Abstract
Purpose:
Lung transplant (LT) centers are increasingly evaluating patients with multiple risk factors for adverse outcomes. Effects of these stacked risks remains unclear. Our aim was to determine the relationship between number of comorbidities and post-transplant outcomes.
Methods:
We performed a retrospective cohort study using the National Inpatient Sample (NIS) and UNOS Starfile (USF). We applied a probabilistic matching algorithm using 7 variables (transplant: month, year and type; recipient: age, sex, race, payer). We matched recipients in the USF to transplant patients in the NIS between 2016 to 2019. The Elixhauser methodology was used to identify comorbidities present on admission. We determined the associations between mortality, LOS, total charges and disposition with comorbidity number using penalized cubic splines, Kaplan-Meier, and linear and logistic regression methods.
Results:
From 28,484,087 NIS admissions, we identified 1,821 LT recipients. Matches were exact in 76.8% of the cohort. While the remaining cohort had a probability match of ≥ 0.94. Penalized splines of Elixhauser comorbidity number identified 3 knots defining 3 groups of stacked risk: low (<3), medium (3-6) and high risk (>6). Inpatient mortality increased from low to medium to high risk categories: (1.6%, 3.9% and 7.0%; p<0.001), as did LOS (16, 21, 29 days, p<0.001), total charges ($553,057, $666,791, $821,641.5; p=0.004) and discharge to skilled nursing facility (15%, 20%, 31%; p <0.001).
Conclusion:
Stacked risks adversely affect post-LT mortality, LOS, charges and discharge disposition. Further study to understand the details of specific stacked risks is warranted.
Keywords: lung transplant, Candidate Selection, Comorbidities, Outcomes
Guidelines for lung transplant candidate selection were first published as a joint statement between International Society for Heart and Lung Transplantation (ISHLT), American Thoracic Society (ATS), and European Respiratory Society (ERS) in 1998.1 Though revised in 2006,2 2014,3 and again in 2021,4 most of the guidelines outlined in these documents are based on expert opinion rather than randomized clinical trials.5 Despite several decades of experience, the evaluation, determination of candidacy, and listing of candidates continue to pose challenges.4
There is variability in acceptance of candidate risk between centers but no system, framework or metric to account for combinations of risk factors that are taken into consideration in candidate selection or continued candidacy on the waitlist after initial listing.6 Current guidelines stress the importance of the cumulative effect of multiple potential risk factors but highlight the difficulty estimating an individual’s post-transplant survival based on published literature.4 The LAS was designed to minimize waitlist death while preventing futile transplants.7 It was not designed to predict long-term survival or define which candidates are at higher risk for graft failure.8 The composite allocation score (CAS) will replace the LAS in early 2023 as part of continuous distribution but is similarly not designed to facilitate center and patient decision making.9 Appropriate selection of candidates and decisions for listing at a particular center rely heavily on center experience rather than universal guidelines.10
Given the subjectivity in candidate decision making, there is significant concern that geographic, racial and socioeconomic disparities present an ongoing challenge to lung transplantation.6,11 Additionally, it is becoming increasingly difficult to make decisions for accepting candidates for transplant with multiple relative contraindications, otherwise known as stacked risks. It is likely that anecdotal experiences overshadow objective assessment as the literature does not address stacked risk in potential candidacy.
The objective of our study was to determine the relationship between number of comorbidities and inpatient mortality, length of stay (LOS), total charges, and disposition using a probabilistic matching algorithm linking two administrative databases.
Methods
Study Population
Data were obtained from the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research (STAR) File and the Healthcare Cost and Utilization Project National Inpatient Sample (NIS). The cohort was composed of all adult lung transplant candidates listed for lung transplant between January 1st, 2016 to December 21st, 2019. Candidates were excluded if their age at transplant was <18 years of age. Institutional review board approval (Protocol #: 848398), data use agreements (DATA0006379) and Health Resources and Services Administration approvals were obtained. All work presented is in compliance with the ISHLT ethics policies.
Description of Administrative Datasets and Matching algorithm
The NIS is a publically available administrative database developed through Federal-State-Industry partnership and sponsored by the Agency for Healthcare Research and Quality (AHRQ).12 The NIS database combines data collection efforts from states, hospital associations, private data organizations, and the federal government to create a national encounter-level sample of healthcare data. The NIS is produced and released annually and engineered to be nationally representative of the total discharges from US hospitals.
The STAR files are limited datasets that contain patient-level information about transplant recipients, deceased and living donors, and waiting list candidates. Unique encrypted donor, candidate, hospital, and organ procurement organization identifiers are internally consistent and used to maintain confidentiality.
There is no individual linking variable to combine the NIS to the UNOS Starfile (USF); therefore, we applied a probabilistic matching algorithm using 7 variables (transplant: month, year and type; recipient: age, sex, race, payer) to match deidentified patients without a linking key. Given the size and the computational requirements for use of the full datasets, a multi-step strategy was employed. The first step retained only index lung transplant admissions identified by 28 ICD-10-CM/PCS codes (see supplement) between 2016 to 2019 in the NIS. The second step, harmonized the 7 matching variable labels. Next, we utilized “reclink2” implemented in Stata which is used for merging two datasets with no linking key.13 After merging the datasets, clerical review of all multiple matches was performed and adjudication was based on length of stay reported in both datasets (only identical or closest match was retained). Sensitivity analyses were performed using deletion of all replicates and retention of all replicates with >90% probability matches.
Primary and secondary endpoints
The primary endpoint was inpatient mortality. Secondary endpoints included hospital length of stay (LOS), total charges for index admission, need for mechanical ventilation and ECMO at 72 hours, and discharge disposition.
Statistical Analysis
We performed all data management and analysis using Stata v14.2 (Stata Corp, College Station, Tx). Summaries of clinical factors are reported with means and standard deviations or medians and interquartile ranges for continuous measures, and percentages for binary measures. All reported P values are 2-sided.
We determined candidate comorbidities using the Elixhauser comorbidity index implemented in Stata (“elixhauser”) which generates 31 categorized comorbidities based on ICD-10-CM codes present on admission and not related to the primary diagnosis of lung transplantation. The Elixhauser methodology was chosen because it outperforms other ICD coding and medication based indices that exclude acute conditions that could be complications of care.14-16 We used penalized splines (“pspline” implemented in STATA 14.2 which fits a penalized spline regression and plots the function to identify knots with equally spaced quantiles) to determine an estimation of the dose response function to the exposure of comorbidity number on inpatient mortality.17,18 We focused on inpatient mortality as the outcome variable because it is extreme and uncommon, thereby reducing the potential for type I error. We used logistic regression to develop a model to predict inpatient mortality to assess the simultaneous effect of multiple variables on the stacked risk classifier following lung transplant. For time to event analysis, we used Kaplan-Meier analysis with logrank test and restricted mean survival time (RMST). Hospital length of stay and inpatient mortality were stratified by transplant type (single, bilateral) for comparison.
Results
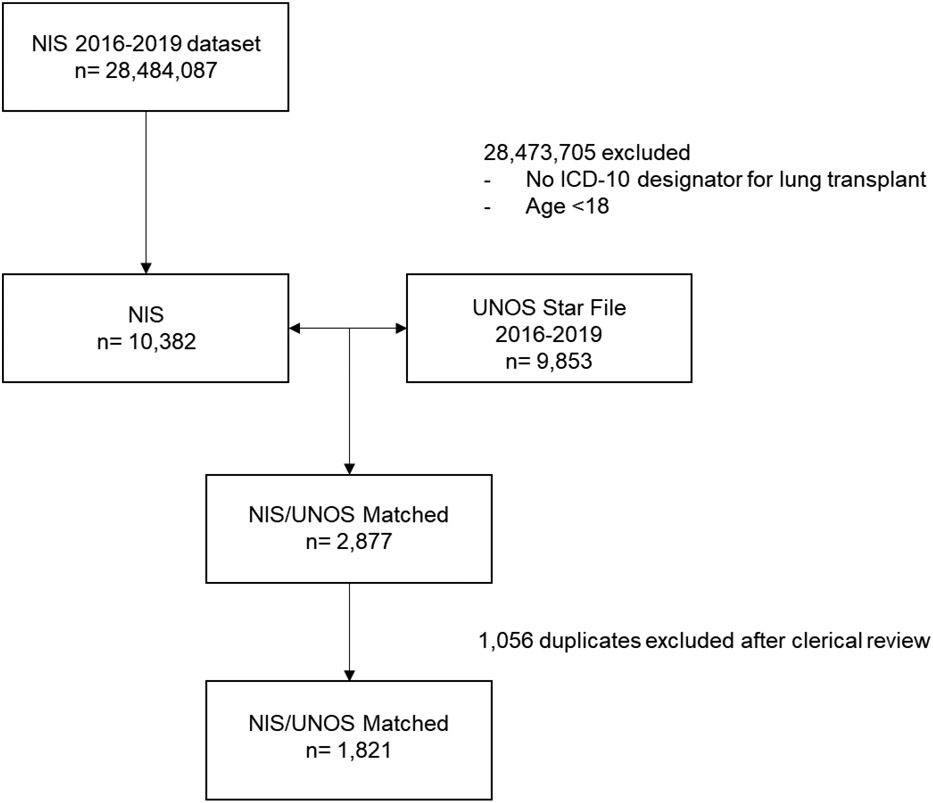
During the study period, there were 28,484,087 hospital admissions captured in the NIS and 9,853 transplants recorded in the USF. From these cohorts, 2,877 lung transplant recipients were matched and 145 (4.8%) were unable to be matched secondary to missing values required for matching. Probabilistic matching identified 76.8% perfect matches, 19.6% with a probability match of 0.97 and 3.5% with a probability match of 0.94. 1,140 (39.6%) patients had only a single match while the remainder (60.4%) had multiple matches with identical probabilities scores which required further clerical review. After adjudication, 1,821 lung transplant recipients were identified representing 18.5% of all lung transplants performed over the study period (CONSORT diagram in Figure 1). Cohort demographics are summarized in Table 1.
Figure 1.
CONSORT diagram
Table 1.
Matched Cohort Demographics
| Factor | Overall |
|---|---|
| N | 1821 |
| Recipient | |
| Age, median (IQR) | 62.0 (54.0, 67.0) |
| Female | 708 (38.9%) |
| Race | |
| White | 1367 (75.1%) |
| Black | 180 (9.9%) |
| Hispanic | 141 (7.7%) |
| Asian or Pacific Islander | 29 (1.6%) |
| Native American | 8 (0.4%) |
| Other | 43 (2.4%) |
| Missing | 53 (2.9%) |
| Diagnosis Grouping | |
| Obstructive | 483 (26.5%) |
| Pulmonary Vascular | 86 (4.7%) |
| Infective | 139 (7.6%) |
| Restrictive | 1113 (61.1%) |
| LAS, median (IQR) | 40.6 (35.2, 51.7) |
| Elective admission | 239 (13.1%) |
| Transplant type | |
| Single | 444 (24.4%) |
| Bilateral | 1377 (75.6%) |
| BMI, median (IQR) | 26.3 (22.5, 29.3) |
| Donor | |
| Donor Age, median (IQR) | 33.0 (23.0, 46.0) |
| Donor Ethnicity | |
| White | 1154 (63.4%) |
| Black | 317 (17.4%) |
| Hispanic | 271 (14.9%) |
| Asian | 57 (3.1%) |
| American Indian/Alaska Native | 7 (0.4%) |
| Native Hawaiian/Pacific Islander | 4 (0.2%) |
| Multiracial | 11 (0.6%) |
| Cigarette Use | |
| No | 1657 (91.0%) |
| Unknown | 31 (1.7%) |
| Yes | 133 (7.3%) |
| Cause of Death | |
| Anoxia | 592 (32.5%) |
| Cerebrovascular | 490 (26.9%) |
| Head Trauma | 697 (38.3%) |
| CNS Tumor | 6 (0.3%) |
| Other | 36 (2.0%) |
| BMI, median (IQR) | 25.6 (22.6, 29.5) |
| PO2, median (IQR) | 415.0 (308.1, 483.0) |
Statistical comparisons are between risk categories overall. Pearson's chi-squared was used for categorical variables and Kruskal-Wallis for continuous factors. Abbreviations: IQR, interquartile range; LAS, Lung Allocation Score; CNS, Central Nervous System; BMI, Body Mass Index; PO2, partial pressure arterial oxygen.
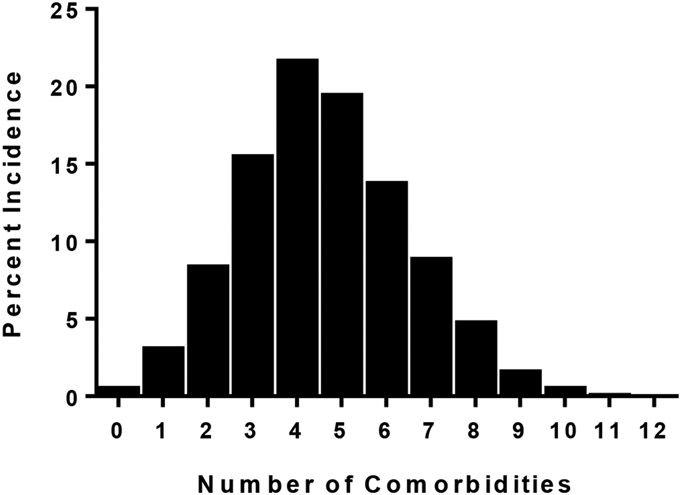
We used the Elixhauser methodology to define comorbid conditions as the score provides the capability to adjust for disease burden in patients. The number of conditions was normally distributed and ranged between 0-12 with the mean being 4.64±1.9 SD (Figure 2). The frequency of each diagnosis is summarized in Table 2. The top five most common comorbid conditions excluding Chronic Lung Disease were Fluid and Electrolyte Disorders (68.1%), Cardiac Arrhythmias (49.6%), Coagulopathy (40.3%), Pulmonary Circulation Disorders (39.5%), and Uncomplicated Hypertension (34.9%).
Figure 2.
Distribution of comorbidity number
Table 2.
Frequency of Elixhauser Defined Comorbidities
| Diagnosis | Frequency (%) |
|---|---|
| Fluid and Electrolyte Disorders | 68.11 |
| Cardiac Arrhythmias | 49.58 |
| Coagulopathy | 40.29 |
| Pulmonary Circulation Disorders | 39.45 |
| Hypertension, Uncomplicated | 34.89 |
| Weight Loss | 34.89 |
| Depression | 17.97 |
| Diabetes, Complicated | 15.08 |
| Congestive Heart Failure | 14.13 |
| Hypothyroidism | 11.96 |
| Hypertension, Complicated | 11.91 |
| Other Neurologic Disorders | 10.18 |
| Obesity | 9.07 |
| Renal Failure | 8.96 |
| Rheumatoid Arthritis/Collagen Vascular Disease | 8.9 |
| Liver Disease | 7.07 |
| Valvular Disease | 5.68 |
| Diabetes, Uncomplicated | 5.23 |
| Peripheral Vascular Disorders | 4.84 |
| Deficiency Anemia | 4.23 |
| Drug Abuse | 1.61 |
| Solid Tumor without Metastasis | 1.45 |
| Blood Loss Anemia | 1.22 |
| Paralysis | 1.11 |
| Alcohol Abuse | 1 |
| Peptic Ulcer Disease excluding Bleeding | 0.78 |
| Psychoses | 0.45 |
| Lymphoma | 0.22 |
| Metastatic Cancer | 0.17 |
| AIDS/HIV | 0.06 |
We utilized penalized splines to define inflection points for dose response effects on inpatient mortality. Risk increased with increasing comorbidities (p=0.012). Three knots were identified at 3, 6 and 9 comorbid conditions which were used to define low (0-3), medium (4-6) and high (>6) risk categories. Demographics of these risk groups are summarized in Table 3. Between risk categories, there were significant differences in age, race, diagnosis group, lung allocation score, elective admission status, body mass index of the recipient and no significant differences in donor characteristics.
Table 3.
Demographics of Elixhauser Defined Risk Category
| Factor | Low Risk | Medium Risk | High Risk | p-value |
|---|---|---|---|---|
| N | 508 | 1010 | 303 | |
| Recipient | ||||
| Age, median (IQR) | 60.0 (50.0, 66.0) | 62.0 (55.0, 67.0) | 62.0 (57.0, 67.0) | <0.001 |
| Female | 180 (35.4%) | 407 (40.3%) | 121 (39.9%) | 0.17 |
| Race | 0.019 | |||
| White | 398 (78.3%) | 759 (75.1%) | 210 (69.3%) | |
| Black | 31 (6.1%) | 106 (10.5%) | 43 (14.2%) | |
| Hispanic | 42 (8.3%) | 74 (7.3%) | 25 (8.3%) | |
| Asian or Pacific Islander | 5 (1.0%) | 16 (1.6%) | 8 (2.6%) | |
| Native American | 4 (0.8%) | 3 (0.3%) | 1 (0.3%) | |
| Other | 12 (2.4%) | 23 (2.3%) | 8 (2.6%) | |
| Missing | 16 (3.1%) | 29 (2.9%) | 8 (2.6%) | |
| Diagnosis Grouping | <0.001 | |||
| Obstructive | 113 (22.2%) | 286 (28.3%) | 84 (27.7%) | |
| Pulmonary Vascular | 19 (3.7%) | 46 (4.6%) | 21 (6.9%) | |
| Infective | 65 (12.8%) | 65 (6.4%) | 9 (3.0%) | |
| Restrictive | 311 (61.2%) | 613 (60.7%) | 189 (62.4%) | |
| LAS, median (IQR) | 39.8 (35.0, 49.0) | 40.6 (35.0, 51.9) | 42.0 (36.9, 53.3) | 0.029 |
| Elective admission | 73 (14.4%) | 114 (11.3%) | 52 (17.2%) | 0.019 |
| Transplant type | 0.055 | |||
| Single | 140 (27.6%) | 243 (24.1%) | 61 (20.1%) | |
| Bilateral | 368 (72.4%) | 767 (75.9%) | 242 (79.9%) | |
| BMI, median (IQR) | 25.8 (22.3, 28.9) | 26.3 (22.5, 29.3) | 26.9 (23.2, 29.7) | 0.008 |
| Donor | ||||
| Age, median (IQR) | 33.0 (23.0, 46.0) | 33.0 (23.0, 46.0) | 33.0 (24.0, 46.0) | 0.98 |
| Ethnicity | 0.64 | |||
| White | 318 (62.6%) | 636 (63.0%) | 200 (66.0%) | |
| Black | 91 (17.9%) | 177 (17.5%) | 49 (16.2%) | |
| Hispanic | 85 (16.7%) | 147 (14.6%) | 39 (12.9%) | |
| Asian | 9 (1.8%) | 37 (3.7%) | 11 (3.6%) | |
| American Indian/Alaska Native | 2 (0.4%) | 5 (0.5%) | 0 | |
| Native Hawaiian/Pacific Islander | 1 (0.2%) | 2 (0.2%) | 1 (0.3%) | |
| Multiracial | 2 (0.4%) | 6 (0.6%) | 3 (1.0%) | |
| Cigarette Use | 0.57 | |||
| No | 461 (90.7%) | 926 (91.7%) | 270 (89.1%) | |
| Unknown | 9 (1.8%) | 14 (1.4%) | 8 (2.6%) | |
| Yes | 38 (7.5%) | 70 (6.9%) | 25 (8.3%) | |
| Cause of Death | 0.38 | |||
| Anoxia | 165 (32.5%) | 327 (32.4%) | 100 (33.0%) | |
| Cerebrovascular | 137 (27.0%) | 273 (27.0%) | 80 (26.4%) | |
| Head Trauma | 200 (39.4%) | 385 (38.1%) | 112 (37.0%) | |
| CNS Tumor | 0 | 3 (0.3%) | 3 (1.0%) | |
| Other | 6 (1.2%) | 22 (2.2%) | 8 (2.6%) | |
| BMI, median (IQR) | 25.5 (22.7, 29.7) | 25.6 (22.6, 29.4) | 25.8 (22.7, 29.8) | 0.98 |
| PO2, median (IQR) | 422.0 (325.0, 487.0) | 410.0 (291.0, 477.0) | 429.0 (296.0, 493.0) | 0.053 |
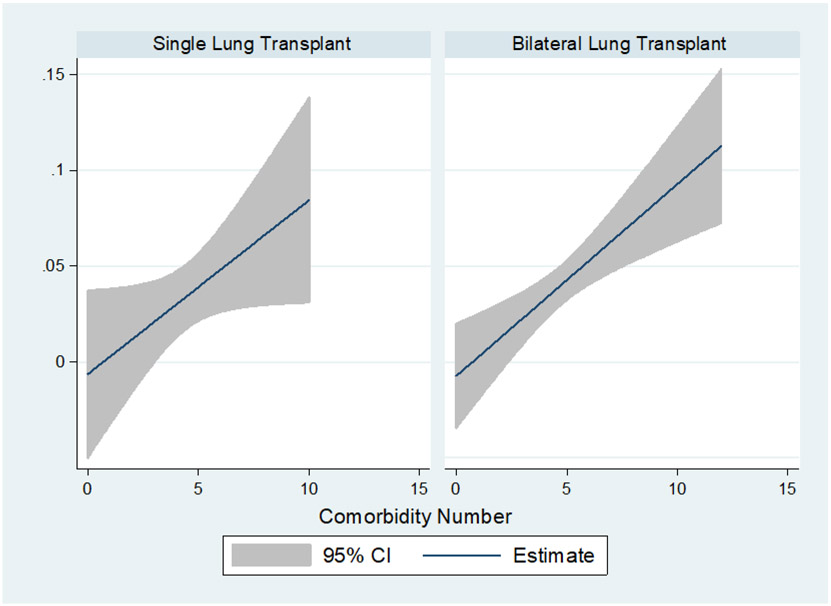
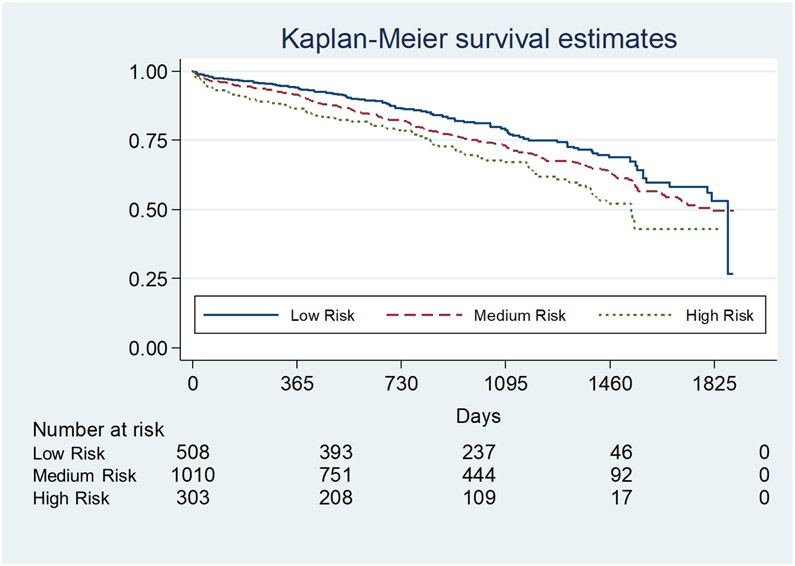
The risk of inpatient mortality increased with the number of comorbidities in a dose dependent fashion (Figure 3, Table 4). By risk categories, inpatient mortality significantly increased from low to high risk (1.6%±12.5 SD, 4.1%±19.7 SD, 6.9%±25.4 SD; p=0.001). This risk persisted and was consistent at 90-days, 1-year, 3-years and 5-years (p=0.0007, Figure 4). Compared to low risk, there was significant decrease in restricted mean survival time (measure of average survival from time 0 to a specified time point) at 3 years demonstrated with medium risk (−44.4 [95% CI: −13.9, −74.9] days, p=0.004) and high risk (−89.6 [95% CI: −42.7, −136.5] days, p<0.0001) categories. Given the significant differences in recipient characteristics identified, multivariate analysis of inpatient mortality by Elixhauser defined risk categories remained a consistent independent predictor of inpatient mortality (odds ratio of 1.9 [95% CI: 1.3; 2.8]; p=0.001). Candidate age (OR 1.01 [95% CI: 0.99; 1.04]; p=0.37), race (OR 1.05 [95% CI: 0.83; 1.32]; p=0.70), diagnostic group (OR 0.92 [95% CI: 0.76; 1.12]; p=0.40), initial LAS (OR 1.00 [95% CI: 0.98; 1.02]; p=0.9), elective admission (OR 0.51 [95% CI: 0.20; 1.29]; p=0.16), transplant type (OR 1.23 [95% CI: 0.65; 2.32]; p=0.52) and BMI (OR 1.03 [95% CI: 0.97; 1.09]; p=0.30) did not predict inpatient mortality. Within this model using the low risk group as a reference, medium risk (OR 2.33 [95% CI: 1.08; 5.05]; p=0.03) and high risk (OR 3.8 [95% CI: 1.63; 8.93]; p=0.002) increased in dose dependent fashion.
Figure 3.
Inpatient mortality risk by number of comorbidities stratified by transplant type
Table 4.
Outcome measure by risk category
| Factor | Overall | Low Risk | Medium Risk | High Risk | p-value |
|---|---|---|---|---|---|
| N | 1821 | 508 | 1010 | 303 | |
| Survival | <0.001 | ||||
| 30-day | 0.98 | 0.99 | 0.98 | 0.97 | |
| 90-day | 0.96 | 0.97 | 0.96 | 0.93 | |
| 1-year | 0.91 | 0.94 | 0.91 | 0.86 | |
| 3-year | 0.74 | 0.79 | 0.73 | 0.67 | |
| Mechanical Ventilation at 72 Hours | <0.001 | ||||
| No | 1315 (72.2%) | 396 (78.0%) | 730 (72.3%) | 189 (62.4%) | |
| Unknown | 4 (0.2%) | 2 (0.4%) | 2 (0.2%) | 0 | |
| Yes | 502 (27.6%) | 110 (21.7%) | 278 (27.5%) | 114 (37.6%) | |
| ECMO at 72 Hours | <0.001 | ||||
| No | 1724 (94.7%) | 492 (96.9%) | 963 (95.4%) | 269 (88.8%) | |
| Unknown | 4 (0.2%) | 1 (0.2%) | 3 (0.3%) | 0 | |
| Yes | 92 (5.1%) | 14 (2.8%) | 44 (4.4%) | 34 (11.2%) | |
| Missing | 1 (0.1%) | 1 (0.2%) | 0 | 0 | |
| iNO at 72 Hours | 0.417 | ||||
| No | 1662 (91.3%) | 471 (92.7%) | 919 (91.0%) | 272 (89.8%) | |
| Unknown | 11 (0.06%) | 3 (0.6%) | 5 (0.5%) | 3 (1.0%) | |
| Yes | 147 (8.1%) | 33 (6.5%) | 86 (8.5%) | 28 (9.2%) | |
| Missing | 1 (0.05%) | 1 (0.2%) | 0 | 0 | |
| Length of Stay | <0.001 | ||||
| Overall | 20 [13; 37] | 16 [11; 27.5] | 21 [14; 37] | 29 [18; 50] | |
| Single | 15 [11; 27] | 12.5 [10; 20] | 15 [11; 25] | 25 [14; 41] | |
| Bilateral | 22 [15; 40] | 17 [12; 30] | 23 [15; 40] | 29 [18; 53] | |
| Disposition | <0.001 | ||||
| Home | 735 (40.4%) | 242 (47.6%) | 397 (39.3%) | 96 (31.7%) | |
| Short Term Hospital | 18 (1.0%) | 7 (1.4%) | 11 (1.1%) | 0 | |
| Skilled Nursing Facility | 375 (20.6%) | 75 (14.8%) | 206 (20.4%) | 94 (31.0%) | |
| Home Health Care | 623 (34.2%) | 176 (34.7%) | 355 (35.2%) | 92 (30.4%) | |
| Died | 70 (3.8%) | 8 (1.6%) | 41 (4.1%) | 21 (6.9%) | |
| Total Charges | <0.001 | ||||
| Overall | $653,024 [454,335; 1,125,028] | $549,593 [397,047; 911,928] | $665,666 [463,642; 1,161,165] | $816,576 [553,376; 1,261,809] | |
| Single | $542,419 [386,252; 971,431] | $504,809 [356,636; 906,914.5] | $502,071 [378,923; 960,064] | $735,919 [526,755; 1,463,928] | |
| Bilateral | $685,880 [483,949; 1,162,435] | $566,470 [414,477; 941,223] | $707,612 [502,246.5; 1,251,858] | $830,990 [574,395; 1,232,098] |
Figure 4.
Post-Transplant Survival
ECMO salvage at 72-hours post-transplant consistently increased from 2.8% for low risk to 4.4% for medium risk to 11.2% for high risk groups (p<0.001). This corresponded to need for mechanical ventilation which also exhibited the same relationship (21.7%; 27.5%; 37.6%; p<0.001).
Hospital length of stay also demonstrated a significant dose response in accordance with comorbid risk (Table 4). Overall length of stay increased from 16 [IQR: 11; 27.5] to 21 [IQR: 14; 37] to 29 [IQR: 18; 50] days among the low medium and high risk groups, respectively (p<0.0001). This was consistent in both single (12.5 [IQR: 10; 20], 15 [IQR: 11; 25]; 25 [IQR: 14; 41]) and bilateral lung transplants (17 [IQR: 12; 30], 23 [IQR: 15; 40], 29 [IQR: 18; 53]).
Consistent with increasing length of stay, total hospital charges for the index admission demonstrated a similar relationship (p=0.0037). Median cost overall between risk groups increased from $549,593 [IQR: 397,047; 911,928] for low risk to $665,666 [IQR: 463,642; 1,161,165] for medium risk to $816,576 [IQR: 553,376; 1,261,809] in the high risk category. This trend remained regardless of transplant type (Single: $504,809 [IQR: 356,636; 906,914.5]; $502,071 [IQR: 378,923; 960,064]; $735,919 [IQR: 526,755; 1,463,928]; Bilateral: $566,470 [IQR: 414,477; 941,223]; $707,612 [IQR: 502,246.5; 1,251,858]; $830,990 [IQR: 574,395; 1,232,098]).
To gain an understanding of resource utilization, discharge disposition was evaluated Table 4. Among the low, medium and high risk groups there was a decrease in discharge to home (47.6%, 39.1% and 31.7%, p <0.001). Conversely, discharge to skilled nursing facility or intermediate care facility increased with increasing risk from 14.8% to 20.4% and 31.0% (p <0.001), respectively. Discharges to short term hospitals was rarely used and only for low and medium risk groups (1.4%; 1.1%; p <0.001, respectively).
Sensitivity analyses were performed to determine if alternate strategies for cohort identification and curation affected results. We contrasted a strategy of keeping all matches when more than one match was possible due to ties in probability score and a strategy of deleting all but the first best match to our presented curation adjudication method based on length of stay matching. Alternative strategies both demonstrated identical cut points for risk categorization and similar adverse effects (data in supplement).
Discussion
Increasing number of recipient comorbidities (stacked risks) increases risk of mortality, need for prolonged mechanical ventilation, ECMO salvage and costs after lung transplantation. Stacked risks further significantly limit discharge disposition to home at the expense of increased placement in skilled nursing facilities, suggesting suboptimal functional short-term outcomes.
Transplant center evaluation of candidates with stacked risks is well recognized but understudied because of the limitations posed on available administrative datasets. This study demonstrated linkage of the USF and the NIS is possible in lung transplant patients despite lack of identifiers.19 Moreover, the ability to link real-world comorbidities to the USF has important and significant applications which can address variability in acceptance of candidate risk between centers.20 The potential to develop a metric to account for stacked risks could improve candidate selection, waitlist and post-transplant survival modeling, and regulatory oversight, particularly if automated methods are employed using ICD-10 coding structures which are uniformly defined. This work is a first step for developing such a metric and more work needs to be done refining and optimizing stacked risk definitions and determining which comorbid conditions are driving the associations identified.
It makes intuitive sense that the absolute number of comorbid conditions increases mortality at any time point and this study confirms that assumption. Surprisingly, the number of diagnoses that contribute to the risk categories were not those routinely considered potential risk factors (e.g. hypertension, diabetes, obesity, weight loss, fluid and electrolyte disorders, anemia, depression, hypothyroidism, cardiac arrhythmias) which, based on our analysis, if present in the same candidate would define a high risk candidate. It is unlikely a transplant center would consider a potential candidate with any combination of the 7 to 9 risk factors listed above as high risk; therefore, a better understanding of associated individual Elixhauser comorbidities and patterns of comorbidities is needed. While the NIS is roughly representative of 20% of annual US inpatient admissions and therefore about 20% of annual lung transplants, the Medicare dataset includes roughly 40% of all lung transplants. Future work will seek to use both the NIS and Medicare data to capture roughly 50% of all annual US transplants.
There is increasing interest in defining outcomes by more than just mortality.21 Alive at one year but institutionalized or unable to function independently is arguably not a positive outcome. Determining the risk of candidates who present for transplant evaluation is complex, subjective and variable.6,11 As a first step in creating an objective candidate assessment metric, we evaluated the disposition of lung transplant recipients based on their stacked risk assessment. We identified an inverse relationship in discharge to home with respect to stacked risks. Consequently, the number of patients requiring discharge to a skilled nursing facility (SNF) doubled from low to high risk. When arbitrarily defining poor outcome as inpatient death or discharge to SNF, about ~40% of the high risk group have a poor outcome compared to ~16% for the low risk group. Better understanding of risk and poor outcomes are needed to facilitate frank discussions with candidates.
There are limitations to this study. The Elixhauser comorbidity methodology is crude and identified patients with diagnoses not normally seen in lung transplant. We acknowledge only 31 comorbidities can be identified using the Elixhauser methods. This method of identifying comorbidities is standardized and commonly used for adjustment for hospital quality comparisons.22 Nonetheless, our future work will utilize the Clinical Classifications Software Refined (CCSR) which is one in a family of databases and software tools developed as part of the Healthcare Cost and Utilization Project. The CCSR may be superior to the Elixhauser methodology because it aggregates ICD-10 codes into 530 clinical categories across 22 body systems giving finer granularity. With respect to candidates identified with diagnoses that would not be transplant candidates (e.g. metastatic cancer, lymphoma or solid tumors), we have assumed that those patients were treated in the past and had met the cancer free survival requirements defined by guidelines.4 Categorical designations of low, medium and high risk based on Elixhauser diagnosis numbers limits granularity in terms of risk prediction. Our decision to use a categorical rather than continuous measure was made to ensure that this relatively small dataset could be used for analysis. Our intention is to refine and optimize this classifier to improve granularity and facilitate discussion in the clinic between providers and patients and at the listing conferences between providers utilizing a larger dataset combining HCUP, Medicare and UNOS administrative data. NIS data prior to 2016 were not included. In late 2015 ICD-10 was implemented in the US making data pre-2016 complicated due to mixed coding structures. We decided to omit all data years prior to 2016 in order to ensure consistent comorbidity identification. Center was not used as a linking variable because the NIS no longer provides State and hospital identifiers in order to protect patient confidentiality. Additionally, there is a correlation of comorbidity number and age (rho 0.125, p<0.001). While this is expected to some degree, we have refrained from suggesting cutoffs or implying certain patient populations not be transplanted because more work will need to be done to further validate and generalize our observations. Lastly, our current metric does not discriminate the level of risk among individually defined diagnoses meaning pulmonary hypertension and uncomplicated diabetes are considered to have similar risk. Future work with an expanded cohort will focus on associated risks of specific diagnoses to better characterize each associated risk and potential interactions.
In summary, stacked risks defined by number of Elixhauser comorbidities are associated with increased mortality, need for prolonged mechanical ventilation, salvage ECMO, hospital length of stay, cost and discharge to a skilled nursing facility. In addition, we successfully demonstrated it is feasible to link the NIS with the USF using probabilistic matching which provides a powerful investigative toolset. Further study of stacked risks is warranted.
Supplementary Material
Acknowledgements
This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This study was supported by NIH grants HL155821, HL116656, HL114626, HL087115, HL115354, and HL145435. None of the authors have any financial relationship with a biotechnology and/or pharmaceutical manufacturer that has an interest in the subject matter or materials discussed in the submitted manuscript.
Abbreviations:
- AHRQ
Agency for Healthcare Research and Quality
- ATS
American Thoracic Society
- CCSR
Clinical Classifications Software Refined
- CAS
Composite Allocation Score
- ECMO
Extracorporeal Membrane Oxygenation
- ERS
European Respiratory Society
- ICD
International Classification of Diseases
- ISHLT
International Society for Heart and Lung Transplantation
- LAS
Lung Allocation Score
- LT
Lung Transplant
- LOS
Length of Stay
- NIS
National Inpatient Sample
- USF
UNOS Starfile
- RMST
Restricted Mean Survival Time
- SNF
Skilled Nursing Facility
- STAR
Standard Transplant Analysis and Research
- UNOS
United Network for Organ Sharing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT authorship contribution statement:
Conception and design: EC, DS
Acquisition of data: EC
Analysis and interpretation of data: EC, JD, MM, JF, JL, AS, JMD, MA, EC, JH, ARL RG, JDC, DS
Drafting or revising the manuscript for important intellectual content: EC, JD, MM, JF, JL, AS, JMD, MA, EC, JH, ARL RG, JDC, DS
Final approval of the version to be published: EC, JD, MM, JF, JL, AS, JMD, MA, EC, JH, ARL RG, JDC, DS
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.International guidelines for the selection of lung transplant candidates. The American Society for Transplant Physicians (ASTP)/American Thoracic Society(ATS)/European Respiratory Society(ERS)/International Society for Heart and Lung Transplantation(ISHLT). Am J Respir Crit Care Med 1998;158(1):335–9. DOI: 10.1164/ajrccm.158.1.15812. [DOI] [PubMed] [Google Scholar]
- 2.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25(7):745–55. DOI: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015;34(1):1–15. DOI: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Leard LE, Holm AM, Valapour M, et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2021;40(11):1349–1379. DOI: 10.1016/j.healun.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orens JB, Merlo CA. Selection of Candidates for Lung Transplantation and Controversial Issues. Semin Respir Crit Care Med 2018;39(2):117–125. DOI: 10.1055/s-0037-1615796. [DOI] [PubMed] [Google Scholar]
- 6.Kizer KW, English RA, Hackmann M. Realizing the Promise of Equity in the Organ Transplantation System. Washington (DC)2022. [PubMed] [Google Scholar]
- 7.Gottlieb J. Lung allocation. Journal of thoracic disease 2017;9(8):2670–2674. DOI: 10.21037/jtd.2017.07.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyu DM, Goff RR, Chan KM. The Lung Allocation Score and Its Relevance. Semin Respir Crit Care Med 2021;42(3):346–356. DOI: 10.1055/s-0041-1729541. [DOI] [PubMed] [Google Scholar]
- 9.Dalton JE, Lehr CJ, Gunsalus PR, Mourany L, Valapour M. Refining the Lung Allocation Score Models Fails to Improve Discrimination Performance. Chest 2022. DOI: 10.1016/j.chest.2022.08.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arjuna A, Olson MT, Walia R. Current trends in candidate selection, contraindications, and indications for lung transplantation. Journal of thoracic disease 2021;13(11):6514–6527. DOI: 10.21037/jtd-2021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wadhwani SI, Lai JC, Gottlieb LM. Medical Need, Financial Resources, and Transplant Accessibility. JAMA 2022. DOI: 10.1001/jama.2022.5283. [DOI] [PubMed] [Google Scholar]
- 12.Databases H. Agency for Healthcare Research and Quality (AHRQ). (www.hcupus.ahrq.gov/databases.jsp.). [DOI] [PubMed]
- 13.Blasnik M. RECLINK: Stata module to probabilistically match records. 2010. [Google Scholar]
- 14.Yurkovich M, Avina-Zubieta JA, Thomas J, Gorenchtein M, Lacaille D. A systematic review identifies valid comorbidity indices derived from administrative health data. J Clin Epidemiol 2015;68(1):3–14. DOI: 10.1016/j.jclinepi.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F, Mohamed MO, Ensor J, Peat G, Mamas MA. Temporal Trends in Comorbidity Burden and Impact on Prognosis in Patients With Acute Coronary Syndrome Using the Elixhauser Comorbidity Index Score. Am J Cardiol 2020;125(11):1603–1611. DOI: 10.1016/j.amjcard.2020.02.044. [DOI] [PubMed] [Google Scholar]
- 16.Sharabiani MTA, Aylin P, Bottle A. Systematic Review of Comorbidity Indices for Administrative Data. Medical Care 2012;50(12):1109–1118. (http://www.jstor.org/stable/41714639). [DOI] [PubMed] [Google Scholar]
- 17.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 2009;47(6):626–33. DOI: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 18.Jann B, Gutierrez RG. PSPLINE: Stata module providing a penalized spline scatterplot smoother based on linear mixed model technology. Statistical Software Components. revised 25 Jan 2009. ed2008. [Google Scholar]
- 19.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant 2014;14(8):1723–30. DOI: 10.1111/ajt.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.In: Hackmann M, English RA, Kizer KW, eds. Realizing the Promise of Equity in the Organ Transplantation System. Washington (DC)2022. [PubMed] [Google Scholar]
- 21.Auriemma CL, Taylor SP, Harhay MO, Courtright KR, Halpern SD. Hospital-Free Days: A Pragmatic and Patient-centered Outcome for Trials among Critically and Seriously Ill Patients. Am J Respir Crit Care Med 2021;204(8):902–909. DOI: 10.1164/rccm.202104-1063PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin SR, Wong YN, Uzzo RG, Beck JR, Egleston BL. Why Summary Comorbidity Measures Such As the Charlson Comorbidity Index and Elixhauser Score Work. Med Care 2015;53(9):e65–72. DOI: 10.1097/MLR.0b013e318297429c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.