Abstract
Objective:
We assessed the feasibility and acceptability of using BACtrack Skyn wearable alcohol monitors for alcohol research in a college student population.
Methods:
We enrolled n=5 (Sample 1) and n=84 (Sample 2) Indiana University undergraduate students to wear BACTrack Skyn devices continuously over a five-day to seven-day study period. We assessed feasibility in both samples by calculating compliance with study procedures, and by analyzing amount and distributions of device output [e.g., transdermal alcohol content (TAC), temperature, motion]. In Sample 1, we assessed feasibility and acceptability with the Feasibility of Intervention Measure (FIM) scale and the Acceptability of Intervention Measure (AIM) scale.
Results:
All participants were able to successfully use the alcohol monitors, producing a total of 11,504 hours of TAC data. TAC data were produced on 567 days of the 602 total possible days of data collection. The distribution of the TAC data showed between-person variation, as would be expected with between-person differences in drinking patterns. Temperature and motion data were also produced as expected. Sample 1 participants (n=5) reported high feasibility and acceptability of the wearable alcohol monitors in survey responses with a mean FIM score of 4.3 (of 5.0 possible score) and mean AIM score of 4.3 (of 5.0 possible score).
Conclusions:
The high feasibility and acceptability we observed underscore the promise of using BACTrack Skyn wearable alcohol monitors to improve our understanding of alcohol consumption among college students, a population at particularly high risk for alcohol-related harms.
Keywords: wearable alcohol monitors, alcohol biosensor, transdermal alcohol content, college students, feasibility, acceptability
INTRODUCTION
The quality of the inference we can make from alcohol research depends on accurate measures of alcohol consumption. Self-reported alcohol consumption, which has historically underpinned much of alcohol research, is limited in its susceptibility to social desirability bias and recall bias. The potential for recall bias is further heightened in cases of alcohol-induced memory loss (1). Objective and passively collected data on alcohol consumption would improve our understanding of the alcohol research landscape and improve our ability to identify effective alcohol risk reduction interventions.
There has thus been sustained interest in developing novel and objective ways to passively collect alcohol use data. Recent decades have seen the development and evaluation of several wearable monitors that measure transdermal alcohol concentration (TAC) (2), including the Secure Continuous Remote Alcohol Monitor (SCRAM) (3) and the Wrist Transdermal Alcohol Sensor (WrisTAS) (4). Both devices have shown good correlation between self-reported alcohol consumption and TAC data from the sensors (3, 5–8). However, both the SCRAM (worn on the ankle) and the WrisTAS (worn on the wrist) are fairly obtrusive, noticeable devices, and have largely been used to monitor alcohol abstinence (3, 9). In particular, the SCRAM, which is commonly used in the criminal justice system, resembles an ankle GPS tracking device worn to monitor people under court-ordered alcohol abstinence as a condition of parole or probation. This similarity may influence willingness to wear such a device in a field-based research context.
The next generation of wearable alcohol monitors includes a device (‘Skyn’) developed by BACTrack©. The BACTrack Skyn has a TAC sensor that is integrated into a small, unobtrusive wristband, and may address some of the limitations of prior models (10–13). Because this technology is newly developed, establishing the feasibility and acceptability of using the devices in research is a critical next step. Previous validity studies have shown preliminary evidence of feasibility in laboratory challenge studies,(10, 12, 14) and one pilot study has demonstrated early evidence of feasibility in naturalistic drinking environments in a small sample.(14) Before these monitors can be adopted widely, it is important to confirm these early pilot findings with studies designed with larger sample sizes, longer follow-up time, and more comprehensive assessments of feasibility and acceptability.
In this study, our objective was to assess the feasibility and acceptability of using BACTrack Skyn wearable alcohol monitors in a college student sample. We chose college students because they are at particularly high risk for heavy alcohol consumption(15) and alcohol-related harms.(16–24) We monitored participants’ natural drinking behaviors over five to seven days, and assessed feasibility and acceptability using both empirical survey questions and operational indicators.
MATERIALS AND METHODS
Study setting and design
To test the feasibility and acceptability of the novel wearable alcohol monitors in a college student sample, we prospectively collected longitudinal alcohol use data on two separate samples of students at Indiana University Bloomington (IUB), a large university in southern Indiana with an undergraduate population of more than 33,000. Data were collected in two independent studies run in separate academic years. Though the studies had some differences in sample size, scope, and study procedures, both were designed to assess feasibility and acceptability of BACtrack Syn wearable alcohol monitors. We combined data across the two studies to maximize the sample size where the survey measures were harmonized, and to maximize inference by triangulating results where survey measures differed.
Study population
Sample 1:
In Fall 2019, we enrolled n=5 undergraduate Indiana University students to wear the devices continuously over a five-day study period. Participants were recruited using on-campus and online flyers to publicize the study. Interested participants contacted study staff who confirmed eligibility and scheduled a baseline visit to obtain written informed consent. Participants were eligible to enroll in the study if they 1) were aged 21 years or older, 2) were English-speaking, 3) typically consumed 1 or more alcoholic drinks per week, and 4) owned an iPhone (wearable alcohol monitors used in this study required syncing with an iPhone) at the time of the study. No further exclusion criteria were applied. Participants were paid $55 for completion of all study procedures. Ethical approval of the study protocol was provided by the Indiana University Human Subjects Office (#1907111038).
Sample 2:
In Spring 2021, we used random cluster sampling and acquaintance sampling techniques to enroll n=84 undergraduate Indiana University students to wear the devices continuously over a seven-day study period. Participants were eligible to enroll in the study if they 1) were aged 18 years or older, 2) were living in Monroe County, IN, 3) typically consumed 1 or more alcoholic drinks per week, 4) owned an iPhone, 5) were enrolled in IUB courses, and 6) reported good general health. Participants were excluded if they were: 1) using medicines contraindicated with alcohol use, or 2) pregnant or breastfeeding. We randomly sampled courses from the full roster of undergraduate courses fulfilling general education requirements and sent study invitations to instructors in the selected classes. Interested instructors forwarded our study invitation to their students. Interested students filled out an eligibility criteria screen and signed the eConsent form to participate in the study. We then asked participants from this random cluster sample to each nominate up to 3 of their friends. We invited nominated participants to the study via email. Participants were paid $75 for completion of all study procedures. Ethical approval of the study protocol was provided by the Indiana University Human Subjects Office (#2008293852).
Study procedures
Participants were asked to wear a ‘BACtrack Skyn’ alcohol monitor for five (Sample 1) or seven (Sample 2) days. The BACtrack Skyn monitors are wristbands equipped with a small sensor to measure transdermal alcohol content (TAC) with measurements recorded approximately every 20 seconds. The sensor pairs via Bluetooth to a smartphone application downloaded onto the participants phone at the baseline study visit. The TAC data are then transmitted from the mobile app to a secure server maintained by BACtrack. During the baseline visit, study staff trained participants to properly use the monitors and instructed them to wear the devices continuously, except for removal for regular charging (at least every other day), and when showering or swimming (because the devices are not waterproof). Participants were asked to complete a baseline survey to provide socio-demographic and behavioral data, short daily surveys querying the drinking of the previous day, and an endline survey focused on participant acceptability of the study procedures. We did not make the alcohol use data collected by Skyn available to the study participants during follow-up to minimize the potential for negative reactivity, but we did provide them with a summary of their alcohol use data at the endline visit.
Key measures
Baseline characteristics:
In the baseline survey, participants provided data on age (continuous, in years), sex (coded as female vs. male), racial identity (coded as white vs. non-white), school year (1st year undergraduate to ≥4th year undergraduate), Greek student organization affiliation (yes vs. no), alcohol consumption frequency (1-2, 3-5, and 6-7 times a week), and average number of drinks per event (1-2, 3-4, and ≥5 drinks per event).
Feasibility measures:
We assessed a variety of interview-based and operational feasibility measures:
Feasibility of Intervention Measure (FIM) scale (Sample 1):
In Sample 1, we formally assessed feasibility with the 4-item FIM scale, collected in the endline survey (25). An example survey item is: “The alcohol monitor seems easy to use.” Each of the four FIM item responses were measured on a five-point Likert scale. The exact survey items and response options are provided in Table S1. The scores were calculated as the mean of the four item responses for a range from 1 to 5. To reduce participant survey burden, we did not include the FIM scale in the endline survey for Sample 2.
Device output on TAC, temperature, and motion (Samples 1 and 2):
We estimated the amount of alcohol data produced by the study participants wearing the alcohol monitors with the number of TAC data points collected. Skyn produces a TAC data point every 20 seconds (3 data points in a minute). We converted the total number of collected TAC data points to hours by dividing the total number by 180 (3 data points per minute times 60 minutes per hour, which equals 180 data points per hour). As a further operational feasibility measure, we calculated the proportion of total study days with TAC data produced by participants. Although there is no consensus threshold to establish feasibility with this measure, we draw from the benchmarks used to interpret correlation coefficients to interpret our results.(26) If 100% represents perfect operational feasibility (all possible study days would have corresponding TAC data), then 70-90% represents strong feasibility, 40-60% represents moderate feasibility, and 10-30% represents weak feasibility.
Using its sensors, Skyn also collects two other continuous variables every 20 seconds: 1) Temperature (°C) and 2) Motion (g). Recent motion records and times with temperature readings above ambient room/environmental temperatures likely indicate times that the device is being worn, though there is not yet consensus around how to leverage them for this purpose. We use these as secondary operational feasibility measures to assess whether the devices were producing output values as expected.
We calculated descriptive statistics (mean, median, interquartile range) for TAC, temperature, and motion in the full data series and for average values calculated within-person. The added value that the within-person average values for TAC, temperature, and motion provide over the statistics from the full data series is information on whether there is variation in device output across individuals.
Battery charge frequency (Sample 2):
In the endline survey for Sample 2, we asked participants to self-report the number of times and dates that they charged the monitor. Alcohol monitors were fully charged when we provided them to participants at the baseline visit. We calculated time difference (in days) between the baseline visit day and the first time participants charged the device to estimate charge frequency.
Acceptability measures:
We assessed participant acceptability of alcohol band use in two ways:
Acceptability of Intervention Measure (AIM) scale (Sample 1):
In Sample 1, we formally assessed acceptability of the wearable alcohol monitors with the 4-item AIM scale, collected in the endline survey (25). An example survey item is “Wearing the alcohol monitor is appealing to me.” Each of the four AIM item responses are measured on a five-point Likert scale. The exact survey items and response options are provided in Table S1. The scores were calculated as the mean of the four item responses for a range from 1 to 5. To reduce participant survey burden, we did not include the AIM scale in the endline survey for Sample 2.
Open-ended survey questions (Samples 1 and 2):
In the endline survey, we asked participants to optionally provide any feedback they may have about their experience with the study procedures. We reported their exact comments and discussed themes that emerged across the responses.
We used Python programming language (version 3.9.1, Python Software Foundation, Beaverton, OR, US) for data analyses and visualization (27).
RESULTS
Overall, we enrolled 89 participants, n=5 from Sample 1 and n=84 from Sample 2. All Sample 1 participants remained in the study for the whole data collection period. However, three participants in Sample 2 joined the study late (on Day 3), and one participant withdrew from the study (on Day 2) because of a positive COVID-19 test.
In Sample 1, two students were male-identified and three were female-identified (Table 1). The mean age was 21.6 years and was diverse with respect to racial/ethnic identity, with White, Hispanic, Black/African-American, and Asian participants represented. At baseline, all participants reported an average alcohol consumption frequency of 3-5 times per week, with most reporting an average number of standard drinks per drinking event between 3 to 4 drinks. In Sample 2, participants were, on average, 19.7 (SD=1.2) years old, mostly female-identified (70%), white (73%), and first year students (32%). About one-third of participants (30%) were affiliated with a Greek student organization. At baseline, most (56%) of participants reported an average alcohol consumption frequency of 1-2 times per week, with most (51%) reporting an average number of standard drinks per drinking event of ≥5 drinks.
Table 1.
Socio-demographic and behavioral characteristics at baseline for the two study samples
| Characteristic | Sample 1 (N=5) September 2019 |
Sample 2 (N=84) March-April 2021 |
|---|---|---|
| N (%) | N (%) | |
| Gender* | ||
| Male | 2 (40.0) | 25 (29.8) |
| Female | 3 (60.0) | 59 (70.2) |
| Mean age (range) | 21.6 (21.2 – 22.3) | 19.7 (18-22) |
| Racial/ethnic identity | ||
| White, non-Hispanic | 2 (40.0) | 61 (72.6) |
| Black or African-American | 1 (20.0) | 1 (1.2) |
| Asian | 1 (20.0) | 10 (11.9) |
| Hispanic, Latinx, or Spanish origin | 1 (20.0) | 2 (2.4) |
| Other | 0 (0) | 10 (11.9) |
| School year | ||
| 1st year undergraduate | 0 (0) | 27 (32.1) |
| 2nd year undergraduate | 0 (0) | 27 (32.1) |
| 3rd year undergraduate | 2 (40.0) | 20 (23.8) |
| ≥4th year undergraduate | 3 (60.0) | 10 (11.9) |
| Greek affiliated | ||
| Yes | 1 (20.0) | 25 (30.5) |
| No | 4 (80.0) | 57 (69.5) |
| Missing | 0 | 2 |
| Alcohol consumption frequency | ||
| 6-7 times a week | 0 (0) | 1 (1.2) |
| 3-5 times a week | 5 (100.0) | 36 (42.9) |
| 1-2 times a week | 0 (0) | 47 (56.0) |
| Average # drinks per event | ||
| 1-2 | 1 (20.0) | 12 (14.3) |
| 3-4 | 3 (60.0) | 29 (34.5) |
| ≥5 | 1 (20.0) | 43 (51.2) |
All participants reported concordance between their sex at birth and current gender identity
FIM scale (Sample 1):
Participants in Sample 1 reported high feasibility of the study procedures in the endline survey (Table 2). The average FIM scale scores were 4.3 out of a maximum of 5, and ranged from 3.3 to 5.0.
Table 2.
Feasibility and acceptability scale data, n=5 participants after 5 days of follow-up
| Scale | Component | Mean | Range |
|---|---|---|---|
| Feasibility of Intervention Measure | Alcohol monitor use | 4.3 | 3.3 – 5.0 |
| Acceptability of Intervention Measure | Alcohol monitor use | 4.3 | 3.3 – 5.0 |
Count of TAC data points (Sample 1 and 2):
Feasibility was further supported by the amount of data collected over the study period (Table 3). In Sample 1, a total of 106,099 TAC datapoints (589 hours) were collected from the 5 participants. Of the total 25 possible days of alcohol monitor data (i.e., five days for each of the five participants), we collected data in 24 days (96%). Comparable results were found in Sample 2. A total of 964,713 (10,915 hours) of TAC data points were collected from the 84 participants. Of the total 577 possible days of alcohol monitor data, we collected data in 543 days (94%). There was modest variability in the amount of data produced between participants. In Sample 1, the median number of TAC data points produced across the five participants was 22,217 (123 hours), with an interquartile range from 22,144 (123 hours) to 22,233 (124 hours). In Sample 2, the median number of TAC data points produced across the 84 participants was 25,553 (142 hours), with an interquartile range from 20,202 (112 hours) to 27,804 (154 hours).
Table 3.
Feasibility process measures, n=5 participants over 5 days of follow-up and n=84 participants over 7 days
| Feasibility item | Sample 1 | Sample 2 |
|---|---|---|
| Overall number of collected TAC data points | 106,099 (589 hours) | 1,964,713 (10,915 hours) |
| Proportion of days of alcohol monitor data produced | 24/25 (96.0%) | 543/577 (94.1%) |
TAC=transdermal alcohol content
TAC level (Sample 1 and 2):
In Sample 1, the mean value across all TAC data points was 5.22 ug/L(air) (Median: 1.14 ug/L(air), IQR: 2.38, Table 4). In Sample 2, the mean value across all TAC data points was 11.17 ug/L(air) (Median: −0.43, IQR: 5.63). There was variation in TAC levels between participants (Figure 1). For Sample 1, the median within-person average TAC level was 6.03 ug/L(air), with an interquartile range from 2.52 to 6.33. For Sample 2, the median within-person average TAC level was 6.25 ug/L(air), with an interquartile range from 0.87 to 12.98. TAC readings were negative in some instances, possibly reflecting sensor error, influences of environmental factors, or user error (e.g., not wearing the device tightly).
Table 4.
Summary statistics on Skyn device output, n=5 participants over 5 days of follow-up and n=84 participants over 7 days
| Sample 1 (N = 5) | Sample 2 (N = 84) | |
|---|---|---|
| TAC ug/L(air), summary statistics for all data points | Mean: 5.22 SD: 31.16 Median: 1.14 IQR: 2.38 |
Mean: 11.17 SD: 58.09 Median: −0.43 IQR: 5.63 |
| TAC ug/L(air), summary statistics across average of within-person data | Mean: 4.98 SD: 3.54 Median: 6.03 IQR: 3.81 |
Mean: 11.99 SD: 22.77 Median: 6.25 IQR: 12.11 |
| Temperature °C, summary statistics for all data points | Mean: 31.24 SD: 5.08 Median: 32.67 IQR: 4.85 |
Mean: 31.00 SD: 5.00 Median: 33.00 IQR: 6.00 |
| Temperature °C, summary statistics across average of within-person data | Mean: 31.27 SD: 3.11 Median: 32.31 IQR: 0.65 |
Mean: 31.37 SD: 3.08 Median: 32.08 IQR: 4.30 |
| Motion g, summary statistics for all data points | Mean: 0.04 SD: 0.09 Median: 0.00 IQR: 0.06 |
Mean: 0.03 SD: 0.05 Median: 0.00 IQR: 0.06 |
| Motion g, summary statistics across average of within-person data | Mean: 0.04 SD: 0.02 Median: 0.03 IQR: 0.00 |
Mean: 0.03 SD: 0.01 Median: 0.03 IQR: 0.01 |
TAC=transdermal alcohol content SD=standard deviation IQR=interquartile range
Figure 1.
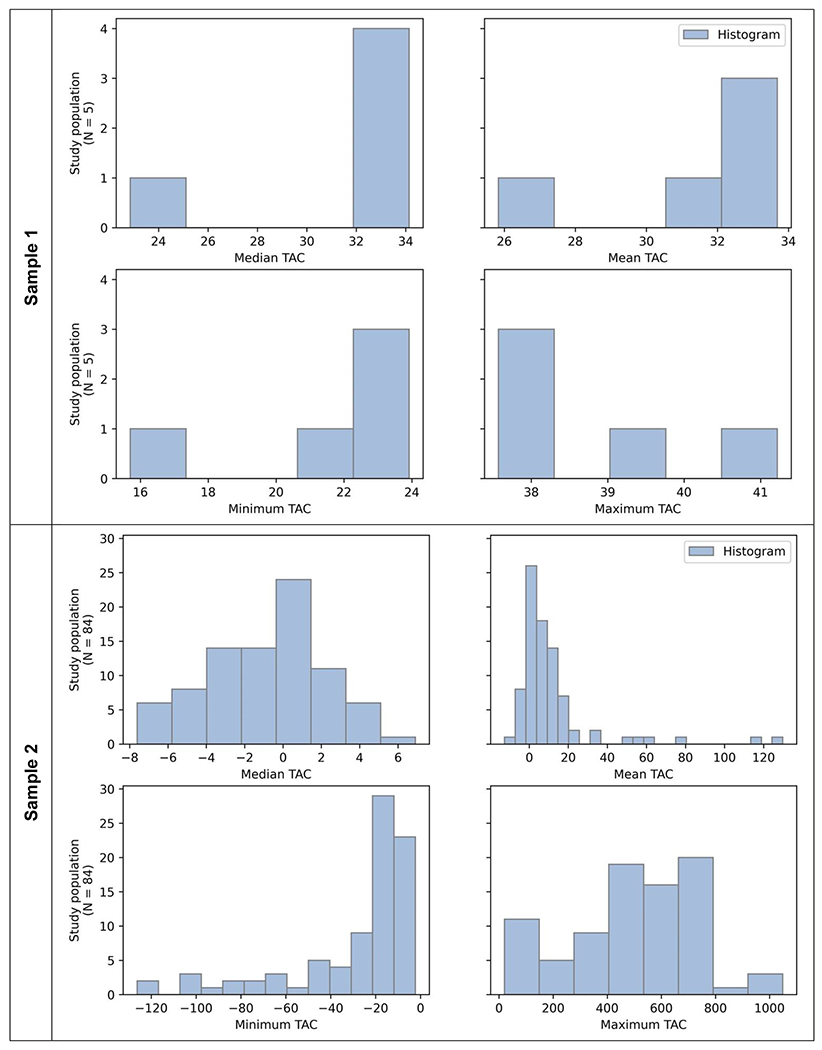
Distribution of TAC data summary measures (in ug/L(air)) for each participant in Sample 1 (n=5, upper panels), and Sample 2 (N=84, lower panels)
Temperature and motion (Sample 1 and 2):
In Sample 1, a total of 106,099 temperature/motion readings were collected. The mean temperature across all temperature data points was 31°C (Median: 33°C, IQR: 5°C). The mean motion reading was 0.04 g (Median: 0.00, IQR: 0.06). In Sample 2, a total of 1,964,713 temperature/motion readings were collected. The mean temperature was 31°C (Median: 33°C, IQR: 6°C). The mean motion reading was 0.03 g (Median: 0.00, IQR: 0.06). There was variation between participants for their temperature readings (Figure S1), but not for their motion readings. For both samples, the median within-person average temperature reading was 31°C, with an interquartile range from 32°C to 33°C in Sample 1, and 29°C to 34°C in Sample 2. The median within-person average motion reading was 0.04 g (IQR 0.00) and 0.03 g (IQR 0.01) in Sample 1 and 2, respectively.
Battery charge frequency (Sample 2):
Of the n=75 Sample 2 participants with complete data on their self-reported charging frequency, on average, participants charged the wristbands 2.5 times during the data collection week (Median: 2, IQR: 1). The device was fully charged at baseline. Most participants (n=34, 53%) first charged the device on the third day of data collection week (i.e., two days after baseline visit day). Because we had instructed participants to charge the device every other day, the true battery life of the device might be longer than two days.
Figure 2 shows an example of (unprocessed) measures that Skyn collected in one participant. Visual inspection of the figure suggests that there could be a correlation between the drinking event start-time from the TAC and self-reported daily survey data, and a correlation between the peak size and number of drinks self-reported.
Figure 2.
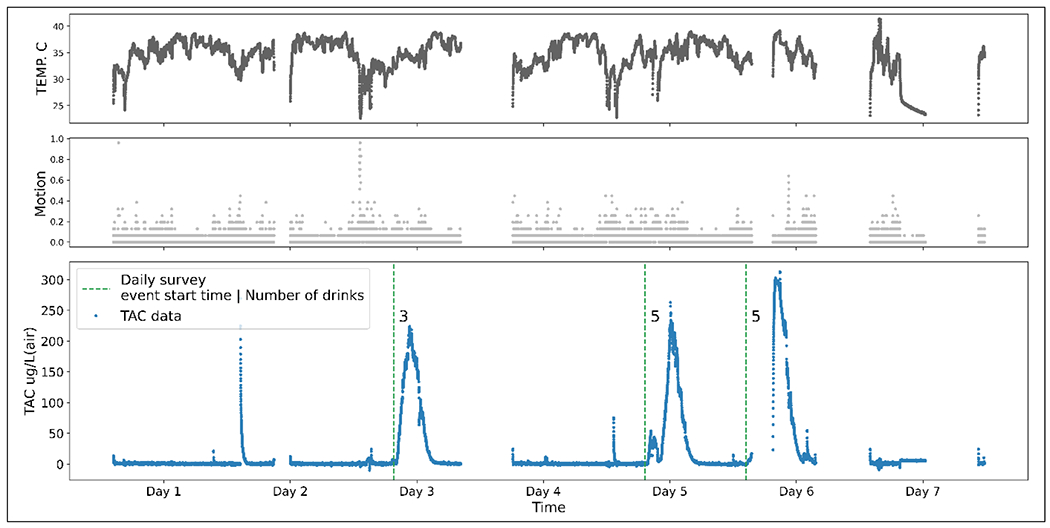
An example of the three variables collected by BACTrack Skyn devices
NOTE: Empty timestamps indicate that the device was off
AIM scale (Sample 1):
Participants in Sample 1 reported high levels of acceptability with the study procedures in the endline survey (Table 2). The average AIM scale scores were 4.3 out of a maximum of 5, and ranged from 3.3 to 5.0.
Open-ended survey questions (Samples 1 and 2):
Participant responses in the open-ended survey questions generally aligned with the high quantitative acceptability scores, but also pointed to some design considerations for improved comfort/fit (Table 5). Three participants from Sample 1 (60%) and 13 participants from Sample 2 (15%) responded to these optional questions. Participants noted positive experiences (“It was a great experience overall!”). Participants also noted that they perceived the alcohol monitors to be accurately measuring their alcohol consumption (“The data that is shown corresponds accurately to the times and amount of drinks that were consumed.”) and described drinking events that aligned with irregularities in the read-out (“The last drinking time, I opened a drink and took a few sips, but ended up doing laundry and then coming back to it, which is demonstrated by how the spike doesn’t occur until a bit later.”). However, five participants reported issues with the wristband comfort (“The only comment I have is that the bracelet itself, although nice and simple in design, irritated the skin on the underside of my wrist with the constant wearing of it.”) or fit (“The bracelet was kinda big on my wrist so it wiggled around a lot.”).
Table 5.
Feedback on the study procedures for participants in Sample 1 (n=5) and Sample 2 (n=84)
|
Feedback question for Sample 1:
Do you have any additional feedback for us about your participation in the current study or ideas for implementing an alcohol band intervention in the future? |
| I think having an application that would run regardless of whether or not the app is on would be tight. But don’t take this too seriously, I know next to nothing on how technology works. |
| No, it was a great experience overall! |
| Ability to allow close friends to see location/BAC |
|
Feedback question for Sample 2:
Please type any other comments that you might have about the study in the box. |
| The wristbands were a little uncomfortable and too large for my wrist. |
| The data that is shown corresponds accurately to the times and amount of drinks that were consumed. The only comment I have is that the bracelet itself, although nice and simple in design, irritated the skin on the underside of my wrist with the constant wearing of it. Maybe cushioning the bottom of the part that touches the wrist would be beneficial! |
| the bracelet was kinda big on my wrist so it wiggled around a lot |
| While the data collection was pretty accurate, I think it would be even more accurate if I knew in advance what I had to record. For example, in the daily surveys, I didn’t know that I had to know the times of my first and last drink until I took the first one. |
| the writstband pinched my wrist the entire week other than that it was fine |
| The data may have been more accurate if the process of submitting a time stamp for each drink was more simple. |
| Times may have been a little off but seem pretty spot on. |
| my wrist is pretty small so I woke up several times to it having fallen off of my wrist/ not being fully secured to my wrist |
| It could be a little bit easier |
| For the second spike, I only had 3 drinks, but they were stronger than a normal drink which will explain the higher spike |
| The last drinking time, I opened a drink and took a few sips, but ended up doing laundry and then coming back to it, which is demonstrated by how the spike doesn’t occur until a bit later |
| Upon receiving the wristband I was instructed to charge it every two days - it never died and when I went to charge it, it was always solid green never blinking |
| I am a nursing student and while in the hospital, I frequently use hand sanitizer so though I was cautious about making sure the hand sanitizer didnt go near the wristband I just wanted to keep you informed. |
DISCUSSION
Overall, we found high acceptability and feasibility of using BACTrack Skyn wearable alcohol monitors to measure drinking behaviors among college students. This was reflected empirically in the high AIM and FIM scores from Sample 1. It is also reflected in the fact that participants from both Sample 1 and 2 were able to use the alcohol monitors to produce nearly complete continuous TAC data over five to seven days of follow-up (>90% of days). Alongside the TAC data, the alcohol monitors produced temperature and motion data as expected (i.e. motion data were captured and average temperature readings were above ambient temperature levels), which may be useful in future applications to identify periods of device non-use. A small number of participants reported issues with comfort and fit of the wristbands, which warrants design considerations to better account for diversity in user wrist size and skin sensitivities.
The high levels of feasibility and acceptability that we observed aligns with findings from a small number of previous studies using BACTrack Skyn devices. Three validity studies used an early version of the device with human participants in laboratory settings (10, 12, 14). Each of these studies reported promising feasibility data, and one reported that the devices were generally acceptable to participants, although acceptability was not formally assessed (10). Only one pilot study has assessed the feasibility and acceptability of using BACTrack Skyn devices in naturalistic drinking environments. This study reported high acceptability using quantitative survey questions from n=10 participants wearing the devices over the course of one drinking event. Our findings build on this prior work, establishing feasibility and acceptability over a larger sample size (n=89) and over a longer period (five to seven days of continuous monitoring). In addition to the high compliance with study protocols in regards to wearing the BACTrack Skyn devices in both our samples, qualitative feedback from participants illustrated that they felt the TAC data produced was fairly accurate. However, some participants did report minor concerns with the comfort of the devices largely due the wristband circumference being too large for smaller wrists, resulting in loose connections and pinching. Future generations of the BACTrack Skyn could incorporate a variety of choices in band sizes to address this concern.
Even so, the BACTrack Skyn seems to overcome some of the feasibility and acceptability concerns faced by the previous generation of wearable alcohol monitors (SCRAM and WrisTAS). Specifically, the BACTrack Skyn device was developed to be sleeker than its predecessors and more closely resembles existing fitness trackers on the market (10, 12). The high levels of acceptability reported by our college student participants is consistent with the fact that young US adults aged 18 to 34 years have the highest levels of engagement with wearable fitness devices of all age groups, with nearly 30% of this age group reporting current use of a wearable fitness tracker (28).
Importantly, the scope of this study was focused on establishing feasibility and acceptability of the BACTrack Skyn devices in naturalistic drinking settings. Thus, we do not present information on processed TAC data and how those data relate to self-reported drinking events. Raw TAC data need to be cleaned and processed to identify TAC peaks that correspond with alcohol drinking events. This process involves development of a model with peak identification properties ideally chosen to align with specific goals of a particular research project (29, 30). Though beyond the scope of this current study, we have completed a validation study with this dataset to assess the performance of the BACTrack Skyn data against self-reported drinking event and drinking magnitude data.(31)
There are important considerations to take into account in assessing the generalizability of our findings. Although our combined study sample was larger than that of prior studies, our modest sample size of n=89 still limits the ability to make broader statistical inference. Further, the small study population underlying Sample 1 (n=5), was the only sample we queried with formal acceptability and feasibility scale questions. However, this concern is attenuated given that other indicators of acceptability and feasibility (e.g., compliance with study procedures, open-ended responses of being pleased with the device experience) were repeated in our larger Sample 2. We also found consistently high operational feasibility measures (e.g. amount and range of TAC, temperature, and motion data produced). These operational feasibility measures could also indirectly indicate amount of time the devices were worn; however, they would underestimate true time worn if users were experiencing issues with poor band fit leading to interrupted data collection. The COVID-19 pandemic is an important contextual variable in interpreting our findings. Sample 1 data were collected prior to the pandemic (Fall 2019). However, Sample 2 data were collected in Spring 2021 when university policies (e.g. mask mandates, group size restrictions, quarantine and isolation protocols) and individual concerns about viral spread could impact study participation and undergraduate student drinking behavior. Although individual drinking behaviors were likely affected, the feasibility and acceptability of the study procedures (primary outcomes for this study) were less likely to be modified by pandemic conditions. More broadly, we limited eligibility in our study to an undergraduate college student population at a predominantly white university in the United States. Important areas to extend our findings in the future will be in establishing feasibility and acceptability of using BACTrack Skyn devices in older populations, in more racially and ethnically diverse populations, in populations with lower educational attainment, and in settings outside the United States.
Conclusions
Our findings underscore the promise of using BACTrack Skyn wearable alcohol monitor technology to improve our understanding of alcohol consumption among college students, a population at particularly high risk for alcohol-related harms. Future applications of this new technology have the potential to overcome some of the biases that arise from traditional self-reported alcohol consumption data and could be leveraged to identify more precise recommendations on the levels of alcohol consumption that confer increased risk for specific alcohol-related harms. Given the high levels of acceptability and feasibility demonstrated in our study, future studies could also consider using these alcohol monitors as a platform to deliver interventions based on real-time alcohol consumption or to give personalized alcohol consumption feedback to users.
Supplementary Material
Highlights.
BACTrack Skyn wearable alcohol devices were feasible and acceptable for use among college students
Data were collected over 5-7 days in naturalistic drinking environments
High feasibility and acceptability were observed in survey responses and operational measures
Acknowledgements
This work was supported by the Vice Provost for Research through the Research Equipment Fund; and the NIH/NIAAA under grant [R01 AA13650 to Peter Finn]. This work was also supported by the National Institute on Alcohol Abuse and Alcoholism [NIAAA grant # R25DA051249, 2021] and Prevention Insights at the Indiana University Bloomington School of Public Health. NIAAA had no role in the design, analysis, interpretation, or publication of this study. The content is solely the responsibility of the authors. The authors are exceedingly grateful to all those involved in the data collection procedure, including Finn Lab staff Lindsay Fisher, Sophie Ideker, and Eli Farmer, and, most importantly, the study participants themselves. The authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT author statement:
Molly Rosenberg: Conceptualization, Methodology, Supervision, Formal analysis, Investigation, Funding acquisition, Writing – Original draft
Sina Kianersi: Investigation, Data analysis, Data curation, Writing – Original draft, Visualization, Project administration
Maya Luetke: Conceptualization, Writing – Editing and review
Kristen Jozkowski: Conceptualization, Writing – Editing and review
Lucia Guerra-Reyes: Conceptualization, Writing – Editing and review
Patrick C. Shih: Conceptualization, Writing – Editing and review
Peter Finn: Conceptualization, Supervision, Resources, Writing – Editing and review
Christina Ludema: Conceptualization, Supervision, Funding acquisition, Writing – Editing and review
Disclosure of interest
The author reports no conflicts of interest.
Data availability statement
Data and code are available upon request.
References
- 1.Rose ME, Grant JE. Alcohol-induced blackout: phenomenology, biological basis, and gender differences. Journal of addiction medicine. 2010;4(2):61–73. [DOI] [PubMed] [Google Scholar]
- 2.Swift R. Transdermal alcohol measurement for estimation of blood alcohol concentration. Alcoholism, clinical and experimental research. 2000;24(4):422–3. [PubMed] [Google Scholar]
- 3.Karns-Wright TE, Dougherty DM, Hill-Kapturczak N, Mathias CW, Roache JD. The correspondence between transdermal alcohol monitoring and daily self reported alcohol consumption. Addictive Behaviors. 2018;85:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sirlanci M, Rosen IG, Wall TL, Luczak SE. Applying a novel population-based model approach to estimating breath alcohol concentration (BrAC) from transdermal alcohol concentration (TAC) biosensor data. Alcohol. 2019;81:117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simons JS, Wills TA, Emery NN, Marks RM. Quantifying alcohol consumption: Self-report, transdermal assessment, and prediction of dependence symptoms. Addict Behav. 2015;50:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fairbairn CE, Rosen IG, Luczak SE, Venerable WJ. Estimating the quantity and time course of alcohol consumption from transdermal alcohol sensor data: A combined laboratory-ambulatory study. Alcohol. 2019;81:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alessi SM, Barnett NP, Petry NM. Objective continuous monitoring of alcohol consumption for three months among alcohol use disorder treatment outpatients. Alcohol. 2019;81:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rash CJ, Petry NM, Alessi SM, Barnett NP. Monitoring alcohol use in heavy drinking soup kitchen attendees. Alcohol. 2019;81:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luczak SE, Ramchandani VA. Special issue on alcohol biosensors: Development, use, and state of the field: Summary, conclusions, and future directions. Alcohol (Fayetteville, NY). 2019;81:161. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Fridberg DJ, Leeman RF, Cook RL, Porges EC. Wrist-worn alcohol biosensors: strengths, limitations, and future directions. Alcohol. 2019;81:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell AS, Kim J, Wang J. Wearable electrochemical alcohol biosensors. Current opinion in electrochemistry. 2018;10:126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairbairn CE, Kang D. Temporal Dynamics of Transdermal Alcohol Concentration Measured via New-Generation Wrist-Worn Biosensor. Alcoholism: Clinical and Experimental Research. 2019;43(10):2060–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairbairn CE, Bosch N. A new generation of transdermal alcohol biosensing technology: practical applications, machine-learning analytics and questions for future research. Addiction. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Fridberg DJ, Shortell DD, Leeman RF, Barnett NP, Cook RL, et al. Wrist-worn alcohol biosensors: Applications and usability in behavioral research. Alcohol. 2021;92:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME. Monitoring the Future national survey results on drug use, 1975–2018: Volume II, College students and adults ages 19–60. Ann Arbor: Institute for Social Research, The University of Michigan; 2019. [Google Scholar]
- 16.Abbey A. Alcohol’s role in sexual violence perpetration: Theoretical explanations, existing evidence and future directions. Drug and alcohol review. 2011;30(5):481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown JL, Vanable PA. Alcohol use, partner type, and risky sexual behavior among college students: Findings from an event-level study. Addictive Behaviors. 2007;32(12):2940–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper ML. Alcohol use and risky sexual behavior among college students and youth: Evaluating the evidence. Journal of Studies on Alcohol. 2002:101–17. [DOI] [PubMed] [Google Scholar]
- 19.Crane CA, Godleski SA, Przybyla SM, Schlauch RC, Testa M. The proximal effects of acute alcohol consumption on male-to-female aggression: a meta-analytic review of the experimental literature. Trauma, Violence, & Abuse. 2016;17(5):520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis KC, Masters NT, Eakins D, Danube CL, George WH, Norris J, et al. Alcohol intoxication and condom use self-efficacy effects on women’s condom use intentions. Addictive Behaviors. 2014;39(1):153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohler-Kuo M, Dowdall GW, Koss MP, Wechsler H. Correlates of rape while intoxicated in a national sample of college women. Journal of studies on alcohol. 2004;65(1):37–45. [DOI] [PubMed] [Google Scholar]
- 22.Fairlie AM, Cadigan JM, Patrick ME, Larimer ME, Lee CM. Unplanned Heavy Episodic and High-Intensity Drinking: Daily-Level Associations With Mood, Context, and Negative Consequences. J Stud Alcohol Drugs. 2019;80(3):331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hingson R, Zha WX, Smyth D. Magnitude and Trends in Heavy Episodic Drinking, Alcohol-Impaired Driving, and Alcohol-Related Mortality and Overdose Hospitalizations Among Emerging Adults of College Ages 18-24 in the United States, 1998-2014. Journal of Studies on Alcohol and Drugs. 2017;78(4):540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patrick ME, Cronce JM, Fairlie AM, Atkins DC, Lee CM. Day-to-day variations in high-intensity drinking, expectancies, and positive and negative alcohol-related consequences. Addict Behav. 2016;58:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiner BJ, Lewis CC, Stanick C, Powell BJ, Dorsey CN, Clary AS, et al. Psychometric assessment of three newly developed implementation outcome measures. Implementation Science. 2017;12(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akoglu H. User’s guide to correlation coefficients. Turkish journal of emergency medicine. 2018;18(3):91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Rossum G, Drake F. Python 3 Reference Manual CreateSpace. Scotts Valley, CA. 2009. [Google Scholar]
- 28.McCarthy J. One in Five U.S. Adults Use Health Apps, Wearable Trackers: Gallup; 2019. [Available from: https://news.gallup.com/poll/269096/one-five-adults-health-apps-wearable-trackers.aspx. [Google Scholar]
- 29.Roache JD, Karns-Wright TE, Goros M, Hill-Kapturczak N, Mathias CW, Dougherty DM. Processing transdermal alcohol concentration (TAC) data to detect low-level drinking. Alcohol. 2019;81:101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kianersi S, Luetke M, Agley J, Gassman R, Ludema C, Rosenberg M. Validation of transdermal alcohol concentration data collected using wearable alcohol monitors: A systematic review and meta-analysis. Drug and Alcohol Dependence. 2020:108304. [DOI] [PubMed] [Google Scholar]
- 31.Kianersi S, Ludema C, Agley J, Ahn YY, Parker M, Ideker S, et al. Development and validation of a model for measuring alcohol consumption from transdermal alcohol content data among college students. Addiction. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code are available upon request.


