Abstract
Recent studies report varying levels of ethanol consumption by rodents maintained on different commercially available laboratory diets. As varied ethanol consumption by dams may impact offspring outcome measures in prenatal ethanol exposure paradigms, we compared ethanol consumption by rats maintained on the Envigo 2920 diet, used in our vivarium, with an isocalorically equivalent PicoLab 5L0D diet used in some alcohol consumption studies. Compared to 5L0D diet, female rats maintained on 2920 diet consumed 14% less ethanol during daily four-hour drinking sessions prior to pregnancy and 28% less ethanol during gestation. Rat dams consuming 5L0D diet gained significantly less weight during pregnancy. However, their pup birth weights were significantly higher. A subsequent study revealed that hourly ethanol consumption was not different between diets during the first two hours, but was significantly lower on 2920 diet at the end of the third and fourth hours. The mean serum ethanol concentration in 5L0D dams after the first two hours of drinking was 46 mg/dL compared to 25 mg/dL in 2920 dams. Further, ethanol consumption at the two-hour blood sampling time point was more variable in 2920 dams compared to 5L0D dams. An in vitro analysis mixing each powdered diet with 5% ethanol in acidified saline revealed that a 2920 diet suspension adsorbed more aqueous medium than 5L0D diet suspension. The total ethanol remaining in aqueous supernatant of 5L0D mixtures was nearly twice the amount of ethanol in supernatants of the 2920 mixtures. These results suggest that the 2920 diet expands to a greater extent in aqueous medium than the 5L0D diet. We speculate that increasing adsorption of water and ethanol by the 2920 diet may reduce or delay the amount of ethanol absorbed and may decrease serum ethanol concentration to a greater extent than would be predicted from the amount of ethanol consumed.
Keywords: Ethanol Consumption, Prenatal Alcohol Exposure, Rodent Diet
INTRODUCTION
It well established that ethanol consumption during pregnancy can cause a wide array of adverse neurobiologic and neurobehavioral consequences described as Fetal Alcohol Spectrum Disorders (FASD; Hoyme et al., 2016; Popova et al., 2023). While no specific behavioral phenotype exists among patients with FASD, most notable among the behavioral problems associated with prenatal alcohol exposure (PAE) are deficits in neurocognition, self-regulation, and adaptive functioning (Matson & Riley, 2011; Kable et al., 2016). Typically, these behavioral consequences are not detectable early in development, but become more apparent as a child matures and can persist into young- and middle-aged adulthood (Glass, Moore & Mattson, 2023; Popova et al., 2023). Further, while the neurobehavioral problems associated with PAE in children and adolescents have been the primary focus of most clinical investigations over the past four decades, it is now apparent that PAE-induced epigenetic modifications in fetal programming (Kobor & Weinberg, 2011; Lussier, Bodnar & Weinberg, 2017) can predispose offspring to an increased risk for so-called secondary medical consequences in adulthood including behavioral health disorders (Streissguth & O’Malley, 2000; Hellemans, Sliwowska, Verma & Weinberg, 2010) along with dysfunction of the neuroendocrine and neuroimmunologic axes (Lussier, Bodnar & Weinberg, 2021).
Efforts to understand the mechanistic consequences of PAE that underlie the adverse neurobiologic and behavioral outcomes associated with FASD have relied primarily on the use of animal models of PAE. Generally speaking, earlier studies of PAE typically employed higher ethanol levels to model chronic heavy or binge drinking patterns to demonstrate ethanol’s teratological effects on morphology (Randall, 1987; Sulik, Cook & Webster, 1988; Berman, Hannigan, Sperry & Zajac, 1996; Chen, Maier, Parnell, West, 2003), often in association with altered rodent behaviors (Riley, 1990; West, Goodlett & Brandt, 1990; Berman & Hannigan, 2000). More recently, work has increasingly involved the use of relatively moderate levels of PAE in an effort to emulate patterns of “social drinking” with a focus on ethanol’s long-term impact on integrative biochemistry and physiology (Weinberg, 1994; Sutherland, McDonald & Savage, 1997; Savage, Cruz, Duran & Paxton, 1998; Costa, Savage & Valenzuela, 2000; Varaschin, Allen, Rosenberg, Valenzuela & Savage, 2018) along with more complex behavioral responses (Noor et al., 2017; Harvey, Berkowitz, Savage, Hamilton & Clark, 2020; Olguin, Thompson, Young & Brigman, 2021).
Each of the various animal models used to study PAE convey certain advantages and some limitations relative to emulating the varied patterns of human ethanol consumption and PAE. In recent years, our laboratory has employed a model of intermittent moderate ethanol consumption in Long-Evans rats (Savage et al., 2010; Davies et al., 2019). This model involves female rats consuming ethanol four hours a day, early in the dark cycle, both prior to and throughout pregnancy. The pre-pregnancy drinking phase provides some translational relevancy to human ethanol consumption as well as an assessment of drinking prior to pregnancy to ensure that pre-pregnancy drinking is similar between rat dams that are subsequently assigned to either a control (no ethanol) group and dams that continue to drink ethanol during pregnancy. However, while oral ethanol consumption and gastric absorption of ethanol also confer translational relevancy to human ethanol consumption, one limitation of this approach is that the amount of ethanol consumed by female Long-Evans can be variable and the total amount of ethanol consumed, on average, is relatively low compared particularly to mouse models of oral ethanol consumption.
Given the relatively low level of ethanol consumption with our model, one recent concern has been whether the use of different rat chow diets may affect the amount and / or the variability of ethanol consumption in a manner that could have a disproportionately greater impact on moderate drinking paradigms. Indeed, recent studies of oral ethanol consumption by rodents indicate that the type of commercially available diet used is one important factor contributing to variable ethanol consumption in mice (Marshall et al.,2015; Quadir et al., 2020; Maphis, Huffman & Linsenbardt, 2022) and rats (Wang, Feltham, Eskin & Suh, 2021). Differences in ethanol consumption is a particular concern in preclinical models of PAE where variable serum ethanol concentrations could affect offspring outcome measures, potentially confounding the interpretation of data sets within a laboratory, as well as across different laboratories if different rodent diets are utilized.
To date, the studies comparing the impact of various commercial diets on ethanol consumption has primarily focused on drinking behavior in adult rodents (Marshall et al., 2015; Maphis et al., 2022). The question of whether commercially available diets affect ethanol consumption by rat dams during pregnancy and the potential for diet-based differences in offspring outcome measures has not been addressed. In the present study, we compared the effects of Teklad 2920 diet, the standard rodent diet used in our Animal Resource Facility, to a PicoLab 5L0D diet reported recently to provide higher levels of ethanol consumption in non-pregnant mice (Quadir et al., 2020; Maphis et al., 2022). Based on these prior reports, we predicted that the rat dams maintained on the 5L0D diet would consume more ethanol on a daily basis, resulting in correspondingly higher serum ethanol concentrations during daily drinking sessions and have a greater impact on maternal weight gain during pregnancy, offspring litter size and pup birth weight.
The diet-based differences in drinking and offspring outcome measures we report here (Figure 1) were drawn from historical data collected over the past four years using our established prenatal ethanol exposure paradigm (Davies et al., 2019). The 2920 diet was employed in earlier breeding rounds and 5L0D diet in more recent breeding rounds. The results of this retrospective review of drinking and offspring outcomes data led to a systematic comparison of the effects of the two diets on hourly ethanol consumption (Figure 2) and resulting serum ethanol concentrations (Figure 3), followed by an examination of aqueous suspensions of each diet in vitro (Figure 4).
FIGURE 1. Effects of 2920 and 5L0D diets on ethanol consumption before and during pregnancy and on rat dam weight gain, litter size and pup birth weight.
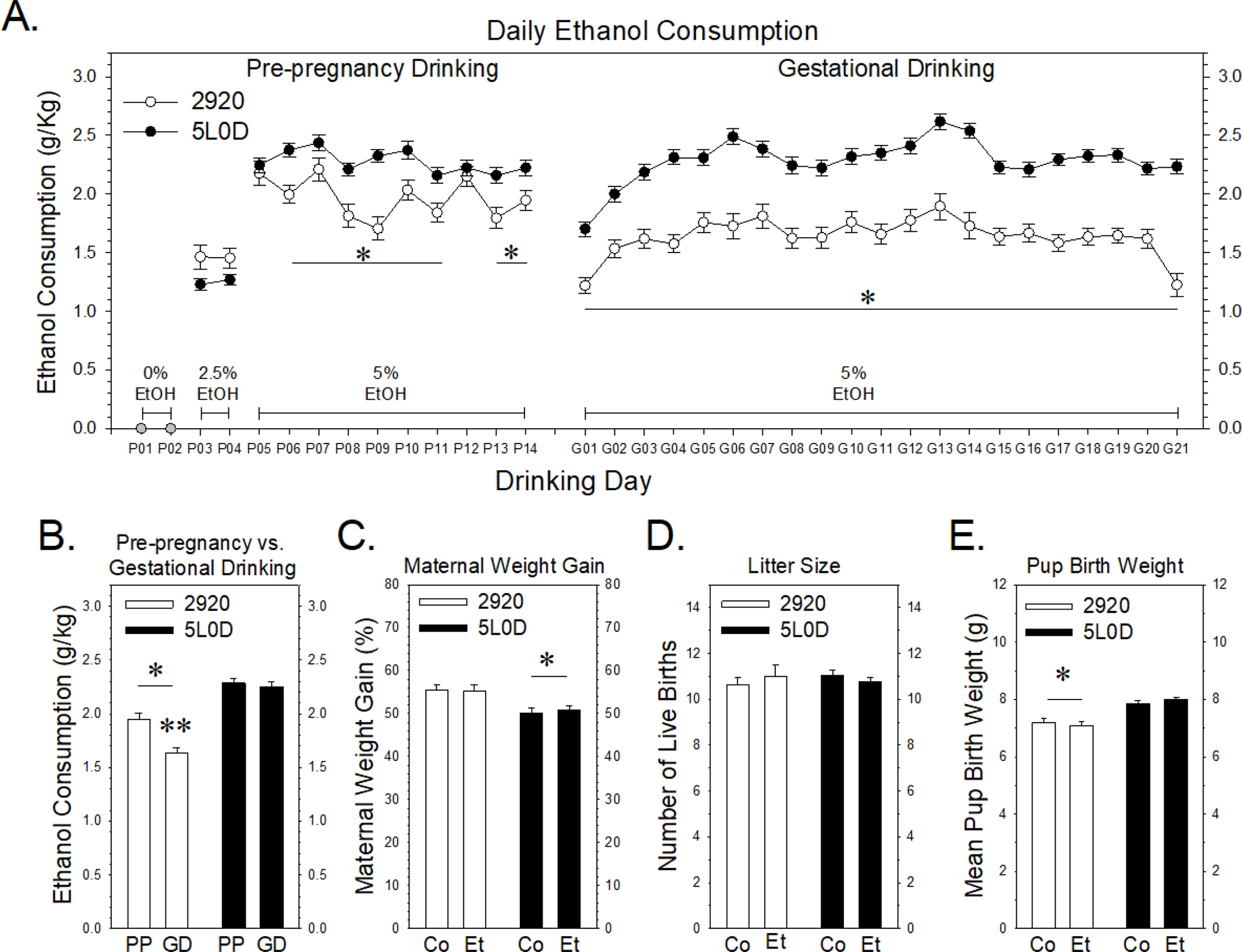
Data points or graph bars in Figures 1A and 1B represent the mean ± SEM grams of ethanol consumed / kg body weight of thirty-seven 2920 females (white) or eighty-four 5L0D females (black). 1A: Pre-pregnancy ethanol consumption. A two-way analysis of variance (ANOVA) revealed a significant interaction between diet and drinking day (F9,1185 = 2.28, p = 0.015). The asterisks denote pre-pregnancy drinking days when 5% ethanol consumption by 2920 females was significantly less compared to 5L0D females (Holm-Sidak post-hoc tests: t values > 2.0, p < 0.045). Gestational ethanol consumption. A two-way ANOVA revealed significant main effects of diet (F1,2494 = 719, p < 0.001) and drinking day (F20,2494 = 8.79, p < 0.001). The asterisk denotes that ethanol consumption by 2920 dams was significantly less compared to 5L0D dams throughout gestation. 1B: Mean daily 5% ethanol consumption prior to and during pregnancy. “PP” denotes pre-pregnancy drinking days and “GD” denotes gestational drinking days. A two-way analysis of variance (ANOVA) revealed a significant interaction between diet and gestational state (F1,238 = 6.84, p = 0.009). The single asterisk denotes that ethanol consumption by 2920 rats was significantly less both before and during pregnancy (Holm-Sidak post-hoc tests: pre-pregnancy t = 4.56, p < 0.001; gestational drinking t = 8.26, p < 0.001). Further, while ethanol consumption by 5L0D rats was not different before or during pregnancy (t = 0.59, p = 0.55), ethanol consumption by 2920 rats decreased significantly during pregnancy, denoted by the double asterisk (t = 3.53, p < 0.001). 1C: Maternal weight gain during pregnancy, expressed as a percentage increase in weight above weight at breeding. In Figures 1C – 1E, “Co” denotes the saccharin (0% ethanol) group and “Et” denotes the 5% ethanol treatment group. Data bars in Figures 1C - 1E represent the mean ± SEM percent change in weight gain (1C), litter sizes (1D) and pup birth weights (1E) of thirty-eight saccharin control 2920 dams and thirty-seven 5% ethanol 2920 dams (white bars) and eighty five saccharin control 5L0D dams and eighty-four 5% ethanol 5L0D dams (black bars). A two-way ANOVA revealed significant main effect of diet on weight gain (F1,240 = 14.4, p < 0.001), with 5L0D dams gaining significantly less weight, denoted by the asterisk, than 2920 dams. Prenatal treatment did not affect maternal weight gain (F1,240 = 0.016, p =0.899). 1D: Offspring litter size, expressed as the number of live births per litter. A two-way ANOVA revealed no significant effects of either diet (F1,240 = 0.064, p = 0.800) or prenatal treatment (F1,240 = 0.0188, p =0.891). 1E: Mean offspring birth weight, expressed in grams, based on total litter weight divided by litter size. A two-way ANOVA revealed significant main effect of diet on pup birth weight (F1,240 = 43.3, p < 0.001), with 2920 dams gaining significantly less weight, denoted by the asterisk, than 5L0D pups. Prenatal treatment did not affect pup birth weight gain (F1,240 = 0.018, p =0.892).
FIGURE 2. Impact of the 2920 and 5L0D diets on hourly ethanol consumption.
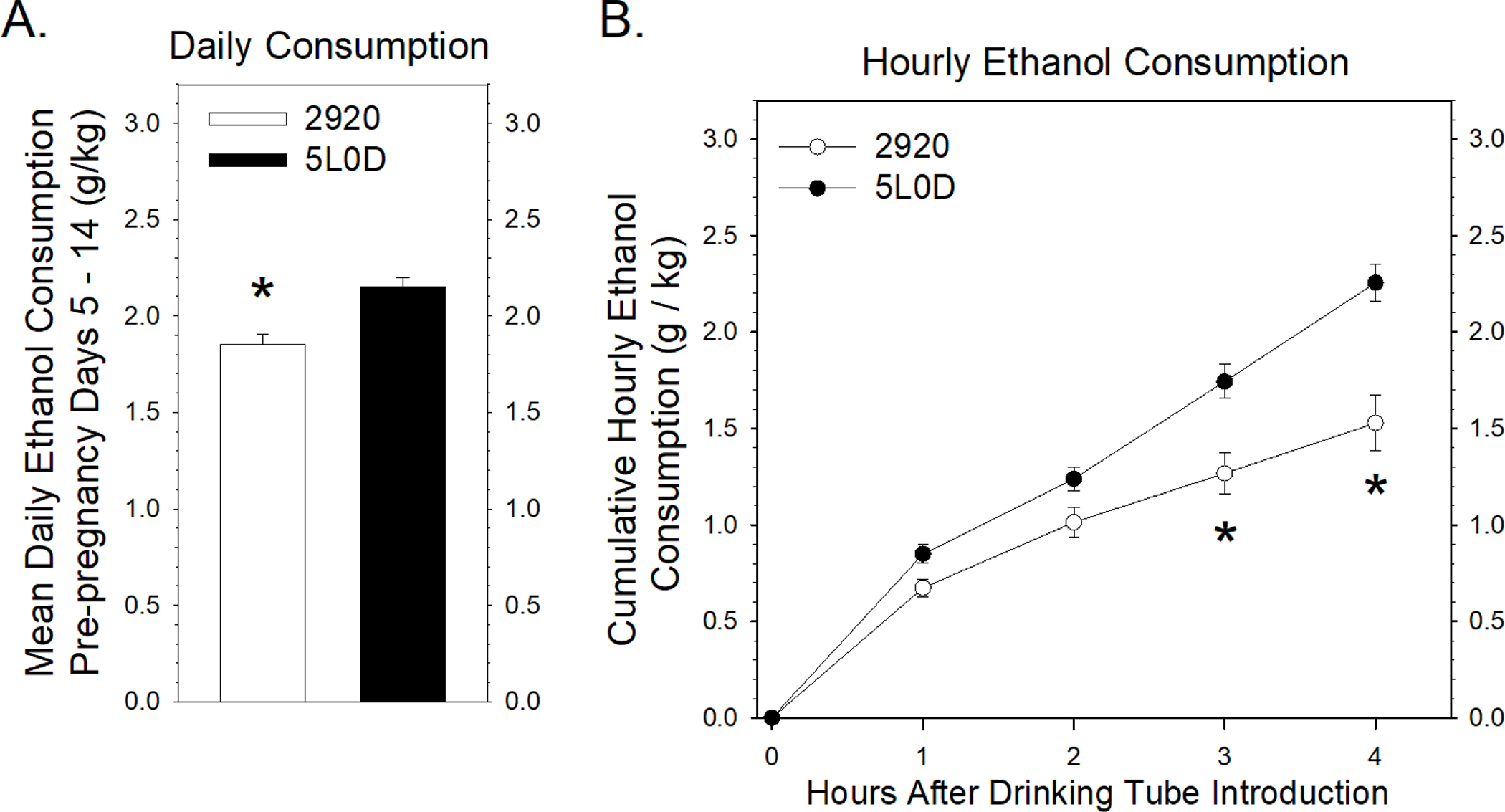
2A: Mean daily 5% ethanol consumption prior to pregnancy. Data points represent the mean ± SEM grams of daily 5% ethanol consumed / kg body weight of 18 pairs of female rats. The asterisk denotes that the 2920 females consumed less ethanol compared to 5L0D females (Student’s two-tailed t-test: t = −4.22 with 34 degrees of freedom; p < 0.001). 2B: Hourly 5% ethanol consumption prior to pregnancy. A two-way ANOVA revealed a significant interaction between the cumulative amount of ethanol consumed and time since the introduction of the drinking tubes (F3,136 = 4.07, p = 0.008). A post-hoc Holm-Sidak test indicated no significant differences in cumulative ethanol consumption after one hour (t = 1.40, p = 0.163) or two hours (t = 1.79, p = 0.075), but significant decreases, denoted by asterisks, in cumulative ethanol consumption by 2920 females compared to 5L0D females after three hours (t = 3.78, p < 0.001) and four hours (t = 5.78, p < 0.001) of ethanol consumption.
FIGURE 3. Effects of the 2920 and 5L0D diets on ethanol consumption and serum ethanol concentrations in rat dams two hours after the introduction of the drinking tube.
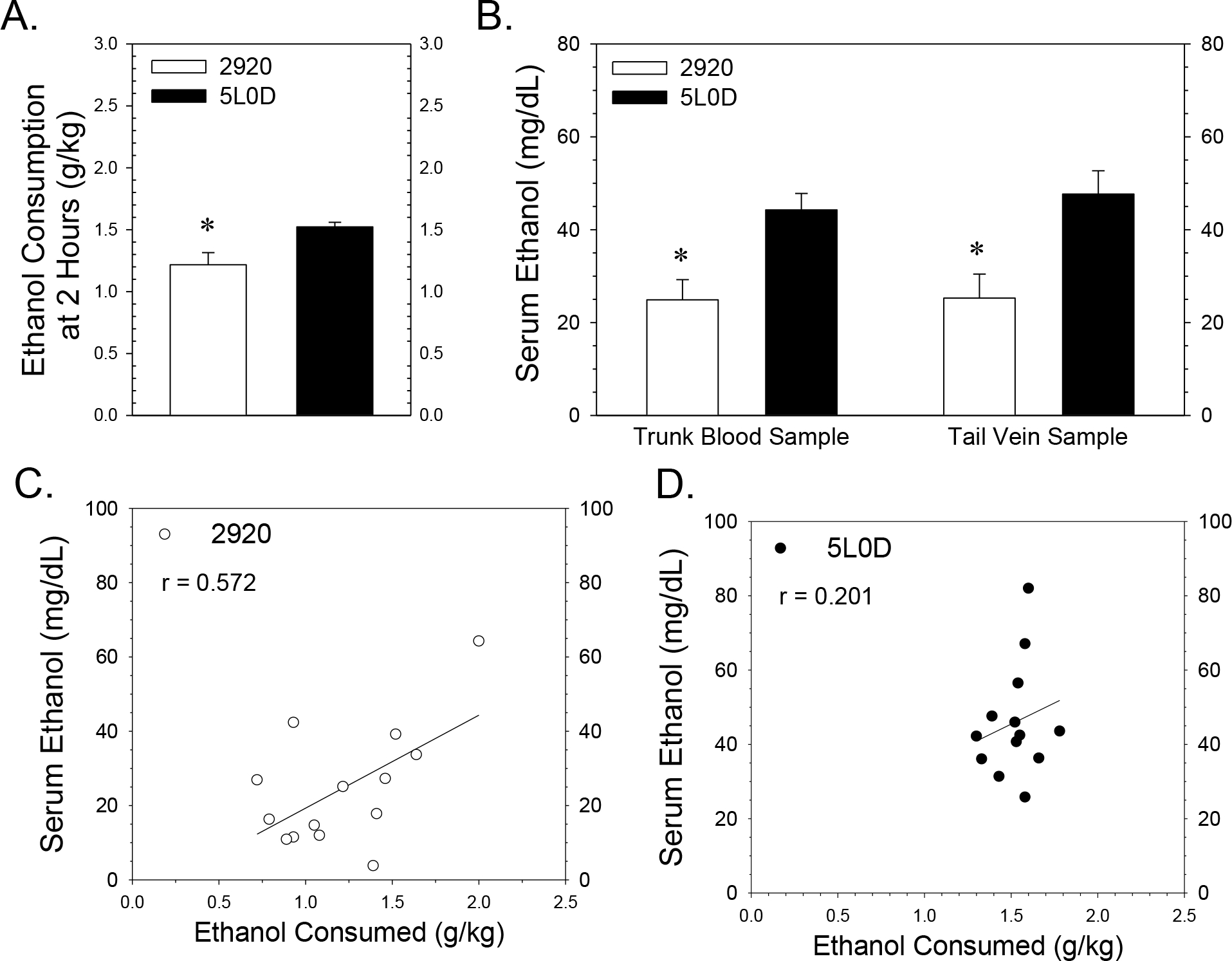
Data points or graph bars in Figure 3 represent the mean ± SEM grams of ethanol consumed / kg body or serum ethanol concentration from fourteen 2920 dams (white) or thirteen 5L0D dams (black). 3A: Mean 5% ethanol consumption two hours after the introduction of the drinking tubes on the gestational day of blood sampling pregnancy. The asterisk denotes that the 2920 dams consumed less ethanol compared to 5L0D dams at the time of blood sampling (Student’s two-tailed t-test: t = −2.83 with 25 degrees of freedom; p = 0.009). 3B: Serum ethanol concentrations from both tail vein and trunk blood samples collected two hours after the introduction of the drinking tubes. Samples were collected sequentially from tail vein and trunk blood from rat dams 110 to 120 minutes after the introduction of drinking tubes either on Gestational Days 15, 16 or 17. A two-way ANOVA revealed significant difference based on diet (F1,47 = 21.1, p < 0.001) with no significant effect of blood ethanol sample source (F1,47 = 0.174, p = 0.678) and no significant interaction between factors (F1,47 = 0.113, p = 0.738). Asterisks denote a significant decrease in serum ethanol concentration in dams consuming the 2920 dams compared to the 5L0D dams. 3C and 3D: Impact of the 2920 and 5L0D diets on the relationship between rat dam ethanol consumption and serum ethanol concentration. Tail vein and trunk blood serum ethanol concentrations were averaged for each rat dam plotted as a function of ethanol consumption data for 2920 dams (3C) and 5L0D dams (3D). The range of ethanol consumption on the 2920 diet was nearly double the range with the 5L0D diet, while the corresponding ranges in serum ethanol concentration were similar between the two diets. Pearson correlation analyses revealed a moderate but significant positive correlation (r = 0.57, p =0.032) between ethanol consumption and resulting serum ethanol levels in 2920 dams (3C), but a lower positive correlation between consumption and ethanol level (r = 0.20, p = 0.511) in 5L0D dams (3D).
FIGURE 4. Effect of the 2920 and 5L0D on liquid adsorption and ethanol recovery from acidified saline suspensions.
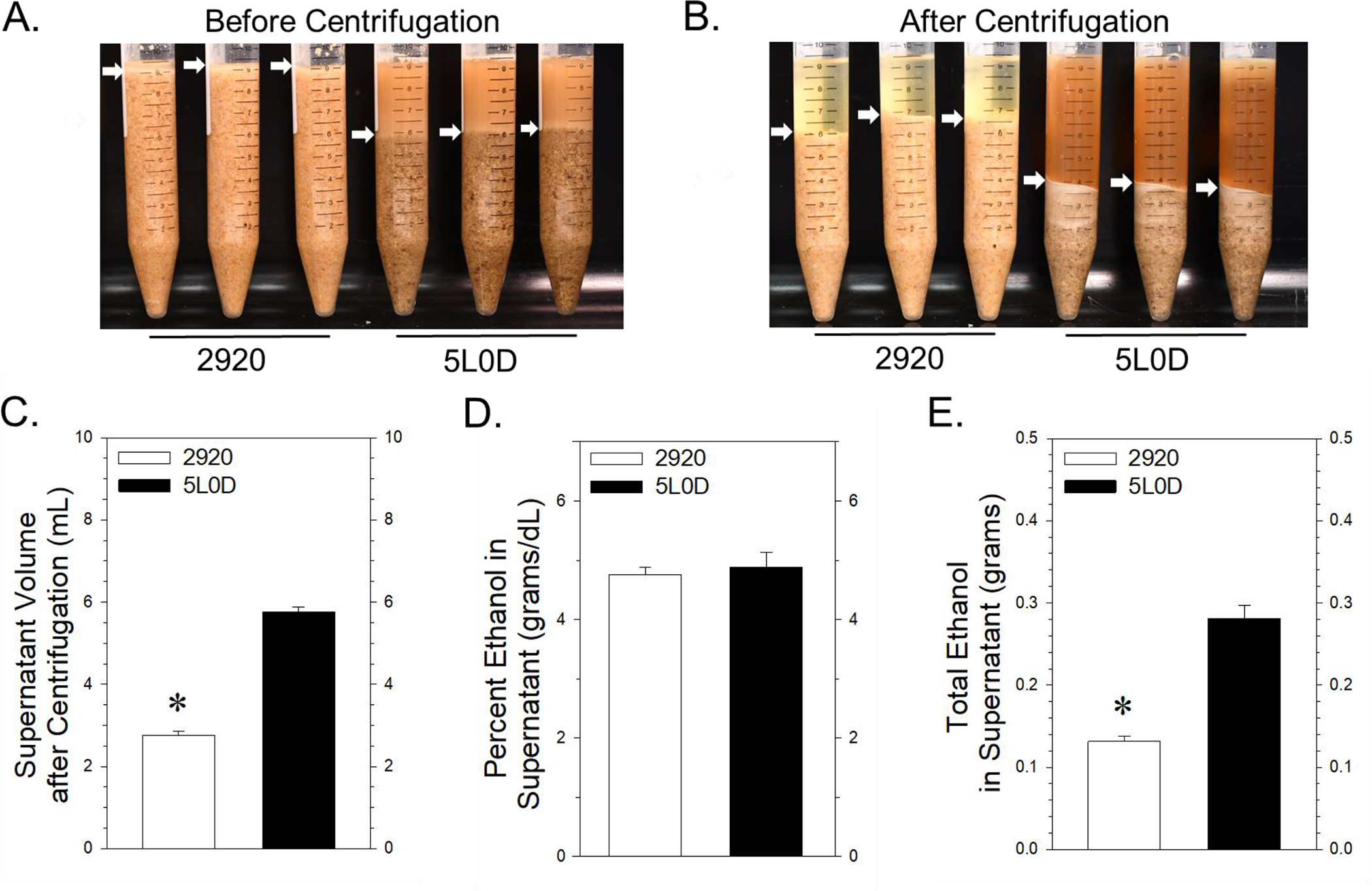
4A: Settled suspensions of 2920 diet (left triplicate of tubes) and 5L0D diet (right triplicate of tubes). Arrows denote the diet suspension - aqueous interface. 4B: Packing density after centrifugation of 2920 diet (left triplicate of tubes) and 5L0D diet suspensions (right triplicate of tubes). Arrows denote the interface between the pelleted fraction and the supernatant fraction. 4C: Volume of supernatant of each diet suspension after centrifugation. Data bars represent the mean ± SEM volume of supernatant, expressed in mL, from four samples of each diet. The asterisk denotes significantly smaller volumes of supernatant from the 2920 mixtures compared to the 5L0D mixtures (Student’s two-tailed t-test: t = −19.2, with 6 degrees of freedom; p < 0.001). 4D: Ethanol concentration in the supernatants of the two diet suspensions. Data bars represent the mean ± SEM mg/dL of ethanol from four samples of each diet supernatant. 4E: The total amount of ethanol in the supernatant from each diet mixture was calculated by dividing the ethanol concentration in each supernatant (from 4D) by the volume of supernatant (from 4C). Data bars represent the mean ± SEM of ethanol, expressed in milligrams, in the supernatant of four samples of each diet mixture. The asterisk denotes a significantly less amount of ethanol in 2920 supernatants compared to 5L0D supernatants (Student’s two-tailed t-test: t = −8.36 with 6 degrees of freedom; p < 0.001).
MATERIALS & METHODS
Subjects.
The University of New Mexico Health Sciences Center (UNM HSC) Institutional Animal Care and Use Committee (IACUC) approved all of the procedures involving the use of live rats. All experiments were in compliance with the ARRIVE guidelines. The Long-Evans rats used in these studies were purchased from Envigo Corporation (Indianapolis, IN, USA) where they had been maintained on Teklad Global Rodent Diet 2018S (Envigo) while at the production facility. Upon arrival at our UNM HSC Animal Resource Facility, the rats were single housed in static micro isolator cages with Envigo Tek-Fresh bedding, housed at 22 °C on a reverse 12-hour dark / 12-hour light schedule (lights on from 21:00 to 09:00 hours) and provided irradiated food and water (chlorine dioxide treated in water bottles, autoclaved prior to use) ad libitum. Pathogen status of the rat housing rooms was monitored using sentinel testing of live rats every four months. Pathogens tested for by serological analysis included MHV, MVM, NS1, MPV 1–5, MNV, TMEV, EDIM, Sendai, M. pulmonis, PVM, REO3, LCMV, Ectro, MAV-1, MAV-2, and Polyoma. Pathogens tested for by PCR analysis included MHV, MVM, NS1, MPV 1–5, MKPV, MNV, TMEV, EDIM, Sendai, M. pulmonis, PVM, REO3, LCMV, Ectro, MAV-1, MAV-2 and Polyoma.
Female rats arrived at around 6–7 weeks of age (125–150 g) and were approximately 9–10 weeks old at the time of breeding. The males were established breeders, 12 weeks old upon arrival and 15 to 16 weeks old at the start of the breeding protocol. Female rats and their respective male breeders were placed either on Teklad Global 2920 Soy protein free rodent diet (Envigo) or PMI Picolab 5L0D laboratory rodent diet (LabDiet Incorporated, St Louis, MO, USA). Table 1 provides the composition of the 2920 and the 5L0D diets, according to the information provided by the manufacturers. After one-week acclimation to the animal facility, including the new diets, baseline body weights for each female were obtained.
TABLE 1.
Diet descriptions and principal characteristics of two commercially available rodent diets.
| Diet name: | Teklad 2920a | PicoLab 5L0Db |
| Description: | Soy protein free diet | PMI Rodent Diet |
| Manufacturer: | Teklad Global | PMI PicoLab |
| Vendor: | Envigo® Labs | Purina LabDiet® Inc. |
| Diet form: | “Extruded” | “Pelleted” / “Compressed” |
| Irradiated: | Yes | Yes |
| Metabolizable energy (kcal/g) | 3.1 | 2.9 |
| Crude protein (%) | 19.1 | 24.6 |
| Protein-derived calories (%) | 24 | 29 |
| Fat-derived calories (%) | 16 | 13 |
| Carbohydrate-derived calories (%) | 60 | 57 |
| Fat (%) | 6.5 | 5.0 |
| Neutral detergent fiber (%) | 12.3 | 16.7 |
| Crude fiber (%) | 2.7 | 5.3 |
| Folic acid/Folate (mg/kg) | 4 | 7.1 |
| Iron (mg/kg) | 200 | 240 |
| Calcium (%) | 0.9 | 0.95 |
| Iodine (mg/kg) | 6 | 0.99 |
| Vitamin D3 (IU/g) | 1.5 | 4.6 |
| Vitamin E (IU/g) | 110 | 4.2 |
| Niacin (nicotinic acid) (mg/kg) | 75 | 130 |
| Choline (mg/kg) | 1200 | 2250 |
| Isoflavone content (mg/kg) | < 20 | 300 – 750 |
– 2920 main ingredients: Ground wheat, ground corn, corn gluten meal, wheat middlings and soybean oil.
– 5L0D main ingredients: Dehulled soybean meal, ground corn, dried beet pulp, fish meal, ground oats, dehydrated alfalfa meal, cane molasses, wheat germ, dried whey, pork fat, wheat middlings, porcine meat and bone meal.
Moderate Prenatal Ethanol Exposure Paradigm.
The breeding and prenatal alcohol exposure (PAE) procedures employed here were the same as described previously (Hamilton et al., 2014, Davies et al., 2019). Pre-pregnancy drinking levels in female rats were evaluated while gradually acclimating them to drinking 5% ethanol in 0.066% saccharin in tap water 4 hours each day. The saccharin water contained 0% ethanol on the first and second day, 2.5% ethanol (v/v) on the third and fourth day, and 5% ethanol on the fifth day and thereafter (see Figure 1). The drinking tubes were placed in the cages 1 hr. after the onset of the dark phase. Water bottles were removed from the cages during the four-hour ethanol consumption session as prior studies had revealed that females drank almost no water while the saccharin water is present in the cage.
Daily ethanol consumption was measured using 55 mL volume conical beaded glass test tubes (25 × 150 mm) (Fisher Scientific International, Waltham, MA, USA) with a paper ruler affixed with millimeter graduated markings, topped with a metal sipper tube with a 45 degree angle bend and a stainless steel ball (Ancare Corporation, Bellmore, NY, USA) inserted in a white #4 rubber stopper (The Plasticoid Company, Elkton, MD, USA). Drinking tubes were filled daily to the 20 mm mark. At the end of each drinking session, the volume consumed was noted as a net change in the mm scale and converted to the volume consumed. The amount of ethanol consumed, expressed as g/kg body weight, was calculated using a daily weight extrapolated from weight data collected at the start and the end of each week. Upon completion of the pre-pregnancy drinking phase, the mean and standard deviation in 5% ethanol consumption from pre-pregnancy Day 5 to Day 14 (PP05 - PP14) was calculated. Any females whose drinking was more than one standard deviation below the group mean (usually less than 10% of the group) was removed from the study at this point.
Subsequently, females were assigned to either a saccharin control or 5% ethanol drinking group and matched such that the mean pre-pregnancy ethanol consumption by each group was similar. Females were then placed with proven male breeders (alcohol naïve) until pregnant, as indicated by the presence of a vaginal plug. Female rats did not consume ethanol during the breeding procedure, which averaged about 2 days in length.
Beginning on Gestational Day 1 (GD1), rat dams were provided saccharin water containing either 0% or 5% ethanol for four hours each day, from 1000 to 1400 hours. The volume of saccharin water provided to the control group (16 mL) was matched to the mean volume of 5% ethanol in saccharin water consumed by the ethanol prenatal treatment group during gestation. Daily four-hour ethanol consumption was recorded for each dam through GD21, after which ethanol consumption was discontinued. No physical signs of ethanol withdrawal, such as hyperexcitability, hyperreactivity, tremors, piloerection or tail stiffness, as described by Becker (2000), were observed either during the one to two days of breeding or after delivery at pup weighing.
Rats were weighed weekly to assess maternal weight gain. At birth, the number of live births were recorded. No birth deaths were observed in this study. The pups were weighed as a group and the litter weight divided by the number of live births to obtain a mean pup birth weight. Subsequently the litters were culled to 10 pups by Postnatal Day 2 (PD2). Offspring were weaned at PD 21–24, at which time the numbers of each sex was confirmed. Subsequently, these offspring were distributed to multiple investigators within our center for a variety of neurochemical, electrophysiological and behavioral experiments. In the drinking and offspring outcome study (Figure 1), a total of 75 rats (38 control and 37 5% ethanol rats) from three separate breeding rounds were maintained on the 2920 diet and 169 rats (85 controls and 84 5% ethanol rats) from five separate breeding rounds were maintained on the 5L0D diet. All of the rat dams in these cohorts successfully delivered litters.
Ethanol Consumption Pattern and Serum Ethanol Levels Study.
A separate set of 2920 and 5L0D female rats were used for a more detailed examination of their ethanol consumption patterns during their pre-pregnancy (PP) drinking phase and resulting serum ethanol concentrations. Eighteen pairs of female rats were used in the hourly consumption study (Figure 2) and 27 of these rats (14 on the 2920 diet and 13 on the 5L0D diet) provided data for the serum ethanol determinations during the third week of gestation (Figure 3). These rats were maintained as described above except that on PP Days 10, 11 and 12, the amount of 5% ethanol consumed by each rat was determined hourly. The hourly measures over the three days were averaged for each rat and then the mean cumulative ethanol consumption each hour was determined for each diet group.
Subsequently, these females were placed with proven male breeders until pregnant. Once pregnant, the dams were placed back on the standard four-hour daily 5% ethanol drinking sessions. On either Gestational Day 15, 16 or 17, two hours after the introduction of the drinking tubes, ethanol consumption was noted and then the rat dam anesthetized with isoflurane and a 350 μL blood sample drawn from the nicked tail vein into SAFE-T-FILL® capillary blood collection tubes (RAM Scientific Inc., Nashville, TN, USA). After collection of the tail vein sample, the rat dam was immediately decapitated and trunk blood collected in heparinized collection tubes. A 200 μL aliquot of each tail and trunk blood samples were transferred to centrifuge tubes, centrifuged at maximum speed for 5 min at 4 °C (Model 5453, Eppendorf US, Enfield, CA, USA). The supernatant was removed and stored at −20 °C until assay for serum ethanol using an AM1 Alcohol Analyzer (Analox Instruments, Stourbridge, UK).
In vitro study of aqueous suspensions of 2920 and 5L0D diet.
Quadruplicate two-gram quantities of the 2920 and 5L0D diet were ground into powder using a mortar and pestle and then transferred to 15 mL graduated polypropylene conical centrifuge tubes. The powders were mixed with 8.0 mL of acidified 0.9% NaCl (pH 4.0) containing 5% ethanol in 0.066% saccharin water. After vortexing, the mixtures were incubated at 37 °C for 30 min in a shaker water bath with occasional vortexing. At the end of the incubation, the diet suspensions were allowed to stand for ten minutes and then the volume of settled suspension measured. Subsequently, the test tubes were centrifuged at 3000 × g for 10 min, and the volume of packed diet suspension in the pellet and the total volume of the mixture measured. Then, a 1.5 mL aliquot of supernatant was centrifuged at 13,000g for 20 min. A 500 μL aliquot of this supernatant was removed and stored at −20 °C until the ethanol concentration was determined using the AM1 Alcohol Analyzer, as described above. The total amount of ethanol in the supernatant from each diet mixture (Figure 4E) was calculated by dividing the ethanol concentration in each supernatant by the volume of supernatant after centrifugation.
Statistical Procedures.
All statistical procedures and graphical illustrations were performed using SigmaPlot® 11 (Systat Software Inc., San Jose, CA, USA). All data are expressed as the mean ± SEM with a p value < 0.05 deemed as statistically significant. Two-way ANOVAs were used to test for differences in all of the measures presented in Figure 1, along with cumulative hourly ethanol consumption (Figure 2B), and serum ethanol concentrations (Figure 3B). A Student’s two-tailed t-test was used to compare overall ethanol consumption in Figures 2A and 3A, along with measures of supernatant volume, ethanol concentration and total supernatant ethanol (Figures 4C - 4E). The Pearson coefficient was computed for the correlations between ethanol consumption and serum ethanol concentration for each diet (Figures 3C and 3D).
RESULTS
Female Long-Evans Rat Ethanol Consumption.
The impact of the 2920 and 5L0D diets on daily four-hour ethanol consumption, both before and during pregnancy is summarized in Figures 1A and 1B. Note that Figures 1A and 1B only illustrate the drinking data for those females that were ultimately assigned to the 5% ethanol group during pregnancy. The pre-pregnancy drinking data for rat dams subsequently assigned to the 0% ethanol control group during gestation are not illustrated in Figures 1A and 1B, but as noted in the methods, these rats consumed similar quantities of ethanol during the pre-pregnancy phase for a given diet. As depicted along the x-axis of Figure 1A, female rats consumed 0% ethanol in saccharin water for two days, followed by two days on 2.5% ethanol and subsequently drank 5% thereafter except during one to two days of breeding. The pre-pregnancy drinking data on the left side of Figure 1A indicates that 2920 females drank significantly less than 5L0D females on most pre-pregnancy drinking days, noted by asterisks, averaging about 14% less ethanol consumption per day overall during this phase of the paradigm. The gestational drinking data shown on the right side of Figure 1A illustrates a more striking difference in drinking in these same females during pregnancy. Overall, 2920 dams consumed 28.4% less ethanol during gestation than 5L0D dams. Figure 1B illustrates the effect of diet on mean daily 5% ethanol consumption throughout the pre-pregnancy period compared to the gestational period. Ethanol consumption by 2920 dams was lower in both the pre-pregnancy and gestational drinking periods compared to 5L0D dams in either phase of pregnancy. Further, 2920 dams consumed significantly less ethanol during gestation than prior to pregnancy, whereas ethanol consumption by 5L0D dams was similar in both phases of the paradigm.
Pregnancy Outcome Measures.
Figures 1C through 1E illustrate the impact of the two diets on three pregnancy outcome measures frequently reported in prenatal ethanol exposure paradigms. Rat dams consuming the 2920 diet gained 55.4% above their pre-pregnancy weight at term, whereas 5L0D dams only gained 50.4%. This 5% diet-based difference was significant, whereas prenatal treatment had no effect on maternal weight gain within diet groups (Figure 1C). Litter sizes shown here represent the total number of live births as no birth deaths were observed in this study of moderate prenatal ethanol exposure. Neither diet nor prenatal treatment affected litter size (Figure 1D). However, the mean pup birth weight of 2920 pups was 7.15 grams compared to 7.93 grams for 5L0D pups. This roughly 10% diet-based decrease in 2920 pup weight was significant, whereas prenatal treatment had no effect on pup birth weight within diet (Figure 1E).
Hourly Ethanol Consumption.
The question of why female rats maintained on the 2920 diet drink less ethanol than 5L0D rats during daily four-hour drinking sessions was subsequently examined in more detail using a separate set of non-pregnant female rats maintained on the two diets. After one-week of acclimation to daily four-hour consumption of 5% ethanol, ethanol consumption was measured at hourly intervals over the four-hour drinking session. Figure 2A illustrates that the diet-based differences in pre-pregnancy ethanol consumption by this separate cohort (Figure 2A) were similar to the differences observed in pre-pregnancy drinking in Figure 1B. Pre-pregnancy ethanol consumption was noted hourly and the cumulative ethanol consumption data plotted as a function of time after introduction of the drinking tubes. In both diet groups, more drinking was observed during the first hour compared to subsequent hours where ethanol consumption was roughly linear within each diet group. A two-way ANOVA of the cumulative ethanol consumption data revealed a significant interaction between diet and time after the introduction of the drinking tubes. Subsequent post-hoc testing indicated no differences in cumulative ethanol consumption between diets during the first or second hours, but a significant reduction in cumulative ethanol consumption at the end of the third and fourth hours for rats consuming the 2920 diet (Figure 2B). These results indicate that the overall reduction in four-hour drinking by 2920 rats (Figures 1B and 2A) is due primarily to a reduction in ethanol consumption during the third and fourth hours of a daily drinking session.
Maternal Serum Ethanol Concentration.
Subsequently, most of these same female rats were successfully bred and blood samples collected either on Gestational Days 15, 16 or 17. The two-hour time point after the introduction of the drinking tubes was selected for blood sampling because, at this time point, ethanol consumption was not significantly different between the two diet groups (Figure 2B). Further, we expected that this window of time was likely to yield a near-peak serum ethanol concentration in dams from both diet groups. At the two-hour time-point, the ethanol consumption was noted for each dam and then blood samples were collected for serum ethanol measurements. As before, the diet-based differences in drinking during pregnancy at two hours in this cohort (Figure 3A) were proportionately similar to the prior study after four hours (Figure 1B). Figure 3B illustrates the serum ethanol data collected from the tail vein and from trunk blood of dams on the two diets. A two-way ANOVA revealed a highly significant effect of diet on the resulting serum ethanol concentrations with no differences based on the blood sample source (tail vein versus trunk blood; Figure 3B). Averaging the tail vein and trunk blood measurements for each dam, the mean serum ethanol concentration in 2920 dams was 25.1 ± 3.3 mg /dL compared to 46.0 ± 3.2 mg / dL in 5L0D dams, a 46% reduction below the serum ethanol concentration in 5L0D dams.
Serum ethanol concentration, based on the combined tail vein and trunk blood sample measurements was also plotted as a function of the amount of ethanol consumed at the blood sample collection time for 2920 dams (Figure 3C) and 5L0D dams (Figure 3D). The range of ethanol consumption values at the two-hour time point was nearly twice as great with the 2920 diet compared to the 5L0D diet, whereas the range of serum ethanol values was similar between diet groups. Pearson correlational analyses indicated a moderate and significant correlation for the 2920 data, whereas a weaker and statistically insignificant correlation existed for the 5L0D data. The moderate to weak correlational strength in these relationships suggests that the ethanol consumption data had limited utility for predicting serum ethanol concentration for a given sample.
In Vitro Measures of Ethanol in Diet Suspensions.
The disproportionately lower serum ethanol concentrations (Figure 3B) relative to lower ethanol consumption in 2920 dams compared to 5L0D dams prompted an in vitro study of the two diets. Figure 4A illustrates samples of graduated conical test tubes containing the 2920 and 5L0D mixtures. Nearly all of the liquid in the 9 mL total volume of the 2920 mixture was adsorbed in the diet suspension. In contrast, only about two-thirds of the 5L0D tube volume contained adsorbed liquid in the diet suspension. After centrifugation of the mixtures, the packing density of particulate matter in the 2920 tubes was about twice the packing density volume in the 5L0D tubes (Figure 4B). Conversely, the resulting volume of supernatant measured in the 2920 tubes was significantly less than the supernatant volume in 5L0D tubes (Figure 4C). While the ethanol concentration in the supernatants, about 4.7%, was not different between diet mixtures (Figure 4D), the total amount of ethanol present in 2920 supernatants was calculated to be less than half of the total ethanol present in 5L0D supernatants (Figure 4E).
DISCUSSION
The diet based differences in ethanol consumption by our female Long-Evans rats reported here (Figures 1A and 1B) are similar to a recent report where non-pregnant C57BL/6 mice consumed significantly less ethanol when maintained on 2920 diet compared to 5L0D diet (Maphis et al., 2022). More striking in our study was the fact that rat dams consumed even less ethanol on the 2920 diet during pregnancy than they did prior to becoming pregnant (Figure 1B). The drinking reduction by 2920 rats compared to 5L0D rats was due to 2920 rats consuming less ethanol during the third and fourth hours of a four-hour drinking session (Figure 2B). Notable also were the observations that the differences in serum ethanol concentrations collected from samples at the two-hour time point (Figure 3B) were disproportionately lower in 2920 dams compared to the differences in overall drinking between diet groups (Figure 1B) at this time point. Further, the strength of the correlations between ethanol consumption and serum ethanol concentration were moderate to weak (Figures 3C and 3D) suggesting that the level of ethanol consumption may not be a good predictor of resulting serum ethanol concentration. In addition, our observation that the 2920 diet expands in aqueous medium to a greater extent than the 5L0D diet (Figure 4A) provides the first evidence suggesting that more water and ethanol are physically adsorbed when mixed with 2920 diet (Figure 4). One interpretation of these latter data is that greater adsorption of ethanol by aqueously suspended 2920 diet may be one factor contributing to disproportionately greater reductions in serum ethanol concentration (Figure 3B) compared to the amount of ethanol consumed (Figures 1B, 2A & 3A) by female rats maintained on 2920 diet.
While these data suggest that different physical characteristics of the two diets in aqueous mixtures may contribute to differences in alcohol consumption and resulting serum ethanol concentrations, this interpretation is limited given a variety of other factors that could have affected ethanol consumption and serum ethanol levels. One factor is the different compositions of the two diets as shown in Table 1. While these diets are described as “isocaloric” and have similar amounts of calories derived from protein, carbohydrates and fat, the main ingredients used in these diets, as footnoted in Table 1, are quite different. How these different mixtures of ingredients might affect ethanol consumption is not known.
Table 1 also indicates that there are notable differences in the amounts of folate, iodine, vitamin D3, vitamin E, niacin, choline and isoflavones in these two diets. The amount of isoflavones present in soy products in nutritionally adequate commercially available diet mixtures has received some attention as one potential factor contributing to variations in ethanol consumption. Marshall and colleagues (2015) reported that mice maintained on one of two diets with relatively low isoflavone levels consumed about one-third less ethanol than mice maintained on one of four other diets containing moderate to high levels of isoflavones. However, a recent meta-analysis by Eduardo & Abrahao (2022) indicated modest correlations between diet isoflavone levels and ethanol consumption, with opposite effects in mice compared to rats. Thus, the impact of isoflavone levels on ethanol consumption remains unclear at present. Further, the extent to which the other diet-based differences in nutrients listed above affect ethanol consumption or whether supplements of these factors affect ethanol consumption is not known.
Aside from nutrient composition, another factor that may have contributed to the diet-based differences in ethanol consumption in our study is the manner in which the diets are prepared. The 2920 is an “extruded” diet whereas the 5L0D diet is “pelleted”. Marshall and colleagues (2015) reported that mice maintained on a H2918 (Teklad 2918) diet, drank about 50% more ethanol than mice maintained on a H2920 (Teklad 2920), the same diet we refer to here as the 2920 diet. Aside from differences in isoflavone content, another salient difference is the fact that H2918 is a pelleted diet whereas the H2920 diet is an extruded diet. While the differences in diet preparation were not discussed as a potential contributing factor in these results in earlier reports (Marshall et al., 2015; Quadir et al., 2020), their results are consistent with Maphis et al., (2022) and our drinking data reported here (Figure 1A and 1B).
As described by Kurtz & Feeney (2020), the diet components in “pelleted” diets, such as the 5L0D diet, are prepared by compressing the mixture into a metal die using a relatively lower level of steam and pressure. In contrast, the diet components in “extruded” diets, such as the 2920 diet, are usually ground finer than pelleted diet components, the mixture subjected to higher temperatures and then the superheated mixture subjected to extremely high pressure before extruding it through a die. Once extruded and, after the form returns to ambient temperature and pressure, water trapped in the diet evaporates and the form expands leaving air pockets in a less dense form. Exactly how these differences in diet preparation might affect these diets in aqueous suspensions and ethanol consumption is unclear at present, but the differences in the amount of water and ethanol adsorbed by the two diets, as illustrated in Figure 4, provide one possible explanation for this difference. One question that remains to be investigated is the extent to which other extruded rodent diet preparations expand in aqueous solution compared to pelleted diets.
Beyond isoflavones and diet preparation, other factors may have affected ethanol consumption and serum ethanol levels in our study. For example, questions remain whether diet-based differences in the amount or pattern of food consumption may have affected the rate or extent of gastrointestinal absorption of ethanol. One manner in which to address this question might have been the use of metabolic cages. However, we have observed that Long-Evans rats find housing in metabolic cages extremely stressful, resulting in weight loss from diminished food consumption.
Another potential factor is whether the two diets differentially affect ethanol metabolism. However, Maphis et al., (2022) reported no diet-based differences in the rate of ethanol metabolism in mice on these two diets. Another complex issue is the question of whether diet-based differences alone, or in the presence of ethanol, differentially alter the gut microbiome in a manner that would affect ethanol consumption and serum ethanol concentration. While there are no studies that have directly addressed this question relative to the two diets used in our study, Wenderlein et al., (2021) have reported that higher starch gelatinization associated with extruded diets shifts the relative abundance of some species of bacteria in the gut, which may affect digestive and metabolic processing. Further, Reyes and colleagues (2020) reported that an antibiotic-induced reduction in the levels of commensal Firmicutes bacteria inversely correlated to elevated ethanol intake levels after antibiotic treatment. Taken together, it becomes clear that assessing the putative impact of the various dietary factors discussed abouve that can affect ethanol consumption using common commercially available diets is limiting. Ultimately, a better approach for addressing the impact of a specific dietary factor would rely on a prospectively-designed study using an inbred strain, maintained on identical diets (except for the variable in question, such as a pellet production process) and simultaneously monitoring additional parameters (food intake / pattern, serum nutrient levels, blood biochemistry and gut microbiota (Bleich & Hansen, 2012) to better interpret the dietrary factor’s impact on ethanol consumption.
Aside from diet-based differences on ethanol consumption and serum ethanol levels, we also examined whether these two diets affect pregnancy outcome measures in ethanol-exposed offspring compared to saccharin control offspring. We have never observed an effect of moderate prenatal ethanol exposure on maternal weight gain, litter size or offspring birth weight in the past (Savage et al., 2010; Hamilton et al., 2014; Davies et al., 2019). These observations were confirmed when comparing the effect of prenatal ethanol exposure within a given diet here (Figures 1C–1E). However, when comparing between these two “isocalorically-equivalent” diets, 2920 rat dams gained significantly more weight during pregnancy than 5L0D dams (Figure 1C). The increased weight gain during pregnancy did not affect litter size (Figure 1D). However, mean pup birth weight was significantly lower for 2920 offspring compared to 5L0D offspring (Figure 1E). These results are similar to a study of Wistar rat dams by Cao et al., 2015, comparing two isocalorically equivalent diets of nearly identical composition, but whose salient difference was isoflavone content. Rat dams consuming a diet containing 400 mg/kg of isoflavones gained less weight, but produced heavier pups, when weighed at Postnatal Day 10, compared to dams that consumed the low-isoflavone Teklad 2020 diet. While the mechanisms by which various “endocrinologically disruptive” isoflavone components affect pregnancy outcome measures is not entirely clear and likely complex (Krishna, Kuriakose & Lakshmi, 2022; Rizzo, Feraco, Storz & Lombardo, 2020), the results suggest that isoflavones influence a shifting of resources from the dam to the offspring leading to heavier birth weights. This speculation is consistent with the Barker Hypothesis (1997) or “thrifty phenotype” which posits that environmental factors can induce changes in fetal programming, making offspring more efficient at scavenging calories and nutrients, but also putting them at higher risk for metabolic disorders later in life. This diet-based effect may be compounded in the presence of prenatal ethanol exposure, which has also been associated with a higher incidence of metabolic disorders in both rodents (Ting & Lautt, 2006; Weinberg, Sliwowska, Lan & Hellemans, 2008) and humans (de la Monte & Wands, 2010; Asiedu, Nyakudya, Lembede & Chivandi, 2021).
Another potential factor contributing to the diet-based differences in offspring birth weights (Figure 1E) may relate to the differences in diet choline levels in the two diets (Table 1). Gestational choline supplementation can mitigate ethanol-induced reductions in maternal weight gain and late-gestational fetal (Kwan et al., 2021) or newborn (Idrus, Breit & Thomas, 2017) effects of higher levels of prenatal alcohol exposure. However, it remains uncertain whether choline may impact outcome measures after more moderate levels of prenatal ethanol exposure, which did not affect either maternal weight gain (Figure 1C) or pup birth weight (Figure 1E). It is doubtful that diet-based differences in choline affected maternal weight gain in this study, but it is possible that the higher levels of choline in the 5L0D diet contributed to the higher offspring birth weights in 5L0D offspring compared to 2920 offspring. Whether maternal serum choline levels are different when dams are maintained on these two diets, both in the absence or presence of ethanol exposure may provide additional insights about the putative role of choline on our pregnancy outcome measures.
Aside from offspring outcome measures at birth, an important question is whether the differences in diet composition or diet preparation method affect other outcome measures of physiological function or behavior as prenatal ethanol-exposed offspring mature. The offspring generated from the rat dams described in the current study (Figures 1D and 1E) were routinely distributed to multiple research collaborators in our center for different types of multidisciplinary studies. Some recent work using 2920 offspring from earlier breeding rounds report neuroimmunological (Sanchez et al., 2019; Sanchez et al., 2019), anatomical (Madden et al., 2019) and behavioral consequences (Harvey et al., 2020) of moderate prenatal ethanol exposure. A single behavioral study using the 5L0D diet (Osterlund-Oltmanns et al., 2022) has been published so far. However, to date, a systematic comparison of the effects of these two diets on specific outcome measures has not been conducted. Thus, the question of whether significantly lower serum ethanol concentrations resulting from consumption of 2920 diet compared to 5L0D diet (Figure 3B) produces differential biological or behavioral outcomes in prenatal ethanol-exposed offspring remains to be investigated.
In conclusion, the results of this current study underscore the critical importance of being aware of the potential impact of different rodent diets on ethanol consumption, along with outcome measures in prenatal ethanol-exposed offspring. These results also highlight the importance of providing details about diet, ethanol concentration and exposure paradigm details when reporting the results of a new or modified prenatal ethanol exposure paradigm.
Highlights.
Female Long-Evans rat dams consumed 28% less ethanol when maintained on Envigo 2920 diet compared to PicoLab 5L0D diet.
Resulting serum ethanol concentrations were 46% lower in the dams maintained on 2920 diet compared to dams on 5L0D diet.
Differences in the physical nature of these two diets in aqueous mixtures may be one factor affecting gastro-intestinal absorption of ethanol resulting in disproportionately lower serum ethanol concentrations than would be predicted from the amount of ethanol consumed.
ACKNOWLEDGEMENTS
This research was supported by NIH NIAAA 1 P50 AA0022534.
The authors wish to thank Ms. Nicole Crandall, Ms. Victoria Sugita and Dr. Kevin O’Hair for their outstanding animal husbandry support, and Dr. David Linsenbardt for access to his Analox Alcohol Analyzer for ethanol measurements in rat serum and diet suspension supernatants.
Grant Support: NIH-NIAAA 1 P50 AA022534
Footnotes
Declaration of Interest
The authors have no conflict of interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Asiedu B, Nyakudya TT, Lembede BW, Chivandi E (2021). Early-life exposure to alcohol and the risk of alcohol-induced liver disease in adulthood. Birth Defects Res. 113: 451–468. doi: 10.1002/bdr2.1881. [DOI] [PubMed] [Google Scholar]
- Barker DJ (1997). Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 13: 807–881. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Becker HC (2000). Animal models of alcohol withdrawal. Alcohol Res Health. 24(2):105–13. No DOI [PMC free article] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH, Sperry MA, Zajac CS (1996). Prenatal alcohol exposure and the effects of environmental enrichment on hippocampal dendritic spine density. Alcohol.13(2): 209–16. doi: 10.1016/0741-8329(95)02049-7. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH (2000). Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 10(1): 94–110. doi: . [DOI] [PubMed] [Google Scholar]
- Bleich A, Hansen AK (2012). Time to include the gut microbiota in the hygienic standardisation of laboratory rodents. Comp Immunol Microbiol Infect Dis. 35(2):81–92. doi: 10.1016/j.cimid.2011.12.006. Epub 2012 Jan 16. [DOI] [PubMed] [Google Scholar]
- Cao J, Echelberger R, Liu M, Sluzas E, McCaffrey K, Buckley B, Patisaul HB (2015). Soy but not bisphenol A (BPA) or the phytoestrogen genistin alters developmental weight gain and food intake in pregnant rats and their offspring. Reprod. Toxicol. 58: 282–294. doi: 10.1016/j.reprotox.2015.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Maier SE, Parnell SE, West JR (2003). Alcohol and the developing brain: neuroanatomical studies. Alcohol Res Health. 27(2): 174–80. No DOI. [PMC free article] [PubMed] [Google Scholar]
- Costa ET, Savage DD, Valenzuela CF (2000). A review of the effects of prenatal or early postnatal ethanol exposure on brain ligand-gated ion channels. Alcohol Clin Exp Res. 24(5):706–15. Erratum in: Alcohol Clin Exp Res 2000 Oct; 24(10):1592. No DOI. [PubMed] [Google Scholar]
- Davies S, Ballesteros-Merino C, Allen NA, Porch MW, Pruitt M,E, Christensen KH, Rosenberg MJ, Savage DD (2019). Impact of moderate prenatal alcohol exposure on histaminergic neurons, histidine decarboxylase levels and histamine H2 receptors in adult rat offspring. Alcohol. 76: 47–57. doi: 10.1016/j.alcohol.2018.07.007. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR (2010). Role of central nervous system insulin resistance in fetal alcohol spectrum disorders. J. Popul. Ther. Clin. Pharmacol. 17: e390–404. No DOI [PMC free article] [PubMed] [Google Scholar]
- Eduardo PMC, Abrahao KP (2022). Food composition can influence how much alcohol your animal model drinks: A mini-review about the role of isoflavones. Alcohol Clin Exp Res 46(1): 6–12. doi: 10.1111/acer.14741. [DOI] [PubMed] [Google Scholar]
- Glass L, Moore EM, Mattson SN (2023). Current considerations for fetal alcohol spectrum disorders: identification to intervention. Curr Opin Psychiatry. 36(3): 249–256. doi: 10.1097/YCO.0000000000000862. Epub 2023 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Magcalas CM, Barto D, Bird CW, Rodriguez CI, Fink BC, Pellis SM, Davies S, Savage DD (2014). Moderate prenatal alcohol exposure and quantification of social behavior in adult rats. J Vis Exp. (94): 52407. doi: 10.3791/52407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RE, Berkowitz LE, Savage DD, Hamilton DA, Clark BJ (2020) Altered hippocampal place cell representation and theta rhythmicity following moderate prenatal alcohol exposure. Curr Biol. 30(18): 3556–3569.e5. doi: 10.1016/j.cub.2020.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J (2010). Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 34(6):791–807. doi: 10.1016/j.neubiorev.2009.06.004. Epub 2009 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais AS, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T, Coles CD, Chambers C, Jones KL, Adnams CM, Shah PE, Riley EP, Charness ME, Warren KR, May PA (2016). Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics. 138(2):2015–4256. e20154256. doi: 10.1542/peds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrus NM, Breit KR, Thomas JD (2017). Dietary choline levels modify the effects of prenatal alcohol exposure in rats. Neurotoxicol Teratol. 59: 43–52. doi: 10.1016/j.ntt.2016.11.007. Epub 2016 Nov 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JA, O’Connor MJ, Olson HC, Paley B, Mattson SN, Anderson SM, Riley EP (2016). Neurobehavioral Disorder Associated with Prenatal Alcohol Exposure (ND-PAE): Proposed DSM-5 Diagnosis. Child Psychiatry Hum Dev. 47(2): 335–46. doi: 10.1007/s10578-015-0566-7. [DOI] [PubMed] [Google Scholar]
- Kobor MS, Weinberg J (2011). Focus on: epigenetics and fetal alcohol spectrum disorders. Alcohol Res Health. 34(1): 29–37. No DOI. [PMC free article] [PubMed] [Google Scholar]
- Krishna SS, Kuriakose BB, Lakshmi PK (2022). Effects of phytoestrogens on reproductive organ health. Arch Pharm Res. 2022 45(12): 849–864. doi: 10.1007/s12272-022-01417-y. [DOI] [PubMed] [Google Scholar]
- Kurtz DM, Feeney WP (2020). The influence of feed and drinking water on terrestrial animal research and study replicability. ILAR J 60(2):175–196. doi: 10.1093/ilar/ilaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan STC, Ricketts DK, Presswood BH, Smith SM, Mooney SM (2021). Prenatal choline supplementation during mouse pregnancy has differential effects in alcohol-exposed fetal organs. Alcohol Clin. Exp. Res: 45(12): 2471–2484. doi: 10.1111/acer.14730. Epub 2021 Nov 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier AA, Weinberg J, Kobor MS (2017). Epigenetics studies of fetal alcohol spectrum disorder: where are we now? Epigenomics. 9(3):291–311. doi: 10.2217/epi-2016-0163. Epub 2017 Feb 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier AA, Bodnar TS, Weinberg J (2021). Intersection of Epigenetic and Immune Alterations: Implications for Fetal Alcohol Spectrum Disorder and Mental Health. Front Neurosci. 3;15:788630. doi: 10.3389/fnins.2021.788630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden JT, Thompson SM, Magcalas CM, Wagner JL, Hamilton DA, Savage DD, Clark BJ, Pentkowski NS (2020). Moderate prenatal alcohol exposure reduces GABAergic interneuron expression in the dorsal hippocampus of adult male and female rat offspring. Neurosci. Lett. 718:134700. doi: 10.1016/j.neulet.2019.134700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maphis NM, Huffman RT, Linsenbardt DN (2022). The development, but not expression, of alcohol front-loading in C57BL/6J mice maintained on LabDiet 5001 is abolished by maintenance on Teklad 2920x rodent diet. Alcohol Clin Exp Res. 27. doi: 10.1111/acer.14876. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Rinker JA, Harrison LK, Fletcher CA, Herfel TM, Thiele TE (2015). Assessment of the effects of 6 standard rodent diets on binge-like and voluntary ethanol consumption in male C57BL/6J mice. Alcohol Clin Exp Res. 39 (8), 1406–1416. doi: 10.1111/acer.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson S,N, Riley EP (2011). The quest for a neurobehavioral profile of heavy prenatal alcohol exposure. Alcohol Res Health. 34(1): 51–5. No DOI. [PMC free article] [PubMed] [Google Scholar]
- Noor S, Sanchez JJ, Vanderwall AG, Sun MS, Maxwell JR, Davies S, Jantzie LL, Petersen TR, Savage DD, Milligan ED (2017). Prenatal alcohol exposure potentiates chronic neuropathic pain, spinal glial and immune cell activation and alters sciatic nerve and DRG cytokine levels. Brain Behav Immun. 61: 80–95. doi: 10.1016/j.bbi.2016.12.016. Epub 2016 Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olguin SL, Thompson SM, Young JW, Brigman JL (2021). Moderate prenatal alcohol exposure impairs cognitive control, but not attention, on a rodent touchscreen continuous performance task. Genes Brain Behav. 20(1):e12652. doi: 10.1111/gbb.12652. Epub 2020 Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund-Oltmanns JR, Schaeffer EA, Goncalves Garcia M, Donaldson TN, Acosta G, Sanchez LM, Savage DD, Wallace DG, Clark BJ (2022). Sexually dimorphic open field behavior organization following moderate prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 46:861–875. doi: 10.1111/acer.14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadir SG, Rohl CD, Zeabi A, Moore CF, Cottone P, Sabino V (2020). Effect of different standard rodent diets on ethanol intake and associated allodynia in male mice. Alcohol 87, 17–23. doi: 10.1016/j.alcohol.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova S, Charness ME, Burd L, Crawford A, Hoyme HE, Mukherjee R,AS, Riley EP, Elliott EJ (2023). Fetal alcohol spectrum disorders. Nat Rev Dis Primers. 23, 9(1):11. doi: 10.1038/s41572-023-00420-x. [DOI] [PubMed] [Google Scholar]
- Randall CL (1987). Alcohol as a teratogen: a decade of research in review. Alcohol Alcohol Suppl. 1:125–32. No DOI. [PubMed] [Google Scholar]
- Reyes REN, Al Omran AJ, Davies DL, Asatryan L (2020). Antibiotic-induced disruption of commensal microbiome linked to increases in binge-like ethanol consumption behavior. Brain Res. 1747:147067. doi: 10.1016/j.brainres.2020.147067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP (1990). The long-term behavioral effects of prenatal alcohol exposure in rats. Alcohol Clin Exp Res. 14(5): 670–3. doi: 10.1111/j.1530-0277.1990.tb01225.x. [DOI] [PubMed] [Google Scholar]
- Rizzo G, Feraco A, Storz MA, Lombardo M (2022) The role of soy and soy isoflavones on women’s fertility and related outcomes: an update. J Nutr Sci. Mar 7;11:e17. doi: 10.1017/jns.2022.15. eCollection 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez LM, Goss J, Wagner J, Davies S, Savage DD, Hamilton DA, Clark BJ (2019). Moderate prenatal alcohol exposure impairs performance by adult male rats in an object-place paired-associate task. Behav Brain Res. 360: 228–234. doi: 10.1016/j.bbr.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JJ, Sanchez JE, Noor S, Ruffaner-Hanson CD, Davies S, Wagner CR, Jantzie LL, Mellios N, Savage DD, Milligan E, D. (2019). Targeting the β2-integrin LFA-1, reduces adverse neuroimmune actions in neuropathic susceptibility caused by prenatal alcohol exposure. Acta Neuropathol Commun. 7(1):54. doi: 10.1186/s40478-019-0701-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DD, Cruz LL, Duran LM, Paxton LL (1998). Prenatal ethanol exposure diminishes activity-dependent potentiation of amino acid neurotransmitter release in adult rat offspring. Alcohol Clin Exp Res. 22(8): 1771–7.No DOI. [PubMed] [Google Scholar]
- Savage DD, Rosenberg MJ, Wolff CR, Akers KG, El-Emawy A, Staples MC, Varaschin RK, Wright CA, Seidel JL, Caldwell KK, Hamilton DA (2010). Effects of a novel cognition-enhancing agent on fetal ethanol-induced learning deficits. Alcohol Clin Exp Res. 34(10), 1793–802. doi: 10.1111/j.1530-0277.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, O’Malley K (2000). Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Semin Clin Neuropsychiatry. 5(3):177–90. doi: 10.1053/scnp.2000.6729. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Cook CS, Webster WS (1988). Teratogens and craniofacial malformations: relationships to cell death. Development. 103 Suppl:213–31. doi: 10.1242/dev.103.Supplement.213. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, Savage DD (1997). Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus. 7(2):232–8. doi: . [DOI] [PubMed] [Google Scholar]
- Ting JW, Lautt WW (2006). The effect of acute, chronic, and prenatal ethanol exposure on insulin sensitivity. J Pharmacol Ther. 111, 346–73. doi: 10.1016/j.pharmthera.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Varaschin RK, Allen NA, Rosenberg MJ, Valenzuela CF, Savage DD (2018). Prenatal Alcohol Exposure Increases Histamine H3 Receptor-Mediated Inhibition of Glutamatergic Neurotransmission in Rat Dentate Gyrus. Alcohol Clin Exp Res. 42(2): 295–305. doi: 10.1111/acer.13574. Epub 2018 Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Feltham BA, Eskin MNA, Suh M (2021). Differential effects of maternal diets on birth outcomes and metabolic parameters in rats after ethanol consumption during pregnancy. Br. J. Nutr. 126(8), 1130–1139. doi: 10.1017/S0007114520005152. [DOI] [PubMed] [Google Scholar]
- Weinberg J (1994). Recent studies on the effects of fetal alcohol exposure on the endocrine and immune systems. Alcohol Alcohol Suppl. 2: 401–9. No DOI. [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG (2008). Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J. Neuroendocrinol. 20(4), 470–88. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderlein J, Böswald LF, Ulrich S, Kienzle E, Neuhaus K, Lagkouvardos I, Zenner C, Straubinger RK (2021). Processing Matters in Nutrient-Matched Laboratory Diets for Mice-Microbiome. Animals (Basel). 11(3):862. doi: 10.3390/ani11030862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JR, Goodlett CR, Brandt JP (1990). New approaches to research on the long-term consequences of prenatal exposure to alcohol. Alcohol Clin Exp Res. 14(5): 684–9. doi: 10.1111/j.1530-0277.1990.tb01227.x. [DOI] [PubMed] [Google Scholar]


