Abstract
Purpose of Review:
The prevalence and incidence of allergic disease have been rising in Westernized countries since the 20th century. Increasingly, evidence suggests that damage to the epithelium initiates and shapes innate and adaptive immune responses to external antigens. The objective of this review is to examine the role of detergents as a potential risk factor for developing allergic disease.
Recent Findings:
Herein, we identify key sources of human detergent exposure. We summarize the evidence suggesting a possible role for detergents and related chemicals in initiating epithelial barrier dysfunction and allergic inflammation. We primarily focus on experimental models of atopic dermatitis, asthma, and eosinophilic esophagitis, which show compelling associations between allergic disease and detergent exposure. Mechanistic studies suggest that detergents disrupt epithelial barrier integrity through their effects on tight junction or adhesion molecules and promote inflammation through epithelial alarmin release.
Summary:
Environmental exposures that disrupt or damage the epithelium may account for the increasing rates of allergic disease in genetically susceptible individuals. Detergents and related chemical compounds represent possible modifiable risk factors for the development or exacerbation of atopy.
Keywords: detergent, surfactants, epithelium, atopy, barrier
Introduction
Allergies are among the most common chronic diseases in industrialized nations and incorporate manifestations at nearly every skin and mucosal surface of the human body. In the United States (US), approximately 30% of the population reports at least one form of atopy. Allergic rhinitis is the most common allergic disease affecting 18.9% of children and 25.7% of adults [1]. Thirteen percent of the US population has asthma [2] and up to 8% of children have food allergy [3]. Alarmingly, rates of allergic disease are increasing, particularly among children. For example, food allergy prevalence increased 50% among children 0-17 years old from 1997-2011 in the US. This increase is paralleled by an almost 70% increase in skin allergies over the same timeframe [4]. Recent decades have also seen the emergence of new allergic diseases such as eosinophilic esophagitis (EoE). First described in the 1990’s [5, 6], this disease now has an estimated prevalence of 0.5-1:1000 and is the leading cause of food impaction in adults [7–9].
The etiology of atopic disease is multifactorial; however, the environment likely plays a significant role. Increases in allergic disease have been attributed to changes in the microbiome linked with the transition from an agrarian lifestyle to living in industrialized urban settings (i.e., hygiene or old friends hypothesis) [10, 11]. As an extension, several alterations in microbial exposure may lead to dysbiosis and have been linked with increased risk of atopy, including the following: antibiotic use, Cesarean delivery, lack of breastfeeding, and changes in food processing [12–14]. Climate change (e.g., increased pollen season duration due to warming temperatures) and environmental pollutants (e.g. microplastics) are additional factors of interest. Mechanistic investigations have identified the epithelium at the center of the immune alterations associated with allergic disease [15, 16]. Indeed, many of the same pathways and disease processes implicated in atopic dermatitis also play a role in asthma. Moreover, a disrupted epithelium provides a logical starting point for understanding the immune dysregulation characteristic of atopy that likely begins as an immuno-protective response. Consequently, it is critical to evaluate the environmental exposures, which may initiate epithelial barrier dysfunction.
Since the 1940’s, detergents have been incorporated into household products such as laundry detergents, industrial cleaners, shampoos, and toothpaste [17]. These products were historically evaluated for their acute toxicity, but their chronic effects on the epithelial barrier are increasingly appreciated. Pothoven and Schleimer proposed the barrier hypothesis, which postulates that allergic sensitization and type 2 inflammation result from epithelial barrier dysfunction and inappropriate exposure to the environment [18]. Akdis et al, extended the barrier theory to suggest that detergents and other compounds provoke epithelial barrier dysfunction and promote dysbiosis and translocation of microbiota through the epithelium [16].
Herein, we provide background information on common household detergents and review epidemiologic and mechanistic studies examining detergents and related compounds as risk factors for allergic disease.
Detergents
History and Definition
Detergents belong to a class of surfactants characterized by an amphiphilic structure with polar and nonpolar moieties. The polar moiety is hydrophilic and nonpolar moiety is hydrophobic, facilitating the mixture of hydrophobic compounds (e.g., oil) with water [17]. Detergents form micelles in water above specific concentrations (i.e. critical micelle concentration, CMC), which can remove grease. Most surfactants employed in everyday household use are anionic, but surfactants may also be cationic, nonionic, or amphoteric.
Detergents are synthetic soap substitutes that were developed for cleaning applications due to critical shortages of the natural fats required to make soap during World War I. German chemists alkylated naphthalene and polynuclear aromatics from coal tar with short-chain and fatty alcohols to produce alkylaromatics which were then sulfonated to produce surfactants. Alkylene sulfates presented unique advantages to traditional soap because they are soluble in hard water and do not leave scum. Further advancements by the chemicals industry led to the production of the first alcohol sulfates in the 1930’s from coconut and palm kernel oil derivatives. Refinements in the petrochemicals industry led to the production of branched chain alkyl benzenes, which were incorporated into laundry detergents in the 1940’s-1950’s. However, branched chain alkyl benzenes were found to be poorly biodegradable, leading to the development of linear alkyl sulfates, including sodium lauryl sulfate (SLS also known as sodium dodecyl sulfate or SDS) and sodium dodecyl benzenesulfonate (SDBS) [19]. These are the most common detergents used in commercial household products and are the focus of recent investigations into the potential link between detergents and atopic disease.
Linear alkyl sulfates have been assessed for safety, and SDS and SDBS are generally considered safe at exposure levels commonly found in the household. Indeed, SDS is even approved as a food additive at low concentrations [20]. Previous assessments have identified these detergents as irritants. Unfortunately, these studies generally focus on toxicity and mutagenicity and do not assess effects on mucosal barrier function or alterations to microbiota or the potential effects of early-life exposure [21].
Sources and Routes of Exposure
Surfactants have been incorporated into most of the cleaning and personal care products used daily. According to a database of 97,370 active personal and home care products, unmodified SDS is contained in 3,990 products. Modified sulfate surfactants are present in a total of 10,564 products [22]. This ubiquitous exposure begins at birth during neonatal resuscitation with laundered cloths or blankets, followed by swaddling and clothing, and then with an infant’s first bath. Table 1 lists common routes and sources of human exposure to detergents.
Table 1.
Routes and sources of human detergent exposure
| Route of Exposure | Surfactant Source or Product |
|---|---|
|
| |
| Skin | Laundry detergent |
| Dishwashing detergent | |
| Hard surface cleaning agents | |
| Detergent residue in clothing | |
| Bubble bath | |
| Cosmetics | |
| Shampoo | |
| Bar and hand soap | |
| Shaving cream | |
|
| |
| Inhalation | Laundry detergent dust |
| Surface cleaning sprays | |
|
| |
| Oral | Toothpaste |
| Medications | |
| Residue on fruits and vegetables | |
| Processed foods (e.g., dairy and baked goods) | |
| Residue on utensils and dishware | |
| Drinking water | |
Effects on Barrier Function
SDS can penetrate the skin [23], enhances intestinal permeability [23], and has been used to facilitate uptake of pharmacological agents [24]. Detergents are capable of solubilizing plasma membranes [25, 26] through saturation of the plasma membrane with detergent monomers followed by fragmentation and release of mixed micelles comprised of detergent monomers and membrane lipids [27]. Notably, SDS can also alter protein structure and function [28, 29].
Association with antigen sensitization
Some detergents can elicit an allergic response; however, SDS and SDBS are largely considered irritants that do not provoke direct sensitization. On the other hand, SDS may serve as a vehicle (skin penetrating/irritant agent) or adjuvant, facilitating sensitization to nickel and chrome when painted on the skin only when mixed with 1% SDS [30]. Indeed, SDS has since been used in multiple skin tests for allergens or irritants in humans, specifically to enhance skin permeability and aid in assessment of sensitization [31]. Recent studies suggest SDS can lower the sensitization threshold by up to a factor of 10 for a weak sensitizer [32]. Studies in mice indicate that SDS can enhance local lymph node responses to sensitizers, possibly by increasing dendritic cell migration [33, 34]. Another noteworthy study showed that mice injected subcutaneously with 1-10 mg/L SDS with ovalbumin (OVA) promoted anti-OVA IgE production [35]. Interestingly, rat peritoneal mast cells release up to 85% of stored histamine at SDS concentrations as low as 0.03 mM [36].
Other cofactors may play an important role. Notably, sensitivity to isothiazolinone, which is often found in liquid laundry detergents as a preservative, is extremely common [37, 38]. It is unclear if this may be linked with co-exposure to surfactants such as SDBS.
Organ-specific Effects
Skin
The irritant properties of SDS on the skin are well appreciated. In fact, SDS serves as a prototypical irritant for pharmacologic investigations of anti-inflammatory topicals and is utilized in routine patch testing to assess skin irritability [39]. SDS causes lipid extraction and swelling in exposed skin [40, 41] and increases absorption of hydrophilic compounds, in particular [42]. Investigations with confocal Raman and infrared microspectroscopy have revealed penetration of SDS to the stratum corneum and, with longer incubation, to the dermal region, suggesting SDS may directly interact with keratinocytes [43]. Penetration of the stratum corneum has also been shown by radiolabeled probe assays [44, 45]. SDS activates the NLRP3 inflammasome and induces caspase-dependent IL-1β secretion by human keratinocytes [46]. Additionally, SDS increases intracellular reactive oxygen species (ROS) and IL-1α secretion by human keratinocytes by increasing intracellular calpain activity and intracellular Ca2+ concentration, promoting prostaglandin E3 (PGE3) release [47, 48]. Notably, SDS also alters protease expression and S100 proteins, including filaggrin, in keratinocytes [49].
Atopic dermatitis
The role of detergents such as SDS and SDBS in promoting atopic dermatitis is unclear; however, clinical studies have shown that atopic dermatitis patients are more susceptible to SDS-induced irritant dermatitis [50] and show increased transepidermal water loss in response to SDS. Moreover, detergent use can trigger flares of atopic dermatitis [51]. In vitro studies identified both SDS and SDBS as having cytotoxic effects on human keratinocytes [52]. Notably, in air-liquid interface (ALI) cultures, both SDS and SDBS affected barrier function at nontoxic doses, reducing transepithelial electrical resistance (TEER) and increasing FITC-dextran permeability. These results were also observed using commercial detergents at a 106 dilution. Moreover, alterations in tight junction proteins claudin-1 and occludin were variously observed with SDS, SDBS, and commercial detergents. Together, these results suggest anionic surfactants can influence skin barrier function, opening the possibility of increased penetration of toxicants and allergens, which may contribute to atopic dermatitis.
Contact Dermatitis
Contact dermatitis encompasses irritant contact dermatitis (ICD: ~80% cases) and allergic contact dermatitis (ACD: ~20%) along with photocontact dermatitis and contact urticaria. ICD irritants can cause damage on the first exposure (i.e. an adaptive immune response is not required), whereas ACD is a type IV delayed hypersensitivity reaction requiring sensitization. SDS is considered an irritant; however, it is commonly used in testing for ACD to facilitate penetration of the skin by potential allergens. For example, a 1955 study found that 1% SDS painted on guinea pig skin could provoke a response to nickel and chrome. Specifically, fur-clipped guinea pig skin was painted with nickel or chrome in the presence or absence of 1% SDS for 8 days and then challenged at day 12. The authors found that, in the absence of SDS, nickel and chrome produced mild to no reactions. However, in the presence of SDS, they observed scaling, erythema, and inflammation. The authors concluded that the eczematogenic properties of the metals were “brought about by the permeability of the skin increasing under the influence of lauryl sulphate” [30].
SDS has also be used to enhance sensitization in humans. Specifically, various methods of altering inflammation were tested to maximize contact sensitization, including tape stripping, UV exposure, cantharidin blistering, freezing, dimethylsulfoxide (DMSO), and SDS. Of all methods tested, SDS was the most effective at inducing contact sensitization [53]. Many early studies are now considered unethical as experiments were performed on a prison population without appropriate respect or protections for the subjects. SDS appears to have a synergistic effect with nickel likely due to increased penetration and proinflammatory effects that lower the threshold for elicitation of an allergic response [54].
Other detergents are more clearly linked with ACD. Namely, cocamidopropyl betaine, the ingredient in no-tears formulations of shampoo, was named Allergen of the Year in 2004 by the American Contact Dermatitis society due to its propensity to induce type IV contact hypersensitivity reactions in up to 7.2% of exposed individuals [55, 56].
Lung
Asthma
Defective epithelial barrier function is associated with asthma [57–59]. Several studies show an increased incidence of allergic respiratory symptoms and risk of asthma development due to occupational exposure to detergents and cleaning products (e.g., detergent factory workers and domestic housekeepers) [60–66]. Most of these reports are related to sensitization to enzymes as components of commercial detergents.
Recently, Wang et al. [67] investigated the effects of SDBS on the human respiratory epithelium using ALI cultures. The goal of this study was to test the effects of laundry detergents on human bronchial epithelial cell barrier function and identify changes in the transcriptomic and epigenomic signatures following detergent exposure. The authors found that the SDBS concentration in rinse residue was approximately a 1:2,500 dilution of the laundry detergent. Using this concentration, they demonstrated cytotoxic and barrier-disruptive effects on human bronchial epithelial cells in vitro. This was evidenced by decreased tight junction protein expression (occludin and zonulin-1), decreased TEER, and increased FITC-dextran flux in ALI cultures. No significant epigenetic changes were identified, and RNAseq revealed downregulation of genes related to cell adhesion and upregulation of genes related to lipid metabolism and apoptosis. Using monolayer cultures, they showed dose-dependent cytotoxicity with two different commercial detergents at dilutions less than 1:20,000-1:40,000. Even at nontoxic detergent concentrations, the authors noted decreased staining intensity of junctional proteins by immunofluorescence. Interestingly, these findings were reproducible with a 1:10 dilution of residual liquid from laundered clothing. These observations suggest that SDBS at low concentrations compromises the barrier function of airway epithelial cells and alters their gene expression.
Gastrointestinal Tract
Oral Ulcers and Oral Allergy Syndrome
SDS is a common component of toothpaste at concentrations up to 3% w/v and can be found in mouthwash as well. As toothbrushing is typically performed multiple times daily for several minutes, the oral mucosa is one of the most highly exposed tissues to SDS. SDS increases oral mucosa permeability in rabbits [68] and has been linked with oral ulceration [69]. Oral desquamation from toothpaste was noted in 1972 [70]. Later studies found that SDS could mediate this activity [71, 72]. A double-blind, cross-over clinical study of 28 females found that toothpaste containing as little as 0.5% SDS significantly increased oral desquamation relative to detergent-free toothpaste or toothpaste containing a less hydrophilic detergent, cocoamidopropyl-betaine [71]. Interestingly, one study found that 1% SDS in mouthwash reduced salivary bacterial counts, which remained significant at 7 hours post rinse. This antimicrobial activity was greater than 0.2% triclosan [73]. In addition, SDS is reported to have direct antimicrobial properties [74, 75]. Specifically, SDS may form pores in the lipid membranes of gram-positive organisms. Gram-negative organisms appear to be resistant to this bacteriostatic property [76, 77]. The significance of the sensitivity to SDS in the oral cavity and whether there could be any link to allergic sensitization and/or oral allergy syndrome requires investigation.
Eosinophilic Esophagitis
Recent investigations in our laboratory revealed that ALI cultures of human esophageal epithelium (i.e., EPC2 cells) exposed to 5,000 ng/mL SDS (~1:600 dilution of the SDS concentration in toothpaste) decreased TEER and increased passage of 4 kDa FITC-dextran, indicating induction of barrier dysfunction. Moreover, expression of DSG-1, an epithelial junction protein dysregulated in active EoE subjects, was markedly decreased while expression of the epithelial alarmin IL-33 was increased. IL-33 can potentiate type 2 immune responses and is upregulated in active EoE [78]. Further, mice exposed to 0.5% SDS in drinking water (~1/6th the concentration in toothpaste) for two weeks exhibited striking EoE-like pathology including eosinophilia, eosinophilic abscesses, dilated intracellular spaces, and basal zone hyperplasia. Interestingly, infiltration of CD4+ cells was increased in the esophagus, and RNAseq pathway analysis on whole esophagus homogenates indicated increased innate and adaptive immune responses along with responses to external biotic stimuli [79]. In unpublished studies, we have found marked alterations of the esophageal microbiome of mice in response to oral SDS exposure. Future studies are needed to understand the extent and contribution of dysbiosis to the observed pathology and to explore the potential for sensitization to food components. Concerningly, we were not the first to note the potential hazards of oral exposure to detergents. In 1939, Epstein et al. noted gastrointestinal irritation of the esophagus and forestomach in rats exposed to low concentrations of SDS over the course of 7 weeks, including edema, loss of keratin, and leukocytic infiltration, warning of the potential injurious effects of replacing traditional soap with SDS in toothpastes [80].
Household cleaning products often contain detergents mixed with other types of cleaning agents. Thus, it may be critically important to evaluate the sensitization potential in combination with proteases. Notably, Tanzer et al [81] found that laundry detergent containing microbial proteases damaged the skin barrier in mice, and, when combined with OVA, epicutaneous exposure (~10% concentrated laundry detergent), induced sensitization to OVA. Markedly increased IL-33 expression was noted in the skin, and sensitized mice challenged intranasally with OVA alone showed eosinophilic inflammation in the esophagus.
Intestinal permeability and food allergy
Ethoxylated alcohols are nonionic surfactants and are a component of rinse aids commonly used in commercial washers to leave dishes with a clean appearance. A recent study by Ogulur et al [82] found that human gut epithelial cell lines exposed to diluted rinse aid showed evidence of toxicity at levels similar to those present on cleaned dishes (e.g., 1:10,000). Moreover, evidence of barrier disruption was observed at a 1:40,000 dilution. Specifically, monolayer cultures of Caco-2 or HT-29 cells exposed to dilutions of rinse aid revealed cellular lysis at dilutions as low as 1:20,000. Liquid-liquid interface cultures of differentiated Caco-2 cells and organoid cultures responded similarly. Rinse aid was also found to induce barrier disruption with decreased TEER following five days of exposure to a 1:10,000 dilution. Increased FITC-dextran permeability and altered immunofluorescent staining of claudin-1 and occludin were observed at three days at a 1:20,000 dilution. Similar results showing barrier disruption were observed using Caco-2 cells in a gut-on-a-chip model. Finally, transcriptome analysis of Caco-2 monolayer cultures exposed to rinse aid showed altered expression of epithelial junction proteins. In addition, pathway analysis showed upregulation of proinflammatory, pattern recognition, and cytokine pathways. Similar results were observed by proteomic analysis. Strikingly, rinse aid residues extracted from cups washed in a professional dishwasher induced toxicity to Caco-2 cells even at a 1:10 dilution, while a 1:5 dilution impaired barrier function.
Notably, the authors also found that 1:20,000 dilutions of multiple common household dishwasher detergents also induced toxicity to Caco-2 cells though this is a higher concentration than the 1:80,000 dilution commonly used in dishwashers. These dishwasher detergents may contain SDS, which was also found to affect barrier function in Caco-2 cell cultures.
Together, these results suggest the gut epithelial barrier is susceptible to commonly used detergents, indicating the potential for barrier dysfunction, increased allergen exposure, and effects on chronic inflammation of the gut.
Conclusions
Allergic disease has increased markedly since the mid-20th century. Although the etiology of allergic disease is unknown, the environment plays a prominent role in disease pathogenesis. Coincident to increases in atopic disease, is the widespread use of detergents in household and personal care products in industrialized nations. The epithelium is central to the pathogenesis of allergic diseases. Common household detergents induce epithelial barrier dysfunction in the skin, lung, and gastrointestinal tract. While detergents are widely considered irritants, they appear to lower the threshold for sensitization, possibly by enhancing epithelial permeability and inducing inflammatory responses. In addition to these direct effects on the epithelium, detergents induce dysbiosis, which is associated with allergic disease. Pervasive and perpetual exposures position detergents prominently among environmental pollutants that may provoke barrier dysfunction. Figure 1 summarizes the potential role of detergents in allergic disease pathogenesis.
Figure 1. Detergent exposure model.
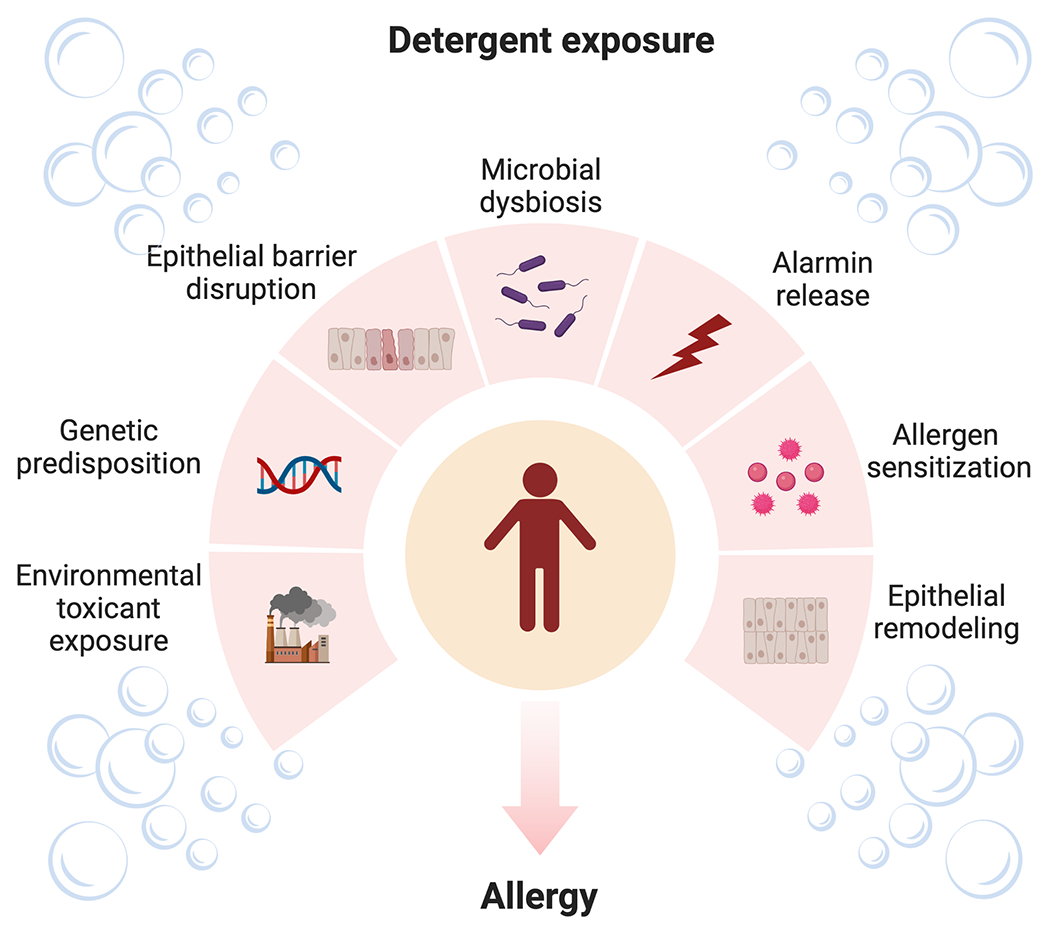
Exposure to detergents and other environmental toxicants, particularly in genetically susceptible individuals, results in epithelial barrier dysfunction, microbial dysbiosis, and alarmin release. This milieu promotes allergic sensitization, epithelial remodeling, and allergic disease.
Additional research is needed to better understand the immunologic and molecular mechanisms involved in detergent-induced barrier dysfunction and inflammation. Studies examining each epithelial surface is needed to understand how timing, route, and co-exposure with potential allergens promote allergic disease. This knowledge must be combined with research on gene-environment interactions as detergent exposure is common, but not all those exposed develop atopy. Further work investigating the effects of other chemical compounds with surfactant properties (i.e., emulsifiers) is needed. It will also be important to examine whether detergent-induced dysregulated barrier function, immunity, and the microbiome possibly contribute to other forms of immune mediated disease, such as autoimmunity.
Acknowledgments:
We would like to thank Huijun Luo, PhD, Arina Putikova, and Jessica Gibson for their scientific contributions to EoE studies referenced in this article. Figure 1 created with BioRender.com
Funding
This work was supported by the Donald R. Levin Family Foundation, M.Y.M. is a member of the Immunology Graduate Program and is supported by the Mayo Clinic Graduate School of Biomedical Sciences. B.L.W. also reports funding from NIH (K23AI158813-01). H.K. was supported by funding from NIH (R37AI71106, R01AI128729).
Abbreviations used:
- ALI
Air-liquid interface
- ACD
allergic contact dermatitis
- CMC
critical micelle concentration
- DMSO
dimethylsulfoxide
- EoE
eosinophilic esophagitis
- FITC
fluorescein isothiocyanate
- ICD
irritant contact dermatitis
- IgE
immunoglobulin E
- OVA
ovalbumin
- PGE3
prostaglandin E3
- ROS
reactive oxygen species
- SDBS
sodium dodecyl benzene sulfonate
- SDS
sodium dodecyl sulfate
- SLS
sodium lauryl sulfate
- TEER
transepithelilal electrical resistance
- US
United States
Footnotes
Disclosure of Potential Conflicts of Interest: Mayo Clinic and Dr. Yiannias have a financial relationship with SkinSAFE.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References:
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Centers for Disease Control and Prevention, National Center for Health Statistics. FastStats: Allergies. 2021. Accessed April 21, 2023; Available from: https://www.cdc.gov/nchs/fastats/allergies.htm.
- 2.Centers for Disease Control and Prevention; Asthma, National Health Interview (NHIS) Data. 2020. Accessed April 21, 2023; Available from: https://www.cdc.gov/asthma/nhis/2020/table2-1.htm.
- 3.Gupta RS, et al. , The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics, 2011. 128(1): p. e9–17. [DOI] [PubMed] [Google Scholar]
- 4.Jackson KD, Howie LD, and Akinbami LJ, Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief, 2013(121): p. 1–8. [PubMed] [Google Scholar]
- 5.Attwood SE, et al. , Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci, 1993. 38(1): p. 109–16. [DOI] [PubMed] [Google Scholar]
- 6.Straumann A, et al. , [Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings]. Schweiz Med Wochenschr, 1994. 124(33): p. 1419–29. [PubMed] [Google Scholar]
- 7.Dellon ES and Hirano I, Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology, 2018. 154(2): p. 319–332 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attwood SE and Furuta GT, Eosinophilic esophagitis: historical perspective on an evolving disease. Gastroenterol Clin North Am, 2014. 43(2): p. 185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spechler SJ, Konda V, and Souza R, Can Eosinophilic Esophagitis Cause Achalasia and Other Esophageal Motility Disorders? Am J Gastroenterol, 2018. 113(11): p. 1594–1599. [DOI] [PubMed] [Google Scholar]
- 10.Strachan DP, Hay fever, hygiene, and household size. BMJ, 1989. 299(6710): p. 1259–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rook GA, et al. , Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Semin Immunopathol, 2004. 25(3–4): p. 237–55. [DOI] [PubMed] [Google Scholar]
- 12.Cho I and Blaser MJ, The human microbiome: at the interface of health and disease. Nat Rev Genet, 2012. 13(4): p. 260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascal M, et al. , Microbiome and Allergic Diseases. Front Immunol, 2018. 9: p. 1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myles IA, Allergy as a Disease of Dysbiosis: Is It Time to Shift the Treatment Paradigm? Front Cell Infect Microbiol, 2019. 9: p. 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellings PW and Steelant B, Epithelial barriers in allergy and asthma. J Allergy Clin Immunol, 2020. 145(6): p. 1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akdis CA, Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol, 2021. 21(11): p. 739–751. [DOI] [PubMed] [Google Scholar]
- 17.Singer MM and Tjeerdema RS, Fate and effects of the surfactant sodium dodecyl sulfate. Rev Environ Contam Toxicol, 1993. 133: p. 95–149. [DOI] [PubMed] [Google Scholar]
- 18.Pothoven KL and Schleimer RP, The barrier hypothesis and Oncostatin M: Restoration of epithelial barrier function as a novel therapeutic strategy for the treatment of type 2 inflammatory disease. Tissue Barriers, 2017. 5(3): p. e1341367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levinson MI, Surfactant Production : Present Realities and Future Perspectives, in Handbook of Detergents: Part F: Production (1st ed.), Zoller U and Sosis P, Editors. 2008, CRC Press. 10.1201/9781420014655. [DOI] [Google Scholar]
- 20.National Archives, Code of Federal Regulations, Title 21, Chapter I, Subchapter B, Part 172, Subpart I, § 172.822, Sodium lauryl sulfate. Accessed April 21, 2023. Available from: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-172/subpart-I/section-172.822.
- 21.HERA Substance Team. Human & Environmental Risk Assessment (HERA) on ingredients of European household cleaning products: Alcohol Sulphates Human Health Risk Assessment. 2002. Accessed March 17, 2022; p. 122. Available from: https://www.heraproject.com/files/3-HH-04-%20HERA%20AS%20HH%20web%20wd.pdf.
- 22.SkinSAFE. Accessed April 24, 2023; Available from: https://www.skinsafeproducts.com/.
- 23.Narkar Y, et al. , Evaluation of mucosal damage and recovery in the gastrointestinal tract of rats by a penetration enhancer. Pharm Res, 2008. 25(1): p. 25–38. [DOI] [PubMed] [Google Scholar]
- 24.de Freitas Araujo Reis MY, et al. , A General Approach on Surfactants Use and Properties in Drug Delivery Systems. Curr Pharm Des, 2021. 27(42): p. 4300–4314. [DOI] [PubMed] [Google Scholar]
- 25.Keller S, et al. , Thermodynamics of lipid membrane solubilization by sodium dodecyl sulfate. Biophys J, 2006. 90(12): p. 4509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.le Maire M, Champeil P, and Moller JV, Interaction of membrane proteins and lipids with solubilizing detergents. Biochim Biophys Acta, 2000. 1508(1-2): p. 86–111. [DOI] [PubMed] [Google Scholar]
- 27.Juan-Colas J, et al. , The Mechanism of Vesicle Solubilization by the Detergent Sodium Dodecyl Sulfate. Langmuir, 2020. 36(39): p. 11499–11507. [DOI] [PubMed] [Google Scholar]
- 28.Winogradoff D, John S, and Aksimentiev A, Protein unfolding by SDS: the microscopic mechanisms and the properties of the SDS-protein assembly. Nanoscale, 2020. 12(9): p. 5422–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otzen DE, et al. , How do surfactants unfold and refold proteins? Adv Colloid Interface Sci, 2022. 308: p. 102754. [DOI] [PubMed] [Google Scholar]
- 30.Nilzen A and Wikstrom K, The influence of lauryl sulphate on the sensitization of guineapigs to chrome and nickle. Acta Derm Venereol, 1955. 35(4-5): p. 292–9. [PubMed] [Google Scholar]
- 31.Kligman AM, The identification of contact allergens by human assay. II. Factors influencing the induction and measurement of allergic contact dermatitis. J Invest Dermatol, 1966. 47(5): p. 375–92. [DOI] [PubMed] [Google Scholar]
- 32.De Rentiis AMA, et al. , Assessment of the different skin sensitization potentials of irritants and allergens as single substances and in combination using the KeratinoSens assay. Contact Dermatitis, 2021. 84(5): p. 317–325. [DOI] [PubMed] [Google Scholar]
- 33.De Jong WH, et al. , Determination of the sensitising activity of the rubber contact sensitisers TMTD, ZDMC, MBT and DEA in a modified local lymph node assay and the effect of sodium dodecyl sulfate pretreatment on local lymph node responses. Toxicology, 2002. 176(1–2): p. 123–34. [DOI] [PubMed] [Google Scholar]
- 34.Cumberbatch M, et al. , Influence of sodium lauryl sulphate on 2,4-dinitrochlorobenzene-induced lymph node activation. Toxicology, 1993. 77(1-2): p. 181–91. [DOI] [PubMed] [Google Scholar]
- 35.Clausen SK, et al. , Study of adjuvant effect of model surfactants from the groups of alkyl sulfates, alkylbenzene sulfonates, alcohol ethoxylates and soaps. Food Chem Toxicol, 2000. 38(11): p. 1065–74. [DOI] [PubMed] [Google Scholar]
- 36.Prottey C and Ferguson TF, The effect of surfactants upon rat peritoneal mast cells in vitro. Food Cosmet Toxicol, 1976. 14(5): p. 425–30. [DOI] [PubMed] [Google Scholar]
- 37.Alexander BR, An assessment of the comparative sensitization potential of some common isothiazolinones. Contact Dermatitis, 2002. 46(4): p. 191–6. [DOI] [PubMed] [Google Scholar]
- 38.Castanedo-Tardana MP and Zug KA, Methylisothiazolinone. Dermatitis, 2013. 24(1): p. 2–6. [DOI] [PubMed] [Google Scholar]
- 39.Bonnekoh H, et al. , Topical inflammasome inhibition with disulfiram prevents irritant contact dermatitis. Clin Transl Allergy, 2021. 11(5): p. e12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SW, et al. , Effects of anionic surfactants on the water permeability of a model stratum corneum lipid membrane. Langmuir, 2014. 30(1): p. 220–6. [DOI] [PubMed] [Google Scholar]
- 41.Ananthapadmanabhan KP, et al. , Cleansing without compromise: the impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol Ther, 2004. 17 Suppl 1: p. 16–25. [DOI] [PubMed] [Google Scholar]
- 42.Chiang A, Tudela E, and Maibach HI, Percutaneous absorption in diseased skin: an overview. J Appl Toxicol, 2012. 32(8): p. 537–63. [DOI] [PubMed] [Google Scholar]
- 43.Mao G, et al. , Imaging the distribution of sodium dodecyl sulfate in skin by confocal Raman and infrared microspectroscopy. Pharm Res, 2012. 29(8): p. 2189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fullerton A, Broby-Johansen U, and Agner T, Sodium lauryl sulphate penetration in an in vitro model using human skin. Contact Dermatitis, 1994. 30(4): p. 222–5. [DOI] [PubMed] [Google Scholar]
- 45.Morris SAV, et al. , The effect of prolonged exposure on sodium dodecyl sulfate penetration into human skin. Toxicol In Vitro, 2021. 77: p. 105246. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe H, et al. , Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol, 2007. 127(8): p. 1956–63. [DOI] [PubMed] [Google Scholar]
- 47.Mizutani T, et al. , Sodium Lauryl Sulfate Stimulates the Generation of Reactive Oxygen Species through Interactions with Cell Membranes. J Oleo Sci, 2016. 65(12): p. 993–1001. [DOI] [PubMed] [Google Scholar]
- 48.Cohen C, et al. , Measurement of inflammatory mediators produced by human keratinocytes in vitro: A predictive assessment of cutaneous irritation. Toxicol In Vitro, 1991. 5(5–6): p. 407–10. [DOI] [PubMed] [Google Scholar]
- 49.Torma H, Lindberg M, and Berne B, Skin barrier disruption by sodium lauryl sulfate-exposure alters the expressions of involucrin, transglutaminase 1, profilaggrin, and kallikreins during the repair phase in human skin in vivo. J Invest Dermatol, 2008. 128(5): p. 1212–9. [DOI] [PubMed] [Google Scholar]
- 50.Agner T, Susceptibility of atopic dermatitis patients to irritant dermatitis caused by sodium lauryl sulphate. Acta Derm Venereol, 1991. 71(4): p. 296–300. [PubMed] [Google Scholar]
- 51.Cork MJ, et al. , Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol, 2009. 129(8): p. 1892–908. [DOI] [PubMed] [Google Scholar]
- 52.Xian M, et al. , Anionic surfactants and commercial detergents decrease tight junction barrier integrity in human keratinocytes. J Allergy Clin Immunol, 2016. 138(3): p. 890–893 e9. [DOI] [PubMed] [Google Scholar]
- 53.Bormann JL and Maibach HI, Draize human repeat insult patch test (HRIPT): Seven decades of pitfalls and progress. Regul Toxicol Pharmacol, 2021. 121: p. 104867. [DOI] [PubMed] [Google Scholar]
- 54.Agner T, et al. , Combined effects of irritants and allergens. Synergistic effects of nickel and sodium lauryl sulfate in nickel- sensitized individuals. Contact Dermatitis, 2002. 47(1): p. 21–6. [DOI] [PubMed] [Google Scholar]
- 55.Jacob SE and Amini S, Cocamidopropyl betaine. Dermatitis, 2008. 19(3): p. 157–60. [PubMed] [Google Scholar]
- 56.Fowler JF Jr., Cocamidopropyl betaine. Dermatitis, 2004. 15(1): p. 3–4. [DOI] [PubMed] [Google Scholar]
- 57.Loxham M and Davies DE, Phenotypic and genetic aspects of epithelial barrier function in asthmatic patients. J Allergy Clin Immunol, 2017. 139(6): p. 1736–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boonpiyathad T, et al. , Immunologic mechanisms in asthma. Semin Immunol, 2019. 46: p. 101333. [DOI] [PubMed] [Google Scholar]
- 59.Heijink IH, et al. , Epithelial cell dysfunction, a major driver of asthma development. Allergy, 2020. 75(8): p. 1902–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cullinan P, et al. , An outbreak of asthma in a modern detergent factory. Lancet, 2000. 356(9245): p. 1899–900. [DOI] [PubMed] [Google Scholar]
- 61.Medina-Ramon M, et al. , Asthma, chronic bronchitis, and exposure to irritant agents in occupational domestic cleaning: a nested case-control study. Occup Environ Med, 2005. 62(9): p. 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zock JP, et al. , The use of household cleaning sprays and adult asthma: an international longitudinal study. Am J Respir Crit Care Med, 2007. 176(8): p. 735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Rooy FG, et al. , A cross-sectional study among detergent workers exposed to liquid detergent enzymes. Occup Environ Med, 2009. 66(11): p. 759–65. [DOI] [PubMed] [Google Scholar]
- 64.Adisesh A, et al. , Occupational asthma and rhinitis due to detergent enzymes in healthcare. Occup Med (Lond), 2011. 61(5): p. 364–9. [DOI] [PubMed] [Google Scholar]
- 65.Laborde-Casterot H, et al. , Occupational rhinitis and asthma due to EDTA-containing detergents or disinfectants. Am J Ind Med, 2012. 55(8): p. 677–82. [DOI] [PubMed] [Google Scholar]
- 66.Le Moual N, et al. , Domestic use of cleaning sprays and asthma activity in females. Eur Respir J, 2012. 40(6): p. 1381–9. [DOI] [PubMed] [Google Scholar]
- 67.Wang M, et al. , Laundry detergents and detergent residue after rinsing directly disrupt tight junction barrier integrity in human bronchial epithelial cells. J Allergy Clin Immunol, 2019. 143(5): p. 1892–1903. [DOI] [PubMed] [Google Scholar]; • This study demonstrated laundry detergent rinse residue (concentration less than 1:20,000 dilution of laundry detergent) has cytotoxic and barrier disruptive effects on human bronchial epithelial cells.
- 68.Siegel IA and Gordon HP, Surfactant-induced alterations of permeability of rabbit oral mucosa in vitro. Exp Mol Pathol, 1986. 44(2): p. 132–7. [DOI] [PubMed] [Google Scholar]
- 69.Herlofson BB and Barkvoll P, Sodium lauryl sulfate and recurrent aphthous ulcers. A preliminary study. Acta Odontol Scand, 1994. 52(5): p. 257–9. [DOI] [PubMed] [Google Scholar]
- 70.Stec IP, A possible relationship between desquamation and dentifrices. A clinical study. J Am Dent Hyg Assoc, 1972. 46(1): p. 42–5. [PubMed] [Google Scholar]
- 71.Herlofson BB and Barkvoll P, Oral mucosal desquamation caused by two toothpaste detergents in an experimental model. Eur J Oral Sci, 1996. 104(1): p. 21–6. [DOI] [PubMed] [Google Scholar]
- 72.Perez-Lopez D, et al. , Oral mucosal peeling related to dentifrices and mouthwashes: A systematic review. Med Oral Patol Oral Cir Bucal, 2019. 24(4): p. e452–e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jenkins S, Addy M, and Newcombe R, Triclosan and sodium lauryl sulphate mouthwashes (I). Effects on salivary bacterial counts. J Clin Periodontol, 1991. 18(2): p. 140–4. [DOI] [PubMed] [Google Scholar]
- 74.Kabara JJ, Structure-function relationships of surfactants as antimicrobial agents. Journal of the Society of Cosmetic Chemists, 1978. 29(11): p. 733–741. [Google Scholar]
- 75.Howett MK, et al. , A broad-spectrum microbicide with virucidal activity against sexually transmitted viruses. Antimicrob Agents Chemother, 1999. 43(2): p. 314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Birkeland JM and Steinhaus EA, Selective Bacteriostatic Action of Sodium Lauryl Sulfate and of “Dreft.”. Proceedings of the Society for Experimental Biology and Medicine, 1939. 40(1): p. 86–88. [Google Scholar]
- 77.Diaz De Rienzo MA, et al. , Antibacterial properties of biosurfactants against selected Gram-positive and -negative bacteria. FEMS Microbiol Lett, 2016. 363(2): p. fnv224. [DOI] [PubMed] [Google Scholar]
- 78.Travers J, et al. , IL-33 is induced in undifferentiated, non-dividing esophageal epithelial cells in eosinophilic esophagitis. Sci Rep, 2017. 7(1): p. 17563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doyle AD, et al. , Detergent exposure induces epithelial barrier dysfunction and eosinophilic inflammation in the esophagus. Allergy, 2023. 78(1): p. 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]; ••This study demonstrated SDS at 1:600 dilution found in toothpaste elicits barrier disruption and inflammatory signals in human esophageal epithelium. In addition, 0.5% SDS (1:6 dilution of toothpaste) in drinking water elicited eosinophilic inflammation in the mouse esophagus.
- 80.Epstein S, et al. , Possible deleterious effects of using soap substitutes in dentrifices. J Am Dent Assoc, 1939. 26: p. 1461–1471. [Google Scholar]
- 81.Tanzer J, et al. , Laundry detergent promotes allergic skin inflammation and esophageal eosinophilia in mice. PLoS One, 2022. 17(6): p. e0268651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ogulur I., et al. , Gut epithelial barrier damage caused by dishwasher detergents and rinse aids. J Allergy Clin Immunol, 2023. 151(2): p. 469–484. [DOI] [PubMed] [Google Scholar]; •This study demonstrated dish detergent rinse aid at levels similar to those on cleaned dishes has cytotoxic and barrier disruptive effects on human gut epithelial cells.


