttime strokes occurring during the sleep since the exact time of symptom onset is unknown.2
The mechanisms underlying faster IG at night remain to be clarified. Emerging preclinical literature suggests that the vascular compartment has circadian regulation: oscillations in resting tone of cerebral arteries display a 24-hour cycle, and blood flow within the penumbra differs during day and night.3,6 In line with these preclinical observations, the HIR was higher –indicating poorer collateral flow– during nighttime in our dataset, which may partly mediate the effect of time-of-day on IG rate. However, the association during nighttime and IG rate remained significant after adjustment for HIR, which suggests that other mechanisms may be involved. For instance, preclinical stroke models have shown that the immune and inflammatory responses demonstrate a circadian regulation, and blood pressure shows circadian variation.6
Our results have implications for the design of neuroprotection trials aiming to limit IG before recanalization is obtained. Substantial benefits are mostly expected in ‘fast progressors’, for whom a large reduction in IG could be achieved with an effective agent. Recent studies in rodents found that the neuroprotective treatment benefits were only seen during their inactive phase, i.e. when IG was faster.3 The circadian mismatch between animal models and clinical trials of neuroprotection may partly explain the difficulties in translating neuroprotection agent effects from the bench into bedside.3,6 Neuroprotection trials may therefore improve their chance of positive findings if they facilitate nighttime enrollment, when fast progressors are particularly frequent. Moreover, our data suggest that reducing time to reperfusion may be particularly important at night, which has implications for acute stroke workflow.7
This study has limitations. First, the sample size was moderate with only 31 patients transferred at night. Larger studies are warranted to confirm our findings. Second, selection biases may have occurred, for instance patients transferred during nighttime may be less frequently re-scanned with an MRI. Last, unlike center 1, centers 2 and 3 data were only available from patients treated with thrombectomy, which may have biased the patient sample. However, the results were similar in the sensitivity analysis limited to center 1. Considering the above-mentioned limitations, our study should be considered hypothesis generating.
CONCLUSION
Our findings indicate that inter-hospital IG appears faster in LVO-related acute stroke patients transferred at night. These results have implications for the design of neuroprotection trials, as well as for optimizing acute stroke workflow.
Supplementary Material
Sources of funding:
Pierre Seners received grants from Edmond de Rothschild Fellowship program, Bettencourt-Schueller Foundation, Philippe Foundation, Servier Institute.
Pierre Seners and Anirudh Sreekrishnan received support from the Leducq Trans-Atlantic Network of Excellence On Circadian Effects in Stroke (CIRCA).
Anirudh Sreekrishnan received salary support from a fellowship grant from StrokeNet (NINDS U24NS107220).
Maarten Lansberg was supported by NIH grants (NINDS U24NS107220 and R01NS075209)
Disclosures:
Dr Henon received speaker fees from Sanofi aventis.
Dr Heit reports consulting fees from Medtronic and MicroVention, and he is a member of the medical and scientific advisory board for iSchemaView.
Dr Olivot reports consultant services for Acticor, Abbvie, Boehringer-Ingelheim, Pfizer, Bristol-Myers Squibb and Medtronic.
Dr Albers reports stock holdings in iSchemaView; compensation from Biogen, iSchemaView and Genentech for consultant services.
The other authors have nothing to disclose.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- DWI
diffusion-weighted imaging
- HIR
hypoperfusion intensity ratio
- LVO
large vessel occlusion
- IG
infarct growth
Appendix: Infarct-Growth collaborators:
Perrine Schmitt, Denis Sablot, Thibault Lalu, Nicolas Bricout, Jean-François Albucher, Christophe Cognard, Charlotte Cordonnier, Soren Christensen, Lauranne Scheldeman.
REFERENCES
- 1.Seners P, Scheldeman L, Christensen S, Mlynash M, Ter Schiphorst A, Arquizan C, Costalat V, Henon H, Bretzner M, Heit JJ, et al. Determinants of infarct core growth during inter-hospital transfer for thrombectomy. Ann Neurol. 2023;93:1117–1129 [DOI] [PubMed] [Google Scholar]
- 2.Reidler P, Brehm A, Sporns PB, Burbano VG, Stueckelschweiger L, Broocks G, Liebig T, Psychogios MN, Ricke J, Dimitriadis K, et al. Circadian rhythm of ischaemic core progression in human stroke. J Neurol Neurosurg Psychiatry. 2023;94:70–73 [DOI] [PubMed] [Google Scholar]
- 3.Esposito E, Li W, E TM, Park JH, Şencan I, Guo S, Shi J, Lan J, Lee J, Hayakawa K, et al. Potential circadian effects on translational failure for neuroprotection. Nature. 2020;582:395–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saver JL, Klerman EB, Buchan AM, Calleja P, Lizasoain I, Bahr-Hosseini M, Lee S, Liebeskind DS, Mergenthaler P, Mun KT, et al. Consensus recommendations for standardized data elements, scales, and time segmentations in studies of human circadian/diurnal biology and stroke. Stroke. 2023. doi: 10.1161/STROKEAHA.122.041394. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–278 [DOI] [PubMed] [Google Scholar]
- 6.Lo EH, Albers GW, Dichgans M, Donnan G, Esposito E, Foster R, Howells DW, Huang YG, Ji X, Klerman EB, et al. Circadian biology and stroke. Stroke. 2021;52:2180–2190 [DOI] [PubMed] [Google Scholar]
- 7.García-Tornel Á, Flores A, Terceño M, Cardona P, Amaro S, Gomis M, Zaragoza J, Krupinski J, Gómez-Choco M, Mas N, et al. Association of time of day with outcomes among patients triaged for a suspected severe stroke in nonurban catalonia. Stroke. 2023;54:770–780 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


