Abstract
The blockade of the CD154-CD40 pathway with anti-CD154 monoclonal antibody has been a promising immunomodulatory approach to prevent allograft rejection. However, clinical trials of immunoglobulin G1 antibodies targeting this pathway revealed thrombogenic properties, which were subsequently shown to be mediated by crystallizable fragment (Fc)-gamma receptor IIa–dependent platelet activation. To prevent thromboembolic complications, an immunoglobulin G4 anti-CD154 monoclonal antibody, TNX-1500, which retains the fragment antigen binding region of ruplizumab (humanized 5c8, BG9588), was modified by protein engineering to decrease Fc binding to Fc-gamma receptor IIa while retaining certain other effector functions and pharmacokinetics comparable with natural antibodies. Here, we report that TNX-1500 treatment is not associated with platelet activation in vitro and consistently inhibits kidney allograft rejection in vivo without clinical or histologic evidence of prothrombotic phenomena. We conclude that TNX-1500 retains efficacy similar to that of 5c8 to prevent kidney allograft rejection while avoiding previously identified pathway-associated thromboembolic complications.
Keywords: immunosuppression, costimulation blockade, kidney transplantation, nonhuman primates
1. Introduction
The development of increasingly effective immunosuppressive medications has enabled organ transplantation to become a standard of care for many patients with end-stage disease. However, long-term graft and patient survival following transplantation of kidneys and other solid organs1 are constrained by nephrotoxicity associated with calcineurin inhibitors (CNI), motivating efforts to develop CNI-free immunosuppressive protocols. To date, belatacept is the only costimulatory blockade currently approved by the Food and Drug Administration as an alternative to CNI protocols in kidney transplantation (KTx).2 Although belatacept is associated with improved glomerular filtration rate (GFR) and a favorable safety profile in a long-term follow-up after KTx relative to CNI-based regimens,3 it has been associated with higher rates of acute cellular rejection at 1 year4 and has not yet been approved for other solid organ transplants.5 The blockade of the CD154-CD40 pathway with an anti-CD154 monoclonal antibody (mAb) has been a promising approach, not only in preventing allograft rejection6,7 but also in promoting the induction of allograft tolerance.8,9 Recent studies in nonhuman primates (NHPs) have shown a more reliable efficacy with anti-CD154 mAb compared with belatacept or anti-CD40 mAb in xenotransplantation.10,11
However, clinical trials of 2 different antibodies targeting CD154, ruplizumab (humanized 5c8 [hu5c8], BG9588), and toralizumab (IDEC-131), were halted because of thromboembolic events. Subsequent in vitro studies revealed that these antibodies’ prothrombotic properties are mediated by crystallizable fragment (Fc)-gamma receptor IIa (FcγRIIa)–dependent platelet activation triggered by high-order immune complexes formed by anti-CD154 antibodies crosslinking soluble CD154.12 Eliminating Fc binding, either by deglycosylation (aglycosyl hu5c8) or by eliminating the Fc region (pegylated fragment antigen binding region [Fab] antibody), prevents platelet activation in vitro and does not induce thrombotic events in Rhesus monkeys.13 However, these modifications were associated with reduced efficacy to prevent transplant rejection relative to the parent immunoglobin (Ig) G1 molecules.14 Treatment with an Fc-silent anti-CD154 domain mAb (BMS-986004, letolizumab) was associated with freedom from clinical thromboembolic events and prolonged renal allograft survival, but additional immunosuppressive agents were required to achieve long-term (>200 days) renal allograft survival.15
To retain the efficacy of the parent 5c8-based molecule, TNX-1500 was developed by engineering the Fc region of IgG4 to decrease FcγRIIa binding. In the current study, we compared TNX-1500 with 5c8 and hu5c8-based IgG1 antibodies in terms of platelet activation in vitro and evaluated the efficacy of TNX-1500 to prevent kidney allograft rejection in an established NHP KTx model.
2. Materials and methods
2.1. Animals and pair selection
Twelve cynomolgus monkey pairs (Charles River Primates) weighing 3 to 8 kg were matched by ABO blood type compatibility and mismatched for the major histocompatibility complex (MHC) (Supplementary Table 1), performed as previously described.16,17 All surgical procedures and postoperative care of animals were performed in accordance with the National Institute of Health guidelines for the care and use of primates and were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee.
2.2. Platelet activation assay
Whole blood from cynomolgus monkeys or humans was used for the platelet activation assay as described previously18 and based on the protocol provided by BD Biosciences. Briefly, whole blood was mixed with anti-CD154 antibody alone or a preformed immune complex of anti-CD154 antibody and soluble CD154 and incubated for 20 minutes at room temperature. The blood was then stained with an antibody against platelet marker CD61 (RUU-PL7F12, BD Biosciences) with activation markers, CD62P (AC1.2, BD Biosciences) and PAC-1 (PAC-1, BD Biosciences), for flow cytometric analysis. Negative control staining was performed using mouse IgG1 isotype control (X40, BD Biosciences) and Arg-Gly-Asp-Ser (Sigma-Aldrich) to inhibit PAC-1 binding to platelets. To generate the immune complex, 1500 nM of soluble human CD154 (Bio-Legend) was mixed with 500 nM of anti-CD154 antibody (mouse 5c8, BioXCell; hu5c8, Nonhuman Primate Reagent Resource; TNX-1500, Tonix Pharmaceuticals) and incubated overnight at 4 °C.
2.3. Binding affinity of anti-CD154 monoclonal antibodies
All surface plasmon resonance assays were carried out using a Biacore T200 instrument (Cytiva Inc) as described by Karlsson.19 Briefly, the protein A capture surfaces were generated on flow cells using the immobilization wizard application within the Biacore T200 software using N-hydroxysuccinimide (NHS), (EDC), and ethanolamine from the Cytiva Amine Coupling Kit. The screening of IgG for binding to Fc-gamma receptors (FcγR) occurs in 2 steps: a direct capture of the IgG of interest onto the protein A surface, followed by an FcγR injection in a single-cycle kinetics method or multicycle kinetics for FcγRIa. This capture step is immediately followed by injections of soluble FcγR ectodomain using a single-cycle kinetics methodology. The sensorgrams were analyzed using the Biacore BiaEvaluation software v3.0. dissociation constant (KD) values are determined from binding isotherms using the equilibrium fit model.
2.4. TNX-1500 serum concentration assay
An enzyme-linked immunosorbent assay technique based on a Cayman Chemical human IgG4 kit (Catalog #500920) was used for determining the concentration of TNX-1500 in cynomolgus monkey serum. Plates were precoated with a human IgG antibody. On the day of the assay, 15 μL of standards, quality controls, and blanks were diluted at a minimum required dilution (MRD) of 20-fold in assay buffer and then added to the plate in duplicates. Any dilution controls that required dilution beyond the MRD were diluted in 5% cynomolgus monkey serum after performing the MRD. TNX-1500 bound to the plate was quantified by incubation with therapeutic IgG4 assay horseradish peroxidase–conjugate followed by tetramethylbenzidine. The plate was periodically monitored at 650 nm on a plate reader until ST01 reached an optical density of approximately 0.800 to 1.000. A stop solution was added, and the plate was read at an optical density of 450 nm using a spectrophotometer. The data for the standards were regressed according to a 5-parameter logistic model with a 1/y2 weighting factor. Both regression and analysis were performed using the Watson LIMS version 7.6.1 software. All concentrations were reported at 4 significant figures.
2.5. Treatment regimens
2.5.1. Standard-dose TNX monotherapy
Six recipients received TNX-1500 (Tonix Pharmaceuticals), 20 mg/kg intravenously (IV) on days 0, 2, 5, and 12 and then weekly for 6 months.
2.5.2. Low-dose TNX + mycophenolate mofetil
Six recipients received 20 mg/kg IV on days 0, 2, 5, and 12 and then weekly for the first 6 weeks, after which the dosing was reduced to 20 mg/kg IV every 2 weeks. Daily mycophenolate mofetil (MMF, Roche Inc; 20 mg/kg orally twice a day) was initiated on day 0 and continued until end of study (EOS) (day 180).
2.5.3. Conventional immunosuppression
Historical reference experiments with conventional immunosuppression (Conv IS): over the past 10 years, at our institution, 37 recipients underwent KTx with a conventional triple drug immunosuppressive regimen consisting of tacrolimus intramuscularly (Astellas Pharma Inc; 2–4 mg/kg intramuscularly daily, titrated to maintain trough levels of 10–15 ng/mL), MMF, and prednisone (100 mg on day 0, tapered to 1 mg by 2 weeks) until EOS (day 120); recipients with surviving renal allografts then underwent conditioning and donor bone marrow transplantation (DBMT) in a 1 of several delayed tolerance protocols.9,20
2.5.4. No immunosuppression
No immunosuppressive medication was given to the 5 transplant recipients.
2.6. Kidney transplantation
The KTx procedure was reported in detail previously.18 The recipients underwent uni- or bilateral native nephrectomy on day 0: the transplanted kidney was monitored using blood chemistry and ultrasound examination twice a week. Bilateral native nephrectomy was delayed until 3 to 6 weeks after transplantation if vigorous urine output was not visually observed during the transplant procedure. In these cases, we carefully monitored the renal allograft with blood chemistry and ultrasound 3 times a week until the remaining native kidney was removed. A serum creatinine rise >25% over the baseline triggered a “for-cause” biopsy.
2.7. Statistical analyses
Statistical analysis was performed with GraphPad PRISM 7.01 (GraphPad Software, Inc). Kaplan-Meir analysis was used to estimate survival time distributions, and the Mantel-Cox log-rank test was used to compare between-group differences.
A linear mixed effects model with random subject level effects and unstructured variance-covariance structure and with fixed terms of treatment, days posttransplant, and treatment × days posttransplant was used to evaluate the differences in TNX-1500 serum levels and white blood cell/platelet counts between standard-dose TNX monotherapy (stTNX/mono) and low-dose TNX + mycophenolate mofetil (loTNX/MMF). Fine-Gray subdistribution hazards model21 was used to evaluate the risk of developing posttransplant lymphoproliferative disorder (PTLD). P values <0.05 were considered statistically significant.
3. Results
3.1. Crystallizable fragment receptor–binding profile of TNX-1500
The prothrombotic properties of anti-CD154 mAB have been demonstrated to be mediated by FcγRIIa receptor-dependent platelet activation triggered by high-order immune complexes formed by anti-CD154 antibodies crosslinking soluble CD154.12 Therefore, TNX-1500 was developed by engineering the Fc region to decrease the binding to FcγRIIa.
Compared with hu5c8, TNX-1500 had an approximately 14-fold weaker affinity to FcγRIIaH; however, affinity to other Fcγ receptors, such as FcγRIa, FcγRIIbF, FcγRIIIaF, and FcγRIIIaV also appeared 4.7 to 264–fold weaker than hu5c8 (Table 1).
Table 1.
Affinity of humanized 5c8 (hu5c8) vs TNX-1500 to crystallizable fragment-gamma receptor (FcγR).
| FcγRI | FcγRIIaH | FcγRIIbF | FcγRIIIaF | FcγRIIIaV | |
|---|---|---|---|---|---|
|
| |||||
| hu5c8 | 0.033 ± 0.005 | 510 ± 10 | 970 ± 70 | 1700 ± 100 | 330 ± 20 |
| TNX-1500 | 8.7 ± 0.3 | 7100 ± 300 | 4900 ± 40 | 8000 ± 1000 | 6000 ± 500 |
Values are in nM.
3.2. Platelet activation by antibody-soluble CD154 immune complexes in vitro
To evaluate platelet activation by anti-CD154 mAbs, freshly collected cynomolgus monkey peripheral blood samples were incubated with anti-CD154 antibody alone or with a preformed immune complex (IC) consisting of anti-CD154 antibody and soluble CD154 (Fig. 1A). Platelets were gated based on CD61 expression (Fig. 1B), and the expression of platelet activation markers, CD62P and PAC-1, were evaluated. Cynomolgus monkey platelet activation (CD62P+PAC-1+) was not observed by antibody alone (5c8 or hu5c8); however, strong activation was observed when cultured with antibody-sCD154 IC (93.3 ± 2.55% by 5c8 IC and 80.75 ± 6.65% by hu5c8 IC). In contrast, no platelet activation was observed in association with TNX-sCD154 IC (Fig. 1C upper panels, D; P < 0.01 in cynomolgus monkeys). Although activation was weaker, a similar profile of human platelet activation was also observed with 5c8-sCD154 IC and hu5c8-sCD154 IC (6.18 ± 4.92% and 9.95 ± 5.35%, respectively) but not with TNX-1500-sCD154 IC (Fig. 1C lower panels, E).
Figure 1.
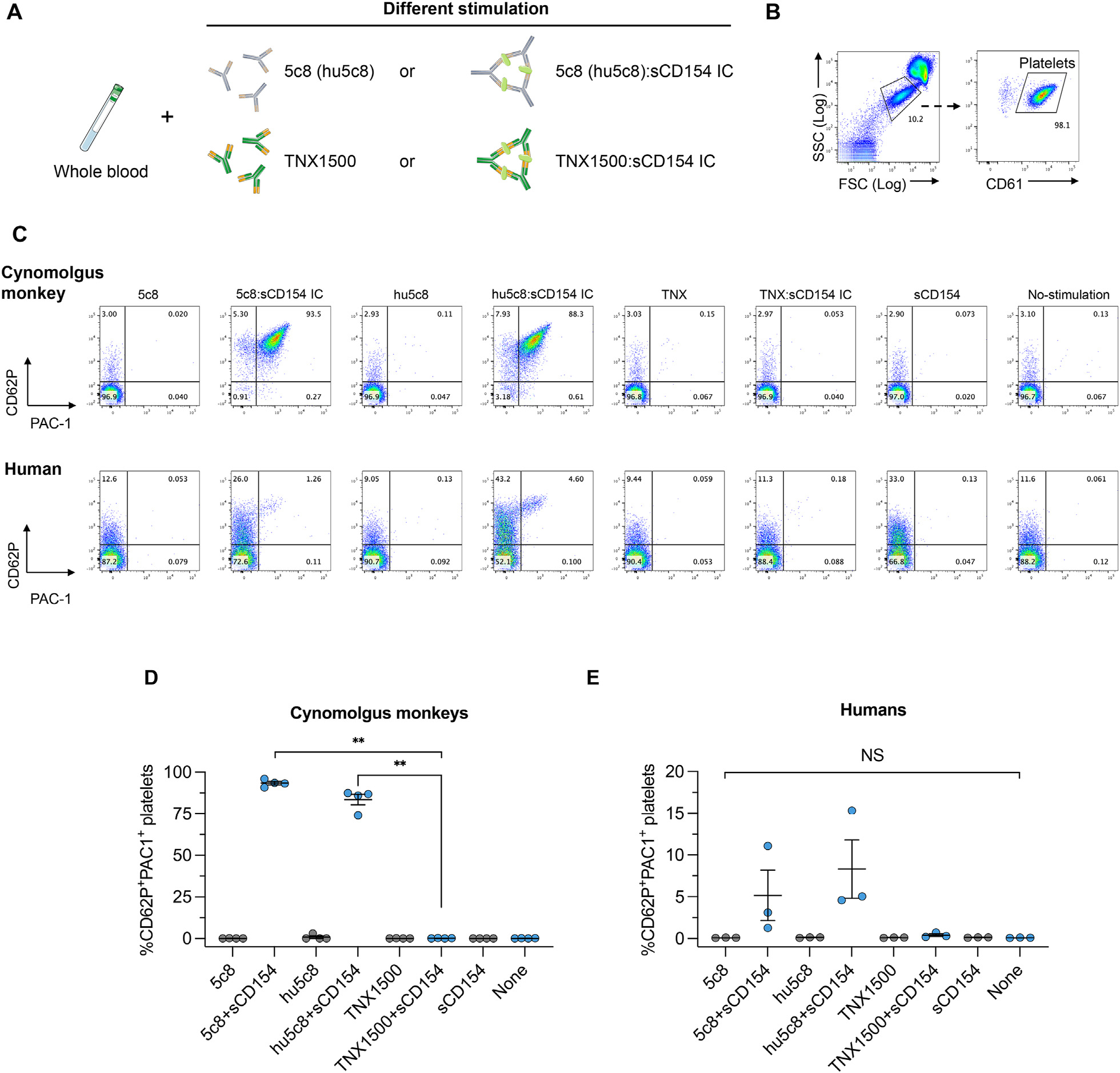
Platelet stimulation by anti-CD154 antibody and soluble CD154. (A) Freshly collected cynomolgus monkey peripheral blood samples were incubated with anti-CD154 antibody (mouse 5c8, humanized 5c8 [hu5c8] or TNX-1500) alone or preformed immune complex (IC) consisting of anti-CD154 antibodies and soluble CD154. (B) Using flow cytometry, platelets were gated on the basis of FSC/SSC scatter and CD61 expression. (C) Platelet activation was then evaluated by the expression of CD62P and PAC-1. Representative dot plots from cynomolgus monkey platelets (upper panels) and human platelets (lower panels) are shown. (D) The mean with standard errors of the mean fluorescence intensity of CD62+PAC-1+ platelets from cynomolgus monkeys (n = 4) and human platelets (n = 3) are shown. **P < 0.01. Platelet activation was observed after incubation with hu5c8-sCD154 ICs in both cynomolgus monkeys and humans. In contrast, no activation was observed with TNX-1500-sCD154 ICs in either cynomolgus or human platelets. FSC, forward scatter; NS, not significant; sCD, soluble CD; SSC, side scatter.
3.3. Pharmacokinetics and serum levels of TNX-1500
3.3.1. Pharmacokinetics
Various doses (30, 100, and 300 mg/kg) of TNX-1500 were administered IV to study the pharmacokinetics (PK) in cynomolgus monkeys (n = 2 for each dose). Monkeys tolerated well up to 300 mg/kg (Fig. 2A), and the half-life of TNX-1500 in cynomolgus monkeys appeared to be approximately 14 days (via the PK modeling). Based on previous studies in nonhuman primates6,15 as well as from these PK studies, 20 mg/kg/d of weekly administration was chosen as the initial dose to test.
Figure 2.
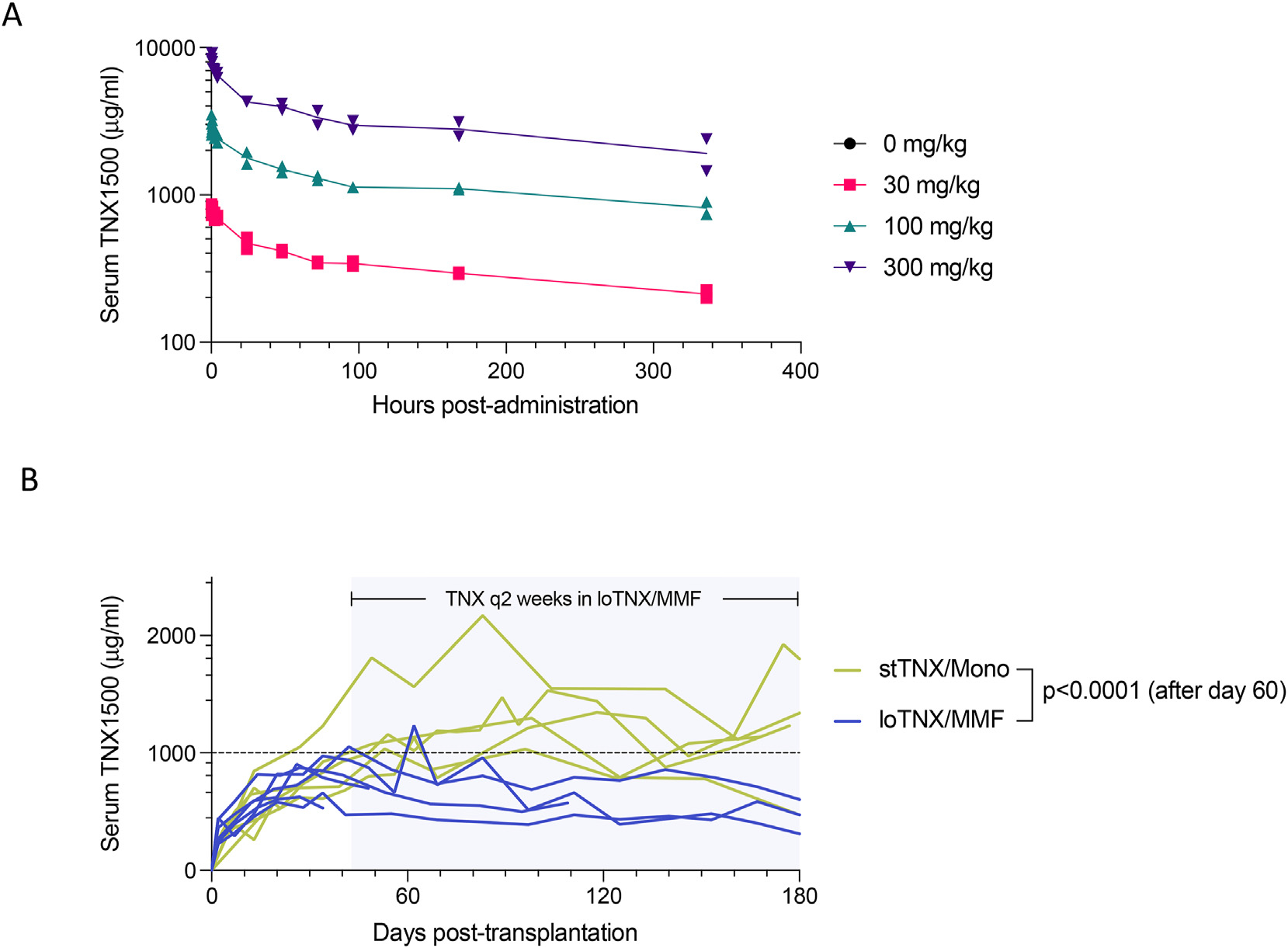
(A) Pharmacokinetics (PK): various doses (0 mg/kg, 30 mg/kg, 100 mg/kg, and 300 mg/kg) of TNX-1500 were administered intravenously to cynomolgus monkeys (n = 2) and their serum levels were measured 5, 15, and 30 minutes and 1, 2, 4, 24, 48, 72, 96, 168, and 336 hours after administration. Drug half-life after intravenous injection appeared to be approximately 14 days based on the PK modeling. (B) Comparison of serum TNX-1500 concentrations between groups: serum levels of TNX-1500 in association with stTNX/mono and loTNX/MMF. Serum levels in stTNX/mono were significantly higher than those in loTNX/MMF (P = 0.016). loTNX/MMF, low-dose TNX + mycophenolate mofetil; stTNX/mono, standard-dose TNX monotherapy.
3.3.2. Serum concentration of TNX-1500 after kidney transplantation
Serum concentrations of TNX-1500 were serially monitored in the 2 treatment groups. In stTNX/mono, recipients received TNX-1500 (20 mg/kg) weekly until EOS, whereas recipients in loTNX/MMF were treated with TNX-1500 weekly until 6 weeks, after which TNX administration was reduced to every other week until EOS. As expected, serum TNX-1500 levels after day 60 were significantly (P = 0.016) higher (1170 ± 325 μg/mL) in association with weekly dosing in stTNX/mono compared with those observed in loTNX/MMF receiving dosing every other week (601 ± 211 μg/mL) (Fig. 2B).
3.4. Renal allograft survival
3.4.1. TNX-1500 monotherapy
To evaluate the immunosuppressive effects of TNX-1500, we first evaluated the effect of monotherapy in MHC-mismatched kidney transplant (Table 2).
Table 2.
Recipient/donor major histocompatibility complex (MHC) disparity and the transplant outcome.
| Group | ID | MHC disparity | Renal allograft survival (d) | Biopsy#1 (D50–70) | Biopsy #2 (D100–120) | Biopsy #3 (D170–180) | DSA at EOS |
|---|---|---|---|---|---|---|---|
|
| |||||||
| stTNX/mono | M10420 | 5/10 | >180 | No rej, C4d0 | No rej, C4d0 | No rej, C4d0 | Negative |
| M10521 | Full | >180 | No rej, C4d0 | NP | No rej, C4d0 | Negative | |
| M3120 | Full | >180 | No rej, C4d0 | No rej, C4d0 | No rej, C4d0 | Negative | |
| M3920 | Full | 28 | TCMR 3, C4d0 | Negative | |||
| M6420 | Full | >180 | No rej, C4d0 | Borderline | No rej, C4d0 | Negative | |
| M1021 | Full | >180 | No rej, C4d0 | No rej, C4d0 | No rej, C4d0 | Negative | |
| loTNX/MMF | M8021 | Full | >111 | No rej, C4d0 | No rej, C4d0 | Negative | |
| M5321 | 5/10 | 48 | TCMR 3 | Positive | |||
| M521 | Full | >180 | No rej, C4d0 | No rej, C4d0 | No rej, C4d0 | Negative | |
| M1421 | 7/10 | 36 | TCMR 2A | Negative | |||
| M11321 | Full | >180 | No rej, C4d0 | NP | No rej, C4d0 | Negative | |
| M8221 | Full | >180 | No rej,C4d0 | NP | No rej, C4d0 | Negative | |
DSA, donor-specific antibody; EOS, end of study; loTNX/MMF, low-dose TNX + mycophenolate mofetil; NP, not performed; rej, rejection; stTNX/mono, standard-dose TNX monotherapy; TCMR, T cell–mediated rejection.
In 6 recipients treated with “standard-dose” TNX-1500 monotherapy (stTNX/mono), 5 of 6 recipients survived >80 days (EOS) with excellent renal function (Fig. 3A). One renal allograft failed because of T cell–mediated rejection (TCMR) on day 28 (Fig. 4A, Table 1, and Supplementary Table 2). Serum TNX-1500 levels plateaued at 800 to 1500 mg/mL by 30 to 60 days posttransplant, and the levels of the recipient that rejected its allograft were not different from those in recipients without rejection (Fig. 3B). Histopathology in the 5 allografts surviving until EOS showed no evidence of rejection (Fig. 4B, Table 2, and Supplementary Table 2) and no C4d deposition (Fig. 4C) without donor-specific antibody (DSA) production (Table 2).
Figure 3.
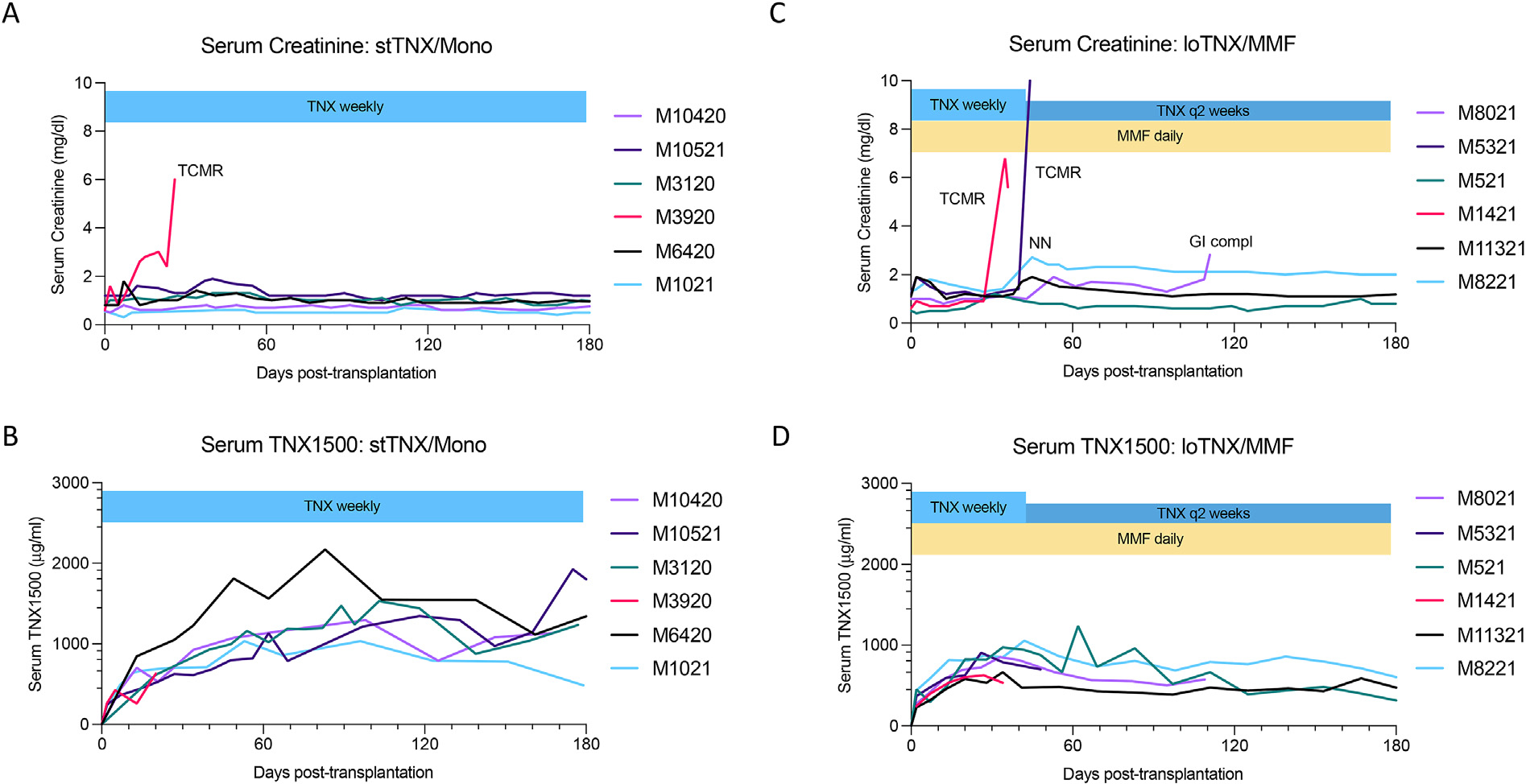
Serum creatinine and TNX-1500 concentrations after transplantation. stTNX/mono: (A) Serum creatinine levels for the recipients in the stTNX/mono group. Five of 6 recipients in the stTNX/mono group did well with normal kidney function until day 180 end of study (EOS). One recipient (M3920) lost his allograft function on day 28 because of rejection. (B) Serum TNX-1500 levels for the recipients in the stTNX/mono group. No significant difference was observed in the TNX-1500 serum levels between 5 recipients without rejection and 1 recipient with rejection. loTNX/MMF: (C) Serum creatinine levels for the recipients treated with loTNX + MMF. Two recipients (M1421 and M5321) developed rejection (T cell–mediated rejection types 2A and 3) on days 36 and 48, respectively. Another recipient (M8021) was euthanized on day 111 because of gastrointestinal complications (ileus) without rejection. The remaining 3 recipients did well with normal kidney function until EOS (180 days). Serum creatinine of M8221 was higher (2.2 mg/dL), after undergoing native nephrectomy on day 40; however, stable without rejection. This was due to the size mismatch of the donor and the recipient. (D) Serum TNX-1500 levels for the recipients in loTNX/MMF. There was no significant difference in the serum levels between the 2 recipients with rejection and the remaining 4 recipients without rejection. loTNX/MMF, low-dose TNX + mycophenolate mofetil; stTNX/mono, standard-dose TNX monotherapy.
Figure 4.
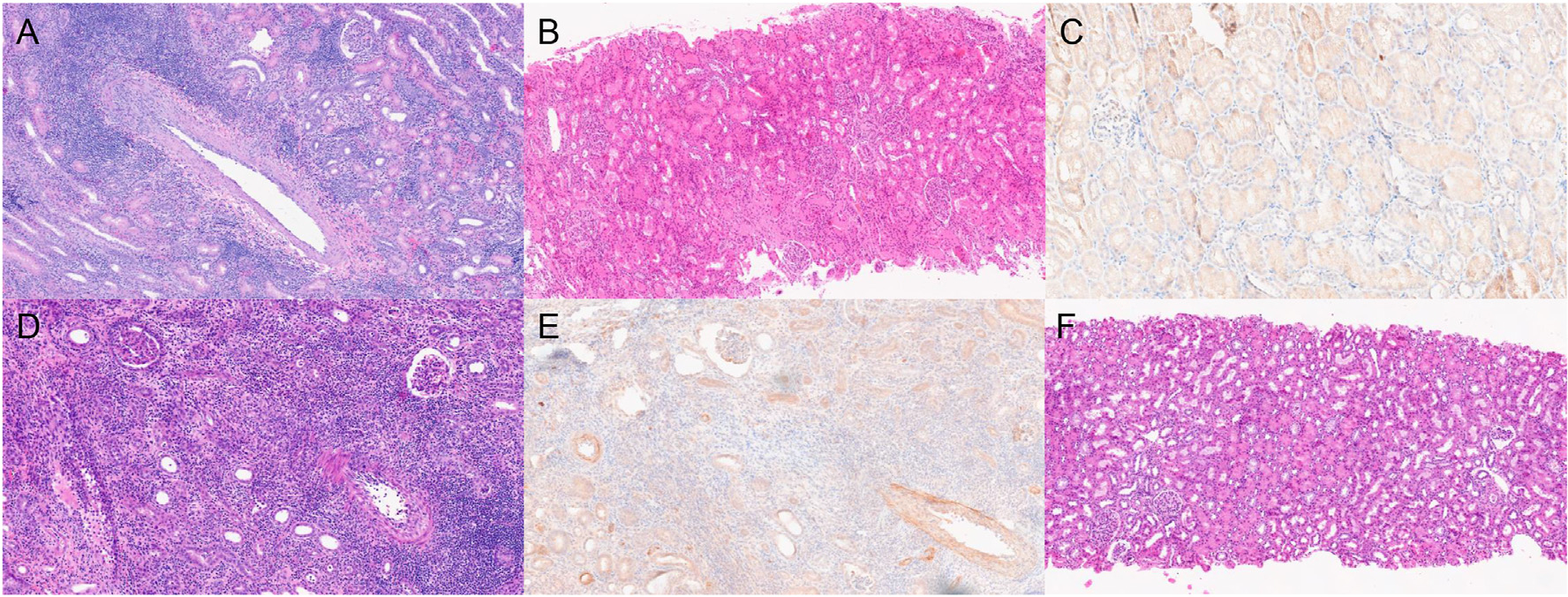
Histopathologic findings. (A) Renal allograft at autopsy on day 28 from a recipient (M3920) in the stTNX/mono group showed T cell–mediated rejection (TCMR) type 3. (B) The remaining 4 recipients did well without rejection. The picture is a representative biopsy from M10521 on day 174, showing no diagnostic abnormality and no C4d staining (C). (D) Biopsy from M5321 (loTNX/MMF) on day 47 showed TCMR type 3 and positive C4d deposition (E). (F) Representative biopsy taken on day 184 from a recipient in the loTNX/MMF group, showed no rejection. loTNX/MMF, low-dose TNX + mycophenolate mofetil; stTNX/mono, standard-dose TNX monotherapy.
3.4.2. TNX-1500 in combination with mycophenolate mofetil
To seek the possibility of reducing the dose of TNX-1500, we evaluated the efficacy of TNX-1500 in conjunction with MMF (loTNX/MMF group), in which TNX-1500 was administered at 20 mg/kg weekly until 6 weeks followed by treatment every other week until EOS. Interestingly, the results were less favorable by combining MMF. Despite daily MMF, 2 recipients developed rejection on days 48 and 36, although they were still receiving TNX-1500 weekly (Fig. 3C). The histopathology of these kidneys showed TCMR types 3 (Fig. 4D) and 2, respectively (Table 2 and Supplementary Table 3). The recipient with TCMR type 3 also developed DSA with C4d deposition (Fig. 4E). TNX-1500 levels in these 2 recipients were between 500 and 800 μg/mL (Fig. 3D), not significantly different from recipients in stTNX/mono (Fig. 3B) or loTNX/MMF animals without rejection episodes (Fig. 3D). An additional loTNX/MMF recipient was euthanized on day 111 (Fig. 3C) because of a gastrointestinal complication (ileus) as a side effect of MMF. No renal allograft rejection was identified during autopsy. The remaining 3 recipients did well until day 180 (EOS) (Fig. 3C), without rejection or DSA (Fig. 4E, Table 2, and Supplementary Table 3).
3.5. Kidney allograft survival compared with conventional immunosuppression or no immunosuppression
The graft survival rates of these 2 TNX groups were significantly higher than our historical results with no immunosuppression (Fig. 5, P < 0.001). Although not statistically significant (P = 0.2), 83% graft survival at EOS (day 180) with stTNX/mono compared favorably with conventional triple drug immunosuppression (ie, Conv IS), with 57% survival at EOS (day 120) (Fig. 5). Overall survival with loTNX/MMF was statistically similar to the graft survival rates with Conv IS. Of note, PTLD was observed in 3 recipients in Conv IS, whereas no PTLD was observed in the current experience with TNX-1500. The risk of developing PTLD appeared significantly lower in TNX-1500 (stTNX/mono + loTNX/MMF) vs Conv IS (Table 3, P < 0.0001).
Figure 5.
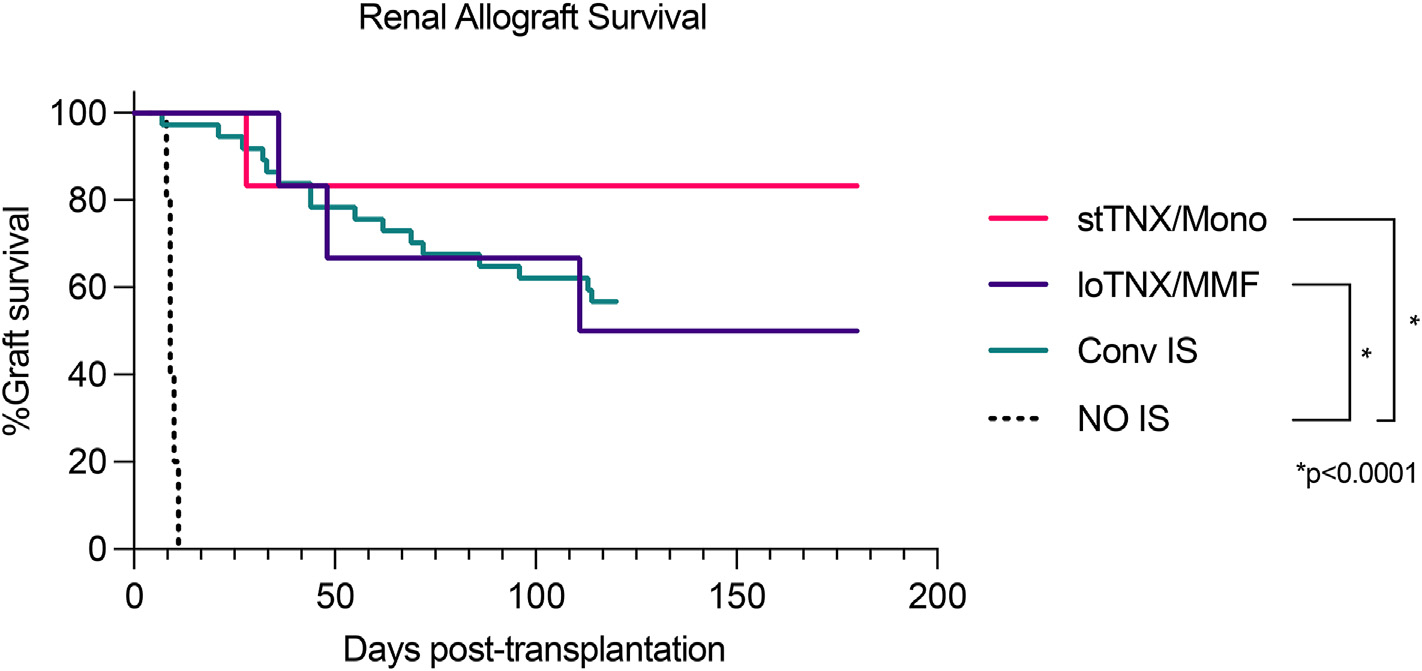
Renal allograft survival. Kaplan-Meier survival comparing stTNX/mono (n = 6), loTNX/MMF (n = 6), conventional immunosuppression (Conv IS) (n = 37), and no immunosuppression (No IS) (n = 5). There was a statistically significant difference between recipients treated with TNX-1500 and No IS (*P < 0.0001) but no significant difference between stTNX/mono vs either loTNX/MMF or Conv IS. loTNX/MMF, low-dose TNX + mycophenolate mofetil; stTNX/mono, standard-dose TNX monotherapy.
Table 3.
Rejections and complications TNX vs conventional.
| Group | n | Rejections and complications |
|---|---|---|
|
| ||
| stTNX/mono | 6 | TCMR (1) |
| loTNX/MMF | 6 | TCMR (2) and GI/ileus (1) |
| Conv IS | 37 | TCMR (11) and PTLD (3) |
Conv IS, conventional immunosuppression; GI, gastrointestinal; loTNX/MMF, low-dose TNX + mycophenolate mofetil; PTLD, posttransplant lymphoproliferative disorder; stTNX/mono, standard-dose TNX monotherapy; TCMR, T cell–mediated rejection.
3.6. Complete Blood Count and lymphocyte subsets in standard-dose TNX monotherapy vs low-dose TNX + mycophenolate mofetil
Although MMF potentially causes bone marrow suppression, there were no significant differences in white blood cell and platelet counts between stTNX/mono and loTNX/MMF (Fig. 6). There were also no significant differences in the naïve and memory T, B, and natural killer lymphocyte subsets between the groups. Although not statistically significant, effector memory T cells (CD4+ or CD8+CD95+CD28−) were more suppressed, whereas regulatory T cells (Treg) were higher in stTNX/mono compared with loTNX/MMF (Fig. 7).
Figure 6.
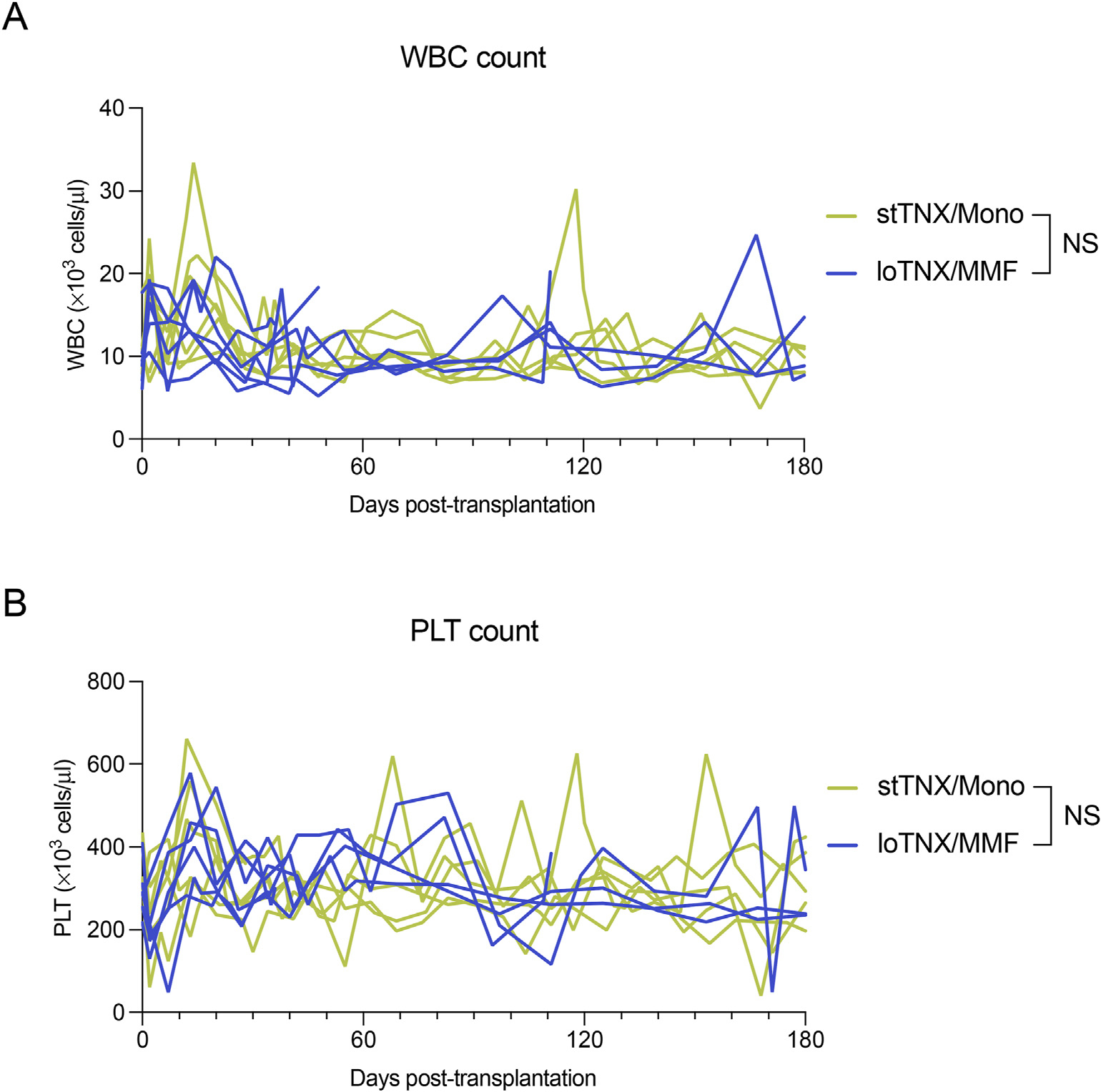
(A) White blood cells (WBCs) and (B) platelet (PLT) count in stTNX/Mono and loTNX/MMF. No statistical difference between the groups. loTNX/MMF, low-dose TNX + mycophenolate mofetil; NS, not significant; stTNX/mono, standard-dose TNX monotherapy.
Figure 7.
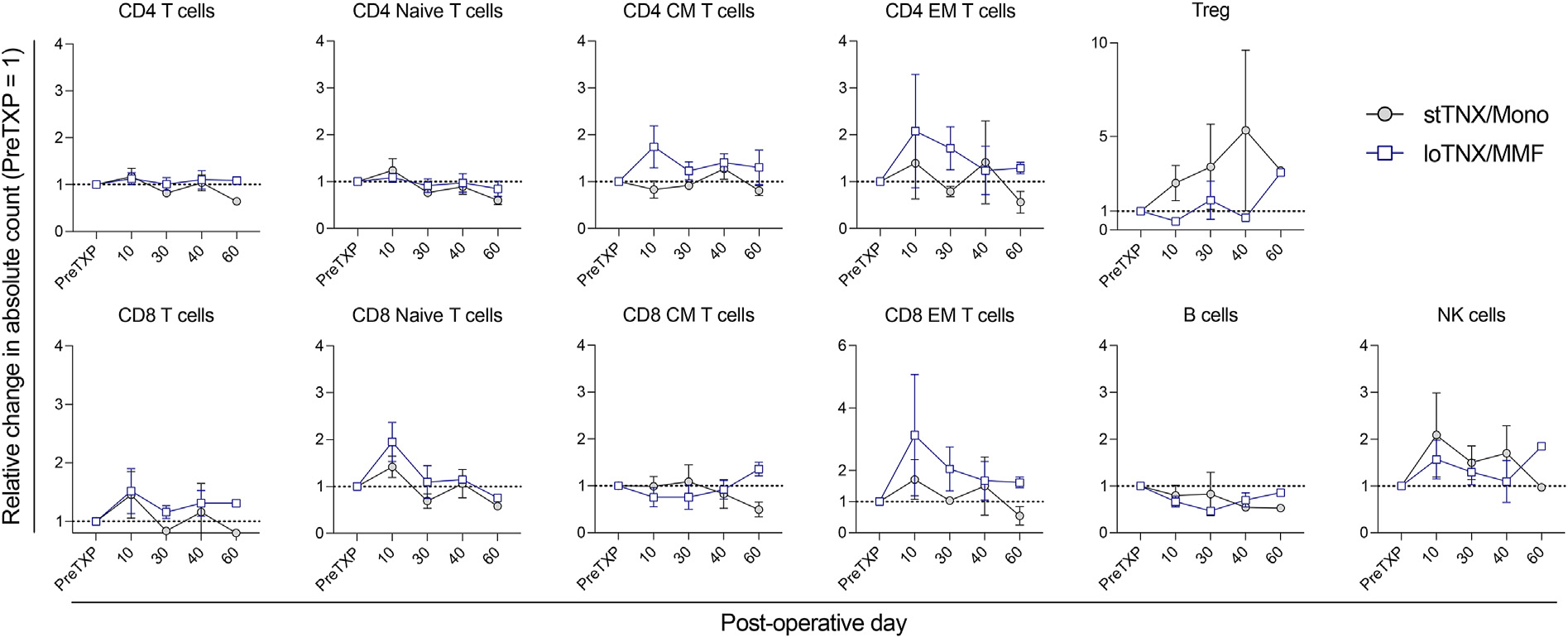
Lymphocyte subsets in stTNX/Mono and loTNX/MMF groups. The posttransplant changes in CD4 T cells (CD3+CD4+), naïve T cells (CD3+CD95−CD28+), central memory (CM) T cells (CD3+CD95+CD28+), effector memory (EM) T cells (CD3+CD95+CD28−), regulatory T (Treg) cells (CD3+CD4+ CD127−Foxp3+), B cells (CD3−CD20+), and natural killer (NK) cells (CD3−, CD8+CD16+NKG2a+) were compared between stTNX/mono and loTNX/MMF by the ratio of posttransplant absolute counts to pretransplant values. There were no significant differences in all lymphocyte subsets between the 2 groups, but EM T cells trended to be more suppressed, whereas Tregs were promoted in stTNX/mono. loTNX/MMF, low-dose TNX + mycophenolate mofetil; stTNX/mono, standard-dose TNX monotherapy.
4. Discussion
TNX-1500 is a novel mAB that contains the hu5c8 Fab domain from ruplizumab22 (hu5c8, BG9588) and an IgG4 Fc region genetically engineered to reduce FcγRIIa binding. Here, we show that TNX-1500 is both efficacious and nonthrombogenic in a clinically relevant NHP KTx model. Our in vitro analysis demonstrated that TNX-1500-sCD154 IC does not activate cynomolgus monkey or human platelets, in clear contrast to 5c8-sCD154 IC and humanized 5c8-sCD154 IC, both of which significantly activated platelets in vitro. Reassuringly, no vascular thrombotic complications were observed in the KTx recipients treated with TNX-1500 in the absence of prophylaxis for thrombosis with platelet inhibitory drugs. The absence of thrombotic events with TNX-1500 in the absence of any prophylaxis for thromboembolism is noteworthy because we previously described a 44% incidence of graft failure when 5c8 was used in conjunction with a tolerogenic conditioning regimen for combined kidney and bone marrow transplantation.23 Around the same time, clinical trials of anti-CD154 mAb for systemic lupus erythematosus and idiopathic thrombocytopenic purpura were halted after unexpected thromboembolic events were associated with ruplizumab.24 In our prior report, 4 of 9 NHP combined kidney and bone marrow transplantation recipients developed either renal arterial or venous thrombosis immediately after transplant. An additional antiplatelet agent, ketorolac, but not heparin, effectively prevented thrombotic complications,25 suggesting a critical role of platelets in thrombosis after CD154 mAb treatment. Several observations in subsequent studies have revealed that immune complexes containing soluble CD154 and anti-CD154 mAb with IgG1 Fc interact with the FcγRIIa/Fc gamma receptor (FCGR2A) on platelets to induce thrombosis in human FCGR2A transgenic mice.12 Eliminating Fc binding (aglycosyl hu5c8) or eliminating the Fc region (pegylated Fab antibody) significantly decreased thromboembolism13 but was also associated with reduced efficacy to prevent transplant rejection.14
A modification to reduce FcγRIIa binding did not appear to diminish the immunosuppressive efficacy of TNX-1500 because allograft survival was 83% at 6 months of EOS in association with standard-dose monotherapy. Although not statistically significant, recipients treated with TNX-1500 monotherapy exhibited a strong trend toward improved transplant survival relative to historical animals treated with conventional triple drug immunosuppression. Importantly, there have been no side effects or infectious complications, especially Epstein-Barr virus–related PTLD, which has often been observed in NHP transplants treated with conventional immunosuppression.
In recipients treated with MMF (loTNX/MMF), there were 2 early graft losses because of rejection when they were still treated with weekly TNX-1500. Although the combination with MMF needs to be evaluated with more animals, a trend toward inferior allograft survival was observed in heart transplant recipients when reduced-intensity TNX-1500 was combined with MMF in concomitantly performed heart transplantation at Massachusetts General Hospital (MGH) (Miura et al, manuscript submitted). In that study, Tregs in MMF-treated recipients appeared significantly lower than in recipients treated with stTNX/mono. Similarly, in our study, Treg expansion observed in stTNX/mono early after transplantation was inhibited in the recipients treated with MMF. In the first report of anti-CD154 mAb tested in NHP KTx, Kirk et al6 also reported that nonspecific immunosuppression may be inhibitory to the salutary effect of anti-CD154 mAb. Should this phenomenon prove reproducible in more transplants, we could speculatively infer that MMF interferes with Treg maturation or function during selective CD154 inhibition, with implications for human trial design.
Optimal TNX-1500 dosing and frequency of administration remain to be defined. In this study, the “standard” dose of TNX was initiated at 20 mg/kg, given 4 times in the first 2 weeks and weekly thereafter. In this study, stTNX/mono did not achieve stable trough levels of approximately 1000 until 30 to 60 days. Because all T cell mediated rejection (TCMRs) were observed within the first 60 days, we speculate that if the schedule of antibody administration was modified to achieve trough levels >500 mg/mL within the first week, the incidence of acute cellular rejection and antibody mediated rejection (AMR) by 2 months might be reduced. The dose of TNX-1500 appears to be safe because we observed no clinically significant infectious complications, such as cytomegalovirus (CMV) and BK virus (BKV). Although the results of Conv IS were historical data, the risk of developing PTLD was significantly lower in TNX-1500 vs Conv IS (P < 0.0001), which may be a potential clinical advantage of using anti-CD154 mAb treatment over Conv IS.
In conclusion, our data demonstrate a favorable safety profile associated with TNX-1500 because neither NHP nor human platelet activation was observed in vitro when exposed to TNX-1500-sCD154 ICs. The therapeutic effects of stTNX-1500 to consistently inhibit rejection of MHC-mismatched kidney allografts were not associated with infectious or thromboembolic complications, suggesting that clinical studies are warranted to evaluate TNX-1500 for transplant indications. Meanwhile, ongoing preclinical work will investigate the mechanisms behind the apparent antagonistic properties of MMF in combination with CD154 blockade and explore TNX-1500 to promote tolerance.
Supplementary Material
Acknowledgments
The authors thank Dr Joanne Morris and Michael Duggan for veterinary supervision and Ashley D’Attilio for transplant and posttransplant management. The research fund was provided by Tonix Pharmaceuticals Inc. The authors would also like to thank Servier Medical Art, provided by Servier and licensed under a Creative Commons Attribution 3.0 Unported License, for partly generating the figures of this article.
Abbreviations:
- ACR
acute cellular rejection
- AMR
antibody mediated rejection
- CKBMT
combined kidney and bone marrow transplantation
- CNI
antigen binding fragment
- DBMT
donor bone marrow transplantation
- DSA
donor specific antibody
- EDC
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride
- EOS
end of study
- Fab
antibody binding fragmen
- FCGR
crystallized fragment gamma receptor
- FcR
crystallized fragment receptor
- GFR
glomerular filtration rate
- IC
immune complex
- IS
immunosuppression
- KD
dissociation constant
- KTx
kidney transplant
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- MMF
mycophenolate mofeti
- NHP
nonhuman primates
- NHS
N-hydroxysuccinimide
- PTLD
post-transplant lymphoproliferative disease
- sCD154
soluble CD154
- TCMR
T cell mediated rejection
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Research funding was provided by Tonix Pharmaceuticals. Tonix Pharma has filed patent applications on the genetic modification of crystallizable fragment portion of 5c8 described in this article. B.D., S.F., and S.L. are employees of Tonix Pharmaceuticals.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajt.2023.03.022.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
- 1.Karolin A, Genitsch V, Sidler D. Calcineurin inhibitor toxicity in solid organ transplantation. Pharmacology. 2021;106(7–8):347–355. [DOI] [PubMed] [Google Scholar]
- 2.Vincenti F, Blancho G, Durrbach A, et al. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol. 2010;21(9):1587–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belatacept Vincenti F. and long-term outcomes in kidney transplantation. N Engl J Med. 2016;374(26):2600–2601. [DOI] [PubMed] [Google Scholar]
- 4.Wen X, Casey MJ, Santos AH, Hartzema A, Womer KL. Comparison of utilization and clinical outcomes for belatacept- and tacrolimus-based immunosuppression in renal transplant recipients. Am J Transplant. 2016;16(11):3202–3211. [DOI] [PubMed] [Google Scholar]
- 5.Kumar D, LeCorchick S, Gupta G. Belatacept as an alternative to calcineurin inhibitors in patients with solid organ transplants. Front Med (Lausanne). 2017;4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5(6):686–693. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Ford ML. CD11b is a novel alternate receptor for CD154 during alloimmunity. Am J Transplant. 2020;20(8):2216–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai T, Sogawa H, Boskovic S, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4(9):1391–1398. [DOI] [PubMed] [Google Scholar]
- 9.Hotta K, Oura T, Dehnadi A, et al. Long-term nonhuman primate renal allograft survival without ongoing immunosuppression in recipients of delayed donor bone marrow transplantation. Transplantation. 2018;102(4):e128–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma D, Hirose T, Lassiter G, et al. Kidney transplantation from triple-knockout pigs expressing multiple human proteins in cynomolgus macaques. Am J Transplant. 2022;22(1):46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22(3):221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robles-Carrillo L, Meyer T, Hatfield M, et al. Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J Immunol. 2010;185(3):1577–1583. [DOI] [PubMed] [Google Scholar]
- 13.Shock A, Burkly L, Wakefield I, et al. CDP7657, an anti-CD40L antibody lacking an Fc domain, inhibits CD40L-dependent immune responses without thrombotic complications: an in vivo study. Arthritis Res Ther. 2015;17(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrant JL, Benjamin CD, Cutler AH, et al. The contribution of Fc effector mechanisms in the efficacy of anti-CD154 immunotherapy depends on the nature of the immune challenge. Int Immunol. 2004;16(11):1583–1594. [DOI] [PubMed] [Google Scholar]
- 15.Kim SC, Wakwe W, Higginbotham LB, et al. Fc-silent anti-CD154 domain antibody effectively prevents nonhuman primate renal allograft rejection. Am J Transplant. 2017;17(5):1182–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connor SL, Blasky AJ, Pendley CJ, et al. Comprehensive characterization of MHC class II haplotypes in Mauritian cynomolgus macaques. Immunogenetics. 2007;59(6):449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pendley CJ, Becker EA, Karl JA, et al. MHC class I characterization of Indonesian cynomolgus macaques. Immunogenetics. 2008;60(7):339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosimi AB, Delmonico FL, Wright JK, et al. Prolonged survival of nonhuman primate renal allograft recipients treated only with anti-CD4 monoclonal antibody. Surgery. 1990;108(2):406–413 [discussion 413–404]. [PubMed] [Google Scholar]
- 19.Karlsson R SPR for molecular interaction analysis: a review of emerging application areas. J Mol Recognit. 2004;17(3):151–161. [DOI] [PubMed] [Google Scholar]
- 20.Yamada Y, Boskovic S, Aoyama A, et al. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. Am J Transplant. 2012;12(2):330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 22.Liossis SN, Sfikakis PP. Costimulation blockade in the treatment of rheumatic diseases. BioDrugs. 2004;18(2):95–102. [DOI] [PubMed] [Google Scholar]
- 23.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6(2):114. [DOI] [PubMed] [Google Scholar]
- 24.Boumpas DT, Furie R, Manzi S, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48(3):719–727. [DOI] [PubMed] [Google Scholar]
- 25.Koyama I, Kawai T, Andrews D, et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation. 2004;77(3):460–462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


