Abstract
BACKGROUND:
Inflammatory bowel disease (IBD) has historically been considered a relative contraindication for pelvic radiotherapy (RT). To date, no systematic review has summarized the toxicity profile of RT for prostate cancer in patients with comorbid IBD.
METHODS:
A PRISMA-guided systematic search was conducted on PubMed/EMBASE for original investigations that reported gastrointestinal (GI; rectal/bowel) toxicity in patients with IBD undergoing RT for prostate cancer. The substantial heterogeneity between patient population, follow-up, and toxicity reporting practices precluded a formal meta-analysis, however a summary of the individual study-level data and pooled rates were described.
RESULTS:
Twelve retrospective studies with 194 patients were included, 5 examined predominantly low-dose-rate (LDR) brachytherapy (BT) monotherapy, 1 predominantly high-dose-rate (HDR) brachytherapy monotherapy, 3 mixed external-beam RT (3D-conformal or intensity-modulated RT [IMRT]) and LDR-BT, 1 intensity-modulated RT and HDR-BT, and 2 stereotactic RT. Amongst these studies, patients with active IBD, patients receiving pelvic RT, and patients with prior surgery were underrepresented. In all but one publication, the rate of late grade 3+ GI toxicities was <5%. The pooled rate of acute and late grade 2+ GI events was 15.3% (n=27/177 evaluable patients, min-max 0-100%) and 11.3% (n=20/177 evaluable patients; min-max: 0-38.5%), respectively. Acute and late grade 3+ GI events was 3.4% (6 cases, min-max: 0-23%) and 2.3% (4 cases, min-max: 0-15%).
CONCLUSIONS:
Prostate RT in patients with comorbid IBD appears to be associated with low rates of grade 3+ GI toxicity; however, the possibility of a higher risk of toxicity remains. Because certain higher-risk subpopulations were underrepresented, overgeneralization is cautioned against. Several strategies should be considered best practice to minimize the probability of toxicity in this susceptible population including careful patient selection, minimizing elective treatment volumes, utilizing rectal sparing techniques, and employing contemporary RT-advancements to minimize exposure to GI organs-at-risk (e.g., IMRT, MRI-based planning, image-guidance).
Keywords: prostate cancer, radiation therapy, brachytherapy, inflammatory bowel disease, Crohn’s disease, ulcerative colitis
INTRODUCTION
Prostate cancer is more common in men with inflammatory bowel disease (IBD),1 which is an umbrella term encompassing ulcerative colitis (UC), Crohn’s disease (CD), and others that are not otherwise specified (IBD-NOS). UC is characterized by episodic inflammation of the colorectal mucosal layer, most commonly involving the rectum. Associated symptoms include bloody diarrhea, tenesmus, and rectal incontinence. Crohn’s disease can affect any part of the gastrointestinal (GI) tract and is characterized by transmural inflammation of the bowel, which can lead to the formation of sinus tracts and fistulae.2
Common therapeutic options for prostate cancer include brachytherapy, external beam radiation therapy (EBRT), both, or radical prostatectomy.3 Historically, IBD has been considered a relative contraindication for radiation therapy (RT) in patients with prostate cancer due to early reports suggesting more frequent and more severe RT-induced toxicities in this subgroup.4 However, this notion is being increasingly questioned in the modern era in which contemporary therapeutic advances have allowed for improved medical management of IBD and higher precision delivery of radiotherapy with resultant lower exposures to surrounding normal gastrointestinal (GI) organs at risk (OAR).
To date, there are no available systematic reviews characterizing the toxicity profile of RT in patients comorbid IBD undergoing treatment for prostate cancer. To address this knowledge gap, we performed a systematic review with the objective to describe the rate of GI toxicity for various radiation therapy techniques including brachytherapy (BT) and EBRT delivered in various fractionation schedules (conventional fractionation, moderate hypofractionation, or stereotactic body RT [SBRT]).
MATERIALS AND METHODS
This systematic review was performed in accordance with published guidelines by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group5 for conducting systematic reviews (Supplemental Table 1). Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Population, Intervention, Control, Outcomes, Study Design (PICOS) framework was used to guide a systematic search of PubMed and EMBASE along with references of studies selected for inclusion6 (Figure 1 and Supplemental Tables 2 and 3). All authors performed searches, with discrepancies resolved by direct communication amongst the authors. Searches were conducted on June 23, 2022, including all studies published in English until the day of the search. The search criteria were as follows: (radi* OR irradi* OR brachytherapy) AND (prostat* OR pelvi*) AND (inflammatory bowel OR IBD OR crohn OR ulcerative OR colitis OR ulcerative colitis). The ROBINS-I7 tool was used to assess the bias of each included study.
Figure 1:
PRISMA diagram illustrating study selection
Original research publications considered for inclusion examined the following target population: patients with IBD (CD, UC, or IBD-NOS) who received any RT for prostate cancer. Since the chief concern for IBD patients pertains to RT-induced GI toxicities, only studies that reported GI toxicities were included. Studies were not excluded based on IBD status with both inactive and active cases permitted. Any combination of RT techniques with or without rectal protection techniques (e.g., rectal spacers or balloons) was permitted. Study authors were contacted if there were any questions regarding pelvic nodal usage or other additional cohort details. Case reports, small case series (≤3 patients), unpublished abstracts, or other publications of non-original research were not included. There was substantial heterogeneity between studies including study population, duration of follow up, and toxicity definition precluding a formal meta-analysis. Crude pooled rates were generated based on the number of patients in whom a specific category (i.e., acute vs. late ) of toxicity was reported. Additionally, a set of exploratory analyses was conducted to assess whether the available published literature supported a relationship between the utilization of EBRT and the various grade (G) and temporal categorization of toxicity on a study level. A similar set of exploratory analyses were conducted between rates of abdominalpelvic surgery and toxicity. Weighted Pearson’s correlation coefficient hypothesis testing was conducted with weights proportional to the each study population size. An α threshold of 0.05 was considered significant, and given the exploratory nature of these tests, no correction was used for the reported p-values. Data management and analyses were conducted using R version 4.2.1.8
RESULTS
The search methology identified 1,805 articles (Figure 1) of which 25 full texts were obtained. Nine articles4, 9–16 were excluded as they were not specific to prostate cancer and did not partition toxicity reporting by cancer type. Three articles were excluded as they did not separately report details for the IBD cohort.17–19 One article was excluded as it was a narrative (non-systematic) review without original data.20 Thus, 12 studies were included in this systematic review. Many papers did not specify if patients were on medications for IBD at the time of RT (n=5 studies21–25) or did not specify the types of medications (n=4 studies26–29). The remaining three studies30–32 reported on IBD medication use at time of RT, mostly mesalamine (n=21), prednisone (n=9) and sulfasalazine (n=6). Most papers did not provide a direct definition of what constituted active IBD, such as signs and symptoms of inflammation (e.g., lab tests such as CRP, which has a high negative predictive value and can essentially rule out active IBD with more than 99% certainty if measured at less than 5 mg/dL).33 The ROBINS-I tool7 was used to assess the bias of each included article in this systematic review. All included studies were deemed to have a high potential for bias due to small sample sizes and the retrospective nature.
Of the 12 included retrospective studies, 5 examined predominantly low-dose-rate (LDR) brachytherapy monotherapy,25, 26, 28, 29, 31 1 predominantly high-dose-rate (HDR) brachytherapy monotherapy,23 1 mixed intensity-modulated RT (IMRT) and HDR brachytherapy,22 3 combination EBRT (3D-conformal RT [3DCRT] or IMRT) and LDR brachytherapy,24, 27, 30 and 2 examined SBRT.21, 32 Only the studies containing more than 7 patients are narratively described below. Publications were divided into two groups based on predominant treatment technique employed (BT [Table 1] vs. EBRT±BT [Table 2]). Utilization of whole pelvic RT (WPRT) was uncommon (4%; n=7).The 12 studies reported on 194 patients with IBD: UC in 105 (54%), CD in 43 (22%), and IBD-NOS in 46 (24%). Most cases were inactive (74%; n=144); eight (4%) were active, and the remainder (n=43, 22%) were not specified. Rectal spacers were utilized in 5% (n=10), and biodegradable rectal balloons inserted between the prostate and the rectum were utilized in 4% (n=8). The number of patients reported to be on medication for IBD at the time of radiotherapy was 32% (n=63). Toxicity outcomes are detailed in Tables 3 (BT) and Table 4 (EBRT±BT).
Table 1.
Baseline characteristics of studies that analyzed predominantly brachytherapy.
| Study | Stage | Sample | Type of IBD | Status of IBD | Number of Patients on Medication for IBD at time of RT (N) | Previous pelvic surgeries for IBD (N) | RT modality* | Brachytherapy dose | Pelvic nodal RT (N) | Rectal Constraints |
|---|---|---|---|---|---|---|---|---|---|---|
| Grann and Wallner | T1c-T2c | 6 | 3 UC, 3 CD | 4 inactive, 2 active, 1 NS | 3 | 3 | I-125 (n=5). I-125 + 45 Gy WPRT (n=1) | 150 Gy monotherapy, 141 Gy combined modality | 1 | Rectal wall V100Gy was kept below 10 mm2 (surface area) |
| Pai et al. | T1c-T2c | 13 | 10 UC, 3 CD | 11 inactive, 2 active | 2 | 4 | I-125 | 144 Gy monotherapy | 0 | Authors suggest scorecard goal of Rectal V100% < 1 cc. |
| Cherian et al. | T1c | 7 | 7 UC | Inactive | 2 | 7 (all had prior J-pouch anastomosis) | I-125 | 144 Gy monotherapy | 0 | No goals stated |
| Williamson et al. | T1c | 5 | 5 UC | Inactive | NR | 5 (all had ileal pouch with anal anastomosis) | I-125 (n=3), Cs-131 (n=2) | 145 Gy or 115 Gy monotherapy, respectively | 0 | No goals stated |
| Peters et al. | T1c-T2b | 24 | 17 UC, 7 CD | Inactive | 11 | 9 | I-125 (n=15), Pd-103 (n=5). Pd-103 + 45 Gy EBRT (n=3) | 160 Gy or 124 Gy monotherapy, respectively; 100 Gy combined modality | 0 | No goals stated |
| Mohammed et al. | T1c-T3b | 11 | 6 UC, 5 CD | 10 inactive, 1 active | NR | 3 | Ir-192 HDR (n=9). Ir-192 + 37.5 Gy IMRT** (n=2) | 19-20 Gy monotherapy, 15 Gy combined modality. | NR | No goals stated |
Abbreviations: CD, Crohn’s disease; EBRT, external beam radiation therapy to prostate ± seminal vesicles (no nodal coverage); Gy, Gray; HDR, high dose rate; IBD, inflammatory bowel disease; IMRT, intensity-modulated radiotherapy; LDR, low dose rate; NOS, not otherwise specified; NR, not reported; RT, radiotherapy; UC, ulcerative colitis; WPRT, whole pelvic (nodal) radiotherapy.
All modalities LDR unless otherwise noted.
Nodal coverage not reported.
Table 2.
Baseline characteristics of studies that analyzed predominantly external beam radiotherapy.
| Study | Stage | Sample | Type of IBD | Status of IBD | Number of Patients on Medication for IBD at time of RT (N) | Previous pelvic surgeries for IBD (N) | RT modality | RT dose* | Pelvic nodal RT (N) | Rectal sparing device | Rectal Constraints |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lehrich et al. | NR | 11 | 6 UC, 1 CD, 4 NOS | NR | NR | 2 | IMRT + Ir-192 HDR | 59.4 Gy in 33 fractions + 16 Gy HDR-BT over 4 fractions | NR | Spacer (n=8) | V60.4<25% V56.3<20% V52.3<25% V48.3<35% V40.3<50%** |
| Murphy et al. | T1-T3 | 21 | 13 UC, 7 CD, 1 NOS | 20 inactive, 1 active | 7 | 5 | 3DCRT (n=6), IMRT (n=11), or I-125 LDR (n=4) | Median 76 Gy (3DCRT/IMRT), I-125: 145 Gy monotherapy | NR | None | V65 < 17% V40 < 35% |
| Gestaut and Swanson | NR | 18 | 16 UC, 2 CD | Inactive | 14 | 2 | 3DCRT (n=6), IMRT (n=6), or LDR (n=4 I-125, n=2 Pd-103) | 69-72 Gy (3DCRT/IMRT), I-125: 144 Gy, Pd-103: 100-110 Gy monotherapy | 3 | None | No goals stated |
| Vanneste et al. | T1-T3 | 8 | 4 UC, 4 CD | 6 inactive, 2 active | NR | 5 | Hypofractionated IMRT (n=5) or LDR (n=3) | 70 Gy in 28 fractions (IMRT), I-125: 145 Gy monotherapy | 1 | Balloon (n=8) | Institutional |
| Lischalk et al. | T1-T2 | 31 | 18 UC, 11 CD, 2 NOS | NR | 24 | NR | SBRT | 35 Gy (n=26) or 36.25 Gy (n=3) in 5 fractions; other (n=2) | 2 | Spacer (n=2) | Institutional |
| Juarez et al. | NR | 39 | 39 NOS | Inactive | NR | NR | SBRT | 35 Gy (n=5), 36.25 Gy (n=6), or 40 Gy (n=28) in 5 fractions | 0 | NR | Various protocols |
Abbreviations: 3DCRT, three-dimensional conformal radiotherapy; CD, Crohn’s disease; Gy, Gray; HDR, high dose rate; IBD, inflammatory bowel disease; IMRT, intensity-modulated radiotherapy; LDR, low dose rate; NOS, not otherwise specified; NR, not reported; RT, radiotherapy; SBRT, stereotactic body radiation therapy; UC, ulcerative colitis.
All radiotherapy was delivered as conventional fractionation (e.g., 1.8-2.0 Gy/fraction) unless otherwise noted.
RTOG 0415 metrics.
Table 3.
Select outcomes of studies that analyzed predominantly brachytherapy.
| Study | Median follow-up (y) | Toxicity | toxicity cutoff | Acute GI toxicities (Grade 2+) | Late GI toxicities (Grade 2+) | Subsequent IBD flares | Comments | Rectal Dosimetry |
|---|---|---|---|---|---|---|---|---|
| Grann and Wallner | 3.7 | Descriptive* | NR | Grade 2 (17%; n=1/6) | Grade 2 (17%; n=1/6) | None | Patient #3 had undiagnosed UC at the time of RT and was started on 45 Gy WPRT. He required a 10-day treatment break at 23 Gy and went on to receive I-125. All GI symptoms for all patients resolved in long term. | Rectal wall V100Gy was kept below 10 mm2 (surface area) |
| Pai et al. | 4.2 | RTOG | 12 mo | Grade 2 (15.4%; n=2/13), Grade 3 (15.4%, n=2/13), Grade 4 (7.7%; n=1/13) | Grade 2 (23%; n=3/13), Grade 3 (7.7%, n=1/13), Grade 4 (7.7%; n=1/13) | 2 | All patients with grade 3+ events had endoscopic rectal biopsy within 3 months of RT. Three out of five patients with grade 2+ late GI toxicity had rectal involvement by IBD. The two cases with active IBD around the time of the implant developed ≥ grade 2 acute and late GI toxicities. Prior bowel surgery did not appear to be a risk factor. | Median rectal V100% was 1.44 cc and 0.56 cc in patients with and without GI toxicities, respectively. The one patient with late grade 4 toxicity had a rectal V100% of 4.24cc and active IBD at time of brachytherapy. |
| Cherian et al. | 1.0 | CTCAE v4 | 6 mo | NS | Grade 2 (28.6%, n=2/7) | None | Authors suggest utilizing caution when deploying the most posterior row of seeds to minimize J-pouch radiation doses | Mean ileal pouch V100% = 0.16 cc. Mean ileal pouch V50% = 1.38 cc. |
| Williamson et al. | 2.9 | Descriptive** | NR | Grade 2 (80%; n=4/5) Grade 3 (20%; n=1/5) | (0%, n=0/5) | NR | Bowel frequency (consistent with grade 2-3 diarrhea) in all patients at two weeks after treatment, but returned to baseline by four months after RT | Mean rectal V100% = 0.76 cc. |
| Peters et al. | 4.0 | CTCAE v4 | 6 mo | Grade 2 (4.2%; n=1/24) | Grade 2 (16.7%; n=4/24) | None | Toxicities not separately mentioned for EBRT cases (n=3/24) | No association between (prescription) BED and rectal toxicity; rectal V100% in the four patients with late grade 2 GI toxicity ranged between 0.54-2.01 cc |
| Mohammed et al. | 0.5 | CTCAE v4 | 3 mo | (0%, n=0/11) | NR | NR | The one patient with active rectal disease at the time of implant suffered no toxicities after HDR brachytherapy | V100% = 0 Mean D2cc 10.0 Gy (min-max 4.4-12.8 Gy) |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; GI, gastrointestinal; IBD, inflammatory bowel disease; NR, not reported; RT, radiation therapy; RTOG, Radiation Therapy Oncology Group; UC, ulcerative colitis; WPRT, whole pelvic (nodal) radiotherapy; cc, cm3.
Acute toxicities: Patient #3 developed blood in stool requiring suppositories for management consistent with CTCAE v4.0 G2 proctitis during treatment; Patient #6 had mild rectal urgency not requiring medical management consistent with CTCAE v4.0 G1 proctitis. Late toxicities: Patient #1 developed increased blood in stool at six months which lasted for two months and resolved without intervention, consistent with CTCAE v4.0 G2 proctitis.
Detailed bowel frequency information in the manuscript. All five patients had at least Grade 2 diarrhea at two weeks (80%, one grade 3), all of which returned to baseline by four months consistent with Grade 0-1 CTCAE toxicity.
Table 4.
Select outcomes of studies that analyzed predominantly external beam radiotherapy.
| Study | Median follow-up (y) | Toxicity | toxicity cutoff | Acute GI toxicities (Grade 2+) | Late GI toxicities (Grade 2+) | Subsequent IBD flares | Comments | Rectal Dosimetry |
|---|---|---|---|---|---|---|---|---|
| Lehrich et al. | 3 | CTCAE v4 | 3 mo | Grade 2 (18.2%; n=2/11) | None | NR | Spacer placement may assist in toxicity reduction | NR |
| Murphy et al. | 4.1 | Institutional | NR | Grade 2 (19.1%, n=4/21) Grade 3 (4.8%, n=1/21) |
Grade 2 (4.8%, n=1/21) Grade 3 (4.8%, n=1/21) |
9 | One patient was treated in a midst of a UC flare as the history was not disclosed until after developing severe diarrhea, abdominal pain, and rectal bleeding after receiving only 10 Gy; RT was stopped and ADT was initiated. Medication use associated with increased acute grade 2+ GI toxicity (8→ 57%, p=0.03) but not late toxicity; no associations with modality or dose thereof; no difference in toxicities compared to non-IBD matched controls. | IMRT cohort (n=11): Median V65 = 11.74% Median V40 = 29.9% LDR cohort (n=4): Median V100% = 3.4%. |
| Gestaut and Swanson | 9.5 | CTCAE v4 | 3 mo | Grade 2 (33.3%; n=3/9) | None. | NR | All cases of grade 2 proctitis were in 3DCRT patients and all cases of grade 2 proctitis resolved within 5 years of treatment with 5-ASA therapy | NR |
| Vanneste et al. | 1.1 | CTCAE v4 | 3 mo | Grade 2 (12.5%; n=1/8) | Grade 2 (12.5%, n=1/8) | 1 | Rectal balloon placement tolerated well and may assist in toxicity reduction | NR |
| Lischalk et al. | 1.8 | CTCAE v5 | 3 mo | Grade 3 proctitis (3.2%, n=1/31) | Grade 2 hemorrhoids (3.2%, n= 1/31), grade 2 proctitis (6.5%, n=2/31) | NR | All patients who developed toxicity were on active IBD medications | Median rectal max dose was 38.5 Gy in patients with ≥G1 toxicity vs. 37.5 Gy in entire cohort |
| Juarez et al. | 7 | CTCAE v4 | NR | Grade 2 (7.7%, n=3/39) | Grade 2 (2.6%; n=1/39), Grade 3 (2.6%, n=1/39) | NR | Compared to non-IBD matched cohort, IBD conferred a significantly higher risk of acute grade 2+ and late grade 1 GI toxicities, but no difference for late grade 2+ toxicities | NR |
Abbreviations: 3DCRT, three-dimensional conformal radiotherapy; BED, biologically effective dose; CTCAE, Common Terminology Criteria for Adverse Events; EBRT, external beam radiotherapy; GI, gastrointestinal; IBD, inflammatory bowel disease; NR, not reported; RT, radiation therapy; RTOG, Radiation Therapy Oncology Group; UC, ulcerative colitis.
Toxicity grading was conducted via various measures (Supplementary Table 4): Common Terminology Criteria for Adverse Events (CTCAE) v4 (n=7), CTCAE v5 (n=1), descriptive (n=2), institutional, and Radiation Therapy Oncology Group (RTOG) (n=1) grading systems. The distribution of the reported Grade 1-4 GI toxicity rates is displayed in Figure 2. Supplementary Figure 1 displays G1+, G2+ and G3+ toxicity. The pooled rate of grade 2+ GI adverse events (AE) was 15.3% (n=27 cases in 177 evaluable patients, min-max: 0-100%) and 11.3% (20 cases in 177 evaluable patients, min-max: 0-38.5%) for acute and late AEs, respectively amongst all patients reported. The pooled rate of grade 3+ GI AEs was 3.4% (n=6/177) and 2.3% (n=4/177) in the acute and late assessment period, respectively. There was one reported acute grade 4 GI AE and one reported late grade 4 GI AE (0.6%; n=1/177). An exploratory, study-level analysis conducted to assess the relationship between toxicity rate and the fraction of patients receiving EBRT in a study showed a trend of decreasing rates of toxicity with increasing rates of EBRT utilization consistent amongst all early and late metrics with exception of isolated grade 1 toxicity (Supplemental Figure 2). A second exploratory analysis suggested a positive relationship between the percentage of patients who had received prior abdominopelvic surgery for IBD in a series and the reported rates of a subset of toxicity measures which was driven primarily by G2 AEs (Supplementary Figure 3).
Figure 2.
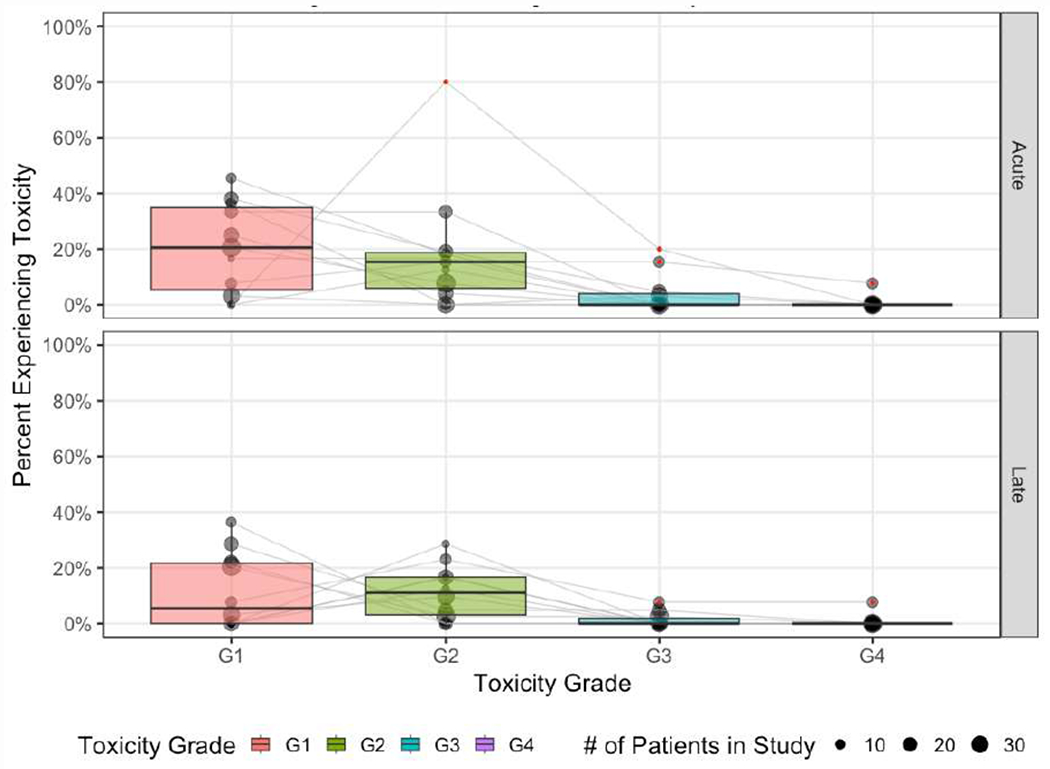
Distribution of reported GI Toxicity Rates
There were five reports (Table 1 & 3) examining patients treated primarily with LDR-BT, and one article examining patients treated primarily with HDR-BT. We discuss the two largest LDR studies here, with more details on smaller studies in Tables 1 & 3 (i.e., Grann and Wallner31, Cherian et al26, and Williamson et al25). The first report with a larger patient population was by Pai and colleagues28 described a rate of 23.1% (n=3/13) and 15.4% (n=2/13) of acute (≤12 month latency) and late (>12 month latency) grade 3+ GI AEs via RTOG grading,34 respectively. These rates were significantly higher compared to matched institutional controls without IBD, who experienced acute and late grade 3+ events at a rate of 0.2% and 1.1%, respectively. Notably, all grade 3+ toxicities developed in patients with UC and all acute grade 3+ toxicities occurred in patients undergoing rectal biopsy within 3 months of completing RT. The median rectal V100% was higher for patients with late grade 2+ toxicity (e.g., 1.44 cc vs. 0.56 cc; Table 3). Peters and colleagues29 used LDR brachytherapy as monotherapy (n=20/24) or in combination with EBRT to the prostate ± seminal vesicles (n=3/24) using either I-125 (n=15/24) or Pd-108 (n=8/24). Acute grade 1 events were experienced by 25% of patients, whereas only 1 patient experienced acute grade 2 AEs by CTCAE v4.0 criteria (4.2%; n=1/24). Late grade 2+ toxicity occurred in 16.7% of patients within 5 years of seed implantation (n=4/24). The rectal V100% in the four patients with late grade 2 GI toxicity ranged between 0.54 and 2.01 cc. No grade 3+ GI AEs were reported.
Two studies reported outcomes for patients treated with HDR brachytherapy monotherapy or combined with EBRT. Mohammed et al.23 treated primarily with HDR-BT. Nine patients underwent HDR-BT alone (19-20Gy in 1 fraction) and 2 in combination with EBRT (15Gy HDR-BT+ 37.Gy IMRT). At six weeks, acute grade 1 AEs included diarrhea in 18% (n=2/11) and proctitis in 27% of patients (n=3/11). No patients experienced grade 3+ acute or late toxicities.23 Of note, goal for rectal D2cc with HDR monotherapy is typically < 75 Gy35. Mean D2cc was 10 Gy (min-max 4.4-12.8 Gy), suggesting great care was utilized when inserting the most posterior row of catheters during HDR implementation. Lehrich et al.22 reported on 11 patients treated with HDR brachytherapy in conjunction with EBRT. In this report three patients had rectal involvement of IBD, two had prior abdominal surgery, and 8 patients (72%) received a hydrogel rectal spacer. Five patients (45%) developed acute grade 1 diarrhea and 1 patient (9%) developed acute grade 2 diarrhea; two patients (18%) and 1 patient (9%) developed acuate grade 1 and grade 2 proctitis, respectively. All late AEs were grade 1 in severity consisting of diarrhea in 3 patients (27%) and proctitis in 2 patients (18%).
There were three articles that reported a combination of EBRT and brachytherapy. Murphy and colleagues27 reported on twenty-one patients receiving 3DCRT, IMRT, or LDR-BT. In this report, one patient experienced severe acute toxicity and one experienced severe late toxicity as defined by an institutional toxicity grading scale. The authors found no statistically significant difference in either acute or late GI toxicity rates for patients with IBD compared to a matched control population without IBD undergoing RT. Furthermore, they found that only IBD medication use at the time of RT predicted for higher grade 2+ AEs (57.1% with vs. 7.7% without use of IBD medication). No predictive factors for late toxicity were identified. Notably, there were no acute grade 2+ or any late toxicities in the four patients who received LDR-BT. In another mixed-modality publication by Gestaut and Swanson30 of eighteen patients, acute grade 2 CTCAE v4.0 GI toxicities were 33.3% (n=3 events / 9 evaluable patients). There were no grade 2+ late events in 18 evaluable patients, and there were no acute or late grade 3+ AEs. A unique finding of this investigation was that grade 2 proctitis only developed in patients who received 3DCRT and did not occur for those receiving IMRT or brachytherapy. The final multi-modality by Vanneste and colleagues24 reported on patients treated with hypofractionated IMRT (70 Gy in 27 fractions) or LDR brachytherapy (145 Gy via I-125 implantation). While this study only had 8 patients, it was of note because it was the only study where patients had a biodegradable rectal spacing balloon. There was one acute grade 2 GI AE (12.5%) and no grade 3+ AEs were reported.
The last two reports21, 32 each characterize the treatment of patients with SBRT. Lischalk and colleagues32 reported on 31 patients and found that of these only one of these developed a CTCAE v5.0 grade 3+ AE, a case of acute grade 3 proctitis (3.2%; n=1/31). Two patients experienced late grade 2 proctitis’ and one patient experienced late grade 2 hemorrhoids’ totaling a 9.7% (n=3/31) proportion of late grade 2 AEs and no late grade 3 AEs. All patients who developed toxicity were on active IBD medications at the time of SBRT. Lastly, the report by Juarez and coworkers21 also exclusively detailed the treatment of patients with SBRT. However, this report had a higher median delivered dose (40 Gy in 5 fractions) than that of the report by Lischalk et al.32 Although IBD patients had significantly higher rates of any acute GI toxicity (28.2% vs 8.5%) and acute grade 2 GI toxicities (7.7% vs 0%) compared to matched controls, there were no acute grade 3+ toxicities in either cohort. The rate of developing any late toxicity among those with IBD was significantly increased with one grade 2 toxicity and one grade 3 toxicity (2.6%; n=1/39), but the rate of grade 2 or 3 events was numerically similar.
DISCUSSION
IBD is an uncommon comorbidity considered a relative contraindication for RT in the treatment of prostate cancer owing to early reports of severe RT-induced toxicities in this subpopulation.17, 20 In this review, we found that the reported cases had a numerically higher level of acute Grade 2+ toxicities compared to those reported in prospective trials of patients without this comorbidity. However, the rates of late Grade 2+ toxicities were numerically lower. Pooled rate of late Grade 2+ events was 11.3% (n=20/177, min-max 0-38.5%). The pooled rate of acute grade 2+ GI events was 15.3% (n=27/177, min-max 0-100%), whereas the pooled rate of acute and late grade 3+ GI events was 3.4% (n=6/177, min-max 0-23%) and 2.3% (n=4/177, min-max 0-15%). With exception of a single study28, the rates of grade 3+ GI AEs were <5%. The explanation for the outlying study is unclear, however the authors note all patients experiencing grade 3+ events received an endoscopic rectal biopsy within 3 months of completing radiotherapy, a practice which is not endorsed by ASTRO in the modern era.36
The summary toxicity rates reported are similar those reported for patients without IBD in prior RCTs. When compared to the 79.2 Gy arm of RTOG 0126, 37 the pooled toxicity rates reported here were numerically higher in terms of acute G2+ GI (7.0% vs. 15.3%) and acute G3+ GI toxicities (0.1% vs. 3.4%) but lower in terms of late G2+ GI (21.5% vs. 11.3%) and late G3+ GI (5.3% vs. 2.3%) toxicities. While this comparison is offered for contextualization, the significance is unclear given the lack of prospective registration and standardized data collection amongst the studies reported in our review. It is reassuring, however, that these pooled rates are not profoundly higher than those in contemporary prospective trials. Other study methodologies such as the two matched-cohort reports21, 27 do seem to suggest an increased risk of G1-G2 toxicity amongst patients with IBD. Prior series have also identified an elevated risk of loose stools and fecal urgency,19 along with bleeding, proctitis, or bowel injury18 when compared to patients without IBD. When interpreted conservatively, the data suggest that patients in the modern era should be counseled regarding a potentially higher risk of GI toxicities, although the true absolute excess risk may be incremental.21
Amongst the studies analyzed, two trends regarding toxicity rates emerged pertaining to (1) the technique of RT delivery and (2) the prior surgical status of patients under study. There was a trend for lower rates of G2+ toxicity as studies included higher rates of patients undergoing EBRT (Supplemental Figure 1). One explanation for this observation is that EBRT utilization is inversely related to BT utilization, and it may be true that the biologically equivalent dose of a standard BT administration to the rectal wall may lead to a higher probability of complication than EBRT. As there was a positive relationship between the average year of treatment and percentage of EBRT utilization as well as a trend towards lower toxicity rates for patients treated in the modern era, it is possible that the treatment epoch may have confounded the relationship between EBRT utilization and toxicity. The addition of treatment year to a linear prediction model of late G2+ toxicity attenuated the effect approximately 20% supporting this claim for this toxicity endpoint.38 A second explanation is that due to the retrospective nature of these data, this was a result of selection bias. Providers may select patients with low risk of RT-induced toxicities to undergo EBRT as opposed to BT (or other management strategy such as surgery) causing the trend observed. The second trend observed was towards higher reported rates of G2+ toxicities amongst studies with higher rates of prior surgical management for IBD amongst patients described (Supplemental Figure 2). This may be due to this population either having a higher baseline risk of IBD-related symptoms as indication by their need for surgery or having post-surgical anatomic configurations which led to more radiosensitive GI organs than the rectum, such as the colon or small bowel, receiving high doses of RT resulting causing toxicity. This result may indicate the need for more stringent dosimetric constraints for GI OARs as discussed below.
These data should be interpreted with caution for subpopulations that were underrepresented in these reports. First, patients with active IBD were severely underrepresented being specifically excluded from some reports and therefore constituting only 4.1% of the total reported patients (n=8/194). Second, only 3.6% of reported patients (n=7/194) underwent whole pelvic RT (WPRT). A recent phase III trial, POP-RT 39 has demonstrated an oncologic benefit to WPRT over prostate only RT (PORT) in intermediate- and high-risk patients. While an older randomized trial40 suggests the potential for higher rates of late GI toxicity in patients who receive WPRT, other randomized trials,41 including POP-RT, 39 have failed to show a significant difference in the population without IBD. As retrospective studies suggest that WPRT may result in acceptable rates of toxicity in patients with IBD10, further investigation is needed to define the risk-to-benefit tradeoff for treatment volume expansion in this subgroup. Given the potential for the increased risk of harms from WPRT in this subpopulation, it may be reasonable to consider stronger recommendations for screening in this subpopulation with the intention of treatment initiation at earlier stages when WPRT is likely of negligible benefit. Third, the relationship between prior surgical intervention for IBD and appropriate candidacy for RT remains unclear. Of the patients reported, 23.2% (n=45/194) underwent prior surgery. Our analysis indicates a positive relationship between rates of reported G2+ AEs and rates of prior surgical intervention (Supplementary Figure 2). Given the contrasting trend in grade 1 AEs, it is possible that this trend in G2+ toxicity represents intensified toxicities amongst patients with prior surgery. While the studies exclusively reporting on toxicity outcomes in patients with prior surgical intervention25, 26 appeared to have acceptable G3+ toxicity rates (n=0 and n=1, respectively, with the single G3 toxicity a self-resolving episode of acute diarrhea), these reports were retrospective in nature and only reported on 12 patients between them. Furthermore, patients in this subgroup may have altered baseline bowel habits because of their post-surgical physiology which may predispose them to higher grade symptoms even outside of the context of RT exposure. Given the absence of outcomes in a non-irradiated subgroup, it is difficult to quantify the excess risk which can be attributed to RT. In sum, despite these efforts to summarize the available literature, there remains substantial uncertainty regarding certain subgroups which require further study in a prospective fashion.
There are several strategies to potentially reduce the incidence of GI adverse events from RT in this population. First, decreasing elective coverage may serve to minimize normal tissue exposure and thereby partially mitigate toxicity risk. This may occur through the omission of elective volumes such as the pelvic lymph nodes or the seminal vesicles when clinically appropriate. Second, the utilization of modern radiotherapy techniques such as image-guided IMRT or HDR-BT with objective-based planning may further help accomplish this purpose. There is evidence demonstrating lower rates of GI toxicity using IMRT over 3DCRT for patients with IBD. One study of 28 patients with IBD undergoing abdominopelvic EBRT for a variety of malignancies reported significantly lower rates of severe late toxicity when utilizing specialized techniques designed to reduce small and large bowel OAR exposure (e.g., split course treatment, proton beam, field minimization, surgical interventions such as omentoplasty or mesh placement) compared to conventional EBRT (25% vs 75%).4 A similar report examining 19 patients with IBD found acute grade 2+ GI toxicity rates of 14% with IMRT versus 100% with 3DCRT.10 Although some authors27, 30 did report the use of 3DCRT in their series, this parameter was not reported with sufficient detail to allow for an analysis of trends. Similarly, evidence exists for lower rates of patient-reported objective toxicity rates with HDR-BT vs. LDR-BT.42 The majority of patients who underwent BT in this report underwent LDR-BT (n = 68/90) possibly indicating that toxicity may be improved if HDR-BT is used. Third, strict attention to dose constraints during the treatment planning process is highly recommended. As discussed, some studies,28, 32 which conducted dosimetric analyses, suggested a relationship between rectal exposure and toxicity, although others did not. 27, 29 Given assumptions regarding baseline susceptibility to rectal injury, it is possible that stricter planning constraints may be appropriate for this subpopulation. Lastly, although rectal sparing devices (e.g., hydrogel spacers or biodegradable rectal balloons) were utilized in a minority of patients summarized here, these interventions may prove beneficial to this subpopulation at high risk of RT-induced GI toxicity via a reduction in rectal organ exposure.43 Currently, clinical evidence for this assertion is lacking, and it is possible that this intervention at least in some cases itself could result in serious toxicity,44 especially in patients with low pelvic adhesions from prior surgery and/or IBD-related inflammation. Finally, judicious patient selection which prioritizes non-radiotherapeutic management strategies such as surgery or surveillance may serve to decrease the population at risk of RT-induced toxicities. Further studies including prospective trials are required to validate the benefit of the aforementioned suggestions in this subpopulation.
LIMITATIONS
There are several limitations of our systematic review. The primary limitation is the quality of heterogeneity of the available reports on which the review is based. Rarely did authors report criteria used to verify the diagnosis of IBD amongst their reported subjects. Additionally, substantial heterogeneity between studies in RT technique was identified (Tables 1 and 2) with many patients (n=87/194) treated with brachytherapy, which is well characterized as having a higher level of operator dependency than EBRT. Moreover, the definition of toxicity grade (Supplemental Table 4) and the acute assessment period (Tables 3 and 4) employed were also heterogeneous. Furthermore, the studies included were retrospective in nature precluding a true understanding of the incidence and prevalence of toxicity given the uncertainty of the size of the true population. While we attempted to enumerate each patient individually from each report, a true patient level analysis was not possible given the lack detail included in the included publications. Finally, the follow-up was limited with 4 studies reporting follow-up of less than 2 years.
CONCLUSION
In a systematic review of the available literature, the rate of serious, grade 3+ GI toxicity appears to be low for patients undergoing RT for prostate cancer treatment who suffer from comorbid IBD. Patients, however, should still be counseled that there may be an increased risk of toxicity, especially grade 2+ toxicity. These conclusions should be interpreted with caution given the quality of the studies available from which to draw inferences. Further investigations are required to validate toxicity mitigation strategies for this vulnerable population. Sensible precautions include the utilization of modern treatment techniques which may serve to decrease the exposure of GI organs-at-risk to RT which is the presumed mechanism for the possible RT-induced toxicity burden in this subpopulation.
Supplementary Material
Funding:
There was no financial support for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Protocol and Registration: There is no registration or protocol associated with this review.
Conflicts of Interest: None
REFERENCES
- 1.Carli E, Caviglia GP, Pellicano R, et al. Incidence of Prostate Cancer in Inflammatory Bowel Disease: A Meta-Analysis. Medicina (Kaunas). 2020;56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019;12:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brawley S, Mohan R, Nein CD. Localized Prostate Cancer: Treatment Options. Am Fam Physician. 2018;97:798–805. [PubMed] [Google Scholar]
- 4.Willett CG, Ooi CJ, Zietman AL, et al. Acute and late toxicity of patients with inflammatory bowel disease undergoing irradiation for abdominal and pelvic neoplasms. Int J Radiat Oncol Biol Phys. 2000;46:995–998. [DOI] [PubMed] [Google Scholar]
- 5.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. [DOI] [PubMed] [Google Scholar]
- 7.Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. [Google Scholar]
- 9.Song DY, Lawrie WT, Abrams RA, et al. Acute and late radiotherapy toxicity in patients with inflammatory bowel disease. Int J Radiat Oncol Biol Phys. 2001;51:455–459. [DOI] [PubMed] [Google Scholar]
- 10.White EC, Murphy JD, Chang DT, Koong AC. Low Toxicity in Inflammatory Bowel Disease Patients Treated With Abdominal and Pelvic Radiation Therapy. Am J Clin Oncol. 2015;38:564–569. [DOI] [PubMed] [Google Scholar]
- 11.Annede P, Seisen T, Klotz C, et al. Inflammatory bowel diseases activity in patients undergoing pelvic radiation therapy. J Gastrointest Oncol. 2017;8:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broussard D, Riviere P, Bonnet J, et al. Impact of abdominal or pelvic radiotherapy on disease activity in inflammatory bowel disease: a multicentre cohort study from the GETAID. Aliment Pharmacol Ther. 2021;53:400–409. [DOI] [PubMed] [Google Scholar]
- 13.Tromp D, Christie DR. Acute and Late Bowel Toxicity in Radiotherapy Patients with Inflammatory Bowel Disease: A Systematic Review. Clin Oncol (R Coll Radiol). 2015;27:536–541. [DOI] [PubMed] [Google Scholar]
- 14.Glick D, Warde P, Su J, Xu W, Milosevic M, Kim J. Gastrointestinal Toxicity in Patients With Inflammatory Bowel Disease Treated With Pelvic Radiation Therapy. International Journal of Radiation Oncology*Biology*Physics. 2014;90:S388–S389. [Google Scholar]
- 15.Rhome RM, Axelrad J, Itzkowitz S, et al. Acute and Chronic Complications After Abdominal/Pelvic Radiation in Patients With Inflammatory Bowel Disease. International Journal of Radiation Oncology*Biology*Physics. 2015;93:E492. [Google Scholar]
- 16.Cantrell JN, Vidal GS, Bitar H, Ahmad S, Gunter TC. Should inflammatory bowel disease be a contraindication to radiation therapy: a systematic review of acute and late toxicities. Journal of Radiotherapy in Practice. 2021;20:480–489. [Google Scholar]
- 17.Leibel SA, Heimann R, Kutcher GJ, et al. Three-dimensional conformal radiation therapy in locally advanced carcinoma of the prostate: preliminary results of a phase I dose-escalation study. Int J Radiat Oncol Biol Phys. 1994;28:55–65. [DOI] [PubMed] [Google Scholar]
- 18.Chen AB, D’Amico AV, Neville BA, Earle CC. Patient and treatment factors associated with complications after prostate brachytherapy. J Clin Oncol. 2006;24:5298–5304. [DOI] [PubMed] [Google Scholar]
- 19.Barnett GC, De Meerleer G, Gulliford SL, Sydes MR, Elliott RM, Dearnaley DP. The impact of clinical factors on the development of late radiation toxicity: results from the Medical Research Council RT01 trial (ISRCTN47772397). Clin Oncol (R Coll Radiol). 2011;23:613–624. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Feagins LA. Managing Patients with Inflammatory Bowel Disease Who Develop Prostate Cancer. Dig Dis Sci. 2020;65:22–30. [DOI] [PubMed] [Google Scholar]
- 21.Juarez JE, Romero T, Mantz CA, et al. Toxicity After Stereotactic Body Radiation Therapy for Prostate Cancer in Patients With Inflammatory Bowel Disease: A Multi-institutional Matched Case-Control Series. Adv Radiat Oncol. 2021;6:100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehrich BM, Moyses HM, Kawakubo A, et al. Long-Term Toxicity of High Dose Rate Brachytherapy in Prostate Carcinoma Patients With Inflammatory Bowel Disease. Clin Oncol (R Coll Radiol). 2019;31:399–400. [DOI] [PubMed] [Google Scholar]
- 23.Mohammed W, Hoskin P, Henry A, Gomez-Iturriaga A, Robinson A, Nikapota A. Short-term Toxicity of High Dose Rate Brachytherapy in Prostate Cancer Patients with Inflammatory Bowel Disease. Clin Oncol (R Coll Radiol). 2018;30:534–538. [DOI] [PubMed] [Google Scholar]
- 24.Vanneste BGL, Van Limbergen EJ, Marcelissen T, et al. Is prostate cancer radiotherapy using implantable rectum spacers safe and effective in inflammatory bowel disease patients? Clin Transl Radiat Oncol. 2021;27:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williamson R, Smaldone MC, Gibbons EP, Smith RP, Beriwal S, Benoit RM. Prostate brachytherapy after ileal pouch-anal anastomosis reconstruction. Urology. 2009;73:369–373. [DOI] [PubMed] [Google Scholar]
- 26.Cherian S, Kittel JA, Reddy CA, et al. Safety and efficacy of iodine-125 permanent prostate brachytherapy in patients with J-pouch anastomosis after total colectomy for ulcerative colitis. Pract Radiat Oncol. 2015;5:e437–e442. [DOI] [PubMed] [Google Scholar]
- 27.Murphy CT, Heller S, Ruth K, et al. Evaluating toxicity from definitive radiation therapy for prostate cancer in men with inflammatory bowel disease: Patient selection and dosimetric parameters with modern treatment techniques. Pract Radiat Oncol. 2015;5:e215–e222. [DOI] [PubMed] [Google Scholar]
- 28.Pai HH, Keyes M, Morris WJ, Christie J. Toxicity after (125)I prostate brachytherapy in patients with inflammatory bowel disease. Brachytherapy. 2013;12:126–133. [DOI] [PubMed] [Google Scholar]
- 29.Peters CA, Cesaretti JA, Stone NN, Stock RG. Low-dose rate prostate brachytherapy is well tolerated in patients with a history of inflammatory bowel disease. Int J Radiat Oncol Biol Phys. 2006;66:424–429. [DOI] [PubMed] [Google Scholar]
- 30.Gestaut MM, Swanson GP. Long term clinical toxicity of radiation therapy in prostate cancer patients with Inflammatory Bowel Disease. Rep Pract Oncol Radiother. 2017;22:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grann A, Wallner K. Prostate brachytherapy in patients with inflammatory bowel disease. Int J Radiat Oncol Biol Phys. 1998;40:135–138. [DOI] [PubMed] [Google Scholar]
- 32.Lischalk JW, Blacksburg S, Mendez C, et al. Stereotactic body radiation therapy for the treatment of localized prostate cancer in men with underlying inflammatory bowel disease. Radiat Oncol. 2021;16:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flynn S, Eisenstein S. Inflammatory Bowel Disease Presentation and Diagnosis. Surgical Clinics of North America. 2019;99:1051–1062. [DOI] [PubMed] [Google Scholar]
- 34.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. [DOI] [PubMed] [Google Scholar]
- 35.Hoskin PJ, Colombo A, Henry A, et al. GEC/ESTRO recommendations on high dose rate afterloading brachytherapy for localised prostate cancer: An update. Radiotherapy and Oncology. 2013;107:325–332. [DOI] [PubMed] [Google Scholar]
- 36.Radiology ASfT, Panel OC. Consensus Statements on Radiation Therapy of Prostate Cancer: Guidelines for Prostate Re-Biopsy After Radiation and for Radiation Therapy With Rising Prostate-Specific Antigen Levels After Radical Prostatectomy. Journal of Clinical Oncology. 1999;17:1155–1155. [DOI] [PubMed] [Google Scholar]
- 37.Michalski JM, Moughan J, Purdy J, et al. Effect of Standard vs Dose-Escalated Radiation Therapy for Patients With Intermediate-Risk Prostate Cancer: The NRG Oncology RTOG 0126 Randomized Clinical Trial. JAMA Oncol. 2018;4:e180039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee PH. Is a cutoff of 10% appropriate for the change-in-estimate criterion of confounder identification? J Epidemiol. 2014;24:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murthy V, Maitre P, Kannan S, et al. Prostate-Only Versus Whole-Pelvic Radiation Therapy in High-Risk and Very High-Risk Prostate Cancer (POP-RT): Outcomes From Phase III Randomized Controlled Trial. J Clin Oncol. 2021;39:1234–1242. [DOI] [PubMed] [Google Scholar]
- 40.Roach M, Moughan J, Lawton CAF, et al. Sequence of hormonal therapy and radiotherapy field size in unfavourable, localised prostate cancer (NRG/RTOG 9413): long-term results of a randomised, phase 3 trial. Lancet Oncol. 2018;19:1504–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pommier P, Chabaud S, Lagrange JL, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol. 2007;25:5366–5373. [DOI] [PubMed] [Google Scholar]
- 42.Paly JJ, Egleston BL, Wong JK, et al. Patient-reported Quality of Life After SBRT, LDR, and HDR Brachytherapy for Prostate Cancer: A Comparison of Outcomes. Am J Clin Oncol. 2021;44:131–136. [DOI] [PubMed] [Google Scholar]
- 43.Hamstra DA, Mariados N, Sylvester J, et al. Continued Benefit to Rectal Separation for Prostate Radiation Therapy: Final Results of a Phase III Trial. Int J Radiat Oncol Biol Phys. 2017;97:976–985. [DOI] [PubMed] [Google Scholar]
- 44.Aminsharifi A, Kotamarti S, Silver D, Schulman A. Major Complications and Adverse Events Related to the Injection of the SpaceOAR Hydrogel System Before Radiotherapy for Prostate Cancer: Review of the Manufacturer and User Facility Device Experience Database. J Endourol. 2019;33:868–871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


