Abstract
OBJECTIVE:
Examine longitudinal associations of history of infertility with menopausal symptoms in midlife.
METHODS:
695 midlife women (≥45 years old or reporting ≥12 months of amenorrhea at the midlife visit) in Project Viva, a prospective cohort enrolled 1999–2002 during pregnancy and followed for 18 years after enrollment (“midlife visit”). Exposure was history of infertility defined as time to pregnancy ≥12 months (≥6 months if ≥35y), use of medical treatment to conceive, or infertility consultation or treatment in the 6 months preceding enrollment. The primary outcome was score below or above the median on the Menopause Rating Scale (MRS). Secondary outcomes included individual symptom score on the MRS and self-reported age of menopause.
RESULTS:
36.6% had a history of infertility in their lifetime. At the time of MRS completion, the women with prior infertility were older (53.4 (SD 3.8) vs. 51.2 (SD 3.7) years) than those without infertility and a larger proportion had reached menopause (62% vs 40%). Women with prior infertility were more likely to score above the median on the MRS (aOR 1.45; 95%CI 1.04–2.01) and had higher odds for reporting any depressive mood (aOR 1.56; 95%CI: 1.12–2.16) and irritability (aOR 1.57 95%CI 1.13–2.19). There was a trend toward greater severity of sleep problems among women with prior infertility. There was no association of prior infertility with report of other menopausal symptoms or age of menopause.
CONCLUSIONS:
Our findings suggest that women with prior infertility are more likely to have an MRS score above the median and experience depressive mood, irritability and sleep problems during midlife than women without infertility. These findings have implications for mental health screening among midlife women.
Keywords: Infertility, menopause, midlife, depressive symptoms, sleep
Introduction:
Menopause represents the end of the reproductive years in women. Women’s quality of life in midlife can be significantly impacted by menopausal symptoms. Although the experience of the menopausal transition is highly variable, most (>85%) women experience symptoms including hot flashes, night sweats, mood changes, reduced libido, genitourinary symptoms, and sleep disturbances1,2. The severity of these symptoms is influenced by a multitude of behavioral, biological, social, psychological and demographic factors2–5.
Reproductive history has been implicated as a factor in the timing of menopause onset and the prevalence of menopause symptoms. For example, nulliparity and diminished ovarian reserve have been associated with earlier age of onset of menopause,6–9 while menstrual cycle characteristics during reproductive years have been related to severity of menopausal symptoms10. Although the literature on the relation of infertility with the onset of menopause or the severity of menopausal symptoms is scarce, there is some indication that women with a history of infertility may experience earlier menopause11 and greater severity of some menopausal symptoms12. Moreover, infertility has been found to be an equivalent life stressor to a cancer diagnosis,13–15 and experiencing stressful life events is associated with report of more menopausal symptoms16–18. Given the limited evidence on this topic, our goal was to characterize the impact of reproductive history, particularly lifetime history of infertility, on the experience of menopausal symptoms in midlife.
Methods:
Study Population:
This analysis is a secondary analysis of a subset of participants in Project Viva, a prospective cohort designed to investigate maternal and child health. Details of this cohort have been previously published.19 Briefly, from 1999 to 2002, women were recruited during their initial prenatal visit at eight participating obstetric offices at Atrius Harvard Vanguard Medical Associates, a multispecialty group practice in Eastern Massachusetts. The eligibility criteria were a singleton pregnancy, ≤22 weeks gestation at enrollment, intention to deliver at a study hospital, and the ability to answer questions in English. Following enrollment, participants were surveyed annually and seen in person every 3–5 years. The most recent completed visit was an ~18-year follow up visit (midlife visit) which included an assessment of menopausal symptoms with the Menopause Rating Scale (MRS)20. There were 2100 participants enrolled in Project Viva. This analysis was restricted to 775 women who attended the midlife visit and completed at least 1 item of the MRS. We further restricted the analysis to women potentially undergoing the menopausal transition, by excluding women <45 years who had not experienced ≥12 months of amenorrhea at the time of the visit (n=80). Eight women who were younger than 45 at the time of the visit but reported having experienced menopause were included. Our analytic sample included 695 participants who had information on menopausal symptoms and exposure data available.
As expected, given the inclusion criteria, participants in the analytic sample (n=695) were older at enrollment (mean age, 33.7 vs 30.9) than those excluded (n=1405). Women in the analytic sample had a lower pre-pregnancy body mass index (BMI, 24.5 vs 25.1 kg/m2) and were more likely to be white (73.2% vs 63.1%), college educated (79.5% vs 57%), married or cohabitating (95.7% vs 89.2%), never smokers (72.2% vs 66.7%) and to have household income >$70,000/year (67.8% vs 56.9%). All participants provided written informed consent. The institutional review board of Harvard Pilgrim Health Care approved all study protocols.
Exposure Assessment:
The primary exposure was history of infertility during a participant’s lifetime (yes/no), assessed via three sources of information, as previously described21. First, we used self-reported data on time-to-conception collected at enrollment about the index pregnancy. Women reporting ≥12 cycles, or ≥ 6 cycles if ≥35 years of age, were classified as having infertility. Second, diagnosis of infertility (International Classification of Diseases-9 code 628.9 entries before the last menstrual period [LMP] + 60 days), a claim for infertility consultation or services, or prescriptions for fertility medications (e.g., clomiphene citrate, gonadotropins, or gonadotropin-releasing hormone agonists before LMP + 14 days) obtained from medical records at the time of enrollment. Third, self-report of ever taking ≥12 months to become pregnant (≥6 months if ≥35 y) or using medical treatment to conceive in any of their past pregnancies as reported in the midlife follow-up questionnaire.
Outcome Assessment:
The primary outcome was presence or absence of menopausal symptoms assessed using the MRS, which we defined categorically as an MRS score below or above the median. The MRS is a validated 11 item questionnaire that was developed to measure the presence and severity of menopausal symptoms20. The participant is asked to report presence or absence and severity of each symptom at that time. Each item is ranked from 0 (no symptoms) to 4 (very severe symptoms). The scale is divided into three subscales: somatic, psychological, and urogenital. The somatic subscale includes items on hot flashes/sweating, heart discomfort, sleep disturbances and muscle or joint discomfort. The psychological subscale includes items on depressive mood, irritability, anxiety, and physical and mental exhaustion. The urogenital subscale includes items on sexual or bladder problems, and vaginal dryness. The score for each item is totaled for a total severity score. The higher the total score, the greater the severity of symptoms. We computed the total score only among women who completed at least 10 items of the MRS. We used the total score divided at the median (≤7 or >7) and also categorized by severity for analysis, using categories previously defined22. The following categories of severity were used: None or minimal severity, score 0–4; Mild severity, score 5–8; Moderate severity, score 9–16; Severe, score ≥17.
As secondary outcomes, we evaluated the presence or absence of each of the 11 individual symptoms and their association with history of infertility. We further explored severity for the symptoms of depressive mood, irritability and sleep difficulties because we observed consistent associations when presence or absence of these symptoms was examined (Categorized as none=0, mild-moderate=1–2, severe/very severe=3–4)22. Another secondary outcome of interest was the age of natural menopause using information reported at the midlife visit. Specifically, participants reported whether they had already experienced menopause (i.e., menstrual periods had stopped for at least 12 months), the reason for this (i.e., natural or secondary [surgical, radiation/chemotherapy]), and age at the onset of menopause.
Covariates:
At enrollment, women reported sociodemographic, reproductive and lifestyle information including their age, parity, race/ethnicity, education level, marital status, annual household income, and prenatal smoking habits via a self-administered questionnaire. We analyzed race and ethnicity as White, Black or other (Other includes Asian or Pacific Islander (n= 41), Hispanic (n= 39), More than one race or ethnicity (n=23) and unclassified (n=1)) given the small number of participants who identified in the additional categories. The other category included Asian or Pacific Islander, Hispanic, more than one race or ethnicity, or unclassified. We calculated pre-pregnancy BMI (kg/m2) from self-reported pre-pregnancy weight and height and again from self-reported height and weight at time of the 18-year follow up. Lifetime gravidity and smoking history were self-reported at the midlife visit. Participants were also asked at baseline and at the midlife visit to report if they had ever been diagnosed with depression by a healthcare provider.
Statistical analysis:
We analyzed the distribution of participant’s characteristics and menopausal symptoms by history of infertility and compared them using the chi-square test for categorical variables, and the t-test or Wilcoxon rank sum for continuous variables.
In multivariable analyses, we used logistic or multinomial logistic regression models to determine the associations between history of infertility and the total MRS score divided at the median (≤7(ref) vs. >7) or categorized by severity (none or minimal (ref), mild, moderate, severe), and the individual items of the MRS categorized as any vs. no symptoms (ref). We constructed a series of models that accounted for potential confounders which we selected based on a priori knowledge, a literature review and considering their bivariate associations with the exposure/outcomes and their impact on the effect estimates. In model 1, we adjusted for maternal age at enrollment and race/ethnicity. In model 2, we further adjusted for enrollment marital status and household income. Adjusting for participant’s education and baseline smoking status did not influence the results as there was a less than 5% change in the OR estimates and the results did not change in direction, magnitude or strength; therefore, we did not include these variables in the final model.
We additionally adjusted models for pre-pregnancy BMI. There was again no change in the direction or strength of the result and given concern that BMI at enrollment may be a mediator of the relationship between infertility and menopausal symptoms this was ultimately not included in the final model. Additionally, participants were asked about history of depression at the time of enrollment. This was included as a covariate in a sensitivity analysis evaluating the odds of depressive symptoms.
We followed the same statistical approach to study the relationship between infertility and the severity of sleep depressive symptoms and irritability reported in the MRS (none (ref), mild-moderate and severe/very severe).
As a secondary outcome, we studied the relationship between history of infertility and time-to-natural menopause using a Cox proportional hazards model. For this analysis we included 678 participants who had complete exposure-event data. The exposure was history of infertility, and the event was the occurrence of natural menopause; age was the time scale for the model. We censored women who had not experienced menopause by the midlife visit or who had experienced secondary menopause (due to surgery, radiation/chemotherapy); they were censored at the age of the midlife visit or age at secondary menopause, accordingly. We adjusted this analysis for the same variables except marital status which did not meet the proportionality assumption.
We conducted multiple imputation with chained equations to generate 50 imputed data sets using an imputation model that included the exposures, outcomes, and covariates under study to reduce bias due to missing values for covariates23,24. The imputed data sets were combined and analyzed using MI ESTIMATE in Stata 16. We conducted all analyses in Stata 16 (StataCorp L.P., College Station, Texas). The variables with the greatest amount of missingness were history of depression at baseline (missing in 11% of participants), household income (6%) and cigarette use in the past 12 months (4%), the remaining covariates were missing in <1% of participants in the sample.
Results:
Approximately 1 in 3 (36.6%) participants reported ever experiencing infertility (Table 1). Participants with a history of infertility were older at the time of enrollment (mean (SD) age: 35.0 (3.7) years vs. 32.9 (3.7) years) than participants without history of infertility. Around 77% of participants with history of infertility were white, compared to 71% of those without infertility. Larger proportions of women with infertility were college graduates (85% vs 76.3%), married or cohabitating (98% vs 94.3%) and nulliparous at enrollment (50.8% vs 43.1%). There were similar rates of prior cigarette use in each group. There was a higher percentage of participants who reported history of depression diagnosis by a healthcare provider among the participants with infertility, compared to those without infertility history (19.3% vs 11.1%). Women with infertility history were older at the midlife visit (53.4 (3.8) years vs. 51.2 (3.7) years) than those without infertility. BMI, number of lifetime pregnancies, and the proportion of participants who had used cigarettes in the past year were similar among those with and without history of infertility. A greater proportion of women in the history of infertility group reported menopause at the midlife visit (61.8% vs 39.9%).
Table 1.
Baseline and Midlife (18-year follow up) characteristics of 695 women enrolled in longitudinal pregnancy cohort by lifetime history of infertilitya
| History of infertility N= 254 (36.6%) |
No infertility N=441 (63.4%) |
||||
|---|---|---|---|---|---|
| Baseline characteristics | mean | SD | mean | SD | b p value |
| Age at enrollment, years | 35.0 | 3.7 | 32.9 | 3.7 | <0.001 |
| Pre-pregnancy BMI, kg/m 2 | 24.8 | 5.3 | 24.4 | 5.0 | 0.30 |
| N | % | N | % | ||
| Race/ethnicity | 0.05 | ||||
| White | 196 | 77.2 | 311 | 70.8 | |
| Black | 20 | 7.9 | 62 | 14.1 | |
| Otherc | 38 | 15.0 | 66 | 15.0 | |
| College graduate | 216 | 85.0 | 335 | 76.3 | 0.01 |
| Married/cohabiting | 249 | 98.0 | 413 | 94.3 | 0.02 |
| Annual household income>$70,000 | 174 | 72.5 | 269 | 65.1 | 0.05 |
| History of depression | 43 | 19.3 | 44 | 11.1 | 0.01 |
| Ever smoker | 71 | 28.0 | 122 | 27.8 | 0.96 |
| Nulliparous d | 129 | 50.8 | 190 | 43.1 | 0.05 |
| Midlife characteristics | mean | SD | mean | SD | *p value |
| Age at midlife visit, years | 53.4 | 3.8 | 51.2 | 3.7 | <0.001 |
| BMI at midlife visit, kg/m2 | 28.2 | 6.5 | 27.7 | 6.6 | 0.30 |
| N | % | N | % | ||
| Lifetime number of pregnancies | 0.95 | ||||
| 1–3 | 162 | 63.8 | 281 | 64.0 | |
| ≥4 | 92 | 36.2 | 158 | 36.0 | |
| Cigarette use in past year | 6 | 2.5 | 12 | 2.8 | 0.79 |
| ≥12 months of amenorrhea | 155 | 61.8 | 174 | 39.9 | <0.001 |
The description was conducted in the non-multiple imputed dataset, N may not add up 695 due to missing values
P value: t-test for continuous ; chi-square test for categorical variables.
Other includes Asian or Pacific Islander (n= 41), Hispanic (n= 39), More than one race or ethnicity (n=23) and unclassified (n=1)
No pregnancy prior to index pregnancy
In bivariate analysis, there was a significantly higher median total MRS score among women with history of infertility (8 vs 7). There was no significant difference in the distribution of MRS score severity by infertility status (see table, Supplemental Digital Content 1). When we dichotomized the total MRS score by the median in the entire population, we observed a higher proportion of women with infertility above the median score compared to those without infertility. Upon comparison of the individual symptoms, there were significant differences in the report of any symptoms of sleep difficulty, sexual discomfort, depressive mood, irritability and joint discomfort between women with and without infertility history in unadjusted analyses. There were no differences in report of hot flashes, bladder discomfort, heart discomfort, anxiety, physical or mental exhaustion or vaginal dryness by history of infertility.
Our results from bivariate analysis, were then confirmed with logistic regression (Table 2). In the unadjusted model, women with a history of infertility had higher odds of a total MRS score above the median (OR 1.52, 95% CI 1.11–2.08) compared to women without a history of infertility. This relationship remained significant after adjustment. There was a trend toward higher odds of severe total MRS score as well with OR of 1.77 (95% CI 1.00–3.15) among those with infertility history. The effect estimate for this association increased in magnitude in the fully adjusted model 2 (OR 1.84, 95% CI 1.00–3.38).
Table 2.
Odds of elevated MRS score in midlife women with lifetime history of infertilitya
| UNADJUSTED | MODEL 1 | MODEL 2 | |
|---|---|---|---|
| Total MRS divided at the median | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| ≤ median (0–7) (n=384) | Ref. | Ref. | Ref. |
| > median (8–33) (n=307) | 1.52 (1.11 to 2.08) | 1.42 (1.02 to 1.96) | 1.45 (1.04 to 2.01) |
| Total MRS severity by category b | |||
| none or minimal (n=211) | Ref. | Ref. | Ref. |
| mild (n=221) | 1.10 (0.74 to 1.64) | 1.03 (0.68 to 1.56) | 1.04 (0.69 to 1.58) |
| moderate (n=197) | 1.27 (0.84 to 1.90) | 1.15 (0.75 to 1.76) | 1.18 (0.77 to 1.81) |
| severe (n=62) | 1.77 (1.00 to 3.15) | 1.78 (0.98 to 3.25) | 1.84 (1.00 to 3.38) |
Estimates obtained from logistic or multinomial regression models. Women without history of infertility are the reference group.
Categories: None or minimal severity, score 0–4; Mild severity, score 5–8; Moderate severity, score 9–16; Severe, score ≥17
Model 1: adjusted for maternal age at enrollment (years), race/ethnicity (White, black, other*). Model 2: model 1 + household income>$70,000 (yes/no) and married/cohabiting (yes/no).
Other includes Asian or Pacific Islander (n= 41), Hispanic (n= 39), More than one race or ethnicity (n=23) and unclassified (n=1)
In the analysis of individual symptoms (Figure 1), women with history of infertility had greater odds of reporting symptoms of sleep problems (OR 1.42, 95% CI 1.00–2.00), depressive mood (OR 1.45, 95% CI 1.07–1.98), irritability (OR 1.40, 95%CI 1.02–1.91), and sexual problems (OR 1.45, 95% CI 1.06–1.98). In the fully adjusted model, the effect size of the association between history of infertility and sleep problems remained significant (OR 1.44, 95% CI 1.00–2.06). The associations with depressive mood and irritability both increased in magnitude in the fully adjusted model and remained significant. Women with a history of infertility had 1.57 times the odds (95% CI 1.13–2.17) of reporting depressive mood symptoms and 1.57 times the odds of reporting irritability (95%CI 1.13–2.19) compared to women without a history of infertility. The association with sexual problems was not significant following adjustment (OR 1.32, 95%CI 0.95–1.83).
Figure 1.
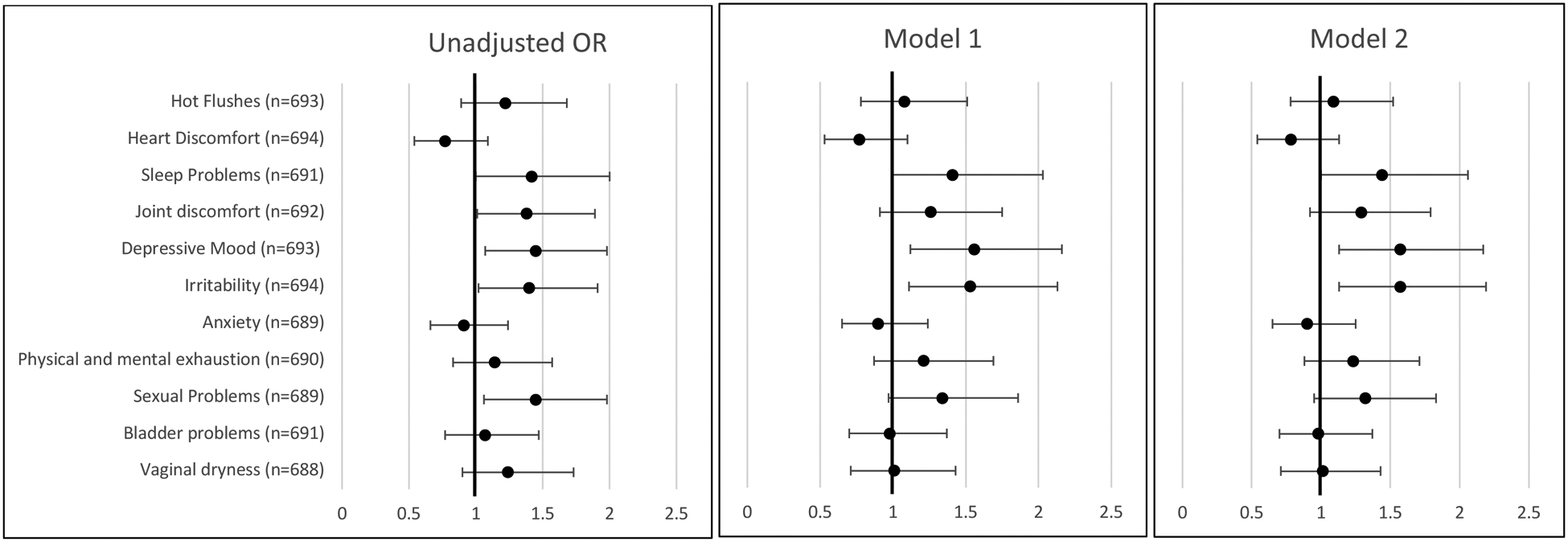
Unadjusted and adjusted odds ratios for report of any menopausal symptoms (vs. none) in midlife women by lifetime history of infertility. Estimates obtained from logistic regression models. Women without history of infertility are the reference group.
Model 1: adjusted for maternal age at enrollment (years), race/ethnicity (White, black, other*). Model 2: model 1 + household income>$70,000 (yes/no) and married/cohabiting (yes/no).
* Other includes Asian or Pacific Islander (n= 41), Hispanic (n= 39), More than one race or ethnicity (n=23) and unclassified (n=1)
At enrollment participants were asked to report if they had ever been diagnosed with depression by a healthcare provider. When adjusting for this, the relationship of history of infertility and depressive symptoms remained though the magnitude of the effect was slightly attenuated (OR 1.45 95% CI 1.03–2.04).
We then further examined the relationship between history of infertility and the severity of depressive symptoms, irritability and sleep problems (Table 3). Individuals with a history of infertility had greater odds of reporting severe/very severe sleep problems (OR 1.91, 95% CI 1.09–3.36) and mild to moderate depressive mood symptoms (OR 1.57, CI 1.13–2.19) than those without infertility history in fully adjusted models. When performing the same analysis for irritability, there were only 15 participants who reported severe symptoms of irritability. The limited sample size did not allow for appropriate model specifications.
Table 3.
Odds of sleep problems and depressive mood assessed by the MRS in midlife women by lifetime history of infertilitya
| Symptom | UNADJUSTED | MODEL 1 | MODEL 2 |
| Sleep Problems severity | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Ref. | Ref. | Ref. | |
| 1.36 (0.96 to 1.94) | 1.34 (0.92 to 1.94) | 1.36 (0.94 to 1.97) | |
| 1.71 (1.00 to 2.91) | 1.87 (1.07 to 3.26) | 1.91 (1.09 to 3.36) | |
| Ref. | Ref. | Ref. | |
| 1.47 (1.07 to 2.02) | 1.57 (1.13 to 2.19) | 1.57 (1.13 to 2.19) | |
| 1.21 (0.46 to 3.14) | 1.33 (0.49 to 3.62) | 1.39 (0.50 to 3.90) | |
Other includes Asian or Pacific Islander (n= 41), Hispanic (n= 39), More than one race or ethnicity (n=23) and unclassified (n=1)
Estimates obtained from multinomial regression models. Women without history of infertility are the reference group.
Model 1: adjusted for maternal age at enrollment (years), race/ethnicity (White, black, other*). Model 2: model 1 + household income>$70,000 (yes/no) and married/cohabiting (yes/no).
Given that we assessed recalled exposure and outcomes at the same timepoint, reverse causality is a possibility. To minimize this concern, we conducted an analysis restricted to individuals who experienced infertility at the time of enrollment (n=146) to evaluate the potential for reverse causality and the impact of infertility earlier rather than later in reproductive life. The adjusted OR for depressive mood on the MRS among these participants (vs. those without infertility) was 1.6 (1.10, 2.34). When further adjusted for history of depression at baseline the OR is attenuated, OR 1.44 (0.97, 2.15). The estimate was consistent in direction and magnitude with the original analysis, though strength of the estimate is attenuated with slightly wider confidence intervals. However, depression at baseline may at least in part be a mediator, since the experience of infertility may have contributed to the symptoms of depression.
We found no difference in time (years) to onset of natural menopause between women with and without a history of infertility (adjusted hazard ratio [95% CI]: 1.17 [0.90–1.52]).
Discussion:
In this cohort of parous midlife women followed longitudinally over 18 years, we found that women with a history of infertility had greater odds of reporting menopausal symptoms, defined as a score above the median on a validated menopause scale, than women without a history of infertility. Women with history of infertility also tended to have more severe menopausal symptoms. When individual symptoms were evaluated, the signal for the overall scale appeared to be explained by an approximately 50% higher odds of both depressive symptoms and irritability and nearly 2-fold higher odds of severe sleep problems. Although these findings need to be confirmed in other studies, they nevertheless suggest that infertility is a risk factor for greater severity of some menopausal symptoms, particularly depressive symptoms, and sleep problems.
Prior work has demonstrated that about 30–60% of women undergoing evaluation and treatment of infertility screen positive for depression15,25–29. However, association of prior infertility with the experience of depressive symptoms in midlife has not, to our knowledge, been previously reported. This represents an important point for counseling of infertility patients on expectations, beyond their immediate family building goals. It also suggests that history of infertility should be considered as a flag for additional screening for depression and sleep disturbances in midlife women.
While there is an extensive literature that women currently experiencing infertility are more likely to have symptoms of depression, it is less clear whether the converse is also the case, that is whether depressive symptoms result in infertility13,15. Most of the literature on this topic has not looked at depression prior to pregnancy attempts began, but rather focused on whether fertility treatment outcomes differ among those with infertility who are or are not experiencing depression28,30,31. Thus, it is hard to generalize from this literature to our study population, in which most did not have infertility and those who did may or may not have received treatment.
Also, in our study population it is somewhat hard to tease out directionality because even if we limit our analysis to baseline measures, the fertility challenges may have predated the depression diagnosis or symptoms – so in that case, baseline depression would be more of a mediator rather than a confounder or upstream variable. There was a higher rate of history of depression at baseline in the group with any history of infertility. To address this, we ran an additional model adjusted for history of depression at enrollment, and the association between infertility and depressive symptoms at midlife persisted, which does somewhat address this concern.
The lack of difference in report of hot flashes is consistent with prior work by Nelson et al12. However, they also reported an increased rate of vaginal dryness and reduced libido in menopause among women with a history of infertility. We did not observe associations of history of infertility with other menopausal symptoms including bladder problems, vaginal dryness, and joint discomfort. The association with sexual problems was present in unadjusted analysis, but this association was attenuated when accounting for covariates. Nelson et al used a different measure to assess menopausal symptoms including assessment of libido in more specific detail and therefore may have been able to better characterize the components of sexual dysfunction than the single item included on the MRS.
Hess et al. observed reduced reports of hot flashes and vaginal dryness among nulliparous and nulligravid menopausal women compared to parous women32. This finding appears to be in an opposite direction to ours, although history of infertility and intention for childbearing was not known in their population. Our cohort did not include any nulliparous women, which limits the generalizability and comparison to the work by Hess et al. However, nulliparous women with history of infertility who attempted and never achieved a live birth may have a different experience of menopause and it would be important to explore in future studies.
Most estimates of the frequency of infertility are estimates of prevalence rather than estimates of lifetime risk of infertility, and these estimates will undercount secondary infertility in most cases. This makes the 36.6% rate of infertility in our population seem higher than would be anticipated. However, it is not far off from estimates of lifetime risk of 12-month infertility from other prospective cohorts in the US33. A study using Nurses Health Study data, found that 27.6% of participants reported a history of infertility. Additionally, most estimates of infertility are for 12-month infertility and the use of 6-months if age ≥35y will increase the frequency of infertility relative to previous estimates.
This analysis is associational in nature and not able to make conclusions regarding causality. However, if confirmed in future causal analysis, the mechanism by which a history of infertility would lead to depressive symptoms and more severe sleep disturbances remains unexplained. We propose two potential hypotheses. One possibility is a physiologic explanation resulting from dysfunction of the hypothalamic pituitary ovarian axis. However, if it were physiologic and related to ovarian reserve and production of ovarian hormones including estrogen, menopausal symptoms such as vaginal dryness, hot flashes or sexual dysfunction would also be likely more prevalent. That was not observed in our cohort. Alternatively, the experience of infertility, even in the past, may be a life stressor which contributes to more severe menopausal symptoms. Negatively perceived life events are associated with greater severity of menopausal symptoms as demonstrated by Pimenta et al18. They evaluated the influence of recent life events, in the preceding month, and the influence which it had on menopausal symptoms. While the experience of infertility is generally more remote from the onset of menopause than a month, the influence of this negative life event may be similar. The MRS is a screening tool for depressive symptoms but is not used to diagnose depression therefore future work should evaluate whether women with history of infertility have greater rates of depression in midlife.
This analysis has several limitations to consider. The definition of the exposure was based partially on recall which allows for recall bias and exposure misclassification. Menopause is known to be a period of transition and symptoms can vary over months and years34,35. We assessed menopausal symptoms at a single time point and the participants were at a variety of menopausal transition states, therefore they may not yet have experienced their most significant symptoms or may have passed the time of their greatest symptoms. Further investigation of this association accounting for stage of menopause would help to further characterize this relationship and is planned as we continue to follow this cohort. Another limitation is the lack of information on the cause of infertility. Individuals with diminished ovarian reserve and PCOS both may have infertility but ultimately different experiences of menopausal symptoms. Subsequent investigations should attempt to characterize menopausal symptoms by infertility diagnosis in order to provide more specific counseling to patients. Based on the design of this prospective cohort, with enrollment during a pregnancy and follow up of only those with a live birth, our population was composed of only parous women. This limits the generalizability of the study to parous women. Additionally, the participants were predominantly white, well-educated, married, and in a single geographic region which limits the generalizability of the findings. Future work should investigate whether menopausal symptoms may be different among women with infertility who do not achieve pregnancy or live birth. Additionally, this was an associational analysis and therefore cannot determine causality or mechanism of the association.
Strengths of this study include the use of prospectively collected data from a well-characterized cohort of women over almost two decades and the use of validated measures to assess menopausal symptoms.
Conclusion:
Our findings suggest that women with a history of infertility are more likely to experience menopausal symptoms during midlife than women who do not have a history of infertility. The individual symptoms that were more common among women with history of infertility included depressive mood, irritability, and greater severity of sleep problems. History of infertility was not associated with age at menopause or any other menopausal symptoms. These findings suggest history of infertility in a woman’s lifetime may be considered as a flag for increased screening for depressive symptoms in midlife.
Supplementary Material
List of Supplemental Digital Content:
Supplemental Digital Content 1: Table of Menopause Rating Scale (MRS) results reported as total score and by symptom for 695 midlife women with and without a history of infertility .docx
Sources of Funding:
This work was supported by grants from the US NIH (R01 HD096032, R01 HD034568, and 1U54 AG062322-01). DCSC is supported by the National Research Service Award T32 HD 104612.
Financial Disclosures/Conflicts of interest:
VWF, DCS, SRS, EO have no relevant disclosures. JLS receives funding from Harvard Pilgrim Health Care for consulting related to research. JEC has no disclosures relevant to this manuscript, however does receive grants from the Food and Drug Administration and National Institutes of Health unrelated to this work. Additionally, IN THE LAST 5 YEARS, JEC HAS RECEIVED royalties from Harvard Health Publications and payment for lectures and/or support for attending conferences from Johns Hopkins University, NIH, Northwestern, Carolinas Medical Center, ASRM, British Dietetic Association, PCRS, Tufts University, ESHRE, Institut Hospital del Mar d’Investigacion Mediques, Japanese Society for Reproductive Medicine, Lund University Faculty of Medicine and Medical University of Vienna
Footnotes
Data from this manuscript was presented as an abstract at the American Society of Reproductive Medicine meeting in October 2021.
REFERENCES
- 1.Thurston RC, Joffe H. Vasomotor Symptoms and Menopause: Findings from the Study of Women’s Health across the Nation. Obstet Gynecol Clin North Am. 2011;38(3):489–501. doi: 10.1016/j.ogc.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woods NF, Mitchell ES. Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118 Suppl 12B:14–24. doi: 10.1016/j.amjmed.2005.09.031 [DOI] [PubMed] [Google Scholar]
- 3.Santoro N, Sutton-Tyrrell K. The SWAN Song: Study of Women’s Health Across the Nation’s Recurring Themes. Obstet Gynecol Clin North Am. 2011;38(3):417–423. doi: 10.1016/j.ogc.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyland A, Piazza K, Hovey KM, et al. Associations between lifetime tobacco exposure with infertility and age at natural menopause: the Women’s Health Initiative Observational Study. Tob Control. 2016;25(6):706–714. doi: 10.1136/tobaccocontrol-2015-052510 [DOI] [PubMed] [Google Scholar]
- 5.Im EO, Chang SJ, Chee E, Chee W. The relationships of multiple factors to menopausal symptoms in different racial/ethnic groups of midlife women: The structural equation modeling. Women Health. 2019;59(2):196–212. doi: 10.1080/03630242.2018.1450321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra GD, Chung HF, Cano A, et al. EMAS position statement: Predictors of premature and early natural menopause. Maturitas. 2019;123:82–88. doi: 10.1016/j.maturitas.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 7.Mishra GD, Pandeya N, Dobson AJ, et al. Early menarche, nulliparity and the risk for premature and early natural menopause. Hum Reprod Oxf Engl. 2017;32(3):679–686. doi: 10.1093/humrep/dew350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Boer EJ. Increased risk of early menopausal transition and natural menopause after poor response at first IVF treatment. Hum Reprod. 2003;18(7):1544–1552. doi: 10.1093/humrep/deg278 [DOI] [PubMed] [Google Scholar]
- 9.de Boer EJ, Tonkelaar I den, Burger CW, van Leeuwen FE. Are cause of subfertility and in vitro fertilization treatment risk factors for an earlier start of menopause? Menopause. 2005;12(5):578–588. doi: 10.1097/01.gme.0000177316.78263.ff [DOI] [PubMed] [Google Scholar]
- 10.Mínguez-Alarcón L, Rifas-Shiman SL, Soria-Contreras DC, et al. Self-reported menstrual cycle length during reproductive years in relation to menopausal symptoms at midlife in Project Viva. Menopause. 2022;29(10):1130–1136. doi: 10.1097/GME.0000000000002042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasui T, Hayashi K, Mizunuma H, et al. Association of endometriosis-related infertility with age at menopause. Maturitas. 2011;69(3):279–283. doi: 10.1016/j.maturitas.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 12.Nelson DB, Sammel MD, Patterson F, Lin H, Gracia CR, Freeman EW. Effects of reproductive history on symptoms of menopause: a brief report. Menopause. 2011;18(10):1143–1148. doi: 10.1097/gme.0b013e318214d69d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol. 1993;14 Suppl:45–52. [PubMed] [Google Scholar]
- 14.Cousineau TM, Domar AD. Psychological impact of infertility. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):293–308. doi: 10.1016/j.bpobgyn.2006.12.003 [DOI] [PubMed] [Google Scholar]
- 15.Rooney KL, Domar AD. The relationship between stress and infertility. Dialogues Clin Neurosci. 2018;20(1):41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binfa L, Castelo-Branco C, Blümel JE, et al. Influence of psycho-social factors on climacteric symptoms. Maturitas. 2004;48(4):425–431. doi: 10.1016/j.maturitas.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 17.Woods NF, Mitchell ES. Pathways to depressed mood for midlife women: observations from the Seattle Midlife Women’s Health Study. Res Nurs Health. 1997;20(2):119–129. doi: [DOI] [PubMed] [Google Scholar]
- 18.Pimenta F, Leal I, Maroco J, Ramos C. Menopausal symptoms: Do life events predict severity of symptoms in peri- and post-menopause? Maturitas. 2012;72(4):324–331. doi: 10.1016/j.maturitas.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 19.Oken E, Baccarelli AA, Gold DR, et al. Cohort Profile: Project Viva. Int J Epidemiol. 2015;44(1):37–48. doi: 10.1093/ije/dyu008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinemann K, Ruebig A, Potthoff P, et al. The Menopause Rating Scale (MRS) scale: A methodological review. Health Qual Life Outcomes. 2004;2(1):45. doi: 10.1186/1477-7525-2-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soria-Contreras DC, Perng W, Rifas-Shiman SL, Hivert MF, Oken E, Chavarro JE. History of infertility and pregnancy outcomes in Project Viva: a prospective study. BMC Pregnancy Childbirth. 2022;22(1):549. doi: 10.1186/s12884-022-04885-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faubion SS, King A, Kattah AG, et al. Hypertensive disorders of pregnancy and menopausal symptoms: a cross-sectional study from the data registry on experiences of aging, menopause, and sexuality. Menopause. 2021;28(1):25–31. doi: 10.1097/GME.0000000000001638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. doi: 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aris IM, Perng W, Dabelea D, et al. Analysis of Early-Life Growth and Age at Pubertal Onset in US Children. JAMA Netw Open. 2022;5(2):e2146873. doi: 10.1001/jamanetworkopen.2021.46873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massarotti C, Gentile G, Ferreccio C, Scaruffi P, Remorgida V, Anserini P. Impact of infertility and infertility treatments on quality of life and levels of anxiety and depression in women undergoing in vitro fertilization. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2019;35(6):485–489. doi: 10.1080/09513590.2018.1540575 [DOI] [PubMed] [Google Scholar]
- 26.Lakatos E, Szigeti JF, Ujma PP, Sexty R, Balog P. Anxiety and depression among infertile women: a cross-sectional survey from Hungary. BMC Womens Health. 2017;17(1):48. doi: 10.1186/s12905-017-0410-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen TH, Chang SP, Tsai CF, Juang KD. Prevalence of depressive and anxiety disorders in an assisted reproductive technique clinic. Hum Reprod Oxf Engl. 2004;19(10):2313–2318. doi: 10.1093/humrep/deh414 [DOI] [PubMed] [Google Scholar]
- 28.Holley SR, Pasch LA, Bleil ME, Gregorich S, Katz PK, Adler NE. Prevalence and predictors of major depressive disorder for fertility treatment patients and their partners. Fertil Steril. 2015;103(5):1332–1339. doi: 10.1016/j.fertnstert.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volgsten H, Skoog Svanberg A, Ekselius L, Lundkvist O, Sundström Poromaa I. Prevalence of psychiatric disorders in infertile women and men undergoing in vitro fertilization treatment. Hum Reprod Oxf Engl. 2008;23(9):2056–2063. doi: 10.1093/humrep/den154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans-Hoeker EA, Eisenberg E, Diamond MP, et al. Major depression, antidepressant use, and male and female fertility. Fertil Steril. 2018;109(5):879–887. doi: 10.1016/j.fertnstert.2018.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holley SR. What is a person with depression who wants to have a baby to do? Fertil Steril. 2018;109(5):797–798. doi: 10.1016/j.fertnstert.2018.02.125 [DOI] [PubMed] [Google Scholar]
- 32.Hess R, Olshansky E, Ness R, et al. Pregnancy and birth history influence women’s experience of menopause. Menopause N Y N. 2008;15(3):435–441. doi: 10.1097/gme.0b013e3181598301 [DOI] [PubMed] [Google Scholar]
- 33.Wang YX, Farland LV, Wang S, et al. Association of infertility with premature mortality among US women: Prospective cohort study. Lancet Reg Health Am. 2022;7:100122. doi: 10.1016/j.lana.2021.100122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soules MR, Sherman S, Parrott E, et al. Executive summary: stages of reproductive aging workshop (STRAW). Fertil Steril. 2001;76(5):874–878. doi: 10.1016/S0015-0282(01)02909-0 [DOI] [PubMed] [Google Scholar]
- 35.El Khoudary SR, Greendale G, Crawford SL, et al. The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health Across the Nation (SWAN). Menopause N Y N. 2019;26(10):1213–1227. doi: 10.1097/GME.0000000000001424 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of Supplemental Digital Content:
Supplemental Digital Content 1: Table of Menopause Rating Scale (MRS) results reported as total score and by symptom for 695 midlife women with and without a history of infertility .docx


