Abstract
Objectives:
While racial and ethnic differences in acute osteomyelitis incidence have been found, evaluation of disparities in outcomes such as length of stay (LOS) by race and ethnicity has been limited. We examined the association between race and ethnicity and LOS for children with acute osteomyelitis in the US.
Study design:
Using the Kids’ Inpatient Database, we conducted a cross-sectional study of children <21 years old hospitalized in 2016 or 2019 with acute osteomyelitis. Using survey-weighted negative binomial regression, we modeled LOS by race and ethnicity, adjusting for clinical and hospital characteristics and socioeconomic status. Secondary outcomes included prolonged LOS, defined as LOS exceeding 7 days (equivalent to LOS in the highest quartile).
Results:
We identified 2,388 children discharged with acute osteomyelitis. Median LOS was 5 days (IQR 3–7). Compared with White children, children of Black race (adjusted incidence rate ratio [aIRR] 1.15, 95%CI 1.05–1.27), Hispanic ethnicity (aIRR 1.11, 95%CI 1.02–1.21), and other race and ethnicity (aIRR 1.12, 95%CI 1.01–1.23) had significantly longer LOS. The odds of Black children experiencing prolonged LOS was 46% higher compared to White children (aOR 1.46, 95%CI 1.01–2.11).
Conclusions:
Children of Black race, Hispanic ethnicity, and other race and ethnicity with acute osteomyelitis experienced longer LOS than White children. Elucidating the mechanisms underlying these race- and ethnicity-based differences—including social drivers such as access to care, structural racism, and bias in provision of inpatient care—may improve management and outcomes for children, and particularly Black and brown children, with acute osteomyelitis.
Keywords: Osteomyelitis, pediatric, health care disparities
Background
The incidence of acute osteomyelitis has increased over the past two decades in the US.1–3 Decreasing hospital length of stay (LOS) has been a primary focus of efforts to improve osteomyelitis care for children.4–6 To date, most efforts aimed at reducing LOS for children with acute osteomyelitis have aimed to decrease use of long-term intravenous antibiotics, supported by data showing comparable outcomes with fewer complications among children receiving oral rather than IV antibiotics.7, 8
Prior studies have identified disparities in hospitalization rates for children with acute osteomyelitis.9, 10 Meanwhile, racial and ethnic disparities in hospital LOS have been found among children with septic arthritis, and these disparities have been found to persist despite broader improvements in LOS for these children.11 Yet despite its importance for children’s well-being, and its relevance as a quality marker for musculoskeletal infection care,5 LOS has not been systematically evaluated as a key outcome in studies evaluating social drivers of health in patients children with acute osteomyelitis.
To fill this gap, we investigated racial and ethnic differences in hospital LOS among children presenting with acute osteomyelitis. Differences in health outcomes are “disparities”—and represent inequity—when they are linked to social and economic disadvantage.12 Importantly, increasing LOS for an individual child with osteomyelitis can be appropriate and equitable care, such as when a patient presents with more severe disease or when discharge safety is not assured. Nonetheless, we interpret race and ethnicity-based population differences in LOS for children with acute osteomyelitis as representing disparities resulting from social drivers of health. Uncovering and describing these population effects is important for directing future research and creating interventions. We hypothesized that racial and ethnic disparities are evident in hospital LOS among US children presenting with acute osteomyelitis, despite overall recent trends towards decreasing LOS. Furthermore, we hypothesized that these disparities would persist even when accounting for debridement procedures and central venous catheter (CVC) insertion.
Methods
We conducted a cross-sectional study of children and adolescents (henceforth “children”) hospitalized in 2016 and 2019 and included in the Kids’ Inpatient Database (KID). These study years were selected to leverage exclusive use of ICD-10 codes in the database. The database uses discharge abstracts for hospitalized children <21 years old, derived from administrative billing data. The KID is nationally representative, with 48 states and the District of Columbia contributing to the database. The KID does not include outpatient or longitudinal data. The database reports data for individual discharges (i.e. the unit of analysis is discharges rather than patients). Our study followed STROBE guidelines for reporting of observational studies.13 The study was reviewed by the Boston Children’s Hospital institutional review board and was deemed exempt.
Study population
We identified all children ≥1 year old with acute osteomyelitis, defined using ICD-10 billing codes M86.0 and M86.1 in the first or second diagnosis code field, as has been previously used to document temporal trends in osteomyelitis incidence in the United States (online table 1)2 We excluded discharges associated with: (1) hardware-related infections; (2) LOS greater than 42 days (i.e., longer than standard course of antibiotics for acute osteomyelitis) or less than 2 days (which likely would not represent hospitalization for acute osteomyelitis based on our clinical experience and previously-reported LOS ranges1, 2); (3) transfer from or to another acute care hospital; (4) debridement procedure performed prior to hospitalization (day of operation listed as before the day of hospitalization in the database); and (5) associated endocarditis, sickle cell disease, or severe sepsis or septic shock14, 15 (i.e., separate disease processes that would independently affect management and prolong hospitalization). Exclusion variables were defined using diagnostic and procedure codes (online table 1), or length of stay and procedure timing data routinely reported in the KID.
Online table 1.
Billing and diagnostic codes used in the analysis.
| Variable | Codes |
|---|---|
| Acute osteomyelitis | ICD-10 M86.0 |
| ICD-10 M86.1 | |
| Severe sepsis | ICD-10 R65.20 |
| ICD-10 R65.21 | |
| Sickle cell disease | ICD-10 D57 |
| Hardware-related infection | ICD-10 T84 |
| ICD-10 T85 | |
| Endocarditis | ICD-10 I33 |
| ICD-10 I38 | |
| ICD-10 I39 | |
| CVC placement | ICD-10-PR 05H503Z |
| ICD-10-PR 05H533Z | |
| ICD-10-PR 05H543Z | |
| ICD-10-PR 05H603Z | |
| ICD-10-PR 05H633Z | |
| ICD-10-PR 05H643Z | |
| ICD-10-PR 02H603Z | |
| ICD-10-PR 02H633Z | |
| ICD-10-PR 02H643Z | |
| ICD-10-PR 02HV33Z | |
| Debridement Procedure | ICD-10-PR 0J[9/B/T/D/C] |
| ICD-10-PR 0K[9/B/T/D/C] | |
| ICD-10-PR 0L[9/B/T/D/C] | |
| ICD-10-PR 0M[9/B/T/D/C] | |
| ICD-10-PR 0N[9/B/T/D/C] | |
| ICD-10-PR 0N[9/B/T/D/C] | |
| ICD-10-PR 0N[9/B/T/D/C] | |
| ICD-10-PR 0P[9/B/T/D/C] | |
| ICD-10-PR 0Q[9/B/T/D/C] | |
| ICD-10-PR 0R[9/B/T/D/C] | |
| ICD-10-PR 0S[9/B/T/D/C] | |
| ICD-10-PR 0W[9/B/T/D/C] | |
| ICD-10-PR 0W[9/B/T/D/C] | |
| ICD-10-PR 0X[9/B/T/D/C] | |
| ICD-10-PR 0Y[9/B/T/D/C] |
Outcomes
The primary outcome was hospital LOS in days, as recorded in the KID database. Secondary outcomes were (1) prolonged LOS, defined as LOS in the highest quartile (equivalent to more than 7 days of hospitalization), (2) placement of a CVC, (3) performance of a debridement procedure, and (4) days to first debridement (among patients who underwent a debridement procedure).
Exposures
The primary exposure was race and ethnicity category. We grouped race and ethnicity into the following categories, as provided in the KID database: White, Black, Hispanic, other race and ethnicity (including Asian or Pacific Islander, Native American, or “other”), and missing. We included a missing race and ethnicity category in the analysis because refusal or failure to report race and ethnicity may be meaningful unto itself, as has been previously used in analyses from the KID.16
Covariates
Covariates were age (in years), sex, insurance status (grouped as: Medicaid or self-pay; private; and Medicare/no charge/other), ZIP code median income quartile, presence of a chronic complex condition,17 hospital location/teaching status (rural, urban non-teaching, urban teaching), hospital region (Northeast, Midwest, South, West), hospital size (small, medium, or large; based on number of beds normalized to hospital region), admission on a weekend vs. weekday, admission quarter (to align our analysis with prior studies that have evaluated seasonality in pediatric osteomyelitis outcomes 18), and year of hospitalization (2016 or 2019). We also included placement of a CVC and use of a debridement procedure in the main model.
Analysis
We used descriptive statistics to present characteristics of the overall cohort, exposures, covariates, and outcomes, and used the chi-squared test to compare proportions of covariates by exposures. We used proportions to summarize categorical variables, and medians and interquartile ranges to summarize continuous variables.
Because LOS was right-skewed and over-dispersed (alpha for the main model was 0.15 [95%CI 0.13–0.18]), we used negative binomial regression with a maximum likelihood estimator to construct univariable and multivariable models of the relationship between exposures, covariates, and LOS.19, 20 Results are presented as unadjusted and adjusted incident rate ratios (IRRs). To facilitate interpretation of results, we computed the marginal mean LOS by race and ethnicity, setting continuous variables at their means and treating categorical variables as having a balanced number of discharges in each category. We used an adjusted Wald test to compare marginal mean predicted LOS between race and ethnicity groups.
To evaluate secondary outcomes, first, we used logistic regression to test whether race and ethnicity was associated with prolonged (i.e. highest quartile) LOS. We used multivariable logistic regression to determine adjusted odds of CVC placement, and then conducted a stratified analysis using multivariable negative binomial regression to test differences in LOS among children who (a) did and (b) did not have a CVC placed. As with the analysis of CVC placement, we used multivariable logistic regression to determine adjusted odds of undergoing debridement, and then conducted stratified analysis using multivariable negative binomial regression to test differences in LOS among children who (a) did and (b) did not have a debridement procedure. We conducted these stratified analyses to isolate the effects of race/ethnicity from the effects of undergoing CVC placement or debridement. Fourth, we used negative binomial regression to model the effect of race and ethnicity on length of time from admission to first debridement procedure, for patients who underwent debridement.
Due to the relatively high proportion of patients with missing race and ethnicity data (8.6%), we conducted multiple sensitivity analyses in addition to the primary analysis. Based on the primary multivariable negative binomial regression model, first, we conducted a complete case analysis in which patients with missing race and ethnicity data were removed. Second, we sequentially classified patients with missing race and ethnicity as White, Black, Hispanic, and other race/ethnicity. Finally, we conducted a sensitivity analysis including patients with sickle cell disease.
All analyses were performed using hospital sampling weights to account for the weighted survey design of the KID database, unless otherwise noted. All analyses were conducted with Stata v17.0 (College Station, Texas).
Results
Out of 6,206,696 (12,168,823 weighted) discharges in the combined KID database, 2388 (3277 weighted) discharges were included in the primary analysis of acute osteomyelitis. Figure 1 illustrates the total number of included and excluded discharges. Among discharges in the primary analysis, median LOS was 5 days (IQR 3–7). Table 2 summarizes covariates by race and ethnicity for discharges included in the primary analysis. The distribution of LOS by race and ethnicity is presented in figure 2.
Figure 1.
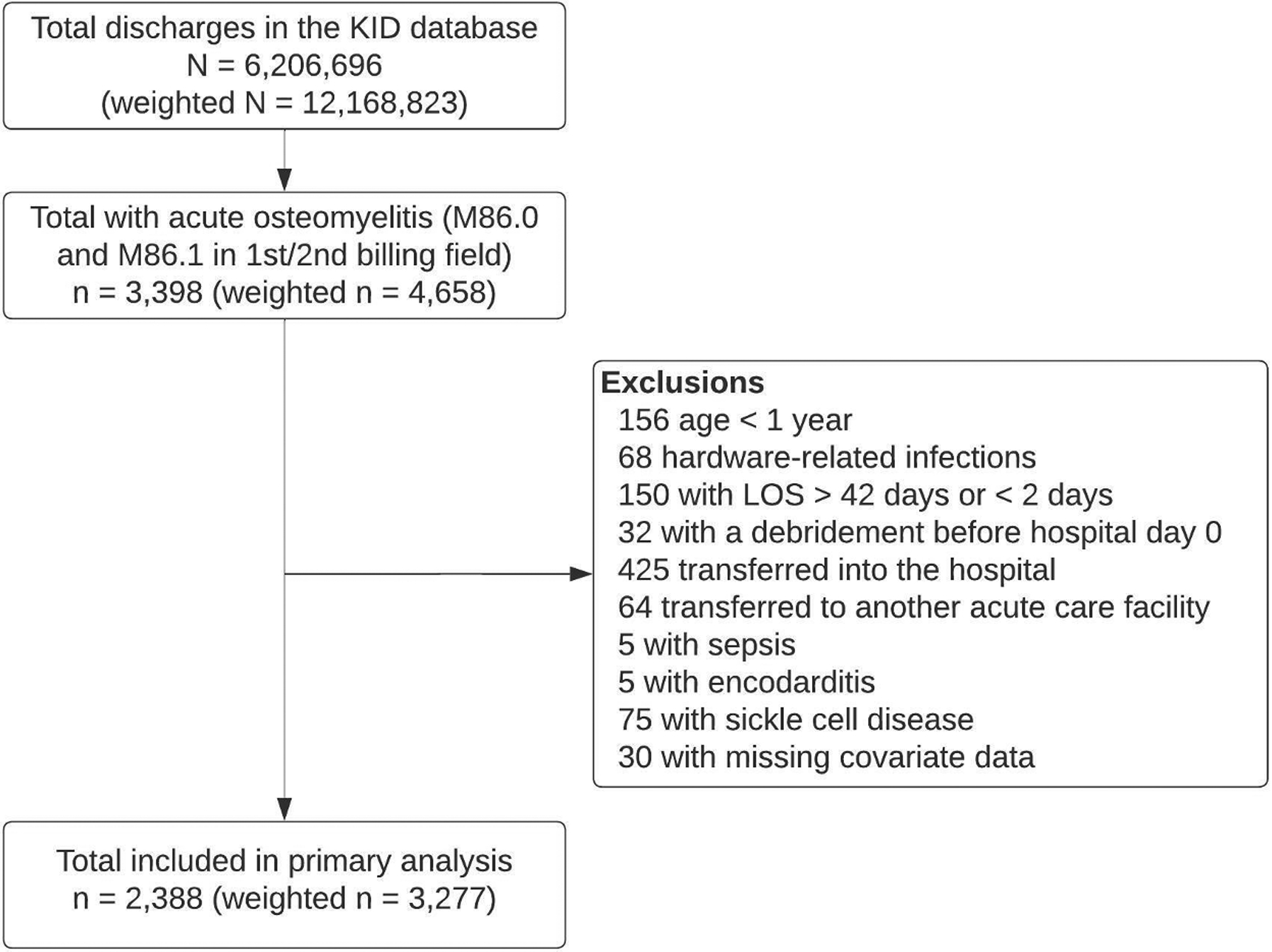
Discharges included in the primary analysis.
Table 2.
Comparison of exposures and covariates. Numbers and percentages reflect sample weighting.
| White (n=1643) | Black (n = 422) | Hispanic (n = 627) | Other (n = 293) | Missing (n = 291) | Total (N=3277) | Chi2 P-value | |
|---|---|---|---|---|---|---|---|
| Age (median, IQR) | 9 (5–13) | 9 (6–13) | 9 (5 – 12) | 8 (5 – 12) | 8 (4 – 12) | 9 (5–12) | - |
| Sex | 0.014 | ||||||
| Male | 985 (60%) | 284 (67%) | 410 (65%) | 161 (55%) | 180 (62%) | 2021 (62%) | |
| Female | 658 (40%) | 138 (33%) | 217 (35%) | 132 (45%) | 111 (38%) | 1256 (38%) | |
| Insurance | <0.001 | ||||||
| Medicaid/uninsured | 523 (32%) | 311 (74%) | 445 (71%) | 140 (48%) | 140 (48%) | 1559 (48%) | |
| Private | 1074 (65%) | 99 (23%) | 121 (19%) | 138 (47%) | 148 (51%) | 1580 (48%) | |
| Medicare/no charge/other | 46.53 (2.8%) | 12 (3%) | 61 (10%) | 15 (5%) | 3 (1%) | 138 (4%) | |
| Complex chronic condition | 214 (13%) | 80 (19%) | 103 (16%) | 27 (9%) | 44 (15%) | 469 (14%) | 0.010 |
| Debridement procedure | 1025 (62%) | 288 (68%) | 348 (56%) | 180 (62%) | 167 (57%) | 2009 (61%) | 0.006 |
| CVC | 413 (25%) | 118 (28%) | 178 (28%) | 73 (25%) | 55 (19%) | 838 (26%) | 0.375 |
| Hospital location | 0.006 | ||||||
| Rural | 25 (2%) | 8 (2%) | 5 (1%) | 0 (0%) | 7 (2%) | 45 (1%) | |
| Urban non-teaching | 80 (5%) | 26 (6%) | 56 (9%) | 8 (3%) | 4 (1%) | 174 (5%) | |
| Urban teaching | 1539 (94%) | 389 (92%) | 565 (90%) | 285 (97%) | 280 (96%) | 3058 (93%) | |
| Hospital region | <0.001 | ||||||
| Northeast | 274 (17%) | 65 (16%) | 72 (12%) | 68 (23%) | 20 (7%) | 500 (15%) | |
| Midwest | 441 (27%) | 101 (24%) | 48 (8%) | 41 (14%) | 77 (26%) | 709 (22%) | |
| South | 623 (38%) | 216 (51%) | 240 (38%) | 81 (28%) | 152 (52%) | 1312 (40%) | |
| West | 305 (19%) | 40 (9%) | 266 (42%) | 103 (35%) | 42 (15%) | 756 (23%) | |
| Hospital size | 0.793 | ||||||
| Small | 236 (14%) | 62 (15%) | 68 (11%) | 59 (20%) | 55 (19%) | 480 (15%) | |
| Medium | 383 (23%) | 98 (23%) | 142 (23%) | 49 (17%) | 58 (20%) | 730 (22%) | |
| Large | 1025 (62%) | 263 (62%) | 417 (67%) | 185 (63%) | 178 (61%) | 2067 (63%) | |
| Weekend admission | 283 (17%) | 89 (21%) | 128 (20%) | 55 (19%) | 49 (17%) | 603 (18%) | 0.323 |
| Discharge quarter | 0.319 | ||||||
| First | 404 (25%) | 88 (21%) | 172 (27%) | 70 (24%) | 67 (23%) | 802 (25%) | |
| Second | 354 (22%) | 104 (25%) | 149 (24%) | 76 (26%) | 73 (25%) | 756 (23%) | |
| Third | 487 (30%) | 125 (30%) | 159 (25%) | 69 (24%) | 68 (23%) | 909 (28%) | |
| Fourth | 398 (24%) | 105 (25%) | 146 (23%) | 77 (26%) | 84 (29%) | 810 (25%) | |
| Discharge year | 0.258 | ||||||
| 2016 | 862 (53%) | 215 (51%) | 325 (52%) | 120 (41%) | 195 (67%) | 1716 (52%) | |
| 2019 | 781 (47%) | 207 (49%) | 302 (48%) | 173 (59%) | 97 (33%) | 1561 (48%) | |
| ZIP code median income quartile | <0.001 | ||||||
| First (poorest) | 314 (19%) | 222 (53%) | 284 (40%) | 59 (10%) | 52 (18%) | 895 (27%) | |
| Second | 376 (23%) | 106 (25%) | 161 (26%) | 44 (15%) | 55 (19%) | 743 (23%) | |
| Third | 418 (25%) | 46 (11%) | 139 (22%) | 58 (20%) | 78 (27%) | 739 (22%) | |
| Fourth (wealthiest) | 536 (33%) | 48 (11%) | 78 (13%) | 132 (45%) | 107 (37%) | 901 (27%) | |
| Outcome | |||||||
| LOS, median (IQR) | 5 (3–6) | 5 (4–9) | 5 (4–8) | 5 (3–7) | 5 (3–7) | 5 (3–7) | |
| Days to first debridement, median (IQR) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | |
| Prolonged LOS [highest quartile], n (%) | 281 (17%) | 126 (30%) | 166 (27%) | 71 (24%) | 59 (20%) | 703 (22%) | <0.001 |
Abbreviations: CVC – central venous catheter; IQR – interquartile range; LOS – length of stay; ZIP code - Zone Improvement Plan code
Figure 2.
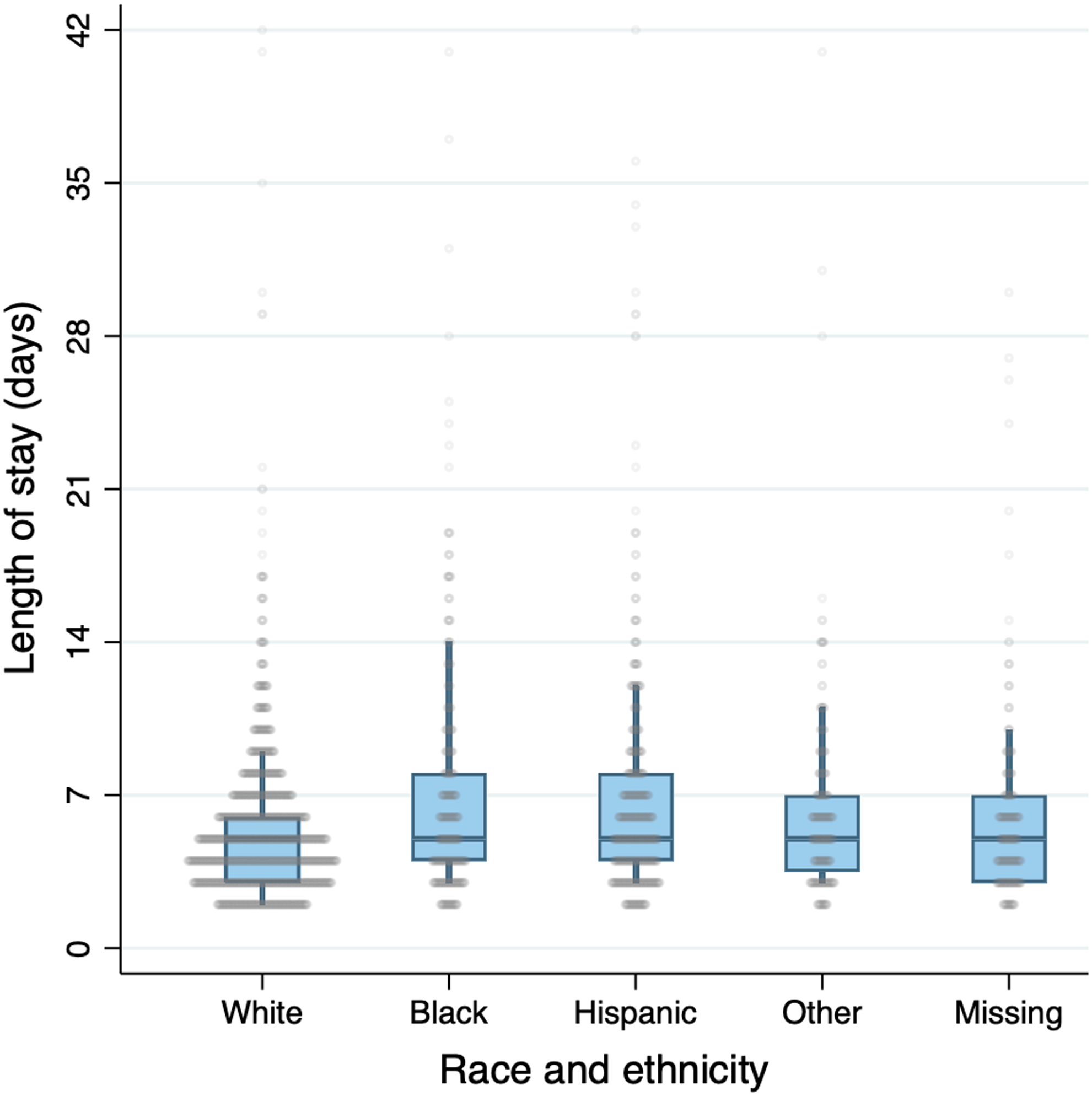
Distribution of LOS by race/ethnicity. Circles represent discharges. Boxes represent median/IQR, and whiskers represent 90%ile range. Unweighted distributions are presented.
Table 3 summarizes length of stay by race and ethnicity, and by covariates in univariable and multivariable analyses. In the multivariable analysis adjusting for CVC placement, debridement, medical complexity, demographic, and hospital variables, children of Black race (aIRR 1.15 [95%CI 1.05–1.27], P = 0.004), Hispanic ethnicity (aIRR 1.11 [95%CI 1.02–1.21], P=0.014), and other race and ethnicity (aIRR 1.12 [95%CI 1.01–1.28], P=0.024) had longer LOS on average, compared to White race. The marginal mean LOS for Black patients (6.6 days [95%CI 5.6–7.6]), Hispanic patients (6.4 days [95%CI 5.5–7.3]), and patients of other race and ethnicity (6.4 days [95%CI 5.5–7.3]) were significantly longer than the mean LOS for White patients (5.7 days [95%CI 5.0–6.4]) (adjusted Wald test P=0.008, P=0.019, and P=0.031 respectively) (online figure 3). The marginal mean LOS for patients with missing race/ethnicity (6.3 days [95%CI 5.3–7.2]) was not significantly different than white race (P=0.090).
Table 3.
Median LOS, and incidence rate ratios (IRR) and confidence intervals from unadjusted and adjusted negative binomial regression models. All analyses account for survey weighting.
| Median LOS (IQR) | Univariable Analysis | Multivariable analysis* | |||||
|---|---|---|---|---|---|---|---|
| IRR | 95%CI | P-value | aIRR | 95%CI | P-value | ||
| Race/ethnicity | |||||||
| White | 5 (3–6) | 1.00 | - | - | 1.00 | - | - |
| Black | 5 (4–9) | 1.29 | 1.17–1.42 | <0.001 | 1.15 | 1.05–1.27 | 0.004 |
| Hispanic | 5 (4–8) | 1.23 | 1.12–1.35 | <0.001 | 1.11 | 1.02–1.21 | 0.014 |
| Other | 5 (3–7) | 1.15 | 1.02–1.30 | 0.025 | 1.12 | 1.01–1.23 | 0.024 |
| Missing | 5 (3–7) | 1.09 | 0.98–1.20 | 0.110 | 1.10 | 0.99–1.21 | 0.077 |
| Age (per year) | - | 1.00 | 0.99–1.01 | 0.256 | 1.00 | 0.99–1.00 | 0.585 |
| Sex | |||||||
| Male | 5 (3–7) | 1.00 | - | - | 1.00 | - | - |
| Female | 5 (3–7) | 0.99 | 0.94–1.05 | 0.831 | 1.00 | 0.95–1.05 | 0.900 |
| Payer | |||||||
| Private | 5 (3–6) | 1.00 | - | - | 1.00 | - | - |
| Medicaid/self-pay | 5 (4–8) | 1.25 | 1.18–1.32 | <0.001 | 1.14 | 1.07–1.21 | <0.001 |
| Medicare/no charge/other | 5 (3–8) | 1.33 | 1.11–1.59 | 0.002 | 1.21 | 1.02–1.45 | 0.033 |
| Hospital location/teaching status | |||||||
| Rural | 4 (2–5) | 1.00 | - | - | 1.00 | - | - |
| Urban nonteaching | 5 (3–7) | 1.40 | 0.97–2.01 | 0.071 | 1.41 | 1.07–1.87 | 0.016 |
| Urban teaching | 5 (3–7) | 1.29 | 0.95–1.74 | 0.106 | 1.36 | 1.09–1.71 | 0.007 |
| Hospital region | |||||||
| Northeast | 5 (3–6) | 1.00 | - | - | 1.00 | - | - |
| Midwest | 5 (3–6) | 1.01 | 0.92–1.11 | 0.877 | 0.99 | 0.91–1.09 | 0.858 |
| South | 5 (4–7) | 1.11 | 1.02–1.22 | 0.017 | 1.09 | 1.00–1.18 | 0.053 |
| West | 5 (4–7) | 1.12 | 0.99–1.26 | 0.067 | 1.08 | 0.98–1.19 | 0.122 |
| KID Vintage Year | |||||||
| 2016 | 5 (3–7) | 1.00 | - | - | 1.00 | - | - |
| 2019 | 5 (3–7) | 0.99 | 0.92–1.07 | 0.767 | 1.01 | 0.95–1.08 | 0.705 |
| Debridement (Ref: no debridement) | |||||||
| No | 4 (3–6) | 1.00 | - | - | 1.00 | - | - |
| Yes | 5 (4–8) | 1.35 | 1.27–1.44 | <0.001 | 1.31 | 1.23–1.38 | <0.001 |
| CVC placed (Ref: no CVC) | |||||||
| No | 4 (3–6) | 1.00 | - | - | 1.00 | - | - |
| Yes | 6 (4–9) | 1.44 | 1.33–1.56 | <0.001 | 1.41 | 1.31–1.51 | <0.001 |
| Complex chronic condition | |||||||
| No | 5 (3–7) | 1.00 | - | - | 1.00 | - | - |
| Yes | 6 (4–9) | 1.25 | 1.14–1.37 | <0.001 | 1.21 | 1.11–1.33 | <0.001 |
| Admission on a weekend | |||||||
| No | 5 (3–7) | 1.00 | - | - | 1.00 | - | - |
| Yes | 5 (3–7) | 1.01 | 0.93–1.09 | 0.866 | 0.99 | 0.93–1.07 | 0.883 |
| Discharge quarter | |||||||
| Quarter 1 | 5 (3–7) | 1.00 | - | - | 1.00 | - | - |
| Quarter 2 | 5 (3–7) | 1.03 | 0.96–1.11 | 0.378 | 1.02 | 0.95–1.10 | 0.515 |
| Quarter 3 | 5 (3–7) | 1.00 | 0.92–1.08 | 0.982 | 1.01 | 0.94–1.08 | 0.866 |
| Quarter 4 | 5 (3–7) | 1.02 | 0.94–1.10 | 0.658 | 1.00 | 0.94–1.08 | 0.902 |
| Hospital size | |||||||
| Small | 5 (3–7) | 1.00 | - | - | 1.00 | - | - |
| Medium | 5 (3–7) | 0.96 | 0.86–1.06 | 0.405 | 1.02 | 0.93–1.11 | 0.714 |
| Large | 5 (3–7) | 1.03 | 0.93–1.14 | 0.605 | 1.07 | 0.98–1.17 | 0.134 |
| ZIP code median income quartile | |||||||
| First (poorest) | 5 (4–8) | 1.00 | - | - | 1.00 | - | - |
| Second | 5 (3–7) | 0.94 | 0.87–1.02 | 0.164 | 1.01 | 0.94–1.10 | 0.732 |
| Third | 5 (3–7) | 0.88 | 0.81–0.96 | 0.003 | 0.97 | 0.89–1.05 | 0.448 |
| Fourth (wealthiest) | 5 (3–6) | 0.84 | 0.78–0.90 | <0.001 | 0.99 | 0.91–1.07 | 0.805 |
Analysis is adjusted for age, sex, insurance, hospital location/teaching status, hospital region, KID year, CVC placement, debridement procedure, complex chronic condition, weekend admission, discharge quarter, hospital size, and ZIP code median income quartile.
Abbreviations: aIRR – adjusted incidence rate ratio; CVC – central venous catheter; IQR – interquartile range; IRR – incidence rate ratio; LOS – length of stay; ZIP code - Zone Improvement Plan code; 95%CI – 95% confidence interval
Online figure 3.
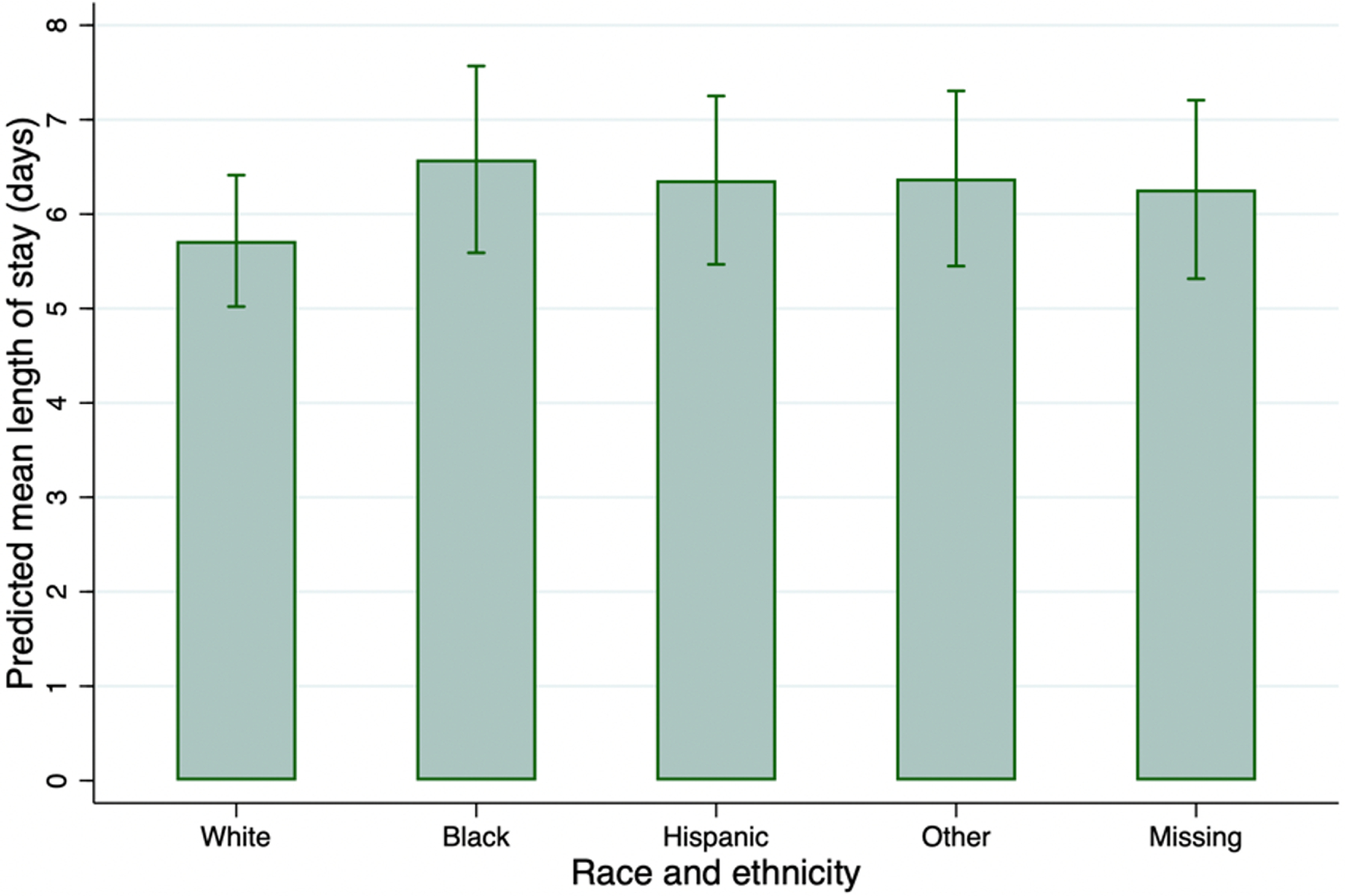
Predicted mean LOS by race and ethnicity, based on results from the main multivariable regression model.
Prolonged LOS
In terms of secondary outcomes, we found that the odds of prolonged LOS (exceeding 7 days) was significantly higher for Black children (aOR 1.45 [95%CI 1.01–2.10], P=0.046) and children of other race and ethnicity (aOR 1.56 [95%CI 1.05–2.31], P=0.029), compared to White children, adjusting for CVC placement, debridement, medical complexity, demographic, and hospital variables. Hispanic ethnicity (aOR 1.25 [95%CI 0.90–1.70], P=0.192) and missing race/ethnicity (aOR 1.30 [95%CI 0.91–1.89], P=0.144) were not significantly associated with prolonged LOS.
Analysis of CVC placement and debridement
In a multivariable model, no race or ethnicity category was associated with increased odds of having a CVC placed (online table 4). There were no differences by race and ethnicity on LOS among patients who did not have a CVC (online table 5). However, Black race was associated with prolonged LOS among children with a CVC (P=0.029) (online table 5).
Online table 4.
Adjusted odds of CVC placement (not adjusted for debridement procedures).*
| CVC placement (weighted n = 3277) | |||
|---|---|---|---|
| Race and ethnicity | aOR | 95%CI | P Value |
| White | 1.00 | - | - |
| Black | 0.97 | 0.70–1.35 | 0.857 |
| Hispanic | 1.14 | 0.84–1.55 | 0.387 |
| Other | 1.02 | 0.68–1.53 | 0.920 |
| Missing | 0.72 | 0.41–1.28 | 0.268 |
Analysis is adjusted for age, sex, insurance, hospital location/teaching status, hospital region, KID year, complex chronic condition, weekend admission, discharge quarter, hospital size, and ZIP code median income quartile.
Online table 5.
Adjusted incidence rate ratios for LOS, stratified by CVC vs. no CVC.*
| No CVC (weighted n = 2,401) | |||
|---|---|---|---|
| Race and ethnicity | aIRR | 95%CI | P Value |
| White | 1.00 | - | - |
| Black | 1.10 | 0.99–1.24 | 0.088 |
| Hispanic | 1.07 | 0.96–1.20 | 0.211 |
| Other | 1.04 | 0.94–1.15 | 0.396 |
| Missing | 1.06 | 0.93–1.21 | 0.369 |
| CVC (weighted n = 838) | |||
| Race and ethnicity | aIRR | 95%CI | P Value |
| White | 1.00 | - | - |
| Black | 1.29 | 1.03–1.63 | 0.029 |
| Hispanic | 1.20 | 0.99–1.45 | 0.064 |
| Other | 1.31 | 1.00–1.71 | 0.052 |
| Missing | 1.24 | 0.91–1/68 | 0.172 |
Analysis is adjusted for age, sex, insurance, hospital location/teaching status, hospital region, KID year, debridement procedure, complex chronic condition, weekend admission, discharge quarter, hospital size, and ZIP code median income quartile.
Abbreviations: aIRR – adjusted incidence rate ratio; CVC – central venous catheter; LOS – length of stay; 95%CI – 95% confidence interval
Hispanic ethnicity (aOR 0.69 [95%CI 0.52–0.91], P=0.008) and missing race/ethnicity (aOR 0.76 [95%CI 0.59–0.98], P=0.035) were significantly associated with lower odds of receiving a debridement procedure (online table 6). Among those patients who did undergo debridement, Black race was significantly associated with increased LOS (P=0.022) (online table 7). However, among patients who did not undergo debridement, Hispanic ethnicity and other race and ethnicity were significantly associated with increased LOS (P=0.012 and P=0.010, respectively) (online table 7).
Online table 6.
Adjusted odds of debridement (not adjusted for CVC placement).*
| Debridement procedure (weighted n = 3277) | |||
|---|---|---|---|
| Race and ethnicity | aOR | 95%CI | P Value |
| White | 1.00 | - | - |
| Black | 1.11 | 0.84–1.47 | 0.461 |
| Hispanic | 0.69 | 0.52–0.91 | 0.008 |
| Other | 1.00 | 0.73–1.37 | 0.999 |
| Missing | 0.76 | 0.59–0.98 | 0.035 |
Analysis is adjusted for age, sex, insurance, hospital location/teaching status, hospital region, KID year, complex chronic condition, weekend admission, discharge quarter, hospital size, and ZIP code median income quartile.
Online table 7.
Adjusted incidence rate ratios for LOS, stratified by debridement vs. no debridement.*
| No debridement (Weighted n = 1,268) | |||
|---|---|---|---|
| Race and ethnicity | aIRR | 95%CI | P Value |
| White | 1.00 | - | - |
| Black | 1.05 | 0.88–1.24 | 0.594 |
| Hispanic | 1.19 | 1.04–1.36 | 0.012 |
| Other | 1.27 | 1.06–1.51 | 0.010 |
| Missing | 1.05 | 0.87–1.27 | 0.588 |
| Debridement (Weighted n = 2,009) | |||
| Race and ethnicity | aIRR | 95%CI | P Value |
| White | 1.00 | - | - |
| Black | 1.20 | 1.03–1.41 | 0.022 |
| Hispanic | 1.06 | 0.92–1.23 | 0.398 |
| Other | 1.06 | 0.92–1.21 | 0.410 |
| Missing | 1.11 | 0.95–1.30 | 0.181 |
Analysis is adjusted for age, sex, insurance, hospital location/teaching status, hospital region, KID year, debridement procedure, complex chronic condition, weekend admission, discharge quarter, hospital size, and ZIP code median income quartile.
Abbreviations: aIRR – adjusted incidence rate ratio; CVC – central venous catheter; LOS – length of stay; 95%CI – 95% confidence interval
Analysis of time to debridement
In a model of LOS that only included patients who underwent debridement, increased time to procedure was significantly associated with increased LOS (IRR 1.10 per 1-day increase in time to debridement [95%CI 1.09–1.12], P<0.001). In an adjusted analysis, no race or ethnicity category was significantly associated with time to first debridement (data not shown).
Sensitivity analysis
Because 8.6% of our sample had missing race/ethnicity data, we conducted a complete case analysis in which patients with missing race and ethnicity were excluded, as well as multiple sensitivity analysis in which patients in the “missing” race and ethnicity category were assigned to the other categories. Results of these sensitivity analyses produced similar effect sizes and significance levels as the main analysis (results not shown). A sensitivity analysis that included patients with sickle cell disease also produced similar effect sizes and significance levels as the main analysis (results not shown).
Discussion
In this analysis of hospital discharges of children with acute osteomyelitis in the US, we found significant differences in LOS based on race and ethnicity. Our results were largely driven by differences in children requiring prolonged LOS: the odds of a Black child being in the highest LOS quartile were 46% higher than a White child. Our results suggest that while LOS is similar for most patients with acute osteomyelitis, there are marked differences in the subset of patients who experience prolonged LOS, likely driving the population-level effects observed. Our results demonstrate that Black children, Hispanic children, and children of other race and ethnicity experience approximately 1-day increased LOS compared to White children with acute osteomyelitis—counteracting the reduction in LOS achieved through biomedical interventions, such as early transitions to oral antibiotics.
The role of LOS in equity-oriented osteomyelitis outcomes research is complex. The population-level associations we identified indicate that underlying social drivers—namely, structural racism—directly or indirectly affect management of patients with acute osteomyelitis. Additional days spent in the hospital may also lead to disparate social and economic consequences for families. However, for many patients with acute osteomyelitis, a longer LOS may be medically appropriate to ensure safe discharge and may represent an “equitable” outcome. We were unable to measure variables that could have yielded insights into need for longer or shorter hospitalization at the individual patient level, such as illness severity on admission or discharge case management complexity. Ultimately, addressing the underlying social drivers of LOS disparities, rather than simply focusing on reducing LOS, should be the focus of equity-oriented research in this field.
Several mechanisms could underlie the observed disparities, including but not limited to (1) access to care, (2) engagement with care teams during hospitalization, and/or (3) discharge co-ordination and preparation. McKay and Parente developed a framework to describe pathways leading to disparities in health outcomes among hospitalized children. This framework posits that race and ethnicity intersect with both social and medical determinants to influence pre-hospitalization access to care, in-hospital family-centeredness and engagement with care teams, and peri-discharge instruction and preparation—all of which contribute to adverse hospitalization-related outcomes such as LOS.21 Differential and delayed access to primary care, urgent care, or emergency care services could lead to delayed diagnosis and more severe disease at time of admission. Delayed access to care leading to increased disease severity at presentation has been hypothesized to link socioeconomic vulnerability to increased LOS for other pediatric conditions, such as bronchiolitis.22 Notably, while a prior study of musculoskeletal infections among children at a single pediatric center (N=173) did not identify differences in disease severity at presentation by race or ethnicity,23 differences in disease severity at presentation leading to differences in LOS could have been amplified in our larger, national dataset. The KID database does not include granular clinical data to characterize severity (e.g. laboratory or imaging markers). CVC placement and debridement procedures, which were both associated with increased LOS, may reflect severity at presentation, though prior research also suggests that institutional norms as well as biomedical indications of disease severity affect decisions to pursue procedures for children with acute hematogenous osteomyelitis.24 A study of children enrolled in a US military insurance program found that Black children were more likely than White children to undergo surgical procedures for osteomyelitis,25 though this was not the case in our sample. Meanwhile, Hispanic patients and patients with missing race and ethnicity were less likely to undergo debridement in our study. Furthermore, we did not identify differences in time to debridement, suggesting that delays in surgical management did not account for observed differences in LOS. Taken together, these findings indicate that further research is needed to determine if observed differences in LOS by race and ethnicity are attributable to delayed access to initial care and more severe illness at presentation.
During the hospitalization, medical complexity and disparities in post-operative complications could have contributed to observed differences in LOS. Complex conditions were more prevalent in Black than White children in our sample, and presence of a pre-existing complex condition was associated with increased LOS. Although we adjusted for a validated marker of medical complexity, unmeasured (i.e. unbilled) medical complexity may have contributed to observed differences in LOS. Differing prevalence of complex conditions has been cited as a potential driver in disparities by race and ethnicity in sepsis outcomes in the KID database.14 In addition, prior research has found disparities in rates of post-operative complications between Black and White children.26 One aspect of our analysis that supports this hypothesis is the finding that LOS was significantly longer among Black children who underwent debridement procedures.
Differences in discharge planning may also have affected LOS. Discharges may have been delayed for families with limited resources or inadequate access to care, who may have experienced difficulties arranging home IV therapies and subspecialty follow-up. Racial and ethnic disparities in access to paid family leave may similarly have affected logistics of safe home discharges.27 We found that Black children with CVCs had significantly increased LOS, potentially attributable to differences in discharge planning needed to safely send children home with intravenous antibiotics. Black children without CVCs did not have significantly increased LOS compared to White children without CVCs; although there was a non-significant trend towards increased LOS in this group, this contrasting finding may suggest discharge factors as a driver of race and ethnicity differences in this study population. In addition, differences in discharge preparation may be exacerbated by cultural or language barriers and could lead to prolonged LOS and/or hospital readmission.21 We were unable to measure readmission rates in our study, though this outcome could be particularly revealing of differences in care at time of discharge.
Factors in addition to race and ethnicity were associated with increased LOS in our study. First, Medicaid/self-pay was associated with increased LOS compared to those with private insurance. In our study, insurance status may have acted both as a specific driver of prolonged hospitalization (e.g. by complicating discharge planning), and as a proxy for socioeconomic status. Second, as expected, children with complex medical conditions have increased LOS. Third, our finding of increased LOS in urban hospitals and teaching hospitals is consistent with those hospitals likely being referral centers for sicker patients. Our results point to the need to understand variability in LOS within different hospital settings.
Our study has several limitations. First, as a cross-sectional study, one cannot infer causality from the associations detected. Second, the KID does not include detailed clinical information that may be relevant to understanding LOS in children with acute osteomyelitis, such as location of infection or causative pathogen. Third, administrative databases are susceptible to billing coding errors and duplications. We applied stringent exclusion criteria to narrow our sample to those children with acute osteomyelitis, and our patient population likely excludes some patients with acute osteomyelitis whose billing information did not match our inclusion criteria. Fourth, race and ethnicity data are provided in the KID in aggregate, and we were not able to account for independent effects of race and ethnicity in children with multiple racial and ethnic identities. Fifth, the KID does not provide patient-level identifiable information, and so we were unable to distinguish initial admissions from re-admissions in our study. Sixth, we excluded patients who were transferred into or out of the hospital, because these transfers would introduce inaccuracies in measurement of LOS. However, children requiring hospital transfer may themselves represent unique social and medical risk groups—for example, patients living in rural areas with severe disease requiring transfer to urban tertiary hospitals may be at risk for increased LOS. Seventh, KID does not include data on need for intensive care unit stay, or data on readmission rates, both of which could provide additional insights into disparities in osteomyelitis outcomes in future analyses with other datasets. Finally, although we adjusted for several sociodemographic factors, we could not adjust for factors such as household income, parental education, or parental employment, which can influence both severity of illness and discharge planning. These factors are also functions of social drivers of health and structural racism.
In conclusion, we identified disparities in LOS by race and ethnicity among children hospitalized with acute osteomyelitis in the United States, as well as disparities in prolonged LOS. Accounting for available covariates, Black children stayed in the hospital approximately 1 day longer than White children—counteracting 1-day reductions in LOS achieved through earlier transitions to oral antibiotics. Targeting social drivers of health, and specifically the possible effects of structural racism on illness and access to care, offers similar potential in reducing LOS as advancing biomedical interventions to improve quality—and equity—of care for children with acute osteomyelitis.
Acknowledgements:
We would like to acknowledge the Boston Children’s Hospital Office of Health Equity and Inclusion for their insights into study design and result interpretation and Dr. Michael Monuteaux and Dr. John Kraemer for their statistical expertise. All authors and acknowledged contributors declare no conflicts of interest.
Funding/Support:
Dr. Campbell and Dr. Allende-Richter were supported by Agency for Healthcare Research and Quality grant number T32 HS000063 as part of the Harvard-wide Pediatric Health Services Research Fellowship Program. J.I.C. and S.A.-R. were supported by the Agency for Healthcare Research and Quality grant number T32HS000063 as part of the Harvard-wide Pediatric Health Services Research Fellowship Program. The funders/sponsors did not participate in the work.
Role of Funder/Sponsor:
The funders/sponsors did not participate in the work.
Abbreviations:
- aIRR
adjusted incident rate ratio
- aOR
adjusted odds ratio
- CI
confidence interval
- CVC
central venous catheter
- ICD-10
International Classification of Diseases, version 10
- ICD-10-PR
International Classification of Diseases, version 10, procedures
- IQR
interquartile range
- IRR
incident rate ratio
- IV
intravenous
- KID
Kids’ Inpatient Database
- LOS
length of stay
- ZIP
Zone Improvement Plan
Footnotes
Conflict of interest disclosures: The authors have no conflicts of interest to disclose.
Prior presentation of data: Results from this study will be presented as an oral abstract at IDWeek 2022 (October 19–23, 2022; Washington, DC)
Contributor Information
Jeffrey I. Campbell, Division of Infectious Diseases, Boston Children’s Hospital, Boston, Massachusetts, USA.
Kristen H. Shanahan, Division of Emergency Medicine, Boston Children’s Hospital, Boston, USA.
Melissa Bartick, Department of Medicine, Mount Auburn Hospital, Cambridge, USA.
Mohsin Ali, Division of Infectious Diseases, The Hospital for Sick Children, Toronto, Canada.
Don Goldmann, Division of Infectious Diseases, Boston Children’s Hospital, Boston, Massachusetts, USA.
Nadia Shaikh, Department of Pediatrics, University of Illinois College of Medicine at Peoria, Peoria, USA.
Sophie Allende-Richter, Department of Pediatrics, Boston Children’s Hospital, Boston, USA.
Data statement:
Data are publicly available from the Agency for Healthcare Research and Quality.
References
- [1].Safdieh G, Silberman J, Nguyen J, Doyle SM, Blanco JS, Scher DM, et al. Pediatric Septic Arthritis and Osteomyelitis in the USA: A National KID Database Analysis. HSS J. 2019;15:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shaikh N, Umscheid J, Rizvi S, Bhatt P, Vasudeva R, Yagnik P, et al. National Trends of Acute Osteomyelitis and Peripherally Inserted Central Catheters in Children. Hosp Pediatr. 2021;11:662–70. [DOI] [PubMed] [Google Scholar]
- [3].Stockmann C, Ampofo K, Pavia AT, Byington CL, Blaschke AJ, Sherwin CM, et al. National trends in the incidence, outcomes and charges of pediatric osteoarticular infections, 1997–2012. Pediatr Infect Dis J. 2015;34:672–4. [DOI] [PubMed] [Google Scholar]
- [4].Truelove JJ, House SA. Reducing PICC Placement in Pediatric Osteomyelitis: A Diamond in the Deimplementation Rough? Hosp Pediatr. 2021;11:e111–e4. [DOI] [PubMed] [Google Scholar]
- [5].Spruiell MD, Searns JB, Heare TC, Roberts JL, Wylie E, Pyle L, et al. Clinical Care Guideline for Improving Pediatric Acute Musculoskeletal Infection Outcomes. J Pediatric Infect Dis Soc. 2017;6:e86–e93. [DOI] [PubMed] [Google Scholar]
- [6].Paakkonen M, Kallio MJ, Kallio PE, Peltola H. Shortened hospital stay for childhood bone and joint infections: analysis of 265 prospectively collected culture-positive cases in 1983–2005. Scand J Infect Dis. 2012;44:683–8. [DOI] [PubMed] [Google Scholar]
- [7].Woods CR, Bradley JS, Chatterjee A, Copley LA, Robinson J, Kronman MP, et al. Clinical Practice Guideline by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America: 2021 Guideline on Diagnosis and Management of Acute Hematogenous Osteomyelitis in Pediatrics. J Pediatric Infect Dis Soc. 2021;10:801–44. [DOI] [PubMed] [Google Scholar]
- [8].Keren R, Shah SS, Srivastava R, Rangel S, Bendel-Stenzel M, Harik N, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169:120–8. [DOI] [PubMed] [Google Scholar]
- [9].Okubo Y, Nochioka K, Testa M. Nationwide survey of pediatric acute osteomyelitis in the USA. J Pediatr Orthop B. 2017;26:501–6. [DOI] [PubMed] [Google Scholar]
- [10].Callinan L, Holman RC, Esposito DH, McDonald M. Racial/Ethnic Disparities in Infectious Disease Hospitalizations in Arizona. Journal of health disparities research and practice. 2013;6:4. [Google Scholar]
- [11].Freedman J, Guller U, Benjamin DK, Higgins LD, Pan D, Cook C, et al. National trends in health care utilization and racial and socioeconomic disparities in pediatric pyogenic arthritis. J Pediatr Orthop. 2006;26:709–15. [DOI] [PubMed] [Google Scholar]
- [12].Braveman P What are health disparities and health equity? We need to be clear. Public Health Rep. 2014;129 Suppl 2:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7. [DOI] [PubMed] [Google Scholar]
- [14].Mitchell HK, Reddy A, Montoya-Williams D, Harhay M, Fowler JC, Yehya N. Hospital outcomes for children with severe sepsis in the USA by race or ethnicity and insurance status: a population-based, retrospective cohort study. The Lancet Child & Adolescent Health. 2021;5:103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lindell RB, Nishisaki A, Weiss SL, Balamuth F, Traynor DM, Chilutti MR, et al. Comparison of Methods for Identification of Pediatric Severe Sepsis and Septic Shock in the Virtual Pediatric Systems Database. Critical care medicine. 2019;47:e129–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pati S, Lorch SA, Lee GE, Sheffler-Collins S, Shah SS. Health insurance and length of stay for children hospitalized with community-acquired pneumonia. J Hosp Med. 2012;7:304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].von Heideken J, Bennet R, Eriksson M, Hertting O. A 10-year retrospective survey of acute childhood osteomyelitis in Stockholm, Sweden. J Paediatr Child Health. 2020;56:1912–7. [DOI] [PubMed] [Google Scholar]
- [19].Shaaban AN, Peleteiro B, Martins MRO. Statistical models for analyzing count data: predictors of length of stay among HIV patients in Portugal using a multilevel model. BMC health services research. 2021;21:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Carter EM, Potts HW. Predicting length of stay from an electronic patient record system: a primary total knee replacement example. BMC medical informatics and decision making. 2014;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McKay S, Parente V. Health Disparities in the Hospitalized Child. Hosp Pediatr. 2019;9:317–25. [DOI] [PubMed] [Google Scholar]
- [22].Slain KN, Shein SL, Stormorken AG, Broberg MCG, Rotta AT. Outcomes of Children With Critical Bronchiolitis Living in Poor Communities. Clin Pediatr (Phila). 2018;57:1027–32. [DOI] [PubMed] [Google Scholar]
- [23].Rocha JL. Are There Disparities in Community-Acquired Pediatric Musculoskeletal S. Aureus Infections? 2014 AAP National Conference and Exhibition: American Academy of Pediatrics; 2014. [Google Scholar]
- [24].Upasani VV, Burns JD, Bastrom TP, Baldwin KD, Schoenecker JG, Shore BJ, et al. Practice Variation in the Surgical Management of Children With Acute Hematogenous Osteomyelitis. J Pediatr Orthop. 2022;42:e520–e5. [DOI] [PubMed] [Google Scholar]
- [25].Young JD, Dee EC, Levine A, Sturgeon DJ, Koehlmoos TP, Schoenfeld AJ. Does Universal Insurance and Access to Care Influence Disparities in Outcomes for Pediatric Patients with Osteomyelitis? Clin Orthop Relat Res. 2020;478:1432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nafiu OO, Mpody C, Kim SS, Uffman JC, Tobias JD. Race, Postoperative Complications, and Death in Apparently Healthy Children. Pediatrics. 2020;146. [DOI] [PubMed] [Google Scholar]
- [27].Bartel AP, Kim S, Nam J. Racial and ethnic disparities in access to and use of paid family and medical leave: evidence from four nationally representative datasets. Monthly Lab Rev. 2019;142:1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are publicly available from the Agency for Healthcare Research and Quality.


