Abstract
The discrimination of cues in the environment that signal danger (“fear cue”) is important for survival but depends critically on the discernment of such cues from ones that pose no threat (“safety cues”). In rodents, we previously demonstrated the underlying neurobiological mechanisms that support fear versus safety discrimination and documented that these mechanisms extend to the discrimination of reward as well. While learning about reward is equally important for survival, it remains an under-studied area of research, particularly in human studies of conditional discrimination. In the present study, we translated our rodent task of fear reward and neutral discrimination (fear, reward, and neutral discrimination [FRND]) for use in humans. Undergraduate students (N = 53) completed the FRND while electrodermal activity was recorded. Skin conductance response (SCR) amplitude, a marker of arousal response, was derived for fear, reward, and neutral cues that signaled no outcome; critical trials assessed conditional discrimination using combined fear + neutral and reward + neutral cues. Participants provided likeability ratings for each cue type. Results demonstrated that participants rated reward cues the best, fear cues the worst, and neutral cues in between, while SCR amplitude was largest for fear and reward cues and lowest for neutral cues. SCR amplitudes were reduced for fear + neutral (compared to fear) and reward + neutral cues (compared to reward). Results demonstrate that the FRND is a useful paradigm for the assessment of psychological and physiological discrimination of fear and reward. Implications and directions for future work are discussed.
Keywords: arousal, conditioning, emotion, fear, reward, skin conductance
1 |. INTRODUCTION
The ability to accurately identify cues in the environment that convey threat is an evolutionarily adaptive mechanism for survival, as it puts into motion complex neurobiological, endocrine, and behavioral responses underlying an appropriate response. Among these is the activation of the hypothalamic–pituitary–adrenal (HPA) axis, the biological stress response that mobilizes energy, regulates heart rate, and facilitates muscle activity for response to threats, such as fleeing and fighting (Smith & Vale, 2006). A suite of emotional-motivational and cognitive processes is also triggered, including selective attention toward the threat stimulus, the emotional response of fear, and the consolidation of memory necessary for stamping down the significance of an acute threat experience to aid future survival (Hamm, 2020). These biological responses are metabolically expensive, such that initiating them in the absence of threat – that is, during situations that are safe – is cost prohibitive. The long-term implications of unnecessary activation of such biological systems are dire: in particular, persistent activation of the HPA axis is associated with diabetes, stroke, cardiovascular disease (Rosmond & Bjorntorp, 2000), and mortality (Chrousos, 2009), as over-engagement of this system contributes to its “wear and tear.” Thus, while accurate detection of threat is important for avoiding danger, it is equally important to accurately discriminate threat from safety in order to preserve long-term health.
The conditional discrimination of threat from safety is a form of Pavlovian learning (Christianson et al., 2012; Krueger & Sangha, 2021b). During fear conditioning, an initially neutral conditioned stimulus (CS) (e.g., colored shape) is repeatedly paired with an unconditioned stimulus (US) (e.g., an aversive noise) such that a conditioned fear response develops to the CS (CS+). Responses to the CS+ are contrasted in comparison to a neutral stimulus which is never paired with the US, referred to as the CS−. The process of fear conditioning relies on the ability to learn which cues in the environment signal threat (CS+) as opposed to safety (CS−). In conditional discrimination, safety learning is assessed using paired cue types, whereby CS+ and CS− are co-presented (Rescorla, 1969). A decrease in the fear response to paired cues (CS+/CS−) compared to the presentation of a stand-alone fear cue (CS+) reflects intact discriminatory evaluation of cue types, while changes in response to the paired CS+/CS− cues are reflective of the influence of the second cue (e.g., CS−). Perceptions of the CS− cues, in that they must be perceived as “non-threatening” even when paired with the CS+, may be responsible for changes in arousal during this process. Absence of this perception or failure to initially learn the CS− may be particularly influential in impairing conditional discrimination (Jovanovic et al., 2006).
In humans, tasks of conditional discrimination have predominantly used fear-potentiated startle (FPS) as a mechanism by which to study response to conditioned fear and safety. FPS uses an acoustic startle probe that elicits an uncontrollable blink response, measured by electromyogram (EMG) of the orbicularis oculi contraction (Grillon & Baas, 2003). Response to the startle probe is measured on its own, and decline in the response to CS− indicates changes in fear based on expectancy (Jovanovic et al., 2006). Conditional discrimination is evidenced by the fact that healthy individuals decrease blink response to combined CS+/CS− cues in comparison to CS+ stand-alone cues (Jovanovic et al., 2005, 2006). Notably, the response to the combined cue is not simply evoked by novelty as the startle response to CS+/CS− combined cues is less than the response to a combined CS+ and novel cue (Jovanovic et al., 2005). The importance of safety discrimination is further evidenced by the fact that contingency awareness is related to fear responding: individuals who cannot self-report which cue is safe (e.g., CS−) exhibit greater FPS (Jovanovic et al., 2006).
Other human paradigms of conditional discrimination have used skin conductance response (SCR), while SCR has long been used as a common index of acquired threat in fear-conditioning studies using the comparison of CS+ > CS− (LaBar et al., 1995; Ohman & Soares, 1993; Orr & Roth, 2000; Phelps et al., 2004). SCR is mediated by the sympathetic nervous system and reflects a change in sweat response measured on the skin surface, commonly at the hand or fingers (Lang et al., 1998). Whereas FPS is measured commonly in the context of the fear–response, SCR measures arousal to both negative and positive stimuli (Bradley et al., 2001). Only a few studies with small sample sizes (n < 30) have examined changes in SCR during conditional discrimination. Meyer et al. (2019) recently demonstrated that SCR decreases in response to combined CS+/CS− cues compared to CS+, while CS− exhibits the lowest SCR (Meyer et al., 2019). An earlier study by Jovanovic, Sakoman, et al. (2013) found differential SCR in response to CS+ versus CS−, but no reduction in SCR to the combined CS+/CS−. Discrepant results indicate that more research is needed on the physiological response during conditional discrimination using SCR.
This is particularly true as the discrimination of threat from safety is an important area of study for biological mechanisms of psychopathology. Generalized hyperarousal as indexed by SCR occurs in individuals with phobic disorders in response to both negative and neutral conditioned stimuli and has been linked to symptoms of avoidance (e.g., avoiding stimuli or contexts) (Hamm, 2020). Trauma-exposed individuals exhibit a deficit in safety learning in particular, as evidenced by identical startle responses to CS+ and CS− cues compared to controls (Grillon & Morgan, 1999), while trauma survivors with greater posttraumatic stress disorder (PTSD) symptoms – compared to trauma-exposed peers with comparatively less symptom severity – exhibit less differential responding between CS+ and CS+/CS− combination cues (Jovanovic et al., 2009). Such responses are indicative of a generalized pattern of responding, made apparent only when comparing responses to conditioned threat and conditioned safety. Further, findings underscore the importance of better understanding conditional discrimination as it relates to a broad array of disorders, as well as studying responses to threat and neutral conditioned cues independently.
One area of investigation that is lacking is the study of conditioned discrimination of reward, as nearly all prior work in humans has been conducted in the context of threat. In rodents, the process of conditioned discrimination translates to reward, whereby the neural mechanisms of safety and reward learning are shared (Ng & Sangha, 2023; Sangha et al., 2013). In our prior work, we found evidence for single cells in the basal amygdala (BA) and infralimbic cortex (IL) responding selectively to safety cues (either CS− alone or CS+/CS− cues) but not fear cues, suggesting that the presence of “safety cells” code for safety discrimination (Sangha et al., 2013). In addition, distinct groups of cells in the BA and IL also respond to both safety and reward signals, suggesting that safety and reward may share underlying neurobiological mechanisms (Ng & Sangha, 2023; Sangha et al., 2013). Further, in rodents, reward conditioning during adolescence accelerated the conditional discrimination of safety from threat in adulthood, while threat conditioning during adolescence hindered discrimination of safety (Müller et al., 2018). We have also shown in rodents a role for dopamine signaling within the amygdala, in that altering D1 receptor activity with either an agonist or antagonist directly into the BA, prevented the reduction in fear normally seen during the CS+/CS− (Ng et al., 2018). Rodent work provides evidence that discrimination of safety is intertwined with discrimination of reward. Nevertheless, there is no translational paradigm presently available in humans to simultaneously study conditional discrimination of threat, safety, and reward.
In the present study, we translated a task previously validated in rodents – the fear, reward, and neutral discrimination (FRND) task – to humans (Greiner et al., 2019; Müller et al., 2018; Ng et al., 2018; Ng & Sangha, 2023; Sangha et al., 2013, 2014). While prior human studies have examined physiological changes associated with conditional discrimination, we know of no study to date that has examined this process through the dual lenses of fear and reward in the same study design. SCR was used as a marker of physiological differentiation (Lang et al., 1998), a measure of salience-mediated arousal that is non-specific to fear (Bradley et al., 2001). Psychological self-report of likeability of cues provided conditional awareness of cue meaning. Further, given the use of a fear probe, we assessed the relationship between conditional discrimination of cues with task-related changes in anxiety through administration of the State and Trait Inventory (STAI; Spielberger, 1988) for the collection of self-reported state anxiety.
2 |. METHOD
Undergraduate students were recruited for completion of the fear, reward, and neutral discrimination (FRND) task in the lab, during which time continuous electrodermal activity (EDA) was collected for quantification of SCR. Potential participants responded to advertisements and were screened over the phone for exclusion criteria. To qualify, participants were between the ages of 18 and 55, able to read and understand English, able to sit for 1-hour, and free of neurological conditions, bipolar disorder, and schizophrenia (assessed via self-report) as these disorders are associated with known neurophysiological abnormalities beyond the interest of this study (Campo et al., 2000). Eligible participants were invited to the lab and completed written informed consent, informed of the general purpose of the study, and briefed on any potential risks and benefits. Following the FRND, all participants completed the Mini International Neuropsychiatric Interview (MINI) with a trained MA-level researcher for quantification of existing psychopathology. As validation of the FRND task in healthy individuals was the primary goal of this study, the presence of psychopathology was controlled for in all analyses. All research procedures were approved by the local Institutional Review Board and all participants received extra credit for their psychology courses for completion of this study. In addition, all participants received $8 cash after study completion based on number of reward cues during the FRND computer task (see Methods below).
2.1 |. Fear, reward, and neutral discrimination (FRND) task
Participants were seated comfortably in a chair approximately 60 cm in front of a computer screen for the duration of the task. Participants were told that they would be viewing various shapes on a computer screen, some of which may be accompanied by sounds. Further, participants were told that some of the shapes may predict a bad outcome, some of the shapes may predict a good outcome, and some of the shapes may result in no outcome.
During the task, participants were shown a series of colored geometric shapes (e.g., yellow circle and blue triangle) presented on a black background. Each shape served as a unique cue type (fear vs. reward). Fear cues were co-presented with an 80-dB white noise burst delivered through headphones (unconditioned stimulus [US]) and reward cues were co-presented with the text $0.25, indicating that the participant would receive $0.25. On some trials, each cue was surrounded by a colored line, indicating the absence of outcome delivery on those trials for creation of combination cue types (e.g., fear + neutral and reward + neutral). Response to the colored line was also measured on its own (e.g., neutral trials). This produced a total of five cue conditions: fear, reward, neutral, fear + neutral, and reward + neutral. Figure 1 shows a sample of six trials of the task.
FIGURE 1.
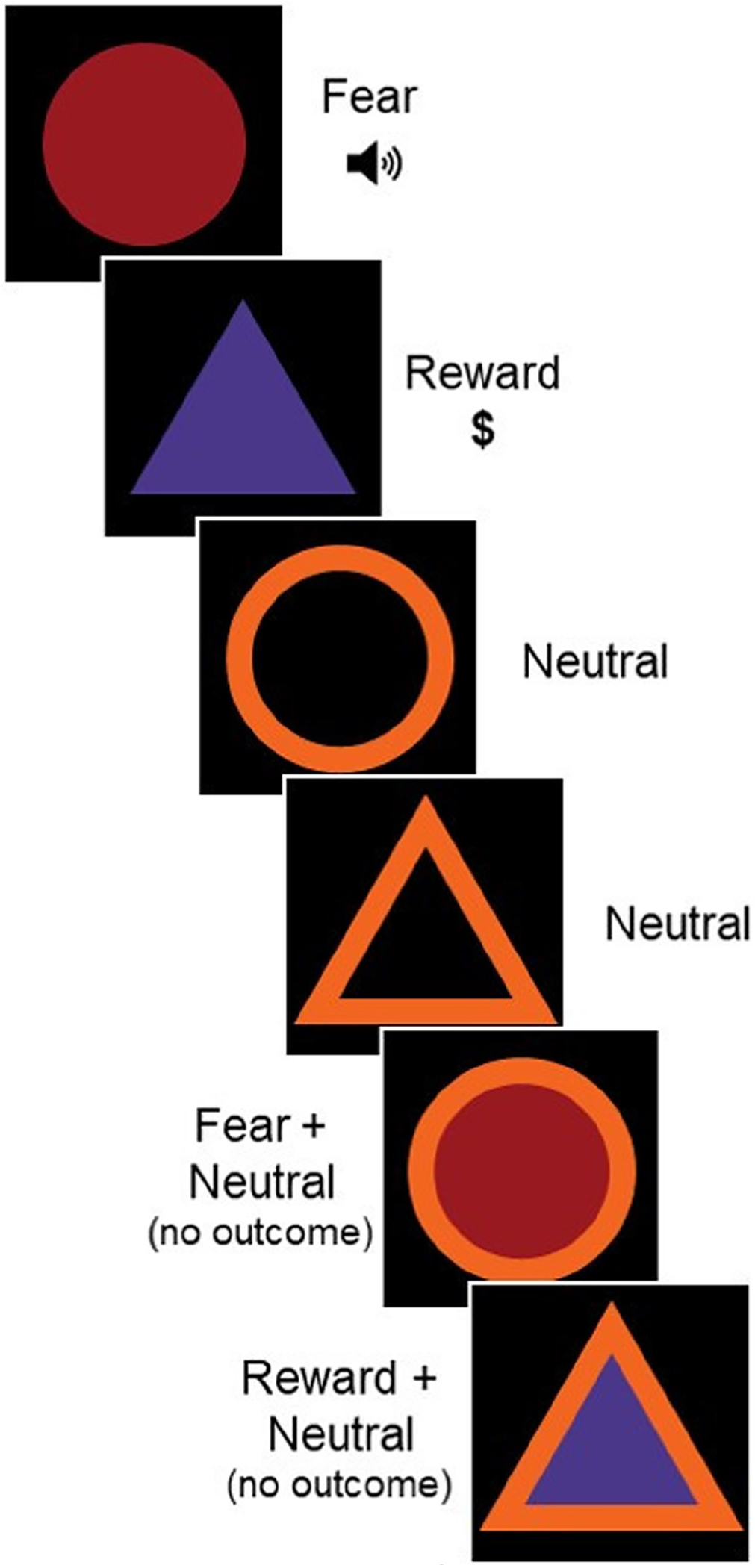
Fear and reward discrimination (FRND) Task. Fear cues were co-p resented with white noise burst (unconditioned stimulus [US]) and reward cues were co-presented with text $0.25. Neutral cues signaled no outcome and were presented independently, as well as in combination with fear cues and reward.
Cue assignment was counter-balanced across participants, such that four versions of the task were used, shuffling cue assignment. In this way, trial order was randomized across participants to balance stimulus sequence and avoid habituation or sensitization effects (Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures, 2012). The geometric shape associated with each outcome was different for each of the four versions and the fear and reward cues differed both in color and geometric shape (e.g., yellow circle vs. blue triangle). In an additional two versions of the task, cues differed only in color (e.g., yellow circle and blue circle). Implementation of different versions was done in an exploratory manner to determine if results differed by number of unique stimulus features. In all versions, eight trials of each of the five cue conditions were presented, for a total of 40 trials (1 trial = 1 cue presentation) and a total task length of 10 minutes. In versions where the fear and reward cues differed in both color and shape, this resulted in the use of two different neutral cues (e.g., circle outline and triangle outline), presented for four trials each to ensure eight neutral cues were consistently used across versions, for a total of 40 trials.
For each trial, the cue was presented first on-screen for 3–5 s, at which point the US was co-presented for fear and reward cues for a duration of 2 s for a total trial length of 5–7 s. For neutral, fear + neutral, and reward + neutral trials, cues remained on-screen for the additional 2 s, again resulting in a total trial length of 5–7 s. Trials were separated by a jittered inter-trial interval of 8–10 s where the US ceased (on fear and reward trials) and the cue was replaced by a generic white cross-hair on a black background.
2.2 |. Likeability ratings
Immediately after the FRND Task, participants were asked to rate their likeability of each cue using the computer and a numerical keypad. Participants were again shown each cue in a random order with the accompanying prompt: “On a scale of 1 through 9, how would you describe this image?”. Likert ratings were anchored in the following manner: 1 = very bad, 5 = neutral, and 9 = very good. Participants were also asked to rate the fear US (white noise burst) immediately following on an identical Likert scale using the prompt: “How would you describe the noise you heard after certain images?”.
2.3 |. Psychophysiological recording and preprocessing
During completion of the FRND task, continuous electrodermal activity (EDA) was collected using the Biopac MP160 wireless EDA system. Prior to EDA setup, participants were asked to wash their hands with soap; following which two 55 mm self-adhesive Ag/AgCl electrodes were placed on the index and middle fingers of the non-dominant hand. EDA was collected at a rate of 2000 Hz. From EDA, skin conductance responses (SCRs) were calculated using standard published guidelines for processing and extracting SCRs from underlying skin conductance (Braithwaite et al., 2015) with respect to cue type: fear, reward, neutral, fear + neutral, reward + neutral, and trial number (1–8). For SCR calculation, EDA was high-pass filtered (0.05 Hz) and tonic and phasic signals separated. SCRs were then measured as the base-to-peak amplitude difference and calculated using a varied scoring window applied individually for each cue given the jittered nature of cue presentation (3–5 s). The lower-bound threshold for scoring was 1 s after cue onset and the upper-bound threshold for scoring was 3 s after cue onset for cues on-screen for 3 s, 4 s after onset for cues on-screen for 4 s, and 5 s after onset for cues on-screen for 5 s. This approach was maximally conservative to ensure SCR amplitudes were not contaminated by response to the USs. Published norms recommend using a minimum amplitude of 0.01–0.05 μS for SCR extraction to minimize the influence of artifact and equipment noise (Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures, 2012); thus, SCRs were extracted if they surpassed >0.03 μS. Responses below this threshold or outside of the scoring windows as described above were excluded.
2.4 |. Anxiety ratings
Just prior to and immediately following completion of the FRND, participants completed the STAI for the quantification of state anxiety. The STAI is a widely used self-evaluation questionnaire that asks participants to rate how they are feeling in the moment across 20 items on a scale of 1 = not at all to 4 = very much so (e.g., “I am tense”). The STAI is considered a highly reliable measure of state anxiety in that it discerns between high-and low-stress situations (Metzger, 1976). The STAI was scored according to measure-specific guidelines, resulting in STAI-S Pre and STAI-S Post scores; greater values indicate greater state anxiety.
2.5 |. Statistical analyses
All analyses were completed using IBM SPSS (v 28.0). Results reported below were the same irrespective of which version of the task was used and thus are reported without reference to task version. For versions that utilized two unique neutral cues (one outline specific to fear cue vs. another outline specific to reward cue), we did not find that either likeability ratings or SCR amplitudes differed between the two unique neutral cues used, and thus conditions were combined in subsequent analyses. We report statistics on likeability ratings and SCR amplitudes by unique neutral cues in Supplemental Material.
2.5.1 |. State anxiety
Difference in state anxiety after FRND compared to before was assessed via a paired-samples t test comparing STAI-S Pre and STAI-S Post scores.
2.5.2 |. Likeability ratings
The effect of cue type on likeability ratings was examined in a repeated-measures ANCOVA (cue condition a within-subjects factor: fear, reward, neutral, fear + neutral, reward + neutral) while controlling for the presence of psychopathology (dichotomous; 0 = absent). Post hoc pairwise comparisons were subsequently completed using a Bonferroni adjustment for multiple comparisons to test relative change in likeability in the following fashion: fear versus fear + neutral, fear + neutral versus neutral, neutral versus reward + neutral, and reward + neutral versus reward. Subsequently, we used Pearson’s partial correlations to test the relationship between likeability ratings for all cues, controlling for the presence of psychopathology. Finally, the relationship between likeability ratings and state anxiety was assessed via Pearson’s partial correlations, controlling for the presence of psychopathology.
2.5.3 |. Physiological response
SCRs were transformed across participants to the z distribution such that all SCRs were merged without reference to cue condition and quantified relative to typical physiological responsiveness (Braithwaite et al., 2015).
Z-transformed SCRs were subsequently entered into a hierarchical mixed-effects model to assess the effect of cue condition (independent variable) on z-transformed SCR amplitude (dependent variable). Given the nested data structure, such that each participant experienced each cue condition and multiple trials of each cue condition, participant was included as a random effect (Kristjansson et al., 2007). In Step 1, the presence of psychopathology (dichotomous; 0 = absent) was entered as a covariate. In Step 2, a predictor signifying cue condition was entered such that cue was coded according to expected linear change in arousal response, such that fear = 5, reward = 4, fear + neutral = 3, reward + neutral = 2, and neutral = 1. Thus, greater values in the condition variable indicated response to fear and reward cues compared to neutral cues. A predictor signifying trial number (values 1–8, subsequently mean centered) was also entered. In Step 3, a Cue Condition x Trial interaction term was added to explore whether there were differences in arousal response to cues across trials (e.g., habituation or sensitization effects).
2.5.4 |. Relationship with individual difference factors
We were also interested in this study on the relationship between SCRs and psychological ratings of the cues (likeability ratings) as well as change in anxiety. Averaged z-transformed SCR amplitudes were calculated for each cue condition. Averaged SCRs were assessed for normality, and Pearson’s partial correlations (controlling for psychopathology) were used to assess the relationship with likeability ratings of the fear US (white noise burst) as well as each cue type (greater likeability ratings = greater positive association). As we were most interested in relative difference in anxiety as a function of the task, we calculated a pre–post difference score for anxiety, represented as Δ STAI-S whereby greater Δ STAI-S indicated a relative increase in anxiety after the task compared to before. The relationship between SCRs and Δ STAI-S was also assessed using a Pearson’s partial correlation, again controlling for psychopathology. Given the total number of correlations that were assessed (see above for planned correlations among likeability ratings), we used a Bonferroni-adjusted correction for multiple comparisons and an adjusted α = .0009 for determining significance in correlational tests.
3 |. RESULTS
3.1 |. Participants
A total of 74 participants were recruited and subsequently completed testing. After completion of the protocol, 15 participants did not have sufficient EDA data to be included for further analyses due to electrode calibration problems (n = 6), missing event markers for determining cue onset (n = 5), missing trials due to coding error (n = 3), or EDA failing to save after collection (n = 1). This left a total of N = 59 available for SCR quantification. After visual inspection of all SCRs, an additional n = 6 participants were dropped from analysis as data contained excessive artifacts due to movement. Artifact detection was done manually by visual inspection and artifacts were identified by a steep rise in EDA (<500 ms). This retained a total of N = 53 available participants for statistical analyses involving EDA. Demographic information for the full sample (N = 74) and the sample retained for EDA analysis (N = 53) is reported in Table 1.
TABLE 1.
Sample demographics.
| Recruited sample | Retained for EDA analysis | |||
|---|---|---|---|---|
| N = 74 | N = 53 | Test statistic | p value | |
| n (%) | n (%) | |||
| Gender (female) | 54 (73.0%) | 39 (73.6%) | 0.04 | .851 |
| Ethnicity (Hispanic or Latinx) | 15 (20.3%) | 12 (22.6%) | 0.71 | .700 |
| Race | ||||
| White | 50 (67.6%) | 35 (66.0%) | 4.43 | .351 |
| Black/African-American | 6 (8.3%) | 6 (11.3% | ||
| Asian | 4 (5.4%) | 4 (7.5%) | ||
| More than one race | 8 (10.8%) | 5 (9.4%) | ||
| Choose not to answer | 4 (5.4%) | 3 (5.7%) | ||
| Diagnosis present | ||||
| Major depressive disorder | 2 (2.7%) | 2 (3.8%) | 0.86 | .353 |
| Mania | 2 (2.7%) | 2 (3.8%) | 0.81 | .367 |
| Panic disorder | 2 (1.4%) | 1 (1.9%) | 0.41 | .522 |
| Social anxiety disorder | 3 (4.1%) | 2 (3.8%) | 0.04 | .846 |
| Alcohol use disorder | 8 (10.8%) | 6 (11.3%) | 0.05 | .822 |
| Substance use disorder | 5 (6.8%) | 5 (9.4%) | 2.03 | .155 |
| Mood disorder | 1 (1.4%) | 1 (1.9%) | 0.40 | .526 |
| Generalized anxiety disorder | 8 (10.8%) | 7 (13.2%) | 1.11 | .292 |
| Any diagnosis present | 20 (27%) | 16 (30.2%) | 0.95 | .331 |
| M (SD) | M (SD) | |||
| Age | 19.16 (1.28) | 19.13 (1.16) | 0.35 | .728 |
| STAI-Trait | 38.68 (9.81) | 38.83 (9.84) | −0.22 | .831 |
| STAI-State Pre | 32.61 (9.56) | 33.85 (10.72) | −1.8 | .016 * |
| STAI-State Post | 32.24 (9.00) | 32.28 (9.56) | −0.06 | .952 |
| ∆ STAI-State | −0.37 (7.57) | −1.56 (7.45) | 2.23 | .029 * |
Note: Concurrent psychiatric conditions are not mutually exclusive.
Abbreviations: M, mean; SD, standard deviation.
p < .05.
Bolded text indicates p < .05.
We did not find differences between those with versus without quantifiable EDA with respect to age (p > .763), gender (p > .850), race (p > .350), ethnicity (p > .699), or in how they rated the fear US (white noise burst) or cue conditions (ps >0.184). In addition, groups did not differ with respect to the presence of psychopathology (p > .330) or in their STAI-S Post scores (p > .951). However, individuals dropped from EDA analysis did experience higher pretask levels of anxiety as indexed by the STAI-S Pre (t(71.63) = 2.47, p = .016 Greenhouse–Geisser corrected) and experienced an increase in anxiety after the task compared to before as indexed by Δ STAI-S (t(72) = 2.23, p = .029).
Of the N = 53 participants retained for analysis, n = 16 met criteria for a DSM-5 disorder as assessed by the MINI. A non-mutually exclusive representation of these disorders is listed in Table 1. Results did not change when these individuals were excluded from analyses; thus, results reported below include the full sample controlling for the presence of diagnosis. For thoroughness, we provide results as they appear in the smaller sample of individuals without psychopathology (n = 37) in Supplemental Material.
3.2 |. State anxiety
STAI-S Pre scores ranged from 20 to 59 (M = 32.61, SD = 9.56), whereas STAI-S Post scores ranged from 20 to 61 (M = 32.24, SD = 9.00). On average, state anxiety did not change as a function of task administration (p > .679). STAI-S Pre and STAI-S Post scores were related to one another, such that individuals who rated experiencing more anxiety before the task also experienced more anxiety after the task (r(70) = .69, p < .001).
3.3 |. Likeability ratings
Analyses involving likeability ratings were completed on n = 73 individuals as one individual was missing task-related files after collection. Results of the repeated-measures ANCOVA examining change in likeability of cue by condition was significant (F(2.85, 202.39) = 75.33, p < .001 Greenhouse–Geisser corrected). Post hoc Bonferroni-adjusted pairwise comparisons demonstrated that participants rated the fear + neutral cues significantly better (M = 4.97, SD = 1.39) than the fear cues (M = 2.52, SD = 2.33; mean difference = 2.45, p < .001), the reward + neutral cues significantly better (M = 5.85, SD = 1.41) than the neutral cues (M = 5.19, SE = 1.39; mean difference = 0.66, p = .001), and the reward cues significantly better (M = 8.12, SD = 1.53) than the reward + neutral cues (mean difference = 2.27, p < .001). Participants did not differ in their likeability rating of the neutral cue compared to the fear + neutral cue (p = 1.000). Figure 2 depicts likeability ratings by cue condition, plotted against likeability of the fear US on the same scale.
FIGURE 2.
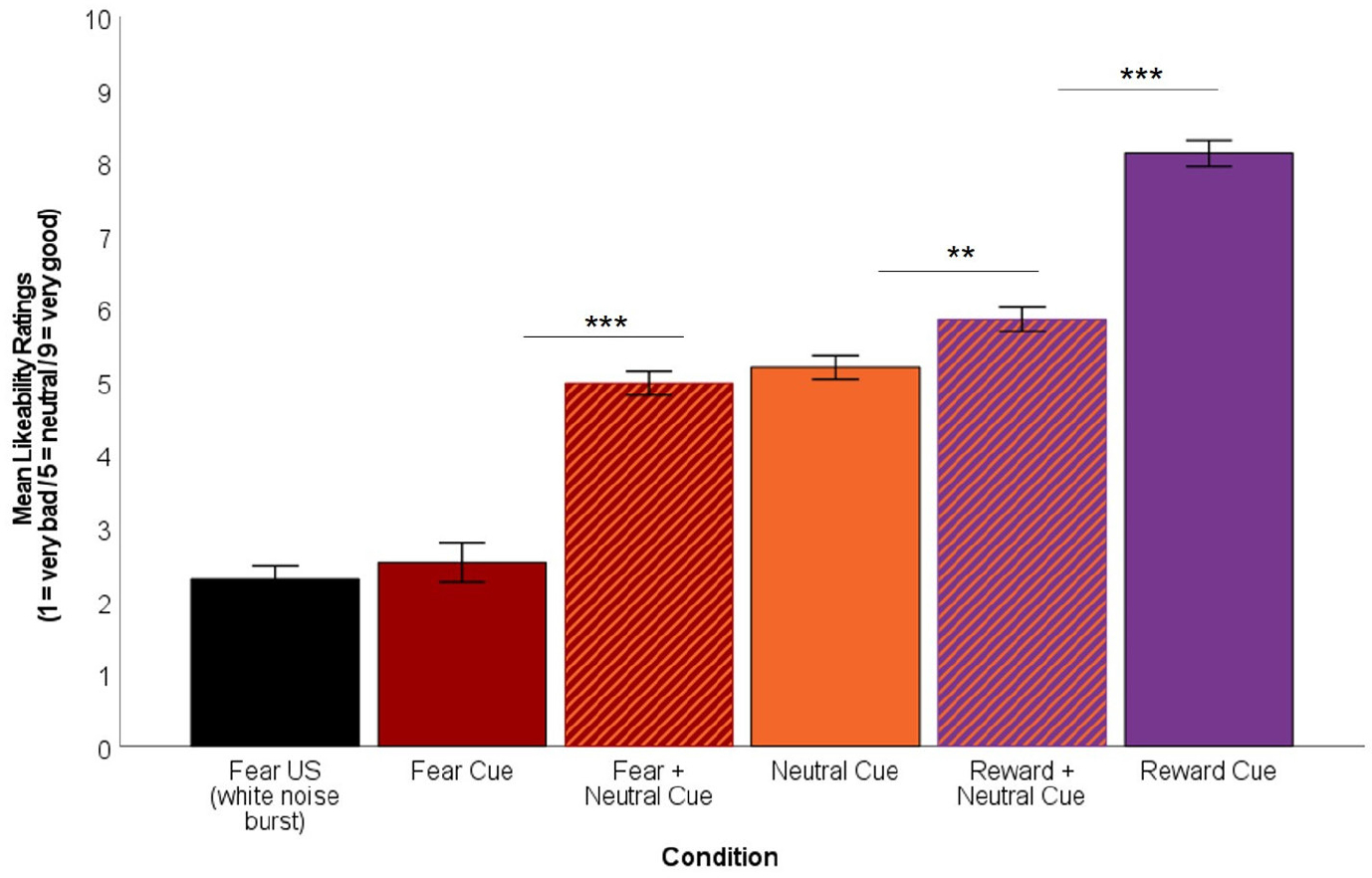
Mean likeability ratings for cues and the unconditioned stimulus (US; white noise burst) using a Likert scale (1 = very bad to 9 = very good with 5 indicating neutral). Ratings of the fear + neutral cue were significantly better compared to the fear cue, while ratings of the reward + neutral cue were significantly worse compared to the reward cue. Ratings of the reward + neutral cue were significantly better compared to the neutral cue. ***p < .001 and **p < .01.
In assessing the relationship among likeability ratings, likeability of the fear US was related to likeability of the fear cue, such that rating the fear US worse was related to perceiving the fear cue worse (r(70) = .52, p = .000003). The US rating was not related to perception of any other cue (ps >.547).
Perception of the neutral cue was also positively related to perception of the fear + neutral cue (r(70) = .40, p = .0005), as well as the reward + neutral cue (r(70) = .51, p = .000004), in that perceiving the neutral cue less favorably was also related to perceiving the combination cues less favorably. No other effects were significant (ps >.136).
3.4 |. Relationship between likeability ratings and state anxiety
No associations between anxiety and likeability ratings were found (ps >.011).
3.5 |. Physiological response
The results of the hierarchical mixed-effects model are presented in Table 2. In Step 1, the presence of diagnosis was not a significant predictor of SCR amplitude (p = .078). In Step 2, cue condition was a significant predictor of SCR amplitude (b = 0.12, SE = 0.03, t(315.16) = 3.78, p < .001), such that SCR amplitude decreased across cue conditions in the following order: Fear > Reward > Fear + Neutral > Reward + Neutral > Neutral. Figure 3 depicts predicted z-transformed SCRs by condition as predicted in the hierarchical model. In Step 3, the Trial x Cue Condition interaction was not significant (b = −0.01, SE = 0.01, t(311.79) = −1.38, p = .170), indicating that there were no cue-specific changes in SCR amplitude over the task trials.
TABLE 2.
Mixed growth models predicting SCR amplitude.
| Variable | SCR amplitude (z transformed) |
|||
|---|---|---|---|---|
| B | SE | t | p value | |
| Step 1 | ||||
| Intercept | −0.06 | 0.11 | −0.55 | .584 |
| Psychopathology (0 = absent) | 0.41 | 0.22 | 1.81 | .078 |
| Step 2 | ||||
| Intercept | −0.45 | 0.16 | −2.89 | .005 ** |
| Psychopathology (0 = absent) | 0.40 | 0.23 | 1.75 | .088 |
| Trial number (1–8) | 0.03 | 0.02 | 1.52 | .130 |
| Condition | 0.12 | 0.03 | 3.78 | <.001 *** |
| Step 3 | ||||
| Intercept | −0.05 | 0.11 | −0.46 | .650 |
| Psychopathology (0 = absent) | 0.43 | 0.23 | 1.88 | .067 |
| Trial number × condition | 0.01 | 0.01 | 1.26 | .209 |
Note: Condition was coded such that smaller values represent neutral cues and larger values represent cues by which arousal response was theorized to be larger, specifically the reward and fear stand-alone cues (1 = neutral, 2 = reward + neutral, 3 = fear + neutral, 4 = reward, and 5 = fear).
Abbreviation: SCR, skin conductance response.
p < .001;
p < .01.
Bolded text indicates p < .05.
FIGURE 3.
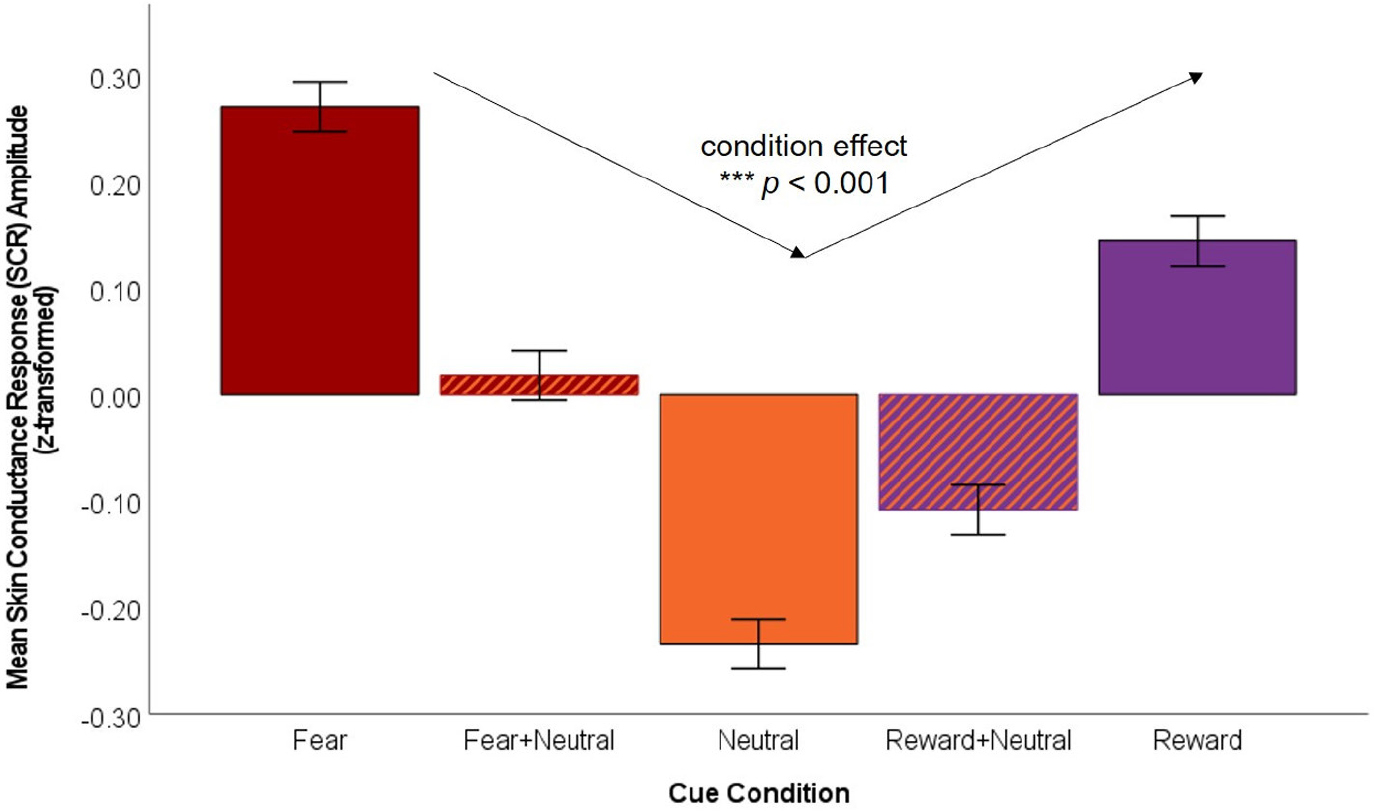
Average SCR (z transformed) differed by cue condition, such that individuals experienced greater arousal to fear and reward cues and less arousal to neutral cues. Neutral cues changed response to fear and reward by declining response. ***p < .001.
3.6 |. Relationship between physiological response, likeability ratings, and state anxiety
Mean SCR amplitudes were unrelated to likeability ratings of the US or conditioned cues (ps >0.025), moreover, mean SCR amplitudes were unrelated to changes in anxiety (p > .075).
4 |. DISCUSSION
In this study, we provide results on the fear, reward, and neutral discrimination (FRND) task designed for humans for the investigation of conditional discrimination of fear and reward from neutral conditioned cues. The FRND was translated from our prior rodent studies of discriminative learning and allows for the assessment of changes in physiological and psychological responding to fear and reward when fear and reward cues are paired with neutral cues that signal no outcome. Results herein demonstrated that individuals accurately discriminated between cues as evidenced by their likeability ratings: perceiving fear cues as the worst, reward cues as the best, and neutral cues as neutral. Discriminative learning was supported by the self-report likeability scores, as we saw a relative increase in likeability to the combined fear + neutral cue compared to the fear cue, and a relative decrease in likeability to the combined reward + neutral cue compared to the reward cue. In assessing physiological response to cues, individuals again exhibited patterns of discriminative learning as evidenced by greater averaged SCR amplitude to fear and reward cues in comparison to neutral cues. As before, changes in SCR amplitudes were evident in the presence of the neutral cue when paired with the fear and reward cues relative to the stand-alone fear and reward cues. That is, in the presence of the neutral cue, we observed a change in the physiological response. Finally, we found several pieces of evidence that perceptions of the neutral cue are important, specifically for psychological discrimination. Here, rating the neutral cue less favorably was related to rating the combination cues less favorably. We also found that perception of the fear US was related to likeability of the fear cue, as may be expected.
Importantly, the current findings mirror our prior work in rodents. We previously demonstrated that rodents conditionally discriminate between cues that signal threat of shock (fear), cues that signal delivery of sucrose (reward), and those that signal no outcome (neutral) (Sangha et al., 2013). In rodents, the presence of neutral cues changes behavior, reducing percent freezing when paired with a conditioned fear cue (Sangha et al., 2013, 2014) and reducing time spent in a port to receive sucrose when paired with a conditioned reward cue (Krueger & Sangha, 2021a). Thus, conditioned neutral cues are associated with changed responses to fear and reward in both rodents and humans. Further, we replicate prior work in humans that found change in SCR to combined cue types (Fear + Neutral) compared to fear cues (Meyer et al., 2019). Our study design was similar to this prior study, in that SCR was used as a marker of differential response and white noise burst served as the aversive US. By contrast, Jovanovic, Sakoman, et al. (2013) did not find evidence that combined cue types decreased SCR when using an FPS paradigm with both a white noise burst and an air blast directed to the larynx. On the contrary, SCR to the combined CS+/CS− cue type increased rather than decreased in that study (Jovanovic, Sakoman, et al., 2013). The fact that we found evidence of changes to SCR amplitudes when neutral cues were present in addition to the results of Meyer et al. (2019) suggests that neutral-mediated change in SCR is evident when using a stand-alone white noise burst US.
The neurobiological mechanisms underlying conditional discrimination are just now coming to light. The majority of work has been conducted in rodents and based on findings from our lab (Greiner et al., 2019; Hackleman et al., 2022; Müller et al., 2018; Ng et al., 2018; Ng & Sangha, 2023; Sangha et al., 2013, 2014; Woon et al., 2020) and others (Foilb et al., 2016; Kong et al., 2014; Kreutzmann et al., 2020; Meyer et al., 2019). Summarizing findings across the body of this work, initial sensory processing about stimuli characteristics is first mediated by relevant cortical regions (auditory and visual) in addition to the thalamus and converge in the lateral amygdala (LA). Afferents from the LA project onto the basal amygdala (BA) for further integration and then onto the central amygdala (CA) for the production of the fear response. Importantly, cells in the CA receive additional information from other brain regions, predominantly the ventral hippocampus for contextual information, while there are dense projections between the amygdala as a whole and dorsal cortical regions important for the modulation and regulation of fear, specifically the infralimbic (IL) cortex (in rodents), homologous to the ventromedial prefrontal cortex (VMPFC) (in humans). While the amygdala is the region instrumental for acquiring conditioned fear, safety learning involves the recruitment of these associated circuits. Specifically, our prior work demonstrates that safety learning is mediated by the IL (Sangha et al., 2014), a finding that was recently replicated (Kreutzmann et al., 2020). Others have shown that safety learning also relies upon ventral hippocampal activation (Meyer et al., 2019). Importantly, cells in the BA and ventral hippocampus that respond to safety signals when presented alone also respond to the combined fear + safety cue (Meyer et al., 2019; Sangha et al., 2013). This provides neurobiological support that perception of a safety cue is the driving mechanism of safety-mediated inhibition, in that particular cells may “code” for the presence of safety. Importantly, although there are comparatively fewer human studies, findings are congruent and there appear to be shared mechanisms across species (Jovanovic, Ely, et al., 2013; Meyer et al., 2019). Specifically, ventral hippocampus activation in humans occurs during safety learning as evidenced by neuroimaging studies (Meyer et al., 2019). As such, future studies exploring underlying neurobiology of safety discrimination in humans using paradigms such as the FRND are warranted.
Previous work on conditional discrimination in humans has exclusively studied this phenomenon in the context of fear; thus, the current study, which examined conditional discrimination with reward, is a novel contribution to the literature. The study of conditioned discrimination in the context of reward is important, particularly considering that reward-related abnormalities exist in disorders that also have known deficits in conditioned discrimination of fear, such as posttraumatic stress disorder (PTSD) (Glover et al., 2011; Jovanovic et al., 2009, 2010; Jovanovic & Norrholm, 2011). Individuals with PTSD experience anhedonia (e.g., reduced pleasure) (Nawijn et al., 2015), while the ability to learn about situations that afford relief may be crucial for adaptive responding to stress (Sangha et al., 2020). Prior work has demonstrated that individuals with PTSD exhibit identical FPS responses to CS+ and CS− cues compared to trauma-exposed controls (Grillon & Morgan, 1999) and exhibit less differential responding between CS+ and CS+/CS− combination cues, as well as a reduction in the transference of safety signals to a novel cue (Glover et al., 2011; Jovanovic et al., 2009). Deficits in conditional discrimination have even been theorized as a biomarker for PTSD, although few studies have examined reward-related discrimination in this population. In addition, individuals with depression have blunted responses to reward; thus, one question is whether the conditioned inhibition effect particular to reward may be stronger in those with depression. Prior work demonstrated that individuals with major depressive disorder (MDD) – as opposed to PTSD+MDD comorbidity – are not impaired in condition inhibition of fear (Jovanovic et al., 2010). However, symptoms of anhedonia may be nevertheless related to deficits in safety learning, as suggested by recent work that found that brain activation during the related process of fear extinction predicts individual differences in anhedonia in a non-psychiatric sample (Rosenberg et al., 2023). Although fear extinction and safety learning are distinct processes, they are related in that subjects learn about contexts that are safe. Notably, safety learning requires learning about how distinct stimuli signal the non-occurrence of an outcome (e.g., absence of threat/absence of reward), and so more work is needed to test the direct relationship between safety learning and anhedonia.
While accurate identification of threat and reward cues is necessary for conditional discrimination, one must also perceive neutral cues correctly. In the present study, we provide data on isolated responses to elemental cues (fear, reward, and neutral) alongside responses to their configuration (fear + neutral and reward + neutral). This approach is advantageous in that it allows one to examine how perception of elemental cues is related to response when cues are combined. Indeed, we found evidence that combined cue types likely retained some properties of the stand-alone cue types, such that the fear + neutral cues were rated more neutral while the reward + neutral cues were rated more positive. This is notable given that combined cues were not reinforced, meaning that the outcome of these two cues was identical. Further, we found several pieces of evidence that perception of the neutral cue (traditionally studied as a safety signal/CS−) was related to the psychological appraisal of combined CS+/CS− cues. Specifically, rating the neutral cue more favorably was related to more favorable ratings of the combined fear + neutral and reward + neutral cues. By contrast, the stand-alone fear and reward cues did not have this same relationship with the combined cues, suggesting that there may be something uniquely informative about the neutral cue that is influential in perception of the combined cues. Such data fit with the first reported results on conditional discrimination in humans, in that perception of the safety cue (vs. a novel cue type in order to test the influence of external inhibition) drove reduced response to CS+/CS− combination cues (Jovanovic et al., 2005). Such findings also align with our rodent findings that specific sub-populations of neurons in the BA and IL respond selectively to fear + neutral cues as well as neutral cues, suggesting that these cells are specific for “neutral coding” (Ng & Sangha, 2023; Sangha et al., 2013). In individuals with PTSD, higher arousal response to a US (shock and white noise burst) drove increased response to CS− and less differential responding between CS+/CS− (Kreutzmann et al., 2021). Therefore, several mechanisms may be at play that contribute to conditional discrimination, such as experiencing the USs as less favorable as well as perceptions of the neutral signal. Mechanisms may further differ by population, although this possibility has yet to be explored.
Although this study presents a unique and novel translational approach to understanding conditional discrimination, it is not without limitations. As the majority of participants (73%) were women, the present study is limited in its ability to test gender differences in conditional discrimination and inhibition. Prior work has found that women may exhibit deficits of inhibition specifically when compared to men and that these effects may be driven by estrogen (Krueger & Sangha, 2021b). Specifically, emerging evidence suggests that low levels of estrogen may contribute to impaired conditional inhibition (Glover et al., 2013), a finding that is largely supported by rodent work (Krueger & Sangha, 2021b). Therefore, a future area of research is to examine the effect of estrogen on findings herein. Second, we included individuals with current psychopathology in an effort to maximize sample size and to increase translation of findings to real-world examples. Even so, we were not powered to investigate differences by specific disorders, although prior work shows that individuals with anxiety disorders may be disrupted in conditional inhibition (Lissek et al., 2009). In addition, our sample size was relatively small, although in line with prior works of conditional discrimination (ns 41–50; Jovanovic et al., 2005, 2010). We also note that the jittered nature of the CS duration made the timing of US presentation unpredictable, although this was a uniform feature throughout the task and thus did not disproportionately affect any one cue type. Nonetheless, predictable versus unpredictable presentation is a feature worth investigating as it relates to conditional discrimination and individual differences (e.g., level of intolerance of uncertainty). In addition, we utilized a relatively short duration for CS presentation (3–5 s). Prior work suggests the majority of SCRs, regardless of stimulus modality, occur between 2 and 3 s poststimulus onset (Sjouwerman & Lonsdorf, 2019). Still, this timing may have been suboptimal for capturing the SCR to the 3 s CS+ if the participant had a late SCR. Finally, we did not collect data on the perceived likeability of the reward US ($0.25), and comparisons between relative perceptions of aversive and appetitive USs could not be made here, although this should be a direction for future work.
In conclusion, findings demonstrate the utility of SCR as a tool for studying conditional discrimination of fear and reward in humans. Novel findings demonstrated that response to reward can be altered in the presence of a neutral cue, and provides promise for studying such effects further, perhaps in the context of reward-related psychopathologies. Results herein showed a robust change in SCR amplitudes across conditioned fear and reward cues as evidenced in a sample of participants controlling for the presence of psychopathology, as well as in a smaller sub-sample of healthy participants without diagnoses. Findings also show that perceptions of the neutral cue were related to psychological response during conditional discrimination, demonstrating the importance of studying neutral cues (i.e., “safety signals”) as stand-alone events, in that perception of the neutrality of such signals is important. Indeed, the perception of what is dangerous and rewarding may be contrasted against a perception of neutral events and thus intertwined.
Supplementary Material
Footnotes
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
Data S1. Supporting information
DATA AVAILABILITY STATEMENT
Study data and analysis scripts are available from the corresponding author upon reasonable request.
REFERENCES
- Bradley MM, Codispoti M, Cuthbert BN, & Lang PJ (2001). Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion, 1(3), 276–298. 10.1037/1528-3542.1.3.276 [DOI] [PubMed] [Google Scholar]
- Braithwaite J, Watson D, Jones R, & Rowe M (2015). A guide for Analysing Electrodermal activity (EDA) & skin conductance responses (SCRs) for psychological experiments. Technical Report. Selective Attention & Awareness Laboratory (SAAL), Behavioural Brain Sciences Centre. [Google Scholar]
- Campo J, Merckelbach HLGJ, Nijman H, Yeates-Frederikx M, & Allertz W (2000). Skin conductance and schizophrenic symptomatology. Acta Neuropsychiatrica, 12(4), 177–182. 10.1017/S0924270800035353 [DOI] [PubMed] [Google Scholar]
- Christianson JP, Fernando ABP, Kazama AM, Jovanovic T, Ostroff LE, & Sangha S (2012). Inhibition of fear by learned safety signals: A Mini-symposium review. Journal of Neuroscience, 32(41), 14118–14124. 10.1523/JNEUROSCI.3340-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP (2009). Stress and disorders of the stress system. Nature Reviews Endocrinology, 5(7), 374–381. 10.1038/nrendo.2009.106 [DOI] [PubMed] [Google Scholar]
- Foilb AR, Flyer-Adams JG, Maier SF, & Christianson JP (2016). Posterior insular cortex is necessary for conditioned inhibition of fear. Neurobiology of Learning and Memory, 134, 317–327. 10.1016/j.nlm.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover E, Mercer K, Norrholm S, Davis M, Duncan E, Bradley B, Ressler K, & Jovanovic T (2013). Inhibition of fear is differentially associated with cycling estrogen levels in women. Journal of Psychiatry & Neuroscience, 38(5), 341–348. 10.1503/jpn.120129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Phifer JE, Crain DF, Norrholm SD, Davis M, Bradley B, Ressler KJ, & Jovanovic T (2011). Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depression and Anxiety, 28(12), 1058–1066. 10.1002/da.20880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner EM, Müller I, Norris MR, Ng KH, & Sangha S (2019). Sex differences in fear regulation and reward-seeking behaviors in a fear-safety-reward discrimination task. Behavioural Brain Research, 368, 111903. 10.1016/j.bbr.2019.111903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, & Baas J (2003). A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology, 114(9), 1557–1579. 10.1016/S1388-2457(03)00202-5 [DOI] [PubMed] [Google Scholar]
- Grillon C, & Morgan CA (1999). Fear-potentiated startle conditioning to explicit and contextual cues in gulf war veterans with posttraumatic stress disorder. Journal of Abnormal Psychology, 108(1), 134–142. 10.1037/0021-843X.108.1.134 [DOI] [PubMed] [Google Scholar]
- Hackleman A, Ibrahim M, Shim K, & Sangha S (2022). Interaction of stress and alcohol on discriminating fear from safety and reward in male and female rats. Psychopharmacology, 240, 609–621. 10.1007/s00213-022-06206-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm AO (2020). Fear, anxiety, and their disorders from the perspective of psychophysiology. Psychophysiology, 57(2), e13474. 10.1111/psyp.13474 [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, Norrholm SD, Bradley B, & Ressler KJ (2013). Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex, 49(7), 1884–1891. 10.1016/j.cortex.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, & Duncan EJ (2005). Fear potentiation and fear inhibition in a human fear-potentiated startle paradigm. Biological Psychiatry, 57(12), 1559–1564. 10.1016/j.biopsych.2005.02.025 [DOI] [PubMed] [Google Scholar]
- Jovanovic T, & Norrholm SD (2011). Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Frontiers in Behavioral Neuroscience, 5, 44. 10.3389/fnbeh.2011.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, & Ressler KJ (2010). Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety, 27(3), 244–251. 10.1002/da.20663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, & Duncan EJ (2009). Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity. Psychiatry Research, 167(1–2), 151–160. 10.1016/j.psychres.2007.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Keyes M, Fiallos A, Jovanovic S, Myers KM, Davis M, & Duncan EJ (2006). Contingency awareness and fear inhibition in a human fear-potentiated startle paradigm. Behavioral Neuroscience, 120(5), 995–1004. 10.1037/0735-7044.120.5.995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Sakoman AJ, Kozarić-Kovačić D, Meštrović AH, Duncan EJ, Davis M, & Norrholm SD (2013). Acute stress disorder versus chronic posttraumatic stress disorder: inhibition of fear as a function of time since trauma: Research article: Fear inhibition and time since trauma. Depression and Anxiety, 30(3), 217–224. 10.1002/da.21991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong E, Monje FJ, Hirsch J, & Pollak DD (2014). Learning not to fear: Neural correlates of learned safety. Neuropsychopharmacology, 39(3), 515–527. 10.1038/npp.2013.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzmann JC, Jovanovic T, & Fendt M (2020). Infralimbic cortex activity is required for the expression but not the acquisition of conditioned safety. Psychopharmacology, 237(7), 2161–2172. 10.1007/s00213-020-05527-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzmann JC, Marin M-F, Fendt M, Milad MR, Ressler K, & Jovanovic T (2021). Unconditioned response to an aversive stimulus as predictor of response to conditioned fear and safety: A cross-species study. Behavioural Brain Research, 402, 113105. 10.1016/j.bbr.2020.113105 [DOI] [PubMed] [Google Scholar]
- Kristjansson SD, Kircher JC, & Webb AK (2007). Multilevel models for repeated measures research designs in psychophysiology: An introduction to growth curve modeling. Psychophysiology, 44(5), 728–736. 10.1111/j.1469-8986.2007.00544.x [DOI] [PubMed] [Google Scholar]
- Krueger JN, & Sangha S (2021a). Sex differences in generalization of a safety cue from fear to reward. Annual Meeting of the International Behavioral Neuroscience Society, Virtual. [Google Scholar]
- Krueger JN, & Sangha S (2021b). On the basis of sex: Differences in safety discrimination vs. conditioned inhibition. Behavioural Brain Research, 400, 113024. 10.1016/j.bbr.2020.113024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar K, LeDoux J, Spencer D, & Phelps E (1995). Impaired fear conditioning following unilateral temporal lobectomy in humans. The Journal of Neuroscience, 15(10), 6846–6855. 10.1523/JNEUROSCI.15-10-06846.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (1998). Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biological Psychiatry, 44(12), 1248–1263. 10.1016/S0006-3223(98)00275-3 [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin SJ, McDowell DJ, Dvir S, Bradford DE, Geraci M, Pine DS, & Grillon C (2009). Impaired discriminative fear-conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behaviour Research and Therapy, 47(2), 111–118. 10.1016/j.brat.2008.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger RL (1976). A reliability and validity study of the state-trait anxiety inventory. Journal of Clinical Psychology, 32(2), 276–278. [DOI] [Google Scholar]
- Meyer HC, Odriozola P, Cohodes EM, Mandell JD, Li A, Yang R, Hall BS, Haberman JT, Zacharek SJ, Liston C, Lee FS, & Gee DG (2019). Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans. Proceedings of the National Academy of Sciences, 116(52), 26970–26979. 10.1073/pnas.1910481116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller I, Brinkman AL, Sowinski EM, & Sangha S (2018). Adolescent conditioning affects rate of adult fear, safety and reward learning during discriminative conditioning. Scientific Reports, 8(1), 17315. 10.1038/s41598-018-35678-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawijn L, van Zuiden M, Frijling JL, Koch SBJ, Veltman DJ, & Olff M (2015). Reward functioning in PTSD: A systematic review exploring the mechanisms underlying anhedonia. Neuroscience & Biobehavioral Reviews, 51, 189–204. 10.1016/j.neubiorev.2015.01.019 [DOI] [PubMed] [Google Scholar]
- Ng KH, Pollock MW, Urbanczyk PJ, & Sangha S (2018). Altering D1 receptor activity in the basolateral amygdala impairs fear suppression during a safety cue. Neurobiology of Learning and Memory, 147, 26–34. 10.1016/j.nlm.2017.11.011 [DOI] [PubMed] [Google Scholar]
- Ng KH, & Sangha S (2023). Encoding of conditioned inhibitors of fear in the infralimbic cortex. Cerebral Cortex, 33, 5658–5670. 10.1093/cercor/bhac450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman A, & Soares J (1993). On the automatic nature of phobic fear: Conditioned electrodermal responses to masked fear-relevant stimuli. Journal of Abnormal Psychology, 102(1), 121–132. [DOI] [PubMed] [Google Scholar]
- Orr SP, & Roth WT (2000). Psychophysiological assessment: Clinical applications for PTSD. Journal of Affective Disorders, 61(3), 225–240. 10.1016/S0165-0327(00)00340-2 [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, & LeDoux JE (2004). Extinction learning in humans: Role of the amygdala and vmPFC. Neuron, 43(6), 897–905. [DOI] [PubMed] [Google Scholar]
- Rescorla RA (1969). Pavlovian conditioned inhibition. Psychological Bulletin, 72(2), 77–94. 10.1037/h0027760 [DOI] [Google Scholar]
- Rosenberg BM, Taschereau-Dumouchel V, Lau H, Young KS, Nusslock R, Zinbarg RE, & Craske MG (2023). A multivoxel pattern analysis of anhedonia during fear extinction: Implications for safety learning. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 8, 417–425. 10.1016/j.bpsc.2021.12.008 [DOI] [PubMed] [Google Scholar]
- Rosmond R, & Bjorntorp P (2000). The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. Journal of Internal Medicine, 247(2), 188–197. 10.1046/j.1365-2796.2000.00603.x [DOI] [PubMed] [Google Scholar]
- Sangha S, Chadick JZ, & Janak PH (2013). Safety encoding in the basal amygdala. Journal of Neuroscience, 33(9), 3744–3751. 10.1523/JNEUROSCI.3302-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Diehl MM, Bergstrom HC, & Drew MR (2020). Know safety, no fear. Neuroscience & Biobehavioral Reviews, 108, 218–230. 10.1016/j.neubiorev.2019.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Robinson PD, Greba Q, Davies DA, & Howland JG (2014). Alterations in reward, fear and safety Cue discrimination after inactivation of the rat Prelimbic and Infralimbic cortices. Neuropsychopharmacology, 39(10), 2405–2413. 10.1038/npp.2014.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjouwerman R, & Lonsdorf TB (2019). Latency of skin conductance responses across stimulus modalities. Psychophysiology, 56(4), e13307. 10.1111/psyp.13307 [DOI] [PubMed] [Google Scholar]
- Smith SM, & Vale WW (2006). The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in Clinical Neuroscience, 8(4), 383–395. 10.31887/DCNS.2006.8.4/ssmith [DOI] [PMC free article] [PubMed] [Google Scholar]
- Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures. (2012). Publication recommendations for electrodermal measurements: Publication standards for EDA. Psychophysiology, 49(8), 1017–034. 10.1111/j.1469-8986.2012.01384.x [DOI] [PubMed] [Google Scholar]
- Spielberger CD (1988). Manual for the state-trait anger expression inventory (STAXI). Psychological Assessment Resources. [Google Scholar]
- Woon EP, Seibert TA, Urbanczyk PJ, Ng KH, & Sangha S (2020). Differential effects of prior stress on conditioned inhibition of fear and fear extinction. Behavioural Brain Research, 381, 112414. 10.1016/j.bbr.2019.112414 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Study data and analysis scripts are available from the corresponding author upon reasonable request.


