Abstract
Summary
Objective:
Transcranial Magnetic Stimulation (TMS) has emerged as a viable non-invasive method for mapping language networks. Little is known about the tolerability of transcranial magnetic stimulation language mapping in children.
Methods:
Children aged 5–18 years underwent bilateral language mapping using repetitive transcranial magnetic stimulation (rTMS) to target 33 sites/hemisphere. Stimulation was delivered at 5 Hz, in 1–2 second bursts, during visual naming and auditory verb generation. Pain unpleasantness and pain intensity were assessed using an unpleasantness visual analog scale (VAS).
Results:
49 participants tolerated motor mapping and had repetitive transcranial magnetic stimulation. 35/49 (71%) completed visual naming and 26/49 (53%) completed both visual naming and verb generation. Mean electrical field per participant was 115 V/m. Young age and lower language ability were associated with lower completion. Visual analogue scale scores were significantly higher (6.1 vs. 2.8) in participants who withdrew early compared to those who completed at least visual naming.
Conclusions:
Pain measured by VAS was a major contributor to early withdrawal. However, a complete bilateral map was obtained with one paradigm in 71% of participants. Future studies designed to reduce pain during repetitive transcranial magnetic stimulation over language cortex will boost viability.
Significance:
This study represents the first attempt to characterize tolerability of bilateral repetitive transcranial magnetic stimulation language mapping in healthy children.
Keywords: transcranial magnetic stimulation, stimulation mapping, repetitive TMS
1. Introduction
When delivered in bursts of repetitive pulses lasting 1–2 seconds over putative language cortex, known as repetitive transcranial stimulation or rTMS, the result is a “virtual lesion” causing observable language disruption.(Tarapore et al., 2013) TMS mapping of language inhibition sites has been reported, mostly in adults undergoing presurgical evaluation for brain tumors or drug resistant epilepsy.(Ille et al., 2015; Schwarzer et al., 2018; Tarapore et al., 2016)
Some advocate rTMS mapping in younger children since, unlike noninvasive functional imaging techniques such as functional MRI and magnetoencephalography (MEG), head movement can be tolerated during TMS while maintaining accuracy.(Narayana et al., 2015; Rezaie et al., 2020) Other authors have reported using rTMS to aid in presurgical planning of peri-eloquent lesion resection in children, resulting in an alteration to surgical plan in many, and some overlap of direct cortical stimulation sites and TMS-identified sites.(Lehtinen et al., 2018; Rosenstock et al., 2020) The rTMS procedure is more analogous to the gold standard electrical stimulation mapping, in contrast to functional MRI and MEG which yield probabilistic maps.(Jeltema et al., 2021) However, TMS functional thresholds have an inverse relationship with age: the youngest patients have the highest functional thresholds, which can often be difficult to obtain.(Garvey et al., 2003; Koh and Eyre, 1988) Repetitive stimulation of the temporalis muscle overlying language cortex has been reported to be more uncomfortable than superior frontal, occipital or parietal stimulation, probably due to more robust musculature and resulting TMS burst-related contractions in this region, but discomfort has been observed to be highly variable between subjects.(Lambon Ralph et al., 2009; Nettekoven et al., 2021)
The use of selective rTMS over language cortex in children undergoing presurgical evaluation for brain tumors or drug resistant epilepsy has been reported.(Babajani-Feremi et al., 2018; Rejno-Habte Selassie et al., 2020; Rosenstock et al., 2019) However the tolerability and relationship to functional thresholds have not been studied prospectively. Given known tolerability issues and potentially higher functional thresholds for language, it is unknown whether clinical language maps being obtained via rTMS in children are viable representations of language cortex, and in what age range this technique can be employed with a reasonable degree of success. We initiated a prospective study of rTMS language mapping in healthy children to determine the tolerability and efficacy of the procedure in order to better predict which patients should be submitted to rTMS. Secondarily, we sought to understand the range of tolerability issues systematically to provide a groundwork for future studies aimed at interventions that reduce discomfort.
2. Materials and methods
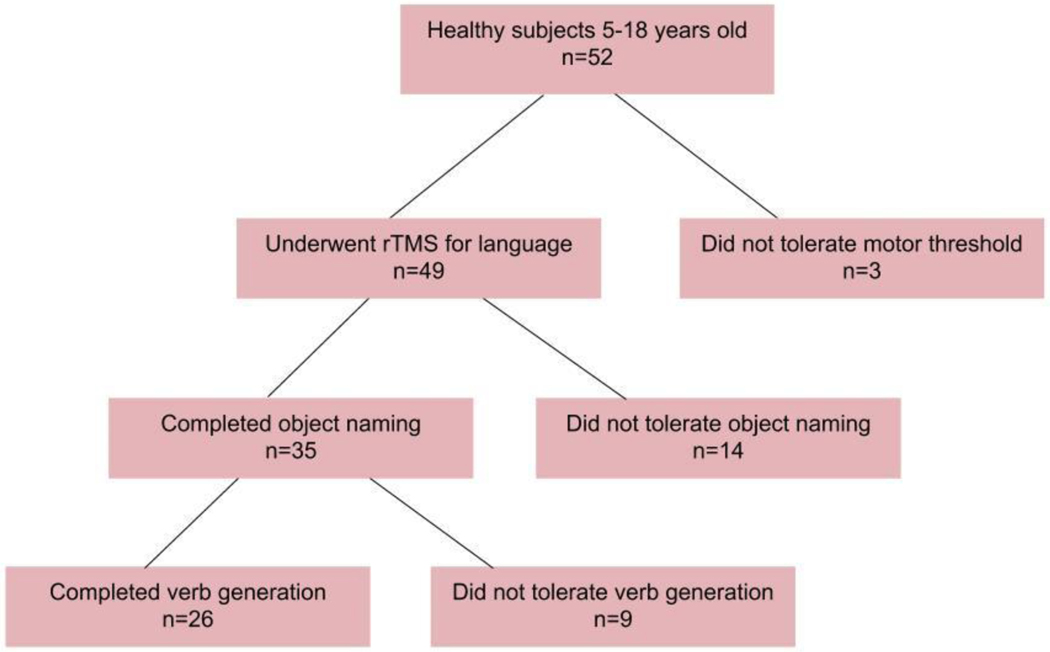
Healthy children ages 5–18 were recruited (Fig 1). Exclusion criteria were hairstyle incompatible with TMS (braids, beads or other piled hair that prevent the coil from being placed near the scalp), standard MRI exclusion criteria, developmental delay/disability, score of >2 standard deviation below mean on total score for Clinical Evaluation of Language Fundamentals (CELF-5) examination and structural abnormality of the supratentorial cortex. The study was approved by the institutional review board and funded by NINDS/NIH, 1 R21 NS106631. During the informed consent/assent process, risks of TMS were discussed including headache, neck stiffness and the rare possibility of seizure.(Pereira et al., 2016) Participants were compensated for study visits.
Figure 1:
Study flow.
2.1. Demographics and baseline neuropsychological assessment
Demographic data including date of birth, stated gender and race/ethnicity were collected. The CELF-5, Wechsler Nonverbal Scale of Ability(Wechsler, 2006) and Edinburgh Handedness Inventory (EHI)(Oldfield, 1971) were administered.
2.2. MRI and neuronavigated TMS
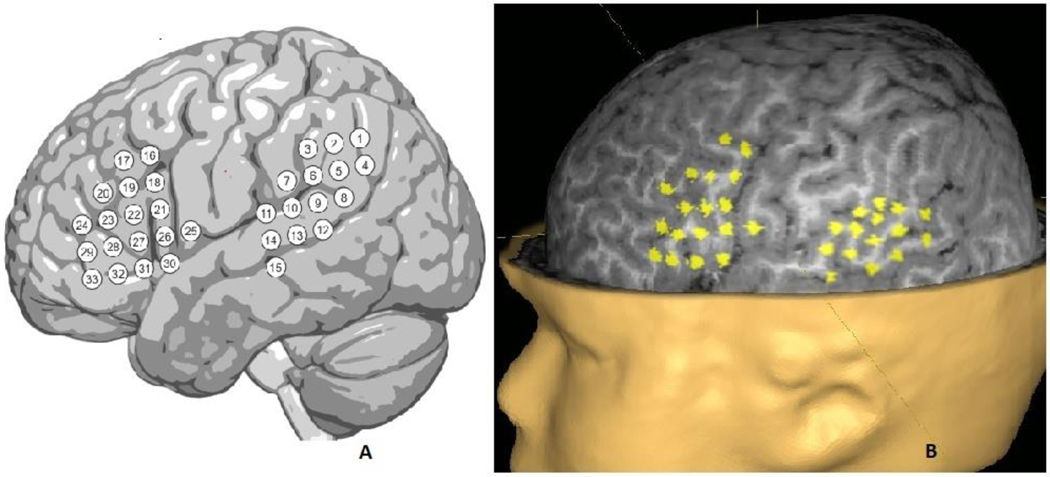
For each participant, multimodal radiographic markers were placed over nasion and preauricular landmarks to facilitate precise coregistration and TMS navigation. High-resolution (1mm isotropic) 3D T1-and T2-weighted volumes, and a diffusion-weighted set of volumes, were acquired on a research-dedicated 3.0T Philips Ingenia Elition scanner (Philips Medical Systems). Standard spatial coordinates corresponding to 33 locations in each hemisphere, to include canonical Broca and Wernicke areas, were marked as targets for stimulation (Fig 2). These positions were nonlinearly warped into participant space and represented as cylindrical visuals (long axis in y-plane), on the participant’s 3D-T1 using AFNI (Fig 2)(Cox, 1996). The resulting images were imported to the Nexstim workstation for co-registration (Nexstim NBS 5.1, Helsinki, Finland). Peeling depth was set at 20 mm at baseline, and modified as needed on an individual basis to visualize all cortical targets. Once set in preparation for stimulation, peeling depth was constant for each participant. A measured E-field (V/m) was resulted at this peeling depth for each stimulation; a mean E-field was calculated by averaging this result across all stimulations for each participant. A surface electromyogram (EMG) electrode (Neuroline 720; Ambu, Ballerup, Denmark) was placed on the belly of the right abductor pollicus brevis (APB) muscle. Co-registration and resting motor threshold (RMT) for the right APB muscle was performed standardly.(Takahashi et al., 2013) During motor mapping, subjects were encouraged to select and watch a video. This was stopped when language mapping was begun, but in some cases for younger children, breaks were employed during testing to increase acceptance of the procedure. In those breaks, the video was resumed.
Figure 2:
a) schematic showing stimulation targets over the left frontal and right temporal language areas b) peeled view of left hemisphere reconstructed brain at a peeling depth of 20 mm, showing the standard 33 stimulation targets locations used. Each site was stimulated three times.
2.3. Language mapping using rTMS
2.3.1. Visual naming
The visual naming task was chosen since it is the current paradigm recommended for clinical rTMS mapping, since it fits within time and space requirements for the rTMS burst and investigates several aspects of language in one task.(Krieg et al., 2017) For visual naming, a set of 137 color images of everyday visuals on a white background, validated in children for use in language tasks, were presented via a monitor.(Kadis et al., 2011) Those images that resulted in a reliable correct response within 1 second of presentation were kept and formed the baseline dataset. Stimulus intensity was set at 100% RMT or 50% maximum stimulator output (MSO), whichever was lower. Pictures were randomly presented for 1 second, in alternation with a black screen with an interpicture interval of 3 seconds. Using a cooled figure-8 configuration coil (Nexstim, Helsinki, Finland), bursts of pulses were triggered beginning 300 ms after the onset of each picture presentation, repeating at 5Hz and lasting 1–2 seconds depending on speed of participant on baseline. Stimulus intensity was adjusted based on participant feedback, with a lower limit of 35% MSO until tolerable and minimum electrical field strength of 100 V/m. For each cortical target (33 targets x 2 hemispheres), three pictures were presented.
2.3.2. Verb generation
The verb generation task was chosen as an adjunct that aligns with the preferred functional MRI and MEG task at our institution, for cross correlation in future experiments. The verb generation task consisted of listening to one or two-syllable nouns for which participants generated a corresponding transitive verb (e.g., cookie -> eat)(Kadis et al., 2011; Pang et al., 2011). The participant’s unique baseline list was adapted for presentation with triggered rTMS. On a parallel workstation, a custom Matlab (Mathworks, Natick, MA) script was used to trigger 5 Hz Nexstim bursts of 1–2 seconds. For the verb generation task, the noun presentation-trigger interval was 500 ms. For each cortical target (33 targets x 2 hemispheres), three nouns were presented.
2.4. Dataset completion
After each quadrant of brain (e.g. left hemisphere posterior 15 targets) was stimulated, the participant was asked whether they wished to continue. In the event of completion of motor threshold and bilateral hemisphere visual naming, this dataset was classified sufficient. In the event of completion of motor threshold and bilateral hemisphere visual naming and verb generation, this dataset was classified complete.
2.5. Measurement of pain intensity and unpleasantness
The participant provided formal ratings of pain using a previously validated Visual Analog Scale (VAS) (Price et al., 1994) at three points: after motor threshold determination and after visual naming for each hemisphere. Participants were introduced to the concepts of pain intensity and pain unpleasantness using the analogy of music volume for intensity and unpleasantness for pain(Price et al., 1983). Participants ≥11 years of age rated pain intensity and pain unpleasantness separately. Participants <11 years rated pain unpleasantness only. At each point, a 15cm long plastic sliding scale VAS was presented with the anchors of no pain intensity/not at all unpleasant at the left extreme, and most intense/unpleasant pain imaginable at the right extreme. Sliding the scale revealed a red bar to the participants and numbers ranging from 0–10 on the back to the experimenter. The participant moved the slider to the desired intensity/unpleasantness rating.
2.6. Annotation
For each subject with sufficient data (completed visual naming), two independent raters (of 4 possible assigned) performed assessment of each trial off-line on a dedicated workstation. Annotations were made based on an agreed-upon classification scheme, consisting of no error, error-semantic, error-performance, error-no response, error-other (included delayed responses).
2.7. Statistics
Descriptive statistics were performed in Excel (Microsoft, Redmond, WA) and Python (SCIKIT). The mean field (V/m) was calculated for all stimulations per participant. Student’s T-test was used to compare means in two sample comparisons. Fisher’s Exact test (2-sided) was used to compare categorical variables and outcome. ANOVA with assumed unequal variance was used to compare means in 3 or more group comparisons. A paired T-test was used to compare pain intensity and unpleasantness scores between hemispheres. The effects of multiple variables as a function of the number of rTMS bursts was determined using a Cox proportional hazards regression model (SAS® version 9.4, SAS Institute, Cary, NC). In order to achieve a parsimonious model, backward elimination variable selection was performed on the following independent variables in the model: age, CELF and WNV scores, RMT, stimulation intensity, mean E-Field and pain score. Only age remained in the regression model; thus the resulting survival curve of continued participation versus the number of rTMS bursts was evaluated at the mean age of the 49 participants. For annotations, subjects with a high error rate (>2 SD) were deemed to be unreliable and excluded from analysis. Interrater reliability was calculated using the Gwet agreement coefficient.(Gwet, 2008). Lateralization index has been used as a global measure of relative hemispheric contribution to the language network in rTMS, and can be correlated across modalities including fMRI and MEG.(Rezaie et al., 2020) Therefore, lateralization index was calculated on a range from −1 to 1, using the formula: −1(# right hemisphere errors) + (#left hemisphere errors)/total trials. The effect of stimulation intensity/RMT difference on number of naming errors was determined using a generalized linear regression model, including age in years, total number of visual naming errors and stimulation intensity/RMT difference. This variable was the calculated difference in percentage stimulator output subtracting RMT from stimulation intensity used for rTMS.
3. Results
Fifty-two participants were enrolled and studied with TMS. Twenty-two (42%) identified as African-American (n=20) or Mixed Race (n=2). Of the 52 participants, 49 attempted motor threshold and then consented to rTMS. The other 3 attempted motor threshold but did not proceed with rTMS. Of the 49 who underwent rTMS, RMT was obtained in 48. One participant tolerated stimulation up to 80% MSO with no MEP; therefore the RMT was marked conservatively as 80% MSO, and the participant continued participation. Sufficient datasets were performed in 35/49 (71%). Twenty-six of 49 (53%) completed all study procedures (Figure 1).
3.1. Demographic and neuropsychological predictors of rTMS completion
The mean age of rTMS participants (n=49) was 11.7 years. Non-white racial status (African-American or Mixed Race), younger age and lower language ability were associated with a lower likelihood to complete rTMS (Table 1). Using a backward elimination model, age and language ability were significant. The likelihood of completing rTMS was significantly lower in younger ages, with a likelihood ratio of 0.838 (95% CI 0.728–0.986). Likelihood ratio for lower CELF was 0.962 (95% CI 0.931–0.993). There was a trend toward lower RMT and mean stimulation intensity (% MSO) in the complete group, but this was not statistically significant.
Table 1:
Demographic and stimulation parameters and tolerability. RMT=resting motor threshold as a percentage of maximum stimulator output. Mean stim=Mean stimulation field, a figure which estimates the delivered current for each stimulus. WNV=Wechsler Nonverbal Scale. CELF=Clinical Evaluation of Language Fundamentals (5th edition) Standard Score. RMT pain=resting motor threshold pain score, scale 0–10 with 10 being highest/most intolerable.
| Age (yr)* | Gender (M:F) | Race (% non-white)# | RMT (%) | Stim Intensity (%) | Mean stim (V/m) | WNV | CELF& | RMT Pain | |
|---|---|---|---|---|---|---|---|---|---|
| Did not tolerate rTMS (n=14) | 10.9 | 6:8 | 12 (79%) | 60.5 | 40 | 110.4 | 102 | 97.3 | 1.5 |
|
| |||||||||
| Tolerated rTMS (n=35) | 12.1 | 19:16 | 8(23%) | 53 | 40 | 116.9 | 106.4 | 110 | 1.1 |
| Completed visual naming only (n=9) | 9.6 | 5:4 | 2(22%) | 60.1 | 39.1 | 122.8 | 104.5 | 111.3 | 1.0 |
| Completed visual naming and verb generation (n=26) | 12.9 | 14:12 | 6(23%) | 50.5 | 40 | 114.9 | 107 | 110 | 1.2 |
|
| |||||||||
| Total (n=49) | 11.7 | 25:24 | 20(41%) | 55.1 | 40 | 115 | 105.1 | 106.6 | 1.3 |
p=0.025, ANOVA
p<0.001, Fisher exact
p<0.001, ANOVA
Using a backwards elimination logistic regression model, age was significant (p=0.016, Chi-Square) and CELF standard scale was significant (p=0.017, Chi-Square) all others n.s.
3.6. Neurophysiology
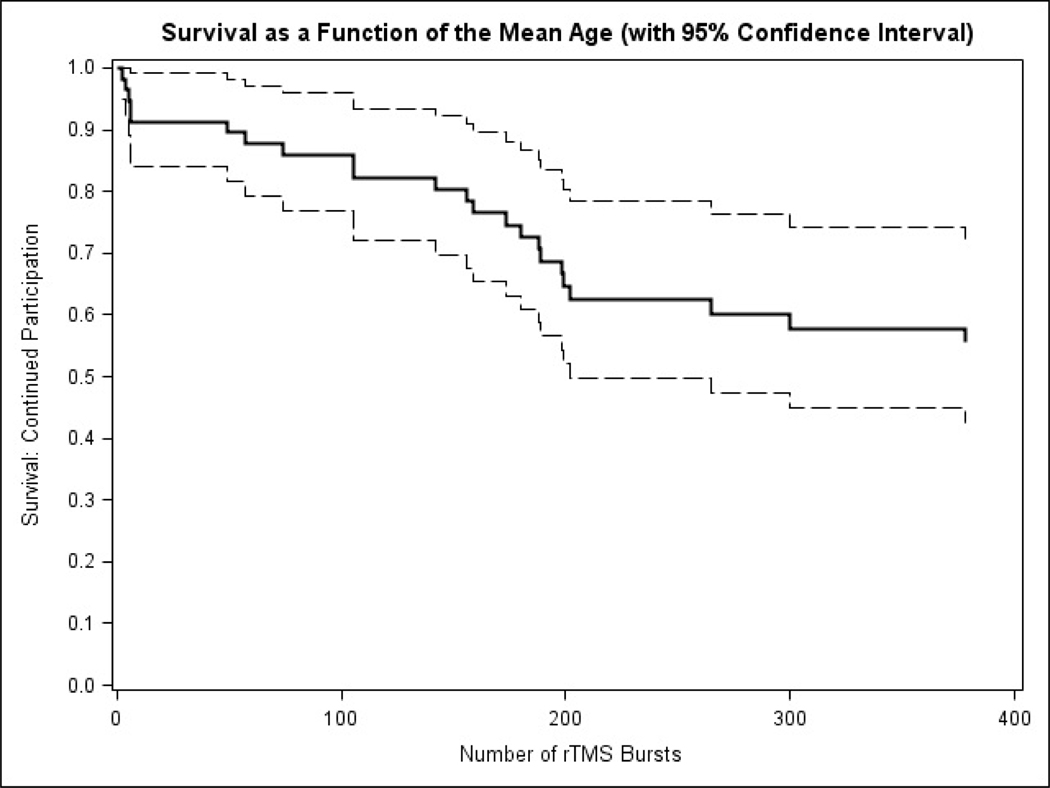
The mean tolerated stimulation intensity was 40% (range 35–52) with a mean E-field (per participant) of 115 (range 82–146) V/m. The number of bursts ranged from 2–456, with a mean of 278 (Fig 3). The RMT was not statistically different between groups, although there was a trend toward lower RMT in the complete group. Mean stimulation E-field and stimulation intensity during rTMS were no different between groups (Table 1). The average difference comparing rTMS intensity minus RMT was 12% (range −14 to 53), with RMT being greater in 25/35 (72% of cases).
Figure 3:
Survival curve as a function of the number of rTMS burst evaluated at the mean age of 11.7 years.
3.7. Pain measures
Of the 14 participants who did not complete a sufficient dataset, five did not participate past the test bursts designed to help gauge tolerable level of stimulation, citing poor tolerability. Nine underwent rTMS in the left hemisphere and were able to rate their experience. The mean pain unpleasantness score in this group was 6.1 (range 0.1–10, median 6).
Since 35 participants completed sufficient datasets, these participants were included in analysis of pain intensity and pain unpleasantness between hemispheres (Table 2). The mean pain unpleasantness score was 2.8 in each hemisphere (median 2.7 range 0–6.8 in left hemisphere, median 2.8 range 0–8.8 in right hemisphere). The mean pain intensity score was 3.3 in the left hemisphere (range 0.3–8.4) and 2.9 in the right hemisphere (range 0.3–8.2). There was no significant difference between hemispheres, and pain did not predict early dropout.
Table 2:
Pain and intensity scores per hemisphere for rTMS. Pain and intensity rating scores were recorded immediately after the hemisphere was mapped. Only participants >age 10 yr had intensity rating score recorded. All scores scaled 0–10 with 10 being highest/most intolerable.
| LH Pain | RH Pain | LH Intensity | RH Intensity | ||
|---|---|---|---|---|---|
|
| |||||
| Completed visual naming only (n=9) | 3.1 | 3.3 | Age>10, n=4 | 4.5 | 3.8 |
|
| |||||
| Completed visual naming and verb generation (n=26) | 2.7 | 2.6 | Age>10, n=21 | 3.1 | 2.8 |
|
| |||||
| Total (n=35) | 2.8 | 2.8 | Age>10, n=25 | 3.3 | 2.9 |
All comparisons n.s., 2 sample T-test
Comparing the 9 raters who did not tolerate rTMS with the raters who had sufficient datasets, those who did not tolerate TMS had a higher mean pain unpleasantness score in the left hemisphere of 6.1, compared to 2.8 in the sufficient group (p=0.015, Student’s T-test). )
Including all 44 left hemisphere pain measurements across all participants, the mean score was 3.5 (median 3).
3.8. Adverse events
Two patients reported headache which resolved within 30 minutes. No significant adverse events were reported.
3.9. Language mapping results
Out of a total 198 trials per subject (33 sites per hemisphere and 3 trials per site), the mean number of errors for visual naming was 13.7 (SD11); for verb generation the mean was 24.2 errors (SD18). Of 35 patients with sufficient datasets undergoing two-rater annotation, 1/35 (2.9%) was an 11 year old subject with > 2SD errors, and was removed from the analysis. Of the remaining 34, the range of errors recorded per annotator for visual naming was between 1 and 27 in left hemisphere and 0–25 in right hemisphere (Gwet agreement coefficient 0.884, 95% CI 0.842–0.926). The effect of stimulation intensity-RMT on visual naming errors was a modest increase in naming errors with increasing positive difference between rTMS intensity and RMT (Spearman rank 0.340, p=0.049). However, this relationship is explained by age, with younger patients having more errors (p=0.0001). With age in the model, there is no significant correlation between stimulation intensity-RMT difference and number of naming errors (p=0.772). Removing the subject with high error rate, there were 24 datasets annotated with verb generation. For verb generation, the range of errors was 0–45 in the left hemisphere and 0–44 in the right hemisphere (Gwet agreement coefficient 0.774, 95% CI 0.670–0.878). All subjects had at least one error recorded by at least one annotator. One subject had only a single error recorded by one annotator during visual naming. Lateralization index was 0.137 for visual naming and 0.083 for verb generation (weakly left lateralized).
4. Discussion
4.1. Overall safety and tolerability
This is the first study of rTMS language mapping tolerability in healthy children. We found that it is possible for children from a broad age range (5–18 years) to tolerate and complete this degree of detailed mapping. In this group of 49 children, 35 (71%) were able to tolerate bilateral mapping with visual naming; 26 (53%) were able to tolerate verb generation also. An additional 9 children, for a total of 44/49 (90%), were able to tolerate at least some left hemisphere mapping.
It is also notable that no serious adverse effects such as seizures occurred. The risk of seizures in repetitive TMS has been estimated only in the epilepsy population; Pereira et al estimated an incidence of atypical seizures of 0.2% across multiple studies.(Pereira et al., 2016) Atypical seizure presentation in an epileptic patient was considered more analogous to the risk of seizures in a non-epileptic patient.
4.6. Demographic predictors of tolerability
We found that younger age and lower language ability were significantly correlated with a lower chance of completing study procedures. Further, reported pain was higher in the participants who dropped out, indicating that a major reason for intolerability was pain. Another potential contribution to dropout is waning attention: the entire study procedure requires approximately 1.5 hours to complete and the repetition of tasks can be tedious.
Race (African-American or Mixed Race) was associated with a lower chance of study completion. This finding is difficult to interpret since other important demographic factors such as ethnicity, cultural affiliations, insurance, family education and income data were not collected. To reflect the racial distribution in our community, 42% of enrolled participants were African-American or Mixed Race.
4.7. Measures of rTMS pain intensity and pain unpleasantness: relevance of location
Even if tolerable and safe, almost all participants reported significant pain unpleasantness and intensity. The median pain score of 3 on the visual analog scale (VAS) observed in our study was actually significantly lower than the median of 4.9 reported in a study of adults.(Tarapore et al., 2016) Comparatively, the adult study had lower mean E-field strength and stimulation intensity.
In our extensive observation, the effect of repetitive stimulation on the temporalis and orbicularis oculi muscles appears to be the reason for the unpleasant sensation. Although motor stimulation is done using a single biphasic pulse, and therefore different than the 1–2 second burst delivered during rTMS, we believe that the higher pain unpleasantness and intensity scores during language mapping compared to motor stimulation are primarily related to location of stimulation. This idea is supported by several researchers who have used theta-burst technique over the supplementary motor area and rTMS with 4 second trains of 10 Hz stimulation over the dorsal prefrontal cortex. In these studies, retention and compliance was >90%. (MacMaster et al., 2019; Wu et al., 2014) Retention across all TMS studies is 91%, with dropout related to adverse effects of 2.5%.(Zis et al., 2020) Our study compares less favorably, since compliance with all study procedures was 50% (26/52). The major difference in our study is location of stimulation. In clinical practice, the typical strategy is to reduce stimulation intensity until it is tolerable.(Braden et al., 2022) However this may result in stimulation below the functional threshold, resulting in inaccurate language maps.
4.8. Effect of mapping parameters
There is insufficient pediatric data to guide effective mapping parameters. Babajani-Feremi and colleagues reported using an E-field of 80–100 V/m in over 70 patients, with the lowest recorded field to generate a speech interruption of 56 V/m.(Babajani-Feremi et al., 2016; Rezaie et al., 2020) Another study of three patients reported fields of 75–100 V/m.(Rejno-Habte Selassie et al., 2020) A study that included 14 pediatric patients reported fields ranging from 59–154 V/m.(Lehtinen et al., 2018) The adult literature generally favors using between 90–110% RMT for stimulation intensity, and 80–120 V/m E-field strength is a typical correlating field strength. (Klaus and Schutter, 2018) In our study the mean field across participants of 115 V/m. Since in our study the average RMT was 55% of MSO, a correlating field would in many cases be between 150–250 V/m and truly intolerable. Therefore, there is a discrepancy between the adult literature and pediatric studies, including the current study: for rTMS in children, the stimulation intensity is typically <90% RMT, particularly in younger children. We found an increase in visual naming errors with younger aged children. This may reflect a wider distributed language network in younger patients, a higher rate of false positive errors, or a combination.
This invites an important question: What is the negative predictive value of rTMS language mapping in children? In adults, it is reported to be 99–100% compared to the gold standard of cortical stimulation mapping, supporting the rTMS indication for diagnostic language mapping. (Krieg et al., 2014; Tarapore et al., 2016) The potential for below-threshold stimulation leading to a false negative result is higher in children given the gap between tolerable rTMS threshold and motor functional threshold.
4.9. Limitations and future directions
There are several limitations in this study. VAS was calculated at 3 time points during the study—a metric that would allow real-time report of discomfort may have better aided localization of pain and relevance to stimulation intensity/E-field strength. However, pain ratings alone may not be sufficient to predict tolerability of the procedure. Further, in order to maximize the number of participants who were able to provide a dominant hemisphere map, the left hemisphere was stimulated first for all subjects. Similarly, visual naming was performed first, rather than a randomized block design between visual naming and verb generation. This is a potential confounder, as fatigue, tolerability or perception of stimulation effects may have been affected by order of stimulation. Lastly, studies of rTMS in future should include additional socioeconomic status information including insurance, family and income data.
In the future, a study introducing an intervention that reduces pain and increases tolerability could be compared with current practice during rTMS language mapping. Such an intervention could include topical analgesia or behavioral modifications. Additional research determining the most effective stimulation parameters and language paradigms should be undertaken. Further, comparison of rTMS data to other noninvasive data may yield a developmental atlas of critical, overlapping brain regions involved in language networks.
5. Conclusions
Repetitive TMS (rTMS) language mapping is feasible for a wide pediatric age range—5–18 years in our study. We present the first study of bilateral hemispheric extensive mapping of healthy children using both visual naming and verb generation. Tolerability is a major factor limiting widespread implementation. Pain/unpleasantness score was clearly correlated with early dropout, though stimulation intensity was not different between those who completed and did not complete rTMS. Therefore, development of specific interventions which increase tolerability of stimulation will be important to increase utility and assure that an adequate functional threshold is met during stimulation.
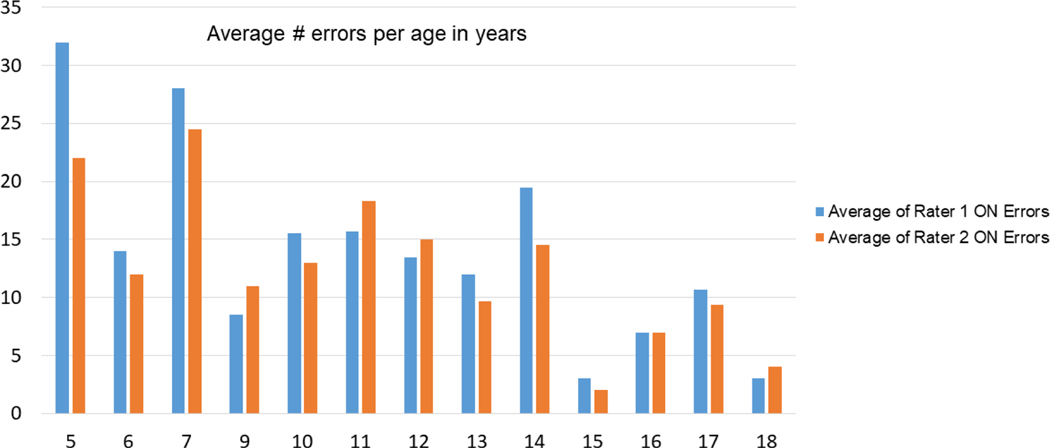
Figure 4.
Average number of errors with visual naming (y-axis) per age bin in years (x-axis). The two bars represent errors rated by each individual rater.
Highlights.
The first prospective study of language mapping using repetitive TMS in healthy children
Most (71%) of children completed stimulation bilaterally during object naming
Discomfort related to stimulation was the major factor associated with withdrawal
Acknowledgements
We want to acknowledge Drs. Don Gilbert and Steve Wu, for their insight into safe practices in TMS research. We acknowledge Dr. Katie Holland for serving as the safety monitor. We also acknowledge the support of the excellent epilepsy surgery team at Cincinnati Children’s Hospital.
Funding:
Research reported in this publication was fully supported by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institute of Health (NIH), under award R21 NS106631 (Greiner/Kadis). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Declarations
Declaration of interests: None of the authors have any conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Availability of Data and Material:
All source data is deidentified and available for review.
References
- Babajani-Feremi A, Holder CM, Narayana S, Fulton SP, Choudhri AF, Boop FA, Wheless JW, 2018. Predicting postoperative language outcome using presurgical fMRI, MEG, TMS, and high gamma ECoG. Clin Neurophysiol 129, 560–571. [DOI] [PubMed] [Google Scholar]
- Babajani-Feremi A, Narayana S, Rezaie R, Choudhri AF, Fulton SP, Boop FA, Wheless JW, Papanicolaou AC, 2016. Language mapping using high gamma electrocorticography, fMRI, and TMS versus electrocortical stimulation. Clin Neurophysiol 127, 1822–1836. [DOI] [PubMed] [Google Scholar]
- Braden AA, Weatherspoon SE, Boardman T, Williard T, Adkins A, Gibbs SK, Wheless JW, Narayana S, 2022. Image-guided TMS is safe in a predominately pediatric clinical population. Clin Neurophysiol 137, 193–206. [DOI] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Garvey MA, Ziemann U, Bartko JJ, Denckla MB, Barker CA, Wassermann EM, 2003. Cortical correlates of neuromotor development in healthy children. Clin Neurophysiol 114, 1662–1670. [DOI] [PubMed] [Google Scholar]
- Gwet KL, 2008. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol 61, 29–48. [DOI] [PubMed] [Google Scholar]
- Ille S, Sollmann N, Hauck T, Maurer S, Tanigawa N, Obermueller T, Negwer C, Droese D, Boeckh-Behrens T, Meyer B, Ringel F, Krieg SM, 2015. Impairment of preoperative language mapping by lesion location: a functional magnetic resonance imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation study. J Neurosurg 123, 314–324. [DOI] [PubMed] [Google Scholar]
- Jeltema HR, Ohlerth AK, de Wit A, Wagemakers M, Rofes A, Bastiaanse R, Drost G, 2021. Comparing navigated transcranial magnetic stimulation mapping and “gold standard” direct cortical stimulation mapping in neurosurgery: a systematic review. Neurosurg Rev 44, 1903–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadis DS, Pang EW, Mills T, Taylor MJ, McAndrews MP, Smith ML, 2011. Characterizing the normal developmental trajectory of expressive language lateralization using magnetoencephalography. J Int Neuropsychol Soc 17, 896–904. [DOI] [PubMed] [Google Scholar]
- Klaus J, Schutter D, 2018. Non-invasive brain stimulation to investigate language production in healthy speakers: A meta-analysis. Brain Cogn 123, 10–22. [DOI] [PubMed] [Google Scholar]
- Koh TH, Eyre JA, 1988. Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex. Arch Dis Child 63, 1347–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg SM, Lioumis P, Makela JP, Wilenius J, Karhu J, Hannula H, Savolainen P, Lucas CW, Seidel K, Laakso A, Islam M, Vaalto S, Lehtinen H, Vitikainen AM, Tarapore PE, Picht T, 2017. Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir (Wien) 159, 1187–1195. [DOI] [PubMed] [Google Scholar]
- Krieg SM, Tarapore PE, Picht T, Tanigawa N, Houde J, Sollmann N, Meyer B, Vajkoczy P, Berger MS, Ringel F, Nagarajan S, 2014. Optimal timing of pulse onset for language mapping with navigated repetitive transcranial magnetic stimulation. Neuroimage 100, 219–236. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Pobric G, Jefferies E, 2009. Conceptual knowledge is underpinned by the temporal pole bilaterally: convergent evidence from rTMS. Cereb Cortex 19, 832–838. [DOI] [PubMed] [Google Scholar]
- Lehtinen H, Makela JP, Makela T, Lioumis P, Metsahonkala L, Hokkanen L, Wilenius J, Gaily E, 2018. Language mapping with navigated transcranial magnetic stimulation in pediatric and adult patients undergoing epilepsy surgery: Comparison with extraoperative direct cortical stimulation. Epilepsia Open 3, 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster FP, Croarkin PE, Wilkes TC, McLellan Q, Langevin LM, Jaworska N, Swansburg RM, Jasaui Y, Zewdie E, Ciechanski P, Kirton A, 2019. Repetitive Transcranial Magnetic Stimulation in Youth With Treatment Resistant Major Depression. Front Psychiatry 10, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana S, Rezaie R, McAfee SS, Choudhri AF, Babajani-Feremi A, Fulton S, Boop FA, Wheless JW, Papanicolaou AC, 2015. Assessing motor function in young children with transcranial magnetic stimulation. Pediatr Neurol 52, 94–103. [DOI] [PubMed] [Google Scholar]
- Nettekoven C, Pieczewski J, Neuschmelting V, Jonas K, Goldbrunner R, Grefkes C, Weiss Lucas C, 2021. Improving the efficacy and reliability of rTMS language mapping by increasing the stimulation frequency. Hum Brain Mapp 42, 5309–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC, 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Pang EW, Wang F, Malone M, Kadis DS, Donner EJ, 2011. Localization of Broca’s area using verb generation tasks in the MEG: validation against fMRI. Neurosci Lett 490, 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LS, Muller VT, da Mota Gomes M, Rotenberg A, Fregni F, 2016. Safety of repetitive transcranial magnetic stimulation in patients with epilepsy: A systematic review. Epilepsy Behav 57, 167–176. [DOI] [PubMed] [Google Scholar]
- Price DD, Bush FM, Long S, Harkins SW, 1994. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain 56, 217–226. [DOI] [PubMed] [Google Scholar]
- Price DD, McGrath PA, Rafii A, Buckingham B, 1983. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 17, 45–56. [DOI] [PubMed] [Google Scholar]
- Rejno-Habte Selassie G, Pegenius G, Karlsson T, Viggedal G, Hallbook T, Elam M, 2020. Cortical mapping of receptive language processing in children using navigated transcranial magnetic stimulation. Epilepsy Behav 103, 106836. [DOI] [PubMed] [Google Scholar]
- Rezaie R, Schiller KK, Embury L, Boop FA, Wheless JW, Narayana S, 2020. The Clinical Utility of Transcranial Magnetic Stimulation in Determining Hemispheric Dominance for Language: A Magnetoencephalography Comparison Study. J Clin Neurophysiol 37, 90–103. [DOI] [PubMed] [Google Scholar]
- Rosenstock T, Picht T, Schneider H, Koch A, Thomale UW, 2019. Left perisylvian tumor surgery aided by TMS language mapping in a 6-year-old boy: case report. Childs Nerv Syst 35, 175–181. [DOI] [PubMed] [Google Scholar]
- Rosenstock T, Picht T, Schneider H, Vajkoczy P, Thomale UW, 2020. Pediatric navigated transcranial magnetic stimulation motor and language mapping combined with diffusion tensor imaging tractography: clinical experience. J Neurosurg Pediatr 26, 583–593. [DOI] [PubMed] [Google Scholar]
- Schwarzer V, Bahrend I, Rosenstock T, Dreyer FR, Vajkoczy P, Picht T, 2018. Aphasia and cognitive impairment decrease the reliability of rnTMS language mapping. Acta Neurochir (Wien) 160, 343–356. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Vajkoczy P, Picht T, 2013. Navigated transcranial magnetic stimulation for mapping the motor cortex in patients with rolandic brain tumors. Neurosurg Focus 34, E3. [DOI] [PubMed] [Google Scholar]
- Tarapore PE, Findlay AM, Honma SM, Mizuiri D, Houde JF, Berger MS, Nagarajan SS, 2013. Language mapping with navigated repetitive TMS: proof of technique and validation. Neuroimage 82, 260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarapore PE, Picht T, Bulubas L, Shin Y, Kulchytska N, Meyer B, Berger MS, Nagarajan SS, Krieg SM, 2016. Safety and tolerability of navigated TMS for preoperative mapping in neurosurgical patients. Clin Neurophysiol 127, 1895–1900. [DOI] [PubMed] [Google Scholar]
- Wechsler D, & Naglieri JA , 2006. Wechsler nonverbal scale of ability. . Pearson, San Antonio TXSW, Maloney T, Gilbert DL, Dixon SG, Horn PS, Huddleston DA, Eaton K, Vannest J, 2014. Functional MRI-navigated repetitive transcranial magnetic stimulation over supplementary motor area in chronic tic disorders. Brain Stimul 7, 212–218. [DOI] [PubMed] [Google Scholar]
- Zis P, Shafique F, Hadjivassiliou M, Blackburn D, Venneri A, Iliodromiti S, Mitsikostas DD, Sarrigiannis PG, 2020. Safety, Tolerability, and Nocebo Phenomena During Transcranial Magnetic Stimulation: A Systematic Review and Meta-Analysis of Placebo-Controlled Clinical Trials. Neuromodulation 23, 291–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All source data is deidentified and available for review.