Abstract
Aims/hypothesis
Type 2 diabetes in people in the healthy weight BMI category (<25 kg/m2), herein defined as ‘normal-weight type 2 diabetes’, is associated with sarcopenia (low muscle mass). Given this unique body composition, the optimal exercise regimen for this population is unknown.
Methods
We conducted a parallel-group RCT in individuals with type 2 diabetes (age 18–80 years, HbA1c 47.5–118.56 mmol/mol [6.5–13.0%]) and BMI <25 kg/m2). Participants were recruited in outpatient clinics or through advertisements and randomly assigned to a 9 month exercise programme of strength training alone (ST), aerobic training alone (AER) or both interventions combined (COMB). We used stratified block randomisation with a randomly selected block size. Researchers and caregivers were blinded to participants’ treatment group; however, participants themselves were not. Exercise interventions were conducted at community-based fitness centres. The primary outcome was absolute change in HbA1c level within and across the three groups at 3, 6 and 9 months. Secondary outcomes included changes in body composition at 9 months. Per adherence to recommended exercise protocol (PP) analysis included participants who completed at least 50% of the sessions.
Results
Among 186 individuals (ST, n=63; AER, n=58; COMB, n=65) analysed, the median (IQR) age was 59 (53–66) years, 60% were men and 83% were Asian. The mean (SD) HbA1c level at baseline was 59.6 (13.1) mmol/mol (7.6% [1.2%]). In intention-to-treat analysis, the ST group showed a significant decrease in HbA1c levels (mean [95% CI] −0.44 percentage points [−0.78, −0.12], p=0.002), while no significant change was observed in either the COMB group (−0.35 percentage points, p=0.13) or the AER group (−0.24 percentage points, p=0.10). The ST group had a greater improvement in HbA1c levels than the AER group (p=0.01). Appendicular lean mass relative to fat mass increased only in the ST group (p=0.0008), which was an independent predictor of HbA1c change (beta coefficient −7.16, p=0.01). Similar results were observed in PP analysis. Only one adverse event, in the COMB group, was considered to be possibly associated with the exercise intervention.
Conclusions/interpretation
In normal-weight type 2 diabetes, strength training was superior to aerobic training alone, while no significant difference was observed between strength training and combination training for HbA1c reduction. Increased lean mass relative to decreased fat mass was an independent predictor of reduction in HbA1c level.
Trial registration
Funding
This study was funded by the National Institutes of Health (NIH; R01DK081371).
Keywords: Body composition, Exercise, HbA1c, Normal weight
Introduction
The prevalence of type 2 diabetes is increasing and it has been associated with a high burden of morbidity and mortality [1]. While the majority of people with type 2 diabetes are overweight/obese, approximately 20% are in the healthy weight BMI category (<25 kg/m2), herein defined as ‘normal-weight type 2 diabetes’, which is recognised as a different phenotype that is more common in Asian populations and older adults [2]. Compared with overweight/obese subgroups, people with a normal weight at diagnosis of type 2 diabetes are shown to have a higher risk of mortality than those who are overweight or obese [2]. Normal-weight type 2 diabetes is associated with sarcopenia or loss of muscle mass [3,4]. In addition, studies suggest that this reduced muscle size mediates the elevated mortality risk in people with normal-weight diabetes compared with overweight people with type 2 diabetes [2,5].
Exercise is generally recommended for people with type 2 diabetes. According to clinical guidelines, exercise recommendations for individuals with type 2 diabetes are similar to those for the general population: 3–5 days per week of aerobic activity at moderate to vigorous intensity, achieving a minimal exercise duration of 150 min per week, and two to three sessions per week of strength training [6–8]. Previous trials have examined the effects of specific types of exercise (strength vs aerobic) on HbA1c levels, but these trials have predominantly been carried out in people with overweight/obesity with type 2 diabetes. For example, two large RCTs, the Diabetes Aerobic and Resistance Exercise (DARE) study and the Health Benefits of Aerobic and Resistance Training in individuals with type 2 diabetes (HART-D) study, evaluated the relative impacts of strength, aerobic, and combination training on HbA1c levels in individuals with obesity and type 2 diabetes (mean BMI 33–35 kg/m2) [9,10]. The DARE study found a combination of aerobic and strength training to be superior to aerobic or strength training alone at lowering HbA1c levels [9]. The HART-D study also reported the superiority of combination (aerobic and strength) training over a non-exercise control intervention for improving HbA1c levels; in contrast, aerobic or strength training alone did not show improvements [10].
While individuals with obesity have both increased fat and increased lean muscle mass [11], normal-weight individuals with type 2 diabetes are more likely to have sarcopenia, especially related to fat mass (relative sarcopenia). Given the relative sarcopenia in people with normal-weight type 2 diabetes compared with those with type 2 diabetes and obesity, the most effective exercise regimen for individuals with overweight/obesity with type 2 diabetes may not be as effective for the normal-weight population. In the Strength Training Regimen for Normal Weight Diabetics (STRONG-D) study, we compared the effects of strength training alone, aerobic training alone and a combination of strength and aerobic training on glycaemic control in individuals with normal-weight (BMI <25 kg/m2) type 2 diabetes. In view of the difference in low lean mass between overweight/obese individuals with type 2 diabetes and normal-weight individuals with type 2 diabetes, we hypothesised that normal-weight individuals with type 2 diabetes would respond better to strength training than aerobic training, with combination training having an intermediate effect. To address this, we explored the changes in body composition and muscle strength resulting from these exercise interventions and assessed the impact of body composition changes on HbA1c.
Methods
Study design
The STRONG-D study was an RCT with three study groups: strength training only (ST), aerobic training only (AER) and combined strength and aerobic training (COMB). Following medical clearance from their primary care physician, all participants were asked to exercise for 3 days per week for 9 months according to their assigned exercise programme. They also received diabetes educational materials via a study website, attended monthly diabetes education meetings and were sent presentations for review at home. The STRONG-D study did not include a control group, as current clinical guidelines recommend exercise for all people with type 2 diabetes, and the data safety monitoring board of a previous study [3] recommended a halt to randomisation ino the control group because of a risk of elevated HbA1c levels [10].
Participants were recruited from the greater San Francisco Bay Area between November 2016 and December 2019. The complete rationale and methodology for the trial have been previously published [12]. In brief, participants were recruited in outpatient clinics or through advertisements. Primary inclusion criteria were age 18–80 years, diagnosis of type 2 diabetes without the use of an insulin pump, HbA1c 47.5–118.56 mmol/mol (6.5–13.0%) and BMI 18.5–25.0 kg/m2. Notable exclusion criteria included any serious medical condition that would contraindicate long-term participation in physical activity. Before randomisation, participants underwent a treadmill exercise test using a standard symptom-limited treadmill ramp protocol. Peak metabolic equivalents of task (MET) were calculated using treadmill peak speed and grade. The protocol was approved by the Institutional Review Board at Stanford University, and all participants provided written informed consent. The STRONG-D study is registered at ClinicalTrials.gov (registration no. NCT02448498).
Randomisation and interventions
The randomisation sequence was computer generated using randomly permuted blocks of equal length each with fixed numbers of treatment allotments to balance enrolments over time. The blocks were stratified by BMI (18.5–21.5 kg/m2 and 21.6–25.0 kg/m2) and HbA1c levels (47.5–69.9 mmol/mol [6.5–8.5%] and 70.0–118.6 mmol/mol [8.6–13.0%]) to ensure balance across the study groups. There were separate intervention and assessment teams and all assessment staff were blinded to participant randomisation assignment.
The primary analysis for this study was a comparison of clinical and anthropomorphic outcomes of the three different exercise regimens (ST, AER, and COMB). To evaluate the clinical effectiveness of the three intervention arms, we examined the overall change from baseline in outcome values (e.g. HbA1c). Using the algebraic properties of log and variance, we estimated the difference between groups as log(HbA1c1)−log(HbA1c2)=log(HbA1c1/HbA1c2), and SD(log(HbA1c))=SD (HbA1c)/mean(HbA1c). Using a two-sample t test between means, a Bonferroni adjusted alpha=0.05/3=0.017, assuming normality of log(HbA1c), equal group variances and a baseline mean HbA1c of 58.5 mmol/mol (7.5%), we had 80% power to detect a 0.5 percentage point difference in HbA1c with 75 participants per arm. Accounting for a 20% attrition rate, this required 94 participants per arm or 282 participants in total.
Exercise interventions were conducted at community-based fitness centres and participants performed the exercises at their own pace. The exercise training staff conducted evaluations at baseline and approximately every 3 months, with exercise prescriptions adjusted on an individual basis. We designed the interventions to have approximately equal time requirements, similar to the HART-D protocol [10]. We standardised the aerobic exercise prescription to body weight at 41.8 (10) (for COMB) and 50.2 (12) (for AER) kJ (kcal) kg body weight−1 week−1 of moderate to vigorous physical activity, distributed over 3 days per week. Participants selected their preferred mode of exercise from a treadmill, an elliptical or a stationary bike. In the ST group, participants exercised 3 days per week, with each session consisting of two sets of four upper body exercises (bench press, seated row, shoulder press and pull down), three sets of three leg exercises (leg press, extension and flexion) and two sets of abdominal crunches and back extensions. The intensity of the strength training programme was low initially to reduce muscle soreness and ensure proper lifting form. The intensity then progressed by increasing the amount of weight lifted until participants could complete eight to 12 repetitions for each muscle group. In the AER group, participants were weighed weekly and asked to expend 50.2 (12) kJ (kcal) kg body weight−1 week−1 on a treadmill, an elliptical or a stationary bike, with a target training intensity between 50% and 80% of their peak MET based on their baseline exercise stress test. In the COMB group, participants were instructed to complete two strength training sessions per week including one set of each of the strength training exercises, plus to expend 41.8 (10) kJ (kcal) kg body weight−1 week−1 through aerobic exercise using the same protocol as for AER. Adherance was evaluated as the ratio of the number of sessions actually attended to the expected number of exercise sessions over the 9 month period.
Outcomes
The primary outcome was the absolute change in HbA1c levels within and across the three groups at 3, 6 and 9 months. Secondary outcomes included changes in body composition and muscle strength at 9 months to identify predictors of change in HbA1c levels. HbA1c levels were analysed using a Siemens DCA Vantage Analyzer (Siemens Healthineers, Erlangen, Germany). Body composition was assessed using the Horizon A system) with Apex software v5.5 (Hologic, Bedford, MA, USA) and standard positioning techniques [13]. Body composition (i.e. fat mass and lean mass) varies with age, sex and race/ethnicity and is also highly correlated with height [14,15]. To adjust for these differences, appendicular lean mass and fat mass were scaled by height squared creating the indices: the appendicular lean mass index (ALMI, kg/m2) and the fat mass index (FMI, kg/m2). These indices were then converted to sex- and race/ethnicity-specific Z scores relative to age (ALMI-Z and FMI-Z) [14,15]. ALMI-Z relative to FMI-Z, which accounts for confounding effects of FMI-Z, was also analysed because this measure has been shown to be a better predictor of sarcopenia than ALMI-Z alone [14]. Muscular strength was assessed using isokinetic dynamometry (Biodex Systems, Shirley, NY, USA). Concentric isokinetic knee flexion and extension were tested to determine peak torque at 60°/s and mean (or total) work at 180°/s. Peak torque was defined as the best of five maximal repetitions and mean or total work was calculated from a set of 30 repetitions performed at maximal speed. Muscle quality was also assessed; calculated as muscle strength divided by muscle mass.
Dietary data were obtained at baseline and follow-up using the online version of the Block Brief Food Frequency Questionnaire [16]. The questionnaire asked about frequency of consumption and portion size for approximately 65 foods over the previous 3 months.
On 16 March 2020, state- and county-wide shelter-in-place restrictions were put in place, closing all intervention (gyms) and assessment (Stanford University campus) locations. Study interventions were adapted to at-home aerobic, strength and combination exercises. Individuals performing at-home exercises did not complete the follow-up assessments due to shelter-in-place restrictions and were thus excluded from the final analysis (Fig. 1). In June 2020, given the increased risks associated with COVID-19 for people with type 2 diabetes [17], the Data Safety Monitoring Board recommended that further study intervention and assessments be stopped for safety reasons.
Fig. 1.
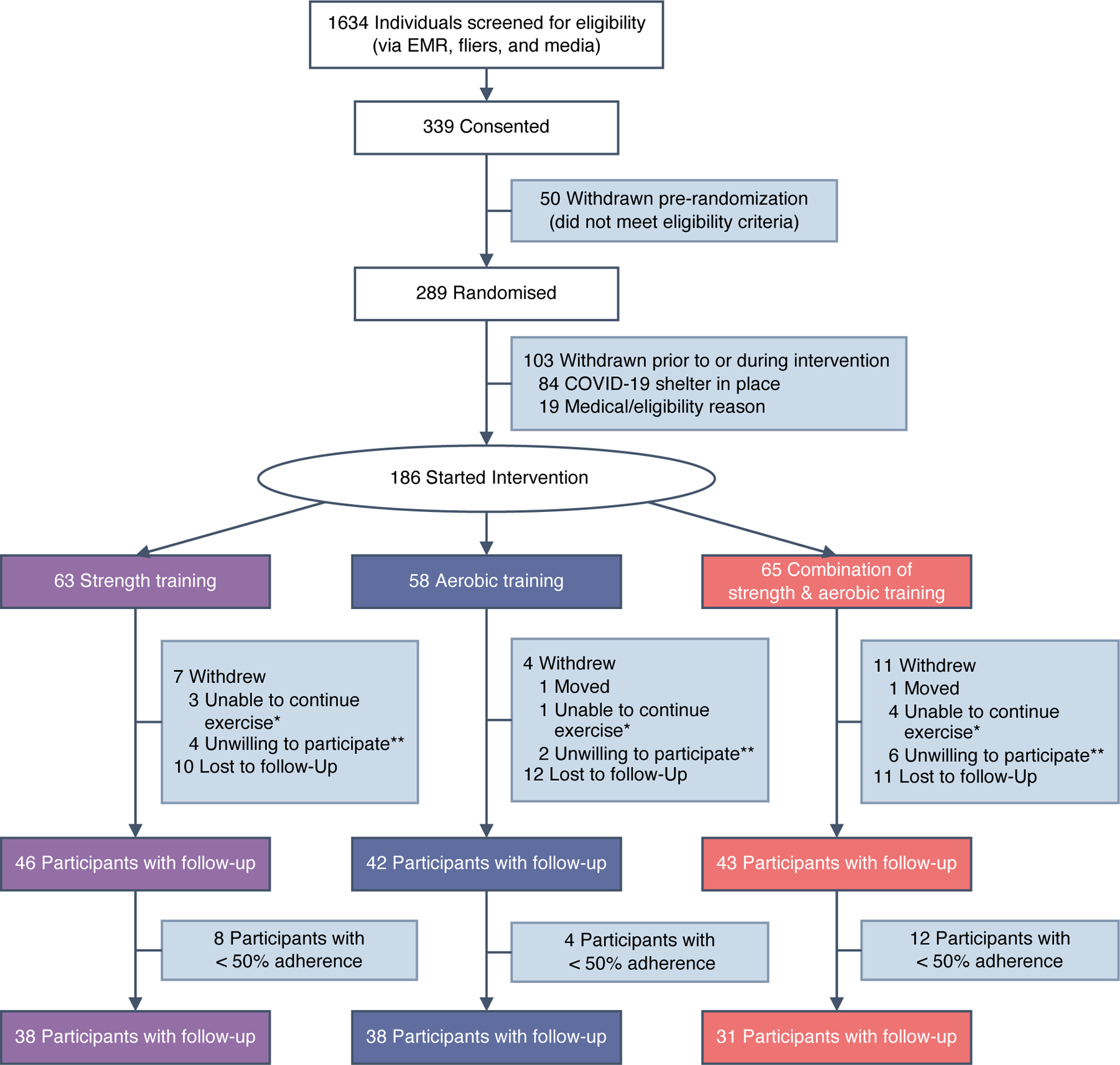
Participant flow chart. aUnable to continue exercise: unable to continue exercise, family illness, stopped exercising and withdrew. bUnwilling to participate: too busy, not convenient, cannot commit, do not want to participate. EMR, electronic medical record
Statistical analysis
Data normality was assessed using the Kolmogorov–Smirnov test. Baseline comparison between groups was performed using Welch’s t test or the Mann-Whitney U test if two groups were compared and one-way ANOVA or the Kruskal–Wallis test if three groups were compared. Intention-to-treat (ITT) analysis included all participants who started their assigned exercise programme. We also performed sensitivity analyses including participants who participated in at least 50% of the expected number of exercise sessions over the 9 month period (per adherence to recommended exercise protocol [PP] analyses). For the primary outcome analysis, we evaluated differences in HbA1c between groups using a repeated ANOVA analysis and differences within groups using paired t tests between baseline and after the 9 month exercise programme. We present the estimated mean HbA1c values at each time point and the p values for the pairwise comparisons between groups from the ANOVA model in Fig. 2. In addition to fat mass and lean mass, we used the validated body composition variables FMI-Z, ALMI-Z and ALMI-Z relative to FMI-Z [15,16] to determine independent predictors of change in HbA1c levels in regression models adjusted for age, sex and baseline HbA1c levels. For within-group comparisons, p<0.05 was used to determine statistical significance. For pairwise comparisons between groups, a Bonferroni corrected p<0.05/3=0.017 was used. All analyses were performed using Stata 16.0 (College Station, TX, USA) or R version 4.0.2 (2020-06-22) [18].
Fig. 2.
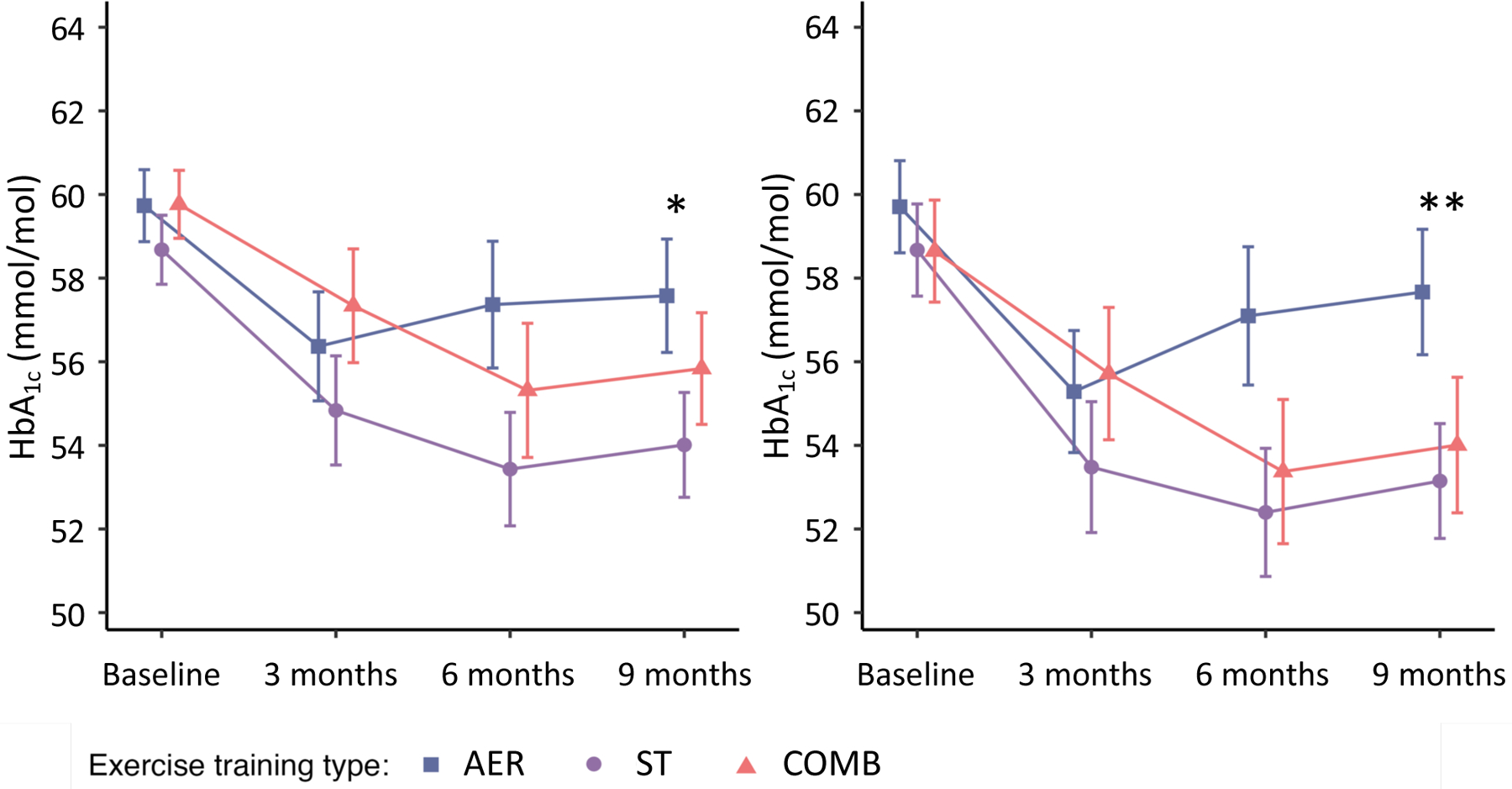
Contrast results from repeated ANOVA. Follow-up mean HbA1c levels derived from a repeated measures ANOVA for the ITT group (n=186) (a) and PP group (n=107) (b). The points correspond to the estimated means from the repeated measures ANOVA model. *p<0.05 AER vs ST, **p<0.01 AER vs ST. The table within each plot shows the results of the pairwise comparisons. Error bars represent SEs
Results
As shown in the CONSORT flow diagram [19] (Fig. 1), out of 1634 people screened, 339 were consented and 289 were randomised, after 50 participants were withdrawn for not meeting the eligibility criteria. Among those randomised, 103 were withdrawn prior to or during the intervention as a result of the shelter-in-place restrictions (n=84) or medical ineligibility (n=19). A total of 186 normal-weight (median [IQR] BMI 23.7 [22.6–24.6] kg/m2) individuals with type 2 diabetes were included in the analysis (ST, n=63; AER, n=58; COMB, n=65).
As shown in Table 1, the median (IQR) age of participants was 59 (53–66) years, 60% were men and 83% were Asian. At baseline the mean (SD) HbA1c level was 59.6 (13.1) mmol/mol (7.6% [1.2%]) and the mean (SD) duration of diabetes was 9.8 (6.8) years. In total, 84% of participants were taking glucose-lowering medication. A comparison of the baseline characteristics of randomised participants who were included (n=186) and those who were excluded (n=103) is shown in ESM Table 1.
Table 1.
Baseline participant characteristicsa
| Characteristic | All participants (N=186) | Exercise training type | |||
|---|---|---|---|---|---|
| ST (N=63) | AER (N=58) | COMB (N=65) | |||
| Age, median (IQR) | 59 (53, 66) | 60 (53, 66) | 59 (54, 65) | 59 (52, 68) | |
| Male, n (%) | 112 (60) | 35 (56) | 33 (57) | 44 (68) | |
| Race/ethnicity, n (%)a | |||||
| White | 17 (9) | 5 (8) | 7 (12) | 5 (8) | |
| Asian | 155 (83) | 52 (83) | 47 (81) | 56 (86) | |
| Black | 5 (3) | 1 (2) | 2 (3) | 2 (3) | |
| Hispanic | 3 (2) | 1 (2) | 2 (3) | 0 (0) | |
| Other | 6 (3) | 4 (6) | 0 (0) | 2 (3) | |
| Bachelor’s degree or higher, n (%) | 147 (79) | 50 (79) | 43 (74) | 54 (83) | |
| Smoking history, n (%) | |||||
| Current | 1 (1) | 0 (0) | 0 (0) | 1 (2) | |
| Former | 29 (16) | 10 (16) | 9 (16) | 10 (15) | |
| Glucose-lowering medication, n (%) | |||||
| Any | 157 (84) | 60 (95) | 48 (83) | 49 (75) | |
| Biguanide | 154 (83) | 60 (95) | 46 (79) | 48 (74) | |
| Sulfonylurea | 61 (33) | 26 (41) | 18 (31) | 17 (26) | |
| Other | 33 (18) | 11 (17) | 10 (17) | 12 (18) | |
| Diabetes factors, mean (SD) | |||||
| HbA1c, mmol/mol | 59.6 (13.1) | 59.6 (14.2) | 59.6 (13.1) | 59.6 (13.1) | |
| HbA1c, % | 7.6 (1.2) | 7.6 (1.3) | 7.6 (1.2) | 7.6 (1.2) | |
| Duration of diabetes, years | 9.8 (6.8) | 10.0 (7.4) | 9.7 (6.8) | 9.8 (6.4) | |
| Anthropometrics | |||||
| Weight, mean (SD), kg | 67.9 (11.8) | 71.2 (17.6) | 69.6 (11.2) | 66.6 (8.2) | |
| BMI, median (IQR), kg/m2 | 23.7 (22.6, 24.6) | 23.5 (22.6, 24.4) | 23.6 (22.2, 24.7) | 23.8 (22.9, 24.7) | |
| Lean mass, mean (SD), kg | 42.8 (7.5) | 42.2 (7.5) | 42.2 (8.2) | 44.0 (6.9) | |
| Fat mass, mean (SD), kg | 21.8 (4.0) | 21.4 (4.1) | 22.3 (3.5) | 21.6 (4.3) | |
| ALMI-Z, mean (SD) | −1.28 (0.77) | −1.26 (0.80) | −1.33 (0.75) | −1.24 (0.76) | |
| FMI-Z, mean (SD) | −0.62 (0.54) | −0.66 (0.52) | −0.60 (0.53) | −0.60 (0.59) | |
| ALMI-Z relative to FMI-Z (SD) | −1.11 (1.19) | −1.03 (1.21) | −1.21 (1.20) | −1.08 (1.19) | |
| Strength extension, mean (SD), N·m | 77.3 (30.0) | 75.7 (33.6) | 77.4 (29.6) | 79.1 (26.5) | |
| Strength flexion, mean (SD), N·m | 34.5 (15.5) | 34.5 (15.7) | 33.9 (15.5) | 34.9 (15.4) | |
| Blood pressure, mean (SD), mmHg | |||||
| Systolic | 132.5 (16.3) | 132.2 (16.1) | 133.0 (18.1) | 132.2 (14.9) | |
| Diastolic | 78.6 (9.7) | 79.4 (10.7) | 78.7 (9.6) | 77.8 (8.7) | |
| Other medication use, n (%) | |||||
| Blood pressure | 107 (58) | 44 (70) | 34 (59) | 29 (45) | |
| Dyslipidaemia | 123 (66) | 41 (65) | 38 (66) | 44 (68) | |
| Antidepressant | 7 (4) | 2 (3) | 4 (7) | 1 (2) | |
Sex, age and race/ethnicity-specific Z scores were used to calculate ALMI and FMI
Percentages may not sum to 100% because of rounding
A total of 131 participants completed the exercise intervention (ST, n=46; AER, n=42; COMB, n=43; Fig. 1). There was no significant difference between the groups in the number of sessions attended per week over the 9 month period (median [IQR] 2.6 [1.9–3.0] for ST, 2.7 [2.2–3.1] for AER and 2.5 [1.4–2.9] for COMB; p=0.14). Among the 131 participants, 107 were classified as being in the per adherence to recommended exercise protocol (PP) group according to the predefined criteria (ST, n=38; AER, n=38; COMB, n=31; Fig. 1). The exercise training data by month for individuals included in the PP analysis are shown in ESM Table 2. Participants in the AER and COMB groups performed their aerobic exercise at ~70% of the maximum MET and this intensity was maintained during the entire exercise intervention period. Overall, 71% of participants had no change in their glucose-lowering medication over the study period (ST: decrease in 14%, no change in 76%, increase in 10%; AER: decrease in 12%, no change in 64%, increase in 24%; COMB: decrease in 19%, no change in 71%, increase in 10%). There was no significant difference across the three groups in medication changes (p=0.62). Additionally, energy and macronutrient intake did not vary at baseline across the treatment groups (ANOVA p values >p=0.05.) In addition, there was no evidence of differences across treatment groups in changes in energy and macronutrient intake.
Figure 2 presents the contrast results from repeated ANOVA for the ITT and PP analyses at each visit. In the ITT analysis (Fig. 2a), participants in the ST group experienced a greater reduction in HbA1c levels than those in the AER group (marginal p=0.01 from repeated ANOVA). Similar results were obtained in the PP analysis (marginal p=0.006 from repeated ANOVA between ST and AER). In the within-group comparisons, HbA1c levels at 9 months were significantly reduced compared with baseline in the ST group, with a mean (95% CI) change of −0.44 (−0.78 to −0.12) percentage points (p=0.02 from t test), while HbA1c levels were not significantly different at 9 months in the COMB group (p=0.10) or the AER group (p=0.13) (ESM Table 3).
At baseline, there were no significant differences across the three groups in weight, lean mass, fat mass, or muscle strength (Table 1). Study participants had a low lean mass (ALMI-Z mean [SD] −1.28 [0.77]), low fat mass (FMI-Z mean [SD] −0.62 [0.54]) and relative sarcopenia (ALMI-Z relative to FMI-Z mean [SD] −1.11 [1.19]). Over the 9 month period, a significant decrease in weight (p=0.04) was observed in the AER group, while there was no statistically significant difference in the ST group (p=0.10) or COMB group (p=0.20). All of these effect sizes were small (about 1–2% of mean body weight) and the change in weight was not associated with changes in HbA1c level. Table 2 presents the results for change in body composition and muscle strength in the ITT and PP populations. In the ITT analysis, ALMI-Z relative to FMI-Z increased significantly only in the ST group (mean [95% CI] 0.27 [0.12, 0.42]), consistent with concurrent gains in ALMI-Z (0.13 [0.01, 0.25]) and declines in FMI-Z (−0.14 [−0.23, −0.05]). No significant within-group changes were observed in the AER or COMB group, except for a decrease in FMI-Z in the AER group (−0.10 [−0.19, −0.02]). Consistent with the ITT analysis, the PP analysis found a significant increase in ALMI-Z relative to FMI-Z only in the ST group (0.26 [0.09, 0.43]), with no difference observed in either the AER group (0.03 [−0.18, 0.23]) or the COMB group (0.07 [−0.14, 0.27]). A significant increase in muscle strength and quality was observed only in the ST group in the PP analysis (muscle strength: 15.82 [2.64, 29.00]; muscle quality: 1.91 [0.37, 3.45]). For all body composition measures, change in ALMI-Z relative to FMI-Z was the only independent predictor of change in HbA1c levels in both the ITT analysis (beta coefficient −7.16, p=0.01) and the PP analysis (beta coefficient −8.77, p=0.006).
Table 2.
Change in HBA1c and body composition post exercise intervention
| Variable | ST | AER | COMB |
|---|---|---|---|
| ITT analysis, mean (95% CI) | |||
| n=63 | n=58 | n=65 | |
| HbA1c, mmol/mol | −4.81 (−8.52, −1.31) | −2.62 (−6.22, 0.98) | −3.83 (−7.98, 0.22) |
| HbA1c, percentage points | −0.44 (−0.78, −0.12) | −0.24 (−0.57, 0.09) | −0.35 (−0.73, 0.02) |
| Body composition | n=34 | n=22 | n=28 |
| Lean mass, kg | 0.30 (−0.13, 0.74) | −0.37 (−0.93, 0.19) | −0.23 (−0.71, 0.24) |
| Fat mass, kg | −0.99 (−1.61, −0.36)* | −0.70 (−1.26, −0.14)* | −0.62 (−1.32, 0.08) |
| ALMI-Z | 0.13 (0.01, 0.25)* | −0.03 (−0.16, 0.11) | −0.02 (−0.12, 0.08) |
| FMI-Z | −0.14 (−0.23, −0.05)* | −0.10 (−0.19, −0.02)* | −0.08 (−0.20, 0.04) |
| ALMI-Z relative to FMI-Z | 0.27 (0.12, 0.42)* | 0.06 (−0.11, 0.23) | 0.04 (−0.10, 0.18) |
| Muscle strength, N·m | 9.70 (−4.48, 23.88) | 1.80 (−7.18, 10.77) | 8.10 (−3.07, 19.26) |
| Muscle quality, N·m/kg | 1.03 (−0.69, 2.76) | 0.11 (−1.27, 1.49) | 1.08 (−0.21, 2.38) |
| PP analysis, mean (95% CI) | |||
| n=38 | n=38 | n=31 | |
| HbA1c, mmol/mol | −5.57 (−9.84, −1.53) | −2.2 (−6.89, 2.40) | −4.92 (−10.16, 0.44) |
| HbA1c, percentage points | −0.51 (−0.90, −0.14) | −0.20 (−0.63, 0.22) | −0.45 (−0.93, 0.04) |
| Body composition | n=28 | n=18 | n=19 |
| Lean mass, kg | 0.15 (−0.27, 0.57) | −0.48 (−1.13, 0.17) | −0.23 (−0.84, 0.38) |
| Fat mass, kg | −1.10 (−1.64, −0.56)* | −0.78 (−1.42, −0.14)* | −0.82 (−1.74, 0.10) |
| ALMI-Z | 0.11 (−0.01, 0.24) | −0.05 (−0.21, 0.10) | −0.01 (−0.15, 0.12) |
| FMI-Z | −0.16 (−0.24, −0.08)* | −0.12 (−0.22, −0.02)* | −0.10 (−0.26, 0.07) |
| ALMI-Z relative to FMI-Z | 0.26 (0.09, 0.43)* | 0.03 (−0.18, 0.23) | 0.07 (−0.14, 0.27) |
| Muscle strength, N·m | 15.82 (2.64, 29.00)* | 3.38 (−7.09, 13.86) | 10.98 (−4.14, 26.10) |
| Muscle quality, N·m/kg | 1.91 (0.37, 3.45)* | 0.39 (−1.18, 1.95) | 1.44 (−0.30, 3.18) |
Individuals with missing data were excluded from the analysis
Sex, age and race/ethnicity-specific Z scores were used to calculate ALMI and FMI. Strength extension and flexion were adjusted for age, sex and height
p<0.05 between pre and post intervention
During the exercise intervention, five events qualified as serious adverse events, with a similar prevalence across groups (ST, n=1; AER, n=2; COMB, n=2). Only one serious adverse event, observed in the COMB group, was considered to be possibly associated with the exercise intervention (rotator cuff repair associated with a previous shoulder injury and potentially exacerbated by exercise during the trial).
Discussion
The primary finding from this RCT is that strength training alone was more effective than aerobic training alone at reducing HbA1c levels in normal-weight individuals with type 2 diabetes, and combination training had an intermediate effect. Furthermore, strength training increased appendicular lean mass relative to fat mass and this was an independent predictor of the reduction in HbA1c level. To our knowledge, this is the first clinical trial of exercise in normal-weight individuals with type 2 diabetes, who make up 20% of the population with type 2 diabetes [2]. While these findings need to be confirmed in further studies, these results could be applied immediately to exercise recommendations for people with type 2 diabetes and a BMI <25 kg/m2.
Previous studies in individuals with overweight/obesity with type 2 diabetes have demonstrated that structured exercise is effective in improving glycaemic control (lowering HbA1c levels) [20–22]. Both the DARE study and the HART-D study reported that COMB training showed a larger reduction in HbA1c levels, followed by AER; of the three interventions, ST ([−3.3 mmol/mol [−0.30 percentage points] in DARE and −0.55 mmol/mol [−0.04 percentage points] in HART-D) was the least effective at lowering HbA1c levels [10,11]. These two clinical trials included individuals with type 2 diabetes and overweight/obesity (mean BMI 33.5 kg/m2 in DARE and 35 kg/m2 in HART-D). No previous clinical trials have been conducted in individuals with normal-weight type 2 diabetes, which is more common among Asian people and older individuals with relative sarcopenia [2].
In contrast to the previous trials, the STRONG-D study was designed to examine normal-weight individuals with type 2 diabetes (mean BMI 23.7 kg/m2). This study found that strength training led to a larger reduction in HbA1c levels (−0.44 percentage points) than aerobic training alone (−0.24 percentage points). In addition, only the ST group showed a significant reduction in HbA1c levels, suggesting a potentially unique benefit of strength training in normal-weight individuals with type 2 diabetes. Compared with the ST group in the HART-D and DARE studies, the ST group in our study achieved a higher absolute mean reduction in HbA1c levels (−4.8 mmol/mol (−0.44 percentage points) in STRONG-D vs −0.55 mmol/mol [−0.04 percentage points] in HART-D vs −3.3 mmol/mol [−0.30 percentage points] in DARE), despite less intensification of glucose-lowering medication than in the ST groups in the HART-D and DARE studies.
Compared with the participants with overweight/obesity in the HART-D study, the normal-weight participants in this study had a lower fat mass (21.8 kg in STRONG-D vs 37.1 kg in HART-D) and lean mass (42.8 kg in STRONG-D vs 57.7 kg in HART-D). When adjusted for age, sex and height, our study participants had a lower fat mass (FMI-Z −0.62) with much lower lean mass (ALMI-Z −1.28). Given that 80% of insulin-mediated glucose uptake occurs in skeletal muscle (lean mass) [23], one should consider the importance of increasing lean mass for improving glycaemic control in this population. An important finding of our study is that body composition change (increase in lean mass with loss of fat mass) was independently associated with a reduction in HbA1c levels, while a decrease in FMI alone or even an increase in ALMI alone was not. We recently showed that ALMI relative to FMI is a more valid construct for redefining lean body mass deficits in the context of fat mass [14,24]. This result, showing the impact of change in ALMI relative to FMI on lowering HbA1c level, is consistent with the growing body of evidence that estimates of muscle mass adjusted for fat mass show stronger associations with metabolic abnormalities than conventional ALMI variables alone [15,25]. Loss of fat mass or weight with AER is usually associated with loss of lean mass [26,27], as we also observed in our study. Strength training led to increased muscle mass relative to decreased fat mass in our study, and this seems to be more beneficial for lowering HbA1c levels in individuals with normal-weight type 2 diabetes, which is associated with relative sarcopenia [3,4]. In contrast, overweight/obese individuals in both the DARE and the HART-D studies had excess fat mass with adequate lean mass; thus, loss of fat mass in individuals with a higher BMI may be more essential for lowering HbA1c levels. In fact, although a significant increase in lean mass (0.8 kg) and a decrease in fat mass (1.4 kg) were also observed in the ST group in the HART-D study, this group ranked third in effectiveness at lowering HbA1c levels, and the highest reduction in HbA1c was observed in the COMB group, in which the largest decrease in fat mass was observed (1.7 kg decrease for COMB and 0.6 kg decrease for AER) [10]. Currently, not enough data are available to support the choice of body composition as a central target for exercise training in type 2 diabetes. However, our findings, along with previous studies that have demonstrated a relationship between body composition and cardiovascular disease mortality [28,29], show that strength training is beneficial in the normal-weight diabetes population.
Weight loss has been associated with a reduction in HbA1c in people with type 2 diabetes with overweight/obesity [30]. In our study, significant weight loss was observed only in the AER group and there was no relationship between weight loss and reduction in HbA1c levels. This result is in contrast to the results of the HART-D study, in which only participants in the COMB group showed weight loss (−1.5 kg) and the largest reduction in HbA1c levels was observed in these participants. This result also supports the view that the most effective exercise regimen for overweight/obese individuals with type 2 diabetes may not necessarily be applicable to normal-weight individuals with type 2 diabetes.
One of the limitations of the STRONG-D study is that it was significantly impacted by the COVID-19 shelter-in-place restrictions introduced in March 2020, which led to early study closure. The follow-up rate was about 45%; therefore, the study was underpowered to obtain conclusive findings. However, even with low power, the STRONG-D study showed signifianct effects of ST exercise on lowering HbA1c levels in people with normal-weight diabetes. This study underscores the value of strength training for glycaemic control, which does not increase the risk of adverse events compared with aerobic training in individuals with normal-weight type 2 diabetes. These results make an important contribution to exercise recommendations for lean individuals with type 2 diabetes and could also feed into the personalised exercise recommendations for different phenotypes. In the current clinical guidelines for individuals with type 2 diabetes, there are no recommended strength training regimens [8]. Therefore, we used a strength exercise regimen based on that used previously in the HART-D study [10]. The intensity of the strength training increased over the 9 month period, while the intensity of the aerobic training based on mean MET did not. This may have influenced the outcome, although there was minimal room to increase the intensity of the aerobic training given that the baseline MET was preserved. Finally, because of the nature of exercise interventions and the higher risks associated with infectious disease in people with type 2 diabetes, future studies should also consider delivering exercise interventions virtually.
In conclusion, our trial showed that strength training alone was effective and superior to aerobic training alone for reducing HbA1c levels in individuals with normal-weight type 2 diabetes, with no significant difference observed between strength training alone and combination training. Normal-weight individuals with type 2 diabetes present with relative sarcopenia, and strength training to achieve increased lean mass relative to decreased fat mass plays an important role in glycaemic control in this population. This study has important implications for the refinement of physical activity recommendations in type 2 diabetes by weight status.
Supplementary Material
Research in context summary:
What is already known about this subject?
A combination of aerobic and resistance training is superior to aerobic or resistance training alone for lowering HbA1c levels in people with overweight/obesity and type 2 diabetes.
What is the key question?
Is strength training alone superior to aerobic training alone for lowering HbA1c levels in individuals with type 2 diabetes and a BMI <25 kg/m2, herein defined as ‘normal-weight type 2 diabetes’?
What are the new findings?
Strength training alone was more effective than aerobic training alone at lowering HbA1c levels in normal-weight individuals with type 2 diabetes. The effect of combination training was intermediate between the effects of strength training and aerobic training.
Increased appendicular lean mass relative to fat mass was an independent predictor of reduction in HbA1c level.
How might this impact on clinical practice in the foreseeable future?
The findings of our study could be applied immediately to exercise recommendations for people with type 2 diabetes and a BMI <25 kg/m2.
Acknowledgements
The authors thank A. Chase and A. Mueller (Stanford Cardiovascular Institute) for their proofreading of the manuscript.
Funding
This study was supported by a grant to LP from the National Institutes of Health (R01DK081371).
Abbreviations
- AER
Aerobic training group
- ALMI
Appendicular lean mass index
- COMB
Combined strength and aerobic training group
- DARE
Diabetes Aerobic and Resistance Exercise
- FMI
Fat mass index
- HART-D
Health Benefits of Aerobic and Resistance Training in individuals with type 2 diabetes
- ITT
Intention to treat
- MET
Metabolic equivalents of task
- PP
Per adherence to recommended exercise protocol
- ST
Strength training group
Footnotes
Authors’ relationship and activities The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Data availability
Data are available from the authors on reasonable request.
References
- 1.Chan JCN, Gregg EW, Sargent J, Horton R. Reducing global diabetes burden by implementing solutions and identifying gaps: a Lancet Commission. The Lancet 2016; 387: 1494–1495. [DOI] [PubMed] [Google Scholar]
- 2.Carnethon MR, De Chavez PJD, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA 2012; 308: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murata Y, Kadoya Y, Yamada S, Sanke T. Sarcopenia in elderly patients with type 2 diabetes mellitus: prevalence and related clinical factors. Diabetol Int 2018; 9: 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang T, Feng X, Zhou J, et al. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci Rep 2016; 6: 38937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy RA, Reinders I, Garcia ME, et al. Adipose tissue, muscle, and function: potential mediators of associations between body weight and mortality in older adults with type 2 diabetes. Diabetes Care 2014; 37: 3213–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanhees L, Geladas N, Hansen D, et al. Importance of characteristics and modalities of physical activity and exercise in the management of cardiovascular health in individuals with cardiovascular risk factors: recommendations from the EACPR. Part II. Eur J Prev Cardiol 2012; 19: 1005–1033. [DOI] [PubMed] [Google Scholar]
- 7.Gibson‐Moore H UK Chief Medical Officers’ physical activity guidelines 2019: what’s new and how can we get people more active? Nutrition Bulletin 2019; 44: 320–328. [Google Scholar]
- 8.American Diabetes Association Professional Practice Committee, et al. 5. Facilitating behavior change and well-being to improve health outcomes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022; 45: S60–82. [DOI] [PubMed] [Google Scholar]
- 9.Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes. Annals of Internal Medicine 2007; 147: 357–369. [DOI] [PubMed] [Google Scholar]
- 10.Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 2010; 304: 2253–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forbes GB, Welle SL. Lean body mass in obesity. Int J Obes 1983; 7: 99–107. [PubMed] [Google Scholar]
- 12.Faroqi L, Bonde S, Goni DT, et al. STRONG-D: strength training regimen for normal weight diabetics: rationale and design. Contemporary Clinical Trials 2019; 78: 101–106. [DOI] [PubMed] [Google Scholar]
- 13.Fung EB, Bachrach LK, Sawyer AJ (2016). Bone health assessment in pediatrics: guidelines for clinical practice Springer, Cham, Switzerland [Google Scholar]
- 14.Weber D, Long J, Leonard MB, Zemel B, Baker JF. Development of novel methods to define deficits in appendicular lean mass relative to fat mass. PLoS One 2016; 11: e0164385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker JF, Long J, Leonard MB, et al. Estimation of skeletal muscle mass relative to adiposity improves prediction of physical performance and incident disability. J Gerontol A Biol Sci Med Sci 2018; 73: 946–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc 1992; 92: 686–693. [PubMed] [Google Scholar]
- 17.Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2020; 14: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team (2020). R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 19.Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials 2010; 11. DOI: 10.1186/1745-6215-11-32. [DOI] [Google Scholar]
- 20.Hu FB, Stampfer MJ, Solomon C, et al. Physical activity and risk for cardiovascular events in diabetic women. Annals of Internal Medicine 2001; 134: 96–105. [DOI] [PubMed] [Google Scholar]
- 21.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 2000; 132: 605–611. [DOI] [PubMed] [Google Scholar]
- 22.Umpierre D, Ribeiro PAB, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011; 305: 1790–1799. [DOI] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009; 32 Suppl 2: S157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziolkowski SL, Long J, Baker JF, Chertow GM, Leonard MB. Relative sarcopenia and mortality and the modifying effects of chronic kidney disease and adiposity. J Cachexia Sarcopenia Muscle 2019; 10: 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JY, Oh S, Park HY, Jun JH, Kim HJ. Comparisons of different indices of low muscle mass in relationship with cardiometabolic disorder. Sci Rep 2019; 9: 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villareal DT, Aguirre L, Burke Gurney A, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. New England Journal of Medicine 2017; 376: 1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beavers KM, Miller ME, Rejeski WJ, Nicklas BJ, Kritchevsky SB. Fat mass loss predicts gain in physical function with intentional weight loss in older adults. J Gerontol A Biol Sci Med Sci 2013; 68: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavie CJ, De Schutter A, Patel DA, Romero-Corral A, Artham SM, Milani RV. Body composition and survival in stable coronary heart disease. Journal of the American College of Cardiology 2012; 60: 1374–1380. [DOI] [PubMed] [Google Scholar]
- 29.Srikanthan P, Horwich TB, Tseng CH. Relation of muscle mass and fat mass to cardiovascular disease mortality. The American Journal of Cardiology 2016; 117: 1355–1360. [DOI] [PubMed] [Google Scholar]
- 30.Gummesson A, Nyman E, Knutsson M, Karpefors M. Effect of weight reduction on glycated haemoglobin in weight loss trials in patients with type 2 diabetes. Diabetes, Obesity and Metabolism 2017; 19: 1295–1305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the authors on reasonable request.


