Abstract
Objective:
This study examines the within- and between-person associations of acute and chronic stress with blood pressure (BP) and heart rate (HR) using an app-based research platform.
Methods:
We examined data from 31,964 adults (aged 18–90) in an app-based ecological momentary assessment study that used a research-validated optic sensor to measure BP.
Results:
Within-person associations revealed that moments with (vs. without) acute stress exposure were associated with higher systolic (SBP: b = 1.54) and diastolic BP (DBP: b = 0.79) and HR (b = 1.53; ps < .001). During moments with acute stress exposure, higher acute stress severity than usual was associated with higher SBP (b = 0.26), DBP (b =0.09), and HR (b = 0.40; ps < .05). During moments without acute stress, higher background stress severity than usual was associated with higher BP and HR (SBP: b = 0.87; DBP: b = 0.51; HR: b = 0.69; ps < .001). Between-person associations showed that individuals with more frequent reports of acute stress exposure or higher chronic stress severity had higher SBP, DBP, and HR (ps < .05). Between-person chronic stress severity moderated within-person physiological responses to stress such that individuals with higher chronic stress severity had higher average BP and HR levels but showed smaller responses to momentary stress.
Conclusion:
Technological advancements with optic sensors allow for large-scale physiological data collection, which provides a better understanding of how stressors of different timescales and severity contribute to momentary BP and HR in daily life.
Keywords: stress, acute stress, chronic stress, blood pressure, ecological momentary assessments
Introduction
Psychological stress is abundant in modern life. Physiological responses to stressors prepare the body for actions and are crucial for daily functioning and survival. However, heightened or prolonged responses to stressors in the long-term can lead to physiological wear and tear—i.e., allostatic load (1). Numerous studies have shown that prolonged stress exposure contributes to inflammation, chronic diseases such as depression and cardiovascular diseases, and accelerated aging (2-5).
Many studies have examined the relationship between stress exposure and blood pressure (BP) changes, given it is a possible contributor to hypertension (6,7). However, prior studies examining physiological responses often use artificial stress manipulations in controlled environments, which have limited ecological validity. Field studies examining these relationships in naturalistic settings have demonstrated the importance of measuring BP and stress in daily life (e.g., 8). However, these studies often have small sample sizes or short study duration (e.g., 24 hours or a few days) (9,10), given the difficulty to scale-up the collection of physiological data.
A prior study overcame these limitations to examine the relationship between stress and BP in daily life in a large sample by using an app-based platform called MyBPLab (11). The authors examined within-person BP and heart rate (HR) reactivity to stress and emotions. The current study uses a subsequent version of the app (MyBPLab 2.0), and a new sample, to examine stress associations with BP and HR responses in daily life across three weeks and features several major advances. First, we distinguish between acute and chronic stress and examined their respective associations with BP and HR. Second, multiple measurements of stress and physiological responses were assessed within a day (as opposed to once every few days) to allow for the examination of associations at the momentary level. Third, this study focused on both between- and within-person associations between stress and physiological responses in daily life, which addresses both individual differences in average BP and HR and within-person changes in momentary BP and HR. Lastly, in an important conceptual advance, we examined whether individual differences in chronic stress severity moderate physiological responses to momentary stress.
From a research perspective, the major advantage of using an app-based platform to collect physiological responses (e.g., BP and HR) and psychological measures is that it is easily scalable and individuals from all over the world can participate. In addition, as smartphones or other mobile devices are increasingly capable of collecting various physiological measures, alongside psychological measures, an app can easily synchronize these measures to better understand individuals and their immediate environment. From a clinical perspective, although the sensors used in smartphones are generally not clinical grade, they may be sufficient and useful given the widespread availability to individuals from a variety of backgrounds and health conditions to gain insights into their physiology, potentially enhancing early indicators of hypertension and heart disease on a large scale.
Acute and Chronic Stress
A critical aspect of stressors is their duration. Acute stressors refer to those that are relatively short-lived, whereas chronic stressors refer to those that are ongoing and may not have a precise ending time. Therefore, acute stressors come and go and generally transpire at a faster timescale than chronic stressors. Individuals’ responses to acute stressors are essential for day-to-day adaptation and survival. The majority of past research that examined the relationship between acute stressors and BP responses has relied on acute stressor manipulations (e.g., evaluated interview or arithmetic tasks), which have been associated with real-time increases in inflammatory markers, HR and BP (12,13). While examining laboratory stressors has its merits, including standard manipulations in a controlled environment, one major limitation is its limited generalizability to real-world stressors. Existing research shows that physiological responses to laboratory stressors generalize poorly to real-life stressors and some research shows that cardiovascular responses are stronger in real-life settings (14). Nevertheless, research examining real-life acute stressors and BP is sparse, with some work suggesting that acute stress exposure is unlikely to be a risk factor for hypertension (15) and other work showing that it is associated with sustained increases in BP (16).
Chronic stress is present even in the absence of acute stressors. It can be thought of as the enduring stress level in the background, even when no events are happening (17). Chronic stress is particularly detrimental to health (3). Numerous studies, including prospective studies, consistently show that chronic stress levels or exposure is associated with higher inflammation and increased risks for hypertension (2,18,19). The effect of chronic stress on health could be due to prolonged activation of physiological stress-response systems and altered responses to everyday stressors (1).
Chronic Stress as a Moderator of Acute Stress
Chronic stress can influence the magnitude of physiological responses to acute stressors, resulting in heightened or blunted responses. Heightened responses could result from a lack of normal habituation to everyday stressors or a lack of recovery when a stressor is over (20). Blunted responses could be due to exhaustion, desensitization, or simply the law of initial values (21) for those experiencing high resting BP and HR. Existing findings on acute laboratoray stressors regarding this interaction effect are inconsistent (17). Some research shows that chronic life stress exposure is associated with exaggerated psychological, hypothalamic-pituitary-adrenal, and inflammatory responses to acute lab stressors (22,23). Other work suggests that chronic stress exposure is associated with reduced BP reactivity to acute stressors (24,25). Still, other research shows no relationship between perceived stress (in the past month) and stressor-induced BP and HR reactivity (26). Nevertheless, both exaggerated and blunted responses to laboratory stressors have been shown to predict future risks for different chronic diseases (see (27) for a review), making it important to understand how chronic stress exposure shapes reactions to acute stressors.
Ecological Momentary Assessments
Real-life stress has been traditionally assessed by asking individuals to recall the presence or absence of stressful experiences or their stress levels over a period of time (e.g., in the past six months). One major limitation of this approach is recall bias. Prior work suggests that negative affect and symptoms tend to be overestimated in recall surveys compared to daily report aggregates (28). Further, as this assessment approach is commonly used in cross-sectional studies, it often only allows for between-person analyses. It is well-known that the associations between constructs often differ within and between persons (29).
With the use of wearable devices, obtaining HR is straightforward but it is insufficient—HR offers some insight but limited psychological and physiological inferences because HR is dually innervated by sympathetic and parasympathetic branches of the nervous system. BP measures provide more diagnostic information regarding health, but the majority of past research has measured BP using cuffs or continuous BP monitors that require individuals to go to the laboratory or the doctor’s office, limiting understanding about individuals’ BP in daily life. Prior studies that examined ambulatory BP tend to use bulky BP devices that must be carried separately, limiting their scalability (e.g., 8).
Studies using ecological momentary assessments (EMA) in which individuals were assessed repeatedly from moment to moment as they go about their daily life have methodological merits (30). Recent technological advances make it possible to measure ambulatory BP using small portable devices (e.g., optic sensor embedded in smartphone), which could be integrated into existing EMA protocols (11). Studying stress and BP using EMA methods offers unique advantages (31). First, individuals are assessed in their natural environment close to real time, reducing recall bias and enhancing ecological validity. Second, the types of stressful situations examined in daily life across weeks are diverse. Finally, the repeated assessments allow for the examination of within-person associations between stress and BP.
The Present Study
This study uses an app-based platform to examine within- and between-person associations of acute and chronic stress with physiological responses in daily life. Examining between-person associations between stress and physiology allows us to answer questions about “who” (e.g., do individuals with higher stress levels also have higher average BP and HR?). Examining within-person associations allows us to answer questions about “when” (e.g., when individuals are more stressed than they usually are, do they have higher BP and HR in those moments?). Although acute and chronic stress differ in duration, they both have within- and between-person components. Individuals can differ in the frequency of acute stress or the mean level of (acute or chronic) stress severity they experience in daily life. Additionally, the occurrence and severity of acute stress and perceived background stress severity are also likely to fluctuate across moments within persons.
In the present study, we first examined whether acute stress exposure, acute stress severity, and chronic stress severity were associated with physiological responses (i.e., BP and HR) within and between persons. We hypothesized that acute stress exposure, acute stress severity, and chronic stress severity would be positively associated with physiological measures both between and within persons. Second, we examined whether individuals’ average level of chronic stress severity moderated within-person associations between momentary stress and physiological measures. Due to inconsistent findings in past research regarding the moderating role of chronic stress on physiological reactivity to acute stressors and the lack of research examining real-life acute stressors, we had competing hypotheses. On the one hand, when average chronic stress severity is high, the cardiovascular stress response system could be sensitized, leading to heightened BP and HR responses to momentary stress. On the other hand, it is also reasonable to anticipate that when average chronic stress severity is high, the cardiovascular system could become more desensitized to stress, leading to smaller (blunted) BP responses.
Method
Participants and Procedure
Individuals enrolled in the study by downloading the MyBPLab app available on the Google Playstore and Samsung websites. The study was approved for global use but was promoted on the Google Playstores in eight countries (US, Canada, India, Singapore, Australia, New Zealand, UK, Hong Kong). The data reported here were from the 2.0 version of the app that was available between March 2019 and December 2021. The only exclusions were participants had to be 18 or older and pass an English proficiency test because the app was only available in English. English proficiency was obtained with a short language test provided prior to reading and signing the informed consent form that was then emailed to the participants. The app required users to have a compatible phone or watch (e.g., Samsung Galaxy 9, Galaxy Watch Active) that had an embedded optic sensor (specifically designed to measure BP and HR; it is a separate sensor from the camera in phones) worked as a photoplethysmograph to measure physiological responses. Upon enrolling in the study, participants watched a video that described how to appropriately provide an optic sensor measurement. For example, one should be seated with the sensors at heart height, limit movement, and stay still during the measurement.
Participants received notifications of check-in surveys three times each day (i.e., morning, afternoon, and evening) and the study was designed for 21 days of check-ins, although participants could continue beyond 21 days if they desired. At each check-in, BP and HR were assessed, and then participants completed measures of their current thoughts and feelings, including measures of stress. Participants received feedback about their current BP and HR as an incentive to participate. They also received summary reports about their stress and emotions at the end of the 21-day study if they actively participated.
The study was approved by the institutional review board at the University of California, San Francisco (19-27169). After applying data cleaning procedures (see below), the final analytic sample for multilevel analyses consisted of 31,964 participants providing 496,214 check-ins. Participants’ characteristics are shown in Table 1.
Table 1.
Demographic Characteristics of the Final Sample
| N Participants | 31,964 | |
| N Observations | 496,214 | |
| Mean (SD) | Range | |
|---|---|---|
| Age | 43.81 (12.84) | 18-90 |
| Socioeconomic Status (SES) | 5.68 (2.24) | 1-10 |
| SBP | 128.40 (15.98) | 80-210 |
| DBP | 79.58 (11.27) | 50-150 |
| HR | 75.13 (13.52) | 30-174 |
| Acute stress | 0.14 | 0-1 |
| Acute stress severity | 2.64 (0.94) | 0-4 |
| Chronic Stress severity | 0.74 (0.91) | 0-4 |
| N | % | |
| Sex | ||
| Female | 9,705 | 30.4 |
| Male | 22,144 | 69.3 |
| Other | 115 | 0.4 |
| Race | ||
| White or European | 20,983 | 67.4 |
| Asian | 3,103 | 10.0 |
| Black or African American | 2,059 | 6.6 |
| Indian | 1,539 | 4.9 |
| American Indian or Alaska Native | 230 | 0.7 |
| Pacific Islander | 142 | 0.5 |
| Other or Mixed | 2,491 | 8.0 |
| Decline to Comment | 598 | 1.9 |
| Missing | 819 | |
| Latino | ||
| Latino | 3,445 | 10.9 |
| Not Latino | 28,150 | 89.1 |
| Missing | 369 | |
| Education | ||
| No high school diploma | 840 | 2.7 |
| High school/GED | 4,995 | 15.9 |
| Some college | 7,396 | 23.5 |
| 2-year college degree | 3,548 | 11.3 |
| 4-year college degree | 7,778 | 24.7 |
| Graduate school degree | 6,910 | 22.0 |
| Missing | 497 | |
| Country | ||
| United States | 21,688 | 67.9 |
| United Kingdom | 2,725 | 8.5 |
| Australia | 2,551 | 8.0 |
| Canada | 1,763 | 5.5 |
| India | 894 | 2.8 |
| Singapore | 590 | 1.8 |
| Hong Kong | 326 | 1.0 |
| New Zealand | 161 | 0.5 |
| Other | 1,235 | 3.9 |
| Missing | 31 | |
| Hypertension diagnosis (self-report) | ||
| Yes | 22,879 | 71.7 |
| No | 9,043 | 28.3 |
| Missing | 42 |
Note. SES was obtained with the MacArthur Scale of Subjective Social Status where participants ranked their perceived standing relative to people in their country on a 10-rung ladder. The ladder was coded from 1 (top of the ladder; wealthiest) to 10 (bottom of the ladder; poorest). It was reversed coded so that higher values indicate higher wealth.
Measures
Physiological Responses
Given the novelty of the optic sensor used in this paper, we first summarize initial data collected to assess the reliability and validity of the optic sensor. We recruited 123 individuals to participate in a multimethod study requiring BP assessments in the lab and in daily life. Participants completed BP assessments with three devices: smartphone sensor on fingertip, smartwatch on wrist, and a validated BP cuff approved by the Food and Drug Administration. Across data collected in the laboratory and in the field, there was moderate to strong agreement between the three devices. For example, when conducting simultaneous measurements with a validated cuff, looking at participant averages across in-lab assessments, the cuff and phone sensor were correlated .75 (SBP) and .87 (DBP) while the cuff and watch sensor were correlated .77 (SBP) and .83 (DBP). The phone sensor and watch sensor were correlated .83 (SBP) and .89 (DBP), indicating high levels of agreement between the two optic sensors (see supplemental materials at https://osf.io/rntmc/). For more details on validation of the phone sensor, which has been previously published, see https://www.pnas.org/doi/suppl/10.1073/pnas.2105573118.
When participants joined the study, they were encouraged to calibrate the sensor with a BP reference device. We encouraged the use of a Bluetooth-enabled BP monitor (A&D Medical [a company]) that directly populated the app with systolic (SBP) and diastolic blood pressure (DBP) values from a measurement, but we accepted any measure from a BP device for the calibration values. These reference values were used in the algorithm to estimate SBP and DBP. Participants were allowed to recalibrate their BP over the course of the study, and their BP estimates at each moment take into account these changes. If participants did not provide calibration values, the app would use default reference values and only display relative percent changes in BP from the last check-in (e.g., SBP decreased 1%; DBP decreased 0.5% since the last check-in), which served as an incentive to calibrate the sensor. In this paper, we only examined SBP and DBP responses that were calibrated (i.e., 75% of all check-ins; 57% of all participants).
At each check-in, participants provided a sensor reading that required them to hold their index finger over the optic sensor for approximately 30s. Using this recording, we estimated SBP, DBP, and HR. The BP and HR values were displayed once this measuring process was completed. The descriptive statistics of BP and HR of the final sample is shown in Table 1. For descriptive statistics of BP and HR separately for phones and watches, see supplemental materials on OSF (https://osf.io/rntmc/). The differences in mean BP and HR between the two types of devices are minimal.
Stress Measures
At each check-in, after providing physiological readings, participants responded to the question “Have you experienced any particularly stressful events since your last check-in?” using a binary response scale (0: No; 1: Yes). This item measured the presence or absence of acute stress exposure. If they responded “Yes,” then they were asked, “How stressful was it?” which assessed acute stress severity. Participants rated the question on a 5-point scale (0: not at all; 1: a little bit; 2: somewhat; 3: moderately; and 4: extremely). For moments without acute stressors reported, acute stress severity rating was treated as missing. This study uses similar items to assess acute stress exposure and acute stress severity as in existing EMA studies of acute stressors (10). If participants responded “No” to the acute stress exposure question, they were directed to respond to the item “I feel stressed, anxious, overwhelmed” using the same 5-point scale, which we used as a measure of stress severity in the absence of a recent acute stressor (for moments with acute stressors, this rating was treated as missing). At the momentary level, we refer this to momentary background stress severity. At the between-person level, we averaged scores within individuals across the study period to indicate chronic stress severity, given that chronic stress is relatively stable across time (32,33). To access the stability of this construct in this study, we computed a measure of reliability for the within-person means of background stress severity using the formula (34) :
in which n is the average cluster size (i.e., the average number of observations of momentary background stress severity per person). The reliability coefficient was .91, indicating excellent reliability (i.e., showing strong evidence of stability across time). As chronic stress is typically defined as lasting for one month or more (35), as a sensitivity analysis, we computed this reliability estimate again by limiting the final sample to individuals who stayed in the study for at least 30 days (51% of individuals in the final sample) to make sure that the observations spanned across at least a month. The reliability coefficient was .94. These findings offer support for operationalizing chronic stress as the within-person means of momentary background stress severity.
Control Variables
The analyses included the following control variables: time of day, check-in number, sex, age, BMI, and device (watch vs. phone). The time of day of the momentary assessment was coded into three categories (0: night, 1: morning, and 2: afternoon); it was included as a covariate because there is a diurnal cycle of BP levels. Check-in number indicates the order in which the check-ins were recorded, which was used to control for possible time trends or the effect of repeated measurements in the study. Also, we controlled for sex, age, and BMI because these are known individual differences that affect BP and HR. Participants’ sex was coded into three categories (0: female, 1: male, and 2: other), with the other category collapsing non-binary, transgender, and open text responders. Although not ideal for collapsing such disparate groups, the percentage of participants falling in these combined groups was relatively small (0.4%), limiting the ability to make any inferences about stress and BP. BMI was calculated using the formula: (weight in pounds × 703) / height in inches2. BMI and age were grand-mean centered. Also, we controlled for whether individuals used phone or watch (coded as 0: phone, 1: watch) as there may be potential selection factors that affect individuals in choosing a specific type of device.
Data Cleaning
We set uncalibrated BP values and extreme values of HR (< 30 or >200), SBP (<80 or >210), and DBP (<50 or >180) to missing. BP values were also set to missing if participants reported that they exercised within the past 30 minutes. We also set BP values into missing if there was zero or negative pulse pressure (SBP minus DBP) in sensor estimates or calibration BP values used for sensor estimates, suggesting an error in input values (consisting of 0.01% of all non-missing BP sensor estimates). Next, demographics with extreme values in age (> 90), weight (< 80 or > 500 pounds), height (< 36 or > 84 inches), or BMI (< 15 or > 60) were also set to missing. Finally, for participants who had more than 100 check-ins, we only retained the first 100 check-ins to limit their influence on the analyses (see (11) for a similar approach).
Analytic Plan
All analyses were conducted in R. The unconditional models were estimated using the lme4 (36) package and all primary analyses models were estimated using nlme package (37). We first computed the intraclass correlation (ICC) (i.e., between-person variance divided by total variance) for each outcome and primary predictor based on unconditional models to examine the degree of within-person and between-person variance.
For our primary analyses, we conducted three sets of multilevel models to examine within- and between-person associations between stress and physiological measures. In each of these analyses, observations were nested within individuals, random intercepts and random slopes of the respective stress variables were allowed, and residuals within person within day were modeled as a first-order autoregressive process [i.e. AR(1)]. In addition, we controlled for time of day, check-in number, and device at level 1, and sex, age, and BMI at level 2. At level 1, categorical predictors (including momentary acute stress exposure) were uncentered and continuous predictors were person-mean centered, except that check-in number was centered at 0. At level 2, categorical predictors were uncentered, and continuous predictors were grand-mean centered.
In the first set of analyses, we examined the within- and between-person associations between acute stress exposure and physiological responses (i.e., SBP, DBP, and HR). Momentary acute stress exposure (level 1) and person-mean acute stress exposure (level 2) were added along with covariates as predictors of each physiological response. As level-1 acute stress exposure was uncentered, including its person means in the models allows for the examination of within-person effect (38). We allowed for random intercepts and random slopes of momentary acute stress exposure. A sample model equation for SBP (covariates not shown) is:
Similarly, in the second set of analyses, we examined the within- and between-person associations between acute stress severity and physiological responses during moments with acute stress exposure. Momentary acute stress severity (level 1) and person-mean acute stress severity (level 2) were added along with covariates as predictors of each physiological response.
In the third set of analyses, we examined the within- and between-person associations between stress severity and physiological responses during moments without acute stress exposure. Momentary background stress severity (level 1) and chronic stress severity (person-mean momentary background stress severity; level 2) were added along with covariates as predictors of each physiological response.
Next, we examined whether chronic stress severity moderated physiological responses to momentary stress (i.e., acute stress exposure, acute stress severity, and background stress severity). To do so, we added chronic stress severity as a level-2 predictor of the intercept (γ02) and slope (γ11) between each momentary stress variable and each physiological response (i.e., nine sets of analyses). Further, although not of focal interest, the level-2 interaction term between the respective person-mean stress variable and chronic stress severity (γ03) was included as a covariate, which was recommended when doing cross-level interactions (38). A sample model equation for chronic stress severity moderating the associations between momentary acute stress exposure predicting SBP (standard covariates not shown) is:
Results
Descriptive Statistics
The original sample consisted of 83,635 participants who provided 832,453 observations. In this sample, 39% of participants had only one check-in, 15% had only two, 9% had three, and 38% had four or more. We examined whether the stress and physiological variables were correlated with participants’ total number of check-ins in the study and found that they indeed were, but the magnitude of correlations was small (∣r∣ ranged .00–.08). Additionally, participants’ total number of check-ins was correlated with their age (r = .26), BMI (r = .05), and sex (r ranged from −.01 to −.02) . These variables were controlled for in the primary analyses. For all multilevel analyses, we only included individuals with three or more valid (i.e., with non-missing data on acute stress exposure, the respective physiological response, and covariates) check-ins. This final analytic sample consisted of 31,964 participants with 496,214 observations. Although our study was designed to be 21 days, many participants stayed beyond this period. In the final sample, participants on average provided data that spanned across 112.8 days, with generally sparse observations over the course of the study. First, we examined the ICC of the primary outcomes and predictors using unconditional models. Results indicated that physiological outcomes showed more between-person variance than within-person variance (ICCs: SBP = .78; DBP = .77; HR = .56). In contrast, stress predictors showed more within-person variance than between-person variance (ICCs: acute stress exposure = .28; acute stress severity = .28; chronic stress severity = .46). Overall, results indicated adequate variance for both within- and between-person analyses.
Within- and Between-Person Associations Between Momentary Stress and Physiological Responses
In the first set of analyses, momentary acute stress exposure (present vs. absent) was significantly associated with higher SBP (b = 1.54, SE = 0.05, p < .001), DBP (b = 0.79, SE = 0.03, p < .001), and HR (b = 1.53, SE = 0.05, p < .001) at the within-person level (Table 2). This suggests that individuals’ SBP, DBP, and HR were higher in moments with reported acute stress exposure, compared to moments without exposure. Looking between persons, individuals’ proportion of acute stress exposure was significantly associated with SBP (b = 2.92, SE = 0.54, p < .001), DBP (b = 0.82, SE = 0.39, p = .037), and HR (b = 4.14, SE = 0.31, p < .001), indicating that individuals who reported a greater proportion of check-ins with recent acute stress exposure had higher average BP and HR.
Table 2.
Within- and Between-Person Associations Between Acute Stress Exposure and Physiological Responses.
| SBP | SBP | DBP | DBP | HR | HR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N participants | 22,015 | 21,690 | 22,015 | 21,690 | 31,963 | 31,370 | ||||||
| N observations | 343,521 | 341,181 | 343,521 | 341,181 | 496,030 | 492,237 | ||||||
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Fixed effects | ||||||||||||
| Intercept | 126.79*** | 0.18 | 126.67*** | 0.18 | 79.01*** | 0.13 | 78.93*** | 0.13 | 77.92*** | 0.11 | 77.84*** | 0.11 |
| Momentary acute stress exposure | 1.54*** | 0.05 | 1.58*** | 0.05 | 0.79*** | 0.03 | 0.82*** | 0.04 | 1.53*** | 0.05 | 1.55*** | 0.05 |
| Person-mean acute stress exposure (contextual) | 1.38* | 0.54 | −0.15 | 0.62 | 0.03 | 0.39 | −1.53*** | 0.45 | 2.61*** | 0.31 | 1.32*** | 0.36 |
| Person-mean acute stress exposure (between) + | 2.92*** | 0.54 | 0.82* | 0.39 | 4.14*** | 0.31 | ||||||
| Chronic stress severity | 1.96*** | 0.15 | 1.66*** | 0.11 | 1.27*** | 0.09 | ||||||
| Momentary acute stress exposure × Chronic stress severity | −0.29*** | 0.07 | −0.17*** | 0.05 | −0.11 | 0.07 | ||||||
| Person-mean acute stress exposure × Chronic stress severity | −0.90 | 0.68 | −0.38 | 0.50 | −0.02 | 0.39 | ||||||
| Time of day 1 (morning) | −0.09** | 0.03 | −0.09** | 0.03 | 0.75*** | 0.02 | 0.75*** | 0.02 | −3.81*** | 0.03 | −3.82*** | 0.03 |
| Time of day 2 (afternoon) | −0.25*** | 0.03 | −0.25*** | 0.03 | 0.08*** | 0.02 | 0.08*** | 0.02 | −0.60*** | 0.03 | −0.61*** | 0.03 |
| Check-in number | −0.03*** | 0.00 | −0.03*** | 0.00 | −0.02*** | 0.00 | −0.02*** | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sex 1 (male) | 4.16*** | 0.21 | 4.32*** | 0.21 | 2.09*** | 0.15 | 2.19*** | 0.15 | −2.32*** | 0.12 | −2.24*** | 0.12 |
| Sex 2 (other) | 0.44 | 1.55 | −0.11 | 1.58 | 0.16 | 1.12 | 0.12 | 1.14 | −0.76 | 0.94 | −0.86 | 0.96 |
| Age | 0.14*** | 0.01 | 0.16*** | 0.01 | −0.03*** | 0.01 | −0.02*** | 0.01 | −0.21*** | 0.00 | −0.20*** | 0.00 |
| BMI | 0.55*** | 0.01 | 0.55*** | 0.01 | 0.28*** | 0.01 | 0.27*** | 0.01 | 0.23*** | 0.01 | 0.23*** | 0.01 |
| Device (watch) | −0.56*** | 0.07 | −0.54*** | 0.07 | −0.88*** | 0.05 | −0.87*** | 0.05 | −0.08 | 0.06 | −0.07 | 0.06 |
| Random effects | ||||||||||||
| Intercept Variance | 185.39 | 183.73 | 98.03 | 96.68 | 87.24 | 86.54 | ||||||
| Slope Variance | 3.53 | 3.43 | 1.60 | 1.58 | 5.99 | 6.01 | ||||||
| Residual Variance | 59.52 | 59.56 | 30.64 | 30.67 | 77.61 | 77.62 |
Note.
p < .05
p < .01
p < .001. The unit of SBP and DBP is mmHg and the unit of HR is beats per minute. Sex 0 (female) was used as the reference group for sex; Time of day 0 (night) was used as the reference group for time of day. Wald tests were conducted on fixed effects only. Effects of primary interests are bolded.
As level-1 acute stress exposure (binary) was uncentered, the between-person effect was calculated as the sum of the within-person effect and the contextual effect. Model with interactions is shown in the second column of each physiological outcome.
In the second set of analyses, for moments with acute stress exposure, higher momentary acute stress severity than usual was significantly associated with higher SBP (b = 0.26, SE = 0.06, p < .001), DBP (b = 0.09, SE = 0.04, p = .049), and HR (b = 0.40, SE = 0.06, p < .001) (Table 3), suggesting when individuals reported more severe acute stress than they usually did during moments with acute stress, their SBP, DBP, and HR were higher in those moments. At the between-person level, individuals with higher average acute stress severity during moments with acute stress exposure had higher average HR (b = 0.99, SE = 0.12, p < .001), but not SBP (b = 0.05, SE = 0.18, p = .78) or DBP (b = −0.01, SE = 0.13, p = .95).
Table 3.
Within- and Between-Person Associations Between Acute Stress Severity and Physiological Responses.
| SBP | SBP | DBP | DBP | HR | HR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N participants | 12,670 | 12,509 | 12,670 | 12,509 | 18,809 | 18,490 | ||||||
| N observations | 41,427 | 41,019 | 41,427 | 41,019 | 65,348 | 64,454 | ||||||
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Fixed effects | ||||||||||||
| Intercept | 127.95*** | 0.27 | 127.67*** | 0.28 | 79.61*** | 0.20 | 79.39*** | 0.20 | 78.96*** | 0.18 | 78.74*** | 0.19 |
| Momentary acute stress severity | 0.26*** | 0.06 | 0.23*** | 0.07 | 0.09* | 0.04 | 0.09 | 0.05 | 0.40*** | 0.06 | 0.37*** | 0.06 |
| Person-mean acute stress severity | 0.05 | 0.18 | −0.20 | 0.18 | −0.01 | 0.13 | −0.26 | 0.13 | 0.99*** | 0.12 | 0.72*** | 0.12 |
| Chronic stress severity | 1.56*** | 0.20 | 1.33*** | 0.15 | 0.97*** | 0.13 | ||||||
| Momentary acute stress severity × Chronic stress severity | 0.15 | 0.09 | 0.03 | 0.06 | 0.11 | 0.08 | ||||||
| Person-mean acute stress severity × Chronic stress severity | −0.12 | 0.25 | 0.08 | 0.18 | 0.42** | 0.16 | ||||||
| Time of day 1 (morning) | −0.01 | 0.12 | −0.01 | 0.12 | 0.74*** | 0.09 | 0.73*** | 0.09 | −3.03*** | 0.11 | −3.03*** | 0.12 |
| Time of day 2 (afternoon) | −0.22* | 0.10 | −0.22* | 0.10 | 0.04 | 0.07 | 0.03 | 0.07 | −0.19* | 0.09 | −0.22* | 0.09 |
| Check-in number | −0.03*** | 0.00 | −0.03*** | 0.00 | −0.02*** | 0.00 | −0.02*** | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sex 1 (male) | 4.49*** | 0.29 | 4.55*** | 0.29 | 2.15*** | 0.21 | 2.17*** | 0.21 | −1.68*** | 0.19 | −1.64*** | 0.19 |
| Sex 2 (other) | 0.68 | 2.11 | 0.25 | 2.17 | 0.32 | 1.52 | 0.65 | 1.56 | −0.07 | 1.32 | 0.13 | 1.33 |
| Age | 0.15*** | 0.01 | 0.16*** | 0.01 | −0.03*** | 0.01 | −0.02* | 0.01 | −0.23*** | 0.01 | −0.22*** | 0.01 |
| BMI | 0.58*** | 0.02 | 0.57*** | 0.02 | 0.28*** | 0.01 | 0.28*** | 0.01 | 0.23*** | 0.01 | 0.23*** | 0.01 |
| Device (watch) | −0.23 | 0.19 | −0.15 | 0.19 | −0.72*** | 0.14 | −0.66*** | 0.14 | −0.15 | 0.15 | −0.11 | 0.15 |
| Random effects | ||||||||||||
| Intercept Variance | 186.48 | 185.41 | 96.19 | 95.03 | 94.48 | 94.29 | ||||||
| Slope Variance | 1.03 | 1.02 | 0.30 | 0.31 | 0.30 | 0.29 | ||||||
| Residual Variance | 62.30 | 62.36 | 33.00 | 33.01 | 87.30 | 87.13 |
Note.
p < .05
p < .01
p < .001. The unit of SBP and DBP is mmHg and the unit of HR is beats per minute. Sex 0 (female) was used as the reference group for sex; Time of day 0 (night) was used as the reference group for time of day. Wald tests were conducted on fixed effects only. Effects of primary interests are bolded. Model with interactions is shown in the second column of each physiological outcome.
In the third set of analyses, for moments without acute stress exposure, momentary background stress severity was significantly associated with higher SBP (b = 0.87, SE = 0.03, p < .001), DBP (b = 0.51, SE = 0.02, p < .001), and HR (b = 0.69, SE = 0.03, p < .001) at the within-person level (Table 4). That is, when participants reported higher background stress severity than usual, they showed higher SBP, DBP, and HR in the moment. At the between-person level, during moments without acute stress exposure, individuals with higher chronic stress severity, had higher average SBP (b = 1.94, SE = 0.14, p < .001), DBP (b = 1.57, SE = 0.10, p < .001), and HR (b = 1.39, SE = 0.09, p < .001).
Table 4.
Within- and Between-Person Associations Between Chronic Stress Severity and Physiological Responses.
| SBP | SBP | DBP | DBP | HR | HR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N participants | 21,687 | 21,687 | 21,687 | 21,687 | 31,361 | 31,361 | ||||||
| N observations | 277,661 | 277,661 | 277,661 | 277,661 | 396,244 | 396,244 | ||||||
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Fixed effects | ||||||||||||
| Intercept | 126.63*** | 0.19 | 126.76*** | 0.20 | 78.88*** | 0.13 | 78.97*** | 0.14 | 77.94*** | 0.12 | 77.85*** | 0.12 |
| Momentary background stress severity | 0.87*** | 0.03 | 0.89*** | 0.03 | 0.51*** | 0.02 | 0.52*** | 0.02 | 0.69*** | 0.03 | 0.72*** | 0.03 |
| Chronic stress severity | 1.94*** | 0.14 | 2.13*** | 0.18 | 1.57*** | 0.10 | 1.71*** | 0.13 | 1.39*** | 0.09 | 1.26*** | 0.11 |
| Momentary background stress severity × Chronic stress severity | −0.09 | 0.05 | −0.07 | 0.03 | −0.12** | 0.04 | ||||||
| Chronic stress severity × Chronic stress severity | −0.27 | 0.15 | −0.20 | 0.11 | 0.18* | 0.09 | ||||||
| Time of day 1 (morning) | −0.11** | 0.04 | −0.11** | 0.04 | 0.74*** | 0.03 | 0.74*** | 0.03 | −3.90*** | 0.04 | −3.90*** | 0.04 |
| Time of day 2 (afternoon) | −0.31*** | 0.03 | −0.31*** | 0.03 | 0.05* | 0.02 | 0.05* | 0.02 | −0.70*** | 0.03 | −0.70*** | 0.03 |
| Check-in number | −0.03*** | 0.00 | −0.03*** | 0.00 | −0.02*** | 0.00 | −0.02*** | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sex 1 (male) | 4.35*** | 0.21 | 4.34*** | 0.21 | 2.27*** | 0.15 | 2.27*** | 0.15 | −2.32*** | 0.13 | −2.31*** | 0.13 |
| Sex 2 (other) | 0.10 | 1.59 | 0.15 | 1.59 | 0.38 | 1.15 | 0.41 | 1.15 | −0.25 | 0.99 | −0.26 | 0.99 |
| Age | 0.16*** | 0.01 | 0.16*** | 0.01 | −0.02*** | 0.01 | −0.02*** | 0.01 | −0.20*** | 0.00 | −0.20*** | 0.00 |
| BMI | 0.55*** | 0.01 | 0.55*** | 0.01 | 0.27*** | 0.01 | 0.27*** | 0.01 | 0.23*** | 0.01 | 0.23*** | 0.01 |
| Device (watch) | −0.50*** | 0.08 | −0.50*** | 0.08 | −0.85*** | 0.06 | −0.85*** | 0.06 | −0.07 | 0.07 | −0.07 | 0.07 |
| Random effects | ||||||||||||
| Intercept Variance | 184.55 | 184.53 | 96.73 | 96.72 | 87.54 | 87.52 | ||||||
| Slope Variance | 2.20 | 2.20 | 1.07 | 1.07 | 1.84 | 1.84 | ||||||
| Residual Variance | 56.98 | 56.98 | 29.65 | 29.65 | 75.00 | 75.00 |
Note.
p < .05
p < .01
p < .001. The unit of SBP and DBP is mmHg and the unit of HR is beats per minute. Sex 0 (female) was used as the reference group for sex; Time of day 0 (night) was used as the reference group for time of day. Wald tests were conducted on fixed effects only. Effects of primary interests are bolded. Model with interactions is shown in the second column of each physiological outcome.
Does Chronic Stress Severity Moderate Physiological Responses to Momentary Stress?
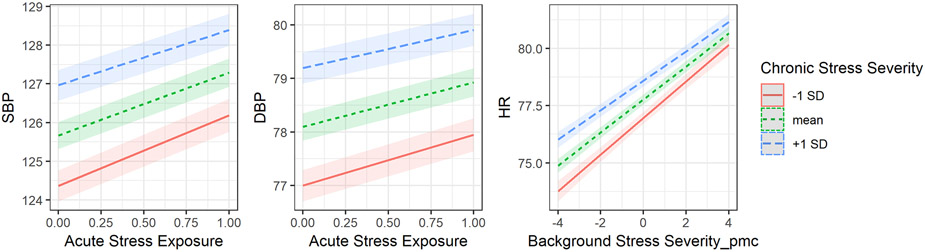
Next, we turned to the question of whether (between-person) chronic stress severity and momentary stress interacted to predict physiology. We found significant interactions between chronic stress severity and momentary acute stress exposure in predicting SBP (b = −0.29, SE = 0.07, p <.001) and DBP (b = −0.17, SE = 0.05, p < .001) (see Figure 1). The model with HR was not significant. The nature of the interaction suggests that for individuals with higher chronic stress severity, exposure to an acute stressor was associated with smaller increases in SBP and DBP than individuals with lower chronic stress severity. We next examined whether chronic stress severity and momentary acute stress severity interacted to influence physiological responses and found no significant moderation across the three physiological responses. Finally, we tested the interaction between chronic stress severity and momentary background stress severity, which yielded significant interaction for HR (b = −0.12, SE = 0.04, p = .006), but not for SBP (b = −0.09, SE = 0.05, p = .05) and DBP (b = −0.07, SE = 0.03, p = .05) (see Figure 1). Similar to the previous interactions, people with higher chronic stress severity, compared to people with lower chronic stress severity, had smaller rises in HR when their momentary background stress was more severe than usual. Taken together, these models support the interpretation that those with higher chronic stress severity may have blunted physiological responses when experiencing an acute stressor (versus not) or when experiencing higher background stress severity than usual.
Figure 1. The moderating effect of chronic stress severity on physiological responses to momentary stress.
Note. The unit of SBP and DBP is mmHg and the unit of HR is beats per minute. Background Stress Severity_pmc = person-mean centered background stress severity. The plotting function was not able to compute 95% confidence intervals for models that included an AR(1) error covariance structure. Therefore, models without this error covariance structure were used only for plotting purpose. The simple slopes are essentially the same.
Discussion
Stress can influence physiological responses such as BP and HR. However, large studies examining these associations in everyday settings are limited. This study examined the within- and between-person associations of acute and chronic stress with BP and HR. There were a few key findings. First, moments with acute stress exposure were associated with higher momentary BP and HR within persons. Further, individuals with a higher proportion of acute stress exposure on average had higher BP and HR. Second, during moments with acute stress, moments with higher acute stress severity than usual were associated with higher momentary SBP, DBP, and HR. Individuals with higher average acute stress severity across moments with acute stress had higher average HR, but not average BP. Third, moments with higher background stress severity than usual were associated with higher momentary BP and HR. Individuals with higher chronic stress severity had higher average BP and HR. Finally, between-person chronic stress severity moderated 1) the within-person associations between acute stress exposure and BP and 2) the within-person associations between background stress severity and HR. Specifically, for individuals with higher chronic stress severity, their average BP and HR were higher, but their momentary physiological responses to acute stress exposure and background stress severity were smaller. Nevertheless, the moderation effect appeared small.
The significant within-person associations between acute stress exposure and physiological responses suggest that individuals’ cardiovascular system is responsive to the occurrence of real-life stressors at the momentary level. Further, between-person finding indicates that individuals with a higher proportion of acute stress exposure have higher BP and HR, suggesting that the frequency of acute stress exposure in daily life may contribute to lasting (rather than transient) differences in BP and HR across individuals. It is possible that frequent exposure to acute stress could become chronic stress; nevertheless, recent work highlights a critical knowledge gap regarding when this transition happens (2), which is an important topic for future research.
The severity of stress also seems to matter. Our results show that BP and HR are sensitive to momentary fluctuations in acute and background stress severity. Chronic stress severity in this study was operationalized as the average momentary background stress severity. Between-person results suggest that the severity of chronic stress (but not acute stress) over time may contribute to individual differences in BP. For acute stress, it appears that the frequency of exposure matters more than its average severity for individual differences in average BP. This is not surprising given the frequency of acute stress exposure captures the cumulative aspect of stressors whereas average acute stress severity does not. Nevertheless, it is possible that the frequency and severity of acute stressors can have a combined influence on BP, a topic for future research.
Regarding the moderating role of chronic stress severity on physiological responses to momentary stress, results indicate that individuals with higher chronic stress severity had higher BP and HR compared to those with lower chronic stress severity, but they showed slightly smaller physiological responses (smaller increases) to momentary acute stress exposure and momentary background stress severity. This finding is consistent with the law of initial values (21), such that the higher the initial values, the smaller the increases in physiological responses. These findings may reflect important regulatory mechanisms in human physiology.
The present study focuses on perceived stress. It is possible that between-person differences in perceived stress (acute or chronic) could be influenced by individuals’ factors such as neuroticism (39). However, as prior work shows that neuroticism is generally unrelated to individuals’ average BP levels (40), it is unlikely that the between-person associations between average acute stress exposure or chronic stress severity and average BP was confounded by neuroticism. Nevertheless, we could not rule out the possibility that the moderating role of chronic stress severity on BP reactivity to momentary stress was confounded by neuroticism. Future work may examine whether chronic stress severity still significantly interacts with momentary stress to predict BP after controlling for neuroticism.
The present research helps highlight a new avenue for collecting psychological and physiological data using an app-based platform. A major challenge in a large field study like this is to accurately measure BP in individuals’ daily life. It is almost inevitable that the errors in measurements in everyday life are larger than in the laboratory due to the uncontrolled nature of the measurement and the diversity of situations and contexts individuals are in. Therefore, a series of validation studies were conducted to determine the validity of BP estimates given the constraints and a large sample was recruited to minimize the effect of noise. The large sample indicates that many people are naturally curious about their physiology, which could be an effective incentive for participation. However, the low compliance rates highlights another challenge, which suggests that providing BP and HR feedback may not be sufficient. Other possible reasons could be the lack of incentives, boredom or lack of interest after initial BP assessment, and/or the burden posed by frequent requests for “check-ins.” Despite these challenges, the return is invaluable given we are able to examine the relationship between naturally-ocurring stressors and BP in individuals’ daily life. Nevertheless, although we observe within- and between-person associations between stress and cardiovascular responses in daily life, we cannot conclude whether these associations are clinically significant.
The present study has several limitations. First, the low compliance rate for the study could introduce bias in the study conclusions. Nevertheless, our results suggest that although the study variables were related to compliance, the effect appeared small. Although financial compensation seems to be a promising incentive in other EMA studies, it might be unrealistic to provide enough financial incentives for participants in such a large-scale study. Future studies should examine whether providing reminders for missed prompts and summaries of additional physiological responses (e.g., heart rate variability) would increase compliance rates. Second, as participants were asked to report acute stressors that happened since their last check-ins, the timeframe in which the stressors occurred may vary across people depending on their compliance. This approach might have underestimated the within-person physiological responses to acute stressors for some participants whose stressors were more distant in time. Third, as participants were not paid to participate in the study, in order to minimize participant burden, we only collected limited information regarding their stress experience (using 1-item measure for each stress construct), and we were not able to collect information regarding the type of stressors and coping techniques. As a result, the psychometric quality of the measures may be compromised, and our understanding of participants’ stress experience is limited. Fourth, we did not measure skin melanin in our study, and optic sensors, in general, have more difficulty with measurements with some skin tones and textures (e.g., darker or thicker skin can impair measurement). Fifth, we did not have information about whether individuals are taking blood pressure medication, it is possible that this may affect the study results. Sixth, although the current sample is large, it is nonetheless a self-selected sample, which may not necessarily be representative of the adult population. In addition, the MyBPLab 2.0 app only works with Samsung phones and could not interact with products without an embedded optic sensor. Finally, BP and HR were measured on discrete occasions, limiting the extent to which we could study the timing of stress reactivity and recovery. Future research using continuous physiological monitoring with closed-loop systems that trigger check-ins when responses increase or decrease relative to typical levels would provide a major step forward to examine stress and physiology in real-time.
In conclusion, our study highlights both opportunities and challenges in scaling EMA studies to examine psychological stress and real-time BP responses in daily life. Our findings indicate that individuals’ BP and HR responses in daily life are sensitive to momentary fluctuations in stress, and between-person differences in stress experiences are associated with individual differences in BP and HR.
Sources of Funding and Conflicts of Interest:
This project was supported by funding from the National Institute on Aging (R24 AG048024), the National Institute of Mental Health (T32 MH019391), and Samsung Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies. The authors declare no conflict of interest.
Glossary
- BP
blood pressure.
- SBP
systolic blood pressure.
- DBP
diastolic blood pressure.
- HR
heart rate.
- BMI
body mass index.
- EMA
ecological momentary assessments.
Footnotes
Data, analytic syntax, and supplemental materials are available online at (https://osf.io/rntmc/).
References
- 1.McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 2.Rohleder N. Stress and inflammation – The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology. 2019;105:164–71. [DOI] [PubMed] [Google Scholar]
- 3.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. Journal of the American Medical Association. 2007;298:1685–87. [DOI] [PubMed] [Google Scholar]
- 4.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17312–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kivimäki M, Virtanen M, Elovainio M, Kouvonen A, Väänänen A, Vahtera J. Work stress in the etiology of coronary heart disease—a meta-analysis. Scandinavian Journal of Work, Environment & Health. 2006;32:431–42. [DOI] [PubMed] [Google Scholar]
- 6.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–78. [DOI] [PubMed] [Google Scholar]
- 7.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: A meta-analysis of prospective evidence. Hypertension. 2010;55:1026–32. [DOI] [PubMed] [Google Scholar]
- 8.Kamarck TW, Li X, Wright AGC, Muldoon MF, Manuck SB. Ambulatory blood pressure reactivity as a moderator in the association between daily life psychosocial stress and carotid artery atherosclerosis. Psychosomatic Medicine. 2018;80:774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues S, Kaiseler M, Queirós C. Psychophysiological assessment of stress under ecological settings: A systematic review. European Psychologist. 2015;20:204–26. [Google Scholar]
- 10.Weber J, Angerer P, Apolinário-Hagen J. Physiological reactions to acute stressors and subjective stress during daily life: A systematic review on ecological momentary assessment (EMA) studies. Boylan JM, editor. PLOS ONE [Internet]. 2022;17:e0271996. Available from: https://dx.plos.org/10.1371/journal.pone.0271996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon AM, Mendes WB. A large-scale study of stress, emotions, and blood pressure in daily life using a digital platform. Proceedings of the National Academy of Sciences. 2021;118:e2105573118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neuroscience and Biobehavioral Reviews. 2014;38:94–124. [DOI] [PubMed] [Google Scholar]
- 13.Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain, Behavior, and Immunity. 2017;64:208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanstra YJ, Johnston DW. Cardiovascular reactivity in real life settings: Measurement, mechanisms and meaning. Biological Psychology. 2011;86:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sparrenberger F, Cichelero FT, Ascoli AM, Fonseca FP, Weiss G, Berwanger O, Fuchs SC, Moreira LB, Fuchs FD. Does psychosocial stress cause hypertension? A systematic review of observational studies. Journal of Human Hypertension. 2009;23:12–19. [DOI] [PubMed] [Google Scholar]
- 16.Gerin W, Chaplin W, Schwartz JE, Holland J, Alter R, Wheeler R, Duong D, Pickering TG. Sustained blood pressure increase after an acute stressor: The effects of the 11 September 2001 attack on the New York City World Trade Center. Journal of Hypertension. 2005;23:279–84. [DOI] [PubMed] [Google Scholar]
- 17.Gump BB, Matthews KA. Do background stressors influence reactivity to and recovery from acute stressors? Journal of Applied Social Psychology. 1999;29:469–94. [Google Scholar]
- 18.Spruill TM. Chronic psychosocial stress and hypertension. Current Hypertension Reports. 2010;12:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spruill TM, Butler MJ, Thomas SJ, Tajeu GS, Kalinowski J, Castañeda SF, Langford AT, Abdalla M, Blackshear C, Allison M, Ogedegbe G, Sims M, Shimbo D. Association between high perceived stress over time and incident hypertension in Black adults: Findings from the Jackson Heart Study. Journal of the American Heart Association. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, Mendes WB. More than a feeling: A unified view of stress measurement for population science. Frontiers in Neuroendocrinology. 2018;49:146–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilder JF. Stimulus and response: The law of initial value. John Wright & Sons; 1967. [Google Scholar]
- 22.Pike JL, Smith TL, Hauger RL, Nicassio PM, Patterson TL, McClintick J, Costlow C, Irwin MR. Chronic life stress alters sympathetic, neuroendocrine, and immune responsivity to an acute psychological stressor in humans. Psychosomatic Medicine. 1997;59:447–57. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35:2617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews KA, Gump BB, Owens JF. Chronic stress influences cardiovascular and neuroendocrine responses during acute stress and recovery, especially in men. Health Psychology. 2001;20:403–10. [PubMed] [Google Scholar]
- 25.Schaubroeck J, Ganster DC. Chronic Demands and Responsivity to Challenge. Journal of Applied Psychology. 1993;78:73–85. [DOI] [PubMed] [Google Scholar]
- 26.Klatzkin RR, Baldassaro A, Rashid S. Physiological responses to acute stress and the drive to eat: The impact of perceived life stress. Appetite. 2019;133:393–99. [DOI] [PubMed] [Google Scholar]
- 27.Turner AI, Smyth N, Hall SJ, Torres SJ, Hussein M, Jayasinghe SU, Ball K, Clow AJ. Psychological stress reactivity and future health and disease outcomes: A systematic review of prospective evidence. Psychoneuroendocrinology. 2020;114:104599. [DOI] [PubMed] [Google Scholar]
- 28.Junghaenel DU, Broderick JE, Schneider S, Wen CKF, Mak HW, Goldstein S, Mendez M, Stone AA. Explaining age differences in the memory-experience gap. Psychology and Aging. 2021;36:679–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voelkle MC, Brose A, Schmiedek F, Lindenberger U. Toward a unified framework for the study of between-person and within-person structures: Building a bridge between two research paradigms. Multivariate Behavioral Research. 2014;49:193–213. [DOI] [PubMed] [Google Scholar]
- 30.Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine. 1994;16:199–202. [Google Scholar]
- 31.Kamarck TW, Shiffman S, Wethington E. Measuring psychosocial stress using ecological momentary assessment methods. In: Contrada RJ, Baum A, editors. The handbook of stress science: Biology, psychology, and health. Springer Publishing Company; 2011. p. 597–617. [Google Scholar]
- 32.Weckesser LJ, Schmidt K, Möschl M, Kirschbaum C, Enge S, Miller R. Temporal Stability and Effect Dynamics Between Executive Functions, Perceived Chronic Stress, and Hair Cortisol Concentrations. Developmental Psychology. 2021;57:1149–62. [DOI] [PubMed] [Google Scholar]
- 33.Lee EH. Review of the psychometric evidence of the perceived stress scale. Asian Nursing Research [Internet]. 2012;6:121–27. Available from: 10.1016/j.anr.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 34.Hox JJ, Moerbeek M, van de Schoot R. Multilevel analysis: Techniques and applications. 3rd ed. New York, NY: Routledge; 2018. [Google Scholar]
- 35.Stoney CM, Niaura R, Bausserman L, Matacin M. Lipid reactivity to stress: I. Comparison of chronic and acute stress responses in middle-aged airline pilots. Health Psychology. 1999;18:241–50. [DOI] [PubMed] [Google Scholar]
- 36.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67:1–48. [Google Scholar]
- 37.Pinheiro J, Bates D, DebRoy S, Sarkar D, Core Team R. nlme: Linear and nonlinear mixed effects models. R package version 3.1–152. 2021. [cited 2021 Dec 28]. Available from: https://cran.r-project.org/package=nlme [Google Scholar]
- 38.Hoffman L. Longitudinal analysis: Modeling within-person fluctuation and change. New York, NY: Taylor & Francis; 2015. [Google Scholar]
- 39.Ebstrup JF, Eplov LF, Pisinger C, Jørgensen T. Association between the five factor personality traits and perceived stress: Is the effect mediated by general self-efficacy? Anxiety, Stress and Coping. 2011;24:407–19. [DOI] [PubMed] [Google Scholar]
- 40.Hozawa A, Ohkubo T, Tsuji I, Kikuya M, Matsubara M, Suzuki T, Nagai K, Kitaoka H, Arai Y, Hosokawa T, Satoh H, Hisamichi S, Imai Y. Relationship between personality and self-measured blood pressure value at home: The Ohasama study. Clinical and Experimental Hypertension. 2002;24:115–23. [DOI] [PubMed] [Google Scholar]