Abstract
Background/objective:
We hypothesize rectal hydrogel spacer (RHS) improves rectal dosimetry in patients undergoing salvage high-dose-rate brachytherapy (HDR-BT) for intact, recurrent prostate cancer (PC).
Methods:
A prospectively collected institutional database was queried for recurrent PC patients treated with salvage HDR-BT from 09/2015 to 11/2021. Patients were offered RHS beginning 06/2019. Dosimetric variables were compared between RHS and no-RHS groups for the average of two fractions using Wilcoxon rank-sum tests. Primary outcomes were rectal volume receiving 75% of prescription dose (V75%) and prostate volume receiving 100% of prescription dose (V100%). Generalized estimating equation (GEE) model was used to evaluate the association between other planning variables and rectal V75%.
Results:
Forty-one PC patients received salvage HDR-BT, of whom 20 had RHS. All patients received 2400cGy in 2 fractions. Median RHS volume was 6.2cm3 [Standard deviation (SD): ± 3.5cm3]. Median follow-up was 4 months and 17 months in the RHS and no-RHS groups, respectively. Median rectal V75% with and without RHS were 0.0cm3 (IQR: 0.0–0.0cm3) and 0.06cm3 (IQR: 0.0–0.14cm3), respectively (p<0.001). Median prostate V100% with and without RHS were 98.55% (IQR: 97.86–99.22%) and 97.78% (IQR: 97.50–98.18%), respectively (p=0.007). RHS, rectum, and prostate volumes did not significantly affect rectal V75% per GEE modeling. There was 10% G1–2 and 5% G3 rectal toxicity in RHS group. There was 9.5% G1–2 and no G3+ rectal toxicities in the no-RHS group.
Conclusion:
Absolute improvement in rectal V75% and prostate V100% was significant with RHS in PC patients undergoing salvage HDR-BT, but clinical benefit is marginal.
Keywords: Rectal hydrogel spacer, high dose-rate brachytherapy, prostate cancer, salvage therapy, re-irradiation, dosimetry
INTRODUCTION
Due to high toxicity rates of salvage therapies, less than 20% of patients with locally recurrent, intact prostate cancer historically underwent salvage therapies and the majority have received androgen deprivation therapy (ADT) alone [1, 2]. The ADT-delaying and potentially curative benefits of radiotherapy salvage options, which have roughly 50% 5-year biochemical control and relapse-free survival rates, must be weighed against their increased risk of toxicities in the reirradiation setting [3, 4]. External beam reirradiation with conventional fractionation results in unacceptably high late toxicity with approximately 30% of patients experiencing grade 4 genitourinary (GU) and gastrointestinal (GI) side effects [5]. Stereotactic body radiotherapy has demonstrated a more favorable late toxicity profile but with short follow-up [6, 7]. High-dose-rate brachytherapy (HDR-BT) has emerged as a favorable modality for reirradiation due to its sharp conformality and steep dose fall-off which limit bladder and rectal dose [8]. Studies with 5 or more years of follow-up have reported rates of G3+ late GU and GI adverse events in the 0–20% range [9–11]. Focal HDR-BT guided by magnetic resonance imaging to target only recurrent lesions instead of whole prostate gland have further improved GU and GI toxicity rates. Grade 3 or higher toxicities with a single 19Gy dose or two 13.5Gy fractions of focal HDR-BT have been reported to be very low with no acute GU and GI G3+ events, no late G3+ GI events, and <5% late G3+ GU toxicity [12, 13]. However, this technique has not been compared to whole-glade HDR-BT and may impose technical and resource limitations. In order to increase the number of patients receiving potentially curative reirradiation, the toxicities of salvage whole-gland HDR-BT should be further diminished.
Rectal hydrogel spacers (RHS) are injected through the perineum between the prostate and anterior rectal wall. They have been shown to significantly reduce acute and late rectal toxicity in the external beam setting due to increased distance for dose fall-off between the prostate and rectum [14]. Patients with RHS receiving first-line external beam radiotherapy for prostate cancer have demonstrated a 5% absolute risk reduction in late rectal toxicity. Late G3 rectal toxicity with combined modality external beam plus HDR-BT boost, in which higher total doses to prostate are achieved, can be limited to <1% with RHS [15, 16]. Currently, toxicity benefits of RHS in salvage prostate reirradiation with HDR-BT are unknown. Limited series have reported improvements in rectal dosimetry of first-line HDR-BT and salvage low-dose-rate brachytherapy with RHS, but the effects of RHS in salvage HDR-BT have not been demonstrated [17, 18]. Herein, we present the first report on the dosimetric and toxicity effects of RHS in the setting of salvage whole-gland HDR-BT for locally recurrent, intact prostate cancer.
METHODS
Patients
This is a single-institution retrospective analysis of a prospectively collected database of prostate cancer patients treated at a high-volume tertiary care center of excellence for brachytherapy. Our database was queried for patients ≥18 years of age who had intact prostate cancer initially treated with radiotherapy and subsequently developed biopsy-proven local recurrence and received salvage whole-gland HDR-BT between September, 2015, to November, 2021. All grade groups and PSA values at the time of recurrence were allowed. Initial radiotherapy could include external beam photon or proton irradiation, as well as permanent low-dose-rate brachytherapy seed implants or HDR-BT. Not all patients necessarily received their first radiotherapy course at our institution. September 2015 was chosen as the cutoff date because that is when we adopted trans-rectal ultrasound-based treatment planning for all of our prostate cancer HDR-BT cases.
Treatment
To evaluate whether prostate size and pelvic anatomy was amenable to HDR-BT, MRI volume study of the pelvis was performed 1–2 weeks prior to the first scheduled HDR-BT fraction. All patients were offered RHS as an elective procedure beginning June, 2019. The main components of RHS were water and polyethylene glycol. Placement of RHS was performed by a urologist. Patients were placed in supine position and external genitalia were retracted out of the operative field. The perineum was prepped with povidone-iodine antiseptic; then local anesthesia was placed superficially to the level of the apex of the prostate. Following mixing of RHS solutions, bi-planar ultrasound guidance was used to inject RHS between the rectum and prostate throughout their cranial-caudal interface. In patients opting for general anesthesia, laryngeal mask airways and operating rooms were used for the procedure. Pelvic MRI was done 1–2 weeks following RHS placement to measure prostate volume, assess pelvic anatomy, and confirm RHS placement position. Patients were given bowel prep instructions to follow prior to each HDR-BT fraction and were started on tamsulosin 0.4 mg twice daily for prophylaxis against bladder outlet obstruction.
All patients received salvage whole-gland HDR-BT to the prostate without simultaneous integrated boost to radiographically identified lesions. All HDR-BT procedures were performed under general anesthesia and in a shielded operating room. Patients were placed in supine position with their lower extremities elevated in stirrups to allow access to their perineum. A trans-rectal ultrasound probe and a needle template were attached to a frame which allowed controlled motion in six axes. This frame was fixed to the operating room table. Ultrasound positioning is adjusted antero-posteriorly to optimize image quality while minimizing pubic arch interference. If needle placement is obstructed by the pubic arch, posterior pressure is applied on the ultrasound probe to bring the prostate posteriorly. Stainless steel interstitial needles (20 cm x 18 G) were guided into each patient’s prostate through the perineum using the needle template which was calibrated to an ultrasound grid. The grid had 169 holes arranged in square 13×13 format with 5mm between each hole. Once an axial slice of the prostate was visualized on ultrasound, needles were placed around the peripheral zone of the prostate with approximately 10 mm between each adjacent needle. Two to four central needles were also placed at least 10 mm from the urethra. The needles were then advanced to the base of the prostate, with the most posterior needles being advanced ~10 mm into the seminal vesicles. Cystoscopy was performed by a urologist to confirm no needles perforated the bladder.
Contouring of the prostate, urethra, rectum, bladder, and RHS (if present) was done on an ultrasound-based treatment planning system. The prostate was contoured directly as a planning target volume. Each patient was prescribed 1200 cGy per fraction to the whole prostate, and two fractions were performed one to two weeks apart. Treatment planning goals included prostate volume receiving 100% of prescription dose (V100%) ≥ 95%, rectum volume receiving 75% of prescription dose (V75%) < 1.0 cm3, bladder V75% < 1.0 cm3, and urethra V125% < 1.0 cm3. Prostate V150% < 35% was a secondary goal. A single iridium-192 source was delivered via an afterloader sequentially through each needle using predetermined dwell times.
Statistical Analysis
Demographic variables and gastrointestinal and genitourinary toxicity outcomes were queried from a prospectively collected database and reported descriptively. Volume of RHS and prostate-rectum separation (defined as the shortest distance between prostate and rectum in each patient with RHS on sagittal MRI at mid-gland) were not recorded in the treatment planning documents and were measured directly in the treatment planning software. Dosimetric variables were retrospectively extracted by reviewing treatment plan documents for every fraction. To account for physical changes in prostate, rectal, and RHS volumes, as well as contouring differences between two fractions for the same patient, dosimetric variables for first and second fractions were averaged.
Patient demographic and clinical characteristics were summarized by RHS and no-RHS groups. Comparison of dosimetry between RHS and no-RHS groups for the average of two fractions was done using Wilcoxon rank-sum tests. First and second fraction dosimetry were also compared between RHS and no-RHS groups without averaging for validation. The primary variables of interest were rectal V75% and prostate V100%. In addition, prostate V150% and V200%, dose to 100% (D100%), D75%, D50%, D25%, and D0.1% of the rectum, homogeneity index, and volumes of the prostate and rectum were also analyzed. Homogeneity index of each treatment plan was a ratio calculated by dividing V150% by V100%. Finally, generalized estimating equation (GEE) model was used to evaluate the effect of inter-fraction changes in rectum, prostate, and RHS volumes on rectum V75%. Analyses were done using R version 4.1.2 with 2-sided p < 0.05 to indicate statistical significance.
RESULTS
There were 475 patients in our prospectively collected database of prostate cancer patients undergoing HDR-BT during September, 2015, to November, 2021. Of these, 41 received salvage, whole-gland HDR-BT for intact, recurrent prostate cancer. There were 20 patients in the RHS group and 21 patients in the no-RHS group. Urologists did not report any technical challenges for RHS placement in any of the operative notes. Rectal infiltration was not documented in operative reports either. On post-RHS pelvic MRI, no patient had definitive radiographic evidence of rectal wall infiltration. Trans-rectal ultrasound visibility of the prostate and interstitial needles was excellent in both the RHS and no-RHS groups. There were no cases of iatrogenic bladder perforation in either group. Median follow-up was 4 months in the RHS group and 17 months in the no-RHS group. Median age was 73 years [Interquartile range (IQR): 67 – 76 years)] in the RHS group and 70 years (IQR:67–77) years in the no-RHS group. Conventionally or moderately hypofractionated intensity modulated radiotherapy was the most commonly used initial treatment modality in both groups, followed by proton beam therapy. Additional patient and treatment-related variables are presented in Table 1.
Table 1.
Baseline patient characteristics
| RHS Group (n=20) | No-RHS Group (N=21) | |
|---|---|---|
|
| ||
| Median age | 73 | 70 |
| IQR | 67–76 | 67–77 |
|
| ||
| Median follow-up (months) | 4 | 17 |
| IQR | 4–10 | 15–30 |
|
| ||
| T-Stage at recurrence | ||
| T1 | 16 (80%) | 18 (86%) |
| T2 | 3 (15%) | 2 (9.5%) |
| T3 | 1 (5) | 1 (4.8%) |
|
| ||
| Modality of first treatment | ||
| Conventional EBRT | 9 (45%) | 16 (76%) |
| SBRT | 2 (10%) | 1 (5%) |
| PBT | 6 (30%) | 4 (19%) |
| LDR-BT | 3 (15%) | 0 |
|
| ||
| Median duration between first RT course and salvage HDR-BT (months) | 93 | 116 |
| IQR | 84–162 | 108–142 |
|
| ||
| Median PSA at recurrence* (ng/mL) | 5.1 | 4.5 |
| IQR | 2.6–6.6 | 2.9–8.8 |
PSA values excluded patients who were on androgen deprivation therapy Abbreviations: EBRT = external beam radiotherapy; HDR-BT = high dose-rate brachytherapy; IQR = interquartile range; LDR-BT = low dose-rate brachytherapy; PBT = proton beam therapy; PSA = prostate specific antigen; RHS = rectal hydrogel spacer; SBRT = stereotactic body radiotherapy
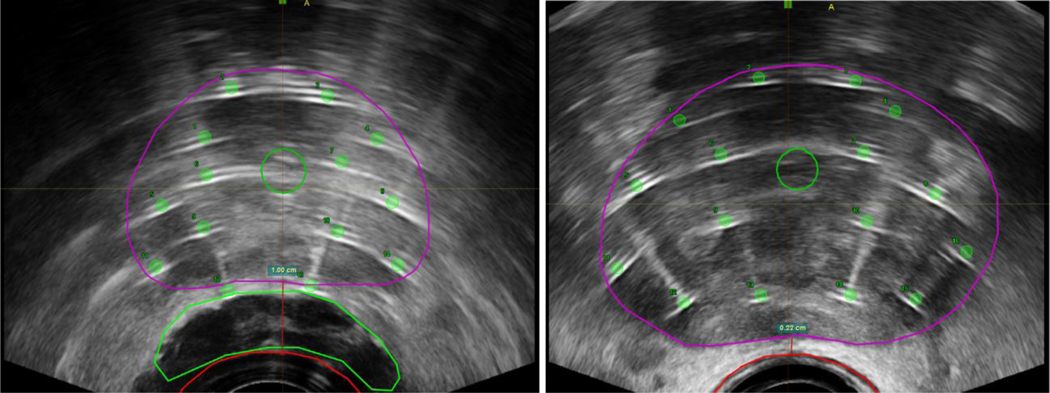
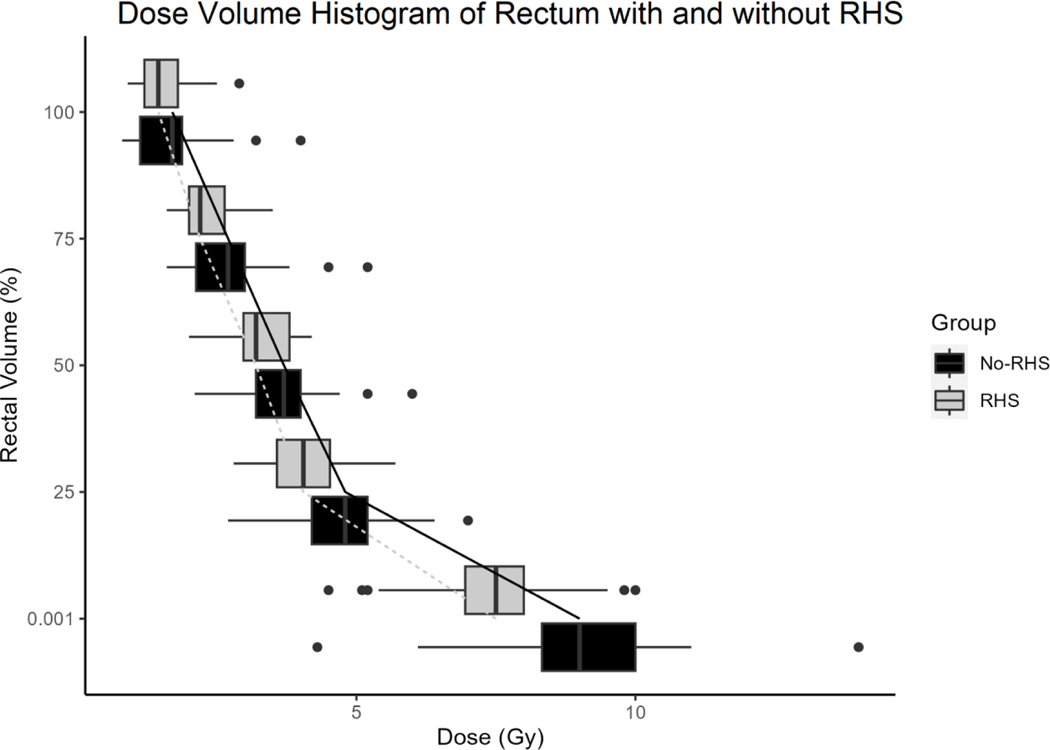
The 41 patients underwent a total of 82 fractions of prostate HDR-BT (2 fractions per patient). Mean RHS volume was 6.2 cm3 [Standard deviation (SD): ± 3.5 cm3] and mean prostate-rectum separation at mid-gland on pre-treatment MRI was 8.0 mm (SD: ± 2.8 mm). In the no-RHS group, all but 3 patients had direct contact between anterior rectal wall and prostate at mid-gland (2 patients with 1 mm fat plane separation and 1 patient with 2.5 mm separation). Axial and 3-dimensional views of target and avoidance structures on ultrasound imaging with and without RHS are shown in Figure 1. Mean prostate volume was 28.1 cm3 (SD: ± 6.4 cm3) in the RHS group and 27.0 cm3 (SD: ± 12.4 cm3) in the no-RHS group. Mean rectum volume was 22.6 cm3 (SD: ± 7.4 cm3) in the RHS group and 22.0 cm3 (SD: ± 7.8 cm3) in the no-RHS group. There was statistically significant improvement in the primary variables of interest in the RHS group. Median rectal V75% was 0.0 cm3 (IQR: 0.0 – 0.0 cm3) in the RHS group and 0.06 cm3 (IQR: 0.0 – 0.1 cm3) in the no-RHS group, p < 0.001. Dose-volume histogram of rectum D100%, D75%, D50%, D25%, and D0.01% in RHS and no-RHS groups is presented in Figure 2. Of these variables, D75%, D25%, and D0.1% were significantly lower in the RHS group at 2.3 Gy (IQR: 2.1 – 2.5 Gy) vs 2.7 Gy (IQR: 2.4 – 3.1 Gy), 4.2 Gy (IQR: 3.6 – 4.7 Gy) vs 4.7 Gy (IQR: 4.5 – 5.2 Gy), and 7.6 Gy (IQR 6.8 – 8.2 Gy) vs 9.0 Gy (8.9 – 9.8 Gy), respectively. Median prostate V100% was 98.6% (IQR: 97.9 – 99.2%) in the RHS group and 97.8% (IQR: 97.5 – 98.2%) in the no-RHS group, p = 0.007. Median homogeneity index in the no-RHS group was 0.38 (IQR: 0.36–0.40) and in the RHS group was 0.35 (IQR: 0.34–0.38), p = 0.10. A summary of dosimetric variables for the prostate and rectum is provided in Supplementary Table 1.
Figure 1.
Axial views of prostate (purple), rectum (red), and urethra and RHS (green) of salvage, whole-gland HDR-BT planned on ultrasound imaging with RHS (left) and without RHS (right). Images are captured at the time of treatment delivery. Prostate-rectum separation at mid-gland was 1.00cm with RHS, versus 0.22cm without RHS, in these cases.
Figure 2.
Dose volume histogram of rectum with and without RHS using D100%, D75%, D50%, D25%, and D0.1% datapoints. Median and interquartile range are demonstrated with box plots. Outliers are represented by bullet points.
Intra-fraction changes in RHS, rectum, and prostate volumes did not significantly affect rectum V75% on GEE modeling. Variation of intra-fraction RHS volume by 1 cm3 results in 0.0015 cm3 change in rectum V75% (95% CI: −0.0018 – 0.0047 cm3, p = 0.38). Changes in intra-fraction rectum and prostate volumes by 1 cm3 also had insignificant effects on rectum V75%: −0.0003 cm3 (95% CI: −0.0011 – 0.0006 cm3, p = 0.54) and 0.0008 cm3 (95% CI: −0.0011 – 0.0027 cm3, p = 0.39), respectively.
Acute and late toxicities were similar between RHS and no-RHS groups. In terms of GI toxicity, there was one grade 1 and one grade 2 acute toxicity, and one grade 3 late toxicity in the RHS group. The single grade 3 late toxicity was in a patient who developed an enterovesicular fistula and required diverting colostomy 4 months following his second salvage HDR-BT fraction. This patient originally received 79.2GyE in 44 fractions via proton beam therapy. In the no-RHS group, there was one grade 1 and one grade 2 acute toxicity, and no patient developed late toxicities. Additionally, there were 7 grade 1–2 acute GU toxicities in the RHS group and 6 grade 1–2 acute GU toxicities in the no-RHS group. One patient in each of the RHS and no-RHS groups developed late GU toxicity. Both late GU toxicities were grade 3 hematuria requiring cystoscopy and fulguration.
DISCUSSION
In our cohort of patients with locally recurrent prostate cancer treated with salvage whole-gland HDR-BT, there was statistically significant improvement in rectal dose constraints in the RHS group. The increased separation between prostate and rectum due to RHS resulted in significant dosimetric improvements including reduction of median rectal V75% from 0.06 cm3 to 0.00 cm3 and increase in prostate V100% from 97.8% to 98.6%. The clinical significance of these dosimetric gains, however, is questionable, as the absolute differences in dosimetric variables were small and descriptive toxicity profiles were similar. In both groups, there was one grade 1 and one grade 2 acute rectal toxicity. However, one patient in the RHS group developed a grade 3 late toxicity involving an enterovesicular fistula requiring diverting colostomy whereas none of the patients in the no-RHS group developed any late toxicity. There are two factors which may have precipitated this enterovesicular fistula. The first being occult infiltration of RHS into the rectal wall at the time of insertion. The second being proton beam therapy as the initial treatment eight years prior to salvage HDR-BT as historical studies have demonstrated increased GI toxicities with proton versus photon irradiation [19]. Nevertheless, we believe our results are meaningful for patients with local failure after definitive radiotherapy.
Rectum V75% is closely associated with rectal toxicities following brachytherapy and is used as the standard rectal constraint in both the definitive and salvage setting [20–22]. All patients readily met the goal of rectum V75% < 1.0cm3 in the absence of RHS. The maximum rectal V75% in the no-RHS group was 0.75 cm3 in our study, which happens to be the mean reported in a historical cohort [17]. Clinically, this translated to the miniscule frequency and severity of acute GI toxicities observed in both groups, as only 4 out of 41 total patients (10%) developed any acute GI toxicity. Assessment of late rectal sequelae is hindered by short follow-up in the RHS group. Nevertheless, only one patient developed any late GI toxicity in our entire cohort so we anticipate fewer than 20% of patients – which is the rate reported in a historical cohort of patients undergoing salvage HDR-BT – will develop late GI toxicity given longer follow-up [22].
Factors explaining our favorable dosimetry include use of real-time, trans-rectal, 3-dimensional ultrasound-based treatment planning and vast HDR-BT case volume at our institution. Accuracy of interstitial needle positioning at the base of the prostate along the bladder interface and in the peripheral zone adjacent to the rectum is challenging without real-time visualization. The prostate-bladder and prostate-rectum interfaces are readily identified on trans-rectal ultrasound which allows accurate placement of interstitial needles close to organs-at-risk but without iatrogenic injury. It has been demonstrated that utilizing 3-dimensional ultrasound guidance for needle placement and planning enhances treatment plan quality by providing sub-millimeter needle precision. This translates to improved dose conformality and rectum and bladder sparing [23]. The trans-rectal ultrasound probe also allows for manipulation of prostate and rectal positioning. Certain cases call for posterior pressure to be applied to the probe to reduce needle placement interference from the pubic arch. In patients receiving de-novo prostate radiotherapy with HDR-BT, the separation between the prostate and rectum may be artificially increased by this technique, thereby reducing dose to the anterior rectal wall in the absence of RHS. In patients undergoing prostate re-irradiation, this separation potential is still possible without RHS, but is greatly reduced by fibrosis of the prostate-rectum interface. Furthermore, in addition to the 41 patients treated with salvage HDR-BT during the time period of this study, 434 patients received either definitive prostate HDR-BT or prostate boost with HDR-BT following external beam irradiation to the pelvis. This accounts for nearly 1000 fractions in a 6-year interval, and the benefits of clinician experience may be intangible. The vast experience with whole-gland prostate HDR-BT aided by 3-dimensional ultrasound guidance at our institution allows our treatment planners to prioritize rectum sparing without sacrificing prostate coverage in patients without RHS. This may account for the low rectum V75% with or without RHS.
The ease of achieving the standardized treatment planning goals in our patients shows that there is room for dose intensification in salvage HDR-BT for improving biochemical control. Focal HDR-BT boost using MRI-guidance has been a recent area of investigation in the upfront prostate cancer setting and may improve efficacy of salvage treatment from historical 5-year biochemical control rates of 50–60% [24, 25]. Further, more stringent constraints may be implemented in addition to the existing rectum V75%. Low-dose to large-volume constraints such as rectum V100% have been associated with grade ≥ 2 proctitis and rectal bleeding [26]. Additionally, high-dose to small-volume constraints are also important to consider, as rectum V25% > 25% and V10% > 40% are significantly associated with late diarrhea [27]. Implementing additional dose constraints and intensifying treatment via focal boost to gross disease may yield improved safety and efficacy of salvage HDR-BT.
There are limitations to our study. Median follow-up was 17 months in the no-RHS group and 4 months in the RHS group. This short follow-up duration, in addition to small cohort size, results in inadequate evaluation of late toxicities. Additionally, the retrospective study design predisposes our patients to selection bias. Patients who received RHS may have had unfavorable anatomy such as large prostate volume or a history of GI comorbidities or toxicities owing to their first course of radiotherapy. All patients were treated with a regimen of 24Gy in 2 fractions which may not be generalizable to the entire population of patients receiving salvage HDR-BT. Other popular prescriptions include 36 Gy in 6 fractions and 32 Gy in 4 fractions [8, 22]. Our dose prescription of 24 Gy in 2 fractions may have played a role in our low rate of rectal toxicities as this two-fraction course can be more sparing for normal tissues while delivering equivalent BED2Gy to the prostate. Prostate-rectum separation was also measured on pre-treatment MRI, and may not accurately represent prostate-rectum separation intraoperatively. It is possible that prostate-rectum separation was artificially increased on a case-by-case basis in the no-RHS group by applying posterior pressure on the ultrasound probe. Lastly, patients with RHS had marginal improvement in dosimetry compared to historical patient cohorts. Relative decreases in rectum V75% have been reported as high as 70% with use of RHS in the external beam setting [28]. In such reports, 10cm3 is a standard RHS volume achieved compared to our cohort in which the median RHS volume was 6.2cm3 [29]. The reduced volume of injected RHS highlights the difficulty of dissecting the prostate-rectum plane in previously radiated fields. To reduce the impact of this obstacle, future studies in salvage HDR-BT may select patients for RHS implantation who had lower rectal doses from initial courses of radiotherapy and avoided treatments with high relative biologic effectiveness.
CONCLUSION
Salvage HDR-BT is an underutilized modality for treatment of locally recurrent, intact prostate cancer but is safe and yields low rates of acute and late GI toxicities when performed at high volume centers. Rectal hydrogel spacer marginally improves rectal sparing and prostate dose coverage in the setting of readily achievable constraints. Larger cohorts with longer follow-up are needed to further elucidate the benefit of RHS for patients undergoing salvage HDR-BT.
Supplementary Material
Funding:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA006927. This grant supports research at all levels of our institution. The authors have not personally received funding for this study.
Footnotes
DISCLOSURES
This work was presented as a poster at the 2022 American Society of Radiation Oncology Annual Meeting in San Antonio, TX.
Ethics: This study has been approved by the Institutional Review Board of our institution (IRB# 20–9074). Waiver of informed consent was granted on the basis that this research involves no more than minimal risk to participants, waiver of informed consent will not adversely affect the rights and welfare of the subjects, and it is not practicable to conduct this retrospective work without such waiver.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability:
All relevant data generated and analyzed during this study are included in this article. Access to data with patient identifiers is prohibited.
REFERENCES
- 1.Cooperberg MR, Vickers AJ, Broering JM, Carroll PR. Comparative risk-adjusted mortality outcomes after primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer 2010; 116: 5226–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haj-Hamed M, Karivedu V, Sidana A. Salvage treatment for radio-recurrent prostate cancer: a review of literature with focus on recent advancements in image-guided focal salvage therapies. Int Urol Nephrol 2019; 51: 1101–1106. [DOI] [PubMed] [Google Scholar]
- 3.Valle LF, Lehrer EJ, Markovic D et al. A Systematic Review and Meta-analysis of Local Salvage Therapies After Radiotherapy for Prostate Cancer (MASTER). Eur Urol 2021; 80: 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong J, Slevin F, Scarsbrook AF et al. Salvage Reirradiation Options for Locally Recurrent Prostate Cancer: A Systematic Review. Frontiers in Oncology 2021; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zilli T, Benz E, Dipasquale G et al. Reirradiation of Prostate Cancer Local Failures After Previous Curative Radiation Therapy: Long-Term Outcome and Tolerance. Int J Radiat Oncol Biol Phys 2016; 96: 318–322. [DOI] [PubMed] [Google Scholar]
- 6.Chen WC, Li YR, Nano T et al. Stereotactic Body Radiotherapy (SBRT) as Salvage Treatment for Locally Recurrent Prostate Cancer. International Journal of Radiation Oncology Biology Physics 2020; 108: E911–E912. [Google Scholar]
- 7.Leroy T, Lacornerie T, Nickers P et al. Robotic SBRT for Locally Advanced Prostate Cancer Recurrence Following Radiation Therapy: Preliminary Results of a Single Institution. International Journal of Radiation Oncology Biology Physics 2015; 93: E200–E200. [Google Scholar]
- 8.Lee B, Shinohara K, Weinberg V et al. Feasibility of high-dose-rate brachytherapy salvage for local prostate cancer recurrence after radiotherapy: the University of California-San Francisco experience. Int J Radiat Oncol Biol Phys 2007; 67: 1106–1112. [DOI] [PubMed] [Google Scholar]
- 9.Chen CP, Weinberg V, Shinohara K et al. Salvage HDR Brachytherapy for Recurrent Prostate Cancer After Previous Definitive Radiation Therapy: 5-Year Outcomes. International Journal of Radiation Oncology Biology Physics 2013; 86: 324–329. [DOI] [PubMed] [Google Scholar]
- 10.Jiang P, van der Horst C, Kimmig B et al. Interstitial high-dose-rate brachytherapy as salvage treatment for locally recurrent prostate cancer after definitive radiation therapy: Toxicity and 5-year outcome. Brachytherapy 2017; 16: 186–192. [DOI] [PubMed] [Google Scholar]
- 11.Lopez IH, Gonzalez-San Segundo C, Vegas JO et al. Salvage brachytherapy for locally-recurrent prostate cancer after radiation therapy: A comparison of efficacy and toxicity outcomes with high-dose rate and low-dose rate brachytherapy. Radiotherapy and Oncology 2019; 141: 156–163. [DOI] [PubMed] [Google Scholar]
- 12.van Son M, Peters M, Moerland M et al. Determining the safety of ultrafocal salvage high-dose-rate brachytherapy for radiorecurrent prostate cancer: A toxicity assessment of 150 patients. Clin Transl Radiat Oncol 2021; 27: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corkum MT, Morton G, Loblaw DA et al. A Prospective Study of Magnetic Resonance Imaging-guided Focal Salvage High-dose-Rate Brachytherapy for Radiorecurrent Prostate Cancer: Updated Results of 30 Patients. Pract Radiat Oncol 2022; 12: e531–e537. [DOI] [PubMed] [Google Scholar]
- 14.Mariados N, Sylvester J, Shah D et al. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. International Journal of Radiation Oncology Biology Physics 2015; 92: 971–977. [DOI] [PubMed] [Google Scholar]
- 15.Yeh J, Lehrich B, Tran C et al. Polyethylene glycol hydrogel rectal spacer implantation in patients with prostate cancer undergoing combination high-dose-rate brachytherapy and external beam radiotherapy. Brachytherapy 2016; 15: 283–287. [DOI] [PubMed] [Google Scholar]
- 16.Chao M, Ow D, Ho H et al. Improving rectal dosimetry for patients with intermediate and high-risk prostate cancer undergoing combined high-dose-rate brachytherapy and external beam radiotherapy with hydrogel space. Journal of Contemporary Brachytherapy 2019; 11: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baghwala A, Khwaja D, Boopathy R. Reduction in Rectal Dose with the Use of SpaceOAR Hydrogel to Treat Prostate Adenocarcinoma with HDR Brachytherapy. Medical Physics 2019; 46: E533–E533. [Google Scholar]
- 18.Taggar AS, Charas T, Cohen GN et al. Placement of an absorbable rectal hydrogel spacer in patients undergoing low-dose-rate brachytherapy with palladium-103. Brachytherapy 2018; 17: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheets NC, Goldin GH, Meyer AM et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 2012; 307: 1611–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gokhale AS, Beriwal S, Smith RP et al. Clinical and dosimetric factors associated with acute rectal toxicity in patients treated with Cs-131 brachytherapy for prostate cancer. Brachytherapy 2010; 9: 328–334. [DOI] [PubMed] [Google Scholar]
- 21.Ohashi T, Yorozu A, Toya K et al. Rectal morbidity following I-125 prostate brachytherapy in relation to dosimetry. Japanese Journal of Clinical Oncology 2007; 37: 121–126. [DOI] [PubMed] [Google Scholar]
- 22.Wu SY, Wong AC, Shinohara K et al. Salvage High-Dose-Rate Brachytherapy for Recurrent Prostate Cancer After Definitive Radiation. Pract Radiat Oncol 2021; 11: 515–526. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee S, Kataria T, Gupta D et al. Use of ultrasound in image-guided high-dose-rate brachytherapy: enumerations and arguments. Journal of Contemporary Brachytherapy 2017; 9: 146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alayed Y, D’Alimonte L, Helou J et al. MRI assisted focal boost integrated with HDR monotherapy study in low and intermediate risk prostate cancer (MARS): Results from a phase II clinical trial. Radiotherapy and Oncology 2019; 141: 144–148. [DOI] [PubMed] [Google Scholar]
- 25.Menard C, Navarro-Domenech I, Liu ZH et al. MRI-guided focal or integrated boost high dose rate brachytherapy for recurrent prostate cancer. Frontiers in Oncology 2022; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price J, Stone NN, Stock RG. Predictive Factors and Management of Rectal Bleeding Side Effects Following Prostate Cancer Brachytherapy. International Journal of Radiation Oncology Biology Physics 2013; 87: S355–S356. [DOI] [PubMed] [Google Scholar]
- 27.Shah JN, Ennis RD. Rectal toxicity profile after transperineal interstitial permanent prostate brachytherapy: Use of a comprehensive toxicity scoring system and identification of rectal dosimetric toxicity predictors. International Journal of Radiation Oncology Biology Physics 2006; 64: 817–824. [DOI] [PubMed] [Google Scholar]
- 28.Te Velde BL, Westhuyzen J, Awad N et al. Late toxicities of prostate cancer radiotherapy with and without hydrogel SpaceAOR insertion (vol 63, pg 836, 2019). Journal of Medical Imaging and Radiation Oncology 2020; 64: 306–306. [DOI] [PubMed] [Google Scholar]
- 29.Babar M, Katz A, Ciatto M. Dosimetric and clinical outcomes of SpaceOAR in men undergoing external beam radiation therapy for localized prostate cancer: A systematic review. Journal of Medical Imaging and Radiation Oncology 2021; 65: 384–397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data generated and analyzed during this study are included in this article. Access to data with patient identifiers is prohibited.